Abstract
Background
Differences in clinical outcomes following a temporary interruption of warfarin or a direct oral anticoagulant (DOAC) for a surgical procedure are not well described. Differences in patient characteristics from practice‐based cohorts have not typically been accounted for in prior analyses.
Aim
To describe risk‐adjusted differences in postoperative outcomes following an interruption of warfarin vs DOACs.
Methods
Patients receiving care at six anticoagulation clinics participating in the Michigan Anticoagulation Quality Improvement Initiative were included if they had at least one oral anticoagulant interruption for a procedure. Inverse probability of treatment weighting (IPTW) was used to balance baseline differences between the warfarin cohort and DOAC cohort. Bleeding and thromboembolic events within 30 days following the procedure were compared between the IPTW cohorts using the Poisson distribution test.
Results
A total of 525 DOAC patients were matched with 1323 warfarin patients, of which 923 were nonbridged warfarin patients and 400 were bridged warfarin patients. The occurrence of postoperative minor bleeding (10.8% vs. 4.7%, p < .001), major bleeding (2.9% vs. 1.1%, p = .01) and clinically relevant nonmajor bleeding (CRNMB) (6.5% vs. 3.0%, p = .002) was greater in the DOAC cohort compared with the nonbridged warfarin cohort. The rates of postoperative bleeding outcomes were similar between the DOAC and the bridged warfarin cohorts.
Conclusion
Perioperative interruption of DOACs, compared with warfarin without bridging, is associated with a higher incidence of 30‐day minor bleeds, major bleeds, and CRNMBs. Further research investigating the perioperative outcomes of these two classes of anticoagulants is warranted.
Keywords: anticoagulant, atrial fibrillation (AF), bleeding, venous thromboembolism (VTE), warfarin
Essentials.
Few studies adjust for cofounders in ‘direct oral anticoagulants (DOAC)’ and warfarin cohorts.
Inverse probability weighting used to balance covariates between our DOAC and warfarin cohorts.
No difference in postoperative adverse events between DOAC cohort vs bridged warfarin cohort.
DOAC cohort associated with higher rates of bleeding/thrombosis vs non‐bridged warfarin cohort.
1. INTRODUCTION
The direct oral anticoagulants (DOACs), which include apixaban, dabigatran, rivaroxaban, and edoxaban, are now first‐line therapy for treatment of venous thromboembolism (VTE) and stroke prevention in atrial fibrillation (AF). Many patients taking chronic anticoagulant therapy will require temporary interruption for a surgical procedure. 1 Management of the anticoagulant in the perioperative period can be complicated because the pharmacokinetic properties of each agent differ, most significantly as compared with warfarin.
Because of its long half‐life, warfarin is often interrupted several days before the procedure. For patients with a high risk of thrombotic complications, “bridging” therapy with low molecular weight heparin or other short‐acting parenteral anticoagulants are frequently used while the anticoagulant activity of warfarin wanes or returns to therapeutic range. This practice, however, is associated with higher risk of bleeding compared with those in whom bridging therapy was not initiated. 2 , 3 , 4 In contrast to warfarin, DOACs have both a short half‐life and rapid onset of action, which make them ideal for perioperative use and removes the necessity of bridging anticoagulation. 5 , 6 Prior studies have compared postoperative outcomes of patients following the perioperative interruption of DOACs vs. warfarin and found no major differences in postoperative rates of major bleeding or thromboembolic events. 7 , 8 , 9 , 10 However, because these were post hoc analyses of clinical trials, the patient samples include a more selective population that is commonly seen in routine clinical practice. Therefore, we aimed to compare 30‐day postoperative bleeding and thromboembolic events associated with DOAC versus warfarin management in the perioperative period in a practice‐based cohort of patients.
2. MATERIALS AND METHODS
The Michigan Anticoagulation Quality Improvement Initiative (MAQI2) is a Blue Cross Blue Shield of Michigan/Blue Care Network‐funded multicenter collaborative of anticoagulation management services in the state of Michigan. MAQI2 was formed in 2008 with the goal of improving patient safety and outcomes by collecting and comparing patient clinical data, identifying best practices, and conducting quality improvement initiatives. Currently, there are six hospitals in Michigan participating in the program. Patients initiated on either a DOAC or warfarin are randomly selected for entry into the MAQI2 database and followed longitudinally so long as they remain on therapy and are managed at the participating health system. All data abstractors undergo training, and each center undergoes regular audits to ensure high‐quality data collection and agreement with predefined data element definitions. More information about MAQI2 has been previously described. 11 Institutional review board approval has been obtained at each site and the coordinating center (University of Michigan).
