Abstract
Insights from epidemiological, clinical and basic research are illuminating the interplay between metabolic disorders, cardiovascular disease (CVD) and kidney dysfunction, termed cardio‐renal‐metabolic (CRM) disease. Broadly defined, CRM disease involves multidirectional interactions between metabolic diseases such as type 2 diabetes (T2D), various types of CVD and chronic kidney disease (CKD). T2D confers increased risk for heart failure, which—although well known—has only recently come into focus for treatment, and may differ by ethnicity, whereas atherosclerotic heart disease is a well‐established complication of T2D. Many people with T2D also have CKD, with a higher risk in Asians than their Western counterparts. Furthermore, CVD increases the risk of CKD and vice versa, with heart failure, notably, present in approximately half of CKD patients. Molecular mechanisms involved in CRM disease include hyperglycaemia, insulin resistance, hyperactivity of the renin‐angiotensin‐aldosterone system, production of advanced glycation end‐products, oxidative stress, lipotoxicity, endoplasmic reticulum stress, calcium‐handling abnormalities, mitochondrial malfunction and deficient energy production, and chronic inflammation. Pathophysiological manifestations of these processes include diabetic cardiomyopathy, vascular endothelial dysfunction, cardiac and renal fibrosis, glomerular hyperfiltration, renal hypoperfusion and venous congestion, reduced exercise tolerance leading to metabolic dysfunction, and calcification of atherosclerotic plaque. Importantly, recognition of the interaction between CRM diseases would enable a more holistic approach to CRM care, rather than isolated treatment of individual conditions, which may improve patient outcomes. Finally, aspects of CRM diseases may differ between Western and East Asian countries such as Japan, a super‐ageing country, with potential differences in epidemiology, complications and prognosis that represent an important avenue for future research.
Keywords: cardiovascular disease, diabetic nephropathy, heart failure, type 2 diabetes
1. INTRODUCTION
In 2021, the International Diabetes Federation estimated that 537 million people aged 20‐79 years had diabetes (11% of the global population) and predicted an increase to 783 million by 2045. 1 The Western Pacific region including East Asia has approximately 206 million diabetes patients, with 11 million in Japan alone, 1 , 2 making it an epicentre of the diabetes pandemic.
The large majority of people with diabetes (approximately 90%) have type 2 diabetes (T2D). 2 It is well established that people with T2D have a high risk for macrovascular and microvascular complications. Heart failure is also a common complication that may be more prevalent than atherosclerotic cardiovascular disease (CVD) in T2D patients. 3 , 4 , 5 However, heart failure has only recently come into focus for therapeutic intervention in people with T2D. 4 In Japan, the number of patients with ischaemic heart disease may have plateaued, 6 whereas the number of patients with heart failure is expected to increase in the future. 7 , 8 Chronic kidney disease (CKD) is also very common among people with T2D, affecting up to 50% of patients. 9
Partly as a consequence of these associations, the increasing prevalence of T2D is paralleled by the rising prevalence of heart failure and CKD. 10 , 11 T2D, heart failure and other types of CVD, and CKD, are closely intertwined at the epidemiological, pathophysiological and molecular levels: so‐called cardio‐renal‐metabolic (CRM) disease. 12 Notably, these relationships seem to not just represent individual diseases being complications of others, but also reflect multidirectional pathophysiological interactions that increase CRM disease risk. However, there may be important differences in the genetics, pathophysiology and epidemiology of CRM disease between Western and East Asian countries such as Japan, which can manifest as differences in complications and prognosis. 13 , 14 , 15 , 16 For example, compared with T2D patients of European descent, East Asians tend to have a lower body mass index but a higher incidence of abdominal obesity, 17 earlier β‐cell dysfunction, and a higher risk of developing renal complications and strokes. 13 Interestingly, a recent meta‐analysis of genome‐wide association studies identified 28 novel loci associated with susceptibility to T2D in the Japanese population. 16 Furthermore, Japan is a super‐aged society and has the longest life expectancy of any country in the Organisation for Economic Co‐operation and Development. 18 Therefore, the profile of CRM disease in Japan may be different from other countries.
This narrative review aims to summarize current knowledge of the epidemiology, molecular mechanisms and pathophysiology of CRM disease, with a focus on Japanese data.
2. METHODS
Articles included in this narrative review were selected based on author expertise, supplemented by searches in PubMed up to 1 May 2022, and citation review of selected primary and review articles. Only articles published in English were considered.
3. CRM DISEASE CONNECTIONS: EPIDEMIOLOGICAL EVIDENCE
Epidemiological studies suggest a multidirectional CRM disease connection. For example, in a cohort study of approximately 1.2 million people with T2D from England, Germany, Japan, the Netherlands, Norway and Sweden that evaluated those initially without concomitant CVD or CKD, 24% of first events were heart failure and 36% were CKD. 19 In the Japanese population in this study, 31% of first events were heart failure and 39% were CKD. 19 In a claims‐based study in the United States of approximately 1.2 million people with T2D initiating oral glucose‐lowering drugs, 16% were diagnosed with CVD or CKD during follow‐up, most commonly heart failure and/or CKD (65%). 20 Furthermore, an analysis of Medicare data from the United States found that the rates of myocardial infarction, heart failure, renal‐replacement therapy and death were higher in patients with either T2D or CKD compared with those without either condition, and were highest in those complicated with both T2D and CKD (Figure 1). 21 Notably, in approximately 530 000 individuals with T2D in a US outpatient registry of 271 primary care, cardiology and endocrinology offices, it was uncommon for patients to have isolated T2D without other CRM conditions (atrial fibrillation, cerebrovascular disease, CKD, coronary artery disease, heart failure, hyperlipidaemia, hypertension, hyperuricaemia/gout or peripheral artery disease). Only 6.4% had no other CRM conditions, while 51% had at least three other conditions. 22
FIGURE 1.
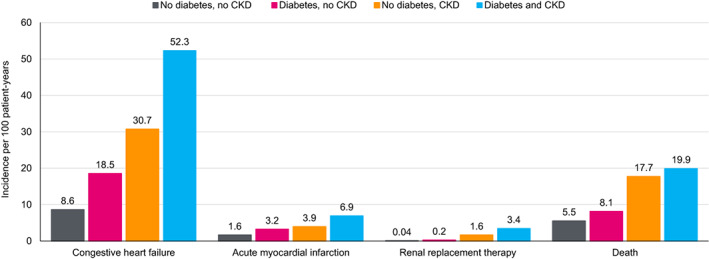
Rate of cardiorenal complications and mortality with diabetes and chronic kidney disease (CKD). Data from Foley et al. J Am Soc Nephrol 2005;16:489‐495 21
As described in the following sections, there is an abundance of epidemiological evidence on bidirectional interactions between CRM diseases (Figure 2).
FIGURE 2.
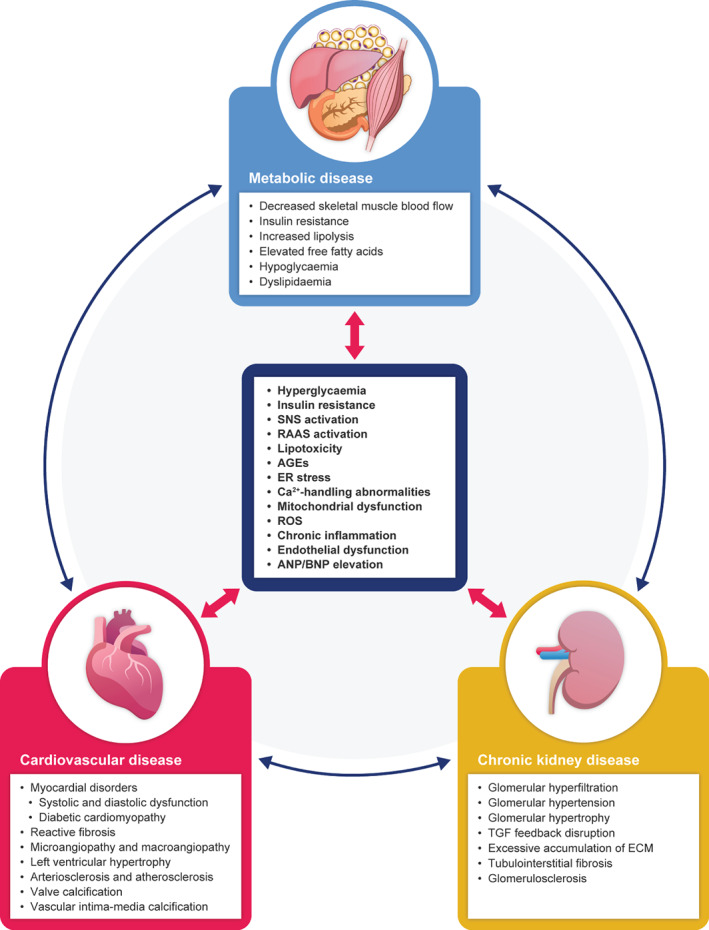
Molecular and pathophysiological interplay between cardiovascular disease, chronic kidney disease and metabolic disease. AGEs, advanced glycated end‐products; ANP, atrial natriuretic peptide; BNP, B‐type natriuretic peptide; ECM, extracellular matrix; ER, endoplasmic reticulum; RAAS, renin‐angiotensin‐aldosterone system; ROS, reactive oxygen species; SNS, sympathetic nervous system; TGF, tubuloglomerular feedback
3.1. T2D and CVD
Prospective studies, mainly in Western and other high‐income countries, found that the risk of CVD is at least twice as high in people with diabetes compared with those without diabetes. 23 A similar level of excess risk was seen in an analysis of large observational studies in countries in the Asia‐Pacific region including Japan 24 and in Japan alone. 25 Important epidemiological studies conducted in Japan are described in the sections below and are summarized in Table 1.
