Abstract
Aims
Renin–angiotensin–aldosterone system inhibitors (RAASi) are guideline‐recommended therapy for individuals with cardiorenal disease. They are associated with increased risk of hyperkalaemia, a common and life‐threatening disorder for this population. RAASi‐induced hyperkalaemia often leads to dose reduction or discontinuation, reducing cardiorenal protection. Guideline recommendations differ between specialties for the clinical management of hyperkalaemia. Using a modified Delphi method, we developed consensus recommendations for optimal management of hyperkalaemia in adults with cardiorenal disease.
Methods and results
An international steering group of cardiologists and nephrologists developed 39 statements regarding hyperkalaemia care, including risk factors and risk stratification, prevention, correction, and cross‐specialty coordination. Consensus was determined by agreement on an online questionnaire administered to cardiorenal specialists across Europe and North America. The threshold for consensus agreement was established a priori by the steering group at 67%. Across November 2021, 520 responses were received from Canada (n = 50), France (n = 50), Germany (n = 54), Italy (n = 58), Spain (n = 57), the UK (n = 49), and the US (n = 202); 268 from cardiologists and 252 from nephrologists. Twenty‐nine statements attained very high agreement (≥90%) and 10 attained high agreement (≥67%–<90%), with strong alignment between cardiologists and nephrologists.
Conclusion
A high degree of consensus regarding hyperkalaemia evaluation and management exists among healthcare professionals. Based on high levels of agreement, the steering group derived six key recommendations for hyperkalaemia prevention and management in people with cardiorenal disease. Future studies examining the quality of hyperkalaemia care delivery are required.
Keywords: Delphi method, Hyperkalaemia, Cardiorenal patients, Renin–angiotensin–aldosterone system inhibitors, Consensus recommendation, Novel potassium binder
Introduction
Hyperkalaemia is a potentially life‐threatening medical condition of elevated blood potassium levels; 3.5–5.0 mEq/L is considered the normokalaemic range in adults, while instances of hyperkalaemia >5.5 mEq/L are more clearly associated with adverse clinical outcomes. 1 There is no universally accepted definition for hyperkalaemia severity. The European Society of Cardiology (ESC) guidelines recommend using a lower cut‐off for mild (>5.0 mEq/L), moderate (>5.5 mEq/L), or severe (>6.0 mEq/L) hyperkalaemia, for closer monitoring of patients who may be at risk of hyperkalaemic complications. 2 Other guidelines, such as those endorsed by the UK Renal Association (UKRA), utilise a more stringent outcome‐associated definition (mild: >5.5 mEq/L, moderate: >6.0 mEq/L, and severe: >6.5 mEq/L). 3 Most individuals with hyperkalaemia (particularly those with mild hyperkalaemia) are asymptomatic but when symptoms do occur, they tend to be non‐specific. Untreated, severe hyperkalaemia can result in life‐threatening cardiac arrhythmias, muscle weakness, paralysis, and mortality. 4
Heart failure (HF) and chronic kidney disease (CKD) often coexist and contribute to poor outcomes. In adults with cardiorenal disease, concomitant HF and CKD increases the risk of hospitalization, need for intensive care or kidney replacement therapy, and mortality 5 ; consequently, median survival in this population is around half that of people with HF alone. 6 Those with cardiorenal disease are also less likely to receive guideline‐directed medical therapy for HF, possibly due to concerns about hypotension, worsening kidney function, and hyperkalaemia. This may lead to worse cardiovascular outcomes. 5
Renin–angiotensin–aldosterone system inhibitors (RAASi) are an evidence‐based treatment for many individuals with both HF and CKD to prevent or slow progression 7 ; however, use of RAASi is also a risk factor for hyperkalaemia. 3 Cardiology‐ (ESC, the Canadian Cardiovascular Society) and nephrology‐based guidelines (Kidney Disease: Improving Global Outcomes [KDIGO]) recommend routine use of RAASi, titrated to a maximum tolerated dose, to improve adverse outcomes in individuals with cardiorenal syndrome. 8 , 9 , 10 , 11 In practice, RAASi therapy optimization is often limited and prevented by the development of hyperkalaemia. 5 This presents a medical quandary regarding the provision of lifesaving RAASi treatment in the context of the increased risk of resultant hyperkalaemia.
In the face of hyperkalaemia, RAASi dose reduction or discontinuation are the most common utilized therapeutic options. Submaximal RAASi dosing or discontinuation is associated with worse cardiorenal outcomes and increased mortality, 4 , 12 and once stopped, treatment is often not reinitiated. 13 The goal must therefore be to maintain treatment with disease‐modifying drugs (e.g. RAASi) for as long as possible, an approach supported by ESC (2021), KDIGO (2020, 2021), and UKRA guidelines (2020). 2 , 3 , 9 , 10 In cases where hyperkalaemia is a barrier to guideline‐directed medical therapy, the ESC HF guidelines (2021) state that administration of potassium‐lowering therapy may enable more individuals to initiate and uptitrate RAASi. 2 Similarly, the KDIGO guidelines for managing diabetes in CKD (2020) and for managing blood pressure in CKD (2021) also state that hyperkalaemia should be managed using potassium‐lowering therapy before considering de‐escalation of RAASi therapy. 9 , 10
As has been shown, people with cardiorenal disease are particularly at risk of complications or mortality and require coordinated and consistent specialist care, but in practice this is not always how they are managed, and individuals may be treated by both cardiology and nephrology functions separately. The lack of unified guidelines for the management of individuals with cardiorenal disease means that cardiologists and nephrologists may be working to different definitions, thresholds, and with a different approach.
Given the available treatment options that can be employed to enable continuation of RAASi therapy, there is an opportunity to define how to optimally manage hyperkalaemia in those with cardiorenal disease.
