Abstract
Background and objective
We have previously described reversal of collateral ventilation (CV) in a severe chronic obstructive pulmonary disease (COPD) patient with endoscopic polymer foam (EPF), prior to endoscopic lung volume reduction (ELVR) with valves. The aim of this study was to investigate the efficacy of this in a larger cohort and compare outcomes with a similar cohort with no CV.
Methods
Patients with severe COPD, with the left upper lobe (LUL) targeted for ELVR, were assessed for CV with high resolution computed tomography (HRCT). If fissure completeness was >95% they were enrolled as controls for valves alone (endobronchial valve control group [EBV‐CTRL]). If fissure completeness was 80%–95%, defects were mapped to the corresponding segment, where EPF was instilled following confirmation of CV with CHARTIS. EBVs were inserted 1 month afterwards.
Results
Fourteen patients were enrolled into both arms. After 6 months, there were significant improvements in both groups in forced expiratory volume in 1 s (FEV1; +19.7% EPF vs. +27.7% EBV‐CTRL, p < 0.05); residual volume (RV; −16.2% EPF vs. −20.1% EBV‐CTRL, p = NS); SGRQ (−15.1 EPF vs. −16.6 EBV‐CTRL p = NS) and 6 min walk (+25.8% EPF [77.2 m] vs. +28.4% [82.3 m] EBV‐CTRL p = NS). Patients with fissural defects mapped to the lingula had better outcomes than those mapped to other segments (FEV1 +22.9% vs. +16.3% p < 0.05). There were no serious adverse reactions to EPF.
Conclusion
EPF successfully reverses CV in severe COPD patients with a left oblique fissure that is 80%–95% complete. Following EBV, outcomes are similar to patients with complete fissures undergoing ELVR with EBV alone. EPF therapy to reverse CV potentially increases the number of COPD patients suitable for ELVR with minimal adverse reactions.
Keywords: bronchoscopy and interventional techniques, chronic obstructive pulmonary disease, collateral ventilation, COPD, endobronchial valve, endoscopic lung volume reduction, polymer foam
In chronic obstructive pulmonary disease (COPD) patients otherwise suitable for endoscopic lung volume reduction (ELVR) with endobronchial valves (EBVs) to the left upper lobe, but previously excluded due to collateral ventilation (CV), this can be successfully reversed with endoscopic polymer foam (EPF) prior to EBV insertion. Clinical improvements are similar to a group undergoing ELVR with no CV.
See related Editorial
INTRODUCTION
Endoscopic lung volume reduction (ELVR) utilizing endobronchial valves (EBVs) is an accepted management option in patients with severe chronic obstructive pulmonary disease (COPD). It is included in the GOLD (Global Initiative for Obstructive Lung Disease) guidelines for COPD management. 1
The predictors of response include severity of gas trapping, and lack of collateral ventilation (CV). CV may be assessed anatomically or functionally (via CHARTIS®). Anatomically, CV is assessed by the integrity of the interlobar fissures using high resolution computed tomography (HRCT) of the chest and predicts successful ELVR using EBV. 2 There is however significant observer variability in the assessment of fissures. Automated software analysis of fissures has therefore been developed and is reported with emphysema destruction scoring at −910 and −950 HU (hounsfield units) (STRATX®). CHARTIS is used to validate the software analysis of fissures. 3 This involves bronchoscopic assessment of CV using a catheter‐based system to measure airflow in the target airway while under balloon occlusion.
European expert panel recommendations for assessing CV suggests that if the targeted lobe has >95% fissure completeness, then ELVR with EBV should proceed. They also suggest that ELVR should not proceed if the targeted lobe has <80% fissure completeness. For fissures 80%–95% complete, CHARTIS assessment is recommended. 4
The polymer foam (EPF) Aeriseal® (polyvinyl alcohol and glutaraldehyde) has been previously studied as means of achieving lung volume reduction. 5 , 6 EPF obstructs small airways and collateral channels causing absorption atelectasis. This reduces lung hyperinflation and improves breathing mechanics. However, initial trials with lobar or multiple bilateral segments targeted, resulted in significant adverse reactions including COPD exacerbations and pneumonia, 7 suggesting an acute inflammatory response. 6 As a result, initial trials were suspended and currently there are trials with varying doses and delivery methods of EPF.
We have previously been first to describe the successful reversal of CV with EPF in a severe COPD patient. 8 The left oblique fissure integrity was only 83.2%, with the left upper lobe (LUL) targeted for ELVR. Positive CV was confirmed on CHARTIS, and EPF (20 ml) was instilled into the lingula segments, after mapping of the fissural defects to these segments. Following EPF, the patient was confirmed CV‐negative via CHARTIS, with successful LUL atelectasis following EBV therapy. FEV1 increased by 48% with improvements in exercise tolerance and quality of life. There were no adverse reactions to EPF, which we postulated was due to the lower volume of EPF used compared to previous studies.