TABLE 1.
Epidemiological studies of cardio‐renal‐metabolic disease in Japan
| Authors (year of publication) | Type of study | Number of subjects | Findings |
|---|---|---|---|
| T2D and CVD | |||
| Shiba et al. (2011) 8 | Prospective, multicentre, cohort study | 10 219 | 22.5% of HF patients had diabetes |
| Cui et al. (2011) 26 | Prospective, multicentre, cohort study | 35 657 | Diabetes associated with increased risk of stroke:
|
| Akao et al. (2013) 27 | Cross‐sectional, multicentre, cohort study | 3183 | 23.2% of patients with atrial fibrillation had diabetes |
| Sato et al. (2013) 28 | Prospective, multicentre, cohort study | 4842 | 33.8% of hospitalized HF patients had diabetes |
| Kato et al. (2015) 29 | Prospective, multicentre, cohort study | 99 584 | Diabetes associated with increased risk of death from IHD:
|
| Takabayashi et al. (2016) 30 | Prospective, multicentre, cohort study | 647 | 31.8% of hospitalized HF patients had diabetes |
| Hirakawa et al. (2017) 25 | Pooled analysis of 8 cohort studies | 38 854 | Diabetes associated with an increased risk of death from:
|
| Yaku et al. (2018) 31 | Prospective, multicentre, cohort study | 4056 | 37% of hospitalized HF patients had diabetes (HFrEF 40%; HFpEF 33%) |
| Kaku et al. (2020) 32 | Retrospective, multicentre, cohort study | 198 861 | 26.2% of hospitalized HF patients had diabetes |
| Sato et al. (2020) 33 | Prospective, multicentre, cohort study | 4876 | 39.5% of elderly HF patients had diabetes |
| Ide et al. (2021) 34 | Retrospective, multicentre, cohort study | 13 238 | 34.2% of hospitalized HF patients had diabetes |
| Kaneko et al. (2021) 35 | Cross‐sectional database study | 1 180 062 | In individuals aged 20 to 49 years, diabetes was associated with an increased risk for:
|
| Seo et al. (2021) 36 | Prospective, single‐centre, cohort study | 349 | 43% of hospitalized HF patients had diabetes (HFrEF 42%; HFpEF 41%) |
| Ohsugi et al. (2021) 37 | Cross‐sectional database study | 10 151 | 2.8% of T2D patients had myocardial infarction, while 7.6% had ischaemic stroke |
| T2D and CKD | |||
| Ohta et al. (2010) 38 | Retrospective, single‐centre, cohort study | 3575 | 46.0% of T2D patients had CKD |
| Iwai et al. (2018) 39 | Prospective, multicentre, cohort study | 2484 | 28.1% of CKD patients had diabetes |
| Yokoyama et al. (2018) 40 | Cross‐sectional, multicentre, cohort study | 7251 | Prevalence of CKD in T2D patients declined from 32.6% to 22.3% from 2004 to 2014 |
| Nitta et al. (2019) 41 | Cross‐sectional, multicentre, cohort study | 1088 | 41.7% of CKD patients had diabetes |
| Yoshida et al. (2020) 42 | Cross‐sectional, multicentre, cohort study | 2385 | 54.0% of T2D patients had CKD |
| CKD and CVD | |||
| Tanaka et al. (2017) 43 | Prospective, multicentre, cohort study | 2966 | 8.6 HF events per 1000 person‐years in CKD patients |
| Kon et al. (2018) 44 | Cross‐sectional, multicentre, cohort study | 132 160 | HR 2.28 (95% CI: 1.28‐4.03) for CV death in elderly patients with eGFR < 45 ml/min/1.73m2 compared with those with eGFR > 90 ml/min/1.73m2 |
| Yaku et al. (2018) 31 | Prospective, multicentre, cohort study | 4056 | 45% of HF patients had CKD |
| Nitta et al. (2019) 41 | Cross‐sectional, multicentre, cohort study | 1088 | 23.4% of CKD patients had left ventricular hypertrophy |
| Sato et al. (2020) 33 | Prospective, multicentre, cohort study | 4876 | 47.7% of elderly HF patients had CKD |
| Ide et al. (2021) 34 | Retrospective, multicentre, cohort study | 13 238 | 38.9% of HF patients had CKD |
Abbreviations: CHD, coronary heart disease; CI, confidence interval; CKD, chronic kidney disease; CV, cardiovascular; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; IHD, ischaemic heart disease; T2D, type 2 diabetes.
3.1.1. Heart failure
Until relatively recently, heart failure was an underrecognized complication of T2D. 4 However, it is now clear that heart failure in people with T2D is a significant co‐morbidity, especially in ageing populations globally. 4 , 45 , 46 , 47 Overall, people with T2D are approximately 2‐4 times more likely to develop chronic heart failure, 48 , 49 and there may be differences by race and ethnicity. 5
Co‐morbid heart failure in people with T2D is associated with poor prognosis. For example, a cohort study of Medicare data found that in T2D patients older than 65 years, the coexistence of heart failure was associated with 10‐fold higher mortality. 50 Conversely, in people with heart failure, the prevalence of T2D is higher than in those without heart failure: up to 4‐fold higher in some studies. Overall, the prevalence appears to be approximately 30%‐40%, irrespective of heart‐failure phenotype (i.e. reduced or preserved ejection fraction), and is even higher in those hospitalized for heart failure. 51 , 52 , 53 , 54 , 55 The risk of all‐cause mortality in people with heart failure may be increased approximately 1.3‐ to 3.2‐fold by co‐morbid T2D, 55 while the risk of all‐cause hospitalization may be up to 50% higher in heart failure patients with diabetes than in those without the disease. 56 , 57 , 58
Furthermore, even prediabetes both increases the risk for heart failure 59 and worsens its prognosis. 60
In Japan, the overall prevalence of heart failure in T2D patients is not firmly established, because of a lack of studies. However, diabetes was associated with a 58% increase in risk for heart failure in individuals in Japan aged 20 to 49 years without established CVD (hazard ratio [HR] 1.58; 95% confidence interval [CI]: 1.41‐1.78), based on data for approximately 1.2 million individuals in a national epidemiology database. 35 More information is available on the incidence of diabetes in patients with heart failure in Japan. For example, Japanese registry data for patients hospitalized for heart failure indicated that the prevalence of T2D was 40% to 42% in patients with reduced ejection fraction and 33% to 41% in those with preserved ejection fraction. 31 , 36
3.1.2. Ischaemic heart disease
Ischaemic heart disease is a well‐established complication of T2D. A meta‐analysis of individual patient data for almost 700 000 patients from 102 studies found that T2D patients in high‐income countries were approximately twice as likely to develop ischaemic heart disease than non‐diabetic patients. 23 Moreover, patients with T2D had a 2.31‐fold increased risk for death from coronary heart disease.
