Abstract
Understanding the factors affecting thermal tolerance is crucial for predicting the impact climate change will have on ectotherms. However, the role developmental plasticity plays in allowing populations to cope with thermal extremes is poorly understood. Here, we meta‐analyse how thermal tolerance is initially and persistently impacted by early (embryonic and juvenile) thermal environments by using data from 150 experimental studies on 138 ectothermic species. Thermal tolerance only increased by 0.13°C per 1°C change in developmental temperature and substantial variation in plasticity (~36%) was the result of shared evolutionary history and species ecology. Aquatic ectotherms were more than three times as plastic as terrestrial ectotherms. Notably, embryos expressed weaker but more heterogenous plasticity than older life stages, with numerous responses appearing as non‐adaptive. While developmental temperatures did not have persistent effects on thermal tolerance overall, persistent effects were vastly under‐studied, and their direction and magnitude varied with ontogeny. Embryonic stages may represent a critical window of vulnerability to changing environments and we urge researchers to consider early life stages when assessing the climate vulnerability of ectotherms. Overall, our synthesis suggests that developmental changes in thermal tolerance rarely reach levels of perfect compensation and may provide limited benefit in changing environments.
Keywords: acclimation, climate vulnerability, critical thermal limits, CTmax , heat tolerance, ontogenetic variation, persistence, phenotypic plasticity, reversibility, systematic review
Understanding the role developmental plasticity plays in allowing populations to cope with thermal extremes is critical. Here, we meta‐analysed how thermal tolerance is impacted by early thermal environments using data from 138 ectothermic species. We found that developmental changes in thermal tolerance are not sufficient to buffer ectotherms from rising temperatures and that embryonic stages may represent a critical window of vulnerability to changing environments.
INTRODUCTION
Ectotherms represent most of the animal diversity on the planet (Zhang, 2013), yet they are particularly vulnerable to extreme heat events (Angilletta, 2009). Extreme heat events are predicted to become fourteen times more likely to occur, and to generate temperatures 2.7°C higher by 2100 relative to the previous century (Arias et al., 2021). As such, it is crucial to understand how ectotherms will adjust to rapidly changing temperatures in the future (Chevin et al., 2010; Hoffmann & Sgrò, 2011; Noble et al., 2019; Seebacher et al., 2015). While genetic adaptation is a key mechanism by which populations can adapt, it can be slow and is constrained by genetic (co)variation (Chevin et al., 2010). Instead, phenotypic changes within an animal's lifetime (i.e., phenotypic plasticity) may be a more effective mechanism to cope with abrupt environmental changes—allowing ectotherms to withstand extreme heat events for longer, buying time for evolutionary rescue to occur (Bush et al., 2016; Morley et al., 2019). Thermal tolerance is a key trait permitting organisms to deal with thermal stress and is known to respond to the environment plastically (Gunderson et al., 2017; Gunderson & Stillman, 2015; Morley et al., 2019; Pottier, Burke, Drobniak et al., 2021; Rohr et al., 2018). Thermal tolerance traits (e.g., CTmax, CTmin) can be used to understand how species distributions will be impacted by climate change (e.g., Sunday et al., 2012, 2014; Comte & Olden, 2017; Pinsky et al., 2019). Nonetheless, broad‐scale ecophysiological models rarely account for plasticity in thermal tolerance (Bush et al., 2016; Huey et al., 2012). In addition, most syntheses examining plasticity in thermal tolerance have not assessed whether embryonic, juvenile and adult stages differ in the extent of their plasticity (Bodensteiner et al., 2021).
Early life stages, however, are crucial periods during development that are often the most impacted by temperature (Bodensteiner et al., 2021; Fawcett & Frankenhuis, 2015; Noble et al., 2018; O'Dea et al., 2019; Refsnider et al., 2019; Truebano et al., 2018; Turriago et al., 2015; While et al., 2018). Neglecting how early (embryonic and/or juvenile) environmental experiences shape thermal tolerance (i.e., developmental plasticity) may be an important oversight given that early life experiences have major and often long‐lasting effects on phenotypes (Bodensteiner et al., 2021; Noble et al., 2018; O'Dea et al., 2019; Refsnider et al., 2019; While et al., 2018). Importantly, examining whether thermal tolerance is persistently shaped by early thermal environments has critical implications for ecophysiological modelling and experimental research. In fact, experimental studies often assume that laboratory acclimation erase the effects of thermal history (Kellermann et al., 2017) and that adult plasticity does not vary with early thermal conditions (Beaman et al., 2016; Healy et al., 2019; Kellermann & Sgrò, 2018). However, early life stages are predicted to differ in their levels of plasticity relative to adults because these stages often coincide with limited mobility—forcing organisms to cope with the environmental conditions in which they settle. Without resort to behavioural thermoregulation, selection for more plastic responses may occur disproportionately in early life stages relative to adults (Bodensteiner et al., 2021; Muñoz, 2021; but see Mitchell et al., 2013). In addition, plastic responses are expected to be costly (DeWitt et al., 1998, but see Murren et al., 2015). As such, the self‐reliance of early life stages on endogenous energy reserves and the costs imposed by developmental processes (Marshall et al., 2020; Pettersen et al., 2018) may constrain the allocation of energy to diverse functions, including plastic responses to temperatures.
Taken together, weaker plastic responses are expected in early life stages if energy allocation trade‐offs have a predominant role, whereas selection for stronger plasticity could occur due to limited thermoregulatory abilities. Importantly, the interplay between basal thermal tolerance and plasticity throughout ontogeny is essential to consider in broad‐scale quantifications of climate vulnerability (Ruthsatz et al., 2022). Ontogenetic variation in absolute thermal tolerance may be mitigated or further exacerbated by varying levels of plasticity throughout the life cycle. For instance, if the lower thermal tolerance of embryos (Truebano et al., 2018; Dahlke et al., 2020; but see Pottier, Burke, Drobniak, et al., 2022 and Dahlke et al., 2022) is associated with low plasticity, then this life stage may be the most sensitive to abrupt climate change. Therefore, it is crucial to investigate whether early life stages can acclimate to new temperatures (i.e., initial effects), whether those responses persist (i.e., persistent effects), and how the magnitude of plastic responses to temperatures vary with ontogeny. Yet, no study has systematically addressed those questions across ectothermic species. A meta‐analysis of the published experimental data could help delineate the initial and persistent effects of developmental temperatures on thermal tolerance, as well as explaining the heterogeneity across studies. For instance, a meta‐analysis may resolve discrepancies between studies by increasing statistical power (Duffy et al., 2021) and highlighting potential differences between species based on their ecology, evolutionary history, or differences in experimental methodology (Gurevitch et al., 2018).
Here, we synthesize the current evidence to quantify the magnitude and variability of developmental plasticity in heat tolerance across ectotherms, using a meta‐analysis of the experimental literature. We hypothesised that, overall, early‐life stages acclimated to higher temperatures would be more heat tolerant than animals acclimated to lower temperatures, reflecting similar patterns as in adult animals (e.g., Gunderson & Stillman, 2015). We also hypothesised that the levels of developmental plasticity would vary with ontogeny. Specifically, we hypothesised that temperatures experienced during embryonic development would have stronger influence on heat tolerance than during juvenile development because the limited ability for embryos to thermoregulate behaviourally may have selected for greater plastic responses. In addition, manipulating the temperature of both embryonic and juvenile development may increase an animal's plasticity by spanning various developmental windows of sensitivity to temperatures. Alternatively, we predicted the opposite pattern if juveniles can invest additional energy into physiological regulation via feeding. Indeed, the reliance of embryos on endogenous energy reserves might constrain the resource investment into plastic responses. For all life stages, we hypothesised that the effects of developmental temperatures on heat tolerance will persist throughout the life of the animals. However, the magnitude of persistent responses should decline as animals are re‐acclimated to common garden conditions for extended periods after the initial acclimation.
We also hypothesised that the developmental plasticity of ectotherms will vary based on their ecology. Because terrestrial habitats tend to have a greater seasonal and daily temperature variability than aquatic habitats, we predicted terrestrial animals to be more developmentally plastic than their aquatic counterparts as greater seasonality may select for greater plastic responses (Janzen, 1967; Ghalambor et al., 2006; Chevin & Hoffmann, 2017). Alternatively, because changes in water temperature result in faster changes in body temperature (Angilletta, 2009; Denny, 1993), plastic responses may be more strongly selected in aquatic taxa because of increased exposure to temperature variability (Chevin & Hoffmann, 2017). Finally, we investigated sources of methodological variation such as differences in thermal tolerance metrics (i.e., LT50 or CTmax) and assay heating rates.
MATERIALS AND METHODS
Protocol, registration, and reporting
We preregistered our predictions (see introduction), methods and planned analyses prior to data extraction and analysis (https://osf.io/zkx6u; Pottier, Burke, Zhang, et al., 2021). We followed the PRISMA‐EcoEvo (Preferred Reporting Items for Systematic reviews & Meta‐Analyses in Ecology and Evolutionary biology; O'Dea et al., 2021) guidelines for reporting this study (Table S4). Data, code, and additional resources are available at https://github.com/p‐pottier/Dev_plasticity_thermal_tolerance (Pottier, Burke, Zhang, et al., 2022).
Literature searches and study selection
We aimed to obtain a relatively comprehensive and representative sample of the experimental literature (published or unpublished) testing for the developmental plasticity of heat tolerance in ectotherms. We accessed Scopus, ISI Web of Science (core collection), and ProQuest (dissertations & theses) on 2021/03/05 and did not apply a timespan limit. Search strings were tailored to each database (full search strings are presented in Supporting Information S1; supplementary methods) to capture studies manipulating developmental temperatures of ectothermic animals, and subsequently measuring their heat tolerance. In addition to database searches, we performed backward searches in Scopus to search for relevant studies citing four influential publications (Schaefer and Ryan (2006); and Bodensteiner et al., 2021, Bowler & Terblanche, 2008 and Refsnider et al., 2019). We also included studies testing CTmax in Table I and Table II of Bodensteiner et al. (2021) and Refsnider et al. (2019), respectively. Finally, we included all studies cited in Bowler and Terblanche (2008) but did not perform a forward search from Schaefer and Ryan (2006) because it was not a literature review.
Our searches found 5996 unique documents. Titles, abstracts, and keywords were screened by PP (90%), SB (5%) and RZ (5%) in Rayyan QCRI (Ouzzani et al., 2016). A total of 571 documents were further assessed for eligibility by PP. Thirty‐five documents were not accessible to the authors, and 32 studies were missing descriptive statistics for their direct inclusion in the meta‐analysis (mean, sample size, and measure of dispersion). We contacted the authors of the original studies to request missing information if the study was published after 1995. We imputed missing standard deviations when authors did not respond but we could not impute missing standard errors (see Data extraction and effect size calculation). One study (Cheung, 2019) was found to be eligible during pilot searches using Google Scholar (i.e., benchmarking, sensu Foo et al., 2021), but was not captured by our search methods. Search methods are summarized in our PRISMA flowchart (Figure 1), and included studies are listed in the Data sources section.
FIGURE 1.
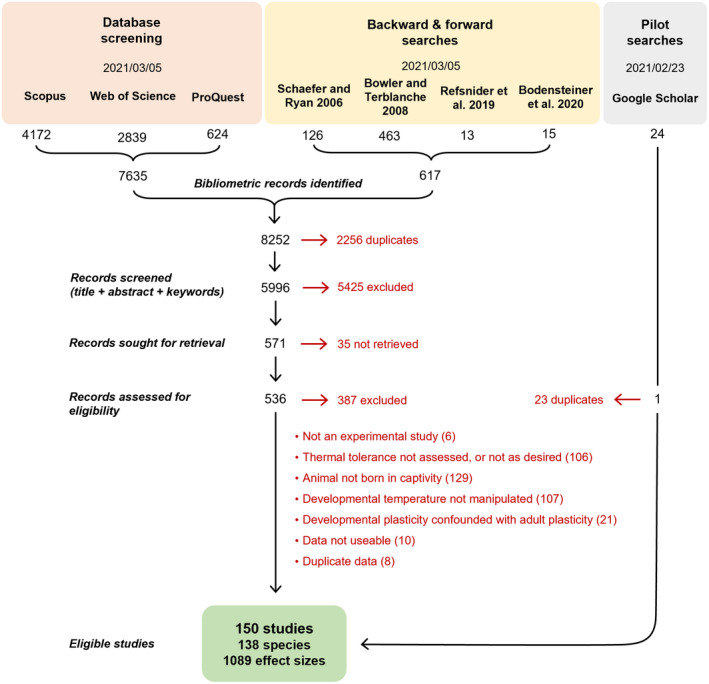
PRISMA flow chart summarizing the search methods, the number of studies excluded, and the reasons for exclusion.
Eligibility criteria
We focused on studies that chronically manipulated the developmental (embryonic or juvenile) temperature of ectothermic animals, and subsequently measured their heat tolerance. We selected studies based on seven eligibility criteria (Figures S1, S2). First, we only included studies on ectothermic animals. Second, we focused our study on manipulative laboratory experiments. Third, we only considered studies using standard and ecologically‐relevant measures of heat tolerance (Terblanche et al., 2011). Eligible heat tolerance metrics were (i) the critical thermal maximum (CTmax), where temperature is incrementally increased until animals reach an endpoint (dynamic method; Lutterschmidt & Hutchison, 2011), and (ii) the temperature lethal for 50% of the animals (LT50), where animals are subjected to constant temperatures and their survival is measured after a given period (static method; Fry, 1947). We also considered studies measuring the time to death (or heat knockdown) at different static temperatures because these measures can be converted to CTmax using regression approaches (see Rezende et al., 2014; Jørgensen et al., 2019, 2021). To increase the comparability of our estimates, we excluded alternative proxies for heat tolerance such as heat knockdown recovery time, or extrapolations from physiological performance curves. Fourth, we only included studies where animals experienced controlled early thermal environments during their embryonic development. Therefore, only data from animals born in captivity were included. Fifth, we included studies using ≥2 developmental constant or fluctuating temperatures differing by their mean and controlled in a laboratory setting. Fluctuating treatments were included provided they were comparable (i.e., differing by their mean, but having a comparable fluctuation). Sixth, we considered any prolonged (≥ 24 h) temperature experienced during the embryonic or juvenile stage as a relevant manipulation of developmental temperature. Hence, we excluded studies solely acclimating adult animals. We also excluded studies where developmental plasticity was confounded with adult acclimation. In other words, adult measures of heat tolerance must have been performed on adults acclimated to the same temperature but differing by their developmental thermal history. For logistical reasons, the developmental thermal exposure may have been continued for hours after the transition to adulthood in some studies (e.g., emergence from pupa). We tolerated such an overlap with adult acclimation when thermal conditions experienced by adults was ≤24 h. Ectotherms can take days to acclimate to new temperatures (e.g., Layne & Claussen, 1982) and 24 h was chosen as cut‐off to separate acclimatory responses from passive physiological plasticity or responses to heat shocks. Decision trees and further details about our inclusion criteria are presented in Figures S1, S2 and Tables S1, S2.
Data extraction and effect size calculations
We extracted mean heat tolerance for developmental temperature along with associated sample sizes and measures of dispersion (i.e., standard deviations and standard errors). Data extractions were performed by PP (72.8%), SB (13.6%) and RZ (13.6%) and all data were further checked for accuracy by PP. Data presented in the text or tables were directly extracted from the study. Data from figures were digitized using the metaDigitise package in R (Pick et al., 2019; version 1.0.1). Means, standard deviations and sample sizes were estimated from the raw data when available. When data were presented in different sources, we prioritized the source having the finest resolution. For studies measuring the time to death (or heat knockdown) at different static temperatures, we performed a linear regression of the logarithm of the time to death against the test temperatures and estimated the temperature the animals could tolerate for 1 h as a proxy for CTmax (following Jørgensen et al., 2019, 2021). We did not use the temperature the animals could tolerate for 1 min because extrapolations beyond thermal death time curves provide less accurate estimates than interpolations of the data (Jørgensen et al., 2019, 2021). In addition to heat tolerance data, we extracted information required to address our a priori hypotheses (see Introduction). We also collected additional data from the studies, such as the origin of the animals, their body mass, body length, sex, or details about the heat tolerance methodology.
We defined our effect size as the developmental acclimation response ratio (dARR), which is analogous to the acclimation response ratio (ARR; Claussen, 1977). Such a metric defines the variation in heat tolerance associated with a one‐degree change in developmental temperature. For instance, a dARR of 0.6 indicates that each degree increase in developmental temperature increases the heat tolerance by 0.6°C. This effect size has the advantage of accounting for the magnitude of temperature difference between the temperature treatments compared (controlling for the “nuisance heterogeneity” sensu Noble et al., 2022).
We defined our effect size as:
| (1) |
where T represents the developmental temperature in Celsius (with T 2 > T 1 ), and HT the heat tolerance estimates in Celsius. When data on >2 developmental temperatures were presented, we calculated dARR for each stepwise comparison (e.g., 20–22°C, 22–25°C, 25–27°C). The sampling variance for this effect size was derived as per Equation 2 (derived from Pottier, Burke, Drobniak et al., 2021):
| (2) |
where s 2 (dARR) is the sampling variance of dARR, sd is the standard deviation and n is the sample size (number of individuals). In cases where sample sizes were unknown and only standard errors were presented, the sampling variance of dARR was calculated as per Equation 3.
| (3) |
Where se is the standard error.