2.1. Patient selection
Patients who had a temporary interruption of DOAC or warfarin therapy for an elective surgical procedure were included if they had follow‐up for at least 3 months after the procedure. Patients with indications for anticoagulant use other than AF or VTE were excluded.
2.2. Data collection
Baseline patient information, including demographics, medications, and comorbidities were abstracted from patient charts at the time of anticoagulation initiation. Information about medication changes, new comorbidities, procedures, and bleeding or thrombotic events were abstracted for each patient interaction with the anticoagulation management service for warfarin‐treated patients and at regular 6‐month follow‐up intervals for DOAC‐treated patients. Procedural data abstracted from the medical record included the date of procedure, anticoagulation interruption and restart dates, whether or not heparin bridging was used, and the type of procedure. The procedures were categorized into having low or high bleed risk based on categories from the BRIDGE trial. 3
2.3. Outcomes
The primary outcomes of our study were 30‐day postoperative thromboembolic or bleeding events. Thromboembolic events included the composite of transient ischemic attacks, ischemic stroke, and VTE. Bleeding events were classified into minor bleeds, major bleeds, and clinically relevant nonmajor bleeds (CRNMB). Criteria for major and CRNMBs were based on International Society on Thrombosis and Haemostasis (ISTH) definitions. 12 , 13
2.4. Statistical analysis
Inverse probability of treatment weighting (IPTW) was used to compare the DOAC and warfarin treatment groups. This statistical method assigns a weight to each patient based on their propensity score, creating a pseudopopulation that allows us to better determine the effects of the two treatments. The IPTW approach allows us to use the entirety of our cohort and maximize the inclusion of as many warfarin‐treated and DOAC‐treated patients as possible while preserving exact matching for sex, anticoagulation indication, and procedural bleeding risk. Other clinical variables were included in the IPTW, and a standardized difference of less than 0.1 was considered negligible (Appendix S1). Bleeding and thromboembolic risk comparisons were adjusted for the average modified HAS‐BLED (excluding time in therapeutic range) scores. 14 , 15 Following IPTW reweighting, multivariate logistic regression was performed to calculate odds ratios that compared 30‐day postoperative outcomes.
3. RESULTS
3.1. Study population
Of a total of 14 168 warfarin and 3253 DOAC patients in the MAQI2 registry, 1323 (9%) warfarin‐treated and 525 (16%) DOAC‐treated patients met inclusion/exclusion criteria for our study (Figure 1). Following IPTW reweighting, differences between the two cohorts were minimal (Appendix S1). Of the warfarin‐treated patients, 400 of 1323 (30%) were bridged during the interruption. Compared with the bridged warfarin cohort, the DOAC cohort was significantly older (71.0 years vs. 65.7 years, p < .001), had a higher concomitant rate of hypertension (80.0% vs. 64.0%, p < .001), had more cases of remote bleeding (6.5% vs. 3.5%, p = .04), were prescribed less antiplatelets (26.5% vs. 41.8%, p < .001) and nonsteroidal anti‐inflammatory drugs (3.6% vs. 10.0%, p < .001), had more bleeding events before the procedure (17.5% vs. 11.2%, p = .007), had a higher CHA2DS2‐VASc score (3.54 vs. 3.12, p = .001), had a lower rate of high bleed risk procedures (30.1% vs. 60.3%, p < .001), had a higher rate of low bleed risk procedures (69.3% vs. 36.5%, p < .001), had a higher proportion of patients being treated for AF (70.7% vs. 35.3%, p < .001), were taking their anticoagulant for more days before the procedure (391 vs. 243, p < .001), and stopped taking their anticoagulant for more days before the procedure (5 vs. 3, p < .001). Compared with the nonbridged warfarin cohort, the DOAC cohort was significantly younger (71.0 years vs. 72.4 years, p = .03), had a lower rate of concomitant abnormal renal function (15.4% vs. 22.1%, p = .002), were prescribed fewer antiplatelets (26.5% vs. 43.1%, p < .001) and nonsteroidal anti‐inflammatory drugs (3.6% vs. 8.3%, p = .001), had more bleeding events before the procedure (20.7% vs. 11.2%, p < .001), had a lower modified HAS‐BLED score (2.58 vs. 3.01, p < .001), and had a lower rate of high bleed risk procedures (30.1% vs. 43.0%, p < .001), had a higher rate of low bleed risk procedures (69.3% vs. 54.1%, p < .001), were taking their anticoagulant for more days before the procedure (480 vs. 243, p < .001), stopped taking their anticoagulant for more days before the procedure (5 vs. 3, p < .001), and had a lower procedure to restart interval (0 vs. 1, p = .002). No other significant differences in patient characteristics or procedure bleed risk between the groups were found (Table 1A). Thirty‐nine bleeding events occurred in patients taking apixaban 5 mg twice daily, and 33 occurred in patients taking rivaroxaban 20 mg each day, which were the two dosages with the highest incidence of bleeding events among the DOAC cohort.
