Abstract
Previous studies have demonstrated that Mycobacterium avium can invade intestinal epithelial cells both in vitro and in vivo. When given to mice orally, M. avium preferentially interacts with the intestinal mucosa at the terminal ileum. We evaluated the mechanism(s) of M. avium binding and invasion of the intestinal mucosa using three different systems: (i) electron microscopy following administration of M. avium into an intestinal loop in mice, (ii) quantitative comparison of the bacterial load in Peyer's patch areas of the terminal ileum versus areas that do not contain Peyer's patches, and (iii) investigation of the ability of M. avium to cause disseminated infection following oral administration using B-cell-deficient mice, lacking Peyer's patches, in comparison with C57BL/6 black mice. By all approaches, M. avium was found to invade the intestinal mucosa by interacting primarily with enterocytes and not with M cells.
The advent of AIDS made it clear that infections caused by Mycobacterium avium are acquired primarily by the gastrointestinal route (6, 12). Although a respiratory route of infection (by aerosol) has also been identified (17), the fact that M. avium is an environmental organism, adapted to live in water and soil, makes oral ingestion the most likely manner in which the host would come into contact with the bacterium (7, 16). In patients with AIDS and disseminated M. avium infection, a large number of organisms are found in the intestinal mucosa and submucosa sometime prior to the identification of bacteremia, suggesting that intestinal colonization precedes the onset of systemic infection (30).
Using a model of oral infection in healthy mice, we determined that 100% of the mice given M. avium orally developed disseminated disease (2). Furthermore, M. avium, when given orally, preferentially colonizes the terminal ileum and ascending colon among all intestinal segments (2). Studies in vitro have shown that M. avium can invade intestinal and respiratory epithelial cells and that the invasion is more efficient when the bacterium is incubated at 37 than at 33°C prior to the assay (3). M. avium is significantly more efficient in entering epithelial cells when grown to the logarithmic phase than when grown to stationary phase (3). In addition, M. avium, when exposed to conditions that mimic the intestinal environment, such as low oxygen tension and hyperosmolarity, prior to incubation with intestinal epithelial cells, can invade intestinal epithelial cells with increased efficiency (1).
It has been demonstrated elsewhere for a number of enteric pathogens that M cells in the Peyer's patches are the portal of entry into the mucosa (5, 10, 14, 18). A few organisms, however, such as Listeria monocytogenes, preferentially use enterocytes to enter the intestinal mucosa (28). Work with two mycobacterial species, Mycobacterium bovis BCG and Mycobacterium paratuberculosis, has shown that both bacteria cross the intestinal mucosa primarily by invading M cells (9, 24).
Little is known about the manner in which M. avium interacts with the intestinal mucosa in the host and specifically whether M cells are the mucosal target for bacterial entry. Therefore, we sought to examine if M cells play any role in the uptake of M. avium, by using three approaches, i.e., the intestinal loop model, oral infection in immunocompetent mice, and oral infection of B-cell-deficient mice that lack Peyer's patches and M cells (11).
MATERIALS AND METHODS
Bacteria.
M. avium strain 101 (serovar 1) and strain 104 (serovar 1) were isolated from the blood of patients with AIDS. Bacteria were cultured in Middlebrook agar 7H10 medium (Difco Laboratories, Detroit, Mich.), supplemented with oleic acid, albumin, dextrose, and catalase (OADC; Difco), for 10 days at 37°C. Morphologically similar transparent colonies (107) of M. avium were transferred to 7H9 broth and cultured for 5 days as previously described (logarithmic-phase growth). The culture was shaken twice daily in order to obtain a homogeneous population of bacteria. Before infection of animals, the bacterial suspension was harvested and vortex agitated for 2 min to disperse possible clumps. The top half of the suspension was removed and stained with Ziehl-Neelsen stain to determine the degree of dispersion of bacteria. Bacteria were plated onto 7H10 agar plates before each experiment to determine the number of CFU in the inoculum.
Mice.
Pathogen-free C57BL/6J black mice used in these experiments (female, 8 to 10 weeks old, weighing an average of 25 g) were obtained from the Jackson Laboratory (Bar Harbor, Maine) and used after 1 to 2 weeks of quarantine. C57BL/6J B-cell-deficient mice (immunoglobulin H6 negative, 8 to 10 weeks old, and 25 g) were purchased from the Jackson Laboratory and used after 1 week of quarantine. These animals have been shown previously to lack Peyer's patches and M cells (11). For the experiments in which it was necessary to identify intestinal segments with Peyer's patches, 10- and 12-week-old mice were used. All experiments were performed according to the guidelines of the Institute's Animal Care Use Committee.