We also included data where the same animals were measured at both T 1 and T 2 . In this case, the sampling variance of dARR was calculated as Equation 4 when standard deviations were available, or Equation 5 when only standard errors were presented.
| (4) |
| (5) |
Where r [T1T2] was taken as 0.5 as a conservative measure (Noble et al., 2017).
The sampling variance for our effect size requires knowledge about the uncertainty around mean estimates (Equations (2), (3), (4), (5)). Therefore, for effect sizes missing standard deviations, we inferred standard deviations using within‐study imputation (Equation 6; Lajeunesse et al., 2013), where the standard deviation to mean ratio was deemed constant across studies.
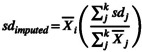 |
(6) |
where sd is the standard deviation, is the mean heat tolerance of the sample, j is the study, k is the total number of studies, and is the mean heat tolerance of the study. Assessments of the accuracy of these imputations and their impact on our analyses are described in Sensitivity Analyses.
Meta‐analysis and meta‐regressions
We performed all statistical analyses in R version 4.1.0 (R Core Team, 2019). We used multi‐level meta‐analytic models using the rma.mv function in the metafor package (Viechtbauer, 2010; version 3.0.2). Test statistics and confidence intervals for the fixed effects were computed using t distributions.
Our data had various sources of non‐independence (Noble et al., 2017). Multiple effect sizes were collected from the same studies (study ID), some species and populations were represented multiple times (species ID and population ID, respectively), species had different levels of phylogenetic relatedness (phylogeny), some animals in different treatments originated from the same parents (family ID), the same data were re‐used in stepwise comparisons when calculating effect sizes (e.g., dARR for groups acclimated to 20–22°C, 22–25°C, 25–27°C; shared treatment ID), and repeated measures were collected on the same group of animals (e.g. 24 h‐LT50 and 48 h‐LT50 measured on the same cohorts; cohort ID).
Family and population ID were confounded, as such, we only included population ID in our models. Similarly, species and study ID were not distinguishable given so few studies had multiple species. As such, we only kept species ID in the models to partition phylogenetic and non‐phylogenetic species effects (Cinar et al., 2022). We inferred phylogenetic relatedness from a phylogenetic tree constructed from the Open Tree of Life using the rotl package (Michonneau et al., 2016; version 3.0.11). We computed branch lengths using Grafen's method and modelled phylogeny as a correlation matrix using the ape package (Paradis & Schliep, 2019; version 5.5). Polytomies were resolved at random, and one species, Villosa delumbis, was manually added to the tree based on information from the Integrated Taxonomic Information System (https://itis.gov). Non‐independence arising from the same cohorts was controlled using Equations 4 and 5. Finally, sampling errors from treatments involved in multiple comparisons were correlated (using a conservative r = 0.5) with a variance covariance matrix using the metaAidR package (“github.com/daniel1noble/metaAidR”; version 0.0.0.9000). To decide on the random effect structure of the models, we first fitted all non‐overlapping random variables (species ID, population ID, and phylogeny) and an observation‐level random effect (effect size ID) in a meta‐analytic (intercept‐only) model. Because population ID explained virtually no variance, it was excluded from further models.
We then estimated the overall meta‐analytic mean and the total amount of heterogeneity (i.e., variation not explained by sampling error; Senior et al., 2016), and decomposed the heterogeneity explained by the different random effect terms. Single‐moderator models were performed with each of our a priori moderators (see Introduction) to address our hypotheses. More complex models with multiple moderators were also built to explain the remaining heterogeneity (see Supporting Information S1; supplementary methods).
For each meta‐regression, we visually assessed assumptions of homogeneity of residual variance and used a heteroscedastic compound symmetric structure with variance components estimated for each level of a categorical variable at the effect size level (”HCS” structure with zero covariance from the rma.mv function in the metafor package). AIC comparisons highlighted that this approach improved model fit (Table S20).
Statistical significance was assumed when 95% confidence intervals did not overlap with zero. We presented the estimates of each moderator category but note that differences between groups (i.e., contrasts) are also presented in Supporting Information S1 (Tables S9‐19).
Sensitivity analyses and publication bias
Publication bias refers to a higher likelihood of statistically significant findings being published than that of non‐significant findings. This bottleneck generates unrepresentative study samples and may impact the robustness of meta‐analytic results (Nakagawa et al., 2022). Publication bias was assessed in four ways. First, we used visual inspections of the relationship between model residuals and the standard error using funnel plots. We note that this method assumes that data heterogeneity is null and may not be appropriate outside of a purely visual tool (see Nakagawa et al., 2022). Second, we performed multilevel meta‐regressions using standard error or sampling variance as moderator variables to detect a small study effect, where small‐sample‐sized studies tend to have larger effect sizes (sensu Nakagawa et al., 2022). Third, we compared whether the estimates obtained from peer‐reviewed publications differed from dissertations and theses in meta‐regressions. Fourth, we assessed the time‐lag bias in our data set using a meta‐regression with publication year. The time lag bias (also known as the ‘decline effect’) refers to cases where studies with larger effects tend to be published earlier than studies with smaller effects (Koricheva & Kulinskaya, 2019).
To assess the robustness of our results, we performed five types of sensitivity analyses. First, we performed leave‐one‐out‐analyses on the meta‐analytic intercept‐only model to determine how robust results were to the exclusion of one study or one species. Second, we performed separate analyses for studies investigating the initial or persistent effects of developmental temperatures. Each moderator variable outlined above (see Introduction) was fitted in single‐moderator models for both data subsets. Third, we fitted a meta‐analytic model without data deemed to be acquired using unusual methods (i.e., risk of bias analysis; Tables S6, S35). Fourth, we fitted a meta‐analytic model without the effect sizes for which sampling variances were imputed. Fifth, because previous syntheses excluded effect sizes under a certain magnitude of response (e.g., excluding effect sizes < −0.15 in Gunderson & Stillman, 2015; or quantifying negative responses as null in Morley et al., 2019), we fitted meta‐analytic models without effect sizes reaching different arbitrary cut‐offs.
Deviations from registration
While we essentially followed our original plans and procedures, we acknowledge minor deviations (details in Supporting Information S1; supplementary methods). Notably, because the distribution of the data was skewed towards aquatic animals (85.7% of effect sizes), we estimated marginal mean estimates for models assessing habitat variation in developmental plasticity. We used the package emmeans (Lenth et al., 2019; version 1.6.2) to obtain marginal means, where data from different habitats were given equal weights (i.e., post‐stratification sensu Gelman et al., 2020). Following recommendations at the peer‐review stage, we examined whether developmental plasticity estimates varied with body mass, age at sexual maturity and the relative time at a common temperature after the initial acclimation, i.e., the proportion of days at a common temperature relative to the age at sexual maturity. We found no evidence that the age at sexual maturity is associated with levels of developmental plasticity in heat tolerance (Table S37). Furthermore, we found no evidence for a significant influence of the (relative) time at a common temperature after the initial acclimation on (i) the magnitude of developmental plasticity, or (ii) the persistence or ontogenetic variation in the reported effects (Tables S22, S23). We also examined two‐ and three‐way interactions between latitudinal origin, body mass, ramping rate, and acclimation duration (Supporting Information S1; supplementary methods; Tables S38–S44). Finally, because the temperature tolerated for 1 h is not a direct proxy for CTmax, and in fact, is more analogous to the death temperature (TKO, cf. Rezende et al., 2014), we demonstrated that the inclusion of the temperature tolerated for 1 h did not influence our results (Table S35).
RESULTS
What is the current state of knowledge?
We collected a total of 1089 effect sizes from 150 studies (1960–2021) and 138 ectothermic species. The mean (±SD) number of effect sizes per study was 7.26 ± 9.63, with a range of 1–80. Developmental plasticity in heat tolerance was tested with several experimental designs in the literature (Figure 2). We combined these designs into two broad categories: “initial” designs, where the heat tolerance was assessed immediately following the period of acclimation, and “persistent” designs, where the heat tolerance of different groups of animals was measured after a period of re‐acclimation to a common temperature after the initial acclimation (Figure 2). Overall, 79.5% of effect sizes represented “initial” effects whereas 20.5% of effect sizes represented “persistent” effects. In total, 57.2% of the effect sizes originated from fish species (Figure 3). Across papers, 79.2% of the effect sizes originated from CTmax data, whereas 20.8% originated from LT50 data (Figure 3). Further visualizations and explorations of the data are included in Supporting Information S2.
FIGURE 2.
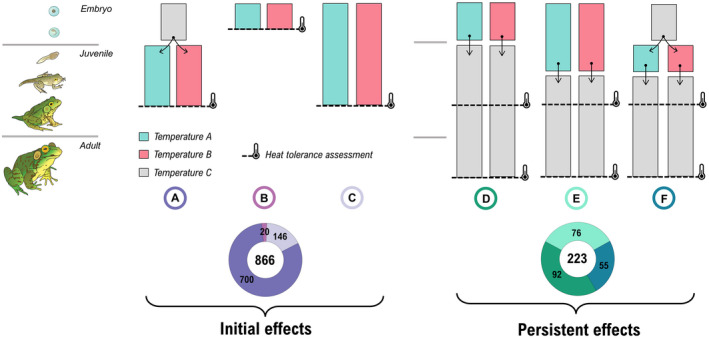
Experimental designs used to assess the developmental plasticity in heat tolerance in ectotherms. Experimental designs are grouped based on whether they assess the initial (a–c; without re‐acclimation to a common garden condition) or persistent (d–f; with re‐acclimation to a common garden condition after the initial acclimation) responses to developmental temperatures. Horizontal dashed lines represent when the heat tolerance was tested. The timing of heat tolerance measurement was positioned arbitrarily within a life history stage. In designs d, e and f, heat tolerance is assessed at either the juvenile or the adult stage following re‐acclimation, as denoted by the two heat tolerance symbols for each experimental design. Three temperatures (pink, green, grey) are presented here, but note that more temperatures can be used, and that the common temperature C can sometimes be identical to temperature a or b. Pie charts denote the number of effect sizes extracted for each type of experimental design.
FIGURE 3.
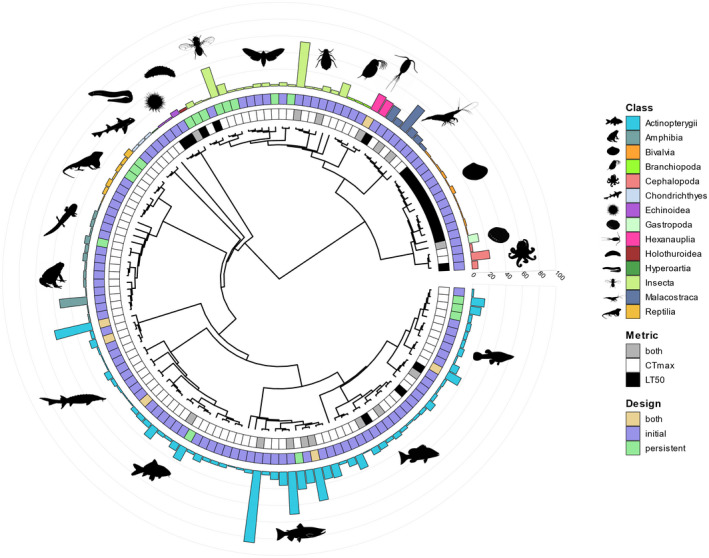
Distribution and characterization of the effect sizes across the phylogeny. The histograms represent the number of effect sizes extracted for each species. The outermost heatmap represent whether the initial or persistent effects of developmental temperatures (or both, cf. Figure 2) were assessed for this species. The innermost heatmap depicts whether the critical thermal maximum (i.e., CTmax), the median lethal temperature (i.e., LT50), or both metrics were assessed for this species. Phylogeny was constructed from the Open Tree of Life (Michonneau et al., 2016), and branch lengths were computed using Grafen's method. Silhouettes were taken from PhyloPic (www.phylopic.org).
How much do early thermal environments impact heat tolerance?
Early thermal environments have a significant but weak overall effect on heat tolerance across ectotherms (dARR = 0.190; 95% confidence interval, CI = 0.015, 0.364; n = 1089; Figure 4). For each degree increase in developmental temperatures, heat tolerance increases by only 0.19°C. Prediction intervals (PI) suggest that 95% of the time, we expect future dARR estimates to fall between −0.444 and 0.823. Adjusting for the over‐representation of aquatic animals in our data set reduced the overall estimate even further (dARR = 0.134; 95% CI = 0.002, 0.266; 95% PI = −0.455, 0.723; n = 1089; Figure 4), pointing to a required 7.5°C shift in developmental temperatures to increase heat tolerance by 1°C. Despite this weak effect, heterogeneity was extremely high (I 2 = 99.5%). Overall, 26.1% of the variation was explained by shared evolutionary history, 10.0% explained by non‐phylogenetic species effects, and 63.4% of the heterogeneity associated with the residuals (i.e., within‐species heterogeneity).
FIGURE 4.
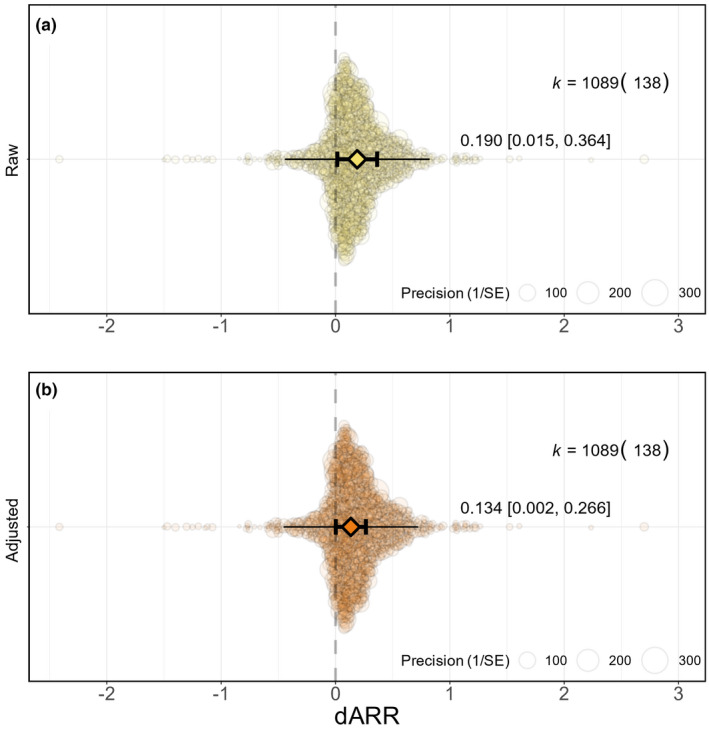
Overall level of developmental plasticity in heat tolerance. Mean meta‐analytic estimates (triangles) with their 95% confidence intervals (thicker bars with whiskers) and prediction intervals (thinner bars without whiskers) are depicted along with individual data points (coloured circles) scaled by precision (inverse of standard error). Results are presented before (a) and after (b) controlling for the over‐representation of data from aquatic vs. terrestrial animals. The graphs were constructed using the orchaRd package (Nakagawa et al., 2021; version 2.0). k: number of effect sizes (number of species). dARR: developmental acclimation response ratio.
Are embryos more plastic than juveniles?
Ectotherms were most plastic when tested immediately following temperature exposure during their juvenile (design A) or both their embryonic and juvenile development (design C) (dARRdesign A = 0.230; 95% CI = 0.085, 0.376; 95% PI = −0.403, 0.864; n = 700; dARRdesign C = 0.250; 95% CI = 0.097, 0.404; 95% PI = −0.166, 0.666; n = 146; R 2 marginal = 0.271; Figure 5). By contrast, embryos held at different temperatures (design B) barely differed in their heat tolerance levels and had highly heterogenous responses to temperature exposures (dARRdesign B = 0.098; 95% CI = −0.210, 0.406; 95% PI = −1.093, 1.290; n = 20; Figure 5).
FIGURE 5.
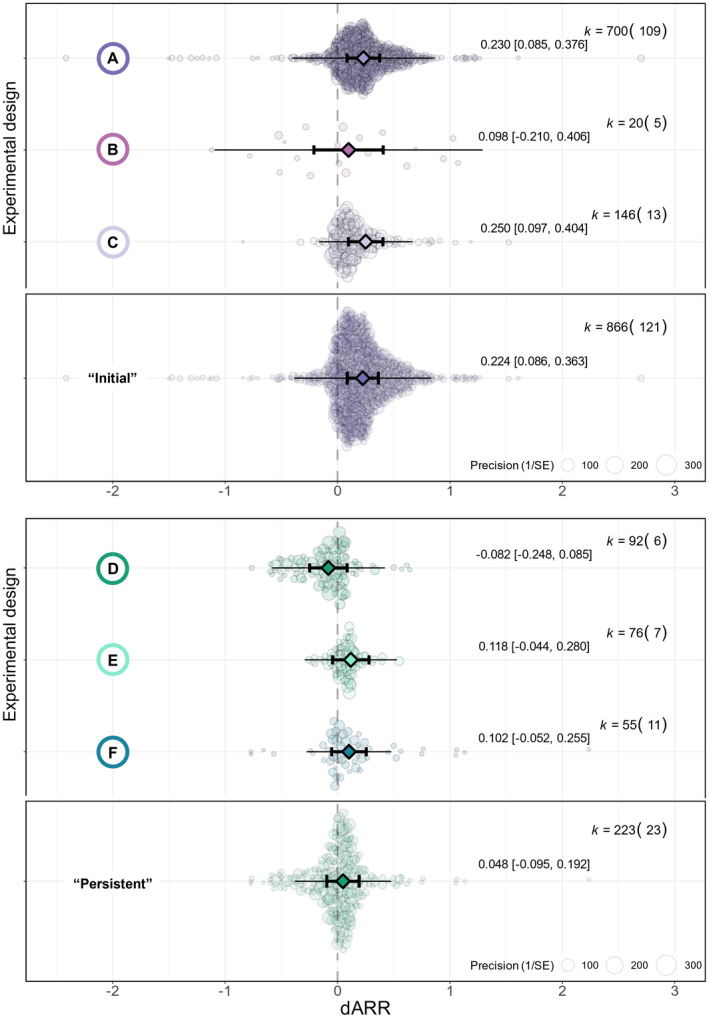
Life history variation and persistence of developmental plasticity. Mean estimates (triangles) with their 95% confidence intervals (thicker bars with whiskers) and prediction intervals (thinner bars without whiskers) are depicted along with individual data points (coloured circles) scaled by precision (inverse of standard error). k: number of effect sizes (number of species). dARR: developmental acclimation response ratio. Experimental design categorisations are presented in Figure 2.
