Abstract
Background
Serologic analysis is an important tool towards assessing the humoral response to COVID-19 infection and vaccination. Numerous serologic tests and platforms are currently available to support this line of testing. Two broad antibody testing categories are point-of-care lateral flow immunoassays and semi-quantitative immunoassays performed in clinical laboratories, which typically require blood collected from a finger-stick and a standard venipuncture blood draw, respectively. This study evaluated the use of dried blood spot (DBS) collections as a sample source for COVID-19 antibody testing using an automated clinical laboratory test system.
Methods
Two hundred and ninety-four participants in the BLAST COVID-19 seroprevalence study (NCT04349202) were recruited at the time of a scheduled blood draw to have an additional sample taken via finger stick as a DBS collection. Using the EUROIMMUN assay to assess SARS-CoV-2 anti-spike IgG status, DBS specimens were tested on 7, 14, 21, and 28 days post- collection and compared to the reference serum sample obtained from a blood draw for the BLAST COVID-19 study.
Results
SARS-CoV-2 anti-spike IgG status from DBS collections demonstrated high concordance with serum across all time points (7–28 days). However, the semi-quantitative value from DBS collections was lower on average than that from serum, resulting in increased uncertainty around the equivocal-to-positive analytical decision point.
Conclusions
DBS collections can be substituted for venipuncture when assaying for COVID-19 IgG antibody, with samples being stable for at least 28 days at room temperature. Finger-stick sampling can therefore be advantageous for testing large populations for SARS-CoV-2 antibodies without the need for phlebotomists or immediate processing of samples. We have high confidence in serostaus determination from DBS collections, although the reduced semi-quantitative value may cause some low-level positives to fall into the equivocal or even negative range.
Keywords: Dried blood spot, COVID-19, COVID-19 antibody, SARS-CoV-2 IgG
Abbreviations: DBS, Dried Blood Spot; PA, Phlebotomist Acquired
1. Introduction
Southeast Michigan was disproportionately affected during the initial wave of COVID-19 infections in the United States (April 2020). To assess seroprevalence within a large healthcare community during that period, a large-scale serology study was initiated (the Beaumont Health Large-Scale Automated Serologic Testing for COVID-19 study, BLAST COVID-19, NCT04349202). This study recruited approximately 22,000 healthcare employee and involved collection of blood samples to assess SARS-CoV-2 anti-spike IgG status (Sims et al., 2020).
Serological testing is commonly performed using serum obtained from patients via venipuncture. In contrast, dried blood spot (DBS) collections, which may be a viable alternative to serum, are collected with a finger stick, are less invasive and require less training to obtain. For DBS testing, blood from a finger stick is dried onto a filter paper card. The protein fraction from the DBS is subsequently extracted manually or using a semi-automated processor. The latter option is routinely used to support new-born screening needs. DBS collections are also stable at room temperature for a prolonged period of time, making this option appealing in circumstances in which electricity and refrigeration are not readily available. Lastly, self-performed DBS collections could provide a more convenient option to individuals for testing compared to standard venipuncture, which typically requires a visit to a healthcare facility (Kuehn, 2020).
DBS testing for SARS-CoV-2 antibodies could allow for larger studies to be performed without the need for phlebotomy and refrigeration. To validate this methodology, a subset of participants in the BLAST COVID-19 study were asked to supply a DBS collection to be tested and compared to a standard venipuncture blood draw obtained at the same time.
2. Materials and methods
2.1. Study design
A total of 300 participants from the BLAST COVID-19 study were consented to provide a DBS card for the purpose of comparing serology results from a DBS collection with a standard venipuncture blood draw (i.e. serum sample analysis). Out of the 300 participants, 200 and 100 individuals were previously known to be positive and negative for SARS-CoV-2 anti-spike IgG, respectively. DBS collections were obtained by the study nurse (Phlebotomist Acquired - PA) from 171 of the IgG positive cohort and 73 of the IgG negative cohort. Twenty-three of the IgG positive cohort and 27 of the IgG negative cohort performed their own finger-stick (self-stick) for purposes of comparing PA to self-stick DBS collections. For self-stick collections, standard instructions from the vendor were provided to each participant. The study nurse did not direct the participants in any way on how to perform the collection but were available to deal with any device-related injury. No injuries or complaints were reported to a study team member in the self-stick cohort. For all participants, only cards that contained at least 3 out of 5 filled circles and passed visual quality inspection were included. Six PA samples were excluded from the study due to inadequate collections, leaving 294 total participants.