Invasion assay in vivo.
To determine M. avium binding to the intestinal mucosa in vivo, we adapted a loop model based on a model originally described for Giardia muris (26). C57BL/6 black mice were anesthetized using intraperitoneal administration of phenobarbital and ether in aerosol. Mice were maintained under profound anesthesia during the whole procedure. Following anesthesia, the abdominal cavity was carefully opened, and an approximately 3-cm-long segment of the small intestine, above the ileocecal area, was identified. A suture line was tied at both segment ends of the intestine, enough to close the intestinal lumen but not to interfere with blood flow. A suspension containing approximately 107 bacteria in Hanks' buffered salt solution (HBSS) was injected into the proximal portion of the isolated intestinal segment. Animals were maintained alive for 1, 2, and 3 h depending on the experiment, after which the intestinal segment was removed, opened longitudinally, and rinsed extensively in HBSS to remove unbound and weakly bound bacteria. Formed feces were never observed in the terminal ileum segment of the intestines. The removed intestine was placed in 5 ml of 7H9 broth with 20% glycerol and homogenized using a sterile glass homogenizer. The suspension was then serially diluted in 7H9 broth before being plated onto 7H11 agar containing antibiotics (polymyxin B, 5 μg/ml; amphotericin B, 4.5 μg/ml; carbenicillin, 22 μg/ml; and trimethoprim, 2.0 μg/ml) to inhibit intestinal biota, for quantitation of viable organisms associated with intestinal mucosa-submucosa. Plates were cultured for 20 days at 37°C in humid air. The CFU per gram of tissue was calculated as follows: CFU per gram of tissue = (average CFU per plate × dilution factor × 5 ml)/intestinal segment weight.
Peyer's patch areas versus non-Peyer's patch areas.
To examine if M. avium enters the intestinal mucosa preferentially at the Peyer's patches or by invading enterocytes in a region without Peyer's patches (or both), we used two approaches. In the first approach, C57BL/6 mice were given M. avium (108 bacteria) orally, and 1, 4, 24, and 48 h later, the mice (eight mice per experimental group and time point) were sacrificed; the abdomen was opened; and four segments each of 1 cm in length comprising a region with Peyer's patches and four segments of intestine comprising a region without Peyer's patches were obtained, opened longitudinally, washed, homogenized, and plated onto 7H10 agar plates to determine the number of bacteria in the mucosa. In the second approach, we used C57BL/6J wild-type (WT) and C57BL/6J B-cell-deficient mice (Jackson Laboratory). These mice have recently been shown to lack Peyer's patches in the terminal ileum of intestine, and therefore they do not possess M cells. Mice were infected with 104 bacteria orally, and at 2 days, 1 week, and 3 weeks, the terminal ileum and spleen were harvested and plated to quantitate the bacterial load. As a control, we used Salmonella enterica serovar Typhimurium (known to invade the intestinal mucosa through Peyer's patches; 104 bacteria) and harvested the mice at 4 and 24 h after oral infection. Salmonella was plated on Luria-Bertani agar.
Electron microscopy.
M. avium (strain 101) was inoculated into the intestinal loop as described above, and at several time points an intestinal segment was obtained, cut longitudinally, and extensively washed in HBSS. Control mice had the intestinal loop injected with HBSS, but not with bacteria. The intestinal segment was cut into small pieces and fixed in ice-cold 1% glutaraldehyde in phosphate buffer for 1 h. The small segments were immersed in 1% OsO4 for 1 h at room temperature, dehydrated through 50 and 80% ethyl alcohol at room temperature, embedded in L.R. White resin, and polymerized at 52°C. Thin sections were cut and stained with uranyl acetate and lead citrate. Electron micrographs were made with a transmission electron microscope. Sections from intestines of approximately 25 mice were examined.
Statistical analysis.
The comparison among the experimental groups was evaluated for statistical significance by using Student's t test.
RESULTS
Intestinal loop model.
To determine whether M. avium would enter the intestinal mucosa by invading M cells in the terminal ileum, we used the intestinal loop technique and injected M. avium into the loop. At 1, 2, and 3 h, the intestinal segment was removed and the number of bacteria associated with it was determined. M. avium strains 101 and 104 became associated with the intestine in a time-related manner. While at 1 h approximately 7% of the inoculum invaded the mucosa, at 3 h the number of bacteria associated with the intestinal mucosa increased to approximately 45% of the inoculum (Table 1).