The magnitude and direction of persistent responses varied based on when in development the animals were acclimated before being re‐acclimated to a common temperature (Figure 5). Specifically, animals which experienced higher temperatures during their juvenile (design F) or both their embryonic and juvenile development (design E) and re‐acclimated to a common garden condition tended to be better at tolerating heat, albeit responses were not significantly different from zero (dARRdesign E = 0.118; 95% CI = −0.044, 0.280; 95% PI = −0.292, 0.528; n = 76; dARRdesign F = 0.102; 95% CI = −0.052, 0.255; 95% PI = −0.276, 0.479; n = 55). By contrast, animals incubated at different temperatures during their embryonic development and raised in a common garden condition after hatching (design D) tended to have relatively reduced heat tolerance levels, albeit not significantly (dARRdesign D = −0.082; 95% CI = −0.248, 0.085; 95% PI = −0.585, 0.421; n = 92). However, we note that the distribution of those effect sizes was skewed towards negative dARR estimates—indicating that higher incubation temperatures persistently reduce the heat tolerance of ectotherms in most instances.
Do early thermal environments have persistent impacts on thermal tolerance?
We found no overall signature for a persistent effect of early thermal environment on thermal tolerance. When animals had been returned to a common temperature after the initial developmental acclimation, the dARRs were not significantly different from zero on average, whereas animals tested immediately after acclimation to higher temperatures had higher thermal tolerance (dARRinitial = 0.224, 95% CI = 0.086, 0.363; 95% PI = −0.383, 0.832; n = 866; dARRpersistent = 0.049, 95% CI = −0.095, 0.192; 95% PI = −0.380, 0.477; n = 223; R 2 marginal = 0.191; Figure 5). However, note that the magnitude and direction of persistent responses varied based on the life‐history stage exposed to temperatures (see above).
Albeit non‐significant, we found a negative association between dARR and the time at a common temperature after the initial acclimation (intercept = 0.050; 95% CI = −0.134, 0.234; slope = −0.009; 95% CI = −0.034, −0.016; n = 204; R 2 marginal = 0.048; Figure S3).
Are terrestrial animals more plastic than aquatic animals?
We found that aquatic animals were more than three times as plastic as terrestrial animals (dARRaquatic = 0.209; 95% CI = 0.079, 0.338; 95% PI = −0.410, 0.827; n = 929; dARRterrestrial = 0.060; 95% CI = −0.091, 0.210; 95% PI = −0.315, 0.434; n = 160; R 2 marginal = 0.113; Figure 6). This variation aligned with differences between functional taxonomic groups (Figure 7). Fish, amphibians, and aquatic invertebrates expressed the largest plastic responses (dARRfish = 0.254, 95% CI = 0.004, 0.504; 95% PI = −0.331, 0.839; n = 623; dARRamphibians = 0.197, 95% CI = −0.152, 0.545; 95% PI = −0.807, 1.200; n = 71; dARRaquatic invertebrates = 0.199, 95% CI = −0.055, 0.454; 95% PI = −0.665, 1.063; n = 221) whereas terrestrial animals had lower, and non‐statistically significant dARR estimates (dARRreptiles = 0.070, 95% CI = −0.273, 0.413; 95% PI = −0.506, 0.647; n = 27; dARRterrestrial invertebrates = 0.049, 95% CI = −0.230, 0.328; 95% PI = −0.457, 0.555; n = 147; R 2 marginal = 0.117).
FIGURE 6.
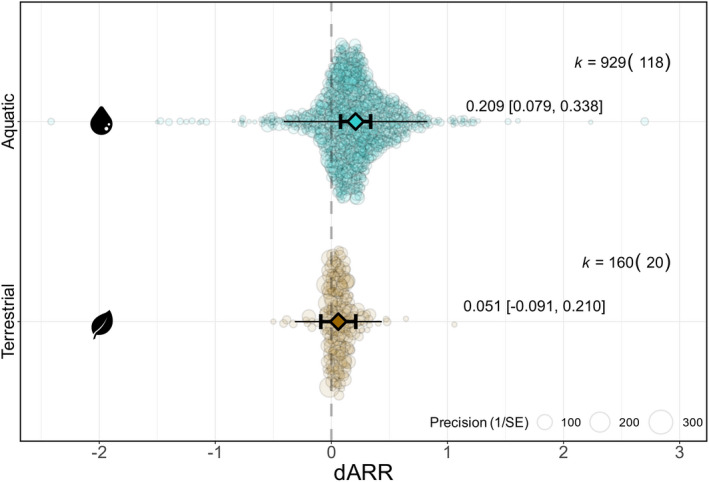
Habitat variation in developmental plasticity. Mean estimates (triangles) with their 95% confidence intervals (thicker bars with whiskers) and prediction intervals (thinner bars without whiskers) are depicted along with individual data points (coloured circles) scaled by precision (inverse of standard error). k: number of effect sizes (number of species). dARR: developmental acclimation response ratio.
FIGURE 7.
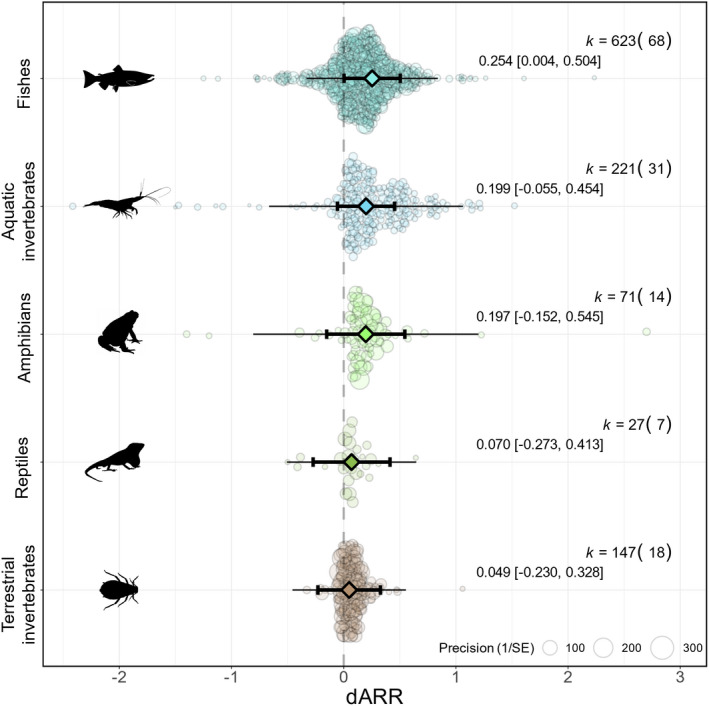
Taxonomic variation in developmental plasticity. Mean estimates (triangles) with their 95% confidence intervals (thicker bars with whiskers) and prediction intervals (thinner bars without whiskers) are depicted along with individual data points (coloured circles) scaled by precision (inverse of standard error). k: number of effect sizes (number of species). dARR: developmental acclimation response ratio. Taxonomic categorisations follow those of Morley et al., (2019). “Reptiles” refer to non‐avian reptiles.
Is experimental methodology influential for estimating plasticity?
Neither the heat tolerance metric (dARRCTmax = 0.195; 95% CI = 0.017, 0.372; 95% PI = −0.435, 0.829; n = 863; dARRLT50 = 0.162; 95% CI = −0.020, 0.343; 95% PI = −0.469, 0.798; n = 226; contrast = −0.033; 95%CI = −0.083, 0.017; R 2 marginal = 0.005) nor the heating rate (intercept = 0.212; 95% CI = 0.005, 0.419; slope = 0.019; 95% CI = −0.045, 0.084; n = 855; R 2 marginal <0.001) had statistically significant influence on developmental responses to temperatures. However, we found a positive association between heating rate and developmental responses to temperature after accounting for differences in body mass (Tables S39–S41). We also found evidence that developmental plasticity estimates were significantly influenced by the interaction between ramping rate and acclimation duration, as well as by a three‐way interaction between body mass, ramping rate, and acclimation duration (Tables S39–S41).
Is there evidence for publication bias?
Visual inspections of the funnel plot of the model's residuals did not suggest evidence for publication bias (Figure S4). We also did not find evidence for publication bias (small‐study effect) when using robust multi‐level meta‐regressions (Table S26). Dissertations and theses provided qualitatively similar estimates to published findings (Table S27), and we found little evidence for a time‐lag bias (Table S28).
How robust are our results?
Our results were robust to the iterative exclusion of one study or one species (Table S29). Investigating initial effects separately yielded higher estimates than previously presented, but generally qualitatively similar results (Tables S31, S32; Supporting Information S1; supplementary results). Analyses of persistent responses sometimes produced contrasting results to previously reported, but those analyses were deemed preliminary (Tables S33, S34; Supporting Information S1; supplementary results). Our results were also robust to the removal of (i) data acquired using uncommon methods, (ii) effect sizes for which sampling variance was imputed, and (iii) extreme negative effect sizes. However, removing extreme effect sizes tended to increase overall estimates (Table S35). Finally, the inclusion of body mass, heating rate, acclimation duration, and their interactions in models did not impact our main conclusions (Table S44).
DISCUSSION
Understanding the extent to which ectotherms can acclimate to temperatures during their development is crucial to assess their vulnerability to rising temperatures. Here, we provide the first systematic review and quantitative synthesis to quantify the initial and persistent influence of developmental temperatures on heat tolerance across 138 ectothermic species.
Early thermal environments have weak overall effects on thermal tolerance
Ectotherms raised at higher developmental temperatures tend to be slightly more tolerant to heat but the effects were weak (Figure 4). This pattern is akin to previous syntheses where data were mostly taken from adults (Gunderson & Stillman, 2015; Morley et al., 2019; Rohr et al., 2018) although early life stages seem to have a lower, and more variable, plasticity than adults. To increase heat tolerance by 1°C in developing ectotherms, it requires a 7.5°C shift in developmental temperatures (adjusted dARR ~0.13); whereas data from a previous synthesis on 278 adult ectothermic species (153 and 183 effect sizes from terrestrial and aquatic animals, respectively) points to a required shift of 4.2°C (ARR ~0.24; Morley et al., 2019). This discrepancy may be due to differences in study methodology and scope. First, previous syntheses often maximized positive ARR values by excluding effect sizes under a certain magnitude of response (e.g., excluding ARR below −0.15; or quantifying negative responses as null; Gunderson & Stillman, 2015; Morley et al., 2019). Such procedures may lead to an overestimation of the magnitude and direction of plastic responses by neglecting the possibility that ectotherms could express “non‐adaptive” (negative) responses to temperature exposures (Terblanche & Hoffmann, 2020). Unsurprisingly, excluding extremely negative effect sizes tended to increase our estimates. Negative responses have been argued to be biologically relevant and should be included in analyses to encompass the diversity of responses to temperatures organisms may exhibit (Terblanche & Hoffmann, 2020). Second, the low plasticity levels we observed may be due to biological and methodological variation. We observed an extremely high heterogeneity within and between species, which certainly contributed to the substantial width of our estimated confidence and prediction intervals. We aim to explain this variation in the next sections.
Embryos respond differently to early thermal environments than juveniles
We found significant variation in degree of plastic responses based on the life‐history stage exposed to temperatures (Figure 5). Initial responses to acclimation during the embryonic stage are extremely heterogeneous. However, acclimation periods overlapping both the embryonic and juvenile stages tend to have similar impacts on heat tolerance compared to acclimation merely constrained to the juvenile stage. The analysis of long‐lasting impacts of developmental temperatures confirms this pattern (Figure 5). Embryonic temperatures differentially impact heat tolerance of later life stages, relative to juvenile developmental temperatures. While juveniles developing at higher temperatures tend to have slightly increased heat tolerance, animals incubated at higher temperatures as embryos and raised in standard conditions after hatching tend to have reduced thermal tolerance. These results suggest an important difference in the ability of embryos to adjust their heat tolerance relative to juvenile stages.
Our results are in favour of our alternative hypothesis that energy allocation trade‐offs may constrain the expression of plastic responses throughout ontogeny. Specifically, embryos, pupae, nymphs, and young larvae rely on endogenous energy reserves, whereas later life stages can resort to feeding to increase their energy intake. This reliance on energy reserves, combined with the important metabolic cost of growth (Marshall et al., 2020; Pettersen et al., 2018), may constrain energy allocation towards diverse functions, including plastic responses to temperatures. If energy allocation trade‐offs are major drivers of the ontogenetic variation in plasticity, then the low plasticity of embryos relative to juveniles may be due to the high energy demands of development and the limited capacity for embryos to increase their energy intake. Investigating whether limited access to nutrients constrain the expression of plastic responses in juveniles would be particularly interesting to confirm this hypothesis.
Persistent responses to early thermal environments are common but not universal
Persistent responses of heat tolerance to developmental temperatures are not universal, which suggests that most of the responses recorded may represent reversible physiological acclimation rather than irreversible developmental thermal plasticity (sensu Beaman et al., 2016). Many ectotherms may successfully re‐acclimate to new environmental conditions, regardless of their early thermal history. However, we also note that only 26 studies investigated persistent responses, which is probably insufficient to reach adequate statistical power given the high heterogeneity in the data. In addition, we emphasize that the magnitude and direction of long‐lasting responses varied based on the life‐history stage exposed to temperatures (Figure 5). Therefore, we draw the reader's attention to the tendency for embryos to express negative responses to increased developmental temperatures, and the numerous cases where juvenile acclimation persistently impacts the heat tolerance of later life stages. We encourage additional research on the persistence of developmental plasticity to unravel whether those effects are robust and recommend prudence when assuming that laboratory acclimation erases the effects of early thermal history. The absence of evidence for a significant decrease in plasticity with re‐acclimation time may indicate that animals were already fully re‐acclimated to common garden conditions when assessed for thermal tolerance. Assessing the course of plasticity reversibility at various time scales is an important direction for future research.
Shared evolutionary history and species ecology affect how species respond to early thermal environments
While we observed weak overall effects of early thermal environments on heat tolerance, effect size heterogeneity was high, suggesting that species exhibit diverse responses to early thermal environments. As predicted, a lot of this variation is due to species‐specific ecology and shared evolutionary history, with ~36% of the variation in effects driven by these two factors alone. Aquatic species were more plastic to thermal developmental environments than terrestrial species (Figures 6, 7). This observation confirms findings from previous syntheses focusing on later life stages (Gunderson & Stillman, 2015; Morley et al., 2019; Rohr et al., 2018) but contradicts our primary hypothesis that larger fluctuations in environmental temperatures may have selected for larger plastic responses in terrestrial animals. Instead, it provides support to our alternative hypothesis that body temperatures equilibrate faster in water, which may select for greater plasticity because of increased exposure to operative thermal fluctuations (Chevin & Hoffmann, 2017; Denny, 1993). Opportunities for behavioural thermoregulation were also hypothesised to be reduced in aquatic environments (Gunderson & Stillman, 2015), which may expose aquatic animals to even larger fluctuations in operative temperatures. In addition, greater selection for developmental plasticity may occur in aquatic environments as a response to limited oxygen availability (Pörtner et al., 2017; but see Jutfelt et al., 2018). On the other hand, terrestrial animals have more thermoregulatory opportunities and the selection for plastic physiological responses may be reduced (Muñoz, 2021). Because marine ectotherms are experiencing operative temperatures closer to their upper thermal limits (Pinsky et al., 2019), increased levels of plasticity seem imperative for their survival in a changing world. Assessing the extent to which plasticity compensates aquatic organisms for the increased exposure to extreme body temperatures is an interesting avenue for future research. While we might expect heavy and slow‐developing animals to be especially responsive to changes in thermal environments (Uno & Stillman, 2020), we found little evidence for a relationship between developmental plasticity in heat tolerance and body mass or age at sexual maturity. The reasons why animals with different life histories respond similarly to early thermal environments are unclear and require biological and methodological considerations (see next section).
Methods for measuring heat tolerance can be influential
Although different metrics (i.e., CTmax or LT50) may yield different absolute levels of heat tolerance, the extent to which heat tolerance varies with developmental acclimation is relatively similar between metrics. While most quantitative syntheses on heat tolerance plasticity focused solely on CTmax (Barley et al., 2021; Gunderson & Stillman, 2015; Morley et al., 2019; Pottier, Burke, Drobniak et al., 2021; Rohr et al., 2018), we recommend, given statistical validation, the inclusion of LT50 in further syntheses. Slow heating rates result in extended time at high temperatures, which reduces thermal limits because of extended physiological stress (Rezende et al., 2011; Terblanche et al., 2007) and allow animals to acclimate during the experimental trials. Therefore, we predicted weak plasticity estimates at slow heating rates because extended heat stress and acclimation during assays reduce differences in thermal tolerance between cool‐ and warm‐acclimated animals. We found that, at equal body mass, animals tested at faster heating rates are usually more plastic, as predicted. Moreover, we detected previously described (Rohr et al., 2018) interactions between heating rate, body mass, and acclimation duration, but did not find evidence for interactions with latitudinal origin, probably due to a lack of statistical power. Our observations support that body size and methodological factors interact to shape the acclimation responses of ectotherms (Rohr et al., 2018).