Methods
A steering group (the study authors) of cardiology and nephrology experts (including researchers, clinical trial leads, and those involved in guideline development) in HF and CKD from across Europe and North America convened in August 2021 to discuss hyperkalaemia management in people with cardiorenal disease, including any current challenges and opportunities for improvement. Using a modified Delphi methodology guided by an independent facilitator (Triducive), the group identified four main topics of focus:
Risk factors and risk stratification for managing hyperkalaemia in people with cardiorenal disease.
Prevention of hyperkalaemia for at‐risk individuals with cardiorenal disease.
Correction of hyperkalaemia for at‐risk individuals with cardiorenal disease with potassium‐lowering therapy.
Cross‐specialty alignment (cardiology and nephrology).
These topics were in turn discussed in greater detail by the group and 39 statements were developed (Appendix Table A1 ). These statements were used to inform an online questionnaire. The questionnaire comprised each statement along with a 4‐point Likert scale (‘strongly disagree’, ‘tend to disagree’, ‘tend to agree’, and ‘strongly agree’) to allow respondents to indicate their corresponding level of agreement. The questionnaire also captured some demographic data to assist further analyses (country, role, and years in role).
The questionnaire was distributed to consultant cardiologists and nephrologists with ≥2 years' experience in role by an independent third party (Sermo). The identity of the respondents was not known to the steering group. Years in role was defined as either 2–5 years or >5 years, and this was stratified to determine any differences based on level of experience. The steering group agreed that due to the complexity of this topic, only those specialists with ≥2 years' experience in role would be able to provide sufficiently expert responses. Anticipating the challenges of generating consensus across two specialties, the threshold for consensus was therefore set at two‐thirds agreement (i.e. 67%) a priori. Consensus would further be defined as ‘high’ (≥67% and <90%) and ‘very high’ (≥90%).
Completed surveys were anonymously collated and analyzed by the independent facilitator to produce an arithmetic agreement score for each statement. This information was then reviewed by the steering group to determine whether specific recommendations could be made, or further rounds of consensus were required. Survey iterations would be performed until the consensus threshold was achieved; however, due to the levels of agreement received after the first round, the steering group agreed that further rounds were unnecessary.
As this study only requested the opinions of physicians, and no patient‐specific data were captured, ethical approval was not sought.
Results
A total of 520 responses from seven countries in Europe and North America were received in the initial distribution round (Figure 1 ). Combined, 268 responses were received from cardiologists and 252 from nephrologists.
Figure 1.
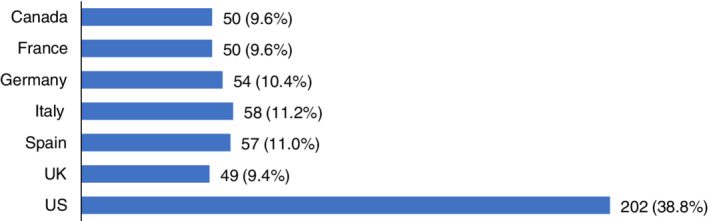
Respondent numbers by country (% of total response).
All statements achieved the threshold for consensus agreement (Figure 2 ); of these, 29/39 statements attained very high agreement (≥90% agreement) and 10/39 attained high agreement (≥67% and <90%).
Figure 2.
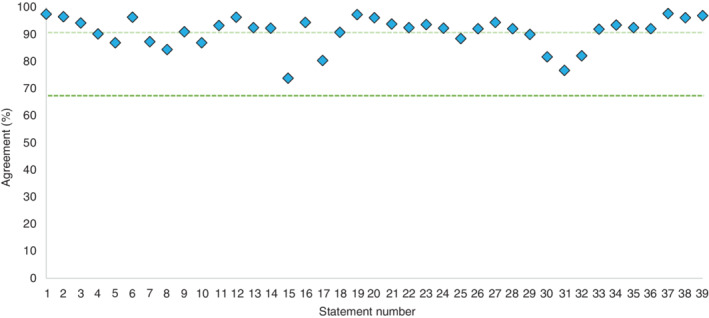
Combined consensus agreement scores. The dark green line represents consensus threshold of 67% and the light green line represents the threshold for very high consensus (90%).
The alignment between cardiologists and nephrologists was clear, with most statements (32/37) showing a difference of ≤5% between the two groups (Figure 3 ). Statement 16 achieved the highest concordance, while statement 31 achieved the lowest, with cardiologists achieving 82% agreement and nephrologists achieving 71%. These low levels of variation suggest that the issues surrounding cardiorenal care are recognized by both specialties with similar perspectives regarding approach to management.
Figure 3.
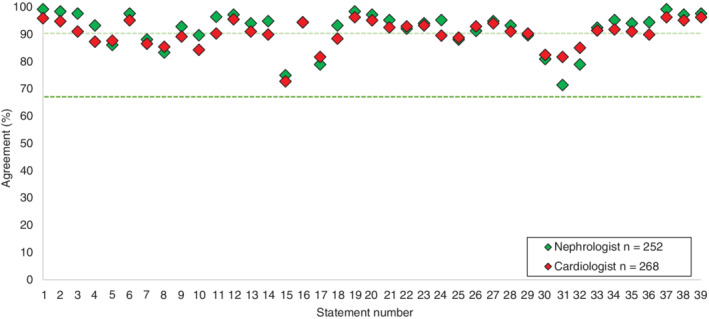
Consensus agreement scores by specialty. The dark green line represents consensus threshold of 67% and the light green line represents the threshold for very high consensus (90%). Values for nephrologists and cardiologists overlap at statement 16.
There was clear agreement with all the statements in Topic A (risk factors and risk stratification for managing hyperkalaemia in adults with cardiorenal disease; 8/12 achieving ≥90% agreement; Table 1 ), demonstrating the recognition from both specialties that hyperkalaemia is a predictable, treatable, and manageable condition in people with cardiorenal disease, and RAASi therapy should be maintained wherever possible to achieve optimal patient outcomes. Notably, there was no significant variation in responses by country or by specialty, suggesting that these are commonly held opinions.
Table 1.