FIGURE 1.
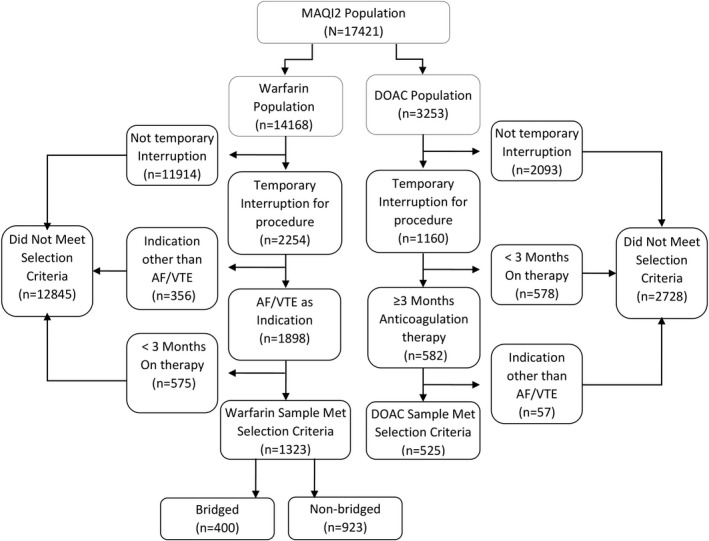
Matching procedure diagram
TABLE 1.
(A) Patient characteristics and procedure bleed risk of warfarin‐ and DOAC‐matched cohorts. (B) Thirty‐day postoperative outcomes
| Warfarin (Bridged) n = 400 | Warfarin (Nonbridged) n = 923 | DOAC n = 525 | p‐value Warfarin Bridged vs. DOAC | p‐value Warfarin Nonbridged vs. DOAC | |
|---|---|---|---|---|---|
| (A) | |||||
| Age (mean ± SD) | 65.7 ± 13.8 | 72.4 ± 11.7 | 71.0 ± 12.2 | <.001 | .03 |
| Sex, n (%), male | 198 (49.5) | 493 (53.4) | 262 (49.9) | .90 | .20 |
| Comorbidities, n (%) | |||||
| HTN | 256 (64) | 717 (77.7) | 420 (80.0) | <.001 | .30 |
| Abnormal renal function | 65 (16.3) | 204 (22.1) | 81 (15.4) | .73 | .002 |
| Abnormal hepatic/liver function | 12 (3) | 37 (4) | 25 (4.8) | .18 | .50 |
| TIA/CVA | 72 (18) | 121 (13.1) | 76 (14.5) | .15 | .47 |
| Recent bleeding | 16 (4) | 54 (5.9) | 27 (5.1) | .41 | .57 |
| Remote bleeding | 14 (3.5) | 42 (4.6) | 34 (6.5) | .04 | .11 |
| Medications, n (%) | |||||
| Antiplatelets | 167 (41.8) | 389 (43.1) | 139 (26.5) | <.001 | <.001 |
| Nonsteroidal anti‐inflammatory drugs | 40 (10) | 77 (8.3) | 19 (3.6) | <.001 | .001 |
| Recurrent adverse events before procedure, n (%) | |||||
| Thrombosis/stroke | 8 (2) | 10 (1.1) | 6 (1.1) | .29 | .92 |
| Bleeding | 70 (17.5) | 191 (20.7) | 59 (11.2) | .007 | <.001 |
| Risk scores (Mean ± SD) | |||||
| TTR | 0.65 ± 0.15 | 0.66 ± 0.14 | – | – | – |
| CHA2DS2‐VASc | 3.12 ± 1.95 | 3.71 ± 1.69 | 3.54 ± 1.78 | .001 | .07 |
| Modified HAS‐BLED | 2.66 ± 1.43 | 3.01 ± 1.34 | 2.58 ± 1.28 | .35 | <.001 |
| Procedure bleed risk, n (%) | |||||
| High/medium | 241 (60.3) | 397 (43.0) | 158 (30.1) | <.001 | <.001 |
| Low | 146 (36.5) | 499 (54.1) | 364 (69.3) | <.001 | <.001 |
| AF as indication | 141 (35.3) | 677 (73.4) | 371 (70.7) | <.001 | .27 |
| Duration of anticoagulation before procedure, median days (IQR) | 391 (706) | 480 (806) | 243 (258) | <.001 | <.001 |
| Days from stop to procedure median (IQR) | 5 (0) | 5 (1) | 3 (2) | <.001 | <.001 |
| Days from procedure to restart median (IQR) | 1 (0, 2) | 0 (0, 1) | 1 (0, 2) | .80 | .002 |
| DOAC dosage at bleed | |||||
