Abstract
Organ damage is a key determinant of poor long‐term prognosis and early death in patients with systemic lupus erythematosus (SLE). Prevention of damage is a key treatment goal of the 2019 update of the European Alliance of Associations for Rheumatology (EULAR) recommendations for SLE management. Belimumab is a monoclonal antibody that inhibits B lymphocyte stimulator (BLyS) and is the only therapy approved for both SLE and lupus nephritis. Here, we review the clinical trial and real‐world data on the effects of belimumab on organ damage in adult patients with SLE. Across 4 phase III studies, belimumab in combination with background SLE therapy demonstrated consistent reductions in key drivers of organ damage including disease activity, risk of new severe flares, and glucocorticoid exposure compared to background therapy alone. Long‐term belimumab use in SLE also reduced organ damage progression measured by the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index, as reported in open‐label extension studies, and propensity score–matched comparative analyses to background therapy alone. Results from a clinical trial showed that in patients with active lupus nephritis, belimumab treatment improved renal response, reduced the risk of renal‐related events, and impacted features related to kidney damage progression compared to background therapy alone. The decrease of organ damage accumulation observed with belimumab treatment in SLE, including lupus nephritis, suggest a disease‐modifying effect.
Introduction
Within 10 years, over half of patients diagnosed as having systemic lupus erythematosus (SLE) develop organ damage (1), of which 30–50% is reported in the first 5 years (2, 3). Damage accumulates over time, affecting multiple organ systems (1, 4, 5), resulting in significant morbidity (6, 7, 8) and mortality (1). Cardiovascular, neuropsychiatric, musculoskeletal, and renal systems are most frequently affected, with steady accumulation over 10–15 years (5, 9, 10). Other organs commonly affected include pulmonary, ocular, skin, and the peripheral vascular system. Damage in the gastrointestinal system, diabetes mellitus, gonadal failure, and malignancies are less frequent (5, 9, 10). Organ damage impacts health‐related quality of life (HRQoL) (11), and 10‐year cumulative health care costs are up to 9‐fold higher in patients with organ damage than in those with no damage (4, 12).
Key drivers of organ damage
Factors associated with organ damage include Black African ancestry or Hispanic race (13, 14), older age, male sex, preexisting organ damage, exposure to glucocorticoids and/or other SLE therapies, severe flares, persistent disease activity, dyslipidemia, hypertension, and active/past nephritis (4, 13, 15). Up to 80% of organ damage is attributable to glucocorticoid use (16), and a clear dose‐dependent relationship has been observed (17).
Assessing organ damage progression
Prevention of damage is a key treatment goal of the European Alliance of Associations for Rheumatology (EULAR) recommendations for SLE and lupus nephritis management (18, 19), highlighting the importance of evaluating the effects of treatment, not only on disease activity, but also directly on the risk of organ damage accrual (14). The Systemic Lupus International Collaborating Clinics (SLICC)/American College of Rheumatology (ACR) Damage Index (SDI) is a validated measure that assesses cumulative damage across multiple organs, regardless of cause (Supplementary Table 1, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24901) (13, 14). The SDI defines organ damage as irreversible tissue injury occurring after SLE diagnosis; to distinguish from disease activity, most features must persist for ≥6 months, although some items are scored immediately (e.g., stroke or avascular necrosis) (4, 13, 15). The SDI has shown both reliability (20) and validity (21) in the real world and as an end point in clinical trials (22, 23, 24). The SLICC, Lupus Foundation of America, and ACR are developing a revised SDI to reflect shifts in the concept of damage. It is intended that the revised SDI will primarily be used as a research tool in clinical trials (25).
Impact of belimumab on organ damage in SLE
Abundant data are available on belimumab's efficacy, tolerability, and real‐world effectiveness for SLE, including lupus nephritis (26). We conducted a literature search (Supplementary Material, available at http://onlinelibrary.wiley.com/doi/10.1002/acr.24901) to identify data on the effects of belimumab in adult patients with SLE, focusing on end points relevant to organ damage.
