Abstract
The chromosomal translocation t(4;11)(q21;q23), a hallmark of an aggressive form of acute lymphoblastic leukemia (ALL), encodes mixed‐lineage leukemia (MLL)‐AF4 oncogenic chimera that triggers aberrant transcription of genes involved in lymphocyte differentiation, including HOXA9 and MEIS1. The scaffold protein 14‐3‐3θ, which promotes the binding of MLL‐AF4 to the HOXA9 promoter, is a target of MiR‐27a, a tumor suppressor in different human leukemia cell types. We herein study the role of MiR‐27a in the pathogenesis of t(4;11) ALL. Reverse transcription quantitative PCR (qPCR) reveals that MiR‐27a and 14‐3‐3θ expression is inversely correlated in t(4;11) ALL cell lines; interestingly, MiR‐27a relative expression is significantly lower in patients affected by t(4;11) ALL than in patients affected by the less severe t(12;21) leukemia. In t(4;11) leukemia cells, ectopic expression of MiR‐27a decreases protein level of 14‐3‐3θ and of the key transcription factor RUNX1. We show for the first time that MiR‐27a also targets AF4 and MLL‐AF4; in agreement, MiR‐27a overexpression strongly reduces AF4 and MLL‐AF4 protein levels in RS4;11 cells. Consequent to AF4 and MLL‐AF4 downregulation, MiR‐27a overexpression negatively affects transcription of HOXA9 and MEIS1 in different t(4;11) leukemia cell lines. In agreement, we show through chromatin immunoprecipitation experiments that MiR‐27a overexpression impairs the binding of MLL‐AF4 to the HOXA9 promoter. Lastly, we found that MiR‐27a overexpression decreases viability, proliferation, and clonogenicity of t(4;11) cells, whereas it enhances their apoptotic rate. Overall, our study identifies the first microRNAthat strikes in one hit four crucial drivers of blast transformation in t(4;11) leukemia. Therefore, MiR‐27a emerges as a new promising therapeutic target for this aggressive and poorly curable form of leukemia.
Keywords: 14‐3‐3θ, AF4, microRNA, MiR‐27a, MLL, t(4;11) acute leukemia, target therapy
Significance statement
A growing body of evidence indicates that microRNAs targeting genes involved in modulating hematopoietic process are dysregulated t(4;11) acute leukemia, a very aggressive and refractory hematologic cancer. Here, we show that MiR‐27a is downregulated both in t(4;11) leukemia cell lines and primary patient samples. We demonstrate that MiR‐27a targets four crucial drivers of blast transformation, namely, 14‐3‐3θ, RUNX1, AF4, and mixed‐lineage leukemia (MLL)‐AF4 in t(4;11) leukemia cells. Thus, MiR‐27a, by striking these crucial oncogenes, functions as a tumor suppressor in t(4;11) leukemia and emerges as a new potential target for the therapy of this still poorly treatable form of leukemia.
1. INTRODUCTION
Genomic rearrangements involving the mixed lineage leukemia (MLL aka KMT2A) gene cause 5%–10% of childhood acute lymphoblastic leukemia (ALL) cases. 1 The translocation t(4;11)(q21;q23) connects the MLL with the AFF1 gene and produces a fusion transcript that encodes the MLL‐AF4 oncogenic chimera. It has been found in about 50% of affected infants with MLL rearrangements and gives rise to an aggressive leukemia form, characterized by early relapse and dismal prognosis. 1 , 2 , 3
The AFF1 gene encodes AF4, a member of the AF4 protein family. The AF4 family includes AF5q31 and LAF4, which also are among the MLL fusion partners in leukemia cases. 2 , 3 Notably, the AF4 family members interact with the ENL family members and with the P‐TEFb elongation factor and form the AEP complex. 3 The MLL‐AF4 oncoprotein constitutively recruits the AEP complex and aberrantly activates transcription of key genes involved in lymphocyte differentiation, including the homeobox A (HOXA) cluster genes and MEIS1. 4 , 5 , 6 , 7 , 8 Recruitment of AEP complex on target genes triggers the aberrant activity of the histone H3 lysine‐79 (H3K79) methyltransferase DOT1L, which tags actively transcribed genes with the epigenetic signature H3K79me2, H3K27ac, and H3K4me3, which are a hallmark of MLL‐AF4 target genes. 7 , 9 Ultimately, the oncogenic potential of MLL‐AF4 is mostly driven by the interaction with AF4 and its protein partners.
Among the numerous proteins belonging to the AF4 interactome, there is the scaffold protein 14‐3‐3θ, which affects MLL‐AF4 aberrant activity through a recently elucidated regulatory mechanism. 10 , 11 In particular, through the direct interaction with AF4, 14‐3‐3θ acts as an oncogene with an important role in the MLL‐AF4 leukemic transformation. Notably, 14‐3‐3θ knockdown decreases MLL‐AF4 target gene expression, induces apoptosis, and hampers proliferation of t(4;11) leukemia cells. 11
A negative regulator of 14‐3‐3θ is MiR‐27a. 12 MicroRNAs (MiRs) are evolutionary conserved, small noncoding RNA molecules that posttranscriptionally regulate gene expression by specific binding to 3′‐untranslated region (UTR) of their targets. Increasing evidence indicates that MiR‐27a plays a key role in tumor biology, including tumorigenesis, proliferation, apoptosis, invasion, migration, and angiogenesis. Moreover, MiR‐27a has clinical significance in drug sensitivity, treatment of cancer, and patient prognosis. 12 , 13 , 14 , 15 Various studies reveal that MiR‐27a is significantly dysregulated in different cancers. In non‐small‐cell lung cancer, liver cancer, colon cancer, and prostate cancer, it acts as an oncogene, whereas it is a tumor suppressor in gastric cancer, bladder cancer, and esophageal squamous cell, as well as in leukemia. 12 , 13 , 14 , 15 In particular, MiR‐27a expression is downregulated in several pre‐B‐ and T‐ALL cell lines, where it functions as a tumor suppressor also through the negative regulation of 14‐3‐3θ. Moreover, MiR‐27a levels are low in primary patient samples, and its cellular replacement results in decreased cell growth and increased cell death. 12
On this basis, we herein analyze the role of MiR‐27a in the molecular pathogenesis of the rare and poorly curable t(4;11) leukemia.
2. MATERIALS AND METHODS
2.1. Antibodies
Anti‐14‐3‐3θ, sc‐632; anti‐α‐tubulin, sc‐5286; anti‐vinculin, sc‐25336 (Santa Cruz Biotechnology); anti‐AF4, #A302‐344A (Bethyl Laboratories); anti‐MLLN, #05‐764; anti‐MLLC, #05‐765 (Merck Millipore); anti‐RUNX1, #4336 (Cell Signaling Technology); horseradish peroxidase‐conjugated anti‐mouse (#NA931) and anti‐rabbit (#NA934) IgG secondary antibodies (GE Healthcare).