TABLE 1.
Abilities of M. avium strains to invade the intestinal mucosa in an intestinal loop
| Bacterial strain | % of the inoculum inside the mucosa over timea
|
||
|---|---|---|---|
| 1 h | 2 h | 3 h | |
| 101 | 6.8 ± 0.3 | 35 ± 7 | 46 ± 10 |
| 104 | 7.1 ± 0.2 | 42 ± 11 | 51 ± 0 |
Bacteria (107) were injected into the intestinal segment and allowed to invade for 1, 2, and 3 h as described in Materials and Methods. The numbers represent the mean percentages (± standard deviations) of the inoculum per gram of tissue that was cultured from the intestinal wall.
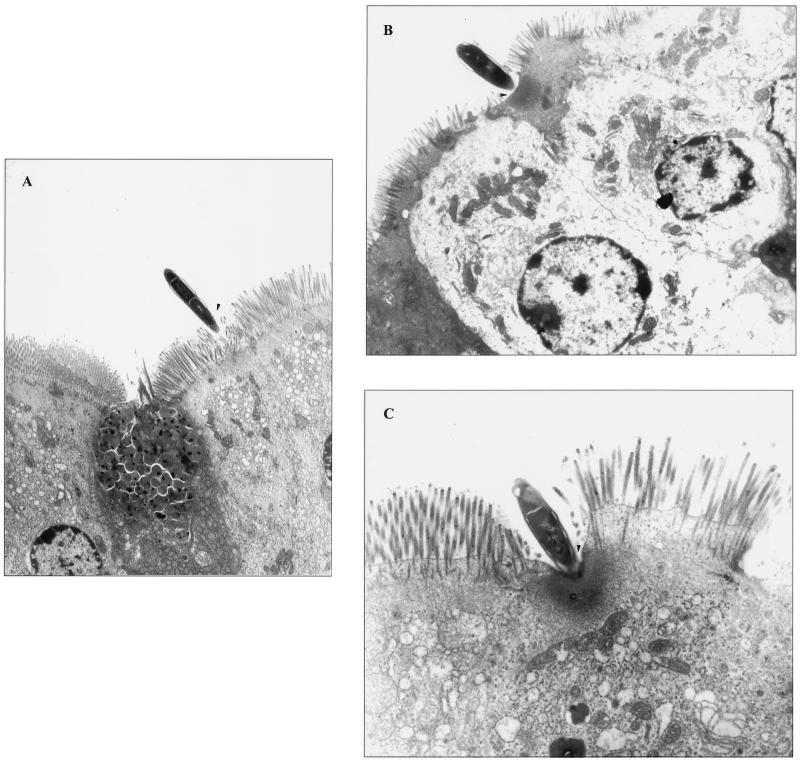
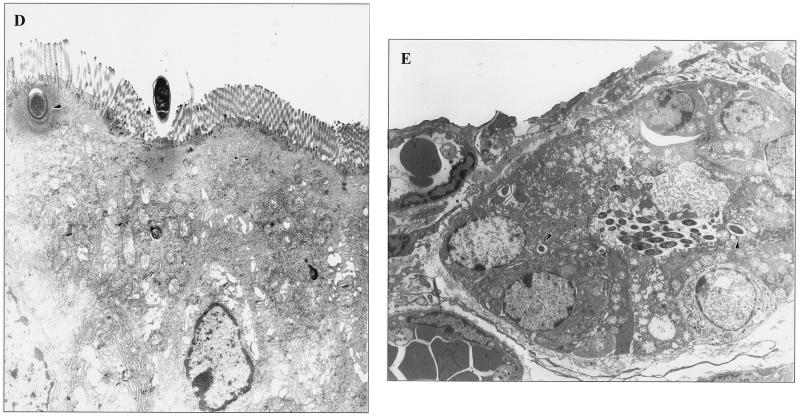
Electron microscopic analysis indicated that M. avium interacts with enterocytes, and only rarely was M. avium observed invading M cells (Fig. 1A). It was also observed that, in the great majority of the enterocytes (>80%), M. avium binding was associated with effacement of the intestinal mucosa (Fig. 1B and C). Once within the intestinal mucosal cells, M. avium was always observed to reside within intracytoplasmic vacuoles (Fig. 1D). Figure 1E shows that M. avium can also invade the intestinal mucosa in the crypts (and not only the villi), and in this case, the bacteria are encountered intracellularly within vacuoles (Fig. 1E).
FIG. 1.