Clinical trials of belimumab
Phase 2 open‐label extension study
In an open‐label extension of the phase II GSK study LBSL02/LBSL99; BEL112626; ClinicalTrials.gov identifier: NCT00583362 [27]), patients with SLE received belimumab for ≤12 years, with a median duration of exposure of 3,334 days (range 260–4,332 days) and total belimumab exposure of 2,294 patient‐years. SDI data were not collected prospectively; however, improvements in drivers of organ damage, including decreased disease activity (Supplementary Figure 1, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24901) and reductions in flares and glucocorticoid exposure, were observed throughout the study. Despite the limitations of an open‐label study, these findings suggested potential benefits of belimumab for organ damage progression.
Phase III randomized controlled trials (RCTs)
The global phase III BLISS‐52 (n = 867; GSK study BEL110752; ClinicalTrials.gov identifier: NCT00424476 [28]), BLISS‐76 (n = 819; GSK study BEL110751; ClinicalTrials.gov identifier: NCT00410384 [29]), BLISS‐NEA (n = 677; GSK study BEL113750; ClinicalTrials.gov identifier: NCT01345253 [30]), and BLISS‐SC (n = 836; GSK study BEL112341; ClinicalTrials.gov identifier: NCT01484496 [31]) RCTs investigated the effects of intravenous (IV) or subcutaneous (SC) belimumab added to background therapy in adults with SLE. Across all 4 studies, statistically significant reductions in Systemic Lupus Erythematosus Responder Index 4 (SRI‐4) scores were observed with belimumab versus background therapy alone (Figure 1). This was accompanied by decreased risks of severe flares in all but the BLISS‐76 study. There were also trends toward reduced glucocorticoid use, reaching statistical significance in the BLISS‐NEA trial. The glucocorticoid‐sparing properties of belimumab were confirmed in a post hoc pooled analysis combining BLISS‐52 and BLISS‐76 data, which found that belimumab treatment was associated with fewer glucocorticoid dose increases and more dose decreases versus background therapy alone (32).
Figure 1.
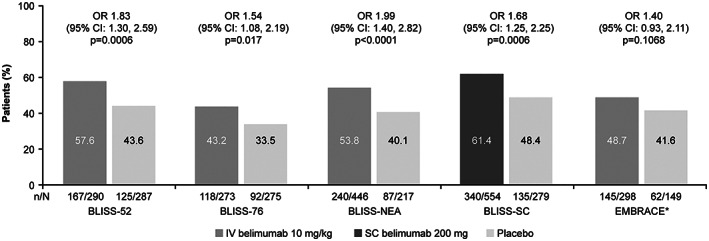
Systemic Lupus Erythematosus Responder Index 4 (SRI‐4) response at 52 weeks in the belimumab systemic lupus erythematosus (SLE) randomized controlled trials (28, 29, 30, 31, 33, 44, 45, 46, 47). The EMBRACE study (asterisk) used the SRI–SLE Disease Activity Index 2000 response, which has modified proteinuria scoring. 95% CI = 95% confidence interval; IV = intravenous; OR = odds ratio; SC = subcutaneous.
The EMBRACE phase III/IV study (GSK study BEL115471; ClinicalTrials.gov identifier: NCT1632241 [33]), a post‐approval commitment RCT in patients of Black African ancestry with SLE (n = 503), demonstrated similar trends in disease activity with belimumab versus placebo as the 4 above‐mentioned trials (Figure 1). Although the primary end point of a modified SRI‐4 score was not met, significant improvements with belimumab versus placebo were observed in patients with high disease activity.
Phase III long‐term, open‐label extension studies
Several long‐term open‐label extension studies of the belimumab RCTs have been conducted, which included evaluation of organ damage progression. All participants received belimumab added to background therapy irrespective of the assigned treatment in the 52‐week, double‐blind phase of the original studies.