The objectives of this study are therefore to determine the feasibility of combining EPF with EBVs in managing patients with severe COPD who are CV‐positive. EPF would be utilized with the aim of ‘sealing’ the defects in the fissure, with the insertion of EBVs subsequently to achieve atelectasis in the targeted lobe. We aimed to establish efficacy of using EPF to target the fissural defect leading to the presence of CV, and its effectiveness in reversing CV in severe COPD patients. Moreover, we aimed to compare the efficacy of ELVR in patients who are CV‐positive and treated with EPF and EBVs, with patients who are CV‐negative and treated with EBVs alone.
METHODS
This is a prospective non‐randomized, unblinded, controlled parallel group study undertaken at Macquarie University Hospital. Patients were recruited between March 2019 and June 2021.
All patients underwent initial baseline screening investigations including spirometry, lung volumes and diffusing capacity for carbon monoxide (DLCO) according to American Thoracic Society guidelines. 9 , 10 , 11 All patients had a 6‐min walk test, quality of life questionnaire (SGRQ), HRCT chest to enable DICOM images for uploading to STRATX®, ECG and echocardiogram.
The inclusion criteria were 40–85 years of age; FEV1 20%–50%; residual volume [RV] > 175%; 6‐min walk test > 150 m; completed pulmonary rehabilitation; STRATX assessment indicating the LUL as the most appropriate lobe for ELVR and heterogenous emphysema (minimum of 10% differential in destruction scores at −950 HU).
The exclusion criteria were acute uncontrolled medical illness; acute respiratory tract infections; bronchiectasis; interstitial lung diseases and active malignancy and pregnancy.
Patients meeting all inclusion and exclusion criteria were stratified into CV‐positive and CV‐negative patients based on the STRATX examination:
CV‐negative group: Left oblique fissure >95% complete.
CV‐positive group: Left oblique fissure 80%–95% complete.
Left oblique fissure <80% complete—EXCLUDED from this study.
All patients were presented and discussed at our Interventional Pulmonology Multidisciplinary Team Meeting to ensure that the most appropriate lobe was being targeted, and to determine the most clinically appropriate therapy.
Protocol for CV‐negative group
Following general anaesthesia and intubation, EBV (Pulmonx) were inserted into the LUL and lingula segments via a therapeutic bronchoscope.
Post‐operative care included admission to ICU for 24 h and a chest x‐ray (CXR) to exclude pneumothorax. Intravenous ceftriaxone 1 g daily was administered for 48 h post‐procedure to prevent post‐operative pneumonia. Routine systemic steroids were not given Patients were discharged after 48 h if clinically stable. If pneumothorax developed, this was managed as per published protocol. 12
Protocol for CV‐positive group
This group had an 80%–95% incomplete left oblique fissure, HRCT chest DICOMs were uploaded to identify the area of anatomical defect in the fissure, and which segmental bronchi subtended that defect (Figures 1 and 2). The feasibility of directing EPF to the appropriate segment (mapping) was then determined, with the aim of delivering 10 ml of EPF to each appropriate segment (maximum of 3 segments or 30 ml). Figure 1 represents a typical single medial left oblique fissure defect mapped to lingula superior segment/lingula inferior segment (LB4/LB5). Figure 2 represents an example of multiple left oblique fissure defects, which involves other segments in addition or instead of LB4/LB5.
FIGURE 1.
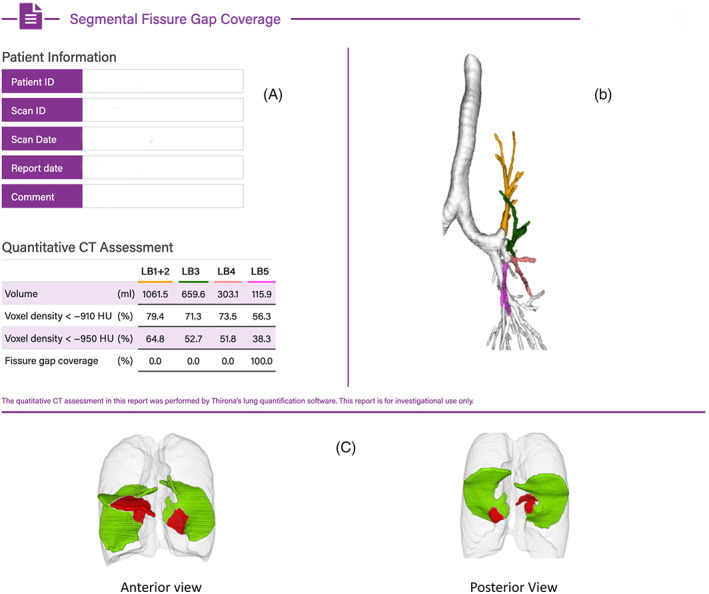
Example of segmental mapping of lingula fissure defect (LB4 and LB5). Single left oblique fissure defect seen, subtended by LB5. (A) LUL segments by volume, voxel density and subtending fissure defects (single defect in LB5 segment). (B) Colour coded graphical representation of mapped segments. (C) Fissure mapping with red representing areas of fissure deficiency and green representing areas where fissures are intact
FIGURE 2.