Binding and invasion of the mouse intestinal mucosa by M. avium. (A) Interaction with an enterocyte 1 h after administration (arrowhead); (B and C) effacement of the mucosa (arrowhead) and alteration of actin-based microvilli (C); (D) M. avium seen inside vacuoles (arrowhead) once within the cell 2 h following administration; (E) M. avium entering the mucosa in an intestinal crypt. Bacteria were observed intracellularly 2 h after administration (arrowheads).
Preferential site of invasion.
Previous work demonstrated that, following ingestion, M. avium organisms preferentially invaded the intestinal mucosa of the terminal ileum (2). To evaluate the accuracy of the observation obtained by electron microscopy suggesting that M. avium enters the intestinal mucosa by interacting primarily with enterocytes, C57BL/6J black mice were given 108 bacteria orally (M. avium strain 104) and the terminal ileum was harvested after 1, 4, 24, and 48 h. The intestinal segment was then cut into regions with Peyer's patches and regions without Peyer's patches. Quantitation of the bacteria in the intestinal segments showed that at all time points the number of bacteria in non-Peyer's patch regions was approximately 100-fold greater than that in Peyer's patch segments. Also, the CFU data for Peyer's patch regions infected with M. avium are represented by only one or two mice out of eight per group. No mycobacteria were detected in the other M. avium-infected mice. Of note was the observation that, after an initial peak in the number of bacteria associated with the intestinal tract at 1 h postingestion, the number of organisms remained constant up to 48 h (Table 2).
TABLE 2.
Interaction between M. avium and the intestinal mucosa in the terminal ileum: Peyer's patch regions versus non-Peyer's patch regions
| Time (h) after oral infection | CFU/g of tissuea
|
|
|---|---|---|
| Peyer's patch region | Non-Peyer's patch region | |
| 0 | 0b | 0b |
| 1 | (4.5 ± 0.3) × 102d | (1.1 ± 0.4) × 104bf |
| 4 | (1.0 ± 0.1) × 101d | (1.5 ± 0.3) × 103cf |
| 24 | (2.7 ± 0.4) × 101e | (3.2 ± 0.3) × 103cf |
| 48 | (1.8 ± 0.3) × 101e | (2.1 ± 0.6) × 103cf |
Four mice were used per experimental group and per experiment (two similar experiments were performed). The results represent numbers of bacteria per four segments of intestine per mouse in eight mice.
Eight out of eight mice.
Seven out of eight mice. Bacteria were not detected in one mouse.
Two out of eight mice. In the other six mice, bacteria were not detected.
One out of eight mice. No bacteria were detected in the other seven mice.
P < 0.05 compared with the number of bacteria in Peyer's patches.
Infection of B-cell-deficient mice.
Recent work has indicated that B-cell-deficient mice do not contain Peyer's patches (11). To examine whether the absence of Peyer's patches and M cells would influence the uptake of M. avium by the intestinal mucosa and consequently the number of organisms in the spleen, C57BL/6J WT and C57BL/6J B-cell-deficient mice were infected orally, and at different time points the bacteria in the terminal ileum and spleen were quantified. Infection with S. enterica serovar Typhimurium was used as the control for invasion by M cells. The results showed that the numbers of M. avium organisms per gram of tissue were approximately the same in both C57BL/6J WT and B-cell-deficient mice, indicating that the absence of M cells has no impact on the ability of the bacterium to translocate (Table 3). In contrast, when S. enterica serovar Typhimurium was used, the absence of Peyer's patches resulted in a significant decrease in the number of bacteria invading the intestinal mucosa (Table 4).
TABLE 3.
Numbers of M. avium bacteria in the spleen and terminal ileum of C57BL/6 WT and B-cell-deficient mice
| C57BL/6Ja | Time point | CFU/g of tissueb
|
|
|---|---|---|---|
| Spleen | Terminal ileum | ||
| WT | 2 days | (1.5 ± 0.1) × 103 | (1.9 ± 0.3) × 103 |
| WT | 1 wk | (4.1 ± 1.1) × 104 | (4.4 ± 0.3) × 103 |
| WT | 3 wk | (1.3 ± 0.2) × 105 | (9.0 ± 0.2) × 104 |
| B-cell KO | 2 days | (6.0 ± 0.8) × 103 | (9.7 ± 0.2) × 102 |
| B-cell KO | 1 wk | (4.7 ± 0.2) × 104 | (3.5 ± 0.3) × 103 |
| B-cell KO | 3 wk | (7.8 ± 0.2) × 104 | (8.1 ± 0.6) × 104 |
Seven mice were used per time point, in each of two experiments. B-cell KO, B-cell-deficient mice.