Consensus statements and levels of agreement displayed by country: risk factors and risk stratification for managing hyperkalaemia in cardiorenal patients (Topic A)
| No. | Statement | Agreement (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All | CA | DE | ES | FR | IT | UK | US | ||
| 1 | Optimizing RAASi therapy provides better outcomes for patients | 98 | 98 | 96 | 95 | 92 | 97 | 100 | 100 |
| 2 | Patients with chronic kidney disease, heart failure, or diabetes are at increased risk of hyperkalaemia | 97 | 96 | 94 | 91 | 94 | 98 | 100 | 98 |
| 3 | RAASi use is a risk factor for hyperkalaemia | 94 | 100 | 91 | 88 | 94 | 93 | 96 | 96 |
| 4 | Hyperkalaemia can be effectively managed to optimize disease‐modifying therapies, which improve morbidity, mortality, and outcomes | 90 | 92 | 94 | 79 | 86 | 93 | 88 | 93 |
| 5 | New risk prediction tools are needed if clinicians are to fully individualize risk assessment for their cardiorenal patients | 87 | 92 | 80 | 91 | 86 | 91 | 82 | 87 |
| 6 | Managing risk of hyperkalaemia should be part of the individualized care plan already in place or planned | 96 | 96 | 93 | 93 | 98 | 97 | 96 | 98 |
| 7 | There is a need for consistent thresholds for defining and treating hyperkalaemia among sub‐specialties | 87 | 90 | 76 | 88 | 94 | 93 | 82 | 88 |
| 8 | Hyperkalaemia is associated with down‐titration or discontinuation of RAASi therapy | 84 | 82 | 76 | 88 | 78 | 86 | 94 | 85 |
| 9 | When managing mild‐to‐moderate hyperkalaemia in cardiorenal patients, RAASis should be maintained due to the inherent benefit in this patient type | 91 | 92 | 89 | 93 | 88 | 86 | 94 | 92 |
| 10 | Mild‐to‐moderate hyperkalaemia should be managed without de‐escalating or discontinuing disease‐modifying drugs, such as RAASis | 87 | 88 | 89 | 84 | 92 | 83 | 84 | 88 |
| 11 | Hyperkalaemia is a known and manageable side effect of RAASi treatment | 93 | 98 | 96 | 91 | 98 | 88 | 94 | 92 |
| 12 | Hyperkalaemia should be recognized as a predictable, treatable, and manageable side effect of optimal heart failure/chronic kidney disease therapy in patients with a history or at high‐risk of hyperkalaemia | 96 | 96 | 96 | 89 | 98 | 98 | 96 | 98 |
CA, Canada; DE, Germany; ES, Spain; FR, France; IT, Italy; RAASi, renin–angiotensin–aldosterone system inhibitor; UK, United Kingdom; US, United States of America.
Whilst all statements in Topic B (prevention of hyperkalaemia for at‐risk individuals with cardiorenal disease) achieved consensus agreement (5/7 achieving ≥90% agreement; Table 2 ), statement 15 had a markedly lower agreement level than the others. Variation in response by country is considerable, with the UK and Canada responses being below the agreement threshold.
Table 2.
Consensus statements and levels of agreement displayed by country: prevention of hyperkalaemia for at‐risk cardiorenal patients (Topic B)
| No. | Statement | Agreement (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All | CA | DE | ES | FR | IT | UK | US | ||
| 13 | For high‐risk patients currently not hyperkalaemic, preventative measures should be considered (e.g. removal of salt substitutes from diet; and considering diuretics for people with hypertension or some volume expansion) | 93 | 94 | 87 | 96 | 92 | 90 | 90 | 94 |
| 14 | For those patients who have a known history of hyperkalaemia preventing optimization of RAASi therapy, a novel K+ binder can be used to enable a trial of RAASi optimization | 92 | 88 | 91 | 95 | 96 | 86 | 90 | 95 |
| 15 | For high‐risk patients currently not hyperkalaemic, the use of a novel K+ binder can be considered when starting/up‐titrating RAASi | 74 | 66 | 67 | 74 | 72 | 83 | 49 | 82 |
| 16 | Non‐disease‐modifying therapies that cause hyperkalaemia should be avoided in patients at high‐risk of hyperkalaemia, e.g. NSAIDs, amiloride, and herbal supplements | 94 | 94 | 94 | 89 | 94 | 93 | 94 | 97 |
| 17 | A low K+ diet is often advised to help manage K+ levels, with no/little evidence to support, and is counter to a healthy diet that is beneficial to cardiorenal patients | 80 | 80 | 74 | 86 | 74 | 84 | 78 | 82 |
| 18 | In people for whom dietary restrictions may not be appropriate or desired, the use of novel K+ binders may enable a balanced diet | 91 | 84 | 96 | 91 | 90 | 88 | 90 | 92 |
| 19 | People at risk should be monitored closely with a strategy in place to manage K+ levels effectively | 97 | 96 | 96 | 96 | 96 | 91 | 100 | 100 |
CA, Canada; DE, Germany; ES, Spain; FR, France; IT, Italy; K+, potassium; NSAID; non‐steroidal anti‐inflammatory drug; RAASi, renin–angiotensin–aldosterone system inhibitor; UK, United Kingdom; US, United States of America.
There were high levels of agreement with the statements in Topic C (correction of hyperkalaemia for at‐risk individuals with cardiorenal disease with potassium‐lowering therapy; 9/13 achieving ≥90% agreement; Table 3 ), except for statement 31, which attained consensus overall but with a marked split in response levels by country. Italy, the UK, and the US responses were all >80% concordant, whereas Canada, Germany, Spain, and France were all <70% concordant.
Table 3.