Globally, the incidence of coronary artery disease in T2D patients varies across countries; 61 the incidence of myocardial infarction appears to be stable or decreasing in many countries, including the United States and Japan. 6 , 62 , 63 Some studies suggest that T2D patients in Asian countries have a lower risk of major coronary events than patients from Europe. 19 , 64 In cohort studies in Japan, diabetes was estimated to increase the risk of coronary heart disease by approximately 2‐fold. 25 , 29
3.1.3. Stroke
T2D is also a well‐established independent risk factor for stroke, with an approximate 2‐ to 3‐fold increase in risk. 23 , 65 , 66 In Asian patients, diabetes is the second‐strongest risk factor for stroke after hypertension. 67
Recent studies have elucidated the epidemiology of stroke in T2D in Japan. In a pooled analysis of eight cohort studies in Japan, diabetes was associated with 40% increased risk for stroke (HR 1.40; 95% CI: 1.05‐1.85). 25 Diabetes was also associated with increased risk for stroke in individuals in Japan aged 20 to 49 years without established CVD (HR 1.31; 95% CI: 1.07‐1.59) in the study of approximately 1.2 million individuals in a national epidemiology database. 35 In the Japan Public Health Study of approximately 36 000 individuals, the risk of stroke was significantly increased in both men (HR 1.64; 95% CI: 1.21‐2.23) and women (HR 2.19; 95% CI: 1.53‐2.12) with diabetes compared with those without diabetes. 26 Comparison of the J‐DREAMS database study of approximately 10 000 T2D patients in Japan with studies conducted in Europe showed that stroke was more common than myocardial infarction in Japanese patients (7.6% and 2.8% of patients, respectively), but less common than myocardial infarction in European patients (5.0%‐9.8% and 7.1%‐12.0% of patients, respectively). 37
3.1.4. Atrial fibrillation
The landmark Framingham Heart Study found an increased risk of atrial fibrillation in patients with diabetes (odds ratio of 1.4 for men, 1.6 for women). 68 Diabetes was also independently associated with atrial fibrillation in a study of individuals in Veterans Health Administration hospitals in the United States (odds ratio 2.13; 95% CI: 2.10‐2.16). 69 A meta‐analysis of prospective cohort and case–control studies in several Western countries and Japan reported an overall 40% increase in risk for atrial fibrillation in patients with diabetes, compared with those without the disease, although it was only 24% in studies that adjusted for multiple potential confounding factors. 70 Among the approximately 1.2 million Japanese individuals aged 20 to 49 years in the epidemiology database study described above, diabetes was associated with an almost 70% increased risk for atrial fibrillation (HR 1.69; 95% CI: 1.35‐2.13). 35
3.2. T2D and CKD
Globally, it is estimated that up to 50% of T2D patients also have CKD, defined as persistent albuminuria (urinary albumin‐to‐creatinine ratio ≥ 30 mg/g), an estimated glomerular filtration rate (eGFR) persistently less than 60 ml/min/1.73m2, or both. 9 In the United States, approximately 25% of patients with T2D also have CKD. 71 In a global study of patients initiating second‐line glucose‐lowering drugs, approximately 49% also had CKD. 72 Large observational studies in both the United States 73 and Japan 74 found that the mortality risk in people with T2D was increased to a similar degree by albuminuria or reduced eGFR (<60 ml/min/1.73m2) and was further doubled in those with both albuminuria and reduced eGFR.
Multiple studies have shown that the risk of CKD is higher in Asians with T2D than their Western counterparts. 13 , 75 One of the first studies to compare diabetes complications across ethnic groups was the World Health Organization Multinational Study of Vascular Disease in Diabetes, which found a higher incidence of albuminuria in Asians than in Europeans. 76 , 77 , 78 In a cross‐sectional study of approximately 32 000 T2D patients from 33 countries, the prevalence of microalbuminuria and macroalbuminuria was 39% and 9.8%, respectively, with Asians having the highest prevalence of albuminuria overall. 79
A recent systematic review of CKD in Asia found that Japan had the second‐highest prevalence in this region (after Singapore). The high prevalence in East Asia was attributed to diabetic nephropathy. 80 Several studies have shown high rates of CKD in T2D patients in Japan. Two studies involving several thousand patients found that approximately 50% had CKD. 38 , 42 Interestingly, a comparison of two independent cohorts found a declining rate of CKD in Japanese T2D patients between 2004 and 2014 from approximately 40% to 30%. 40
Unsurprisingly, given the previously described studies showing a high prevalence of CKD in T2D patients, the reverse has also been found: high rates of diabetes in CKD patients in both non‐Asian and Asian countries. Among approximately 5200 patients with CKD in the German Chronic Kidney Disease cohort, 50% also had T2D. 81 In patients with CKD in China, 19.1% of those with eGFR less than 60 ml/min/1.73m2 had diabetes and 17.3% of those with albuminuria had diabetes. 82 In the KNOWCKD study of approximately 2200 CKD patients in South Korea, 33.7% also had T2D. 83 In recent cohorts of CKD patients in Japan, the prevalence of diabetes was 28% to 42%. 39 , 41
3.3. CKD and CVD
CKD is a key co‐morbidity in T2D patients, partly because of the important role played by kidney dysfunction in the pathophysiology of CVD, particularly heart failure, 84 as described later in sections 6.1 and 6.2. A meta‐analysis of studies involving approximately 1.1 million patients with heart failure found an overall CKD prevalence of 49%, which was higher in patients with acute heart failure (53%) than in those with chronic heart failure (42%). 85
From the opposite perspective, it is well‐established that CVD, including heart failure, is more prevalent among patients with CKD than in the general population, and its prevalence increases as kidney function decreases. 84 , 86 In the United States, CKD patients had double the risk of heart failure in the ARIC study, 87 while the HOPE study found that the presence of microalbuminuria approximately doubled the risk of hospitalization for heart failure. 88 In the CRIC study in the United States, the rate of heart failure events in CKD patients was 26 per 1000 person‐years. 89 In Japan, the large, prospective CKD‐JAC cohort study found a rate of heart failure in CKD patients of 8.6 per 1000 person‐years. 43
In the CRIC study, the rate of major adverse cardiovascular events (heart failure, myocardial infarction, stroke) in CKD patients was 38 per 1000 person‐years, while myocardial infarction alone was 13 per 1000 person‐years. 89 In the CKD‐JAC study, the rate of CVD in CKD patients was 22.8 per 1000 person‐years, while myocardial infarction occurred in 1.6 per 1000 person‐years. 43
4. T2D IN CRM DISEASE: MECHANISMS AND PATHOPHYSIOLOGY
4.1. Mechanisms for the effect of T2D on the cardiovascular system and kidneys
4.1.1. Hyperglycaemia and advanced glycation end‐products
Hyperglycaemia causes non‐enzymatic glycation of lipids and proteins to produce advanced glycation end‐products (AGEs). 90 AGEs can directly cause cross‐linking of proteins such as collagen and laminin in the extracellular matrix, resulting in vascular stiffening. 90 Furthermore, AGEs bind to the cell surface receptor for AGEs (RAGE), which can promote fibrosis by activating signalling pathways such as nuclear factor‐κB (NF‐κB), resulting in excessive accumulation of extracellular matrix proteins. 91 These effects are thought to in part underlie the pathophysiology of diabetic cardiomyopathy 92 and diabetic nephropathy. 93
4.1.2. Insulin resistance
Impaired insulin metabolic signalling is associated with cardiac fibrosis/stiffness and diastolic dysfunction, 94 and insulin resistance in the heart is considered a major pathophysiological abnormality in diabetic cardiomyopathy. In renal podocytes, defective insulin receptor signalling also leads to a pathology reminiscent of diabetic nephropathy, even with normoglycaemia. 90
4.1.3. Lipotoxicity
In T2D, reduced levels of intracellular glucose resulting from insulin resistance, in cells that take up glucose in an insulin‐dependent manner, may shift metabolism towards free fatty acid oxidation, a less efficient process. In the diabetic heart, decreased adenosine triphosphate (ATP) production from glucose metabolism may cause compensatory increases in free fatty acid uptake and accumulation of triacylglycerols, which may exceed mitochondrial respiratory capacity, resulting in a build‐up of toxic lipid metabolites and mitochondrial dysfunction. 90 , 92 , 95 Insulin metabolic signalling is inhibited by certain lipid metabolites, including diacylglycerol and ceramides, which also promotes diabetic cardiomyopathy. 96 Abnormalities in cellular energy production have been documented in both microvascular and macrovascular diabetic complications. 90
4.1.4. Hyperactivity of the renin‐angiotensin‐aldosterone system
Hyperglycaemia is associated with activation of the systemic renin‐angiotensin‐aldosterone system (RAAS) and increased arterial pressure and vascular resistance. 96 RAAS activation promotes oxidative stress via elevating NADPH oxidase activity and induces insulin resistance both systematically and in the heart via the mammalian target of rapamycin (mTOR)/S6 kinase 1 (S6K1) signalling pathway. 96 In addition, local RAAS signalling in the myocardium also increases inflammation. 96
4.1.5. Other mechanisms
Other pathways in T2D that appear to cause cardiovascular and kidney complications include endoplasmic reticulum (ER) stress, abnormal calcium handling, oxidative stress and chronic inflammation.