2.2. Cell lines
Leukemia cell lines were obtained from the Cell Culture Facility of CEINGE—Advanced Biotechnologies and from IRCCS SDN (Naples, Italy). RS4;11 ALL cells were grown in minimum essential medium (MEM) (Sigma‐Aldrich), supplemented with 10% fetal bovine serum (FBS) (Lonza); SEM ALL cells in Iscove's modified Dulbecco's medium (Lonza) supplemented with 10% FBS; MV4‐11 acute monocytic leukemia (AML), 697 ALL, REH B‐cell precursor leukemia, and HL‐60 AML cells in RPMI supplemented with 20% FBS and 10 ml/L penicillin/streptomycin (Sigma‐Aldrich). RS4;11, SEM, and MV4‐11 harbor the t(4;11) chromosomal rearrangement. REH harbors the t(12;21) chromosomal rearrangement.
2.3. Molecular cloning and site‐directed mutagenesis
The AFF1‐3′‐UTR was amplified from genomic DNA in three overlapping fragments, by using specific primer pairs (Supporting Information: Figure S1 and Table S1). Primers used to amplify the central fragment of AFF1‐3′‐UTR (584 bp) are as follows: F—XhoI: 5′‐GCCGC/TCGAGTTCCCAAAGGCAAAATCTGT‐3′ and R—NotI: 5′‐ GCCGGC/GGCCGCATAAGTGCGGTCCAATCTGT‐3′. Amplified fragments were cloned in psiCHECK2™ vector (#C8021; Promega) after digestion with SgF1 #R7103 (Promega) or XhoI #10703770001, and NotI #11014714001 (Roche). Mutant psiCHECK2‐AFF1‐3′‐UTR construct was obtained by QuickChange Mutagenesis Kit (#200518; Agilent) and primers: forward, 5′‐GTGTTTAATGTTTCTGTCCTTTATCTGTATTATTGAATTTAAGAGCCCTGC‐3′ and reverse, 5′‐GCAGGGCTCTTAAATTCAATAATACAGATAAAGGACAGAAACATTAAACAC‐3′.
2.4. Cell transfection
Hsa‐pre‐miR‐27a‐3p‐ (#AM17100, assay ID PM10939; Ambion) or has‐pre‐miR‐negative control (#AM17110; Ambion), and hsa‐anti‐miR‐27a‐3p‐ (#AM17000, assay ID AM10939; Ambion) or has‐anti‐miR‐negative control (#AM17010; Ambion) was added to 1 × 107 cells at a final concentration of 50 nM in 0.1 ml of opti‐MEM (Thermo Fisher Scientific Inc.). Cells were transfected by electroporation.
2.5. RNA isolation, reverse transcription, and quantitative PCR
Total RNA from the bone marrow of nine patients affected by t(4;11) ALL and seven patients with t(12;21) ALL was obtained from the CEINGE Biobank. All patients signed informed consent and agreed to use their samples for scientific research purposes, in an anonymous form. The Ethics Committee of the University of Naples Federico II approved the study (protocol code 77/21, March 26, 2021).
Total RNA was extracted with an RNeasy Mini Kit (ID: 74104; Qiagen). MiR‐27a was reverse‐transcribed (RT) with a small RNA–specific primer and TaqMan MicroRNA Reverse Transcription Kit (#4366596, Thermo Fisher Scientific Inc.). Complementary DNA (cDNA) was synthesized with SuperScript™III First‐Strand Synthesis System (#12574; Thermo Fisher Scientific Inc.). 16
MiR‐27a quantitative PCR (qPCR) was performed in TaqMan® Universal Master Mix II with 1 µl of TaqMan probe (TaqMan® MicroRNA Assays; Thermo Fisher Scientific Inc.), specific for MiR‐27a (#4427975, assay ID 000408) or for U18 small nucleolar RNA (#4351372, assay ID Hs06637271_g1). MiR‐27a relative expression was normalized to U18. qPCR of gene‐specific cDNA was performed with iQ™ SYBR® Green Supermix (#1708880, Bio‐Rad Laboratories s.r.l.) and gene‐specific primer pairs. 11 , 17 Relative gene expression was normalized to POLR2A and ACTB. Data were analyzed with methods.
For patients' data analysis, relative quantification of normalized gene expression was determined using the Bio‐Rad CFX Manager Gene Expression tool (Bio‐Rad Laboratories); machinery tool information: normalized expression mode → , without the control sample. 18 , 19 Relative gene expression was normalized to POLR2A and ACTB. Graphically reported values were averages from at least three independent experiments.
2.6. Western blot analysis
Whole protein extracts (WCEs) were prepared from leukemia cells incubated in the lysis buffer (50 mM Tris/HCl pH 8, 150 mM NaCl, 0.5% NP‐40, 2 mM EDTA pH 8, 1 mM Na3VO4, 10 mM NaF), and 0.1% (v/v) protease inhibitor cocktail (Sigma‐Aldrich). WCEs were clarified at 16,000g and protein concentration was measured by Bio‐Rad protein assay (#5000001; Bio‐Rad Laboratories). Forty micrograms of WCEs were loaded, resolved on 8% or 10% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis, and transferred onto the nitrocellulose membrane. Membranes were incubated with appropriate primary and secondary antibodies, and protein signals were detected with the ECL Plus detection system (#RPN2132; GE Healthcare) and quantified by a scanner equipped with a sun spark classic densitometric workstation. 20 , 21
2.7. Bioinformatic analysis
MiRNA putative seed sequence was predicted by using TargetScan, 22 microRNA.org (www.microrna.org), DIANA Tools' softwares (http://carolina.imis.athena-innovation.gr).
2.8. Luciferase reporter assay
RS4;11 cells (1 × 107) were cotransfected with pre‐MiR‐27a and the psiCHECK2‐AFF1‐3′‐UTR constructs (each with the appropriate controls). After 24 h, cells were harvested and assays were performed using the Dual‐Luciferase® Reporter (DLR) Assay System (#16185; Thermo Fisher Scientific Inc.).
2.9. Chromatin immunoprecipitation (ChIP)
RS4;11 cells (30 × 106) were transfected with pre‐MiR‐27a or pre‐MiR‐negative control and, after 36 h, fixed in optiMEM containing 1% formaldehyde; the reaction was stopped by glycine quenching (125 mM). ChIP and DNA extraction, as well as qPCR, were performed as already described. 17 , 23 A pool of nonspecific IgG was used as ChIP‐negative control; ACTB gene promoter (ACTB pr) represented the negative control chromatin.