Consensus statements and levels of agreement displayed by country: correction of hyperkalaemia for at‐risk cardiorenal patients with the potassium‐lowering therapy (Topic C)
| No. | Statement | Agreement (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All | CA | DE | ES | FR | IT | UK | US | ||
| 20 | A reduction in emergency department visits and unplanned hospitalizations due to complications associated with hyperkalaemia should be a goal of good management | 96 | 96 | 94 | 100 | 94 | 91 | 98 | 97 |
| 21 | A goal for the management of high‐risk cardiorenal patients should be to utilize the maximum recommended dose of RAASi therapy | 94 | 92 | 89 | 89 | 96 | 88 | 98 | 97 |
| 22 | RAASi‐induced hyperkalaemia should not be considered intolerance until other strategies to reduce K+ have been exhausted | 93 | 92 | 91 | 96 | 94 | 91 | 96 | 91 |
| 23 | De‐escalation or discontinuation of RAASi therapy is associated with worse cardiovascular and renal outcomes in cardiorenal patients | 94 | 92 | 91 | 96 | 94 | 84 | 100 | 95 |
| 24 | Permanent discontinuation of a RAASi should only be considered as a last resort strategy for chronic hyperkalaemia | 92 | 94 | 87 | 88 | 98 | 95 | 96 | 92 |
| 25 | Hyperkalaemia should no longer be seen as a barrier to optimization of guideline‐directed therapy | 88 | 88 | 93 | 91 | 96 | 88 | 82 | 87 |
| 26 | Novel K+ binders enable guideline‐recommended RAASi dosing and the proven benefits that this brings to patients | 92 | 90 | 91 | 89 | 90 | 95 | 94 | 93 |
| 27 | Use of novel K+ binders in patients with mild hyperkalaemia can enable guideline‐recommended doses of RAASi therapy | 94 | 90 | 93 | 98 | 94 | 100 | 92 | 94 |
| 28 | RAASi use should not be de‐escalated or discontinued due to hyperkalaemia unless alternative measures of hyperkalaemia management have been optimized, including initiation of K+ binder therapy | 92 | 94 | 94 | 95 | 92 | 91 | 90 | 91 |
| 29 | Novel K+ binders can enable optimization of RAASi therapy in a similar way that antiemetics can enable optimization of chemotherapy | 90 | 84 | 89 | 88 | 94 | 93 | 88 | 91 |
| 30 | Novel K+ binders should not need to show mortality benefit; they enable RAASis, which have an already proven mortality benefit | 82 | 86 | 74 | 79 | 86 | 86 | 86 | 80 |
| 31 | The use of SPS should be avoided due to concerns with GI toxicity, low compliance due to poor palatability, and is only indicated in severely oliguric or anuric patients | 77 | 60 | 69 | 68 | 66 | 86 | 92 | 82 |
| 32 | SPS should not be used in the medium‐ or long‐term as it may cause severe GI side effects, including bowel necrosis | 82 | 76 | 81 | 75 | 70 | 86 | 90 | 86 |
CA, Canada; DE, Germany; ES, Spain; FR, France; GI, gastrointestinal; IT, Italy; K+, potassium; RAASi, renin–angiotensin–aldosterone system inhibitor; SPS, sodium polystyrene sulfonate; UK, United Kingdom; US, United States of America.
Responses from all countries demonstrated strong agreement in Topic D (cross‐specialty alignment; 7/7 achieving ≥90% agreement; Table 4 ) with the need for a cross‐specialty approach to managing people with cardiorenal disease, supported by consistent guidelines and evidence‐based decision making. Strong agreement levels for Topic D suggest that there is a clear case for the implementation of joint clinics to manage those with cardiorenal disease.
Table 4.
Consensus statements and levels of agreement displayed by country: cross‐specialty alignment (cardiology and nephrology) (Topic D)
| No. | Statement | Agreement (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All | CA | DE | ES | FR | IT | UK | US | ||
| 33 | Patients with cardiorenal comorbidities should be managed by a multidisciplinary team with an agreed management plan | 92 | 92 | 89 | 95 | 88 | 91 | 90 | 94 |
| 34 | Cross‐specialty alignment can enable optimal doses of disease‐modifying drugs (RAASi) to be maintained | 93 | 96 | 93 | 96 | 92 | 93 | 86 | 95 |
| 35 | Cross‐specialty management improves patient satisfaction, patient outcomes, and quality of life | 93 | 90 | 89 | 86 | 88 | 98 | 96 | 95 |
| 36 | Cross‐specialty management is a good use of resources and should improve patient outcomes | 92 | 88 | 96 | 91 | 94 | 97 | 88 | 92 |
| 37 | Enhanced communication between interdisciplinary teams could improve patient outcomes | 98 | 96 | 98 | 96 | 98 | 95 | 98 | 99 |
| 38 | Cardiology and nephrology guidelines should contain consistent recommendations for the management of hyperkalaemia | 96 | 96 | 94 | 96 | 98 | 97 | 92 | 97 |
| 39 | Collaborative care and evidence‐based decision making (based on guidelines and expert consensus) is an example of best practice and patient centered care | 97 | 98 | 94 | 96 | 98 | 97 | 98 | 97 |
CA, Canada; DE, Germany; ES, Spain; FR, France; IT, Italy; RAASi, renin–angiotensin–aldosterone system inhibitor; UK, United Kingdom; US, United States of America.
Discussion
Recommendations
Based on the results obtained, the authors offer the recommendations presented in Table 5 .
Table 5.
Consensus recommendations for the optimal management of hyperkalaemia in people with cardiorenal disease
|
Recommendations Based on the results obtained, the authors offer the following recommendations:
|
Hyperkalaemia and renin–angiotensin–aldosterone system inhibitor use
The management of hyperkalaemia in people with cardiorenal disease is complex and requires a consistent and informed approach. In their responses to this consensus, both cardiologists and nephrologists agree that hyperkalaemia is a predictable, treatable, and manageable condition. Amongst non‐specialists or those less experienced in managing hyperkalaemia, a lack of knowledge of current guidelines and treatment options may drive a decision to discontinue or down‐titrate RAASi therapy. However, data have shown that although reductions in RAASi treatment may reduce the risk of hyperkalaemia, they are also associated with worsening of the underlying cardiorenal condition and subsequent increase in mortality. 12 , 14 , 15 , 16 , 17 , 18
The results show strong recognition that hyperkalaemia is manageable and when it occurs, treatment optimization to maintain the efficacy of disease‐modifying therapies is imperative. In mild‐to‐moderate hyperkalaemia, RAASi therapy should be maintained without dose reduction as a rule. Individuals with HF and CKD or diabetes should be assessed for risk of hyperkalaemia and an individualized care plan put in place to manage this risk, using universally agreed thresholds for intervention.