ER stress—where the capacity of the ER to fold proteins is overwhelmed—appears to play an important role in the pathophysiology of diabetes, and is initiated by several pathways, including the RAAS and AGE‐RAGE signalling. 90 ER stress has been implicated in cardiomyocyte apoptosis, atherosclerosis in diabetes and diabetic kidney disease. 90
Lowered glucose uptake by the heart reduces activity of the Ca2+ ATPase, increasing intracellular Ca2+ levels. 96 In addition, excessive Ca2+ uptake opens the mitochondrial permeability transition pore, resulting in energy deficiency and oxidative stress. 97 The interaction of aberrant Ca2+ handling with ER stress and oxidative stress increases autophagy, apoptosis and necrosis, leading to cardiomyocyte death. 92
Reactive oxygen species (ROS) are produced spontaneously during the formation of AGEs; furthermore, AGE‐RAGE signalling also leads to the generation of ROS by NADPH oxidase and mitochondria. 90 Furthermore, in the hyperglycaemic, hyperlipidaemic and inflammatory environment characteristic of diabetes, the activity of mitochondrial enzymes producing ROS is upregulated. 98 In turn, the increased levels of ROS reduce fatty acid oxidation capacity, drive further mitochondrial dysfunction and apoptosis, and lead to lipid accumulation, fibrosis and cardiac dysfunction in patients with diabetes. 96
In tissues with diabetic complications, various mechanisms mentioned above activate the endothelium, leading to NF‐κB activation and increased expression of cytokines and cell adhesion factors, which in turn promotes the recruitment of leukocytes and activation of inflammatory immune cells. 90 , 92 Renal inflammation may be involved in the pathogenesis of diabetic kidney disease via several pathways, including those mediated by inflammasomes. 99
4.2. Pathophysiology of T2D in the cardiovascular system
4.2.1. Diabetic cardiomyopathy and heart failure
Diabetic cardiomyopathy is associated with metabolic disturbances such as impaired insulin metabolic signalling and insulin resistance, increased myocardial free fatty acid uptake and mitochondrial dysfunction. 92 , 96 , 100 Together, these pathogenic processes promote fibrosis and diastolic dysfunction. 92 , 96 , 100 In later stages, fibrosis and cardiac remodelling become more advanced, associated with impaired insulin signalling, reduced nitric oxide levels, RAAS activation and oxidative stress, which further impairs diastolic and systolic function. 92 , 96 , 100
4.2.2. Vascular endothelial dysfunction and coronary artery disease
The uptake of glucose by endothelial cells is proportional to plasma glucose concentration. Therefore, during hyperglycaemia, energy production in endothelial cells (which mainly occurs via glycolysis) may become uncontrolled as a result of excess substrate availability, leading to increased production of ROS and, consequently, endothelial dysfunction. 90 Furthermore, vascular repair of the endothelium is impaired because of reduction in endothelial progenitor cells. 90 As described above, various mechanisms in diabetic tissues also result in endothelial activation, disrupting vascular endothelial cells and allowing immune cells such as macrophages and T cells to attach to the vessel wall, eventually resulting in the formation of complex atherosclerotic plaques that can rupture, leading to myocardial infarction and unstable angina. 90
4.3. Pathophysiology of T2D in the kidneys
4.3.1. Increased glomerular filtration
Haemodynamic changes associated with systemic and intrarenal changes in blood pressure occur early in diabetes and are characterized by glomerular hyperfiltration, which is observed in up to 40% of patients with T2D. 90 , 101 In early diabetes, the kidneys increase in size as a result of both hyperplasia and hypertrophy; most of this growth occurs in the proximal tubule, resulting in increased reabsorption of glomerular filtrate, which increases GFR via tubuloglomerular feedback. 90 Furthermore, the loss of nephrons as CKD progresses results in hyperfiltration by the remaining nephrons. 102 Hyperfiltration is thought to be the main cause of damage to the glomeruli and anterior glomerular vessels, the filtration components of the kidney. 103
4.3.2. Structural changes in the glomerulus and tubulointerstitial fibrosis
Glomerular structural changes that occur in diabetic kidney disease include increased thickness of the glomerular basement membrane, fusion of the foot processes, loss of podocytes and expansion of the mesangial matrix. 101 In late stages, glomerulosclerosis develops as a consequence of excessive accumulation of extracellular matrix proteins in the mesangial interstitial space, resulting in fibrosis. 101 , 104 These changes can lead to initial glomerular hyperfiltration followed by decreased eGFR and/or albuminuria, and eventually kidney failure. 101
5. CVD IN CRM DISEASE: MECHANISMS AND PATHOPHYSIOLOGY
5.1. Mechanisms for the effect of CVD on renal and metabolic disease
In patients with heart failure, a complex and incompletely understood interplay between several haemodynamic and non‐haemodynamic pathways causes kidney dysfunction, including activation of the RAAS, stimulation of the sympathetic nervous system, oxidative stress, inflammation, fibrosis and natriuretic peptides. 105 , 106 , 107 , 108 Increased renal vein pressure or decreased renal blood flow activates the RAAS, which worsens kidney function and stimulates the sympathetic nervous system. Suppression of inhibitory cardiovascular reflexes and augmentation of excitatory cardiovascular reflexes also stimulates the sympathetic nervous system, which in turn can activate the RAAS in a vicious cycle. The sympathetic nervous system can damage kidney function through both hypertensive and non‐hypertensive mechanisms such as increasing oxidative stress. RAAS activation can also increase oxidative stress, and chronically high aldosterone levels seem to contribute to fibrosis in the myocardium and kidneys. Inflammation and oxidative stress‐related endothelial dysfunction can also induce myocardial and kidney fibrosis. Furthermore, elevated levels of atrial natriuretic peptide or brain natriuretic peptide may also contribute to kidney damage in patients with heart failure.
There is evidence that heart failure can induce insulin resistance and glucose intolerance. 109 Although the exact mechanisms are unclear, myocardial uptake of free fatty acids is normally increased in heart failure, which can cause lipotoxicity and contribute to insulin resistance, as described in section 4.1.
5.2. Pathophysiology of CVD in the kidneys
Renal hypoperfusion because of decreased cardiac output was previously considered the main reason for declining kidney function in cardiorenal syndrome; however, venous congestion is now known to play an important role and may be the main haemodynamic contributor. 85 , 110 , 111 , 112 , 113 , 114 Venous congestion also activates endothelial cells via circumferential stretch, leading to increased levels of proinflammatory cytokines such as tumour necrosis factor and interleukin‐6 that may worsen cardiac and kidney dysfunction. 115 , 116
5.3. Pathophysiology of CVD in metabolism
Atrophy of slow muscle fibres, reduced aerobic metabolic capacity and decreased muscle glycogen content have been documented in the skeletal muscle of patients with heart failure, 117 as has decreased muscle power output, 118 suggesting that reduced exercise tolerance may lead to further metabolic dysfunction.
6. CKD IN CRM DISEASE: MECHANISMS AND PATHOPHYSIOLOGY
6.1. Mechanisms for the effect of CKD on the cardiovascular system and metabolism
Physiological responses to decreasing GFR can elicit activation of compensatory mechanisms including activation of the RAAS, the sympathetic nervous system and the calcium‐parathyroid axis (perturbing calcium‐phosphate homeostasis), with potentially deleterious cardiovascular effects. 119 Calcium‐phosphate imbalance appears to play a role in vascular calcification in CKD patients. In addition to calcification of atherosclerotic plaque in the intimal layer of the artery, CKD is characterized by calcification of the media wall (Mönckeberg‐type tunica media calcification), which further stiffens the arterial wall and reduces vascular compliance. 120
Declining eGFR in CKD is also associated with renal anaemia, chronic inflammation, oxidative stress, increased extracellular fluid and electrolyte abnormalities. 121 , 122 Insulin resistance occurs early during the course of kidney dysfunction, although the aetiology is unclear. 123
6.2. Pathophysiology of CKD in the cardiovascular system
As described in sections 4.1.5 and 4.2.2, various pathological mechanisms such as hyperglycaemia, AGEs, RAAS activation, excessive lipid accumulation and oxidative stress can activate the endothelium. Furthermore, as described in section 5.2, venous congestion in CVD can also activate endothelial cells. Endothelial dysfunction triggers atherogenesis, 90 which may be a common link in the pathological interplay between CKD and CVD. Subclinical atherosclerosis may begin at early stages of CKD 124 and may be more prevalent in CKD patients with diabetes than in non‐diabetes patients. 125 Calcification may be important in vascular disease in CKD. In haemodialysis patients, mitral annular calcification was found in 74% and aortic valve calcification in 64%, and the risk of death was significantly higher in both groups than in non‐calcified valve patients. 126
6.3. Pathophysiology of CKD in metabolism
CKD‐associated insulin resistance is linked to oxidative stress, endothelial dysfunction and inflammation, which can lead to vascular dysfunction and atherosclerosis. 123 CKD also increases the risk of hypoglycaemia via reduced renal clearance of plasma insulin arising from decreased eGFR, reduced hepatic clearance of plasma insulin (thought to be a consequence of toxic uraemic effects on the liver) and impairment of counter‐regulatory renal gluconeogenesis. 127 CKD is also associated with dyslipidaemia characterized by increased levels of triglyceride‐rich apoB lipoproteins; impaired lipolysis of these lipoproteins appears to be a fundamental underlying reason for this atherogenic lipid profile. 128 , 129 , 130
7. ETHNIC DIFFERENCES IN CRM DISEASE: POTENTIAL GENETIC CONTRIBUTORS
Interethnic differences in CRM disease may arise partly from genetic variation. A meta‐analysis of genome‐wide association studies (GWASs) of approximately 900 000 individuals of European ancestry found over 240 loci associated with T2D. 131 A subsequent meta‐analysis of GWAS data for approximately 190 000 Japanese people identified 28 novel loci, including those associated with genes related to pancreatic acinar cells and insulin secretion. 16 Furthermore, a structure–function analysis of ethnic‐specific variants of another identified locus (PAX4), which encodes a transcription factor involved in pancreatic β‐cell development, found decreased expression of a gene involved in pancreatic β‐cell survival, suggesting a pathogenic role for these variants. 132 A similar GWAS of a wider East Asian population of over 400 000 individuals found 61 novel loci, including some with lipodystrophy‐like traits. 15 These variations may have relevance for clinical differences between ethnic groups, such as the observation that East Asians tend to develop T2D at a lower body mass index than White individuals. 13 , 14
Genetic variation may also contribute to interethnic differences in diabetic kidney disease, such as its greater prevalence in Asians than Europeans (section 3.2). In fact, GWASs have found that some loci appear to be associated with diabetic kidney disease in Japanese but not in Europeans. 133 , 134
Similarly, genetic variation may play a role in differences in CVD complications of T2D between Asians and non‐Asians. 135 Studies have shown various genotypes to be significantly associated with CVD in Asians with T2D, including Japanese, but not in Whites, including alleles encoding proteins involved in glucose and lipid metabolism. 135
Overall, the contribution of genetic variation to interethnic differences in CRM disease is a nascent field that is an important avenue for future research.