2.10. Cell viability assay
The proliferation of cells transfected with pre‐MiR‐27a or pre‐MiR‐negative control was assessed using the Cell Counting Kit 8 (CCK8) (#CK04‐11; Dojindo EU GmbH). Absorbance at 540 nm was measured 24, 48, and 72 h after transfection, for each analyzed cell line, using a Spectramax spectrophotometer (Molecular Devices). Cell viability was normalized versus nonelectroporated (untransfected) cells. The percentage of viable cells is expressed as:
2.11. 5‐Bromo‐2′‐deoxyuridine (BrdU) cell proliferation assay
Forty‐eight hours after transfection with pre‐MiR‐27a or pre‐MiR‐negative control, cells (1 × 106/ml) were treated with 1 mM BrdU, fixed, permeabilized, and stained with a fluorescein isothiocyanate (FITC)‐conjugated anti‐BrdU antibody and with 7‐aminoactinomycin D (#559619; BD Pharmingen). Cytofluorimetric analysis was carried out by FACS.
2.12. Apoptosis assay
Seventy‐two hours after transfection with MiR‐27a or pre‐MiR‐negative control, cells (4 × 106) were incubated in annexin binding buffer (10 mM HEPES, pH 7.4; 140 mM NaCl; 2.5 mM CaCl2) and centrifuged at 300g at 4°C for 10 min. A solution of Annexin V‐FITC (#130‐092‐052; Miltenyi Biotec) and propidium iodide (#556463, BD Pharmingen) was added. Positive staining was detected by FACS.
2.13. Colony formation assay
Twenty‐four hours after transfection with MiR‐27a or pre‐MiR‐negative control, cells (1.2 × 104) were seeded in MethoCult™ (#H4034; STEMCELL Technologies). After2 weeks, colony morphology and number were determined under an inverted microscope (Leica, Germany), with a ×20 objective.
2.14. Statistical analysis
All data are expressed as mean ± SD and are representative of three or more independent experiments. The data of repeated experiments were analyzed using one‐way two‐tailed paired Student's t‐test. p Values were presented as *<.05, **<.01, and ***<.001. Pearson's R value was calculated, for all the tested cell lines, to assess the correlation between MiR‐27a and 14‐3‐3θ transcript levels (Supplementary Figure S2)
3. RESULTS
3.1. MiR‐27a regulates the expression of 14‐3‐3θ and RUNX1 in t(4;11) leukemia cells
We performed RT‐qPCR analysis to compare transcript levels of MiR‐27a and 14‐3‐3θ in different ALL and AML cell lines, and in peripheral blood lymphocytes from healthy donors. We observed relatively low expression of MiR‐27a in the ALL cells (Figure 1A), and relatively high transcript levels of 14‐3‐3θ only in t(4;11) cells. In agreement, the value of Pearson's R = −.961 ± .032 indicated a strong inverse correlation between MiR‐27a and 14‐3‐3θ expression in t(4;11) cell lines (Supporting Information: Figure S2); notably, MiR‐27a expression was relatively higher in MV4‐11, the t(4;11) AML cell line, with respect to RS4;11 and SEM, which are t(4;11) ALL cell lines (Figure 1A).
Figure 1.
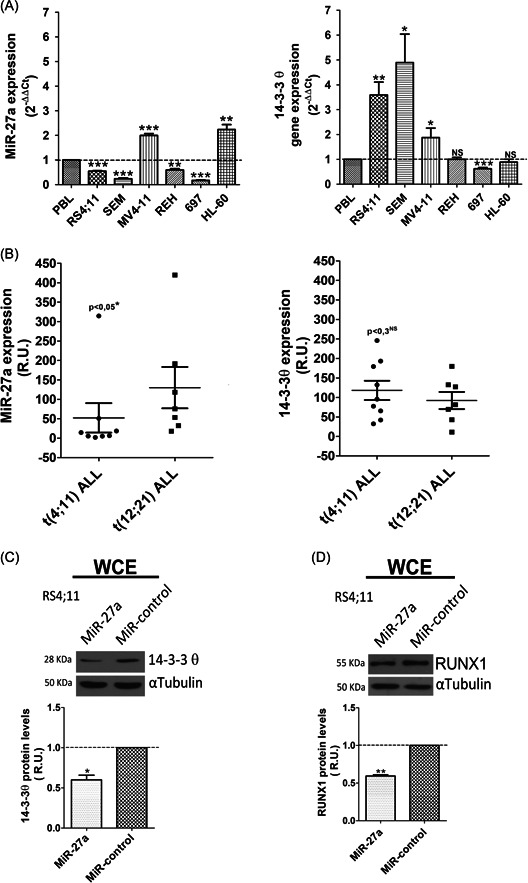
MiR‐27a affects the expression of 14‐3‐3θ and RUNX1 in t(4;11) leukemia cells. (A) MiR‐27a and 14‐3‐3θ transcript levels in different leukemia cell lines; PBLs are used as reference. Relative gene expression was determined by the method. (B) MiR‐27a and 14‐3‐3θ transcript level in t(4;11) versus t(12;21) ALL patients. Relative gene expression was determined by the method, without the control sample. Western blot analysis of (C) 14‐3‐3θ and (D) RUNX1 protein level in RS4;11 cells transfected with MiR‐27a or MiR‐negative control; αTubulin, loading control. Error bars indicate standard deviations of triplicate experiments. MiR, microRNA; NS, not statistically significant; PBL, peripheral blood leukocytes; R.U., relative units; WCE, whole‐cell extract. *p< .05; **p< .01; ***p < .005.
Low MiR‐27a expression levels correlate with poor prognosis and low relapse‐free survival in ALL patients. 24 Therefore, we evaluated MiR‐27a and 14‐3‐3θ transcript levels, by RT‐qPCR, in lymphocytes of patients with t(4;11) and of patients with t(12;21)(p13;q22) translocation, an indicator of good prognosis. 25 Notably, MiR‐27a levels were significantly lower in t(4;11) than in t(12;21) patients (Figure 1B, left). In contrast, although 14‐3‐3θ transcript levels were slightly higher in t(4;11) than in t(12;21) patients, the difference was not statistically significant (Figure 1B, right). Also, Pearson's R value did not confirm the inverse correlation between MiR‐27a and 14‐3‐3θ expression previously observed in t(4;11) leukemia cell lines, likely because other mechanisms can influence 14‐3‐3θ expression in the patients. However, despite interindividual variability and the relatively small number of patients, these overall results supported the relationship between low MiR‐27a expression and poor prognosis in t(4;11) ALL.