Limitations and future directions
While we aimed at performing a comprehensive systematic review, existing taxonomical and methodological biases in the literature (Figures 2, 3) constrain the generalisability of our findings. Notably, nearly 60% of the data eligible for our synthesis were on fish species, whereas we could only extract 27 relevant effect sizes in non‐avian reptiles. We encourage further research efforts on invertebrates and the herpetofauna for a more uniform distribution of data across the tree of life. We also observed a great disparity in the experimental designs employed to assess developmental plasticity in the literature (Figure 2). Most studies assessed the initial effects of developmental temperatures, with only 26 studies assessing whether those effects persist when animals are re‐acclimated to common garden conditions after the initial acclimation. Our synthesis also highlighted that only five studies tested for the initial plasticity of embryos. We stress the need for a greater standardization and unification of experimental approaches in the field, with a priority on the responses of embryos to varying temperatures. Importantly, we did not inspect whether there exist intrinsic differences in developmental plasticity within a life stage (e.g., between larval stages). However, basal thermal tolerance and plasticity may follow complex patterns throughout ontogeny (Klockmann et al., 2017; Pincebourde & Casas, 2015; Ruthsatz et al., 2022; Ruthsatz, Dausmann, et al., 2018) that need to be further investigated. We also encourage the use of state‐of‐the‐art meta‐analytic approaches to increase the reproducibility and comparability of evidence syntheses in comparative physiology (cf. Noble et al., 2022; Vetter et al., 2013). Particularly, a formal statistical comparison of the level of plasticity of adults relative to earlier life stages would represent an important advancement towards understanding and modelling how ectotherms will respond to rising temperatures.
Implications for climate change impacts
Our study provides evidence that the capacity for ectotherms to adjust their heat tolerance is remarkably limited throughout their life cycle. Strikingly, nearly none of the 95% prediction intervals of the estimated effect sizes overlapped with unity. In other words, future changes in thermal phenotypes will rarely be expected to reach levels of perfect compensation, i.e., when heat tolerance perfectly tracks changes in environmental temperatures. We also observed numerous cases of reduced heat tolerance at higher developmental temperatures, particularly when acclimation occurred during the embryonic development (Figure 7). In fact, previous syntheses (Przeslawski et al., 2015; Collin et al., 2021; Dahlke et al., 2020; but see Pottier, Burke, Drobniak, et al., 2022 and Dahlke et al., 2022) and empirical work (e.g., Hall & Warner, 2019; Klockmann et al., 2017; Truebano et al., 2018; Turriago et al., 2015) suggest that embryos may have reduced thermal tolerance relative to other life stages. Non‐adaptive responses to developmental acclimation may represent a signature of physiological stress imposed upon embryos, possibly because of the inherent lower heat tolerance of this life stage. Low thermal tolerance combined with low, and sometimes non‐adaptive plasticity, brings embryos to the forefront of climate vulnerability. With rising temperatures, most animals may endure significant heat stress long before they reach the adult stage, although adults are often the focus of empirical studies and evidence syntheses. Assuming sufficient heritable variation, the strength of selection is expected to be stronger in embryos expressing non‐adaptive developmental plasticity. Investigating whether non‐adaptive plasticity may lead to rapid evolutionary change or extinction in a warming climate is thus a particularly interesting avenue for research (Gibert et al., 2019). We urge ecophysiologists to consider early life stages when assessing the vulnerability of ectotherms to changing temperatures.
Finally, although thermal tolerance limits are useful and intensively studied, evidence points to these metrics as not being perfect predictors of climate change vulnerability (Clusella‐Trullas et al., 2021). While thermal tolerance is relatively constrained, decreases in thermal sensitivity may help ectotherms tolerate heat waves for longer and ensure their survival (Seebacher et al., 2015; Rezende et al., 2020). Investigating how thermal tolerance and sensitivity are both impacted by early thermal environments within the same framework will represent a significant advancement towards understanding how ectotherms will navigate through changing environments. Thermal fertility limits, the temperatures at which animals lose fertility, may also represent better proxies (David et al., 2005; Parratt et al., 2021; van Heerwaarden & Sgrò, 2021; Walsh et al., 2019). In fact, fertility limits are much lower than standard thermal limits, and recent research suggest they may correlate better with global species distributions (Parratt et al., 2021; van Heerwaarden & Sgrò, 2021). Therefore, we may underestimate the impacts of rising temperatures by studying thermal tolerance limits. Notably, the development and maintenance of sexual organs and function may be sensitive to temperatures, and fertility loss may not be promptly reversible (Sales et al., 2021). Assessing the initial and persistent impacts of temperatures on fertility loss throughout ontogeny will be crucial to understand how ectotherms will navigate through changing environments.
CONCLUSIONS
We found evidence for developing ectotherms to possess the ability to adjust their heat tolerance. Animals inhabiting aquatic environments tend to be more than three times as plastic as terrestrial animals, possibly because of their increased exposure to operative temperature fluctuations. Strikingly, we found evidence that embryos express a reduced, and more heterogenous plasticity than later life stages, with numerous responses appearing as non‐adaptive. Our study adds to the evidence that the embryonic stage may represent a critical window of vulnerability to changing temperatures. While we did not find universal evidence for developmental acclimation to have long‐lasting impacts on heat tolerance, persistent effects are common, and we call for increased consideration of those effects in future research. We also encourage a standardization of empirical studies and evidence syntheses, and we formally highlight important knowledge gaps in the literature. Overall, the capacity for developing ectotherms to adjust their thermal tolerance is limited and may provide minimal benefit in changing environments. Examining the combined impacts of developmental temperatures on thermal tolerance, sensitivity, and fertility will provide important insights into the future of most animals on the planet.
AUTHORSHIP
Conceptualisation: PP, SB, RZ, DWAN, LES, SMD, SN. Methodology: PP, SB, RZ, DWAN, LES, SMD, SN. Software: PP, DWAN, SMD, SN. Formal Analysis: PP, DWAN, SMD, SN. Investigation: PP, SB, RZ. Data Curation: PP. Visualization: PP. Writing – Original Draft: PP. Writing – Review and Editing: PP, SB, RZ, DWAN, LES, SMD, SN. Project administration: PP. Supervision: SMD, SN. All authors gave final approval for publication.
CONFLICT OF INTEREST
We declare no conflict of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ele.14083.
OPEN RESEARCH BADGES
This article has earned Open Data, Open Materials and Preregistered Research Design badges. Data, materials and the preregistered design and analysis plan are available at [https://osf.io/zkx6u].
Supporting information
Appendix S1
Appendix S2
ACKNOWLEDGEMENTS
We acknowledge that financial support for this study was provided to P.P. and S.B. by a UNSW Scientia Doctoral Scholarship. S.N. and D.N. are supported by the Australian Research Council (ARC) Discovery Projects (S.N.: DP200100367; D.N.: DP210101152). S.M.D. is supported by the ARC Discovery Early Career Award (DE180100202). We are grateful to all authors of the studies included in our systematic review; without their hard work this project would not be possible. Finally, we would like to thank Sylvain Pincebourde and two anonymous reviewers for their very constructive comments on previous drafts. Open access publishing facilitated by University of New South Wales, as part of the Wiley ‐ University of New South Wales agreement via the Council of Australian University Librarians.
Pottier, P. , Burke, S. , Zhang, R.Y. , Noble, D.W.A. , Schwanz, L.E. & Drobniak, S.M. et al. (2022) Developmental plasticity in thermal tolerance: Ontogenetic variation, persistence, and future directions. Ecology Letters, 25, 2245–2268. Available from: 10.1111/ele.14083
Szymon M. Drobniak and Shinichi Nakagawa supervised this work equally.
Editor: Cameron Ghalambor
DATA AVAILABILITY STATEMENT
Data and analysis code are available at https://github.com/p‐pottier/Dev_plasticity_thermal_tolerance and archived in Zenodo (https://doi.org/10.5281/zenodo.6818559; Pottier, Burke, Zhang, et al., 2022).
References
REFERENCES
- Angilletta, M.J. (2009) Thermal Adaptation: A Theoretical and Empirical Synthesis. United Kingdom: Oxford University Press, Oxford, p. 304. [Google Scholar]
- Arias, P.A. , Bellouin, N. , Coppola, E. , Jones, R.G. , Krinner, G. , Marotzke, J. et al. (2021) Technical summary. In: Climate change 2021: The physical science basis. Cambridge, United Kingdom: Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press. [Google Scholar]
- Barley, J.M. , Cheng, B.S. , Sasaki, M. , Gignoux‐Wolfsohn, S. , Hays, C.G. , Putnam, A.B. et al. (2021) Limited plasticity in thermally tolerant ectotherm populations: evidence for a trade‐off. Proceedings of the Royal Society B: Biological Sciences, 288, 20210765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman, J.E. , White, C.R. & Seebacher, F. (2016) Evolution of Plasticity: mechanistic Link between Development and Reversible Acclimation. Trends in Ecology & Evolution, 31, 237–249. [DOI] [PubMed] [Google Scholar]
- Bodensteiner, B.L. , Agudelo‐Cantero, G.A. , Arietta, A.Z.A. , Gunderson, A.R. , Muñoz, M.M. , Refsnider, J.M. et al. (2021) Thermal adaptation revisited: how conserved are thermal traits of reptiles and amphibians? Journal of Experimental Zoology Part A Ecological and Integrative Physiology, 335, 173–194. [DOI] [PubMed] [Google Scholar]
- Bowler, K. & Terblanche, J.S. (2008) Insect thermal tolerance: what is the role of ontogeny, ageing and senescence? Biological Reviews, 83, 339–355. [DOI] [PubMed] [Google Scholar]
- Bush, A. , Mokany, K. , Catullo, R. , Hoffmann, A. , Kellermann, V. , Sgrò, C. et al. (2016) Incorporating evolutionary adaptation in species distribution modelling reduces projected vulnerability to climate change. Ecology Letters, 19, 1468–1478. [DOI] [PubMed] [Google Scholar]
- Chevin, L.‐M. & Hoffmann, A.A. (2017) Evolution of phenotypic plasticity in extreme environments. Philosophical Transactions of the Royal Society, B: Biological Sciences, 372, 20160138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevin, L.‐M. , Lande, R. & Mace, G.M. (2010) Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biology, 8, e1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinar, O. , Nakagawa, S. & Viechtbauer, W. (2022) Phylogenetic multilevel meta‐analysis: a simulation study on the importance of modelling the phylogeny. Methods in Ecology and Evolution, 13, 383–395. [Google Scholar]
- Claussen, D.L. (1977) Thermal acclimation in Ambystomatid salamanders. Comparative Biochemistry and Physiology, Part A: Molecular & Integrative Physiology, 58, 333–340. [Google Scholar]
- Clusella‐Trullas, S. , Garcia, R.A. , Terblanche, J.S. & Hoffmann, A.A. (2021) How useful are thermal vulnerability indices? Trends Ecol. Evolution, 36, 1000–1010. [DOI] [PubMed] [Google Scholar]
- Collin, R. , Rebolledo, A.P. , Smith, E. & Chan, K.Y.K. (2021) Thermal tolerance of early development predicts the realized thermal niche in marine ectotherms. Functional Ecology, 35, 1679–1692. [Google Scholar]
- Comte, L. & Olden, J.D. (2017) Climatic vulnerability of the world's freshwater and marine fishes. Nature Climate Change, 7, 718–722. [Google Scholar]
- Dahlke, F.T. , Butzin, M. , Wohlrab, S. & Pörtner, H.‐O. (2022) Reply to: methodological inconsistencies define thermal bottlenecks in fish life cycle. Evolutionary Ecology, 36, 293–298. [Google Scholar]
- Dahlke, F.T. , Wohlrab, S. , Butzin, M. & Pörtner, H.‐O. (2020) Thermal bottlenecks in the life cycle define climate vulnerability of fish. Science, 369, 65–70. [DOI] [PubMed] [Google Scholar]
- David, J.R. , Araripe, L.O. , Chakir, M. , Legout, H. , Lemos, B. , Pétavy, G. et al. (2005) Male sterility at extreme temperatures: a significant but neglected phenomenon for understanding Drosophila climatic adaptations. Journal of Evolutionary Biology, 18, 838–846. [DOI] [PubMed] [Google Scholar]
- Denny, M.W. (1993) Thermal Properties: Body temperatures in Air and Water. In: Air and Water. Princeton, New Jersey, USA: The Biology and Physics of Life's Media. Princeton University Press, pp. 145–173. [Google Scholar]
- Dewitt, T.J. , Sih, A. & Wilson, D.S. (1998) Costs and limits of phenotypic plasticity. Trends in Ecology & Evolution, 13, 77–81. [DOI] [PubMed] [Google Scholar]
- Duffy, G.A. , Kuyucu, A.C. , Hoskins, J.L. , Hay, E.M. & Chown, S.L. (2021) Adequate sample sizes for improved accuracy of thermal trait estimates. Functional Ecology, 35, 2647–2662. [Google Scholar]
- Fawcett, T.W. & Frankenhuis, W.E. (2015) Adaptive explanations for sensitive windows in development. Frontiers in Zoology, 12, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo, Y.Z. , O'Dea, R.E. , Koricheva, J. , Nakagawa, S. & Lagisz, M. (2021) A practical guide to question formation, systematic searching and study screening for literature reviews in ecology and evolution. Methods in Ecology and Evolution, 12, 1705–1720. [Google Scholar]
- Fry, F. (1947) Effects of the environment on animal activity. Publications of the Ontario Fisheries Research Laboratory, 55, 1–62. [Google Scholar]
- Gelman, A. , Hill, J. & Vehtari, A. (2020) Poststratification and missing‐data imputation. In: Regression and Other Stories. Cambridge, United Kingdom: Cambridge University Press, pp. 313–336. [Google Scholar]
- Ghalambor, C.K. , Huey, R.B. , Martin, P.R. , Tewksbury, J.J. & Wang, G. (2006) Are mountain passes higher in the tropics? Janzen's hypothesis revisited. Integrative and Comparative Biology, 46, 5–17. [DOI] [PubMed] [Google Scholar]
- Gibert, P. , Debat, V. & Ghalambor, C.K. (2019) Phenotypic plasticity, global change, and the speed of adaptive evolution. Current Opinion in Insect Science, 35, 34–40. [DOI] [PubMed] [Google Scholar]
- Gunderson, A.R. , Dillon, M.E. & Stillman, J.H. (2017) Estimating the benefits of plasticity in ectotherm heat tolerance under natural thermal variability. Functional Ecology, 31, 1529–1539. [Google Scholar]
- Gunderson, A.R. & Stillman, J.H. (2015) Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proceedings of the Royal Society B: Biological Sciences, 282, 20150401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevitch, J. , Koricheva, J. , Nakagawa, S. & Stewart, G. (2018) Meta‐analysis and the science of research synthesis. Nature, 555, 175–182. [DOI] [PubMed] [Google Scholar]
- Hall, J.M. & Warner, D.A. (2019) Thermal tolerance in the urban heat Island: thermal sensitivity varies ontogenetically and differs between embryos of two sympatric ectotherms. The Journal of Experimental Biology, 222, jeb210708. [DOI] [PubMed] [Google Scholar]
- Healy, T.M. , Bock, A.K. & Burton, R.S. (2019) Variation in developmental temperature alters adulthood plasticity of thermal tolerance in Tigriopus californicus. The Journal of Experimental Biology, 222, jeb213405. [DOI] [PubMed] [Google Scholar]
- Hoffmann, A.A. & Sgrò, C.M. (2011) Climate change and evolutionary adaptation. Nature, 470, 479–485. [DOI] [PubMed] [Google Scholar]
- Huey, R.B. , Kearney, M.R. , Krockenberger, A. , Holtum, J.A.M. , Jess, M. & Williams, S.E. (2012) Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Proceedings of the Royal Society B: Biological Sciences, 367, 1665–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen, D.H. (1967) Why mountain passes are higher in the tropics. The American Naturalist, 101, 233–249. [Google Scholar]
- Jørgensen, L.B. , Malte, H. , Ørsted, M. , Klahn, N.A. & Overgaard, J. (2021) A unifying model to estimate thermal tolerance limits in ectotherms across static, dynamic and fluctuating exposures to thermal stress. Scientific Reports, 11, 12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen, L.B. , Malte, H. & Overgaard, J. (2019) How to assess Drosophila heat tolerance: Unifying static and dynamic tolerance assays to predict heat distribution limits. Functional Ecology, 33, 629–642. [Google Scholar]
- Jutfelt, F. , Norin, T. , Ern, R. , Overgaard, J. , Wang, T. , McKenzie, D.J. et al. (2018) Oxygen‐and capacity‐limited thermal tolerance: blurring ecology and physiology. The Journal of Experimental Biology, 221, jeb169615. [DOI] [PubMed] [Google Scholar]
- Kellermann, V. & Sgrò, C.M. (2018) Evidence for lower plasticity in CTMAX at warmer developmental temperatures. Journal of Evolutionary Biology, 31, 1300–1312. [DOI] [PubMed] [Google Scholar]
- Kellermann, V. , van Heerwaarden, B. & Sgrò, C.M. (2017) How important is thermal history? Evidence for lasting effects of developmental temperature on upper thermal limits in Drosophila melanogaster. Proceedings of the Royal Society B: Biological Sciences, 284, 20170447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockmann, M. , Günter, F. & Fischer, K. (2017) Heat resistance throughout ontogeny: body size constrains thermal tolerance. Global Change Biology, 23, 686–696. [DOI] [PubMed] [Google Scholar]
- Koricheva, J. & Kulinskaya, E. (2019) Temporal instability of evidence base: A threat to policy making? Trends Ecology Evolution, 34, 895–902. [DOI] [PubMed] [Google Scholar]
- Lajeunesse, M.J. (2013) Recovering missing or partial data from studies: A survey of conversions and imputations for meta‐analysis. In: Hanbook of meta‐analysis in ecology and evolution. Princeton, NJ: Princeton University Press, pp. 195–206. [Google Scholar]
- Layne, J.R. & Claussen, D.L. (1982) The time courses of CTmax and CTmin acclimation in the salamander Desmognathus fuscus. Journal of Thermal Biology, 7, 139–141. [Google Scholar]
- Lenth, R. , Singmann, H. , Love, J. , Buerkner, P. , & Herve, M. (2019). Emmeans: Estimated marginal means, aka least‐squares means. R package version 1.7.2‐9000003.