Cards were stored in a sealed plastic bag at room temperature. Seven days after the date of DBS collection, three punches from each card were tested for IgG to assess test reproducibility. No more than two punches were taken from each circle. The card was then stored in the same manner and tested for SARS-CoV-2 anti-spike IgG on days 14, 21, or 28 post-collection. One third of the positive and negative samples chosen at random were tested by obtaining 3 additional punches per card at each of those time points for triplicate measurements.
2.2. DBS extraction and antibody testing
The automated DBS extraction procedure and immunoassay for SARS-CoV-2 anti-spike IgG antibody detection used for the study was developed by EUROIMMUN. For analyte extraction, a single 4.7 mm round punch using the PerkinElmer DBS Puncher was submerged into a well of an uncoated microtiter deep well plate containing 250 μL of sample buffer obtained from the EUROIMMUN SARS-CoV-2 anti-spike IgG test kits. The plate was incubated for 1 h at 37 ± 1 °C without agitation. After incubation, the extract was mixed by pipetting up and down for a minimum of 3 times, and 200 μL of the eluate was transferred to an uncoated U-bottom microtiter plate. The sample plate was subsequently loaded onto the EUROIMMUN Eurolab Workstation, an automated high-throughput analyzer designed for clinical labs to support ELISA-based testing. The EUROIMMUN SARS-CoV-2 anti-spike IgG assay was performed according to the package insert for all samples tested (DBS, serum). Each serology test result from a DBS collection was compared to the corresponding reference serum result collected from the same study participant on the same day.
2.3. Serum reproducibility study
To determine antibody status using the EUROIMMUN SARS-CoV-2 anti-spike IgG assay, the OD (optical density) value from a tested sample is divided by the OD of the test calibrator. This OD ratio is subsequently used to determine antibody status based on cut-off values established by the assay manufacturer. To assess imprecision of serum OD ratios relative to a benchmark, specimens from 16 de-identified patients were tested for SARS-CoV-2 anti-spike IgG (benchmark or reference result). The same cohort of samples were subsequently tested in triplicate on a different day. The triplicate OD ratios was compared to the original serum test result to determine variation relative to a reference result for serum-based testing.
2.4. Data aggregation and statistical analysis
All demographic data were summarized using number or percent for categorical data or mean and standard deviation for continuous data. Initial analyses focused solely on data from the DBS study. The qualitative result of the EUROIMMUN assay for anti-SARS-CoV-2 anti-spike IgG was evaluated first. Agreement in the qualitative results of DBS relative to the reference serum sample (i.e. standard venipuncture blood draw) collected on the same day was evaluated using contingency tables and Cohen's kappa. Pearson's correlation coefficient was used to quantitatively compare OD ratios between DBS and reference serum samples. We evaluated systematic differences between DBS and reference serum sample OD ratios using Bland-Altman analyses (Bland and Altman, 1986; Zou, 2011).
Systematic differences between DBS and reference serum sample OD ratio were evaluated using linear mixed models, with data from the DBS and Serum Reproducibility (SR) studies. First, we examined the systematic differences between the three repeat OD ratios and the original serum sample, comparing DBS and serum OD ratio repeats. The difference between the repeated value (DBS OD ratio of PA subjects for a given day for DBS study; repeated measurement of serum OD ratio for SR study) and the reference serum OD ratio was included as the dependent variable in a linear mixed model. This model included the fixed effects of a restricted cubic spline of reference serum OD ratio, study (DBS vs SR), and the interaction between these two effects. The model also included a random intercept for study subject within study. Second, we evaluated how the systematic difference between DBS and serum reference OD ratio (DBS study only) varied by day of the assay using the difference between the DBS and the reference serum OD ratios as the dependent variable. The model included the fixed effects of day of assay (7, 14, 21, or 28 days; linear relationship assumed), collector (PA vs self-stick), the interaction of day and collector, and a restricted cubic spline of reference serum OD ratio. A random intercept for subject within collector was also included in the model. Lastly, we compared within-subject variability of DBS (DBS study) and serum (SR study) OD ratios using observed OD ratios as the dependent variable. The model included the fixed effects of study (DBS vs SR), a restricted cubic spline of reference serum OD ratio and the interaction of these two. The model also included a random intercept for subject within study. We used the Akaike information criterion (AIC) and the Schwarz Bayesian information criterion (BIC) to evaluate the structure of the variance of the random effects and the residual variance, evaluating whether the variance in the random intercept depended on study. We also evaluated whether the residual variance depended on study reference serum OD ratio group (OD ratio < 0.8, 0.8≤OD ratio < 2, 2≤OD ratio < 5, OD ratio≥5) or the combination of study and reference serum OD ratio group. Both AIC and BIC favored a model with one variance for the random intercept and eight residual variances for study by reference serum OD ratio group.