8. CONCLUSIONS
Cardiovascular, renal and metabolic diseases such as T2D interact at the pathophysiological level, resulting in clinical overlap between these conditions. Increased recognition of this overlap by clinicians, particularly the role of metabolic disorders such as T2D, would facilitate a more holistic approach to CRM care, rather than isolated treatment of individual diseases, which may improve patient outcomes.
Mechanisms by which T2D exerts pathophysiological effects on the heart and kidneys include hyperglycaemia, production of AGEs, insulin resistance, hyperactivity of the RAAS, lipotoxicity, ER stress, abnormalities in calcium handling, mitochondrial malfunction and deficits in energy production, oxidative stress and chronic inflammation. Several of these processes, as well as others, also arise in CVD and CKD. The resulting pathophysiological interplay between metabolic disease, the heart and the kidneys forms a vicious cycle of CRM disease (Figure 2).
Compared with T2D patients in Europe and the United States, those in Japan seem to have more heart failure and CKD events, while stroke seems to occur more frequently than ischaemic heart disease complications such as myocardial infarction (in contrast to Western countries). These apparent differences in the epidemiology of CRM disease in Japan compared with other countries may reflect underlying genetic, biochemical and pathophysiological characteristics.
AUTHOR CONTRIBUTIONS
All the authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE) and were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development and have approved the final version.
CONFLICT OF INTEREST
T.K. reports consulting/lecture fees from Abbott, Asahi Mutual Life Insurance, Astellas Pharma Inc., AstraZeneca K.K., Bayer, Boehringer Ingelheim, Cosmic, Daiichi Sankyo Company, Limited, Eli Lilly and Company, Fujifilm, FUJIREBIO, Johnson & Johnson Co., Ltd, Kissei Pharmaceutical Co., Ltd, Kowa Co., Ltd, Kyowa Hakko Kirin Co., Ltd, Medical Review, Medscape Education, Medtronic Sofamor Danek, Mitsubishi Tanabe Pharma Corporation, MSD, Musashino Foods, Nipro, Novartis International AG, Novo Nordisk Pharma Ltd, Ono Pharmaceutical Co., Ltd, Sanofi S.A., SANWA KAGAKU KENKYUSHO CO., LTD, Sumitomo Dainippon, Taisho Pharmaceutical Co., Ltd, Takeda Pharmaceutical Company Limited and Terumo; grants from Astellas Pharma Inc., Daiichi Sankyo Company, Limited, Eli Lilly and Company, Kissei Pharmaceutical Co., Ltd, Mitsubishi Tanabe Pharma Corporation, MSD, Novo Nordisk Pharma Ltd, Ono Pharmaceutical Co., Ltd, Sanofi S.A., Sumitomo Dainippon, Taisho Pharmaceutical Co., Ltd, and Takeda Pharmaceutical Company Limited; contracted research from AstraZeneca K.K. and Takeda Pharmaceutical Company Limited; joint research from Daiichi Sankyo Company, Limited; endowed chair from Asahi Mutual Life Insurance, Boehringer Ingelheim, Kowa Co., Ltd, Mitsubishi Tanabe Pharma Corporation, MSD, Novo Nordisk Pharma Ltd, Ono Pharmaceutical Co., Ltd, and Takeda Pharmaceutical Company Limited. H.M. has received lecture fees from MSD K.K., Nippon Boehringer Ingelheim Co. Ltd, Astellas Pharma Inc., Mitsubishi‐Tanabe Pharma Corporation, Sanofi K.K., Takeda Pharmaceutical Co. Ltd, Daiichi Sankyo Co. Ltd, AstraZeneca K.K., Novo Nordisk Pharma Ltd, Sumitomo Dainippon Pharma Co. Ltd and Eli Lilly; research support from Astellas Pharma Inc., AstraZeneca K.K., Nippon Boehringer Ingelheim Co. Ltd, Sunstar Inc., Mitsubishi Tanabe Pharma Corporation, Kyowa Kirin Co. Ltd, Nissan Chemical Corporation and MIKI Corporation; and research grants from Takeda Pharmaceutical Co. Ltd, Astellas Pharma Inc., MSD K.K., Nippon Boehringer Ingelheim Co. Ltd, Kyowa Kirin Co. Ltd, Taisho‐Toyama Pharm Co. Ltd, Kowa Pharmaceutical Co. Ltd, Ono Pharmaceutical Co. Ltd, Daiichi Sankyo Co. Ltd, Sanofi K.K., Mitsubishi‐Tanabe Pharma Corporation, Sanwa Kagaku Kenkyusho Co. Ltd, Eli Lilly Japan K.K., Sumitomo Dainippon Pharma Co. Ltd, Novo Nordisk Pharma Ltd, Bayer Yakuhin Ltd, Teijin Ohama Co. Ltd, Novartis Pharma K.K. and Nipro Corporation. H.W. has received grants from Kowa, Sanofi, Yakult, Eli Lilly, Novartis, Sanwa Kagaku Kenkyusho, Abbott Japan, Astellas Pharma, Boehringer Ingelheim, Daiichi Sankyo, Dainippon Sumitomo Pharma, Pfizer, Kissei Pharma, Kyowa Hakko Kirin, Mitsubishi Tanabe Pharma, Merck Sharp & Dohme, Novo Nordisk, Ono Pharmaceutical, Teijin, Taisho‐Toyama and Souiken; and has received personal fees from Astellas Pharma, AstraZeneca, Boehringer Ingelheim, Dainippon Sumitomo Pharma, Eli Lilly, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma, Novo Nordisk, Ono Pharmaceutical, Sanofi, Sanwa Kagaku Kenkyusho, Kyowa Hakko Kirin, Terumo Corp, Fuji Film and Takeda. D.Y. has received consulting/lecture fees from Eli Lilly Japan K.K., Novo Nordisk Pharma Ltd, Ono Pharmaceutical Co. Ltd and Takeda Pharmaceutical Company Limited, and grants from Arkray Inc., Novo Nordisk Pharma Ltd, Nippon Boehringer Ingelheim, Ono Pharmaceutical Co. Ltd, Taisho Pharmaceutical Co. Ltd, Takeda Pharmaceutical Company Limited and Terumo Corporation during the conduct of the study. K.N. has received lecture fees from Boehringer Ingelheim, Daiichi Sankyo, Mitsubishi Tanabe, Astellas, Bayer, MSD K.K., Takeda, Ono, Eli Lilly and Company and Otsuka; and research support from Boehringer Ingelheim, Teijin Pharma, Mitsubishi Tanabe, Asahi‐kasei, Terumo, Astellas, Bayer and Daiichi Sankyo. T.M. has received contributions from Boehringer Ingelheim, and honorariums from Boehringer Ingelheim and Eli Lilly and Company. J.W. receives speaker honoraria from Astra Zeneca, Daiichi Sankyo, Novartis, Novo Nordisk Pharma and Tanabe Mitsubishi, and receives grant support from Astellas, Baxter, Bayer, Chugai, Dainippon Sumitomo, Kyowa Kirin, Novo Nordisk Pharma, Ono, Otsuka, Tanabe Mitsubishi and Teijin.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14829.
ACKNOWLEDGEMENTS
Medical writing assistance was provided by Giles Brooke, PhD, of Elevate Scientific Solutions, during the preparation of this manuscript, and was funded by Nippon Boehringer Ingelheim Co. Ltd and Eli Lilly Japan K.K.
Kadowaki T, Maegawa H, Watada H, et al. Interconnection between cardiovascular, renal and metabolic disorders: A narrative review with a focus on Japan. Diabetes Obes Metab. 2022;24(12):2283‐2296. doi: 10.1111/dom.14829
Funding information
Nippon Boehringer Ingelheim Co. Ltd. and Eli Lilly Japan K.K.
Eli Lilly Japan
Boehringer Ingelheim
DATA AVAILABILITY STATEMENT
All data discussed in this review are available in the public domain in the cited literature.