- Lutterschmidt, W.I. & Hutchison, V.H. (2011) The critical thermal maximum: history and critique. Canadian Journal of Zoology, 75, 1561–1574. [Google Scholar]
- Marshall, D.J. , Pettersen, A.K. , Bode, M. & White, C.R. (2020) Developmental cost theory predicts thermal environment and vulnerability to global warming. Nature Ecology and Evolution, 4, 406–411. [DOI] [PubMed] [Google Scholar]
- Michonneau, F. , Brown, J.W. & Winter, D.J. (2016) rotl: an R package to interact with the Open Tree of Life data. Methods in Ecology and Evolution, 7, 1476–1481. [Google Scholar]
- Mitchell, K.A. , Sinclair, B.J. & Terblanche, J.S. (2013) Ontogenetic variation in cold tolerance plasticity in Drosophila: is the Bogert effect bogus? Science of Nature, 100, 281–284. [DOI] [PubMed] [Google Scholar]
- Morley, S.A. , Peck, L.S. , Sunday, J.M. , Heiser, S. & Bates, A.E. (2019) Physiological acclimation and persistence of ectothermic species under extreme heat events. Global Ecology and Biogeography, 28, 1018–1037. [Google Scholar]
- Muñoz, M.M. (2021) The Bogert effect, a factor in evolution. Evolution, 76, 4. [DOI] [PubMed] [Google Scholar]
- Murren, C.J. , Auld, J.R. , Callahan, H. , Ghalambor, C.K. , Handelsman, C.A. , Heskel, M.A. et al. (2015) Constraints on the evolution of phenotypic plasticity: limits and costs of phenotype and plasticity. Heredity, 115, 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa, S. , Lagisz, M. , Jennions, M.D. , Koricheva, J. , Noble, D.W. , Parker, T.H. et al. (2022) Methods for testing publication bias in ecological and evolutionary meta‐analyses. Methods in Ecology and Evolution, 13, 4–21. [Google Scholar]
- Nakagawa, S. , Lagisz, M. , O'Dea, R.E. , Rutkowska, J. , Yang, Y. , Noble, D.W.A. et al. (2021) The orchard plot: Cultivating a forest plot for use in ecology, evolution, and beyond. Research Synthesis Methods, 12, 4–12. [DOI] [PubMed] [Google Scholar]
- Noble, D.W.A. , Lagisz, M. , O'dea, R.E. & Nakagawa, S. (2017) Nonindependence and sensitivity analyses in ecological and evolutionary meta‐analyses. Molecular Ecology, 26, 2410–2425. [DOI] [PubMed] [Google Scholar]
- Noble, D.W.A. , Pottier, P. , Lagisz, M. , Burke, S. , Drobniak, S.M. , O'Dea, R.E. et al. (2022) Meta‐analytic approaches and effect sizes to account for ‘nuisance heterogeneity’ in comparative physiology. The Journal of Experimental Biology, 225, jeb243225. [DOI] [PubMed] [Google Scholar]
- Noble, D.W.A. , Radersma, R. & Uller, T. (2019) Plastic responses to novel environments are biased towards phenotype dimensions with high additive genetic variation. PNAS, 116, 13452–13461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble, D.W.A. , Stenhouse, V. & Schwanz, L.E. (2018) Developmental temperatures and phenotypic plasticity in reptiles: a systematic review and meta‐analysis. Biological Reviews, 93, 72–97. [DOI] [PubMed] [Google Scholar]
- O'Dea, R.E. , Lagisz, M. , Hendry, A.P. & Nakagawa, S. (2019) Developmental temperature affects phenotypic means and variability: a meta‐analysis of fish data. Fish and Fisheries, 20, 1005–1022. [Google Scholar]
- O'Dea, R.E. , Lagisz, M. , Jennions, M.D. , Koricheva, J. , Noble, D.W.A. , Parker, T.H. et al. (2021) Preferred reporting items for systematic reviews and meta‐analyses in ecology and evolutionary biology: a PRISMA extension. Biological Reviews, 96, 1695–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouzzani, M. , Hammady, H. , Fedorowicz, Z. & Elmagarmid, A. (2016) Rayyan—a web and mobile app for systematic reviews. Systematic Reviews, 5, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis, E. & Schliep, K. (2019) ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics, 35, 526–528. [DOI] [PubMed] [Google Scholar]
- Parratt, S.R. , Walsh, B.S. , Metelmann, S. , White, N. , Manser, A. , Bretman, A.J. et al. (2021) Temperatures that sterilize males better match global species distributions than lethal temperatures. Nature Climate Change, 11, 481–484. [Google Scholar]
- Pettersen, A.K. , White, C.R. , Bryson‐Richardson, R.J. & Marshall, D.J. (2018) Does the cost of development scale allometrically with offspring size? Functional Ecology, 32, 762–772. [Google Scholar]
- Pick, J.L. , Nakagawa, S. & Noble, D.W.A. (2019) Reproducible, flexible and high‐throughput data extraction from primary literature: the metadigitise R package. Methods in Ecology and Evolution, 10, 426–431. [Google Scholar]
- Pincebourde, S. & Casas, J. (2015) Warming tolerance across insect ontogeny: influence of joint shifts in microclimates and thermal limits. Ecology, 96, 986–997. [DOI] [PubMed] [Google Scholar]
- Pinsky, M.L. , Eikeset, A.M. , Mccauley, D.J. , Payne, J.L. & Sunday, J.M. (2019) Greater vulnerability to warming of marine versus terrestrial ectotherms. Nature, 569, 108–111. [DOI] [PubMed] [Google Scholar]
- Pörtner, H.O. , Bock, C. & Mark, F.C. (2017) Oxygen‐and capacity‐limited thermal tolerance: bridging ecology and physiology. The Journal of Experimental Biology, 220, 2685–2696. [DOI] [PubMed] [Google Scholar]
- Pottier, P. , Burke, S. , Drobniak, S.M. , Lagisz, M. & Nakagawa, S. (2021) Sexual (in)equality? A meta‐analysis of sex differences in thermal acclimation capacity across ectotherms. Functional Ecology, 35, 2663–2678. [Google Scholar]
- Pottier, P. , Burke, S. , Drobniak, S.M. & Nakagawa, S. (2022) Methodological inconsistencies define thermal bottlenecks in fish life cycle: a comment on Dahlke et al. 2020. Evolutionary Ecology, 36, 287–292. [Google Scholar]
- Pottier, P. , Burke, S. , Zhang, R.Y. , Noble, D.W.A. , Schwanz, L.E. , Drobniak, S.M. et al. (2021) Developmental plasticity of heat tolerance in ectotherms: a systematic review and meta‐analysis. Open Science Framework Registries. 10.17605/osf.io/zkx6u [DOI] [Google Scholar]
- Pottier, P. , Burke, S. , Zhang, R.Y. , Noble, D.W.A. , Schwanz, L.E. , Drobniak, S.M. et al. (2022) Data from: Developmental plasticity in thermal tolerance: ontogenetic variation, persistence, and future directions. Zenodo. 10.5281/zenodo.6818559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przeslawski, R. , Byrne, M. & Mellin, C. (2015) A review and meta‐analysis of the effects of multiple abiotic stressors on marine embryos and larvae. Global Change Biology, 21, 2122–2140. [DOI] [PubMed] [Google Scholar]
- R Core Team . (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing. Retrieved from https://www.r‐project.org/
- Refsnider, J.M. , Clifton, I.T. & Vazquez, T.K. (2019) Developmental plasticity of thermal ecology traits in reptiles: Trends, potential benefits, and research needs. Journal of Thermal Biology, 84, 74–82. [DOI] [PubMed] [Google Scholar]
- Rezende, E.L. , Bozinovic, F. , Szilágyi, A. & Santos, M. (2020) Predicting temperature mortality and selection in natural Drosophila populations. Science, 369, 1242–1245. [DOI] [PubMed] [Google Scholar]
- Rezende, E.L. , Castañeda, L.E. & Santos, M. (2014) Tolerance landscapes in thermal ecology. Functional Ecology, 28, 799–809. [Google Scholar]
- Rezende, E.L. , Tejedo, M. & Santos, M. (2011) Estimating the adaptive potential of critical thermal limits: methodological problems and evolutionary implications. Functional Ecology, 25, 111–121. [Google Scholar]
- Rohr, J.R. , Civitello, D.J. , Cohen, J.M. , Roznik, E.A. , Sinervo, B. & Dell, A.I. (2018) The complex drivers of thermal acclimation and breadth in ectotherms. Ecology Letters, 21, 1425–1439. [DOI] [PubMed] [Google Scholar]
- Ruthsatz, K. , Dausmann, K.H. , Peck, M.A. & Glos, J. (2022) Thermal tolerance and acclimation capacity in the European common frog (Rana temporaria) change throughout ontogeny. Journal of Experimental Zoology Part A: Ecological and Integrative Physiology, 337, 477–490. [DOI] [PubMed] [Google Scholar]
- Ruthsatz, K. , Peck, M.A. , Dausmann, K.H. , Sabatino, N.M. & Glos, J. (2018) Patterns of temperature induced developmental plasticity in anuran larvae. Journal of Thermal Biology, 74, 123–132. [DOI] [PubMed] [Google Scholar]
- Sales, K. , Vasudeva, R. & Gage, M.J.G. (2021) Fertility and mortality impacts of thermal stress from experimental heatwaves on different life stages and their recovery in a model insect. Royal Society Open Science, 8, 201717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer, J. & Ryan, A. (2006) Developmental plasticity in the thermal tolerance of zebrafish Danio rerio. Journal of Fish Biology, 69, 722–734. [Google Scholar]
- Seebacher, F. , White, C.R. & Franklin, C.E. (2015) Physiological plasticity increases resilience of ectothermic animals to climate change. Nature Climate Change, 5, 61–66. [Google Scholar]
- Senior, A.M. , Grueber, C.E. , Kamiya, T. , Lagisz, M. , O'Dwyer, K. , Santos, E.S.A. et al. (2016) Heterogeneity in ecological and evolutionary meta‐analyses: its magnitude and implications. Ecology, 97, 3293–3299. [DOI] [PubMed] [Google Scholar]
- Sunday, J.M. , Bates, A.E. & Dulvy, N.K. (2012) Thermal tolerance and the global redistribution of animals. Nature Climate Change, 2, 686–690. [Google Scholar]
- Sunday, J.M. , Bates, A.E. , Kearney, M.R. , Colwell, R.K. , Dulvy, N.K. , Longino, J.T. et al. (2014) Thermal‐safety margins and the necessity of thermoregulatory behavior across latitude and elevation. PNAS, 111, 5610–5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terblanche, J.S. , Deere, J.A. , Clusella‐Trullas, S. , Janion, C. & Chown, S.L. (2007) Critical thermal limits depend on methodological context. Proceedings of the Royal Society B: Biological Sciences, 274, 2935–2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terblanche, J.S. & Hoffmann, A.A. (2020) Validating measurements of acclimation for climate change adaptation. Current Opinion in Insect Science, 41, 7–16. [DOI] [PubMed] [Google Scholar]
- Terblanche, J.S. , Hoffmann, A.A. , Mitchell, K.A. , Rako, L. , le Roux, P.C. & Chown, S.L. (2011) Ecologically relevant measures of tolerance to potentially lethal temperatures. The Journal of Experimental Biology, 214, 3713–3725. [DOI] [PubMed] [Google Scholar]
- Truebano, M. , Fenner, P. , Tills, O. , Rundle, S.D. & Rezende, E.L. (2018) Thermal strategies vary with life history stage. The Journal of Experimental Biology, 221, jeb171629. [DOI] [PubMed] [Google Scholar]
- Turriago, J.L. , Parra, C.A. & Bernal, M.H. (2015) Upper thermal tolerance in anuran embryos and tadpoles at constant and variable peak temperatures. Canadian Journal of Zoology, 93, 267–272. [Google Scholar]
- Uno, H. & Stillman, J.H. (2020) Lifetime eurythermy by seasonally matched thermal performance of developmental stages in an annual aquatic insect. Oecologia, 192, 647–656. [DOI] [PubMed] [Google Scholar]
- van Heerwaarden, B. & Sgrò, C.M. (2021) Male fertility thermal limits predict vulnerability to climate warming. Nature Communications, 12, 2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter, D. , Rücker, G. & Storch, I. (2013) Meta‐analysis: A need for well‐defined usage in ecology and conservation biology. Ecosphere, 4, 1–24. [Google Scholar]
- Viechtbauer, W. (2010) Conducting meta‐analyses in R with the metafor package. Journal of Statistical Software, 36, 1–48. [Google Scholar]
- Walsh, B.S. , Parratt, S.R. , Hoffmann, A.A. , Atkinson, D. , Snook, R.R. , Bretman, A. et al. (2019) The impact of climate change on fertility. Trends in Ecology & Evolution, 34, 249–259. [DOI] [PubMed] [Google Scholar]
- While, G.M. , Noble, D.W.A. , Uller, T. , Warner, D.A. , Riley, J.L. , Du, W.‐G. et al. (2018) Patterns of developmental plasticity in response to incubation temperature in reptiles. Journal of Experimental Zoology Part A: Ecological and Integrative Physiology, 329, 162–176. [DOI] [PubMed] [Google Scholar]
- Zhang, Z.‐Q. (2013) Animal biodiversity: an outline of higher‐level classification and survey of taxonomic richness (Addenda 2013). Zootaxa, 3703, 1–82. [DOI] [PubMed] [Google Scholar]
DATA SOURCES
- Abayarathna, T. , Murray, B.R. & Webb, J.K. (2019a) Higher incubation temperatures produce long‐lasting upward shifts in cold tolerance, but not heat tolerance, of hatchling geckos. Biology Open, 8, bio042564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abayarathna, T. , Murray, B.R. & Webb, J.K. (2019b) Data from: higher incubation temperatures produce long‐lasting upward shifts in cold tolerance, but not heat tolerance, of hatchling geckos. Dryad Digital Repository. 10.5061/dryad.k4pf189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel Salam, A. , Ghanim, A. , Serafi, H.E. , El‐Heneidy, A. & El‐Sherbeni, M. (2009) Biological and life table parameters of Myzus persicae (Salz.) (Hemiptera: Aphididae) in relation to host plants and thermal requirements. Journal of Plant Protection and Pathology, 34, 8251–8262. [Google Scholar]
- Abou Shabana, N.M. , Abd El Rahman, S.H. , Al Absawy, M.A. & Assem, S.S. (2012) Reproductive biology of Argyrosomus regius (Asso, 1801) inhabiting the South Eastern Mediterranean Sea. Egyptian Journal of Aquatic Research, 38, 147–156. [Google Scholar]
- Adamczuk, M. (2020) Population dynamics and life history traits of Daphnia magna across thermal regimes of environments. Science of the Total Environment, 723, 137963. [DOI] [PubMed] [Google Scholar]
- Agnew, P. , Bedhomme, S. , Haussy, C. & Michalakis, Y. (1999) Age and size at maturity of the mosquito Culex pipiens infected by the microsporidian parasite Vavraia culicis. Proceedings of the Royal Society B: Biological Sciences, 266, 947–952. [Google Scholar]
- Akhtar, M.S. , Pal, A.K. , Sahu, N.P. , Ciji, A. & Mahanta, P.C. (2013) Thermal tolerance, oxygen consumption and haemato‐biochemical variables of Tor putitora juveniles acclimated to five temperatures. Fish Physiology and Biochemistry, 39, 1387–1398. [DOI] [PubMed] [Google Scholar]
- Alford, L. (2010). The thermal macrophysiology of core and marginal populations of the aphid Myzus persicae in Europe. PhD thesis. The University of Birmingham, United Kingdom, pp. 169.