There are a range of treatment options recommended for use in managing hyperkalaemia in adults with cardiorenal disease:
Loop and thiazide diuretics can be used to increase potassium excretion by increasing the delivery of sodium to the collecting ducts. Diuretics are recommended for use in treating mild‐to‐moderate hyperkalaemia in individuals with adequate renal function. 2 Diuretics also have utility in those with hyperkalaemia and concomitant volume overload or hypertension. 9
Oral sodium bicarbonate may be considered in individuals with hyperkalaemia and metabolic acidosis, particularly in those with a serum bicarbonate level of <22 mmol/L, but it is important to consider sodium load, particularly in those at risk of fluid overload. 2 In those at risk of fluid overload, concurrent diuretic use should be considered. 9
Two novel potassium binders have recently been developed, patiromer and sodium zirconium cyclosilicate (SZC). 2 Both have demonstrated efficacy, 19 , 20 , 21 and the ESC HF guidelines (2021) and the KDIGO guidelines for managing diabetes in CKD (2020) and for managing blood pressure in CKD (2021) recommend their use for treatment of RAASi‐associated hyperkalaemia. 2 , 9 , 10
Sodium polystyrene sulfonate (SPS) has traditionally been used to treat hyperkalaemia, but there are questions regarding the safety and efficacy of this agent. 22
Regarding the mixed response to statement 31, the reasons for this variation are unclear but could be due to greater acceptance/experience of SPS in Canada, Germany, Spain, and France. Statement 32 also displayed a similar (though less marked) pattern of variation, although this is perhaps a less refutable statement amongst those specialists that use SPS. People with cardiorenal disease may be on disease‐modifying therapy for years with multiple episodes of hyperkalaemia, and due to the questions regarding the safety and efficacy of SPS, novel potassium binders may be the natural choice for managing hyperkalaemia in this population.
Preventative measures should be implemented in those considered to be at high‐risk of hyperkalaemia. Cessation of non‐disease‐modifying therapies that contribute to hyperkalaemia should be implemented where appropriate and diuretics should be considered for those with hypertension or volume expansion. However, an element of caution is recommended as dietary restrictions to reduce the risk of hyperkalaemia have been shown to have limited impact and may deprive the individual of many heart‐healthy foods. 23 In individuals at high risk, there is limited clinical trial evidence to support the use of potassium binders as a preventative measure upon RAASi initiation; this may explain the more variable response to statement 15. This variation may also be driven by differences in access to novel potassium binders between individual health economies. For example, the UK health system makes decisions based on the perceived cost‐effectiveness of interventions, therefore there may be limited support for ‘pre‐emptive’ prescribing in the absence of strong evidence of cost‐effectiveness. A trial evaluating the use of patiromer to enable treatment with spironolactone in individuals with resistant hypertension and CKD found that patiromer enabled more people to continue treatment with spironolactone, with less hyperkalaemia. 24 A phase 3b trial has recently completed to assess the effects of patiromer compared with placebo on serum potassium in people with HF, and results are expected to be published in 2022. 25 Two clinical trials are currently in progress to evaluate the use of SZC as an adjunct to enable RAASi (a phase 3 trial in CKD and a phase 4 trial in HF). 26 , 27 The results of these trials should provide insight into the clinical value of this approach in adults with cardiorenal disease.
Collaborative care and guidelines
Due to the need for both cardiology and nephrology input in the care of individuals with cardiorenal disease, alignment in treatment approach and collaborative care is vital for optimal patient management, and this is recognized by respondents. Individuals with cardiorenal comorbidities should be managed by a multidisciplinary team (MDT) and an agreed management plan put in place. The use of cardio‐nephrology MDT meetings to devise a unified management plan may provide several benefits, including improved care outcomes, better utilization of healthcare resources, and increased satisfaction for team members. 28 Modern technology also provides opportunities for closer working through the use of telehealth, virtual meetings, and digital management systems; these should be explored where geographic or logistic barriers to face‐to‐face working exist.
There is clear support for joint cardiology and nephrology clinics to manage people with cardiorenal disease, but this requires support from both clinicians and wider business functions. If joint/collaborative clinics were adopted, this would improve the impact of support team members, such as physician extenders and nurse practitioners. Findings from the implementation of a cardiorenal clinic in the UK resulted in an increase in the number of individuals on single or dual RAASi, with higher proportions of those being on higher dosages, while having no associated clinically significant deterioration in renal function and hyperkalaemia. As the clinic provided anaemia nurse specialist input and same‐day intravenous iron administration, a more efficient use of healthcare resource, minimization of patients' waiting time, transport time, and expense were also identified. 29
There are initiatives in place to support the cross‐specialty approach, including the UK Cardio‐Renal‐Metabolic (CaReMe) partnership 30 and the Global Multidisciplinary Teams In Cardiometabolic Care Global Program (UNITE). 31 In line with any cross‐specialty approach, local and national guidelines should be updated and published.
Regarding guidelines, there are many guidelines covering HF and CKD at both the local, national, and international level, of varying complexity and modernity. Any guidelines for people with cardiorenal disease should ideally be co‐created by cardiology and nephrology specialists to reflect best practice and insights, and to agree on a common approach. This approach supports evidence‐based management of hyperkalaemia, and aligns with the implementation of joint clinics and cardiorenal‐specific MDTs.