The multicenter, long‐term extension studies BEL112233 (ClinicalTrials.gov identifier: NCT00724867 [22,34]) and BEL112234 (ClinicalTrials.gov identifier: NCT00712933 [23,35]), which were pooled in study GSK201223 (34, 36, 37), enabled patients from the BLISS‐52 and BLISS‐76 studies to continue receiving open‐label IV belimumab plus background therapy over 8 years of follow‐up. Organ damage was evaluated every 48 weeks using the SDI. At year 7, 69.8% (88 of 126) and 86.9% (185 of 213) of patients receiving long‐term IV belimumab treatment had no changes (index of <1) in SDI scores (mean ± SD change of 0.4 ± 0.68 and 0.2 ± 0.45 from baseline SDI scores of 1.2 ± 1.51 and 0.6 ± 1.02) (Supplementary Figure 2, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24901) (22, 23, 34, 35). Similarly, among patients with high disease activity (anti–double‐stranded DNA positivity [≥30 IU/ml] and either low C3 [<0.9 gm/liter] or C4 [<0.16 gm/liter] levels) at baseline receiving long‐term treatment with IV belimumab, 75.5% (n = 37 of 49) and 86.4% (n = 89 of 103) had no changes in SDI scores by year 7 (mean ± SD change 0.3 ± 0.54 and 0.2 ± 0.41 from the baseline index scores of 0.7 ± 1.26 and 0.6 ± 0.95) (Supplementary Figure 2) (22, 23, 34, 35). Values were similar for year 8, but with fewer patients (22, 23, 34, 35).
GSK201223, a pooled interim analysis of the BEL112233 and BEL112234 studies from 5 to 6 years follow‐up, allowed insight across a wider data sample. At years 5–6, 85.1% of patients (n = 343 of 403) receiving belimumab had no change in SDI score compared to baseline (Figure 2A and Supplementary Table 2, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24901) (34). Patients without organ damage at baseline were more likely to remain free of organ damage progression than those with organ damage at baseline (Figure 2B) (36). The effect of IV belimumab on organ damage was similar among patients at years 5–6, irrespective of differences in baseline disease activity, proteinuria status (Figures 2C and D), background medication or presence of high disease activity at baseline (Supplementary Figures 3A and B, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24901) (34, 36).
Figure 2.
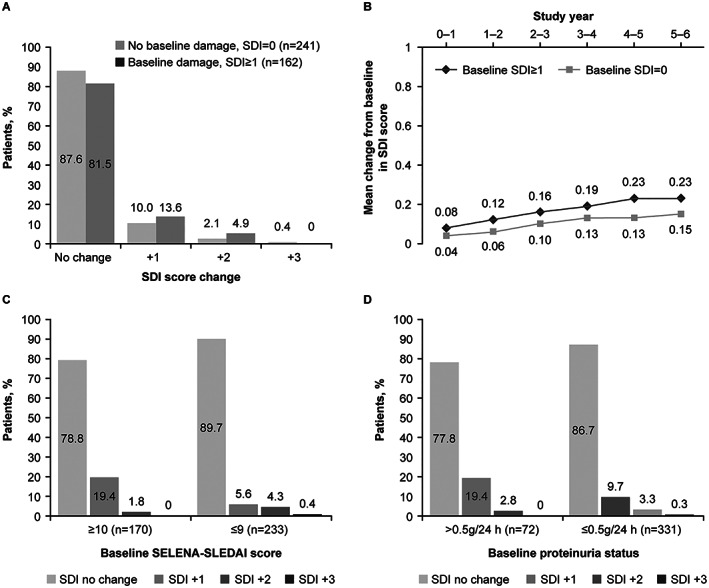
Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI) response in patients receiving intravenous belimumab therapy from a pooled interim analysis of BLISS‐52 and BLISS‐76 long‐term extension studies (BEL112233 and BEL112234), overall at study years 5–6 (A), and stratified by baseline SDI score (B), disease activity at study years 5–6 (C), and proteinuria status at study years 5–6 (D) (34, 36, 37). SELENA–SLEDAI = Safety of Estrogens in Lupus Erythematosus National Assessment–SLE Disease Activity Index. Panel (B) reproduced, with permission, from ref. 36.
Study BEL114333 (ClinicalTrials.gov identifier: NCT01597622) was carried out in Japanese and South Korean populations (24) and is a 7‐year open‐label study of IV belimumab in 142 Japanese and South Korean patients with SLE, recruited from BLISS‐NEA (BEL113750) and BLISS‐SC (BEL112341; patients in Japan only). Over 7 years, there was little change in SDI score from baseline; ≤4 patients from BEL113750 and ≤2 patients from BEL112341 had organ damage that had worsened (change of >0) compared to baseline (Supplementary Table 2).