| Apixaban 2.5 mg twice daily | ‐ | – | 9 | – | – |
| Apixaban 5 mg twice daily | – | – | 29 | – | – |
| Apixaban 10 mg twice daily | – | – | 1 | – | – |
| Dabigatran 150 mg twice daily | – | – | 1 | – | – |
| Edoxaban 60 mg every day | – | – | 1 | – | – |
| Rivaroxaban 15 mg every day | – | – | 2 | – | – |
| Rivaroxaban 20 mg every day | – | – | 28 | – | – |
| DOAC dosage at clot | |||||
| Apixaban 5 mg twice daily | – | – | 3 | – | – |
| Edoxaban 60 mg twice daily | – | – | 1 | – | – |
| n (%) | Warfarin (Bridged) n = 400 | Warfarin (Nonbridged) n = 923 | DOAC n = 525 | p‐value Warfarin Bridged vs. DOAC | p‐value Warfarin Nonbridged vs. DOAC |
|---|---|---|---|---|---|
| (B) | |||||
| Any minor bleeds | 34 (8.5) | 43 (4.7) | 57 (10.8) | .23 | <.001 |
| Major bleeds | 13 (3.3) | 10 (1.1) | 15 (2.9) | .73 | .01 |
| TIA/stroke/VTE | 3 (0.8) | 5 (0.5) | 4 (0.8) | .98 | .61 |
| CRNMBs | 26 (6.5) | 28 (3) | 34 (6.5) | .99 | .002 |
Abbreviations: AF, atrial fibrillation; CRNMB, clinically relevant nonmajor bleeding; CVA, cardiovascular accident; DOAC, direct oral anticoagulant; HTN, hypertension; IQR, interquartile range; TIA, transient ischemic accident; TTR, time in therapeutic range.
3.2. Postoperative outcomes
Among the 1323 warfarin patients and 525 DOAC patients in the treatment cohorts, 260 bleeding events and 12 thrombotic events were reported. Compared with the nonbridged warfarin cohort, the DOAC cohort had a significantly higher postoperative rate of both minor bleeds (10.8% vs. 4.7%, p < .001), major bleeds (2.9% vs. 1.1%, p = .01), and CRNMBs (6.5% vs. 3.0%, p = .002) (Table 1B). There was no difference in the rate of thromboembolic events between the nonbridged warfarin cohort and the DOAC cohort. Similarly, there was no difference in any bleeding or thromboembolic events between the bridged warfarin cohort and DOAC cohort.
3.3. IPTW outcomes
Between the nonbridged warfarin cohort and the DOAC cohort, IPTW analysis indicates that DOAC use contributed most greatly to the differences in the rates of minor bleeds (OR 2.546, 95% CI 1.656–3.912), major bleeds (OR 3.712, 95% CI 1.605–8.584), and CRNMBs (OR 2.928, 95% CI 1.718–4.991) (Figure 2).
FIGURE 2.