3. Results
The study included 244 PA and 50 self-stick samples (Table 1 ). Demographics were similar to the BLAST COVID-19 study (Sims et al., 2020).
Table 1.
Study Demographics.
| Category | Description | Phlebotomist Acquired | Self-Stick |
|---|---|---|---|
| N | 244 | 50 | |
| Gender | Female | 197 (80.7) | 43 (86.0) |
| Male | 47 (19.3) | 7 (14.0) | |
| Race | White | 189 (77.5) | 40 (80.0) |
| Black | 17 (7.0) | 4 (8.0) | |
| Asian/Pacific Islander | 25 (10.3) | 4 (8.0) | |
| Other/Prefer not to answer | 13 (5.3) | 2 (4.0) | |
| Age | 20–29 | 46 (18.9) | 9 (18.0) |
| 30–39 | 57 (23.4) | 8 (16.0) | |
| 40–49 | 54 (22.1) | 14 (28.0) | |
| 50–59 | 60 (24.6) | 11 (22.0) | |
| 60–69 | 25 (10.3) | 8 (16.0) | |
| 70–79 | 2 (0.8) | 0 (0.0) | |
| Comorbidity | Diabetes | 14 (5.7) | 1 (2.0) |
| Cardiovascular Disease | 5 (2.1) | 0 (0.0) | |
| Chronic Lung Disease | 13 (5.3) | 1 (2.0) | |
| Hypertension | 34 (13.9) | 8 (16.0) | |
| Any Comorbidity | 64 (26.2) | 9 (18.0) |
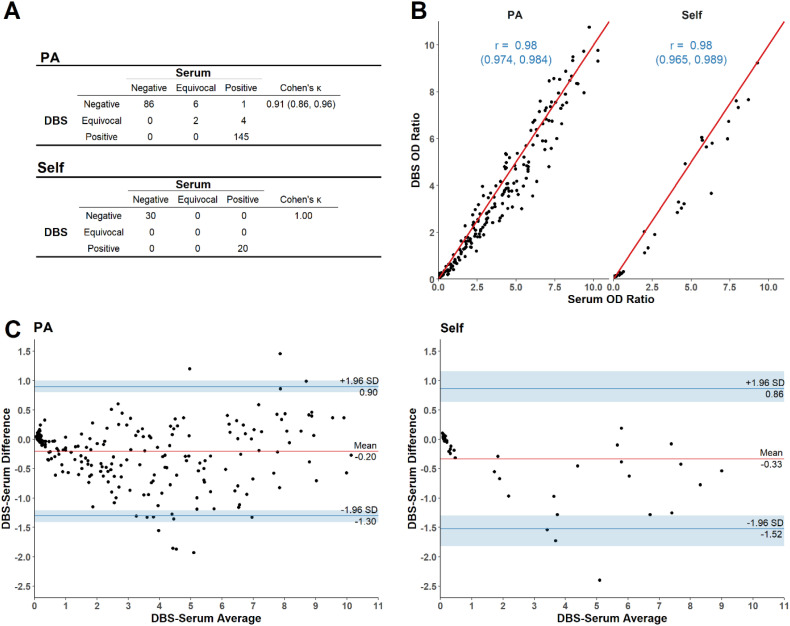
Comparing qualitative serology test determinations, the DBS result obtained from the first punch after 7 days showed high agreement with the reference serum result. Samples from both the PA and self-stick cohorts demonstrated large Cohen's κ (Fig. 1A; PA: 0.91, 95% CI – 0.86-0.96; Self-stick: 1.00, with no discordant samples). Likewise, DBS and reference serum OD ratios showed a strong quantitative relationship for both PA and self-stick groups (Fig. 1B; r = 0.98). Bland-Altman analysis of the DBS OD ratio after 7 days showed that DBS OD ratios tended to be smaller than serum OD ratios, although the limits of agreement include 0 (lower limit of agreement ∼ −1.5; upper limit of agreement ∼1.0; for both PA and self-stick samples; Fig. 1C). The results also suggest that the largest difference between DBS and reference serum OD ratios is at an average OD ratio of ∼5. Similar results were obtained for DBS samples assayed on day 14, 21 and 28 post-collection (Supplementary Figs. S1-S3).