The numbers represent means ± standard deviations. P < 0.05 for all comparisons.
TABLE 4.
Numbers of Salmonella bacteria in the spleen and terminal ileum of C57BL/6J WT and B-cell-deficient mice
| C57BL/6J micea | Time point (h) | CFU/g of tissueb
|
|
|---|---|---|---|
| Spleen | Terminal ileum | ||
| WT | 4 | (3.2 ± 0.4) × 104c | (5.3 ± 0.6) × 105c |
| WT | 24 | (4.9 ± 0.3) × 103c | (6.3 ± 0.4) × 101 |
| B-cell KO | 4 | Undetectable | (4.2 ± 0.3) × 101 |
| B-cell KO | 24 | (1.4 ± 0.2) × 101 | (2.9 ± 0.1) × 101 |
Five mice were used per time point, per experiment. Two experiments were performed. B-cell KO, B-cell-deficient mice.
The numbers represent means ± standard deviations.
P < 0.05 for the comparison of the number of bacteria between WT mice and B-cell-deficient mice.
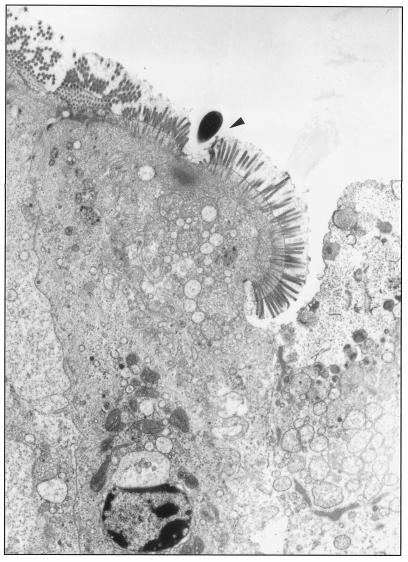
Figure 2 shows a transmission electron micrograph of the intestine of a C57BL/6J B-cell-deficient mouse with M. avium at the host cell surface causing effacement.
FIG. 2.
Interaction between M. avium and enterocytes in a C57BL/6 B-cell-deficient mouse. The bacterium (arrowhead) is observed interacting with an enterocyte in the villi 2 h after administration.
DISCUSSION
We have presented evidence using three different approaches (electron microscopy, culture of specific sites in the intestine that do or do not contain Peyer's patches, and oral challenge of B-cell-deficient mice) that M. avium, in contrast to S. enterica serovar Typhimurium, invades the intestinal mucosa by targeting enterocytes and not M cells. This observation contrasts with data obtained with other mycobacteria. For example, M. bovis BCG has been shown elsewhere to interact with Peyer's patch tissue following oral infection (9). In addition, M. paratuberculosis invasion of the intestinal tract has been associated with M cells (24). More recently, M. tuberculosis was shown to translocate the bronchial mucosa by entering M cells, although this observation could not be extrapolated in regard to possible intestinal invasion mechanisms of M. tuberculosis (36). These characteristics of M. bovis BCG might be associated with the inability to cause infection through the intestinal route, since this organism would be transported to submucosal macrophages following M-cell translocation and very likely eliminated or suppressed. It is certainly an important question whether virulent M. bovis uses the same path to cross the intestinal barrier in cattle.
Enteropathogens interact with the intestinal mucosa in many ways, but M cells have been shown elsewhere to be the target for Yersinia pseudotuberculosis (although it also invades enterocytes), S. enterica serovar Typhimurium, and Shigella flexneri (8, 10, 19, 27). The fact that M. avium invades the intestinal mucosa primarily through enterocytes may have evolutionary implications and is likely to be important for the pathogenesis of the infection. One of the evolutionary aspects to be considered is why M. avium, but not Mycobacterium intracellulare, is efficient in crossing the intestinal mucosal barrier (N. Hsu, J. R. Goodman, L. S. Young, and L. E. Bermudez, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. B25, p. 26, 1996). We are currently attempting to characterize genomic differences between these two organisms to better understand the difference in pathogenesis.