- Alford, L. , Blackburn, T.M. & Bale, J.S. (2012) Effect of latitude and acclimation on the lethal temperatures of the peach‐potato aphid Myzus persicae . Agricultural and Forest Entomology, 14, 69–79. [Google Scholar]
- Archambault, J.M. , Cope, W.G. & Kwak, T.J. (2014a) Influence of sediment presence on freshwater mussel thermal tolerance. Freshwater Science, 33, 56–65. [Google Scholar]
- Archambault, J.M. , Cope, W.G. & Kwak, T.J. (2014b) Survival and behaviour of juvenile unionid mussels exposed to thermal stress and dewatering in the presence of a sediment temperature gradient. Freshwater Biology, 59, 601–613. [Google Scholar]
- Ashaf‐Ud‐Doulah, M. , Mamun, A.A. , Rahman, M.L. , Islam, S.M.M. , Jannat, R. , Hossain, M.A.R. et al. (2020) High temperature acclimation alters upper thermal limits and growth performance of Indian major carp, rohu, Labeo rohita (Hamilton, 1822). Journal of Thermal Biology, 93, 102738. [DOI] [PubMed] [Google Scholar]
- Åsheim, E.R. , Andreassen, A.H. , Morgan, R. & Jutfelt, F. (2020a) Rapid‐warming tolerance correlates with tolerance to slow warming but not growth at non‐optimal temperatures in zebrafish. The Journal of Experimental Biology, 223, jeb229195. [DOI] [PubMed] [Google Scholar]
- Åsheim, E.R. , Andreassen, A.H. , Morgan, R. & Jutfelt, F. (2020b) Data from: Rapid‐warming tolerance correlates with tolerance to slow warming but not growth at non‐optimal temperatures in zebrafish. Figshare Digital Repository. 10.6084/m9.figshare.12311102.v2 [DOI] [PubMed] [Google Scholar]
- Azra, M.N. , Chen, J.‐C. , Ikhwanuddin, M. & Abol‐Munafi, A.B. (2018) Thermal tolerance and locomotor activity of blue swimmer crab Portunus pelagicus instar reared at different temperatures. Journal of Thermal Biology, 74, 234–240. [DOI] [PubMed] [Google Scholar]
- Bai, C.‐M. , Ma, G. , Cai, W.‐Z. & Ma, C.‐S. (2019a) Independent and combined effects of daytime heat stress and night‐time recovery determine thermal performance. Biology Open, 8, bio038141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, C.‐M. , Ma, G. , Cai, W.‐Z. & Ma, C.‐S. (2019b). Data from: Independent and combined effects of daytime heat stress and night‐time recovery determine thermal performance. Mendeley Digital Repository. Available at: 10.17632/kvj8k99y3f.1 [DOI] [PMC free article] [PubMed]
- Barker, M.F. (2001) The ecology of Evechinus chloroticus . In: Developments in Aquaculture and Fisheries Science, Edible Sea Urchins: Biology and Ecology. Amsterdam, Netherlands: Elsevier, pp. 245–260. [Google Scholar]
- Baroudy, E. & Elliott, J.M. (1994) The critical thermal limits for juvenile Arctic charr Salvelinus alpinus . Journal of Fish Biology, 45, 1041–1053. [Google Scholar]
- Becker, C.D. & Genoway, R.G. (1979) Evaluation of the critical thermal maximum for determining thermal tolerance of freshwater fish. Environmental Biology of Fishes, 4, 245–256. [Google Scholar]
- Benedict, M.Q. , Cockburn, A.F. & Seawright, J.A. (1991) Heat‐shock mortality and induced thermotolerance in larvae of the mosquito Anopheles albimanus . Journal of the American Mosquito Control Association, 7, 547–550. [PubMed] [Google Scholar]
- Billman, E.J. , Wagner, E.J. , Arndt, R.E. & Vandyke, E. (2008) Optimal temperatures for growth and upper thermal tolerance of juvenile northern leatherside chub. Western North American Naturalist, 68, 463–474. [Google Scholar]
- Bishai, H.M. (1960) Upper lethal temperatures for larval salmonids. ICES Journal of Marine Science, 25, 129–133. [Google Scholar]
- Blair, S.D. & Glover, C.N. (2019) Acute exposure of larval rainbow trout (Oncorhynchus mykiss) to elevated temperature limits hsp70b expression and influences future thermotolerance. Hydrobiologia, 836, 155–167. [Google Scholar]
- Bowden, A.J. , Andrewartha, S.J. , Elliott, N.G. , Frappell, P.B. & Clark, T.D. (2018) Negligible differences in metabolism and thermal tolerance between diploid and triploid Atlantic salmon (Salmo salar). The Journal of Experimental Biology, 221, jeb166975. [DOI] [PubMed] [Google Scholar]
- Britton, D.K. (2005). The nature of thermal tolerance in the western mosquitofish, Gambusia affinis, exposed to heated effluents. PhD thesis. The University of Texas at Arlington, Texas, USA, pp. 223.
- Bugg, W.S. , Yoon, G.R. , Schoen, A.N. , Laluk, A. , Br, T.C. , Brandt, C. et al. (2020) Effects of acclimation temperature on the thermal physiology in two geographically distinct populations of lake sturgeon (Acipenser fulvescens). Conservation Physiology, 8, coaa087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, Z.‐P. & Chen, H.‐R. (2005) Thermal effects of temperature on two commercially important shrimp species in Daya Bay. Shengtai Xuebao, 25, 1115–1122. [Google Scholar]
- Call, D. & Malon, M. (2014). Conservation Plan for the Black Sandshell and Butterfly Mussels. Report to the U.S. Fish & Wildlife Service and Illinois Department of Natural Resources.
- Carbonell, J.A. & Stoks, R. (2020) Thermal evolution of life history and heat tolerance during range expansions toward warmer and cooler regions. Ecology, 101, e03134. [DOI] [PubMed] [Google Scholar]
- Cavalheri, H.B. , Symons, C.C. , Schulhof, M. , Jones, N.T. & Shurin, J.B. (2019) Rapid evolution of thermal plasticity in mountain lake Daphnia populations. Oikos, 128, 692–700. [Google Scholar]
- Chatterjee, N. , Pal, A.K. , Manush, S.M. , Das, T. & Mukherjee, S.C. (2004) Thermal tolerance and oxygen consumption of Labeo rohita and Cyprinus carpio early fingerlings acclimated to three different temperatures. Journal of Thermal Biology, 29, 265–270. [Google Scholar]
- Chen, J.‐C. & Chen, W.‐C. (1999) Temperature tolerance of Haliotis diversicolor supertexta at different salinity and temperature levels. Comparative Biochemistry and Physiology ‐ Part A: Molecular & Integrative Physiology, 124, 73–80. [Google Scholar]
- Chen, P. (2008). Ecological niche modeling as a predictive tool: Asiatic freshwater fishes in North America. PhD Thesis. University of Kansas, Kansas, USA, pp. 195.
- Chen, Z. , Anttila, K. , Wu, J. , Whitney, C.K. , Hinch, S.G. & Farrell, A.P. (2013) Optimum and maximum temperatures of sockeye salmon (Oncorhynchus nerka) populations hatched at different temperatures. Canadian Journal of Zoology, 91, 265–274. [Google Scholar]
- Cheung, K. (2019). The effects of embryonic incubation temperature on subsequent development, growth, and thermal tolerance through early ontogeny of White Sturgeon. MSc Thesis. University of British Columbia, Canada, pp. 92.
- Chidawanyika, F. & Terblanche, J.S. (2011) Costs and benefits of thermal acclimation for codling moth, Cydia pomonella (Lepidoptera: Tortricidae): implications for pest control and the sterile insect release programme. Evolutionary Applications, 4, 534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, A.M. , Duston, J. & Bradford, R.G. (2006) Thermal tolerance of a northern population of striped bass Morone saxatilis . Journal of Fish Biology, 69, 1482–1490. [Google Scholar]
- CSIRO . (2014) Draft Assessment Report: application to amend the List of Specimens Suitable for Live Import (Haliotis discus hannai). CSIRO Australian Animal Health. The Laboratory. [Google Scholar]
- Currie, R. , Bennett, W. & Beitinger, T. (1998) Critical thermal minima and maxima of three freshwater game‐fish species acclimated to constant temperatures. Environmental Biology of Fishes, 51, 187–200. [Google Scholar]
- Dang, W. , Hu, Y.‐C. , Geng, J. , Wang, J. & Lu, H.‐L. (2019) Thermal physiological performance of two freshwater turtles acclimated to different temperatures. Journal of Comparative Physiology B, 189, 121–130. [DOI] [PubMed] [Google Scholar]
- Das, T. , Pal, A. , Chakraborty, S. , Manush, S. , Chatterjee, N. & Mukherjee, S. (2004) Thermal tolerance and oxygen consumption of Indian Major Carps acclimated to four temperatures. Journal of Thermal Biology, 29, 157–163. [Google Scholar]
- Das, T. , Pal, A.K. , Chakraborty, S.K. , Manush, S.M. , Sahu, N.P. & Mukherjee, S.C. (2005) Thermal tolerance, growth and oxygen consumption of Labeo rohita fry (Hamilton, 1822) acclimated to four temperatures. Journal of Thermal Biology, 30, 378–383. [Google Scholar]
- Dash, G. , Koya, M. , Dash, S.S. , Sreenath, K.R. , Vase, V.K. , Pradhan, R.K. et al. (2018) Fishery, population dynamics and stock status of Jinga shrimp, Metapenaeus affinis (Milne‐Edwards, 1837) from Gujarat waters of India. Indian Journal of Marine Sciences, 47, 2267–2277. [Google Scholar]
- Dayananda, B. , Murray, B.R. & Webb, J.K. (2017a) Hotter nests produce hatchling lizards with lower thermal tolerance. The Journal of Experimental Biology, 220, 2159–2165. [DOI] [PubMed] [Google Scholar]
- Dayananda, B. , Murray, B.R. & Webb, J.K. (2017b) Data from: Hotter nests produce hatchling lizards with lower thermal tolerance. Dryad Digital Repository. 10.5061/dryad.dp1fh [DOI] [PubMed] [Google Scholar]
- de Beeck, L.O. , Verheyen, J. & Stoks, R. (2017) Integrating both interaction pathways between warming and pesticide exposure on upper thermal tolerance in high‐ and low‐latitude populations of an aquatic insect. Environmental Pollution, 224, 714–721. [DOI] [PubMed] [Google Scholar]
- de Beeck, L.O. , Verheyen, J. & Stoks, R. (2018a) Competition magnifies the impact of a pesticide in a warming world by reducing heat tolerance and increasing autotomy. Environmental Pollution, 233, 226–234. [DOI] [PubMed] [Google Scholar]
- de Beeck, L.O. , Verheyen, J. & Stoks, R. (2018b) Strong differences between two congeneric species in sensitivity to pesticides in a warming world. Science of the Total Environment, 618, 60–69. [DOI] [PubMed] [Google Scholar]
- Debnath, D. , Pal, A.K. , Sahu, N.P. , Baruah, K. , Yengkokpam, S. , Das, T. et al. (2006) Thermal tolerance and metabolic activity of yellowtail catfish Pangasius pangasius (Hamilton) advanced fingerlings with emphasis on their culture potential. Aquaculture, 258, 606–610. [Google Scholar]
- Del Rio, A.M. , Davis, B.E. , Fangue, N.A. & Todgham, A.E. (2019) Combined effects of warming and hypoxia on early life stage Chinook salmon physiology and development. Conservation Physiology, 7, coy078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme Juri, N. (2017). Thermal biology of the New Zealand sea urchin Evechinus chloroticus . PhD thesis. University of Auckland, New Zealand, pp. 181.
- Deslauriers, D. , Heironimus, L. & Chipps, S.R. (2016) Lethal Thermal Maxima for Age‐0 Pallid and Shovelnose Sturgeon: Implications for Shallow Water Habitat Restoration. River Research and Applications, 32, 1872–1878. [Google Scholar]
- Dhillon, R.S. & Fox, M.G. (2004) Growth‐independent effects of temperature on age and size at maturity in japanese medaka (Oryzias latipes). Copeia, 2004, 37–45. [Google Scholar]
- Díaz Herrera, F. , Uribe, E.S. , Ramirez, L.F.B. & Mora, A.G. (1998) Critical thermal maxima and minima of Macrobrachium rosenbergii (Decapoda: Palaemonidae). Journal of Thermal Biology, 23, 381–385. [Google Scholar]
- Donelson, J.M. (2015) Development in a warm future ocean may enhance performance in some species. Journal of Experimental Marine Biology and Ecology, 472, 119–125. [Google Scholar]
- Dülger, N. , Kumlu, M. , Türkmen, S. , Ölçülü, A. , Tufan Eroldoĝan, O. , Asuman Yilmaz, H. et al. (2012) Thermal tolerance of European Sea Bass (Dicentrarchus labrax) juveniles acclimated to three temperature levels. Journal of Thermal Biology, 37, 79–82. [Google Scholar]
- Duponchelle, F. & Panfili, J. (1998) Variations in age and size at maturity of female Nile tilapia, Oreochromis niloticus, populations from man‐made lakes of Côte D'ivoire. Environmental Biology of Fishes, 52, 453–465. [Google Scholar]
- Ebel, W. , Dawley, E. & Monk, B. (1971) Thermal tolerance of juvenile pacific salmon and steelhead trout in relation to supersaturation of nitrogen gas. Fishery Bulletin‐ National Oceanic and Atmospheric Administration, 69, 833. [Google Scholar]
- Edenbrow, M. & Croft, D.P. (2013) Environmental and genetic effects shape the development of personality traits in the mangrove killifish Kryptolebias marmoratus . Oikos, 122, 667–681. [Google Scholar]
- Elliott, J.M. (1991) Tolerance and resistance to thermal stress in juvenile Atlantic salmon, Salmo salar . Freshwater Biology, 25, 61–70. [Google Scholar]
- Elliott, J.M. & Klemetsen, A. (2002) The upper critical thermal limits for alevins of Arctic charr from a Norwegian lake north of the Arctic circle. Journal of Fish Biology, 60, 1338–1341. [Google Scholar]
- Esquer Mendez, J.L. , Mónica Hernández, R. & Bückle Ramirez, L.F. (2010) Thermal tolerance and compatibility zones as a tool to establish the optimum culture condition of the halibut Paralichthys californicus (Ayres, 1859). Aquaculture Research, 41, 1015–1021. [Google Scholar]
- Faleiro, F. , Pimentel, M. , Pegado, M.R. , Bispo, R. , Lopes, A.R. , Diniz, M.S. et al. (2016) Small pelagics in a changing ocean: Biological responses of sardine early stages to warming. Conservation Physiology, 4, cow017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields, R. , Lowe, S.S. , Kaminski, C. , Whitt, G.S. & Philipp, D.P. (1987) Critical and chronic thermal maxima of northern and Florida largemouth bass and their reciprocal F1 and F2 hybrids. Transactions of the American Fisheries Society, 116, 856–863. [Google Scholar]
- Floyd, R. (1985) Effects of photoperiod and starvation on the temperature tolerance of larvae of the giant toad. Bufo marinus. Copeia, 1985, 625–631. [Google Scholar]
- Floyd, R.B. (1983) Ontogenetic change in the temperature tolerance of larval Bufo marinus (Anura: Bufonidae). Comparative Biochemistry and Physiology ‐ Part A: Molecular & Integrative Physiology, 75, 267–271. [Google Scholar]
- Gervais, C.R. , Huveneers, C. , Rummer, J.L. & Brown, C. (2021) Population variation in the thermal response to climate change reveals differing sensitivity in a benthic shark. Global Change Biology, 27, 108–120. [DOI] [PubMed] [Google Scholar]
- Gibson, D.J. , Sylvester, E.V.A. , Turko, A.J. , Tattersall, G.J. & Wright, P.A. (2015) Out of the frying pan into the air‐emersion behaviour and evaporative heat loss in an amphibious mangrove fish (Kryptolebias marmoratus). Biology Letters, 11, 20150689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez Isaza, D.F. , Cramp, R.L. & Franklin, C.E. (2020) Thermal acclimation offsets the negative effects of nitrate on aerobic scope and performance. The Journal of Experimental Biology, 223, jeb224444. [DOI] [PubMed] [Google Scholar]
- Grabowski, T.B. , Young, S.P. , Isely, J.J. & Ely, P.C. (2012) Age, growth, and reproductive biology of three Catostomids from the Apalachicola River, Florida. Journal of Fish and Wildlife Management, 3, 223–237. [Google Scholar]
- Gray, E.M. (2013) Thermal acclimation in a complex life cycle: The effects of larval and adult thermal conditions on metabolic rate and heat resistance in Culex pipiens (Diptera: Culicidae). Journal of Insect Physiology, 59, 1001–1007. [DOI] [PubMed] [Google Scholar]
- Grunert, L.W. , Clarke, J.W. , Ahuja, C. , Eswaran, H. & Nijhout, H.F. (2015) A quantitative analysis of growth and size regulation in Manduca sexta: the physiological basis of variation in size and age at metamorphosis. PLoS One, 10, e0127988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson, A.R. , Fargevieille, A. & Warner, D.A. (2020) Egg incubation temperature does not influence adult heat tolerance in the lizard Anolis sagrei . Biology Letters, 16, 20190716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag, W.R. & Rypel, A.L. (2011) Growth and longevity in freshwater mussels: evolutionary and conservation implications. Biological Reviews of the Cambridge Philosophical Society, 86, 225–247. [DOI] [PubMed] [Google Scholar]
- He, Y. , Wu, X. , Zhu, Y. , Li, H. , Li, X. & Yang, D. (2014) Effect of rearing temperature on growth and thermal tolerance of Schizothorax (Racoma) kozlovi larvae and juveniles. Journal of Thermal Biology, 46, 24–30. [DOI] [PubMed] [Google Scholar]
- Healy, T.M. , Bock, A.K. & Burton, R.S. (2019) Variation in developmental temperature alters adulthood plasticity of thermal tolerance in Tigriopus californicus . The Journal of Experimental Biology, 222, jeb213405. [DOI] [PubMed] [Google Scholar]
- Healy, T.M. , Bock, A.K. & Burton, R.S. (2019) Data from: Variation in developmental temperature alters adulthood plasticity of thermal tolerance in Tigriopus californicus . Dryad Digital Repository. 10.5061/dryad.np5hqbzp0 [DOI] [PubMed] [Google Scholar]
- Huang, J. , Yang, Q. , Ma, Z. , Chen, X. , Zhou, F. , Wen, W. et al. (2013) The growth, development and sexual maturity of pond‐reared Penaeus monodon . Journal of Fisheries of China, 37, 397–406. [Google Scholar]
- Ibarra, L. , Alvarez‐Lajonchere, L. , García Aguilar, N. , María, I. , Parra, A. & Rodríguez‐Ibarra, L. (2012) Generation cycle closure of the spotted rose snapper, Lutjanus guttatus, in captivity. Revista de Biología Marina y Oceanografía, 47, 333–337. [Google Scholar]
- Illing, B. , Downie, A.T. , Beghin, M. & Rummer, J.L. (2020a) Critical thermal maxima of early life stages of three tropical fishes: Effects of rearing temperature and experimental heating rate. Journal of Thermal Biology, 90, 102582. [DOI] [PubMed] [Google Scholar]
- Illing, B. , Downie, A.T. , Beghin, M. & Rummer, J.L. (2020b) Data from: Critical thermal maxima of early life stages of three tropical fishes: Effects of rearing temperature and experimental heating rate. Dryad Digital Repository. 10.5061/dryad.z8w9ghx7z [DOI] [PubMed] [Google Scholar]
- Ineno, T. , Tsuchida, S. & K, a, M. & Watabe, S. (2005) Thermal tolerance of a rainbow trout Oncorhynchus mykiss strain selected by high‐temperature breeding. Fisheries Science, 71, 767–775. [Google Scholar]
- Joshi, K.D. , Das, S.C.S. , Pathak, R.K. , Khan, A. , Sarkar, U.K. & Roy, K. (2018) Pattern of reproductive biology of the endangered golden mahseer Tor putitora (Hamilton 1822) with special reference to regional climate change implications on breeding phenology from lesser Himalayan region, India. Journal of Applied Animal Research, 46, 1289–1295. [Google Scholar]
- Kamilov, B. , Yuldashov, M. , Khakimova, R. & Ibodova, M. (2021) Age and growth of two bream species in the Tudakul reservoir of Uzbekistan. E3S Web Conference, 244, 02041. [Google Scholar]
- Kavanagh, K.D. (1996). The early life history of the brooding damselfish Acanthochromis polycanthus: effects of environment and ancestry. PhD thesis. James Cook University, Townsville, Australia.