Fig. 1.
Agreement between dried blood spot (DBS) and reference serum at 7 days post-collection A. Agreement between qualitative results, where each OD ratio was categorized as being IgG negative, IgG positive or equivocal based on the manufacturer's test cutoff values. Only the first DBS spot analyzed 7 days post-collection is presented, and the agreement is summarized using Cohen's κ. B. Association between quantitative results, showing the Pearson correlation between DBS and reference serum OD ratio for the first DBS spot analyzed 7 days post-collection. C. Differences between DBS and reference serum OD ratios versus the average ratios using all spots per study participant 7 days post-collection. PA = phlebotomist acquired sample; Self = self-stick acquired sample.
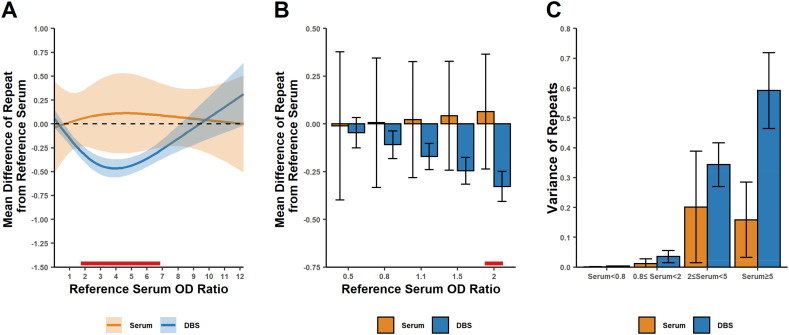
The observed difference between DBS and reference serum OD ratios (Fig. 1C) led us to examine systematic differences in replicate measurements of the same serum sample using our test system. We modeled the difference between DBS and reference serum OD ratios as well as the difference between serum replicates and corresponding reference serum values (Fig. 2 ). The DBS/reference serum difference in OD ratio on day 7 post-collection showed a non-linear relationship with reference serum OD ratio (Fig. 2A). In contrast, the serum reproducibility study did not show this relationship, in which a cohort of 16 serum samples were re-tested in triplicate and compared against the reference results from the same cohort of samples originally tested a few days prior. The DBS OD ratio showed a small, but statistically significant, difference from the serum reference OD ratio when the serum OD ratio was at least 0.8 (Fig. 2B). Similar results were observed on days 14, 21 and 28 post-collection (Supplementary Figs. S4-S6).
Fig. 2.
Differences between repeat and reference serum OD ratios at 7 days post-collection for dried blood spots (DBS) and serum measurements. A. The difference between the mean OD ratio from a DBS collection (three measurements) and the OD ratio of the reference serum from the same subject (y-axis values) as a function of the reference serum OD ratio (x-axis). The results shown are based on a linear mixed model analyzing the three repeated OD ratios for each sample from both the DBS and Serum Repeat (SR) studies. The means (dark lines) were estimated using a linear mixed model, and 95% confidence intervals are shown as the shaded area. The red line at the bottom indicates the region where the difference from the reference serum OD ratio differs significantly between the DBS and SR studies. B. Estimated mean differences as indicated in panel A, but at reference serum measurements near the analytical decision points (OD ratios of <0.8, 0.80–1.09 and ≥ 1.1 are interpreted as negative, equivocal, and positive, respectively). Estimated means and 95% confidence intervals are shown. The red line at the bottom indicates a statistically significant difference between serum and DBS. C. The estimated variance among replicate OD ratio values for all subjects, with the variance depending on the reference serum OD ratio. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The repeatability of OD ratio measurements was compared between DBS and serum. In general, the variance among repeats increased for both DBS and serum repeats as the OD ratio increased (Fig. 2C). However, the variance among repeat DBS measurement was always larger compared to serum measurements. These results indicate that the repeatability for DBS is slightly worse compared to serum collections.