Real‐world evidence of belimumab
Effect of belimumab versus background therapy on organ damage outcomes in SLE (study 206347)
To overcome some of the limitations of clinical and open‐label extension studies, propensity score matching (described in the Supplementary Materials, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24901) was used to evaluate the impact of belimumab versus background therapy on organ damage. Study 206347 was a post hoc 5‐year longitudinal analysis that compared organ damage outcomes in a cohort of patients receiving belimumab therapy in the BEL112233 study to a separate cohort of patients who had SLE treated with background therapy only (The Toronto Lupus Cohort [TLC]), using propensity score matching (Supplementary Figure 4, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24901). Of 567 patients (belimumab cohort [n = 195] and TLC [n = 372]), 99 from each cohort were propensity score–matched at a ratio of 1:1 in this analysis. Over the 5‐year period, patients receiving belimumab plus background therapy had a smaller change in SDI score than those receiving background therapy only, with an SDI score of –0.434 (95% confidence interval [95% CI] −0.667, −0.201; P < 0.001). Of the patients with an SDI score increase of ≥1, those treated with belimumab had a 5‐fold lower likelihood of experiencing a ≥2‐point increase in SDI score (6.1% [2 of 33]) than those treated with background therapy only (30.6% [22 of 72]; P = 0.006). Belimumab treatment was also associated with a 61% reduction in the risk of progressing to a higher SDI score over any given year of follow‐up compared to background therapy (Figure 3), with a hazard ratio (HR) of 0.391 (95% CI 0.253, 0.605; P < 0.001) (38).
Figure 3.
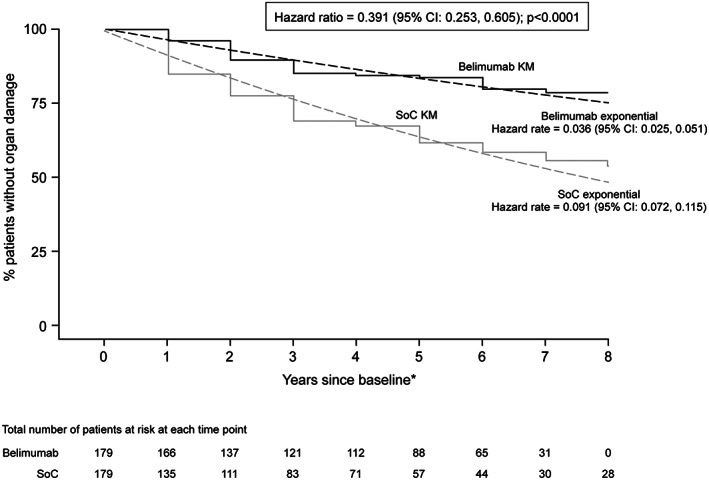
Post hoc longitudinal propensity score matching comparative analysis of time to organ damage progression. Years are 48 weeks in length (asterisk). 95% CI = 95% confidence interval; KM = Kaplan‐Meier curve; SoC = standard of care. Reproduced, with permission, from ref. 38.
Another post hoc longitudinal analysis of 5‐year data from study BEL112234, which enrolled US and non‐US patients (592 subjects from the belimumab cohort and 381 subjects from the TLC study, with 181 subjects selected from each cohort after propensity score matching using the same covariates as study 206347), replicated the above findings, with a difference in 5‐year SDI score change of −0.453 (95% CI −0.646, −0.260; P < 0.001) and a 60% risk reduction of progressing to a higher SDI score over any given year of follow‐up for belimumab plus background therapy versus background therapy alone (HR 0.397 [95% CI 0.275, 0.572]; P < 0.001) (39).