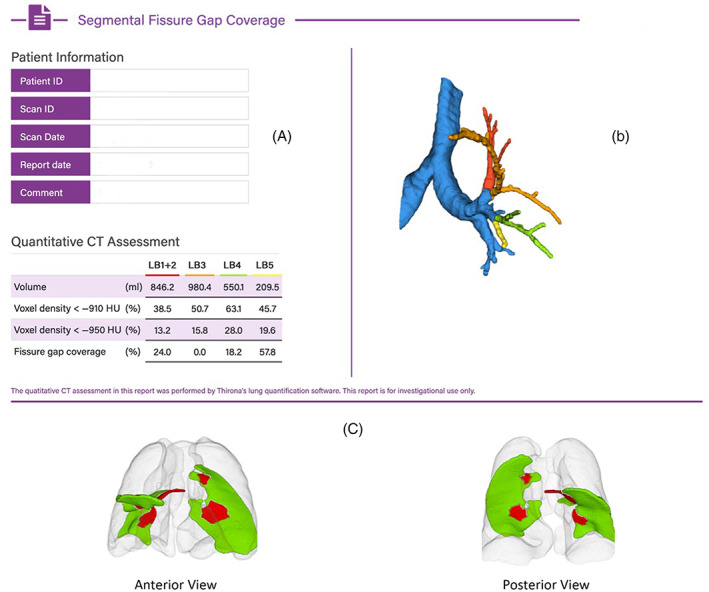
Example of segmental mapping of fissural defects (involving other segments in addition to lingula segments). Multiple defects seen in Left Oblique fissure with defects corresponding to left upper lobe apical segment/left upper lobe posterior segment (LB1/2) and lingula superior segment (LB4) and lingula inferior segment (LB5). (A) LUL segments by volume, voxel density, and subtending fissure defects (multiple defects subtended by LB 1 + 2, LB4 and LB5). (B) Colour coded graphical representation of mapped segments. (C) Fissure mapping with red representing areas of fissure deficiency and green representing areas where fissures are intact
The patient then underwent two separate procedures. The first was performed following general anaesthesia, and intubation. A therapeutic bronchoscope was used to perform CHARTIS evaluation of the LUL. The tidal volume was recorded so that future CHARTIS assessments were consistent. If confirmed as CV‐positive, EPF was instilled into the appropriate LUL segment(s) via a CHARTIS balloon catheter, which also prevented the reflux of the foam into more proximal airways. The patient was subsequently admitted for 24‐h and had a CXR performed prior to discharge.
If the CHARTIS evaluation was CV‐negative, the patient went onto have EBV inserted into the LUL at the first procedure without EPF.
The second procedure was performed for CV‐positive patients 4 weeks after the first. CHARTIS evaluation of the LUL was repeated utilizing the same tidal volume as per the first procedure. If the CHARTIS evaluation was either CV‐negative or equivocal, the patient went onto have EBVs inserted into the LUL. Post‐operative care was identical to the CV‐negative group.
If the CHARTIS evaluation was CV‐positive at the second procedure, the patient was to be considered for other ELVR techniques and would not have EBV inserted.
All patients were followed up with a telemedicine consult at 1‐week post‐procedure. A physical visit was routinely scheduled for 1‐ and 3‐months. Repeat baseline investigations including CT Thorax to determine LUL volume loss, would be performed at the 3‐month visit. Unfortunately, due to the Covid‐19 pandemic, elective procedures at our institution were postponed and clinic visits were curtailed. This adversely affected our ability to achieve timely follow‐up. However, we were able to finish recruitment for both arms of our study in June 2021, and we report follow‐up data at 6‐months rather than 3‐months.
Sample size calculation
Using FEV1 with the minimal difference we determined to find significant as 5%, a population variance from previous studies of 2.5%, and with an alpha of 0.05 and a beta of 0.10, we calculated we required 14 patients in each arm of our study.
Statistical analysis
All analyses were performed using the SPSS version 20.0 software (IBM, Armonk, NY). Continuous data are expressed as mean ± SD. Comparisons of baseline and post‐ELVR data were made using paired‐samples t‐test. Correlations of the changes in non‐parametric data such as SGRQ were assessed by the Pearson correlation method. A p‐value of <0.05 was deemed statistically significant.