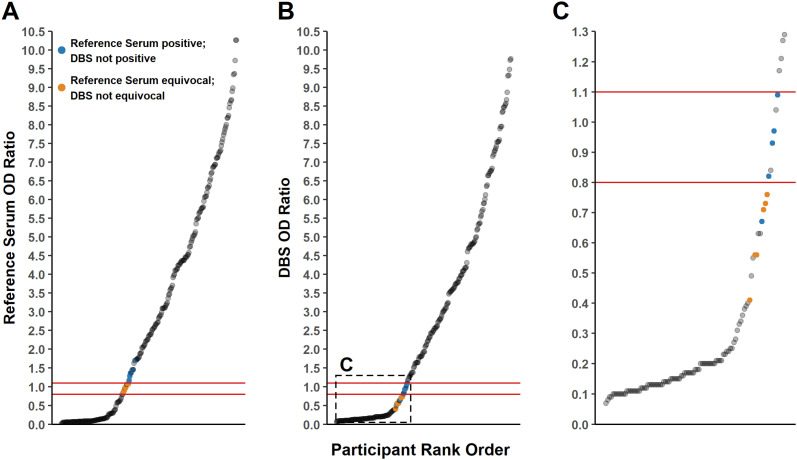
We next examined how differences in OD ratios from parallel DBS and serum collections would impact serological status determination. As established by the test manufacturer, OD ratios of <0.8, 0.80–1.09 and ≥ 1.1 are interpreted as negative, equivocal, and positive, respectively. The systematic difference estimated in Fig. 2 indicates that OD ratios >1.6 are unlikely to result in different serostatus determinations between DBS and serum collections (i.e. all would be considered IgG positive). However, smaller OD ratios near the analytical decision points of the assay are more likely to yield discordant results. For example, a sample with a reference serum OD ratio of 1.1 (positive) could yield a DBS OD ratio of ∼0.85 (equivocal), since the systematic difference at a reference serum OD ratio of 1.1 is ∼ − 0.25 (Fig. 2B). In these circumstances, the described systematic differences did result in samples being mis-called between DBS and serum collections (Fig. 3 ). Some samples with reference serum OD ratios in the equivocal range yielded OD ratios from DBS collections that were interpreted as negative. Likewise, some samples with positive reference serum OD ratios yielded results in the equivocal range for DBS collections. Similar findings were observed for samples tested on days 14, 21 and 28 (Supplementary Figs. S7-S9).
Fig. 3.
Comparison of DBS vs. serum collection on serostatus determination for specimens tested 7 days after collection. A. Serum OD ratios highlighting discordant qualitative results when measured using DBS. B. DBS OD ratios highlighting discordant qualitative results when measured using serum. C. DBS OD ratio for OD ratios ≤ 1.3. Orange and blue dots represent discordant qualitative results between serum and DBS. All other data points are concordant between the two cohorts. Red lines indicate the assay's analytical decision points (0.8, 1.1). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
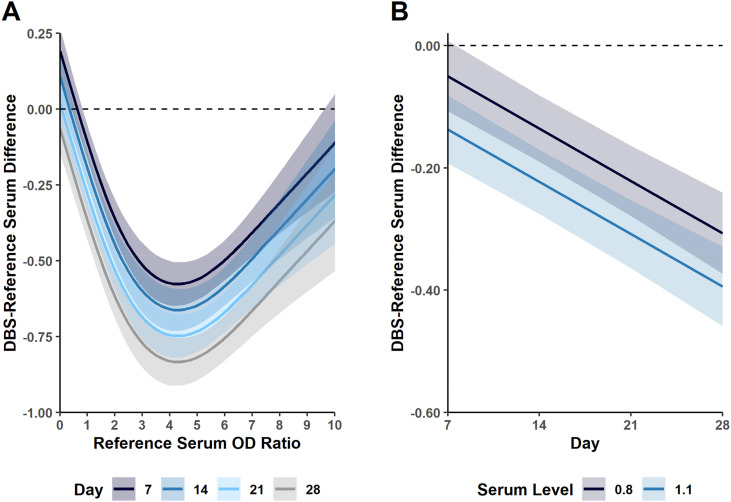
We next examined the impact of time between sample collection and processing on assay OD ratios between DBS and standard venipuncture blood draws (i.e. reference serum samples). Similar to what was initially observed with samples tested on day 7 post-collection, the OD ratios were lower for DBS samples tested on days 14, 21 and 28 compared to serum samples. For PA samples, the maximum absolute difference observed between DBS and reference serum OD ratios at 28 days post-collection was approximately 0.75 (Fig. 4 ). The difference at this time-point between DBS and the corresponding reference serum sample with an OD ratio of 0.8 and 1.1 (analytical decision cutoff points) was approximately −0.3 and − 0.4, respectively. Samples from the self-fingerstick cohort did not show as strong an association with duration of time between sample collection and processing (Supplementary Fig. S10). However, those samples demonstrated a more consistent but larger decrease in OD ratios for DBS compared to serum collections.