To test whether MiR‐27a actually targeted 14‐3‐3θ also in t(4:11) leukemia, we transiently transfected RS4;11 ALL cells with pre‐MiR‐27a or the pre‐MiR‐negative control and evaluated 14‐3‐3θ protein expression. Western blot analysis indicated that MiR‐27a overexpression (Supporting Information: Figure S3A) decreased the 14‐3‐3θ level (Figure 1C), thereby confirming the inverse relationship existing between 14‐3‐3θ and MiR‐27a in basal conditions. An additional known target of MiR‐27a with a key role in t(4;11) leukemia is the transcription factor RUNX1. 4 , 26 , 27 , 28 In agreement with and similar to 14‐3‐3θ, MiR‐27a overexpression significantly decreased the RUNX1 level (Figure 1D).
3.2. AF4 and MLL‐AF4 are novel targets of MiR‐27a
To look for new potential MiR‐27a targets involved in t(4;11) leukemia, we interrogated various miRNA prediction tools that detected a 7‐mer seed sequence in 3′‐UTR of the AFF1 gene, 2884–2890 nucleotides downstream of the stop codon. Since AFF1 and KMT2A/AFF1 share their 3′‐UTR, we considered also the fusion transcript a putative target of MiR‐27a.
We therefore evaluated AF4 and MLL‐AF4 protein levels in RS4;11 cells that expressed ectopic MiR‐27a and found a strong decrease in both AF4 and MLL‐AF4 protein amount (Figure 2A). In agreement, RS4;11 cells transfected with anti‐MiR‐27a, which binds MiR‐27a and inhibits its function, showed a twofold increase of AF4 and MLL‐AF4 protein amounts with respect to the control cells (Figure 2A).
Figure 2.
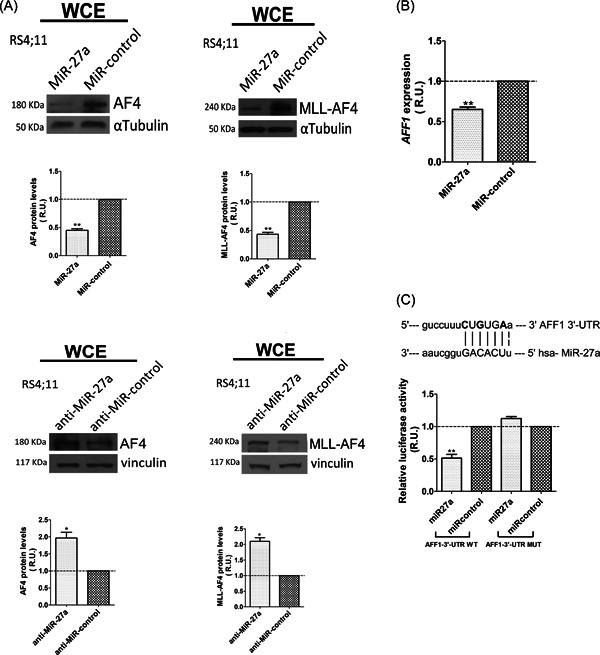
AF4 and MLL‐AF4 are novel targets of MiR‐27a. (A) Western blot analysis of AF4 and MLL‐AF4 levels in RS4;11 cells transfected with MiR‐27a and with MiR‐negative control; αTubulin, loading control (upper panel). Western blot analysis of AF4 and MLL‐AF4 levels in RS4;11 cells transfected with anti‐MiR‐27a and the anti‐MiR‐negative control; vinculin, loading control (lower panel). (B) AFF1 transcript levels were measured 24 h after cell transfection with MiR‐27a or MiR negative control. Relative gene expression was determined by the method. (C) Sequence alignment of MiR‐27a and its putative seed within AFF1‐3′‐UTR, and dual‐luciferase reporter assay results in RS4;11 cells cotransfected with MiR‐27a and the psiCHECK‐2 vector containing AFF1‐3′‐UTR WT or MUT, and the relative controls. In bold are the nucleotides mutated in the MUT construct. Error bars indicate standard deviations. miR, microRNA; MLL, mixed‐lineage leukemia; MUT, mutant; R.U., relative units; UTR, untranslated region; WCE, whole cell extract; WT, wild type. *p< .05; **p< .01.
Notably, MiR‐27a overexpression decreased the AFF1 transcript level by about 40%, thereby suggesting posttranscriptional repression of AFF1 through messenger RNA destabilization rather than translation inhibition (Figure 2B). Since AFF1 and KMT2A/AFF1 share their 3′‐UTR, we assumed MiR‐27a repressed MLL‐AF4 through the same mechanism.
To functionally identify MiR‐27a seeds within the AFF1 transcript, as previously reported for RUNX1 and 14‐3‐3θ, we performed DLR assays. 12 , 26 Three different constructs, covering the whole AFF1‐3′‐UTR (Supporting Information: Figure S1 and Table S1), were cotransfected with pre‐miR‐27a or pre‐MiR‐negative control in RS4;11 cells. Construct 2‐AFF1‐3′‐UTR, which contained the predicted 7‐mer seed identified by bioinformatic analysis, caused a significant decrement in Renilla activity (Supporting Information: Figure S4). Therefore, we subcloned a 584‐bp‐long fragment (AFF1‐3′‐UTR wild type [WT]) that contained this 7‐mer seed and generated the corresponding negative control, AFF1‐3′‐UTR mutant (MUT), by site‐directed mutagenesis (Figure 2C). AFF1‐3′‐UTR WT transfection decreased Renilla activity by about 50%, whereas no effect was observed with AFF1‐3′‐UTR MUT (Figure 2C).
3.3. MiR‐27a affects MLL‐AF4 chimera's activity in t(4;11) cell lines
14‐3‐3θ, RUNX1, AF4, and MLL‐AF4 have a crucial role in t(4;11)‐driven leukemic transformation through deregulation of gene expression. 11 , 29 We found that MiR‐27a transfection (Supporting Information: Figure S3) significantly decreased HOXA9, HOXA7, and MEIS1 transcript levels in different t(4;11) cell lines (Figure 3A). This effect was specific because MiR‐27a overexpression did not reduce the transcript level of these genes in REH cells, a non‐MLL‐related ALL cell line (Figure 3A).
Figure 3.