BeRLiSS: a real‐world cohort study of Italy (40)
BeRLiSS was a retrospective study of 466 patients with active SLE who received IV belimumab plus background therapy (median follow‐up of 18 months [range 1–60 months]). SDI score was assessed yearly, and 309 patients had available organ damage data. Across 7,983 person‐months, ~90% of belimumab‐receiving patients remained free of new organ damage events, with 36 new events observed in 29 patients (9.4%) and a rate of 0.54 events per 10 person‐years. Patients without baseline organ damage (SDI score of 0) remained free of damage progression for up to 3 years after belimumab initiation, with mean ± SD SDI scores of 0.02 ± 0.14 (P = 0.083), 0.05 ± 0.28 (P = 0.182), and 0.10 ± 0.38 (P = 0.103) versus baseline for years 1, 2, and 3, respectively. In a multivariate model, following belimumab treatment, achievement of low disease activity for ≥50% of the follow‐up period was associated with significantly less organ damage (odds ratio 0.442 [95% CI 0.199, 0.983]; P = 0.045), while baseline organ damage (SDI score of ≥1) was an independent predictor of further damage accumulation (odds ratio 3.22 [95% CI 1.25, 8.33]; P = 0.016). Patients treated with belimumab who were in remission for ≥25% of follow‐up or had low disease activity for ≥50% of follow‐up had less damage accumulation than those who did not achieve either outcome (40).
Impact of belimumab on organ damage in lupus nephritis
The BLISS‐LN study (GSK study BEL114054; ClinicalTrials.gov identifier: NCT01639339) was a phase III RCT that evaluated the efficacy and safety of IV belimumab versus placebo, both added to background induction and maintenance therapy, on renal outcomes for up to 104 weeks in patients with active lupus nephritis (n = 448). Belimumab reduced the risk of a renal‐related event or death by ~50% compared to placebo (41).
In a post hoc analysis of the BLISS‐LN study, therapy with belimumab reduced the risk of a 30% decline (HR 0.47 [95% CI 0.27, 0.83]) or 40% decline (HR 0.35 [95% CI 0.17, 0.74]) in estimated glomerular filtration rate (eGFR) and also reduced the risk of a lupus nephritis flare from week 24 by 55% (HR 0.45 [95% CI 0.28, 0.72]) compared to placebo (42).
Conclusions
Organ damage was assessed using the SDI in patients with SLE across the 5 phase III RCTs—BLISS‐52 (28), BLISS‐76 (29), BLISS‐NEA (30), BLISS‐SC (31), and EMBRACE (33)—with no difference between treatment groups (Supplementary Table 2, available on the Arthritis Care & Research website at http://onlinelibrary.wiley.com/doi/10.1002/acr.24901). However, clinical trials of 1 year are too short to detect changes in the SDI, and therefore, longer studies are necessary. Nevertheless, as with the LBSL02/LBSL99 study, the BLISS RCTs demonstrated improvements from baseline to week 52 in known drivers of organ damage in patients with SLE. The level of damage progression was lower in the phase III long‐term, open‐label extension studies (studies BEL112233 [22, 34] and BEL112234 [23, 35]) than expected from observational cohorts; this could, in part, be explained by different baseline disease characteristics such as nephritis or central nervous system disease (36).
Limitations of open‐label extension studies include the absence of a control group and patient selection bias. Randomized, double‐blind, placebo‐controlled studies over a sufficiently long period (≥5 years of follow‐up) would be the gold standard for directly assessing the effects of an SLE therapy on organ damage progression via the SDI. However, such studies pose significant ethical challenges (long‐term exposure to placebo treatment) and feasibility challenges (maintaining patient participation enrolled in long‐term trials). Long‐term comparison studies are feasible using propensity score matching, which is a valid and established methodology. Propensity score matching studies showed that use of belimumab was associated with a smaller increase in SDI over 5 years compared to background therapy alone, supporting the beneficial effect of belimumab therapy on organ damage progression in patients with SLE (38, 39).
For assessment of organ damage, eGFR (one component of the BLISS‐LN composite primary end point [primary efficacy renal response]) has been reported as an acceptable surrogate end point for kidney disease progression by European and US regulatory authorities. Specifically, a decline of >30% in eGFR or a reduction in eGFR slope of 0.5–1.0 ml/minute/1.73 m2 over a total of 2–3 years are recommended thresholds for defining chronic kidney disease progression in phase III RCTs of SLE/lupus nephritis (43). The reduction in risk of a renal‐related event and/or death and renal flare, and less decline in eGFR, with belimumab versus background therapy alone in the post hoc analysis of BLISS‐LN study suggests beneficial effects of belimumab on organ damage progression in patients with active lupus nephritis.