Another important aspect is the question of virulence. One hypothesis is that M. avium, which appears to have less intrinsic virulence than M. tuberculosis, evolved to follow an entry path that would delay the exposure of the bacterium to the immune system of the host. All the evidence so far agrees with this hypothesis. For example, a previous study by our laboratory (20) has shown that oral-gastrointestinal infection by M. avium in mice is not accompanied by the inflammatory response until approximately 1 week after infection. This finding suggests that the bacterium may initially hide from the immune system of the host within the epithelial cell. In addition, we showed that infection of intestinal epithelial cells in vitro with M. avium does not trigger chemokine production and that M. avium infection suppresses Salmonella-triggered interleukin-8 production by epithelial cells (33). A similar observation was made using a central nervous system model of M. avium infection in mice, in which invasion of the brain parenchyma is not accompanied by an inflammatory response (37) and the mice with M. avium infection in the brain can live for long periods without any apparent symptoms (37). In line with this hypothesis, after 2 to 3 days inside intestinal epithelial cells, M. avium is capable of expressing an invasive phenotype that enhances the ability of the bacterium to enter macrophages and survive intracellularly (31).
M. avium entry into the intestinal mucosa was observed to be associated with effacement. Several other organisms, such as enteropathogenic Escherichia coli (EPEC), enterohemorrhagic E. coli, Citrobacter rodentium, Hafnia alvei, and Helicobacter pylori, have been shown previously to induce attachment-effacement of the epithelial cell upon contact (4, 23, 35). EPEC does not enter the host cell (26), and the fate of H. pylori is still arguable (13). Effacement triggered by EPEC is dependent on the presence of EspA, EspB, and EspD, and mutation in each of those factors prevents adhering-effacing lesion formation (25). Whether M. avium-induced effacement is needed to uncover the epithelial cell membrane and expose the cell membrane receptor(s) is a possibility currently being investigated.
In the process of invasion, alteration of the actin was observed, confirming previous in vitro data (3, 22, 29). The bacteria were always observed to be inside cytoplasmic vacuoles, supporting findings in different model systems (31, 32).
Although not much is currently known about the M. avium adhesins, a recent study by Labo and colleagues that sequenced a large region of the M. avium genome has identified two genes (invA and invB) with homology to the p60 invasion protein of L. monocytogenes (21). Furthermore, work by Hess and colleagues suggests that the p60-dependent entry of Listeria into the mucosa occurs through the enterocyte, which would support a role for the p60 homolog in the binding of M. avium to the intestinal mucosa (15). Another putative adhesin, a fibronectin attaching protein, has been characterized for M. avium and other mycobacteria (34). This adhesin binds integrin and is expected to connect the bacterium to the β1 integrin receptor. Because β1 integrin is present only on M cells in the intestinal tract, this mechanism should not be involved in the invasion of enterocytes.
More recently, we have identified several genes that appear to participate in intestinal epithelial cell invasion, though future confirmation in an animal model is required (E. Miltner, A. Parker, F. J. Sangari, and L. E. Bermudez, Abstr. 100th Gen. Meet. Am. Soc. Microbiol., abstr. U-29, p. 648, 2000).
In conclusion, we have shown that M. avium invades the intestinal mucosa in vivo by using enterocytes as target cells, a characteristic that might be associated with the ability to cause disease.
ACKNOWLEDGMENTS
We thank Karen Allen for preparing the manuscript and Dirk Wagner and Martin Wu for help with the art montage. We also thank Stanley Falkow for very fruitful discussions; Robert Owen for his help with the work with M cells; and Dirk Wagner, Jeffery McGarvey, and Lowell S. Young for critically reviewing the manuscript.
This work was supported by grant AI43199 from the National Institutes of Health and grant R99-CHSF-091 from the U.C. Task Force on AIDS.