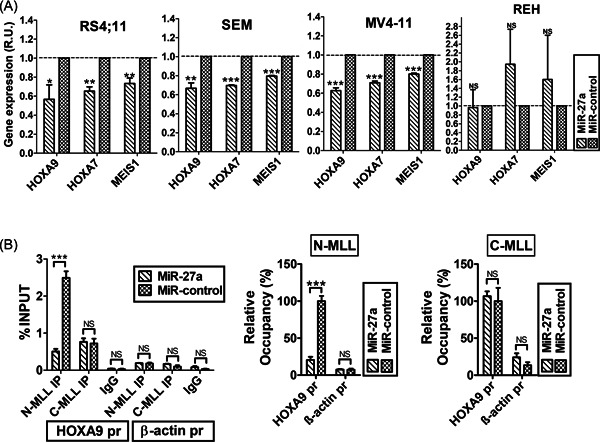
MiR‐27a impairs the expression of MLL‐AF4 target genes in t(4;11) cell lines. (A) HOXA9, HOXA7, and MEIS1 transcript expression in RS4;11, SEM, MV4‐11, and REH, 24 h after transfection with MiR‐27a or MiR‐negative control. Relative gene expression was determined by the method. (B) Interaction of MLL‐AF4 and wild‐type MLL with HOXA9 gene promoter (HOXA9 pr) after RS4;11 cell transfection with MiR‐27a or MiR negative control, determined by ChIP. ACTB promoter (β‐actin pr) is the negative control. Data are expressed as a percentage of HOXA9 and ACTB promoters in immunoprecipitated chromatin with respect to the INPUT. IgG is the mock (left panel). Error bars indicate standard deviations. ChIP, chromatin immunoprecipitation; MLL, mixed‐lineage leukemia; NS, not statistically significant; R.U., relative units. *p< .05; **p< .01, ***p < .005.
We performed ChIP to evaluate the strength of MLL‐AF4 binding to the HOXA9 promoter (HOXA9 pr), after transfection of pre‐MiR‐27a or pre‐MiR‐negative control, in RS4;11 cells. We used an anti‐MLLN antibody, targeting both WT MLL and MLL‐AF4 chimera, and an anti‐MLLC that targeted WT MLL. By qPCR, we measured the amount of HOXA9 pr and ACTB promoter (chromatin negative control) in DNA immunoprecipitated with anti‐MLLN and anti‐MLLC. 11 , 17 , 23 Percentage of HOXA9 pr in chromatin immunoprecipitated with anti‐MLLN was significantly lower in cells transfected with pre‐MiR‐27a compared to the negative control cells (Figure 3B); the absence of ACTB demonstrated the assay specificity.
3.4. MiR‐27a affects cell viability, proliferation, apoptosis, and clonogenic capacity of t(4:11) leukemic cells
We showed that MiR‐27a targeted a set of relevant genes for MLL‐AF4‐driven leukemia cell transformation. Consistently, we also showed, using MTT assay, that MiR‐27a overexpression decreased the viability of the tested t(4;11) cells by at least 20% (Figure 4A). Furthermore, in the representative RS4;11 ALL cell line, 48 h after pre‐MiR‐27a transfection, BrdU assay showed, coherently with cell viability results, decreased number of cells in the S phase of the cell cycle, that is, proliferating cells (Figure 4B).
Figure 4.
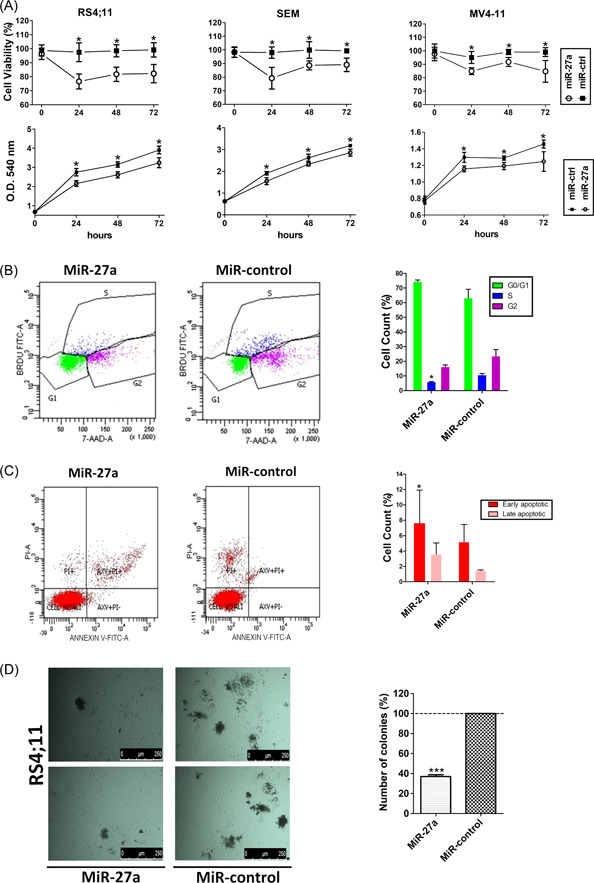
MiR‐27a affects cell viability and proliferation of t(4:11) leukemic cells. (A) Viability of RS4;11, SEM, and MV4‐11 leukemia cells at 24, 48, and 72 h after transfection with MiR‐27a or MiR‐negative control. Results are reported both as viability percentage and as cell growth increase expressed as spectrophotometric absorbance (O.D.) at 540 nm. (B) RS4;11 cell proliferation analyzed using BrdU assay, 48 h after cell transfection with MiR‐27a or MiR‐negative control. Results are represented as cytograms and bar graphs. (C) Cell apoptosis was determined by cytofluorimetric analysis of annexin‐V‐FITC (AXV) and propidium iodide (PI) staining at 72 h after cell transfection with MiR‐27a or MiR negative control. Early apoptotic cells are AXV positive, and late apoptotic cells show AXV and PI staining. Results are graphically represented as cytograms and bar graphs. (D) Colony formation assay performed by culturing RS4;11 cells in a semisolid methylcellulose‐based media at 24 h after transfection with MiR‐27a or MiR‐negative control. Pictures illustrate differences in size and number of colonies after 2 weeks in culture; the bar graph represents colony percentage (%). Error bars indicate the standard deviations. 7‐AAD, 7‐aminoactomycin D; BrdU, 5‐bromo‐2′‐deoxyuridine; PI, propidium iodide. *p< .05; ***p < .005.
HOXA9 knockdown induces apoptosis in the MLL‐leukemia cell lines, 30 and 14‐3‐3 proteins belong to a family of antiapoptotic proteins. 31 To test whether overexpression of MiR‐27a had a proapoptotic effect, we performed apoptosis assays in RS4;11 cells transfected with MiR‐27a. Seventy‐two hours after transfection, the apoptotic rate of cells transfected with MiR‐27a was about twofold higher than in MiR‐control cells (Figure 4C). We also tested whether MiR‐27a overexpression affected the clonogenic capacity of t(4;11) cells through colony formation assay. After 2 weeks in culture, colonies of cells transfected with pre‐MiR‐27a were smaller and less numerous than the control ones (Figure 4D).