- Kelly, N.I. , Burness, G. , Mcdermid, J.L. & Wilson, C.C. (2014) Ice age fish in a warming world: minimal variation in thermal acclimation capacity among lake trout (Salvelinus namaycush) populations. Conservation Physiology, 2, cou025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerfoot, J.R. (2012) Thermal tolerance of the invasive belonesox belizanus, pike killifish, throughout ontogeny. Journal of Experimental Zoology Part A: Ecological and Integrative Physiology, 317, 266–274. [DOI] [PubMed] [Google Scholar]
- Kern, P. , Cramp, R.L. , Seebacher, F. , Ghanizadeh Kazerouni, E. & Franklin, C.E. (2015) Plasticity of protective mechanisms only partially explains interactive effects of temperature and UVR on upper thermal limits. Comparative Biochemistry and Physiology ‐ Part A: Molecular & Integrative Physiology, 190, 75–82. [DOI] [PubMed] [Google Scholar]
- Kingsolver, J.G. , Maclean, H.J. , Goddin, S.B. & Augustine, K.E. (2016) Plasticity of upper thermal limits to acute and chronic temperature variation in Manduca sexta larvae. The Journal of Experimental Biology, 219, 1290–1294. [DOI] [PubMed] [Google Scholar]
- Kır, M. (2020) Thermal tolerance and standard metabolic rate of juvenile gilthead seabream (Sparus aurata) acclimated to four temperatures. Journal of Thermal Biology, 93, 102739. [DOI] [PubMed] [Google Scholar]
- Kır, M. & Demirci, Ö. (2018) Thermal tolerance and standard metabolic rate of juvenile European sea bass (Dicentrarchus labrax, Linnaeus, 1758) acclimated to four temperatures. Journal of Thermal Biology, 78, 209–213. [DOI] [PubMed] [Google Scholar]
- Kır, M. , Sunar, M.C. & Altındağ, B.C. (2017) Thermal tolerance and preferred temperature range of juvenile meagre acclimated to four temperatures. Journal of Thermal Biology, 65, 125–129. [DOI] [PubMed] [Google Scholar]
- Komoroske, L.M. , Connon, R.E. , Lindberg, J. , Cheng, B.S. , Castillo, G. , Hasenbein, M. et al. (2014) Ontogeny influences sensitivity to climate change stressors in an endangered fish. Conservation Physiology, 2, cou008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumlu, M. , Türkmen, S. & Kumlu, M. (2010) Thermal tolerance of Litopenaeus vannamei (Crustacea: Penaeidae) acclimated to four temperatures. Journal of Thermal Biology, 35, 305–308. [Google Scholar]
- Larios Soriano, E. , Re‐Araujo, A.D. , Díaz, F. , Sánchez, C.G. , López‐Galindo, L. , Castro, L.I. et al. (2020) Effect of acclimation temperature on thermoregulatory behaviour, thermal tolerance and respiratory metabolism of Lutjanus guttatus and the response of heat shock protein 70 (Hsp70) and lactate dehydrogenase (Ldh‐a) genes. Aquaculture Research, 51, 1089–1100. [Google Scholar]
- Lawrence, C. , Adatto, I. , Best, J. , James, A. & Maloney, K. (2012) Generation time of zebrafish (Danio rerio) and medakas (Oryzias latipes) housed in the same aquaculture facility. Laboratory Animals, 41, 158–165. [DOI] [PubMed] [Google Scholar]
- Lee, S.‐K. , Park, B. , Jeon, S.‐W. , Jeong, I.‐H. , Park, S.‐K. , Kim, J.‐H. et al. (2017) The temperature‐dependent development characteristic of predatory natural enemy, Propylea japonica Thunberg (Coleoptera: Coccinellidae). Korean Journal of Environmental Agriculture, 25, 861–873. [Google Scholar]
- León Palomino, C. , Flores‐Mego, J. , Dionicio‐Acedo, J. , Rosado‐Salazar, M. , Flye‐Sainte‐Marie, J. & Aguirre‐Velarde, A. (2017) Thermal preference and tolerance of peruvian grunt Anisotremus scapularis juveniles (Pisces: Haemulidae). Revista de Biología Marina y Oceanografía, 52, 581–589. [Google Scholar]
- Li, A.J. , Leung, P.T.Y. , Bao, V.W.W. , Lui, G.C.S. & Leung, K.M.Y. (2015) Temperature‐dependent physiological and biochemical responses of the marine medaka Oryzias melastigma with consideration of both low and high thermal extremes. Journal of Thermal Biology, 54, 98–105. [DOI] [PubMed] [Google Scholar]
- Linton, T.K. , Morgan, I.J. , Reid, S.D. & Wood, C.M. (1998) Long‐term exposure to small temperature increase and sublethal ammonia in hardwater acclimated rainbow trout: Does acclimation occur? Aquatic Toxicology, 40, 171–191. [Google Scholar]
- Llewelyn, J. , Macdonald, S.L. , Moritz, C. , Martins, F. , Hatcher, A. & Phillips, B.L. (2018) Adjusting to climate: acclimation, adaptation and developmental plasticity in physiological traits of a tropical rainforest lizard. Integrative Zoology, 13, 411–427. [DOI] [PubMed] [Google Scholar]
- Lohr, S.C. , Byorth, P.A. , Kaya, C.M. & Dwyer, W.P. (1996) High‐temperature tolerances of fluvial arctic grayling and comparisons with summer river temperatures of the big hole river, montana. Transactions of the American Fisheries Society, 125, 933–939. [Google Scholar]
- Lu, H. , Hu, Y. , Li, S. , Dang, W. & Zhang, Y. (2020) Acclimatory responses of thermal physiological performances in hatchling yellow pond turtles (Mauremys mutica). Animal Biology, 70, 55–65. [Google Scholar]
- Ma, G. & Ma, C.‐S. (2012) Climate warming may increase aphids' dropping probabilities in response to high temperatures. Journal of Insect Physiology, 58, 1456–1462. [DOI] [PubMed] [Google Scholar]
- Mahmood, F. (1997) Life‐table attributes of Anopheles albimanus (Wiedemann) under controlled laboratory conditions. Journal of Vector Ecology, 22, 103–108. [PubMed] [Google Scholar]
- Manriquez, P.H. , Gonzalez, C.P. , Brokordt, K. , Pereira, L. , Torres, R. , Lattuca, M.E. et al. (2019) Ocean warming and acidification pose synergistic limits to the thermal niche of an economically important echinoderm. Science of the Total Environment, 693, 133469. [DOI] [PubMed] [Google Scholar]
- Mascaró, M. , Amaral‐Ruiz, M. , Huipe‐Zamora, I. , Martínez‐Moreno, G. , Simões, N. & Rosas, C. (2016) Thermal tolerance and phenotypic plasticity in juvenile Hippocampus erectus Perry, 1810: effect of acute and chronic exposure to contrasting temperatures. Journal of Experimental Marine Biology and Ecology, 483, 112–119. [Google Scholar]
- McCauley, R. (1963) Lethal temperatures of the developmental stages of the sea lamprey, Petromyzon marinus l. Journal of the Fisheries Research Board of Canada, 20, 483–490. [Google Scholar]
- McDermid, J.L. , Wilson, C.C. , Sloan, W.N. & Shuter, B.J. (2013) Intraspecific differences in thermal biology among inland lake trout populations. Transactions of the American Fisheries Society, 142, 756–766. [Google Scholar]
- McDonnell, L.H. , Reemeyer, J.E. & Chapman, L.J. (2019) Independent and interactive effects of long‐term exposure to hypoxia and elevated water temperature on behavior and thermal tolerance of an equatorial cichlid. Physiological and Biochemical Zoology, 92, 253–265. [DOI] [PubMed] [Google Scholar]
- Medina‐Romo, E.Z. , Díaz, F. , Re‐Araujo, A.D. , Ibarra‐Castro, L. , Garduño‐Lugo, M. , Latorre‐Pozos, E.R. et al. (2018) Thermal tolerance and aerobic scope of tetra‐hybrid tilapia Pargo‐UNAM. Latin American Journal of Aquatic Research, 46, 935–944. [Google Scholar]
- Meng, X.‐I. , Ji, T.T. , Dong, Y.W. , Wang, Q.l. & Dong, S.l. (2009) Thermal resistance in sea cucumbers (Apostichopus japonicus) with differing thermal history: The role of Hsp70. Aquaculture, 294, 314–318. [Google Scholar]
- Mitchell, J.D. , Hewitt, P.H. & van der Linde, T.C.D.K. (1993) Critical thermal limits and temperature tolerance in the harvester termite Hodotermes mossambicus (Hagen). Journal of Insect Physiology, 39, 523–528. [Google Scholar]
- Moreno, A. , Pereira, J. & Cunha, M. (2005) Environmental influences on age and size at maturity of Loligo vulgaris . Aquatic Living Resources, 18, 377–384. [Google Scholar]
- Moyano, M.C. , Ebat, C. , Ruhbaum, Y. , Álvarez‐Fernández, S. , Claireaux, G. , Candebat, C. et al. (2017) Effects of warming rate, acclimation temperature and ontogeny on the critical thermal maximum of temperate marine fish larvae. PLoS One, 12, e0179928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyano, M.C. , Ebat, C. , Ruhbaum, Y. , Álvarez‐Fernández, S. , Claireaux, G. et al. (2017) Data from: Effects of warming rate, acclimation temperature and ontogeny on the critical thermal maximum of temperate marine fish larvae . Dryad Digital Repository. 10.5061/dryad.t5453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, C.A. , Bucsky, J. , Korito, L. & Manzanares, S. (2019) Immediate and persistent effects of temperature on oxygen consumption and thermal tolerance in embryos and larvae of the Baja California chorus frog. Frontiers in Physiology, 10, 754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz, N.J. , Farrell, A.P. , Heath, J.W. & Neff, B.D. (2018) Hematocrit is associated with thermal tolerance and modulated by developmental temperature in Juvenile Chinook Salmon. Physiological and Biochemical Zoology, 91, 757–762. [DOI] [PubMed] [Google Scholar]
- Mutamiswa, R. , Chidawanyika, F. & Nyamukondiwa, C. (2018a) Superior basal and plastic thermal responses to environmental heterogeneity in invasive exotic stemborer Chilo partellus Swinhoe over indigenous Busseola fusca (Fuller) and Sesamia calamistis Hampson. Physiological Entomology, 43, 108–119. [Google Scholar]
- Mutamiswa, R. , Chidawanyika, F. & Nyamukondiwa, C. (2018b) Thermal plasticity potentially mediates the interaction between host Chilo partellus Swinhoe (Lepidoptera: Crambidae) and endoparasitoid Cotesia flavipes Cameron (Hymenoptera: Braconidae) in rapidly changing environments. Pest Management Science, 74, 1335–1345. [DOI] [PubMed] [Google Scholar]
- Myrick, C. & Cech, J. (2000) Temperature influences on California rainbow trout physiological performance. Fish Physiology and Biochemistry, 22, 245–254. [Google Scholar]
- Myrick, C.A. & Cech, J.J., Jr. (2005) Effects of temperature on the growth, food consumption, and thermal tolerance of age‐0 nimbus‐strain steelhead. North American Journal of Aquaculture, 67, 324–330. [Google Scholar]
- Myrick, C.A.E.R (1998). Temperature, genetic, and ration effects on juvenile rainbow trout (Oncorhynchus mykiss) bioenergetics. PhD thesis. University of California Davis, California, USA, pp. 170.
- Noyola, J. , Caamal‐Monsreal, C. , Díaz, F. , Re, D. , Sánchez, A. & Rosas, C. (2013) Thermopreference, tolerance and metabolic rate of early stages juvenile Octopus maya acclimated to different temperatures. Journal of Thermal Biology, 38, 14–19. [DOI] [PubMed] [Google Scholar]
- OECD . (2018) Reproductive biology of the mosquito Aedes aegypti. Paris, France: OECD. [Google Scholar]
- Ogino, Y. , Furumitsu, K. , Kiriyama, T. , Yamaguchi, A. , Ogino, Y. , Furumitsu, K. et al. (2019) Using optimised otolith sectioning to determine the age, growth and age at sexual maturity of the herbivorous fish Kyphosus bigibbus: with a comparison to using scales. Marine and Freshwater Research, 71, 855–867. [Google Scholar]
- Opuszyňski, K. , Lirski, A. , Myszkowski, I. & Wolnicki, J. (1989) Upper lethal and rearing temperatures for juvenile common carp, Cyprinus carpio L., and silver carp, Hypophthalmichthys molitrix (Valenciennes). Aquaculture Research, 20, 287–294. [Google Scholar]
- Orille, A.C. , McWhinnie, R.B. , Brady, S.P. & Raffel, T.R. (2020) Positive Effects of Acclimation Temperature on the Critical Thermal Maxima of Ambystoma mexicanum and Xenopus laevis . Journal of Herpetology, 54, 289–292. [Google Scholar]
- Oyamaguchi, H.M. , Vo, P. , Grewal, K. , Do, R. , Erwin, E. , Jeong, N. et al. (2018a) Thermal sensitivity of a Neotropical amphibian (Engystomops pustulosus) and its vulnerability to climate change. Biotropica, 50, 326–337. [Google Scholar]
- Oyamaguchi, H.M. , Vo, P. , Grewal, K. , Do, R. , Erwin, E. , Jeong, N. et al. (2018b) Data from: Thermal sensitivity of a Neotropical amphibian (Engystomops pustulosus) and its vulnerability to climate change . Dryad Digital Repository. 10.5061/dryad.rv982 [DOI] [Google Scholar]
- Pandolfo, T. , Cope, W.G. & Arellano, C. (2010) Thermal tolerance of juvenile freshwater mussels (Unionidae) under the added stress of copper. Environmental Toxicology and Chemistry, 29, 691–699. [DOI] [PubMed] [Google Scholar]
- Pandolfo, T. , Cope, W.G. , Arellano, C. , Bringolf, R.B. , Barnhart, M.C. & Hammer, E. (2010) Upper thermal tolerances of early life stages of freshwater mussels. Journal of the North American Benthological Society, 29, 959–969. [Google Scholar]
- Peng, J. , Cao, Z.‐D. & Fu, S.‐J. (2014) The effects of constant and diel‐fluctuating temperature acclimation on the thermal tolerance, swimming capacity, specific dynamic action and growth performance of juvenile Chinese bream. Comparative Biochemistry and Physiology ‐ Part A: Molecular & Integrative Physiology, 176, 32–40. [DOI] [PubMed] [Google Scholar]
- Pereira, R.J. , Sasaki, M.C. & Burton, R.S. (2017a) Adaptation to a latitudinal thermal gradient within a widespread copepod species: the contributions of genetic divergence and phenotypic plasticity. Proceedings of the Royal Society B: Biological Sciences, 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira, R.J. , Sasaki, M.C. & Burton, R.S. (2017b) Data from: Adaptation to a latitudinal thermal gradient within a widespread copepod species: The contributions of genetic divergence and phenotypic plasticity. Dryad Digital Repository. 10.5061/dryad.bp76g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez, E. , Díaz, F. & Espina, S. (2003) Thermoregulatory behavior and critical thermal limits of the angelfish Pterophyllum scalare (Lichtenstein) (Pisces: Cichlidae). Journal of Thermal Biology, 28, 531–537. [Google Scholar]
- Pimentel, M.S. , Faleiro, F. , Dionísio, G. , Repolho, T. , Pousão‐Ferreira, P. , Machado, J. et al. (2014) Defective skeletogenesis and oversized otoliths in fish early stages in a changing ocean. The Journal of Experimental Biology, 217, 2062–2070. [DOI] [PubMed] [Google Scholar]
- Piyaphongkul, J. , Pritchard, J. & Bale, J. (2012) Heat stress impedes development and lowers fecundity of the brown planthopper Nilaparvata lugens (Stål). PLoS One, 7, e47413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piyaphongkul, J. , Pritchard, J. & Bale, J. (2014) Effects of acclimation on the thermal tolerance of the brown planthopper Nilaparvata lugens (Stal). Agricultural and Forest Entomology, 16, 174–183. [Google Scholar]
- Piyaphongkul, J. , Suraksakul, P. , Tangchitsomkid, N. & Sahaya, S. (2018) Thermal acclimation capacity of Jack Beardsley mealybug (Pseudococcus jackbeardsleyi) to survive in a warming world. Journal of Asia‐Pacific Entomology, 21, 737–742. [Google Scholar]
- Porter, Z.C. (2020) Thermal Niche Requirements of the White‐Spotted Bamboo Shark Chiloscyllium plagiosum. MSc thesis. Florida, USA: The University of West Florida, p. 36. [Google Scholar]
- Powlik, J.J. (1996). Ecology of Tigriopus californicus (Copepoda, Harpacticoida) in Barkley Sound, British Columbia. PhD thesis. University of British Columbia, Canada, pp. 232.