The use of novel potassium binders to manage hyperkalaemia is gaining traction, and recent guidelines from ESC, KDIGO, and the National Institute for Health and Care Excellence reflect this. These guidelines are also well aligned regarding hyperkalaemia management, and could help to close any ‘treatment gap’ between cardiologists and nephrologists. The agreement levels achieved in this consensus provide further support for this approach. The impetus now is to ensure that local guidelines (country or region specific) are adapted to reflect this.
Whilst the immediate (and understandable) response to hyperkalaemia may be to reduce RAASi dose or stop treatment entirely, healthcare professionals should be aware of the options available to manage hyperkalaemia in people with cardiorenal disease and maintain their outcomes. Novel potassium binders should be considered for hyperkalaemia to enable individuals to access disease‐modifying therapies. In terms of benefit, this approach is akin to the use of antiemetics to enable chemotherapy in people with cancer.
Conclusions
This consensus document is based on expert opinion from 520 cardiologist and nephrologist respondents across five European countries, the US, and Canada. Specialists agree that RAASi therapy should be maintained where possible (certainly for those with mild‐to‐moderate hyperkalaemia) and novel potassium binders utilized to enable individuals to access the maximal dose of their disease‐modifying therapies. This provides an opportunity to manage hyperkalaemia without compromising the therapeutic benefits of RAASi therapy in people with cardiorenal disease. Collaboration between cardiology and nephrology specialties in the treatment of individuals with cardiorenal disease should be formalized (ideally in the form of a cardiorenal MDT), and treatment approach aligned based on evidence‐based medicine. The strong agreement levels achieved from many experienced specialists support the collaborative, optimal management of hyperkalaemia in individuals with comorbid cardiorenal disease. A key first step would be for local guideline writers and institutions to adopt a coordinated approach across cardiorenal specialists to review and align local guidance for hyperkalaemia management.
Strengths and limitations
The large number of experienced cardiology and nephrology specialists that responded to the consensus questionnaire lends weight to the validity of the recommendations proposed by the steering group. Responses were sought from seven countries across Europe and North America, reducing any country‐specific bias, although the large number of responses from the US in comparison to other countries will undoubtedly have the greatest influence on results. The clearly defined inclusion criteria ensured that only the views of experts within their field were sought. The use of a third party to manage the anonymous questionnaire distribution and subsequent collection of results reduces any bias from the steering group.
As this study only undertook one round of consensus with no adjustments to the statements, it is possible that some of the statements were too agreeable and did not sufficiently challenge the status quo. Further research on this question should refine the statements generated herein to determine any greater variance that may exist. Convenience sampling may have introduced motivation bias into the study. However, this was mitigated by seeking responses from across the countries represented by the steering group and by permitting responses at any time during the study period to allow the most reflective response to the statements.
Funding
The study was initiated and funded by AstraZeneca. All authors, except CP Kovesdy, received funding from AstraZeneca while undertaking this study. AstraZeneca commissioned Triducive Partners Ltd. to facilitate the project and analyzed the responses to the consensus statements in line with the Delphi methodology. Editorial support was provided by Jake Fox of Core Medica, London, UK, and funded by AstraZeneca.
Conflict of interest: J.O.B. received research grant support, served on advisory boards for, or speaker engagements with Astellas, AstraZeneca, Bayer, Bristol‐Myers Squibb‐Pfizer, Boehringer Ingelheim, Napp, Novo Nordisk, and Vifor Pharma; and serves on a clinical trial steering committee for studies sponsored by AstraZeneca and Vifor Pharma. A.J.S.C. received honoraria and/or lecture fees from Abbott, Actimed, Arena, AstraZeneca, Boehringer Ingelheim, Cardiac Dimensions, Corvia, CVRx, Enopace Biomedical Ltd., ESN Cleer, Faraday, Impulse Dynamics, Menarini, Novartis, Respicardia, Servier, Viatris, and Vifor Pharma. C.P.K. received honoraria as consultant for Abbott, Akebia, AstraZeneca, Bayer, Boehringer Ingelheim, CSL Behring, Cara Therapeutics, Rockwell, and Vifor Pharma. M.M.S. received honoraria for speaker engagements and consulting for AstraZeneca. I.P. has served on advisory boards for AstraZeneca and Vifor Pharma. S.Z. received research grant support, served on advisory boards for, or speaker engagements with Abbott, Akcea Therapeutics, Inc., AstraZeneca, Amgen, Alnylam, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novartis, Novo Nordisk, Otsuka, Pfizer, Servier, and Vifor Pharma; and serves on a clinical trial steering committee as a national lead for studies sponsored by AstraZeneca, Bayer, Boehringer Ingelheim, Merck, and Novartis. B.F.P. and G.R. have nothing to disclose.
Acknowledgements
The authors thank Triducive Partners Ltd. for their assistance in analyzed the results, writing the manuscript, and reviewing the final draft.
Appendix A.
Table A1.