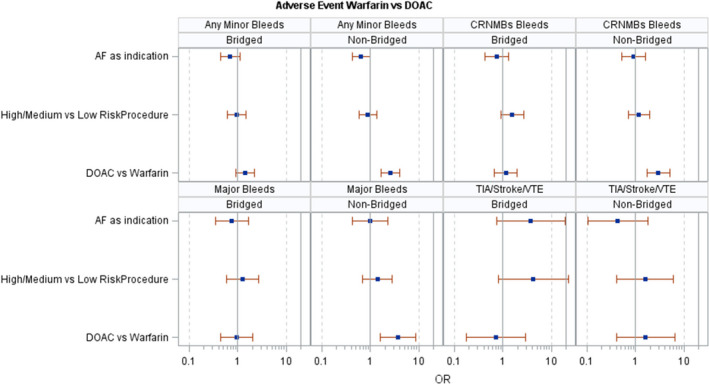
Inverse probability of treatment weighting outcomes
4. DISCUSSION
Among the 1323 warfarin patients and 525 DOAC patients who underwent surgical procedure requiring perioperative interruption, rates of minor bleeding, major bleeding, and bleeding requiring an emergency department visit were lower for patients treated with warfarin and no bridging therapy than for DOAC‐treated patients following IPTW weighting. There were no differences in thromboembolic events between warfarin‐treated patients without bridging and DOAC‐treated patients, and no differences between warfarin‐treated patients with bridging and DOAC‐treated patients. IPTW analysis indicates that DOAC use contributed most greatly to the differences in bleeding rates between the two cohorts.
Given the large body of evidence associating bridging therapy with increased bleeding risk, it was unsurprising that the bridged warfarin cohort had a numerically higher bleeding rate than to our unbridged warfarin cohort. 2 , 3 , 4 Our bridged warfarin cohort saw similar outcomes with our DOAC cohort with regard to bleeding and thromboembolic events. In contrast to warfarin, DOACs have both a short half‐life and fast onset of action and thus do not require bridging. 16 , 17 This leads to a shortened time between the surgical procedure and when a DOAC medication is stopped or restarted as compared to warfarin. It is thus not unexpected that the addition of a fast‐acting bridging agent in our bridged warfarin cohort to result in similar rates of bleeding compared to our DOAC cohort.
DOAC‐treated patients in our study experienced a postoperative major bleed rate of 2.9%, which is slightly higher (2.9% vs. 0.9%–1.85%) than the rates of the cohorts in PAUSE. 18 However, our rate of minor bleeding was 10.8%, which is notably higher than the rates of 4.3%–5.7% of the cohorts of the PAUSE study. Two potential contributing factors why our DOAC cohort had a higher rate of major bleeding are the higher rate of antiplatelet use 19 , 20 , 21 (26.5% vs. 9.1%–14.7%) and a higher modified HAS‐BLED score 22 , 23 , 24 (2.6 vs. 1.9).
Shaw and colleagues also performed a retrospective analysis of warfarin and DOAC interruption for surgical procedures. In addition to the multivariable logistic‐regression model that they utilized, we also implemented IPTW before logistic regression to adjust for confounding variables. 25 Their analysis also found that postoperative rates of major bleeding were significantly higher in the warfarin cohort, which we did find in our analysis of our nonbridged warfarin cohort. However, there are various differences between the two studies that led to this shared finding. First, our study included patients whose primary indication for anticoagulation was either AF or VTE, whereas the Shaw study focused on AF only, although our study's IPTW analysis indicates that an indication of AF does not contribute to the differences in bleeding rates in the DOAC versus nonbridged warfarin cohorts (Figure 2). Additionally, we differentiated our warfarin patients into cohorts based on the use of bridging low molecular weight heparin. Last, we used an IPTW statistical approach, which may have resulted in different analysis of the variables and affected outcomes since IPTW sets our groups to be similar in our covariates before logistics regression. Further efforts are needed to reconcile the differences between these two important studies of perioperative anticoagulation care.