REFERENCES
- 1.Bermudez L E, Petrofsky M, Goodman J. Exposure to low oxygen tension and increased osmolarity enhance the ability of Mycobacterium avium to enter intestinal epithelial (HT-29) cells. Infect Immun. 1997;65:3768–3773. doi: 10.1128/iai.65.9.3768-3773.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bermudez L E, Petrofsky M, Kolonoski P, Young L S. An animal model of Mycobacterium avium complex disseminated infection after colonization of the intestinal tract. J Infect Dis. 1992;165:75–79. doi: 10.1093/infdis/165.1.75. [DOI] [PubMed] [Google Scholar]
- 3.Bermudez L E, Young L S. Factors affecting invasion of HT-29 and HEp-2 epithelial cells by organisms of the Mycobacterium avium complex. Infect Immun. 1994;62:2021–2026. doi: 10.1128/iai.62.5.2021-2026.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celli J, Deng W, Finlay B B. Enteropathogenic Escherichia coli (EPEC) attachment to epithelial cells: exploiting the host cell skeleton from the outside. Cell Microbiol. 2000;2:1–9. doi: 10.1046/j.1462-5822.2000.00033.x. [DOI] [PubMed] [Google Scholar]
- 5.Clark A M, Jepson M A, Simmons N L, Hirst B H. Preferential interaction of Salmonella typhimurium with mouse Peyer's patch M cells. Res Microbiol. 1994;145:543–552. doi: 10.1016/0923-2508(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 6.Damsker B, Bottone E J. Mycobacterium avium-Mycobacterium intracellulare from the intestinal tracts of patients with the acquired immunodeficiency syndrome: concepts regarding acquisition and pathogenesis. J Infect Dis. 1985;151:179–181. doi: 10.1093/infdis/151.1.179. [DOI] [PubMed] [Google Scholar]
- 7.Falkinham J O., III Epidemiology of infection by nontuberculous mycobacteria. Clin Microbiol Rev. 1996;9:177–215. doi: 10.1128/cmr.9.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujimora Y. Functional morphology. Gastroenterol Jpn. 1986;21:325–330. [PubMed] [Google Scholar]
- 10.Fujimora Y, Kihara T, Mine H. Membranous cells as a portal of Yersinia pseudotuberculosis entry into rabbit ileum. J Clin Electron Microsc. 1992;25:34–45. [Google Scholar]
- 11.Golovkina T V, Shlomchik M, Hannum L, Chervonsky A. Organogenic role of B lymphocytes in mucosal immunity. Science. 1999;286:1965–1968. doi: 10.1126/science.286.5446.1965. [DOI] [PubMed] [Google Scholar]
- 12.Gray J R, Rabeneck L. Atypical mycobacterial infection of the gastrointestinal tract in AIDS patients. Am J Gastroenterol. 1989;84:1521–1524. [PubMed] [Google Scholar]
- 13.Guruge J L, Falk P G, Lorenz R G, Dans M, Wirth H P, Blaser M J, Berg D E, Gordon J I. Epithelial attachment alters the outcome of Helicobacter pylori infection. Proc Natl Acad Sci USA. 1998;95:3925–3930. doi: 10.1073/pnas.95.7.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanski C, Kutschka U, Schmoranzer H P, Naumann M, Stallmach A, Hahn H, Menge H, Riecken E O. Immunohistochemical and electron microscopic study of interaction of Yersinia enterocolitica serotype O8 with intestinal mucosa during experimental enteritis. Infect Immun. 1989;57:673–678. doi: 10.1128/iai.57.3.673-678.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hess J, Dreher A, Gentschev I, Goebel W, Ladel C, Miko D, Kaufmann S H. Protein p60 participates in intestinal host invasion by Listeria monocytogenes. Zentbl Bakteriol. 1996;284:263–272. doi: 10.1016/s0934-8840(96)80102-2. [DOI] [PubMed] [Google Scholar]
- 16.Inderlied C B, Kemper C A, Bermudez L E. The Mycobacterium avium complex. Clin Microbiol Rev. 1993;6:266–310. doi: 10.1128/cmr.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobson A M, Hopewell P C, Yajko D M, Hadley W K, Lazarus E, Mohanty P K, Modin G W, Feigal D W, Cusick P S, Sande M A. Natural history of disseminated Mycobacterium avium complex infection in AIDS. J Infect Dis. 1991;164:994–998. doi: 10.1093/infdis/164.5.994. [DOI] [PubMed] [Google Scholar]
- 18.Jones B, Pascopella L, Falkow S. Entry of microbes into the host: using M cells to break the mucosal barrier. Curr Opin Immunol. 1995;7:474–478. doi: 10.1016/0952-7915(95)80091-3. [DOI] [PubMed] [Google Scholar]
- 19.Jones B D, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J Exp Med. 1994;180:15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S Y, Goodman J R, Petrofsky M, Bermudez L E. Mycobacterium avium infection of gut mucosa in mice associated with late inflammatory response and intestinal cell necrosis. J Med Microbiol. 1998;47:725–731. doi: 10.1099/00222615-47-8-725. [DOI] [PubMed] [Google Scholar]