Consensus statements
| No. | Statement |
|---|---|
| A) Risk factors and risk stratification for managing hyperkalaemia in cardiorenal patients | |
| 1 | Optimizing RAASi therapy provides better outcomes for patients |
| 2 | Patients with chronic kidney disease, heart failure, or diabetes are at increased risk of hyperkalaemia |
| 3 | RAASi use is a risk factor for hyperkalaemia |
| 4 | Hyperkalaemia can be effectively managed to optimize disease‐modifying therapies, which improve morbidity, mortality, and outcomes |
| 5 | New risk prediction tools are needed if clinicians are to fully individualize risk assessment for their cardiorenal patients |
| 6 | Managing risk of hyperkalaemia should be part of the individualized care plan already in place or planned |
| 7 | There is a need for consistent thresholds for defining and treating hyperkalaemia among sub‐specialties |
| 8 | Hyperkalaemia is associated with down‐titration or discontinuation of RAASi therapy |
| 9 | When managing mild‐to‐moderate hyperkalaemia in cardiorenal patients, RAASis should be maintained due to the inherent benefit in this patient type |
| 10 | Mild‐to‐moderate hyperkalaemia should be managed without de‐escalating or discontinuing disease‐modifying drugs, such as RAASis |
| 11 | Hyperkalaemia is a known and manageable side effect of RAASi treatment |
| 12 | Hyperkalaemia should be recognized as a predictable, treatable, and manageable side effect of optimal heart failure/chronic kidney disease therapy in patients with a history or at high‐risk of hyperkalaemia |
| B) Prevention of hyperkalaemia for at‐risk cardiorenal patients | |
| 13 | For high‐risk patients currently not hyperkalaemic, preventative measures should be considered (e.g. removal of salt substitutes from diet; and considering diuretics for people with hypertension or some volume expansion) |
| 14 | For those patients who have a known history of hyperkalaemia preventing optimization of RAASi therapy, a novel K+ binder can be used to enable a trial of RAASi optimization |
| 15 | For high‐risk patients currently not hyperkalaemic, the use of a novel K+ binder can be considered when starting/up‐titrating RAASi |
| 16 | Non‐disease‐modifying therapies that cause hyperkalaemia should be avoided in patients at high‐risk of hyperkalaemia, e.g. NSAIDs, amiloride, and herbal supplements |
| 17 | A low K+ diet is often advised to help manage K+ levels, with no/little evidence to support, and is counter to a healthy diet that is beneficial to cardiorenal patients |
| 18 | In people for whom dietary restrictions may not be appropriate or desired, the use of novel K+ binders may enable a balanced diet |
| 19 | People at risk should be monitored closely with a strategy in place to manage K+ levels effectively |
| C) Correction of hyperkalaemia for at‐risk cardiorenal patients with K+ lowering therapy | |
| 20 | A reduction in emergency department visits and unplanned hospitalizations due to complications associated with hyperkalaemia should be a goal of good management |
| 21 | A goal for the management of high‐risk cardiorenal patients should be to utilize the maximum recommended dose of RAASi therapy |
| 22 | RAASi‐induced hyperkalaemia should not be considered intolerance until other strategies to reduce K+ have been exhausted |
| 23 | De‐escalation or discontinuation of RAASi therapy is associated with worse cardiovascular and renal outcomes in cardiorenal patients |
| 24 | Permanent discontinuation of RAASi therapy should only be considered as a last resort strategy for chronic hyperkalaemia |
| 25 | Hyperkalaemia should no longer be seen as a barrier to optimization of guideline‐directed therapy |
| 26 | Novel K+ binders enable guideline‐recommended RAASi dosing and the proven benefits that this brings to patients |
| 27 | Use of novel K+ binders in patients with mild hyperkalaemia can enable guideline‐recommended doses of RAASi therapy |
| 28 | RAASi use should not be de‐escalated or discontinued due to hyperkalaemia unless alternative measures of hyperkalaemia management have been optimized, including initiation of K+ binder therapy |
| 29 | Novel K+ binders can enable optimization of RAASi therapy in a similar way that antiemetics can enable optimization of chemotherapy |
| 30 | Novel K+ binders should not need to show mortality benefit; they enable RAASi therapy, which have an already proven mortality benefit |
| 31 | The use of SPS should be avoided due to concerns with GI toxicity, low compliance due to poor palatability, and is only indicated in severely oliguric or anuric patients |
| 32 | SPS should not be used in the medium‐ or long‐term as it may cause severe GI side effects, including bowel necrosis |
| D) Cross‐specialty alignment (cardiology and nephrology) | |
| 33 | Patients with cardiorenal comorbidities should be managed by a multidisciplinary team with an agreed management plan |
| 34 | Cross‐specialty alignment can enable optimal doses of disease‐modifying drugs (RAASi) to be maintained |
| 35 | Cross‐specialty management improves patient satisfaction, patient outcomes, and quality of life |
| 36 | Cross‐specialty management is a good use of resources and should improve patient outcomes |
| 37 | Enhanced communication between interdisciplinary teams could improve patient outcomes |
| 38 | Cardiology and nephrology guidelines should contain consistent recommendations for the management of hyperkalaemia |
| 39 | Collaborative care and evidence‐based decision making (based on guidelines and expert consensus) is an example of best practice and patient centered care |
GI, gastrointestinal; K+, potassium; NSAID, non‐steroidal anti‐inflammatory drug; RAASi, renin–angiotensin–aldosterone system inhibitor; SPS, sodium polystyrene sulfonate.