REFERENCES
- 1. Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country‐level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2021;183:109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. International Diabetes Federation. IDF Diabetes Atlas. 10th ed. Brussels, Belgium: International Diabetes Federation; 2021. https://www.diabetesatlas.org/en/. Accessed December 6, 2021. [Google Scholar]
- 3. Juhaeri J, Gao S, Dai WS. Incidence rates of heart failure, stroke, and acute myocardial infarction among type 2 diabetic patients using insulin glargine and other insulin. Pharmacoepidemiol Drug Saf. 2009;18:497‐503. [DOI] [PubMed] [Google Scholar]
- 4. McMurray JJ, Gerstein HC, Holman RR, et al. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol. 2014;2:843‐851. [DOI] [PubMed] [Google Scholar]
- 5. Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007‐2017. Cardiovasc Diabetol. 2018;17:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cui Y, Hao K, Takahashi J, et al. Age‐specific trends in the incidence and in‐hospital mortality of acute myocardial infarction over 30 years in Japan ‐ report from the Miyagi AMI registry study. Circ J. 2017;81:520‐528. [DOI] [PubMed] [Google Scholar]
- 7. Shiba N, Shimokawa H. Chronic heart failure in Japan: implications of the CHART studies. Vasc Health Risk Manag. 2008;4:103‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shiba N, Nochioka K, Miura M, et al. Trend of Westernization of etiology and clinical characteristics of heart failure patients in Japan ‐ first report from the CHART‐2 study. Circ J. 2011;75:823‐833. [DOI] [PubMed] [Google Scholar]
- 9. Thomas MC, Cooper ME, Zimmet P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat Rev Nephrol. 2016;12:73‐81. [DOI] [PubMed] [Google Scholar]
- 10. Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail. 2020;22:1342‐1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. GBD Chronic Kidney Disease Collaboration . Global, regional, and national burden of chronic kidney disease, 1990‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moser M, Sowers JR. Cardio‐Renal‐Metabolic Interactions between Diabetes, Cardiometabolic Syndrome, and Cardiovascular and Renal Disease. Chapter 4. Clinical management of cardiovascular risk factors in diabetes. Caddo, OK: Professional Communications, Inc; 2009. [Google Scholar]
- 13. Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281:64‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129‐2140. [DOI] [PubMed] [Google Scholar]
- 15. Spracklen CN, Horikoshi M, Kim YJ, et al. Identification of type 2 diabetes loci in 433,540 East Asian individuals. Nature. 2020;582:240‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Suzuki K, Akiyama M, Ishigaki K, et al. Identification of 28 new susceptibility loci for type 2 diabetes in the Japanese population. Nat Genet. 2019;51:379‐386. [DOI] [PubMed] [Google Scholar]
- 17. Williams R, Periasamy M. Genetic and environmental factors contributing to visceral adiposity in Asian populations. Endocrinol Metab. 2020;35:681‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. OECD . Health at a Glance 2019: OECD Indicators. Paris: OECD Publishing; 2019. [Google Scholar]
- 19. Birkeland KI, Bodegard J, Eriksson JW, et al. Heart failure and chronic kidney disease manifestation and mortality risk associations in type 2 diabetes: a large multinational cohort study. Diabetes Obes Metab. 2020;22:1607‐1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Olufade T, Jiang L, Israni R, Huang J, Gosmanov AR. Cardiovascular and renal disease manifestation and healthcare resource utilization in patients on first‐line oral therapy for type 2 diabetes: a claims‐based observational cohort study. Diabetes Obes Metab. 2021;23:2741‐2751. [DOI] [PubMed] [Google Scholar]
- 21. Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489‐495. [DOI] [PubMed] [Google Scholar]
- 22. Arnold SV, Kosiborod M, Wang J, Fenici P, Gannedahl G, LoCasale RJ. Burden of cardio‐renal‐metabolic conditions in adults with type 2 diabetes within the diabetes collaborative registry. Diabetes Obes Metab. 2018;20:2000‐2003. [DOI] [PubMed] [Google Scholar]
- 23. Emerging Risk Factors Collaboration , Sarwar N, Gao P, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta‐analysis of 102 prospective studies. Lancet. 2010;375:2215‐2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Woodward M, Zhang X, Barzi F, et al. The effects of diabetes on the risks of major cardiovascular diseases and death in the Asia‐Pacific region. Diabetes Care. 2003;26:360‐366. [DOI] [PubMed] [Google Scholar]
- 25. Hirakawa Y, Ninomiya T, Kiyohara Y, et al. Age‐specific impact of diabetes mellitus on the risk of cardiovascular mortality: an overview from the Evidence for Cardiovascular Prevention from Observational Cohorts in the Japan Research Group (EPOCH‐JAPAN). J Epidemiol. 2017;27:123‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cui R, Iso H, Yamagishi K, et al. Diabetes mellitus and risk of stroke and its subtypes among Japanese: the Japan public health center study. Stroke. 2011;42:2611‐2614. [DOI] [PubMed] [Google Scholar]
- 27. Akao M, Chun YH, Wada H, et al. Current status of clinical background of patients with atrial fibrillation in a community‐based survey: the Fushimi AF registry. J Cardiol. 2013;61:260‐266. [DOI] [PubMed] [Google Scholar]
- 28. Sato N, Kajimoto K, Keida T, et al. Clinical features and outcome in hospitalized heart failure in Japan (from the ATTEND registry). Circ J. 2013;77:944‐951. [DOI] [PubMed] [Google Scholar]
- 29. Kato M, Noda M, Mizoue T, et al. Diagnosed diabetes and premature death among middle‐aged Japanese: results from a large‐scale population‐based cohort study in Japan (JPHC study). BMJ Open. 2015;5:e007736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takabayashi K, Ikuta A, Okazaki Y, et al. Clinical characteristics and social frailty of super‐elderly patients with heart failure ‐ the Kitakawachi clinical background and outcome of heart failure registry. Circ J. 2016;81:69‐76. [DOI] [PubMed] [Google Scholar]
- 31. Yaku H, Ozasa N, Morimoto T, et al. Demographics, management, and in‐hospital outcome of hospitalized acute heart failure syndrome patients in contemporary real clinical practice in Japan‐observations from the prospective, multicenter Kyoto Congestive Heart Failure (KCHF) registry. Circ J. 2018;82:2811‐2819. [DOI] [PubMed] [Google Scholar]
- 32. Kaku H, Funakoshi K, Ide T, et al. Impact of hospital practice factors on mortality in patients hospitalized for heart failure in Japan‐ an analysis of a large number of health records from a nationwide claims‐based database, the JROAD‐DPC. Circ J. 2020;84:742‐753. [DOI] [PubMed] [Google Scholar]
- 33. Sato M, Sakata Y, Sato K, et al. Clinical characteristics and prognostic factors in elderly patients with chronic heart failure ‐ a report from the CHART‐2 study. Int J Cardiol Heart Vasc. 2020;27:100497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ide T, Kaku H, Matsushima S, et al. Clinical characteristics and outcomes of hospitalized patients with heart failure from the large‐scale Japanese Registry Of Acute Decompensated Heart Failure (JROADHF). Circ J. 2021;85:1438‐1450. [DOI] [PubMed] [Google Scholar]
- 35. Kaneko H, Itoh H, Kiriyama H, et al. Fasting plasma glucose and subsequent cardiovascular disease among young adults: analysis of a nationwide epidemiological database. Atherosclerosis. 2021;319:35‐41. [DOI] [PubMed] [Google Scholar]
- 36. Seo M, Yamada T, Tamaki S, et al. Prognostic significance of cardiac I‐123‐metaiodobenzylguanidine imaging in patients with reduced, mid‐range, and preserved left ventricular ejection fraction admitted for acute decompensated heart failure: a prospective study in Osaka Prefectural Acute Heart Failure Registry (OPAR). Eur Heart J Cardiovasc Imaging. 2021;22:58‐66. [DOI] [PubMed] [Google Scholar]
- 37. Ohsugi M, Eiki JI, Iglay K, Tetsuka J, Tokita S, Ueki K. Comorbidities and complications in Japanese patients with type 2 diabetes mellitus: retrospective analyses of J‐DREAMS, an advanced electronic medical records database. Diabetes Res Clin Pract. 2021;178:108845. [DOI] [PubMed] [Google Scholar]
- 38. Ohta M, Babazono T, Uchigata Y, Iwamoto Y. Comparison of the prevalence of chronic kidney disease in Japanese patients with type 1 and type 2 diabetes. Diabet Med. 2010;27:1017‐1023. [DOI] [PubMed] [Google Scholar]
- 39. Iwai T, Miyazaki M, Yamada G, et al. Diabetes mellitus as a cause or comorbidity of chronic kidney disease and its outcomes: the Gonryo study. Clin Exp Nephrol. 2018;22:328‐336. [DOI] [PubMed] [Google Scholar]