Fig. 4.
Impact of time between sample collection and processing on assay OD ratios for phlebotomist acquired DBS collections. A. Mean difference between DBS and reference serum collections from testing performed in triplicate as a function of reference serum OD ratio. Each time point (i.e. 7, 14, 21 and 28 days post-collection) is denoted by a different colored line. B. Mean difference between DBS and reference serum collections from testing performed in triplicate at OD ratios of 0.8 and 1.1 (analytical decision cutoff values).
4. Discussion
In this study, we evaluated the ability to substitute standard venipuncture blood draws with DBS collections obtained by finger stick for the evaluation of SARS-CoV-2 anti-spike IgG antibody status. The ability to use DBS as an option for this line of testing compared to a standard blood draw has a number of advantages including reduced dependency of phlebotomy staff to perform the collection, decreased time necessary to collect the sample, and no need for centrifuges for specimen processing and refrigerators for specimen storage. These factors allow for large scale collection of samples for seroprevalence studies and the ability to conduct such studies in areas where such resources may not be readily available. Furthermore, specimens could potentially be obtained at home using a self-finger-stick kit and a pre-prepared mailer (Kuehn, 2020; Centers ForDisease Control and Prevention, 2017).
The results from this study demonstrate that DBS collections can serve as an acceptable alternative to standard venipuncture blood draws for SARS-CoV-2 anti-spike IgG antibody testing. DBS collections can be stored for at least 28 days and yield comparable results to those processed on day 7 after collection. The ELISA method used for antibody testing in this study also offers a semi-quantitative metric to assess antibody levels, which can be useful to assess the magnitude of the humoral response to COVID-19 infection and vaccination. For example, including a quantitative evaluation of antibody responses in longitudinal studies designed to determine how long the SARS-CoV-2 anti-spike IgG response is sustained in various patient populations if of high clinical value. For the ELISA assay manufactured by EUROIMMUN, the absorbance value for each reaction is divided by the absorbance of a calibrator tested on each plate. That OD ratio is proportional to antibody levels within certain limits. Testing using DBS collections demonstrated slight systematic differences relative to the reference serum (Fig. 2C). The OD ratios of DBS specimens tested on day 7 post-collection averaged a decrease of up to 0.5 compared to the reference serum samples from standard venipuncture blood draws. This systematic difference can lead to a difference in antibody status determination for specimens with a reference serum result slightly above the equivocal or positive cut-off points. In our study cohort, five positive reference serum specimens with OD ratios slightly above the positive cut-off point yielded an equivocal (four specimens) or negative (one specimen) result when the corresponding DBS collections were tested. Furthermore, six reference serum specimens with equivocal OD ratios yielded a negative result when the corresponding DBS collections were tested. None of the specimens between the serum and DBS collections shifted from negative to equivocal/positive, respectively.
A number of other studies have compared DBS to serum for detecting SARS-CoV-2 antibodies. These studies used different kits for antibody detection with variable results and conclusions (Weisser et al., 2021; Amendola et al., 2021; Zava and Zava, 2020; Morley et al., 2020; Moat et al., 2020; Valentine-Graves et al., 2020; Brown et al., 2021; Mulchandani et al., 2021). Three of these studies were performed using the EUROIMMUN assay (Weisser et al., 2021; Amendola et al., 2021; Zava and Zava, 2020). Of note, none used an automated punching device for subsequent extraction and testing as in this study. Weisser et al. examined DBS collections using a single timepoint for each specimen processed between day 6 and 14 post-collection (Weisser et al., 2021). Similar to our study, a strong correlation between standard venipuncture blood draws and DBS collections was observed. However, the quantitative correlations were different (i.e. positive bias for DBS vs. serum). One potential concern was that nearly half of the DBS collection cards were noted to be of sub-optimal quality. Another study by Amendola included a cohort of 52 participants (Amendola et al., 2021). That group also used the EUROIMMUN assay for SARS-CoV-2 antibody evaluation with similar overall qualitative concordance to what is described here. Our study provides further details regarding how DBS collections compares to standard blood draws for SARS-CoV-2 anti-spike IgG testing from the analytical standpoint.