4. DISCUSSION
In t(4;11) leukemia, the oncogenic chimera MLL‐AF4 is necessary and sufficient to activate hematopoietic progenitor transformation. 28 , 32 In many patients, survival of leukemic blasts depends on the maintenance of high expression levels of a set of MLL‐AF4 target genes, including HOXA9, HOXA8, HOXA7, and MEIS1 6 ; notably, HOXA9 silencing alone is sufficient to promote apoptotic death of t(4;11)(q21;q23) lymphoblasts. 33
To exert its aberrant function, MLL‐AF4 exploits numerous protein interactors, with very heterogeneous cellular roles, that acquire an opportunistic oncogenic function. 11 , 23 As a consequence, a wide plethora of proteins are dysregulated in the leukemia context. 3 , 34 , 35
MiRNAs are a class of small noncoding RNAs that regulates gene expression in eukaryotes. More importantly, a single miRNA can regulate very large sets of genes, thereby influencing almost every metabolic and regulatory pathway in individuals who are healthy as well asdisease. 15 , 36 , 37 , 38 A growing number of MiRNAs that target genes involved in the hematopoietic process are dysregulated in human leukemia. 39 , 40 , 41 , 42 In t(4;11) ALL, MLL‐AF4 activates aberrant expression of the oncogenic miR‐130b and miR‐128a; 43 in contrast, the tumor suppressor miR‐142‐3p, miR‐142‐3p, and miR‐205 target and downregulate MLL‐AF4 and AF4. 44 , 45 , 46
The scaffold protein 14‐3‐3θ, a direct interactor of AF4 and MLL‐AF4, acquires an opportunistic oncogenic role in t(4;11) leukemia. Indeed, 14‐3‐3θ knockdown negatively affects HOXA9 and MEIS1 expression, induces apoptosis, and hampers proliferation of cells that constitutively express MLL‐AF4. 11 Intriguingly, MiR‐27a functions as a tumor suppressor in different acute leukemia forms by downregulating 14‐3‐3θ; moreover, it is downregulated in leukemia cell lines and in patients' primary samples compared to hematopoietic progenitor cells. 12
Interestingly, we found a statistically significant inverse correlation between transcript levels of MiR‐27a and 14‐3‐3θ in SEM and RS4;11, the t(4;11) ALL cell lines, where relatively low levels of MiR‐27a associated with relatively high expression of 14‐3‐3θ (Figure 1A). Notably, despite Pearson's value revealing an inverse correlation between MiR‐27a and 14‐3‐3θ expression also in MV4‐11, MiR‐27a level was higher in t(4;11) AML cell line compared to SEM and RS4;11. This observation is in agreement with previous expression microarray analysis performed on a large number of leukemia samples, which found no significant differences in MiR‐27a expression between CD34+ hematopoietic stem/progenitor cells and AML cases. 12
MiR‐27a is expressed at lower levels tends to relapse in infant and childhood ALL patients compared with primary patients at diagnosis; in contrast, patients with high levels of MiR‐27a at diagnosis have longer relapse‐free survival than those with low levels, suggesting that MiR‐27a dysregulation might contribute to leukemia relapse. 24 , 47 In ALL, the t(12;21) translocation is a good‐risk prognostic biomarker, whereas t(4;11) has a very poor prognosis. 48 , 49 , 50 Interestingly, we show that MiR‐27a levels are significantly lower in patients with t(4;11) ALL than in t(12;21) patients (Figure 1B), thereby supporting the negative prognostic value of this miRNA in MLL‐AF4‐driven ALL.
We also show that MiR‐27a plays an important role in the proliferation and survival of t(4;11) blasts because its ectopic expression in an MLL‐AF4‐positive ALL cell line significantly decreases the protein level of 14‐3‐3θ and of the transcription factor RUNX1 (Figure 1C,D), another MiR‐27a target involved in this type of leukemia. 4 , 27 Indeed, MLL‐AF4 transactivates the gene encoding RUNX1 that in turn dysregulates MLL‐AF4 downstream genes, thereby contributing to the leukemic transcription program. 4 , 26 , 28 , 29
A single miRNA is able to posttranscriptionally regulate the expression of multiple genes by targeting regulatory regions within their 3′‐UTR. We demonstrate that MiR‐27a, besides 14‐3‐3θ and RUNX1, downregulates AF4 and MLL‐AF4 expression (Figure 2A). Indeed, DLR assays demonstrate that 3′‐UTR of AFF1 and KMT2A/AFF1 contain at least one seed sequence of MiR‐27a. Accordingly, expression of a MiR‐27a inhibitor in RS4;11 cells recovers protein levels of both AF4 and MLL‐AF4 (Figure 2D). Intriguingly, AF4 and MLL‐AF4, the master players in t(4;11) ALL molecular pathogenesis, are molecular partners of 14‐3‐3θ and RUNX1. 4 , 11 , 17 , 28
As mutual interactors, 14‐3‐3θ, RUNX1, AF4, and MLL‐AF4 take part in a protein complex that deregulates the expression of various genes, including the direct chimera targets. 28 Significantly, MiR‐27a overexpression downregulates HOXA9, HOXA7, and MEIS1 expression, the main chimera targets, in all the tested t(4;11) cell lines (Figure 3A), whereas it does not affect the expression of these genes in REH, a non‐MLL‐related leukemia cell line (Figure 3A). Consistently, ChIP experiments reveal that MiR‐27a overexpression actually decreases the binding of MLL‐AF4 to HOXA9pr, whereas the binding of MLL WT is unaffected, in agreement with the target specificity of MiR‐27a.
Consistently with previous studies demonstrating that HOXA9 knockdown hampers the proliferative potential and promotes apoptotic death of t(4;11) leukemia cells, 33 MiR‐27a overexpression reduces viability and proliferative capability of t(4;11) leukemia cells (Figure 4A). Similar to other known miRNAs that target MLL‐AF4 and AF4, 44 , 45 , 46 and with the antiapoptotic role of many protein members of the 14‐3‐3 family, including 14‐3‐3θ, 11 , 30 , 51 MiR‐27a overexpression also increases the apoptotic rate of RS4;11 leukemia cells (Figure 4C). Since the blast's capability to form colonies depends on the potential of hematopoietic progenitors to differentiate and proliferate, 52 the significantly reduced clonogenic potential observed for RS4;11 leukemic blasts that overexpress MiR‐27a (Figure 4D) is consistent with their reduced proliferative capability and increased apoptosis. As loss of differentiation and gain of uncontrolled proliferation are leukemic blast crucial features that are reversed, in t(4;11) cell lines, by ectopic expression of MiR‐27a, our overall results strongly indicate that MiR‐27a functions as a tumor suppressor in t(4;11) leukemia context.