- Probst, W.N. , Tan, D. , Gao, Y. , Drossou, A. , Petereit, C. , Wecker, B. et al. (2006) Rearing of Procypris rabaudi during early life‐history stages. Journal of Applied Ichthyology, 22, 530–535. [Google Scholar]
- Procarione, L.S. & King, T.L. (1993) Upper and lower temperature tolerance limits for juvenile red drums from Texas and South Carolina. Journal of Aquatic Animal Health, 5, 208–212. [Google Scholar]
- Re, A.D. , Diaz, F. & o & Valdez, G. (2006) Effect of salinity on the thermoregulatory behavior of juvenile blue shrimp Litopenaeus stylirostris Stimpson. Journal of Thermal Biology, 31, 506–513. [Google Scholar]
- Re, A.D. , Díaz, F. , Ponce‐Rivas, E. , Giffard, I. , Muñoz‐Marquez, M. & Sigala‐Andrade, H.M. (2012) Combined effect of temperature and salinity on the Thermotolerance and osmotic pressure of juvenile white shrimp Litopenaeus vannamei (Boone). Journal of Thermal Biology, 37, 413–418. [Google Scholar]
- Rebouças, R. , da Silva, H.R. , Sanuy, D. & Solé, M. (2019) Sexual maturity and growth of male toads (Rhinella ornata): A comparison between insular and mainland populations. Zoologischer Anzeiger, 283, 12–19. [Google Scholar]
- Reyes, I. , Díaz, F. , Re, A.D. & Pérez, J. (2011) Behavioral thermoregulation, temperature tolerance and oxygen consumption in the Mexican bullseye puffer fish, Sphoeroides annulatus Jenyns (1842), acclimated to different temperatures. Journal of Thermal Biology, 36, 200–205. [Google Scholar]
- Rinke, K. (2005). Species‐oriented model approaches to Daphnia spp.: linking the individual level to the population level. PhD thesis. Universität Dresden, Dresden, Germany, pp. 164.
- Rodgers, E.M. , Todgham, A.E. , Connon, R.E. & Fangue, N.A. (2019) Stressor interactions in freshwater habitats: Effects of cold water exposure and food limitation on early‐life growth and upper thermal tolerance in white sturgeon, Acipenser transmontanus . Freshwater Biology, 64, 348–358. [Google Scholar]
- Romero‐Carvajal, A. , Sáenz‐Ponce, N. , Venegas‐Ferrín, M. , Almeida‐Reinoso, D. , Lee, C. , Bond, J. et al. (2009) Embryogenesis and laboratory maintenance of the foam‐nesting túngara frogs, genus Engystomops (= Physalaemus). Developmental Dynamics, 238, 1444–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa, R. , Trubenbach, K. , Pimentel, M.S. , Boavida‐Portugal, J. , Faleiro, F. , Baptista, M. et al. (2014) Differential impacts of ocean acidification and warming on winter and summer progeny of a coastal squid (Loligo vulgaris). The Journal of Experimental Biology, 217, 518–525. [DOI] [PubMed] [Google Scholar]
- Ruthsatz, K. , Dausmann, K.H. , Peck, M.A. , Drees, C. , Sabatino, N.M. , Becker, L.I. et al. (2018) Thyroid hormone levels and temperature during development alter thermal tolerance and energetics of Xenopus laevis larvae. Conservation Physiology, 6, coy059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthsatz, K. , Dausmann, K.H. , Reinhardt, S. , Robinson, T. , Sabatino, N.M. , Peck, M.A. et al. (2020) Post‐metamorphic carry‐over effects of altered thyroid hormone level and developmental temperature: physiological plasticity and body condition at two life stages in Rana temporaria. Journal of Comparative Physiology B, 190, 297–315. [DOI] [PubMed] [Google Scholar]
- Sakurai, G. , Takahashi, S. , Yoshida, Y. , Yoshida, H. , Shoji, J. & Tomiyama, T. (2021) Importance of experienced thermal history: Effect of acclimation temperatures on the high‐temperature tolerance and growth performance of juvenile marbled flounder. Journal of Thermal Biology, 97, 102831. [DOI] [PubMed] [Google Scholar]
- Salachan, P.V. & Sørensen, J.G. (2017a) Critical thermal limits affected differently by developmental and adult thermal fluctuations. The Journal of Experimental Biology, 220, 4471–4478. [DOI] [PubMed] [Google Scholar]
- Salachan, P.V. & Sørensen, J.G. (2017b) Data from: Critical thermal limits affected differently by developmental and adult thermal fluctuations. Dryad Digital Repository. 10.5061/dryad.25b8f [DOI] [PubMed] [Google Scholar]
- Salinas, S. , Irvine, S.E. , Schertzing, C.L. , Golden, S.Q. & Munch, S.B. (2019) Trait variation in extreme thermal environments under constant and fluctuating temperatures. Philosophical Transactions of the Royal Society B: Biological Sciences, 374, 20180177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki, M. , Hedberg, S. , Richardson, K. & Dam, H.G. (2019) Complex interactions between local adaptation, phenotypic plasticity and sex affect vulnerability to warming in a widespread marine copepod. Royal Society Open Science, 6, 182115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki, M.C. & Dam, H.G. (2019) Integrating patterns of thermal tolerance and phenotypic plasticity with population genetics to improve understanding of vulnerability to warming in a widespread copepod. Global Change Biology, 25, 4147–4164. [DOI] [PubMed] [Google Scholar]
- Sasaki, M.C. & Dam, H.G. (2020) Genetic differentiation underlies seasonal variation in thermal tolerance, body size, and plasticity in a short‐lived copepod. Ecology and Evolution, 10, 12200–12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasmita, H.I. , Tu, W.‐C. , Bong, L.‐J. & Neoh, K.‐B. (2019) Effects of larval diets and temperature regimes on life history traits, energy reserves and temperature tolerance of male Aedes aegypti (Diptera: Culicidae): optimizing rearing techniques for the sterile insect programmes. Parasites & Vectors, 12, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer, J. & Ryan, A. (2006) Developmental plasticity in the thermal tolerance of zebrafish Danio rerio . Journal of Fish Biology, 69, 722–734. [Google Scholar]
- Shinner, R. , Terblanche, J.S. & Clusella‐Trullas, S. (2020) Across‐stage consequences of thermal stress have trait‐specific effects and limited fitness costs in the harlequin ladybird, Harmonia axyridis . Evolutionary Ecology, 34, 555–572. [Google Scholar]
- Shrode, J. (1975) Developmental temperature tolerance of a death‐valley pupfish (Cyprinodon nevadensis). Physiological Zoology, 48, 378–389. [Google Scholar]
- Simon, M.N. , Ribeiro, P.L. & Navas, C.A. (2015) Upper thermal tolerance plasticity in tropical amphibian species from contrasting habitats: Implications for warming impact prediction. Journal of Thermal Biology, 48, 36–44. [DOI] [PubMed] [Google Scholar]
- Slotsbo, S. , Schou, M.F. , Kristensen, T.N. , Loeschcke, V. & Sørensen, J.G. (2016) Reversibility of developmental heat and cold plasticity is asymmetric and has long‐lasting consequences for adult thermal tolerance. The Journal of Experimental Biology, 219, 2726–2732. [DOI] [PubMed] [Google Scholar]
- Sniegula, S. , Raczyński, M. , Golab, M.J. & Johansson, F. (2020) Effects of predator cues carry over from egg and larval stage to adult life‐history traits in a damselfly. Freshwater Science, 39, 804–811. [Google Scholar]
- Spinks, R.K. , Munday, P.L. & Donelson, J.M. (2019a) Developmental effects of heatwave conditions on the early life stages of a coral reef fish. The Journal of Experimental Biology, 222, jeb202713. [DOI] [PubMed] [Google Scholar]
- Spinks, R.K. , Munday, P.L. & Donelson, J.M. (2019b). Data from: Developmental effects of heatwave conditions on the early life stages of a coral reef fish. James Cook University Repository. Available at: 10.25903/5d01d448c3756 [DOI] [PubMed]
- Stitt, B.C. (2012). Brook trout (Salvelinus fontinalis) thermal adaptive potential: Physiological implications for climate change. MSc. thesis. Trent University, Canada, pp. 122.
- Stoler, S.R. (2015). Affects of acclimation temperature on the critical thermal maxima and minima of the little skate, Leucoraja erinacea . MSc. Thesis. The University of West Florida, Florida, USA, pp. 42.
- Tatum, A. (2020). Thermal Tolerance Determination of the Red‐eared Slider, Trachemys scripta elegans . MSc. thesis. The University of West Florida, Florida, USA, pp. 40.
- Terblanche, J.S. & Chown, S.L. (2006) The relative contributions of developmental plasticity and adult acclimation to physiological variation in the tsetse fly, Glossina pallidipes (Diptera, Glossinidae). The Journal of Experimental Biology, 209, 1064–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troia, M.J. , Whitney, J.E. & Gido, K.B. (2015) Thermal performance of larval longfin dace (Agosia chrysogaster), with implications for climate change. Environmental Biology of Fishes, 98, 395–404. [Google Scholar]
- Tsuchida, S. & Setoguma, T. (1997) Temperature responses of young Schlegel's black rockfish Sebastes schlegeli . Nippon Suisan Gakkaishi, 63, 317–325. [Google Scholar]
- Underwood, Z.E. , Myrick, C.A. & Rogers, K.B. (2012) Effect of acclimation temperature on the upper thermal tolerance of Colorado River cutthroat trout Oncorhynchus clarkii pleuriticus: Thermal limits of a North American salmonid. Journal of Fish Biology, 80, 2420–2433. [DOI] [PubMed] [Google Scholar]
- Uriarte, I. , Rosas, C. , Espinoza, V. , Hernández, J. & Farías, A. (2018) Thermal tolerance of paralarvae of Patagonian red octopus Enteroctopus megalocyathus . Aquaculture Research, 49, 2119–2127. [Google Scholar]
- Van Heukelem, W. (1977) Laboratory maintenance, breeding, rearing, and biomedical research potential of the Yucatan octopus (Octopus maya). Laboratory Animal Science, 27, 852–859. [PubMed] [Google Scholar]
- Vanvelk, H. , Govaert, L. , van den Berg, E.M. , Brans, K.I. & De Meester, L. (2020) Interspecific differences, plastic, and evolutionary responses to a heat wave in three co‐occurring Daphnia species. Limnology and Oceanography, 66, 1201–1220. [Google Scholar]
- Wagner, E.J. , Arndt, R.E. & Brough, M. (2001) Comparative tolerance of four stocks of cutthroat trout to extremes in temperature, salinity, and hypoxia. Western North American Naturalist, 61, 434–444. [Google Scholar]
- Walsh, S.J. , Haney, D.C. , Timmerman, C.M. & Dorazio, R.M. (1998) Physiological tolerances of juvenile robust redhorse, Moxostoma robustum: Conservation implications for an imperiled species. Environmental Biology of Fishes, 51, 429–444. [Google Scholar]
- Wang, Q.‐L. , Dong, Y.‐W. , Qin, C.‐X. , Yu, S.‐S. , Dong, S.‐L. & Wang, F. (2013) Effects of rearing temperature on growth, metabolism and thermal tolerance of juvenile sea cucumber, Apostichopus japonicus selenka: Critical thermal maximum (CTmax) and hsps gene expression. Aquaculture Research, 44, 1550–1559. [Google Scholar]
- Wang, L. (2014) The effects of constant and variable thermal acclimation on thermal tolerance of the common giant toad tadpoles (Bufo gargarizans). Shengtai Xuebao, 34, 1030–1034. [Google Scholar]
- Wang, Q. , Yu, S. & Dong, Y. (2015) Parental effect of long acclimatization on thermal tolerance of juvenile sea cucumber Apostichopus japonicus . PLoS One, 10, e0143372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warriner, T.R. , Semeniuk, C.A.D. , Pitcher, T.E. , Heath, D.D. & Love, O.P. (2020) Mimicking transgenerational signals of future stress: thermal tolerance of juvenile chinook salmon is more sensitive to elevated rearing temperature than exogenously increased egg cortisol. Frontiers in Ecology and Evolution, 368. [Google Scholar]
- Webb, J.K. , Pike, D.A. & Shine, R. (2008) Population ecology of the velvet gecko, Oedura lesueurii in South Eastern Australia: implications for the persistence of an endangered snake. Austral Ecology, 33, 839–847. [Google Scholar]
- White, D.P. & Wahl, D.H. (2020) Growth and physiological responses in largemouth bass populations to environmental warming: effects of inhabiting chronically heated environments. Journal of Thermal Biology, 88, 102467. [DOI] [PubMed] [Google Scholar]
- Williams, D.G. & McDonald, G. (1982) The duration and number of the immature stages of codling moth Cydia Pomonella (l.) (Tortricidae: Lepidoptera). Australian Journal of Entomology, 21, 1–4. [Google Scholar]
- Wong, J.M. & Hofmann, G.E. (2020) The effects of temperature and pco2 on the size, thermal tolerance and metabolic rate of the red sea urchin (Mesocentrotus franciscanus) during early development. Marine Biology, 167, 1–15. [Google Scholar]
- Wu, M.‐X. , Hu, L.‐J. , Dang, W. , Lu, H.‐L. & Du, W.‐G. (2013) Effect of thermal acclimation on thermal preference, resistance and locomotor performance of hatchling soft‐shelled turtle. Current Zoology, 59, 718–724. [Google Scholar]
- Xu, W. , Dang, W. , Geng, J. & Lu, H.‐L. (2015) Thermal preference, thermal resistance, and metabolic rate of juvenile Chinese pond turtles Mauremys reevesii acclimated to different temperatures. Journal of Thermal Biology, 53, 119–124. [DOI] [PubMed] [Google Scholar]
- Xue, Q. & Ma, C.‐S. (2020) Aged virgin adults respond to extreme heat events with phenotypic plasticity in an invasive species, Drosophila suzukii. Journal of Insect Physiology, 121, 104016. [DOI] [PubMed] [Google Scholar]
- Yoon, G.R. , Deslauriers, D. & Gary Anderson, W. (2019) Influence of a dynamic rearing environment on development of metabolic phenotypes in age‐0 Lake Sturgeon, Acipenser fulvescens. Conservation Physiology, 7, coz055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. & Kieffer, J.D. (2014) Critical thermal maximum (CTmax) and hematology of shortnose sturgeons (Acipenser brevirostrum) acclimated to three temperatures. Canadian Journal of Zoology, 92, 215–221. [Google Scholar]
- Zhou, L.‐Y. , Fu, S.‐J. , Fu, C. , Ling, H. & Li, X.‐M. (2019) Effects of acclimation temperature on the thermal tolerance, hypoxia tolerance and swimming performance of two endangered fish species in China. Journal of Comparative Physiology B, 189, 237–247. [DOI] [PubMed] [Google Scholar]
- Zhu, T. , Li, X. , Wu, X. & Yang, D. (2019) Growth and thermal tolerance of a Tibet fish Schizopygopsis younghusbandi juveniles acclimated to three temperature levels. Journal of Applied Ichthyology, 35, 1281–1285. [Google Scholar]
- Ziegeweid, J.R. , Jennings, C.A. & Peterson, D.L. (2008) Thermal maxima for juvenile shortnose sturgeon acclimated to different temperatures. Environmental Biology of Fishes, 82, 299–307. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Data Availability Statement
Data and analysis code are available at https://github.com/p‐pottier/Dev_plasticity_thermal_tolerance and archived in Zenodo (https://doi.org/10.5281/zenodo.6818559; Pottier, Burke, Zhang, et al., 2022).