RESULTS
Twenty‐two consecutive patients were screened for the EPF group, following initial STRATX assessment that determined the LUL as the most appropriate target, with the left oblique fissure 80%–95% complete. Six were excluded for varying reasons including interstate COVID‐19 travel restrictions, COPD exacerbations and suitability of alternative therapies (thermal ablation and transplantation). Sixteen patients went onto undergo procedure one. Two were determined to be CV‐negative via CHARTIS, (fissure completeness 94.5% and 90.7%) and underwent ELVR with EBVs at the same procedure. These two patients were not included in either group. All 14 patients in the EPF group proceeding onto procedure two had EBVs. Nine patients were CV‐negative at procedure 2, and five patients were inconclusive. The average fissure integrity in the EPF group was 88.6 ± 4.7%.
The 14 patients in the EBV‐CTRL group were consecutively recruited from March 2019 with the LUL determined as the most appropriate target. The average fissure integrity this group was 98.7 ± 1.8%.
The baseline characteristics of both groups are shown in Table 1. The only significant difference is that the EPF group had a greater pre‐ELVR LUL volume which may reflect a higher number of male subjects (9 vs. 5).
TABLE 1.
Baseline demographics of our EBV‐CTRL and EPF group
| CV negative EBV‐CTRL | CV positive EPF group | p value | |
|---|---|---|---|
| Subjects (N) | 14 | 14 | |
| Female (N) | 9 | 5 | |
| Age (years) | 69.2 (3.8) | 71.8 (3.8) | NS |
| Body mass index (kg/m2) | 25.1 (4.5) | 26.7 (5.2) | NS |
| Smoking history (pack‐years) | 51.1 (18.7) | 49.6 (10.4) | NS |
| Post‐BD FEV1 (% predicted) | 31.2 (8.9) | 28.2 (5.4) | NS |
| Post‐BD FVC (% predicted) | 76.2 (24.8) | 73.5 (14.0) | NS |
| TLC (% predicted) | 127.1 (17.7) | 129.1 (16.3) | NS |
| RV (% predicted) | 207.1 (32.8) | 217.2 (39) | NS |
| DLCO (ml/min/mm Hg) | 7.97 (3.20) | 9.94 (3.64) | NS |
| KCO (ml/min/mm Hg/L) | 2.04 (0.58) | 2.15 (0.74) | NS |
| 6MWD (m) | 289.7 (100.8) | 298.8 (97.3) | NS |
| SGRQ | 57.3 (9.7) | 62.2 (12.8) | NS |
| Left upper lobe volume (ml) | 1561.7 (429.5) | 1957.5 (544.2) | <0.05 |
Note: Data are presented as n (%) or mean ± SD, unless otherwise stated.
Abbreviations: 6MWD, 6 minute walk distance; BD, bronchodilator; CV, collateral ventilation; DLCO, diffusing capacity for carbon monoxide; EBV‐CTRL, endobronchial valve control group; EPF, endobronchial polymer foam; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; KCO, carbon monoxide transfer coefficient; RV, residual volume; SGRQ, St George Respiratory Questionnaire; TLC, total lung capacity.
The EPF group had mean follow‐up at 5.8 ± 1.7 months, and the EBV‐CTRL group at 6.1 ± 2.2 months (p = NS). The results of the 6‐month follow‐up in both groups are depicted in Table 2. In the EPF group there were significant improvements documented in all lung function parameters except DLCO. FEV1 improved by 19.7 ± 6.3% or 150(100) ml, RV fell by 16.2 ± 4.7% or 690(383) ml, and LUL volume (on CT) reduced by 895 ± 347 ml. Six had complete lobar atelectasis and eight partial. There were also significant improvements in 6‐min walk test and SGRQ. In the EBV‐CTRL group, there were also significant improvement across all parameters apart from DLCO. When the improvements in the EPF and EBV‐CTRL groups were compared as percent changes (Table 3), the EBV‐CTRL group had significantly greater improvement in FEV1. Although there was a trend towards greater improvements in other parameters including RV and SGRQ, these were not significant.
TABLE 2.