In conclusion, we demonstrate that MiR‐27a can negatively regulate four crucial drivers of blast transformation in t(4;11) leukemia pathogenesis, thereby emerging as a new potential target for the treatment of this still poorly curable and aggressive leukemia.
AUTHOR CONTRIBUTIONS
Tiziana Fioretti and Mariateresa Zanobio performed experiments, and prepared and analyzed figures. Maddalena Raia and Fabio Cattaneo performed partial experiments. Santa Errichiello and Barbara Izzo provided materials. Fabio Cattaneo and Rosario Ammendola revised the manuscript and the figures. Gabriella Esposito and Armando Cevenini conceived the project, supervised experiments, analyzed data, and wrote the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
We are grateful to AIL Onlus for supporting research in the field of human leukemia. This work was partially funded by: Italian Ministry of Health [RF‐2011‐02349269 to GE]; Italian Ministry of University and Research [“Fondo di finanziamento per le attività base di ricerca” (FFABR) 2017] to Open Access Funding provided by Universita degli Studi di Napoli Federico II within the CRUI‐CARE Agreement.
Fioretti T, Zanobio M, Raia M, et al. MiR‐27a downregulates 14‐3‐3θ, RUNX1, AF4, and MLL‐AF4, crucial drivers of blast transformation in t(4;11) leukemia cells. Cell Biochem Funct. 2022;40:706‐717. 10.1002/cbf.3736
Tiziana Fioretti and Mariateresa Zanobio contributed equally to this study.
Contributor Information
Armando Cevenini, Email: armando.cevenini@unina.it.
Gabriella Esposito, Email: gabriella.esposito@unina.it.
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article and its Supporting Information: Materials.
REFERENCES
- 1. El Chaer F, Keng M, Ballen KK. MLL‐rearranged acute lymphoblastic leukemia. Curr Hematol Malig Rep. 2020;15:83‐89. [DOI] [PubMed] [Google Scholar]
- 2. Meyer C, Burmeister T, Gröger D, et al. The MLL recombinome of acute leukemias in 2017. Leukemia 2018;32:273‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Takahashi S, Yokoyama A. The molecular functions of common and atypical MLL fusion protein complexes. Biochim Biophys Acta. 2020;1863(7):194548. [DOI] [PubMed] [Google Scholar]
- 4. Wilkinson AC, Ballabio E, Geng H, et al. RUNX1 is a key target in t(4;11) leukemias that contributes to gene activation through an AF4‐MLL complex interaction. Cell Rep. 2013;3:116‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marschalek R. Mechanisms of leukemogenesis by MLL fusion proteins. Br J Haematol. 2011;152:141–154. [DOI] [PubMed] [Google Scholar]
- 6. Collins C.T, Hess JL. Deregulation of the HOXA9/MEIS1 axis in acute leukemia. Curr Opin Hematol. 2016;23:354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Godfrey L, Kerry J, Thorne R, et al. MLL‐AF4 binds directly to a BCL‐2 specific enhancer and modulates H3K27 acetylation. Exp Hematol. 2016;47:64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aryal S, Zhang Y, Wren S, Li C, Lu R. Molecular regulators of HOXA9 in acute myeloid leukemia. FEBS J . 2021; 1‐19. 10.1111/febs.16268 [DOI] [PubMed]
- 9. Dafflon C, Craig VJ, Méreau H, et al. Complementary activities of DOT1L and Menin inhibitors in MLL‐rearranged leukemia. Leukemia 2017;31:1269‐1277. [DOI] [PubMed] [Google Scholar]
- 10. Esposito G, Cevenini A, Cuomo A, et al. Protein network study of human AF4 reveals its central role in RNA Pol II‐mediated transcription and in phosphorylation‐dependent regulatory mechanisms. Biochem J 2011;438:121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fioretti T, Cevenini A, Zanobio M, et al. Crosstalk between 14‐3‐3θ and AF4 enhances MLL‐AF4 activity and promotes leukemia cell proliferation. Cell Oncol. 2019;42:829‐845. [DOI] [PubMed] [Google Scholar]
- 12. Scheibner KA, Teaboldt B, Hauer MC, et al. MiR‐27a functions as a tumor suppressor in acute leukemia by regulating 14‐3‐3θ. PLoS One. 2012;7:e50895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Colangelo T, Polcaro G, Ziccardi P, et al. The miR‐27a‐calreticulin axis affects drug‐induced immunogenic cell death in human colorectal cancer cells. Cell Death Dis. 2016;7:e2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li X, Xu M, Ding L, Tang J. MiR‐27a: A novel biomarker and potential therapeutic target in tumors. J Cancer. 2019;10:2836‐2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barisciano G, Colangelo T, Rosato V, et al. MiR‐27a is a master regulator of metabolic reprogramming and chemoresistance in colorectal cancer. Br J Cancer. 2020;122:1354‐1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Di Iorio V, Esposito G, De Falco F, et al. CHM/REP1 transcript expression and loss of visual function in patients affected by choroideremia. Invest Ophthalmol Vis Sci. 2019;60:1547‐1555. [DOI] [PubMed] [Google Scholar]
- 17. Yokoyama A, Lin M, Naresh A, Kitabayashi I, Cleary ML. A higher‐order complex containing AF4 and ENL family proteins with P‐TEFb facilitates oncogenic and physiologic MLL‐dependent transcription. Cancer Cell. 2010;17:198‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(−Delta Delta C(T)). Methods. 2001;25(4):402‐408. [DOI] [PubMed] [Google Scholar]
- 19. Busiello T, Ciano M, Romano S, et al. Role of ZNF224 in cell growth and chemoresistance of chronic lymphocytic leukemia. Hum Mol Genet. 2017;26(2):344‐353. [DOI] [PubMed] [Google Scholar]
- 20. Cattaneo F, Russo R, Castaldo M, et al. Phosphoproteomic analysis sheds light on intracellular signaling cascades triggered by Formyl‐Peptide receptor 2. Sci Rep. 2019;9:17894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Castaldo M, Zollo C, Esposito G, Ammendola R, Cattaneo F. NOX2‐dependent reactive oxygen species regulate formyl‐peptide receptor 1‐mediated TrkA transactivation in SH‐SY5Y cells. Oxid Med Cell Longev. 2019;2019:2051235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lewis BP, Shih IH, Jones‐Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell 2003;115:787–798. [DOI] [PubMed] [Google Scholar]