Accumulated data from a range of studies demonstrate that belimumab reduces key drivers of organ damage including disease activity, severe flares, and glucocorticoid exposure. Long‐term open‐label extension and propensity score matching analysis studies of patients with SLE treated with belimumab have demonstrated decreased organ damage progression using the SDI. In patients with active lupus nephritis, a decrease in renal‐related events and less decline in eGFR was seen with belimumab treatment versus background therapy alone. These observed impacts of belimumab on organ damage imply a disease‐modifying effect on SLE, including lupus nephritis, suggesting potential benefits of belimumab treatment earlier in the disease course.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication.
ROLE OF THE STUDY SPONSOR
GSK was involved in designing the study; contributed to the collection, analysis, and interpretation of the data; supported the authors in the development of the manuscript; and funded the medical writing assistance provided by Casmira Brazaitis, PhD, of Fishawack Indicia Ltd, part of Fishawack Health. All authors, including those employed by GSK, approved the content of the submitted manuscript and were involved in the decision and to submit the manuscript for publication.
ADDITIONAL DISCLOSURES
Authors Bass, Chauhan, Gilbride, Hammer, Levy, Quasny, and Roth are employees of GSK. Authors Asukai, Fox, and Gonzalez‐Rivera were employed by GSK at the time the study was conducted.
Supporting information
Disclosure Form
Appendix S1 Supporting Information
ACKNOWLEDGMENTS
The authors thank the patients, clinical investigators, and staff for their participation in the clinical trials involving belimumab. The USC Clinical Trials Unit is funded in part through grants UL1‐TR‐001855 and UL1‐TR‐000130 from the National Center for Advancing Translational Science (NCATS) of the US National Institutes of Health. Medical writing support was provided by Casmira Brazaitis, PhD, of Fishawack Indicia Ltd, UK, part of Fishawack Health, and was funded by the University of Southern California Clinical Trials Unit, which is funded in part through NIH grants UL1‐TR‐001855 and UL1‐TR‐000130 from the National Center for Advancing Translational Sciences.
Supported by GSK.
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Facr.24901&file=acr24901‐sup‐0001‐Disclosureform.pdf.
REFERENCES
- 1. Murimi‐Worstell IB, Lin DH, Nab H, Kan HJ, Onasanya O, Tierce JC, et al. Association between organ damage and mortality in systemic lupus erythematosus: a systematic review and meta‐analysis. BMJ Open 2020;10:e031850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chambers SA, Allen E, Rahman A, Isenberg D. Damage and mortality in a group of British patients with systemic lupus erythematosus followed up for over 10 years. Rheumatology (Oxford) 2009;48:673–5. [DOI] [PubMed] [Google Scholar]
- 3. Urowitz MB, Gladman DD, Ibanez D, Fortin PR, Bae SC, Gordon C, et al. Evolution of disease burden over five years in a multicenter inception systemic lupus erythematosus cohort. Arthritis Care Res (Hoboken) 2012;64:132–7. [DOI] [PubMed] [Google Scholar]
- 4. Doria A, Gatto M, Zen M, Iaccarino L, Punzi L. Optimizing outcome in SLE: treating‐to‐target and definition of treatment goals. Autoimmun Rev 2014;13:770–7. [DOI] [PubMed] [Google Scholar]
- 5. Gladman DD, Urowitz MB, Rahman P, Ibanez D, Tam LS. Accrual of organ damage over time in patients with systemic lupus erythematosus. J Rheumatol 2003;30:1955–9. [PubMed] [Google Scholar]
- 6. Fialho SC, Bonfa E, Vitule LF, D'Amico E, Caparbo V, Gualandro S, et al. Disease activity as a major risk factor for osteonecrosis in early systemic lupus erythematosus. Lupus 2007;16:239–44. [DOI] [PubMed] [Google Scholar]
- 7. Hanly JG, Urowitz MB, Gordon C, Bae SC, Romero‐Diaz J, Sanchez‐Guerrero J, et al. Neuropsychiatric events in systemic lupus erythematosus: a longitudinal analysis of outcomes in an international inception cohort using a multistate model approach. Ann Rheum Dis 2020;79:356–62. [DOI] [PubMed] [Google Scholar]
- 8. Tselios K, Urowitz MB. Cardiovascular and pulmonary manifestations of systemic lupus erythematosus. Curr Rheumatol Rev 2017;13:206–18. [DOI] [PubMed] [Google Scholar]