The key strength of our study is the use of our IPTW statistical analysis, which is able to account for various potential confounding variables in the warfarin and DOAC cohorts while maintaining statistical power and generalizability. This approach was not taken with most other studies comparing DOAC and warfarin outcomes in the perioperative period. 7 , 8 , 9 , 10 Although each of those analyses were conducted on randomized, controlled trial populations and thus reduce the effect of confounding variables on outcomes, the samples for these studies are limited to patients who are eligible for randomization. In contrast, our data were not limited to patients eligible for randomization, therefore representing a potentially more generalizable estimate of adverse event rates. Last, our analysis distinguishes between warfarin‐treated patients into both a bridged and a nonbridged cohort, which is critical given the strong evidence associating use of bridging therapy with increased bleeding risk. 2 , 3 , 4
Despite these strengths, certain limitations must be acknowledged. First, the modest sample sizes and inclusion of patients from one geographic region may limit generalizability. Second, data on exact perioperative management of DOACs was not available for this analysis. Therefore, it is unclear whether our DOAC perioperative interruption practices are fully consistent with the PAUSE protocol. Third, as with any retrospective cohort study, our analysis is only as accurate as what is depicted in our electronic health records and cannot account for things such as misinformation or lapses in data. Fourth, although the use of IPTW methodology significantly reduces the impact of confounding in the analysis, covariate balance between the two groups are notably different before and after the probability weighting is applied. Fifth, data on the rationale for why bridging was or was not given were not available in the MAQI2 dataset. Sixth, we do not have data on the use of prophylactic‐dose heparin in the periprocedural period. Seventh, we are only able to comment on association, not causation. Last, although we used a fairly comprehensive list of confounding variables for our matching procedure, it is possible that there are other confounding factors that were not included and influenced our results.
5. CONCLUSION
Our study suggests that the periprocedural interruption of DOACs may be associated with a higher incidence of 30‐day minor bleeds, major bleeds, and bleeds requiring medical therapy when compared with the interruption of nonbridged warfarin. However, no difference in bleeding or thromboembolic events was noted between patients with warfarin who received bridging therapy and DOAC‐treated patients. Further studies that investigate the effects of perioperative interruption of DOACs and warfarin on postoperative outcomes while addressing potential issues of confounding variables are warranted.
AUTHOR CONTRIBUTIONS
J. Lee: conceived the project, reviewed analyses, and drafted the manuscript. X. Kong: conducted analyses and critically reviewed the manuscript. B. Haymart: conceived the project, reviewed analyses, and critically reviewed the manuscript. G. Barnes: conceived the project, reviewed analyses, and critically reviewed the manuscript. All other authors critically reviewed the manuscript.
CONFLICT OF INTEREST
E. Kline‐Rogers: Janssen (personal fees), American College of Physicians (personal fees). S. Kaatz: Osmosis Research (grant), Janssen (grant), Bristol Myers Squibb (grant), NIH (grant), Janssen (personal fees), Pfizer/Bristol Myers Squibb (personal fees), Astra Zeneca (personal fees), Phase Bio (personal fees), Gilead (personal fees), Board of Directors for Anticoagulation Forum (other), Scientific Advisory Board for National Blood Clot Alliance (other), PERT Consortium (other). J. Froehlich: Merck (personal fees), Janssen (personal fees), Novartis (personal fees), Boehringer‐Ingelheim (personal fees), Pfizer (personal fees), Blue Cross Blue Shield of Michigan (grant), Fibromuscular Disease Society of America (grant). G. Barnes: Pfizer/Bristol‐Myers Squibb (personal fees), Janssen (personal fees), Acelis (personal fees), AMAG Pharmaceuticals (personal fees), Connected Health (personal fees), Blue Cross Blue Shield of Michigan (grant), Board of Directors: Anticoagulation Forum (other), Board of Directors: National Certification Board of Anticoagulation Providers (other). The remaining authors have no conflicts of interest to report.