Results at 6 months post ELVR
| EPF group N = 14. | Baseline | 6 months post ELVR | p value |
|---|---|---|---|
| Panel 1—EPF (CV positive group) | |||
| Post‐BD FEV1 (% predicted) | 28.2 (5.4) | 34.7 (7.9) | <0.01 |
| Post‐BD FEV1 (ml) | 0.75 (0.18) | 0.90 (0.28) | <0.01 |
| Post‐BD FVC (% predicted) | 73.5 (14.0) | 82.7 (14.7) | <0.01 |
| TLC (% predicted) | 129.1 (16.3) | 122.9 (16.4) | <0.01 |
| RV (% predicted) | 217.2 (39.0) | 190.62 (34.3) | <0.01 |
| RV (L) | 5.22 (0.91) | 4.53 (0.68) | <0.01 |
| DLCO (% predicted) | 41.2 (14.2) | 43.6 (13.6) | NS |
| DLCO (ml/min/mm Hg) | 9.94 (3.64) | 10.33 (3.62) | NS |
| KCO (% predicted) | 53.9 (17.2) | 61.0 (18.9) | <0.01 |
| KCO (ml/min/mm Hg/L) | 2.15 (0.74) | 2.38 (0.83) | <0.01 |
| 6MWD (m) a | 298.8 (97.3) | 376.0 (123) | <0.01 |
| SGRQ | 62.2 (12.8) | 47.1 (13.7) | <0.01 |
| Left upper lobe volume (ml) | 1957.5 (544.2) | 1062.9 (547.8) | <0.01 |
| EBV‐CTRL group N = 14 | Baseline | 6 months post ELVR | p value |
|---|---|---|---|
| Panel 2—EBV‐CTRL group (CV negative) | |||
| Post‐BD FEV1 (% predicted) | 31.2 (8.9) | 39.0 (7.5) | <0.01 |
| Post‐BD FEV1 (ml) | 0.76 (0.28) | 0.96 (0.30) | <0.01 |
| Post‐BD FVC (% predicted) | 76.2 (24.8) | 81.3 (11.9) | <0.01 |
| TLC (% predicted) | 127.1 (17.7) | 119.8 (19.9) | <0.01 |
| RV (% predicted) | 207.1 (32.8) | 172.9 (38.2) | <0.01 |
| RV (L) | 4.36 (0.96) | 3.68 (0.67) | <0.01 |
| DLCO (% predicted) | 34.8 (11.8) | 36.6 (8.7) | NS |
| DLCO (ml/min/mm Hg) | 7.97 (3.20) | 8.22 (2.41) | NS |
| KCO (% predicted) | 47.8 (13.9) | 52.2 (14.2) | <0.01 |
| KCO (ml/min/mm Hg/L) | 2.04 (0.58) | 2.21 (0.65) | <0.01 |
| 6MWD (m) | 289.7 (100.8) | 372.0 (67.6) | <0.01 |
| SGRQ | 57.3 (9.7) | 40.7 (10.6) | <0.01 |
| Left upper lobe volume (ml) | 1561.7 (429.5) | 862.3 (492.1) | <0.01 |
Note: Data are presented as n (%) or mean ± SD, unless otherwise stated.
Abbreviations: BD, bronchodilator; CV, collateral ventilation; DLCO, diffusing capacity for carbon monoxide; EBV‐CTRL, endobronchial valve control group; ELVR, endoscopic lung volume reduction; EPF, endobronchial polymer foam; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; RV, residual volume; TLC, total lung capacity.
n = 12 ‐ as 2 subjects from rural areas were unable to travel to have 6MWD performed in an appropriate timeframe.
TABLE 3.
EPF versus EBV‐CTRL group—% changes post ELVR
| N = 14 both groups | CV negative EBV‐CTRL—difference at 6 months | CV positive EPF group—difference at 6 months | p value |
|---|---|---|---|
| Post‐BD FEV1 (% change) | 27.7 (5.3) | 19.7 (6.3) | <0.05 |
| Post‐BD FVC (% change) | 15.9 (5.6) | 14.7 (5.2) | NS |
| TLC (% change) | −5.8 (1.5) | −4.0 (1.8) | NS |
| RV (% change) | −20.1 (5.6) | −16.2 (4.7) | NS |
| DLCO (% predicted) | 6.5 (5.0) | 6.1 (3.4) | NS |
| KCO (% change) | 9.4 (3.8) | 7.0 (3.6) | NS |
| 6MWD (% change) | 28.4 (11.0) | 25.8 (4.9) a | NS |
| SGRQ (% change) | −28.8 (4.7) | −22.8 (5.6) | NS |
| LUL volume (% change) | −46.1 (6.0) | −43.4 (7.2) | NS |
Note: Data are presented as n (%) or mean ± SEM, unless otherwise stated.
Abbreviations: BD, bronchodilator; CV, collateral ventilation; DLCO, diffusing capacity for carbon monoxide; EBV‐CTRL, endobronchial valve control group; ELVR, endoscopic lung volume reduction; EPF, endobronchial polymer foam; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; LUL, left upper lobe; RV, residual volume; TLC, total lung capacity.
n = 12—as two subjects from rural areas were unable to travel to have 6MWD performed in an appropriate timeframe.