Our results indicate that the DBS/reference serum OD ratio difference increases with each additional week of storage before the specimen was extracted and tested. This difference becomes progressively more negative each week (average weekly decrease of ∼0.09 for PA cohort, Fig. 4A). As observed on day 7, some reference serum low-level positive OD ratios obtained 14, 21 and 28 days after collection yielded either negative or equivocal result when the corresponding DBS collections were tested. Storing samples in a sealed bag containing a desiccant or at a low temperature may mitigate the noted issue with each additional week of storage. Overall, DBS collections produced some false negatives when compared to reference serum samples. However, the risk of a false negative result from a DBS specimen only applies to reference serum specimen that would yield an OD ratio close to the positive cut-off of the assay. Given this finding, we suggest that the equivocal range for DBS-based testing would need to be expanded appropriately through further validation studies. Using our data as a derivation set, the equivocal range would expand from 0.80 to 1.09 to approximately 0.40–1.09. From a clinical standpoint, an equivocal result indicates the testing is insufficient to determine antibody status with high confidence. Re-testing in 2–3 weeks is typically advised in these circumstances to determine if antibody seroconversion has occurred.
We also evaluated repeatability of DBS collections relative to serum obtained from standard venipuncture blood draws. Serum samples measured multiple times show significantly less variability among the repeats when compared to DBS. The increased variability among DBS repeats is likely due to slight sample inconsistencies in the extraction process. Small differences in the amount of dried blood recovered from a punch might lead to a corresponding difference in the amount of antibody recovered. Using an automated DBS card puncher and automated platform for testing can certainly minimize analytical variability compared to any manual procedure introduced into the workflow. Although the variability in antibody measurements for DBS collections was higher compared to serum, the assay imprecision using DBS is considered acceptable for all clinical applications.
Our data also suggest that DBS specimens could be obtained through a self-fingerstick collection kit, with the individual mailing the blood spot card to a clinical laboratory for testing. The primary benefits of the self-fingerstick option are there would be no need for phlebotomy or nursing support to perform the collection, and it obviates the need for an individual to make a visit to a clinic or healthcare facility. Results from this study demonstrates self-fingerstick collections were comparable to those obtained by a nurse. Further evaluations are needed to determine the quality of self-fingerstick collections using at-home kits. Such kits can be supplemented with online resources and instructions for how to perform the collections appropriately.
Using an automated workflow amenable to a clinical laboratory, this study provides a critical analysis comparing DBS collections with standard venipuncture blood draws for SARS-CoV-2 IgG antibody testing. With a point of caution regarding test result values close to the analytical decision point of the assay, DBS collections can serve as an acceptable and convenient option for COVID-19 antibody evaluations.
Financial support
This project was supported with funds from EUROIMMUN and institutional funds through the Beaumont Health Foundation. Philanthropic gifts were provided by Sidney & Madeline Forbes, Nathan & Catherine Forbes, Edward C. Levy & the Linda Dresner Foundation, Stephen & Bobbi Polk, Warren & Carol Ann Rose & Family, Elizabeth Rose, Mickey Shapiro & Family, S. Evan & Gwen Weiner and The Hearst Foundations. The study sponsors had no role in the design of the study, collection of data, or interpretation of results.
Declaration of Competing Interest
Matthew Sims reports grants as Site Principal Investigator from Aridis Pharmaceuticals Inc., Cidara Therapeutics, ContraFect, Cubist Pharmaceuticals Inc. (now Merck), Curetis Ag, Curetis GmBM, CutisPharma (Advisory Board), DiaSorin Molecular LLC, Epigenomics Inc. (Site Sub-Investigator), EUROIMMUN US, Finch Therapeutics, Genentech USA Inc., Gilead Sciences, IBIS Biosystems, Iterum Therapeutics, Janssen Research and Development, LLC, Kinevant Sciences GmBH, Leonard-Meron Biosciences, Merck, Nabriva Therapeutics, NeuMoDx Molecular, Paratek Pharmaceuticals (Advisory Board), Pfizer, Prenosis, Regeneron Pharmaceuticals, Sanofi Pasteur Inc., Seres Therapeutics Inc., Shire (Site Sub-investigator), and Summit Therapeutics. Gabriel Maine reports grants from EUROIMMUN, during the conduct of the study. Kevin Heinrich reports being a co-founder and equity holder of Quire Inc. The company was contracted by Beaumont to perform data analysis Hans Keil reports that he worked for PerkinElmer, Inc. from September 2001 to January 2020. PerkinElmer is the parent company of EUROIMMUN. Beaumont Health used EUROIMMUN instrumentation and reagents in this study. Ramin Homayouni reports being a co-founder, equity holder and serves on the Board of Directors of Quire Inc. and has received consulting fees from Quire Inc. outside the submitted work. All other authors have no conflicts of interest.