- 9. Cassano G, Roverano S, Paira S, Bellomio V, Lucero E, Berman A, et al. Accrual of organ damage over time in Argentine patients with systemic lupus erythematosus: a multi‐centre study. Clin Rheumatol 2007;26:2017–22. [DOI] [PubMed] [Google Scholar]
- 10. Stoll T, Seifert B, Isenberg DA. SLICC/ACR Damage Index is valid, and renal and pulmonary organ scores are predictors of severe outcome in patients with systemic lupus erythematosus. Br J Rheumatol 1996;35:248–54. [DOI] [PubMed] [Google Scholar]
- 11. Bjork M, Dahlstrom O, Wettero J, Sjowall C. Quality of life and acquired organ damage are intimately related to activity limitations in patients with systemic lupus erythematosus. BMC Musculoskelet Disord 2015;16:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barber MR, Hanly JG, Su L, Urowitz MB, St Pierre Y, Romero‐Diaz J, et al. Economic evaluation of damage accrual in an international systemic lupus erythematosus inception cohort using a multistate model approach. Arthritis Care Res (Hoboken) 2020;72:1800–8. [DOI] [PubMed] [Google Scholar]
- 13. Feld J, Isenberg D. Why and how should we measure disease activity and damage in lupus? Presse Med 2014;43:e151–6. [DOI] [PubMed] [Google Scholar]
- 14. Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996;39:363–9. [DOI] [PubMed] [Google Scholar]
- 15. Segura BT, Bernstein BS, McDonnell T, Wincup C, Ripoll VM, Giles, I , et al. Damage accrual and mortality over long‐term follow‐up in 300 patients with systemic lupus erythematosus in a multi‐ethnic British cohort. Rheumatology (Oxford) 2020;59:524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fava A, Petri M. Systemic lupus erythematosus: diagnosis and clinical management. J Autoimmun 2019;96:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Al Sawah S, Zhang X, Zhu B, Magder LS, Foster SA, Iikuni N, et al. Effect of corticosteroid use by dose on the risk of developing organ damage over time in systemic lupus erythematosus‐the Hopkins Lupus Cohort. Lupus Sci Med 2015;2:e000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019;78:736–45. [DOI] [PubMed] [Google Scholar]
- 19. Fanouriakis A, Kostopoulou M, Cheema K, Anders HJ, Aringer M, Bajema I, et al. 2019 update of the joint European League Against Rheumatism and European Renal Association–European Dialysis and Transplant Association (EULAR/ERA–EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis 2020;79:713–23. [DOI] [PubMed] [Google Scholar]
- 20. Gladman DD, Urowitz MB, Goldsmith CH, Fortin P, Ginzler E, Gordon C, et al. The reliability of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index in patients with systemic lupus erythematosus. Arthritis Rheum 1997;40:809–13. [DOI] [PubMed] [Google Scholar]
- 21. Gladman DD, Goldsmith CH, Urowitz MB, Bacon P, Fortin P, Ginzler E, et al. The Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) damage index for systemic lupus erythematosus international comparison. J Rheumatol 2000;27:373–6. [PubMed] [Google Scholar]
- 22. Furie RA, Wallace DJ, Aranow C, Fettiplace J, Wilson B, Mistry P, et al. Long‐term safety and efficacy of belimumab in patients with systemic lupus erythematosus: a continuation of a seventy‐six‐week phase III parent study in the United States. Arthritis Rheumatol 2018;70:868–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van Vollenhoven RF, Navarra SV, Levy RA, Thomas M, Heath A, Lustine T, et al. Long‐term safety and limited organ damage in patients with systemic lupus erythematosus treated with belimumab: a Phase III study extension. Rheumatology (Oxford) 2020;59:281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tanaka Y, Bae SC, Bass D, Curtis P, Chu M, DeRose K, et al. Long‐term open‐label continuation study of the safety and efficacy of belimumab for up to 7 years in patients with systemic lupus erythematosus from Japan and South Korea. RMD Open 2021;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johnson SR, Gladman DD, Brunner HI, Isenberg D, Clarke AE, Barber MR, et al. Evaluating the construct of damage in systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2021. doi: 10.1002/acr.24849. E‐pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26. Levy RA, Gonzalez‐Rivera T, Khamashta M, Fox NL, Jones‐Leone A, Rubin B, et al. 10 years of belimumab experience: what have we learnt? Lupus 2021;0:09612033211028653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wallace DJ, Ginzler EM, Merrill JT, Furie RA, Stohl W, Chatham WW, et al. Safety and efficacy of belimumab plus standard therapy for up to thirteen years in patients with systemic lupus erythematosus. Arthritis Rheumatol 2019;71:1125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Navarra SV, Guzman RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo‐controlled, phase 3 trial. Lancet 2011;377:721–31. [DOI] [PubMed] [Google Scholar]