The six EPF patients who had the lingula segments targeted, had significantly better outcomes than EPF patients who had other LUL segments targeted (Table 4). The lingula segment group had significantly greater improvements in FEV1 and DLCO, and significantly greater reductions in RV, SGRQ and LUL volume post ELVR.
TABLE 4.
EPF group: Lingula LB4/LB5 fissural defects analysis
| Lingula fissural defects (n = 6) | Other LUL segment fissural defects (n = 8) | p value | |
|---|---|---|---|
| Panel 1: Comparison with fissural defects in other LUL segments—% changes post ELVR | |||
| Post‐BD FEV1 (% change) | 22.9 (16.3) | 16.3 (7.8) | < 0.05 |
| Post‐BD FVC (% change) | 15.9 (7.9) | 13.7 (7.3) | NS |
| TLC (% change) | −7.5 (2.1) | ‐ 3.8 (2.1) | NS |
| RV (% change) | −22.1 (7.4) | −9.4 (4.6) | < 0.05 |
| DLCO (% predicted) | 10.3 (4.7) | 2.4 (4.7) | < 0.05 |
| KCO (% change) | 15.5 (6.7) | 4.1 (3.7) | < 0.05 |
| 6MWD (% change) | 23.9 (4.2) | 21.3 (11) a | NS |
| SGRQ (% change) | −34.9 (9.0) | −16.8 (6.7) | < 0.05 |
| LUL volume (% change) | −53.91 (9.9) | −34.5 (9.6) | < 0.05 |
| Lingula fissural defects (n = 6) | EBV‐CTRL group (n = 14) | p value | |
|---|---|---|---|
| Panel 2: Comparison with EBV‐CTRL group—% changes post ELVR | |||
| Post‐BD FEV1 (% change) | 22.9 (16.3) | 27.7 (5.3) | NS |
| Post‐BD FVC (% change) | 15.9 (7.9) | 15.9 (5.6) | NS |
| TLC (% change) | −7.5 (2.1) | −5.8 (1.5) | NS |
| RV (% change) | −22.1 (7.4) | −20.1 (5.6) | NS |
| DLCO (% predicted) | 10.3 (4.7) | 6.5 (5.0) | NS |
| KCO (% change) | 15.5 (6.7) | 9.4 (3.8) | NS |
| 6MWD (% change) | 23.9 (4.2) | 28.4 (11.0) | < 0.05 |
| SGRQ (% change) | −34.9 (9.0) | −28.8 (4.7) | NS |
| LUL volume (% change) | −53.91 (9.9) | −46.1 (6.0) | NS |
Note: Data are presented as n (%) or mean ± SEM, unless otherwise stated.
Abbreviations: BD, bronchodilator; CV, collateral ventilation; DLCO, diffusing capacity for carbon monoxide; EBV‐CTRL, endobronchial valve control group; ELVR, endoscopic lung volume reduction; EPF, endobronchial polymer foam; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; LUL, left upper lobe; RV, residual volume; TLC, total lung capacity.
n = 6—as two subjects from rural areas were unable to travel to have 6MWD performed in an appropriate timeframe.
When the EPF group who had lingula segments targeted with foam were compared to the EBV‐CTRL group, there were no significant differences in post ELVR parameters apart from 6‐min walk test (Table 4).
In the EPF group, there were no significant difference in outcomes between patients who were confirmed as CV‐negative and those who were CV inconclusive.
There was one patient with a post‐ELVR pneumothorax in the EPF group and two in the EBV‐CTRL group. All pneumothoraces occurred on the left and within 48 h of ELVR. All three patients required management with a small‐bore intercostal catheter and made full recoveries.
There were no recorded serious adverse reactions to EPF. Two patients developed a low‐grade fever and cough. Both had radiological changes of inflammation when investigated (Figure S1 in the Supporting Information) and raised CRP. Both were managed with oral steroids for 3 days with complete resolution of symptoms. No antibiotics were required. There was no haemoptysis, chest pain or COPD exacerbations post EPF instillation.
DISCUSSION
In this controlled parallel group study, patients with severe COPD in both groups had significant improvements in lung function, exercise tolerance and quality of life parameters at 6‐months post‐ELVR targeting the LUL. Both groups also demonstrated significant reduction in targeted lobe volume. However, there were greater improvements in FEV1 in the EBV‐CTRL group. There was also a trend towards greater improvements in SGRQ scores, and reduction in RV. When a subset of six patients in the EPF group with lingula segments targeted with EPF prior to ELVR were compared to the EBV‐CTRL group, there were no significant differences in response apart from the 6‐min walk test.