Supporting information
Appendix S1
Lee J, Kong X, Haymart B, et al. Outcomes in patients undergoing periprocedural interruption of warfarin or direct oral anticoagulants. J Thromb Haemost. 2022;20:2571‐2578. doi: 10.1111/jth.15850
Manuscript handled by: Sabine Eichinger
Final decision: Sabine Eichinger, 08 August 2022
REFERENCES
- 1. Rechenmacher SJ, Fang JC. Bridging anticoagulation: primum non nocere. J Am Coll Cardiol. 2015;66:1392‐1403. [DOI] [PubMed] [Google Scholar]
- 2. Yong JW, Yang LX, Ohene BE, Zhou YJ, Wang ZJ. Periprocedural heparin bridging in patients receiving oral anticoagulation: a systematic review and meta‐analysis. BMC Cardiovasc Disord. 2017;17:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barnes GD, Li Y, Gu X, et al. Periprocedural bridging anticoagulation in patients with venous thromboembolism: a registry‐based cohort study. J Thromb Haemos. 2020;18:2025‐2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood. 2012;120:2954‐2962. [DOI] [PubMed] [Google Scholar]
- 6. Shaw J, de Wit C, Le Gal G, Carrier M. Thrombotic and bleeding outcomes following perioperative interruption of direct oral anticoagulants in patients with venous thromboembolic disease. J Thromb Haemost. 2017;15:925‐930. [DOI] [PubMed] [Google Scholar]
- 7. Healey JS, Eikelboom J, Douketis J, et al. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long‐Term Anticoagulation Therapy (RE‐LY) randomized trial. Circulation. 2012;126:343‐348. [DOI] [PubMed] [Google Scholar]
- 8. Sherwood MW, Douketis JD, Patel MR, et al. Outcomes of temporary interruption of rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: results from the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stro. Circulation. 2014;129:1850‐1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garcia D, Alexander JH, Wallentin L, et al. Management and clinical outcomes in patients treated with apixaban vs warfarin undergoing procedures. Blood. 2014;124:3692‐3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Douketis JD, Murphy SA, Antman EM, et al. Peri‐operative adverse outcomes in patients with atrial fibrillation taking warfarin or edoxaban: analysis of the ENGAGE AF‐TIMI 48 trial. Thromb Haemost. 2018;118:1001‐1008. [DOI] [PubMed] [Google Scholar]
- 11. Barnes GD, Kaatz S, Winfield J, et al. Warfarin use in atrial fibrillation patients at low risk for stroke: analysis of the Michigan Anticoagulation Quality Improvement Initiative (MAQI2). J Thromb Thrombolysis. 2014;37:171‐176. [DOI] [PubMed] [Google Scholar]
- 12. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3:692‐694. [DOI] [PubMed] [Google Scholar]
- 13. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S. Subcommittee on Control of Anticoagulation. Definition of clinically relevant non‐major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non‐surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13:2119‐2126. [DOI] [PubMed] [Google Scholar]
- 14. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093‐1100. [DOI] [PubMed] [Google Scholar]
- 15. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263‐272. [DOI] [PubMed] [Google Scholar]
- 16. Douketis JD, Healey JS, Brueckmann M, et al. Perioperative bridging anticoagulation during dabigatran or warfarin interruption among patients with an elective surgery or procedure. Substudy of the RE‐LY trial. Thromb Haemost. 2014;113:625‐632. [DOI] [PubMed] [Google Scholar]
- 17. Heidbuchel H, Verhamme P, Alings M, et al. Updated European heart rhythm association practical guide on the use of non‐vitamin K antagonist anticoagulants in patients with non‐valvular atrial fibrillation. Europace. 2015;17:1467‐1507. [DOI] [PubMed] [Google Scholar]
- 18. Douketis JD, Spyropoulos AC, Duncan J, et al. Perioperative management of patients with atrial fibrillation receiving a direct oral anticoagulant. JAMA Intern Med. 2019;179:1469‐1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gallego P, Roldán V, Torregrosa JM, et al. Relation of the HAS‐BLED bleeding risk score to major bleeding, cardiovascular events, and mortality in anticoagulated patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2012;5:312‐318. [DOI] [PubMed] [Google Scholar]
- 20. Roldan V, Marin F, Manzano‐Fernandez S, et al. The HAS‐BLED score has better prediction accuracy for major bleeding than the CHADS or CHADS‐VASc scores in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2013;62:2199‐2204. [DOI] [PubMed] [Google Scholar]
- 21. Zhu W, He W, Guo L, Wang X, Hong K. The HAS‐BLED score for predicting major bleeding risk in anticoagulated patients with atrial fibrillation: a systematic review and meta‐analysis. Clin Cardiol. 2015;38:555‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Davidson BL, Verheijen S, Lensing AWA, et al. Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti‐inflammatory drugs or aspirin. JAMA Intern Med. 2014;174:947‐953. [DOI] [PubMed] [Google Scholar]
- 23. Alexander JH, Lopes RD, James S, et al. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med. 2011;365:699‐708. [DOI] [PubMed] [Google Scholar]
- 24. Oldgren J, Budaj A, Granger CB, et al. Dabigatran vs. placebo in patients with acute coronary syndromes on dual antiplatelet therapy: a randomized, double‐blind, phase II trial. Eur Heart J. 2011;32:2781‐2789. [DOI] [PubMed] [Google Scholar]
- 25. Shaw JR, Zhang T, Le Gal G, Douketis J, Carrier M. Perioperative interruption of direct oral anticoagulants and vitamin K antagonists in patients with atrial fibrillation: a comparative analysis. Res Pract Thromb Haemost. 2020;4:131‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