- 40. Yokoyama H, Araki SI, Kawai K, et al. Declining trends of diabetic nephropathy, retinopathy and neuropathy with improving diabetes care indicators in Japanese patients with type 2 and type 1 diabetes (JDDM 46). BMJ Open Diabetes Res Care. 2018;6:e000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nitta K, Iimuro S, Imai E, et al. Risk factors for increased left ventricular hypertrophy in patients with chronic kidney disease: findings from the CKD‐JAC study. Clin Exp Nephrol. 2019;23:85‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yoshida Y, Kashiwabara K, Hirakawa Y, et al. Conditions, pathogenesis, and progression of diabetic kidney disease and early decliner in Japan. BMJ Open Diabetes Res Care. 2020;8:e000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tanaka K, Watanabe T, Takeuchi A, et al. Cardiovascular events and death in Japanese patients with chronic kidney disease. Kidney Int. 2017;91:227‐234. [DOI] [PubMed] [Google Scholar]
- 44. Kon S, Konta T, Ichikawa K, et al. Association between renal function and cardiovascular and all‐cause mortality in the community‐based elderly population: results from the Specific Health Check and Guidance Program in Japan. Clin Exp Nephrol. 2018;22:346‐352. [DOI] [PubMed] [Google Scholar]
- 45. Maack C, Lehrke M, Backs J, et al. Heart failure and diabetes: metabolic alterations and therapeutic interventions: a state‐of‐the‐art review from the Translational Research Committee of the Heart Failure Association‐European Society of Cardiology. Eur Heart J. 2018;39:4243‐4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Birkeland KI, Bodegard J, Norhammar A, et al. How representative of a general type 2 diabetes population are patients included in cardiovascular outcome trials with SGLT2 inhibitors? A large European observational study. Diabetes Obes Metab. 2019;21:968‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Braunwald E. Diabetes, heart failure, and renal dysfunction: the vicious circles. Prog Cardiovasc Dis. 2019;62:298‐302. [DOI] [PubMed] [Google Scholar]
- 48. Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628‐1637. [DOI] [PubMed] [Google Scholar]
- 49. van Melle JP, Bot M, de Jonge P, de Boer RA, van Veldhuisen DJ, Whooley MA. Diabetes, glycemic control, and new‐onset heart failure in patients with stable coronary artery disease: data from the heart and soul study. Diabetes Care. 2010;33:2084‐2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC Jr. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27:699‐703. [DOI] [PubMed] [Google Scholar]
- 51. Lombardi C, Spigoni V, Gorga E, Dei CA. Novel insight into the dangerous connection between diabetes and heart failure. Herz. 2016;41:201‐207. [DOI] [PubMed] [Google Scholar]
- 52. Ambrosy AP, Fonarow GC, Butler J, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123‐1133. [DOI] [PubMed] [Google Scholar]
- 53. Greenberg BH, Abraham WT, Albert NM, et al. Influence of diabetes on characteristics and outcomes in patients hospitalized with heart failure: a report from the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE‐HF). Am Heart J. 2007;154:277. [DOI] [PubMed] [Google Scholar]
- 54. Dhingra A, Garg A, Kaur S, et al. Epidemiology of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2014;11:354‐365. [DOI] [PubMed] [Google Scholar]
- 55. Seferovic PM, Petrie MC, Filippatos GS, et al. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20:853‐872. [DOI] [PubMed] [Google Scholar]
- 56. Dunlay SM, Redfield MM, Weston SA, et al. Hospitalizations after heart failure diagnosis: a community perspective. J Am Coll Cardiol. 2009;54:1695‐1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chaudhry SI, McAvay G, Chen S, et al. Risk factors for hospital admission among older persons with newly diagnosed heart failure: findings from the cardiovascular health study. J Am Coll Cardiol. 2013;61:635‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lawson CA, Jones PW, Teece L, et al. Association between type 2 diabetes and all‐cause hospitalization and mortality in the UK general heart failure population: stratification by diabetic glycemic control and medication intensification. JACC Heart Fail. 2018;6:18‐26. [DOI] [PubMed] [Google Scholar]
- 59. Cai X, Liu X, Sun L, et al. Prediabetes and the risk of heart failure: a meta‐analysis. Diabetes Obes Metab. 2021;23:1746‐1753. [DOI] [PubMed] [Google Scholar]
- 60. Mai L, Wen W, Qiu M, et al. Association between prediabetes and adverse outcomes in heart failure. Diabetes Obes Metab. 2021;23:2476‐2483. [DOI] [PubMed] [Google Scholar]
- 61. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88‐98. [DOI] [PubMed] [Google Scholar]
- 62. Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62:3‐16. [DOI] [PubMed] [Google Scholar]
- 63. Gregg EW, Li Y, Wang J, et al. Changes in diabetes‐related complications in the United States, 1990‐2010. N Engl J Med. 2014;370:1514‐1523. [DOI] [PubMed] [Google Scholar]
- 64. Clarke PM, Glasziou P, Patel A, et al. Event rates, hospital utilization, and costs associated with major complications of diabetes: a multicountry comparative analysis. PLoS Med. 2010;7:e1000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tun NN, Arunagirinathan G, Munshi SK, Pappachan JM. Diabetes mellitus and stroke: a clinical update. World J Diabetes. 2017;8:235‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035‐2038. [DOI] [PubMed] [Google Scholar]
- 67. Venketasubramanian N, Yoon BW, Pandian J, Navarro JC. Stroke epidemiology in South, East, and South‐East Asia: a review. J Stroke. 2017;19:286‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population‐based cohort. The Framingham Heart Study. JAMA. 1994;271:840‐844. [PubMed] [Google Scholar]
- 69. Movahed MR, Hashemzadeh M, Jamal MM. Diabetes mellitus is a strong, independent risk for atrial fibrillation and flutter in addition to other cardiovascular disease. Int J Cardiol. 2005;105:315‐318. [DOI] [PubMed] [Google Scholar]
- 70. Huxley RR, Filion KB, Konety S, Alonso A. Meta‐analysis of cohort and case‐control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol. 2011;108:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Afkarian M, Zelnick LR, Hall YN, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988‐2014. JAMA. 2016;316:602‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Khunti K, Charbonnel B, Chen H, et al. Prevalence and progression of chronic kidney disease among patients with type 2 diabetes: insights from the DISCOVER study. Diabetes Obes Metab. 2021;23:1956‐1960. [DOI] [PubMed] [Google Scholar]
- 73. Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24:302‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yamamoto Y, Hanai K, Mori T, et al. Kidney outcomes and all‐cause mortality in people with type 2 diabetes exhibiting non‐albuminuric kidney insufficiency. Diabetologia. 2022;65:234‐245. [DOI] [PubMed] [Google Scholar]
- 75. Kong AP, Xu G, Brown N, et al. Diabetes and its comorbidities—where East meets West. Nat Rev Endocrinol. 2013;9:537‐547. [DOI] [PubMed] [Google Scholar]
- 76. Chi ZS, Lee ET, Lu M, et al. Vascular disease prevalence in diabetic patients in China: standardised comparison with the 14 centres in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44(Suppl 2):S82‐S86. [DOI] [PubMed] [Google Scholar]
- 77. Lee ET, Keen H, Bennett PH, et al. Follow‐up of the WHO Multinational Study of Vascular Disease in Diabetes: general description and morbidity. Diabetologia. 2001;44(Suppl 2):S3‐S13. [DOI] [PubMed] [Google Scholar]
- 78. Lee ET, Lu M, Bennett PH, et al. Vascular disease in younger‐onset diabetes: comparison of European, Asian and American Indian cohorts of the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44(Suppl 2):S78‐S81. [DOI] [PubMed] [Google Scholar]
- 79. Parving HH, Lewis JB, Ravid M, Remuzzi G, Hunsicker LG. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int. 2006;69:2057‐2063. [DOI] [PubMed] [Google Scholar]
- 80. Liyanage T, Toyama T, Hockham C, et al. Prevalence of chronic kidney disease in Asia: a systematic review and analysis. BMJ Glob Health. 2022;7:e007525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Titze S, Schmid M, Kottgen A, et al. Disease burden and risk profile in referred patients with moderate chronic kidney disease: composition of the German Chronic Kidney Disease (GCKD) cohort. Nephrol Dial Transplant. 2015;30:441‐451. [DOI] [PubMed] [Google Scholar]
- 82. Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross‐sectional survey. Lancet. 2012;379:815‐822. [DOI] [PubMed] [Google Scholar]
- 83. Kang E, Han M, Kim H, et al. Baseline general characteristics of the Korean chronic kidney disease: report from the KoreaN cohort study for Outcomes in patients With Chronic Kidney Disease (KNOW‐CKD). J Korean Med Sci. 2017;32:221‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143:1157‐1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Damman K, Valente MA, Voors AA, et al. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta‐analysis. Eur Heart J. 2014;35:455‐469. [DOI] [PubMed] [Google Scholar]