- 23. Fioretti T, Cevenini A, Zanobio M, et al. Nuclear FGFR2 interacts with the MLL‐AF4 oncogenic chimera and positively regulates HOXA9 gene expression in t(4;11) leukemia cells. Int J Mol Sci. 2021;22:4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Han BW, Feng DD, Li ZG, et al. A set of miRNAs that involve in the pathways of drug resistance and leukemic stem‐cell differentiation is associated with the risk of relapse and glucocorticoid response in childhood ALL. Hum Mol Genet. 2011;20:4903‐4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alvarez Y, Caballín MR, Gaitán S, et al. Presenting features of 201 children with acute lymphoblastic leukemia: comparison according to presence or absence of ETV6/RUNX1 rearrangement. Cancer Genet Cytogenet. 2007. Sep;177:161‐163. [DOI] [PubMed] [Google Scholar]
- 26. Ben‐Ami O, Pencovich N, Lotem J, Levanon D, Groner Y. A regulatory interplay between miR‐27a and Runx1 during megakaryopoiesis. Proc Natl Acad Sci USA. 2009;106:238‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stavast CJ, Leenen PJM, Erkeland SJ. The interplay between critical transcription factors and microRNAs in the control of normal and malignant myelopoiesis. Cancer Lett. 2018;427:28‐37. [DOI] [PubMed] [Google Scholar]
- 28. Harman JR, Thorne R, Jamilly M, et al. A KMT2A‐AFF1 gene regulatory network highlights the role of core transcription factors and reveals the regulatory logic of key downstream target genes. Genome Res. 2021;31:1159‐1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prange KHM, Mandoli A, Kuznetsova T, et al. MLL‐AF9 and MLL‐AF4 oncofusion proteins bind a distinct enhancer repertoire and target the RUNX1 program in 11q23 acute myeloid leukemia. Oncogene 2017;36:3346‐3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Faber J, Krivtsov AV, Stubbs MC, et al. HOXA9 is required for survival in human MLL‐rearranged acute leukemias. Blood 2009;113:2375‐2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aghazadeh Y, Papadopoulos V. The role of the 14‐3‐3 protein family in health, disease, and drug development. Drug Discovery Today. 2016;21:278‐287. [DOI] [PubMed] [Google Scholar]
- 32. Malouf C, Ottersbach K. Molecular processes involved in B cell acute lymphoblastic leukaemia. Cell Mol Life Sci. 2018;75:417–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Orlovsky K, Kalinkovich A, Rozovskaia T, et al. Down‐regulation of homeobox genes MEIS1 and HOXA in MLL‐rearranged acute leukemia impairs engraftment and reduces proliferation. Proc Natl Acad Sci USA 2011;108:7956–7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Okuda H, Stanojevic B, Kanai A, et al. Cooperative gene activation by AF4 and DOT1L drives MLL‐rearranged leukemia. J Clin Investig. 2017;127:1918–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Takahashi S, Kanai A, Okuda H, et al. HBO1‐MLL interaction promotes AF4/ENL/P‐TEFb‐mediated leukemogenesis. eLife 2021;10:e65872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009;136:215‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jie M, Feng T, Huang W, et al. Subcellular localization of miRNAs and implications in cellular homeostasis. Genes. 2021;12:856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hanna J, Hossain GS, Kocerha J. The potential for microRNA therapeutics and clinical research. Front Genet. 2019;10:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scourzic L, Salataj E, Apostolou E. Deciphering the complexity of 3D chromatin organization driving lymphopoiesis and lymphoid malignancies. Front Immunol. 2021;12:669881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Organista‐Nava J, Gómez‐Gómez Y, Illades‐Aguiar B, Leyva‐Vázquez MA. Regulation of the miRNA expression by TEL/AML1, BCR/ABL, MLL/AF4 and TCF3/PBX1 oncoproteins in acute lymphoblastic leukemia. Oncol Rep. 2016;36:1226‐1232. [DOI] [PubMed] [Google Scholar]
- 41. Gonzales‐Aloy E, Connerty P, Salik B, et al. miR‐101 suppresses the development of MLL‐rearranged acute myeloid leukemia. Haematologica 2019;104:e296‐e299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Anelli L, Zagaria A, Specchia G, Musto P, Albano F. Dysregulation of miRNA in leukemia: exploiting miRNA expression profiles as biomarkers. Int J Mol Sci. 2021;22:7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Malouf C, Antunes ETB, O'Dwyer M, et al. MiR‐130b and miR‐128a are essential lineage‐specific co‐drivers of t(4;11) MLL‐AF4 acute leukemia. Blood. 2021;138:2066‐2092. [DOI] [PubMed] [Google Scholar]
- 44. Dou L, Li J, Zheng D, et al. MicroRNA‐142‐3p inhibits cell proliferation in human acute lymphoblastic leukemia by targeting the MLL‐AF4 oncogene. Mol Biol Rep. 2013;40:6811‐6819. [DOI] [PubMed] [Google Scholar]
- 45. Dou L, Li J, Zheng D, et al. MicroRNA‐205 downregulates mixed‐lineage‐AF4 oncogene expression in acute lymphoblastic leukemia. OncoTargets Ther. 2013;6:1153‐1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dou L, Zheng D, Li J, et al. Methylation‐mediated repression of microRNA‐143 enhances MLL‐AF4 oncogene expression. Oncogene 2012;31(4):507‐517. [DOI] [PubMed] [Google Scholar]
- 47. Feng DD, Zhang H, Zhang P, et al. Down‐regulated miR‐331‐5p and miR‐27a are associated with chemotherapy resistance and relapse in leukaemia. J Cell Mol Med. 2011;15:2164‐2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moorman AV. New and emerging prognostic and predictive genetic biomarkers in B‐cell precursor acute lymphoblastic leukemia. Haematologica 2016;101:407‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Winters AC, Bernt KM. MLL‐rearranged Leukemias—an update on science and clinical approaches. Front Pediatr. 2017;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Britten O, Ragusa D, Tosi S, Kamel YM. MLL‐rearranged acute leukemia with t(4;11)(q21;q23)—current treatment options. Is there a role for CAR‐T cell therapy? Cells 2019;8:1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cau Y, Valensin D, Mori M, Draghi S, Botta M. Structure, function, involvement in diseases and targeting of 14‐3‐3 proteins: an update. Curr Med Chem. 2018;25:5‐21. [DOI] [PubMed] [Google Scholar]
- 52. Sarma NJ, Takeda A, Yaseen NR. Colony forming cell (CFC) assay for human hematopoietic cells. J Vis Exp. 2010;46:2195. 10.3791/2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its Supporting Information: Materials.