References
- 1. Luo J, Brunelli SM, Jensen DE, Yang A. Association between serum potassium and outcomes in patients with reduced kidney function. Clin J Am Soc Nephrol. 2016;11:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail. 2022;24:4–131. [DOI] [PubMed] [Google Scholar]
- 3. Renal Association . Clinical Practice Guideline. Treatment of acute hyperkalaemia in adults. 2020. https://ukkidney.org/sites/renal.org/files/RENAL%20ASSOCIATION%20HYPERKALAEMIA%20GUIDELINE%202020.pdf (11 May 2022). [Google Scholar]
- 4. Wang AY. Optimally managing hyperkalemia in patients with cardiorenal syndrome. Nephrol Dial Transplant. 2019;34:iii36–44. [DOI] [PubMed] [Google Scholar]
- 5. House AA, Wanner C, Sarnak MJ, Piña IL, McIntyre CW, Komenda P, et al. Heart failure in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2019;95:1304–17. [DOI] [PubMed] [Google Scholar]
- 6. Lawson CA, Seidu S, Zaccardi F, McCann G, Kadam UT, Davies MJ, et al. Outcome trends in people with heart failure, type 2 diabetes mellitus and chronic kidney disease in the UK over twenty years. eClinicalMedicine. 2021;32:100739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosano GM, Spoletini I, Vitale C, Agewall S. Hyperkalemia and renin‐angiotensin‐aldosterone system inhibitors dose therapy in heart failure with reduced ejection fraction. Card Fail Rev. 2019;5:130–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al.; ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200. [DOI] [PubMed] [Google Scholar]
- 9. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group . KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;98:S1–S115. [DOI] [PubMed] [Google Scholar]
- 10. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group . KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99:S1–S87. [DOI] [PubMed] [Google Scholar]
- 11. Ezekowitz JA, O'Meara E, McDonald MA, Abrams H, Chan M, Ducharme A, et al. 2017 comprehensive update of the Canadian Cardiovascular Society guidelines for the management of heart failure. Can J Cardiol. 2017;33:1342–433. [DOI] [PubMed] [Google Scholar]
- 12. Epstein M, Reaven NL, Funk SE, McGaughey KJ, Oestreicher N, Knispel J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin‐angiotensin‐aldosterone system inhibitors. Am J Manag Care. 2015;21:S212–20. [PubMed] [Google Scholar]
- 13. Trevisan M, de Deco P, Xu H, Evans M, Lindholm B, Bellocco R, et al. Incidence, predictors and clinical management of hyperkalaemia in new users of mineralocorticoid receptor antagonists. Eur J Heart Fail. 2018;20:1217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosano GMC, Tamargo J, Kjeldsen KP, Lainscak M, Agewall S, Anker SD, et al. Expert consensus document on the management of hyperkalaemia in patients with cardiovascular disease treated with renin angiotensin aldosterone system inhibitors: coordinated by the Working Group on Cardiovascular Pharmacotherapy of the European Society of Cardiology. Eur Heart J Cardiovasc Pharmacother. 2018;4:180–8. [DOI] [PubMed] [Google Scholar]
- 15. Halliday BP, Wassall R, Lota AS, Khalique Z, Gregson J, Newsome S, et al. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED‐HF): an open‐label, pilot, randomised trial. Lancet. 2019;393:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gosmanova EO, Molnar MZ, Naseer A, Sumida K, Potukuchi P, Gaipov A, et al. Longer predialysis ACEi/ARB utilization is associated with reduced postdialysis mortality. Am J Med. 2020;133:1065–73.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rossignol P, Lainscak M, Crespo‐Leiro MG, Laroche C, Piepoli MF, Filippatos G, et al.; Heart Failure Long‐Term Registry Investigators Group. Unravelling the interplay between hyperkalaemia, renin‐angiotensin‐aldosterone inhibitor use and clinical outcomes. Data from 9222 chronic heart failure patients of the ESC‐HFA‐EORP Heart Failure Long‐Term Registry. Eur J Heart Fail. 2020;22:1378–89. [DOI] [PubMed]
- 18. Gilstrap LG, Fonarow GC, Desai AS, Liang L, Matsouaka R, DeVore AD, et al. Initiation, continuation, or withdrawal of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers and outcomes in patients hospitalized with heart failure with reduced ejection fraction. J Am Heart Assoc. 2017;6:e004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weir MR, Bakris GL, Gross C, Mayo MR, Garza D, Stasiv Y, et al. Treatment with patiromer decreases aldosterone in patients with chronic kidney disease and hyperkalemia on renin‐angiotensin system inhibitors. Kidney Int. 2016;90:696–704. [DOI] [PubMed] [Google Scholar]
- 20. Pitt B, Bushinsky DA, Kitzman DW, Ruschitzka F, Metra M, Filippatos G, et al.; Patiromer‐204 Investigators . Evaluation of an individualized dose titration regimen of patiromer to prevent hyperkalaemia in patients with heart failure and chronic kidney disease. ESC Heart Fail. 2018;5:257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Packham DK, Rasmussen HS, Lavin PT, El‐Shahawy MA, Roger SD, Block G, et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015;372:222–31. [DOI] [PubMed] [Google Scholar]
- 22. Batterink J, Lin J, Au‐Yeung SHM, Cessford T. Effectiveness of sodium polystyrene sulfonate for short‐term treatment of hyperkalemia. Can J Hosp Pharm. 2015;68:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bernier‐Jean A, Wong G, Saglimbene V, Ruospo M, Palmer SC, Natale P, et al. Dietary potassium intake and all‐cause mortality in adults treated with hemodialysis. Clin J Am Soc Nephrol. 2021;16:1851–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agarwal R, Rossignol P, Romero A, Garza D, Mayo MR, Warren S, et al. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double‐blind, placebo‐controlled trial. Lancet. 2019;394:1540–50. [DOI] [PubMed] [Google Scholar]
- 25. ClinicalTrials.gov . NCT03888066: Patiromer for the management of hyperkalemia in subjects receiving RAASi medications for the treatment of heart failure (DIAMOND). 2019. https://www.clinicaltrials.gov/ct2/show/NCT03888066 (3 February 2022).
- 26. ClinicalTrials.gov . NCT05056727: A study to evaluate the effect of sodium zirconium cyclosilicate on chronic kidney disease (CKD) progression in participants with CKD and hyperkalaemia or at risk of hyperkalaemia (STABILIZE‐CKD). 2021. https://www.clinicaltrials.gov/ct2/show/NCT05056727. (3 February 2022).
- 27. ClinicalTrials.gov . NCT04676646: Study to assess efficacy and safety of SZC for the management of high potassium in patients with symptomatic HFrEF receiving spironolactone (REALIZE‐K). 2020. https://clinicaltrials.gov/ct2/show/NCT04676646 (3 February 2022).
- 28. Sankaranarayanan R, Douglas H, Wong C. Cardio‐nephrology MDT meetings play an important role in the management of cardiorenal syndrome. BJC. 2020;27:80–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nguyen M, Rumjaun S, Lowe‐Jones R, Ster IC, Rosano G, Anderson L, et al. Management and outcomes of heart failure patients with CKD: experience from an inter‐disciplinary clinic. ESC Heart Fail. 2020;7:3225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. British Cardiovascular Society . CaReMe. Improving the management of patients with diabetes, cardiovascular and renal disease. Latest updates. 2021. https://www.britishcardiovascularsociety.org/resources/careme (12 May 2022).
- 31. American College of Cardiology . UNITE: multidisciplinary teams in cardiometabolic care global program. 2022. https://www.acc.org/UNITE (12 May 2022).