- 21.Labo M, Gusberti L, Rossi E D, Speziale P, Riccardi G. Determination of a 15437 bp nucleotide sequence around the inhA gene of Mycobacterium avium and similarity analysis of the products of putative ORFs. Microbiology. 1998;144:807–814. doi: 10.1099/00221287-144-3-807. [DOI] [PubMed] [Google Scholar]
- 22.Mapother M E, Songer J G. In vitro interaction of Mycobacterium avium with intestinal epithelial cells. Infect Immun. 1984;45:67–73. doi: 10.1128/iai.45.1.67-73.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDaniel T K, Kaper J B. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol Microbiol. 1997;23:399–407. doi: 10.1046/j.1365-2958.1997.2311591.x. [DOI] [PubMed] [Google Scholar]
- 24.Momotani E, Whipple D L, Thiermann A B, Cheville N F. Role of M cells and macrophages in the entrance of Mycobacterium paratuberculosis into domes of ileal Peyer's patches in calves. Vet Pathol. 1988;25:131–137. doi: 10.1177/030098588802500205. [DOI] [PubMed] [Google Scholar]
- 25.Nataro J P, Kaper J B. Diarrheagenic Escherichia. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owen R L, Nemanic P C, Stevens D P. Ultrastructural observations on giardiasis in a murine model. I. Intestinal distribution, attachment, and relationship to the immune system of Giardia muris. Gastroenterology. 1979;76:757–769. [PubMed] [Google Scholar]
- 27.Perdomo O J, Cavaillon J M, Huerre M, Ohayon H, Gounon P, Sansonetti P J. Acute inflammation causes epithelial invasion and mucosal destruction in experimental shigellosis. J Exp Med. 1994;180:1307–1319. doi: 10.1084/jem.180.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Racz P, Tenner K, Mero E. Experimental Listeria enteritis. I. An electron microscopic study of the epithelial phase in experimental listeria infection. Lab Investig. 1972;26:694–700. [PubMed] [Google Scholar]
- 29.Reddy V M, Kumar B. Interaction of Mycobacterium avium complex with human respiratory epithelial cells. J Infect Dis. 2000;181:1189–1193. doi: 10.1086/315327. [DOI] [PubMed] [Google Scholar]
- 30.Roth R I, Owen R L, Keren D F, Volberding P A. Intestinal infection with Mycobacterium avium in acquired immune deficiency syndrome (AIDS). Histological and clinical comparison with Whipple's disease. Dig Dis Sci. 1985;30:497–504. doi: 10.1007/BF01318186. [DOI] [PubMed] [Google Scholar]
- 31.Sangari F, Goodman J, Bermudez L E. Mycobacterium avium enters intestinal epithelial cells through the apical membrane but not by the basolateral surface, activates small GTPase Rho and once within the epithelial cells expresses an invasive phenotype. Cell Microbiol. 2000;2:561–568. doi: 10.1046/j.1462-5822.2000.00080.x. [DOI] [PubMed] [Google Scholar]
- 32.Sangari F J, Goodman J R, Bermudez L E. Ultrastructural study of Mycobacterium avium infection of HT-29 human intestinal epithelial cells. J Med Microbiol. 2000;49:139–147. doi: 10.1099/0022-1317-49-2-139. [DOI] [PubMed] [Google Scholar]
- 33.Sangari F J, Petrofsky M, Bermudez L E. Mycobacterium avium infection of epithelial cells results in inhibition or delay in the release of interleukin-8 and RANTES. Infect Immun. 1999;67:5069–5075. doi: 10.1128/iai.67.10.5069-5075.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schorey J S, Holsti M A, Ratliff T L, Allen P M, Brown E J. Characterization of the fibronectin-attachment protein of Mycobacterium avium reveals a fibronectin-binding motif conserved among mycobacteria. Mol Microbiol. 1996;21:321–329. doi: 10.1046/j.1365-2958.1996.6381353.x. [DOI] [PubMed] [Google Scholar]
- 35.Segal E D, Falkow S, Tompkins L S. Helicobacter pylori attachment to gastric cells induces cytoskeletal rearrangements and tyrosine phosphorylation of host cell proteins. Proc Natl Acad Sci USA. 1996;93:1259–1264. doi: 10.1073/pnas.93.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teitelbaum R, Schubert W, Gunther L, Kress Y, Macaluso F, Pollard J W, McMurray D N, Bloom B R. The M cell as a portal of entry to the lung for the bacterial pathogen Mycobacterium tuberculosis. Immunity. 1999;10:641–650. doi: 10.1016/s1074-7613(00)80063-1. [DOI] [PubMed] [Google Scholar]
- 37.Wu H S, Kolonoski P, Chang Y Y, Bermudez L E. Invasion of the brain and chronic central nervous system infection after systemic Mycobacterium avium complex infection in mice. Infect Immun. 2000;68:2979–2984. doi: 10.1128/iai.68.5.2979-2984.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]