This study confirms the proof of concept and case report, 8 that EPF may successfully reverse CV in COPD patients with incomplete fissures prior to ELVR. However, this conclusion has specific pre‐requisites. First, the fissural defect studied is in the left oblique fissure, and the degree of completeness is 80%–95%. The success of EPF in reversing CV in other fissures, or when fissural completeness is <80% is not established. Secondly, we have evidence that the best response occurs when there is a single defect in the fissure. EPF patients with a single defect in the medial portion of the left oblique fissure (subtended by LB4/LB5) responded best to EPF, with post ELVR response being no different to the EBV‐CTRL group apart from the 6 min walk test.
Successful conversion from a CV‐positive to CV‐negative status was confirmed with CHARTIS in 9 of the 14 patients (64%). While the remaining five patients had an inconclusive CV assessment on CHARTIS, they had meaningful improvements in all outcomes at 6‐months after ELVR with EBV, suggesting that the CHARTIS assessment was limiting. Thus, the CV conversion rate is likely higher than 64%.
Follow‐up CTs reveal volume loss across all LUL segments, not just segments instilled with EPF, implying that volume loss is not just from EPF alone.
There were no serious adverse reactions to EPF. Two patients (14.3%) presented with fever and cough which resolved within 7 days after a short course of oral steroids. The adverse reactions to EPF are therefore significantly less than in previous studies, 6 where 44% of patients required hospitalization for adverse reactions, with two deaths (6%). The likely explanation of this is our EPF group only had segmental instillation of EPF, with an average of 2.1 segments treated (min 1 and max 3). In previous trials, multiple segments in both upper lobes were targeted at the same session. 5 , 6 As a result, significantly less volume of EPF was used in our study.
The magnitude of improvement in lung function, exercise capacity and volume loss in both our groups is similar to other published studies of ELVR with EBV, targeting patients with heterogenous emphysema with absent CV. 13 , 14
This study is a small, single centre study, but has been adequately powered to determine that the use of EPF may successfully reverse CV in patients with severe COPD prior to ELVR with EBV. This is applicable to patients with heterogenous emphysema, with the LUL being the appropriate target lobe, and with an 80%–95% complete left oblique fissure. The best response occurs with a single fissure defect subtended by lingula segments. The procedure is safe with significant volume loss in the targeted lobe which predicts improved prognosis. 15
This study therefore suggests that EPF therapy to reverse CV is a worthwhile technique to increase the number of severe COPD patients who may be suitable for ELVR with EBVs. This potentially opens a therapeutic modality to many patients that previously were deemed to be not suitable. More research however needs to be undertaken to determine if lobar targets other than the LUL are suitable, and to determine if multiple fissure defects should be treated.
AUTHOR CONTRIBUTION
Alvin J. Ing: Conceptualization (lead); data curation (equal); formal analysis (equal); funding acquisition (lead); investigation (equal); methodology (equal); project administration (lead); resources (lead); supervision (lead); writing – original draft (lead); writing – review and editing (lead). Anand Jayapadman: Data curation (equal); formal analysis (equal); investigation (equal); software (supporting); writing – review and editing (equal). Woo‐Veen Kim: Data curation (equal); formal analysis (equal); investigation (equal); software (supporting); writing – review and editing (equal). Chelsea Ly: Data curation (equal); investigation (equal); software (lead); writing – review and editing (equal). Kevin Ho‐Shon: Data curation (equal); investigation (equal); methodology (equal); writing – review and editing (equal). Paul Lilburn: Data curation (equal); formal analysis (equal); investigation (equal); supervision (supporting); writing – review and editing (equal). Alan Carew: Data curation (equal); investigation (equal); supervision (supporting); writing – review and editing (equal). Benjamin J. H. Ng: Data curation (equal); investigation (equal); supervision (supporting); writing – review and editing (equal). Tajalli Saghaie: Conceptualization (supporting); data curation (equal); formal analysis (equal); funding acquisition (supporting); investigation (equal); methodology (supporting); project administration (supporting); supervision (supporting); writing – review and editing (equal). Jonathan P. Williamson: Conceptualization (supporting); data curation (equal); formal analysis (equal); funding acquisition (supporting); investigation (supporting); methodology (supporting); project administration (supporting); supervision (supporting); writing – review and editing (equal).
CONFLICTS OF INTEREST
This is an investigator‐initiated trial funded by Pulmonx Australia. Alvin J. Ing has received consultancy fees in the past from Pulmonx Australia, Olympus Australia and Morair Medical. The other authors have made no disclosures.
HUMAN ETHICS APPROVAL DECLARATION
The study was approved by the Macquarie University Hospital Human Research Ethics Committee (Ethics Reference No: 5201937547764). All subjects gave their informed written consent.
Supporting information
Figure S1 Chest x‐ray series of EPF patient with fever post polymer instillation.