Acknowledgements
We would like to acknowledge the invaluable support of the Beaumont Health Institutional Review Board for their rapid review of this project. In particular, we wish to acknowledge Lynne Paul, Jake Sarzynski, and Trish Walker who were always available to handle anything that needed review by the IRB.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jim.2022.113420.
Appendix A. Supplementary data
Supplemental figure 1: Revised SNA to PA (phlebotomist acquired) as indicated in the figure. -Supplemental figure 2: Revised SNA to PA (phlebotomist acquired) as indicated in the figure. -Supplemental figure 3: Revised SNA to PA (phlebotomist acquired) as indicated in the figure.
References
- Amendola A., Bianchi S., Gori M., et al. Dried blood spot as an alternative to plasma/serum for SARS-CoV-2 IgG detection, an opportunity to be sized to facilitate COVID-19 surveillance among schoolchildren. Pediatr. Infect. Dis. J. 2021;40 doi: 10.1097/INF.0000000000002955. https://journals.lww.com/pidj/Fulltext/2021/01000/Dried_Blood_Spot_as_an_Alternative_to_Plasma_Serum.32.aspx Available at: [DOI] [PubMed] [Google Scholar]
- Bland J.M., Altman D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet (London, England) 1986;1:307–310. [PubMed] [Google Scholar]
- Brown L., Byrne R.L., Fraser A., et al. Self-sampling of capillary blood for SARS-CoV-2 serology. Sci. Rep. 2021;11:7754. doi: 10.1038/s41598-021-86008-5. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers ForDisease Control and Prevention Shipping Guidelines for Dried-Blood Spot Specimens. 2017. https://www.cdc.gov/labstandards/nsqap_resources.html Available at:
- Kuehn B.M. Dried blood spots may offer route to wider antibody testing. JAMA. 2020;324:1933. doi: 10.1001/jama.2020.21109. . Available at: [DOI] [PubMed] [Google Scholar]
- Moat S.J., Zelek W.M., Carne E., et al. Development of a high-throughput SARS-CoV-2 antibody testing pathway using dried blood spot specimens. Ann. Clin. Biochem. 2020;58:123–131. doi: 10.1177/0004563220981106. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley G.L., Taylor S., Jossi S., et al. Sensitive detection of SARS-CoV-2-specific antibodies in dried blood spot samples. Emerg. Infect. Dis. 2020;26:2970–2973. doi: 10.3201/eid2612.203309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulchandani R., Brown B., Brooks T., et al. Use of dried blood spot samples for SARS-CoV-2 antibody detection using the Roche Elecsys ® high throughput immunoassay. J. Clin. Virol. 2021;136 doi: 10.1016/j.jcv.2021.104739. https://www.sciencedirect.com/science/article/pii/S1386653221000068 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims M.D., Maine G.N., Childers K.L., et al. Coronavirus disease 2019 (COVID-19) seropositivity and asymptomatic rates in healthcare workers are associated with job function and masking. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1684. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine-Graves M., Hall E., Guest J.L., et al. At-home self-collection of saliva, oropharyngeal swabs and dried blood spots for SARS-CoV-2 diagnosis and serology: post-collection acceptability of specimen collection process and patient confidence in specimens. PLoS One. 2020;15 doi: 10.1371/journal.pone.0236775. . Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisser H., Steinhagen K., Höcker R., Borchardt-Lohölter V., Anvari Ö., Kern P.M. Evaluation of dried blood spots as alternative sampling material for serological detection of anti-SARS-CoV-2 antibodies using established ELISAs. Clin. Chem. Lab. Med. 2021;59:979–985. doi: 10.1515/cclm-2020-1436. Available at: [DOI] [PubMed] [Google Scholar]
- Zava T.T., Zava D.T. Validation of dried blood spot sample modifications to two commercially available COVID-19 IgG antibody immunoassays. Bioanalysis. 2020;13:13–28. doi: 10.4155/bio-2020-0289. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou G.Y. Confidence interval estimation for the Bland–Altman limits of agreement with multiple observations per individual. Stat. Methods Med. Res. 2011;22:630–642. doi: 10.1177/0962280211402548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1: Revised SNA to PA (phlebotomist acquired) as indicated in the figure. -Supplemental figure 2: Revised SNA to PA (phlebotomist acquired) as indicated in the figure. -Supplemental figure 3: Revised SNA to PA (phlebotomist acquired) as indicated in the figure.