- 86. Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17‐28. [DOI] [PubMed] [Google Scholar]
- 87. Kottgen A, Russell SD, Loehr LR, et al. Reduced kidney function as a risk factor for incident heart failure: the Atherosclerosis Risk In Communities (ARIC) study. J Am Soc Nephrol. 2007;18:1307‐1315. [DOI] [PubMed] [Google Scholar]
- 88. Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421‐426. [DOI] [PubMed] [Google Scholar]
- 89. Denker M, Boyle S, Anderson AH, et al. Chronic Renal Insufficiency Cohort study (CRIC): overview and summary of selected findings. Clin J Am Soc Nephrol. 2015;10:2073‐2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93:137‐188. [DOI] [PubMed] [Google Scholar]
- 91. Zhao J, Randive R, Stewart JA. Molecular mechanisms of AGE/RAGE‐mediated fibrosis in the diabetic heart. World J Diabetes. 2014;5:860‐867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol. 2016;12:144‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yamamoto Y, Kato I, Doi T, et al. Development and prevention of advanced diabetic nephropathy in RAGE‐overexpressing mice. J Clin Invest. 2001;108:261‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kim JA, Jang HJ, Martinez‐Lemus LA, et al. Activation of mTOR/p70S6 kinase by ANG II inhibits insulin‐stimulated endothelial nitric oxide synthase and vasodilation. Am J Physiol Endocrinol Metab. 2012;302:E201‐E208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED. Cardiac energy metabolism in heart failure. Circ Res. 2021;128:1487‐1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Jia G, Whaley‐Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia‐ and insulin‐resistance‐induced heart disease. Diabetologia. 2018;61:21‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bertero E, Maack C. Calcium signaling and reactive oxygen species in mitochondria. Circ Res. 2018;122:1460‐1478. [DOI] [PubMed] [Google Scholar]
- 98. Kaludercic N, Di Lisa F. Mitochondrial ROS formation in the pathogenesis of diabetic cardiomyopathy. Front Cardiovasc Med. 2020;7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tang SCW, Yiu WH. Innate immunity in diabetic kidney disease. Nat Rev Nephrol. 2020;16:206‐222. [DOI] [PubMed] [Google Scholar]
- 100. Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213‐3223. [DOI] [PubMed] [Google Scholar]
- 101. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12:2032‐2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int. 1996;49:1774‐1777. [DOI] [PubMed] [Google Scholar]
- 103. O'Bryan GT, Hostetter TH. The renal hemodynamic basis of diabetic nephropathy. Semin Nephrol. 1997;17:93‐100. [PubMed] [Google Scholar]
- 104. Qian Y, Feldman E, Pennathur S, Kretzler M, Brosius FC III. From fibrosis to sclerosis: mechanisms of glomerulosclerosis in diabetic nephropathy. Diabetes. 2008;57:1439‐1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Rangaswami J, Bhalla V, Blair JEA, et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2019;139:e840‐e878. [DOI] [PubMed] [Google Scholar]
- 106. Delgado‐Valero B, Cachofeiro V, Martinez‐Martinez E. Fibrosis, the bad actor in cardiorenal syndromes: mechanisms involved. Cell. 2021;10:1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bongartz LG, Cramer MJ, Doevendans PA, Joles JA, Braam B. The severe cardiorenal syndrome: 'Guyton revisited'. Eur Heart J. 2005;26:11‐17. [DOI] [PubMed] [Google Scholar]
- 108. Yogasundaram H, Chappell MC, Braam B, Oudit GY. Cardiorenal syndrome and heart failure‐challenges and opportunities. Can J Cardiol. 2019;35:1208‐1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Doehner W, Rauchhaus M, Ponikowski P, et al. Impaired insulin sensitivity as an independent risk factor for mortality in patients with stable chronic heart failure. J Am Coll Cardiol. 2005;46:1019‐1026. [DOI] [PubMed] [Google Scholar]
- 110. Hatamizadeh P, Fonarow GC, Budoff MJ, Darabian S, Kovesdy CP, Kalantar‐Zadeh K. Cardiorenal syndrome: pathophysiology and potential targets for clinical management. Nat Rev Nephrol. 2013;9:99‐111. [DOI] [PubMed] [Google Scholar]
- 111. Nohria A, Hasselblad V, Stebbins A, et al. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol. 2008;51:1268‐1274. [DOI] [PubMed] [Google Scholar]
- 112. Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582‐588. [DOI] [PubMed] [Google Scholar]
- 113. Ljungman S, Laragh JH, Cody RJ. Role of the kidney in congestive heart failure. Relationship of cardiac index to kidney function. Drugs. 1990;39(Suppl 4):10‐21. discussion 22‐24. [DOI] [PubMed] [Google Scholar]
- 114. Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Gnanaraj JF, von Haehling S, Anker SD, Raj DS, Radhakrishnan J. The relevance of congestion in the cardio‐renal syndrome. Kidney Int. 2013;83:384‐391. [DOI] [PubMed] [Google Scholar]
- 116. Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358:2148‐2159. [DOI] [PubMed] [Google Scholar]
- 117. Drexler H, Riede U, Munzel T, et al. Alterations of skeletal muscle in chronic heart failure. Circulation. 1992;85:1751‐1759. [DOI] [PubMed] [Google Scholar]
- 118. Lipkin DP, Jones DA, Round JM, Poole‐Wilson PA. Abnormalities of skeletal muscle in patients with chronic heart failure. Int J Cardiol. 1988;18:187‐195. [DOI] [PubMed] [Google Scholar]
- 119. Tumlin JA, Costanzo MR, Chawla LS, et al. Cardiorenal syndrome type 4: insights on clinical presentation and pathophysiology from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol. 2013;182:158‐173. [DOI] [PubMed] [Google Scholar]
- 120. London GM, Guerin AP, Marchais SJ, et al. Arterial media calcification in end‐stage renal disease: impact on all‐cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731‐1740. [DOI] [PubMed] [Google Scholar]
- 121. Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154‐2169. [DOI] [PubMed] [Google Scholar]
- 122. Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137‐147. [DOI] [PubMed] [Google Scholar]
- 123. Spoto B, Pisano A, Zoccali C. Insulin resistance in chronic kidney disease: a systematic review. Am J Physiol Renal Physiol. 2016;311:F1087‐F1108. [DOI] [PubMed] [Google Scholar]
- 124. Valdivielso JM, Rodriguez‐Puyol D, Pascual J, et al. Atherosclerosis in chronic kidney disease: more, less, or just different? Arterioscler Thromb Vasc Biol. 2019;39:1938‐1966. [DOI] [PubMed] [Google Scholar]
- 125. Palanca A, Castelblanco E, Perpinan H, et al. Prevalence and progression of subclinical atherosclerosis in patients with chronic kidney disease and diabetes. Atherosclerosis. 2018;276:50‐57. [DOI] [PubMed] [Google Scholar]
- 126. Raggi P, Bellasi A, Gamboa C, et al. All‐cause mortality in hemodialysis patients with heart valve calcification. Clin J Am Soc Nephrol. 2011;6:1990‐1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Alsahli M, Gerich JE. Hypoglycemia, chronic kidney disease, and diabetes mellitus. Mayo Clin Proc. 2014;89:1564‐1571. [DOI] [PubMed] [Google Scholar]
- 128. Attman PO, Samuelsson O, Alaupovic P. Lipoprotein metabolism and renal failure. Am J Kidney Dis. 1993;21:573‐592. [DOI] [PubMed] [Google Scholar]
- 129. Chan DT, Irish AB, Dogra GK, Watts GF. Dyslipidaemia and cardiorenal disease: mechanisms, therapeutic opportunities and clinical trials. Atherosclerosis. 2008;196:823‐834. [DOI] [PubMed] [Google Scholar]
- 130. Lee DM, Knight‐Gibson C, Samuelsson O, Attman PO, Wang CS, Alaupovic P. Lipoprotein particle abnormalities and the impaired lipolysis in renal insufficiency. Kidney Int. 2002;61:209‐218. [DOI] [PubMed] [Google Scholar]
- 131. Mahajan A, Taliun D, Thurner M, et al. Fine‐mapping type 2 diabetes loci to single‐variant resolution using high‐density imputation and islet‐specific epigenome maps. Nat Genet. 2018;50:1505‐1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Hosoe J, Suzuki K, Miya F, et al. Structural basis of ethnic‐specific variants of PAX4 associated with type 2 diabetes. Hum Genome Var. 2021;8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Taira M, Imamura M, Takahashi A, et al. A variant within the FTO confers susceptibility to diabetic nephropathy in Japanese patients with type 2 diabetes. PLoS One. 2018;13:e0208654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. van Zuydam NR, Ahlqvist E, Sandholm N, et al. A genome‐wide association study of diabetic kidney disease in subjects with type 2 diabetes. Diabetes. 2018;67:1414‐1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Zarkasi KA, Abdul Murad NA, Ahmad N, Jamal R, Abdullah N. Coronary heart disease in type 2 diabetes mellitus: genetic factors and their mechanisms, gene‐gene, and gene‐environment interactions in the Asian populations. Int J Environ Res Public Health. 2022;19:647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data discussed in this review are available in the public domain in the cited literature.