Visual Abstract Reversal of collateral ventilation using endoscopic polymer foam (EPF) in COPD patients undergoing endoscopic lung volume reduction (ELVR) with endobronchial valves (EBV): A controlled parallel group trial
ACKNOWLEDGEMENTS
We wish to acknowledge the support and work of the Macquarie University Hospital Clinical Trials Unit and specifically Nicola Chapman, Hung Tran and Manny Marquez. We also wish to thank Pulmonx Australia for their unrestricted support and contributions to this study, in particular Sarah Coxon, Elhassan Elghirani and Narinder Shargill. Open access publishing facilitated by Macquarie University, as part of the Wiley ‐ Macquarie University agreement via the Council of Australian University Librarians.
Ing AJ, Jayapadman A, Kim W‐V, Ly C, Ho‐Shon K, Lilburn P, et al. Reversal of collateral ventilation using endoscopic polymer foam in COPD patients undergoing endoscopic lung volume reduction with endobronchial valves: A controlled parallel group trial. Respirology. 2022;27(12):1064–1072. 10.1111/resp.14338
Associate Editor: Chi Chiu Leung; Senior Editor: Phan Nguyen
See related Editorial
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. GOLD . 2020. Global Strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease (2020 report). www.goldcopd.org
- 2. Koster DT, van Rikxoort EM, Huebner RH, Doellinger F, Klooster K, Charbonnier JP, et al. Predicting lung volume reduction after endobronchial valve therapy is maximized using a combination of diagnostic tools. Respiration. 2016;92(3):150–7. [DOI] [PubMed] [Google Scholar]
- 3. Carew A, Williamson JP, Philips MJ, Saghaie T, Garah CS, Ing AJ. Interventional bronchoscopy for chronic obstructive pulmonary disease: more than a pipe dream. Med J Aust. 2021;215(6):280–5. [DOI] [PubMed] [Google Scholar]
- 4. Herth FJF, Slebos DJ, Criner GJ, Valipour A, Sciurba F, Shah PL. Endoscopic lung volume reduction: an expert panel recommendation—update 2019. Respiration. 2019;97:548–57. [DOI] [PubMed] [Google Scholar]
- 5. Herth FJ, Gompelmann D, Stanzel F, Bonnet R, Behr J, Schmidt B, et al. Treatment of advanced emphysema with emphysematous lung sealant (AeriSeal®). Respiration. 2011;82:36–45. [DOI] [PubMed] [Google Scholar]
- 6. Come CE, Kramer MR, Dransfield MT, Abu‐Hijleh M, Berkowitz D, Bezzi M, et al. A randomised trial of lung sealant versus medical therapy for advanced emphysema. Eur Respir J. 2015;46:651–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O'Donnell DE, Webb KA, Neder JA. Lung hyperinflation in COPD: applying physiology to clinical practice. COPD Res Prac. 2015;1:4–12. [Google Scholar]
- 8. Ing AJ, Sullivan C, Hersch N, Saghaie T, Williamson J. Reversal of collateral ventilation using endobronchial polymer sealant in a patient with emphysema undergoing endoscopic lung volume reduction (ELVR) with valves: a case report and proof of concept. J Bronchology Interv Pulmonol. 2020;27(1):14–6. [DOI] [PubMed] [Google Scholar]
- 9. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. [DOI] [PubMed] [Google Scholar]
- 10. Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511–22. [DOI] [PubMed] [Google Scholar]
- 11. Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, et al. Standardisation of the single‐breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26(4):720–35. [DOI] [PubMed] [Google Scholar]
- 12. Van Dijk M, Sue R, Criner GJ, Gompelmann D, Herth FJ, Hogarth DK, et al. Expert statement: pneumothorax associated with one‐way valve therapy for emphysema: 2020 update. Respiration. 2021;100(10):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klooster K, ten Hacken NH, Hartman JE, Kerstjens HA, van Rikxoort EM, Slebos DJ. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med. 2015;373:2325–35. [DOI] [PubMed] [Google Scholar]
- 14. Davey C, Zoumot Z, Jordan S, McNulty WH, Carr DH, Hind MD, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR‐HIFi study): a randomised controlled trial. Lancet. 2015;386:1066–73. [DOI] [PubMed] [Google Scholar]
- 15. Gompelmann D, Benjamin N, Bischoff E, Hoffmann H, Heussel C‐P, Herth FJF, et al. Survival after endoscopic valve therapy in patients with severe emphysema. Eur Respir J. 2019;97:145–52. 10.1159/000492274 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Chest x‐ray series of EPF patient with fever post polymer instillation.
Visual Abstract Reversal of collateral ventilation using endoscopic polymer foam (EPF) in COPD patients undergoing endoscopic lung volume reduction (ELVR) with endobronchial valves (EBV): A controlled parallel group trial
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