- 29. Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzova D, et al. A phase III, randomized, placebo‐controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 2011;63:3918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang F, Bae SC, Bass D, Chu M, Egginton S, Gordon D, et al. A pivotal phase III, randomised, placebo‐controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan and South Korea. Ann Rheum Dis 2018;77:355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stohl W, Schwarting A, Okada M, Scheinberg M, Doria A, Hammer AE, et al. Efficacy and safety of subcutaneous belimumab in systemic lupus erythematosus: a fifty‐two‐week randomized, double‐blind, placebo‐controlled study. Arthritis Rheumatol 2017;69:1016–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Van Vollenhoven RF, Petri M, Wallace DJ, Roth DA, Molta CT, Hammer AE, et al. Cumulative corticosteroid dose over fifty‐two weeks in patients with systemic lupus erythematosus: pooled analyses from the phase III belimumab trials. Arthritis Rheumatol 2016;68:2184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ginzler E, Barbosa LS, D'Cruz D, Furie R, Maksimowicz‐McKinnon K, Oates J, et al. Phase III/IV, randomized, fifty‐two–week study of the efficacy and safety of belimumab in patients of Black African ancestry with systemic lupus erythematosus. Arthritis Rheumatol 2022;74:112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. GSK . Clinical study report 112233. 2021. URL: https://www.gsk-studyregister.com/en/trial-details/?id=112233.
- 35. GSK . Clinical study report 112234. 2021. URL: https://www.gsk-studyregister.com/en/trial-details/?id=112234.
- 36. Bruce IN, Urowitz M, van Vollenhoven R, Aranow C, Fettiplace J, Oldham M, et al. Long‐term organ damage accrual and safety in patients with SLE treated with belimumab plus standard of care. Lupus 2016;25:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. GSK . Clinical study report 201223. 2021. URL: https://www.gsk-studyregister.com/en/trial-details/?id=201223.
- 38. Urowitz MB, Ohsfeldt RL, Wielage RC, Kelton KA, Asukai Y, Ramachandran S. Organ damage in patients treated with belimumab versus standard of care: a propensity score‐matched comparative analysis. Ann Rheum Dis 2019;78:372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Urowitz MB, Ohsfeldt RL, Wielage RC, Dever JJ, Zakerifar M, Asukai Y, et al. Comparative analysis of long‐term organ damage in patients with systemic lupus erythematosus using belimumab versus standard therapy: a post hoc longitudinal study. Lupus Sci Med 2020;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gatto M, Saccon F, Zen M, Regola F, Fredi M, Andreoli L, et al. Early disease and low baseline damage as predictors of response to belimumab in patients with systemic lupus erythematosus in a real‐life setting. Arthritis Rheumatol 2020;72:1314–24. [DOI] [PubMed] [Google Scholar]
- 41. Furie R, Rovin BH, Houssiau F, Malvar A, Teng YK, Contreras G, et al. Two‐year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med 2020;383:1117–28. [DOI] [PubMed] [Google Scholar]
- 42. Rovin BH, Furie R, Teng YK, Contreras G, Malvar A, Yu X, et al. A secondary analysis of the Belimumab International Study in Lupus Nephritis trial examined effects of belimumab on kidney outcomes and preservation of kidney function in patients with lupus nephritis. Kidney Int 2022;101:403–13. [DOI] [PubMed] [Google Scholar]
- 43. Levey AS, Gansevoort RT, Coresh J, Inker LA, Heerspink HL, Grams ME, et al. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the National Kidney Foundation in collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis 2020;75:84–104. [DOI] [PubMed] [Google Scholar]
- 44. GSK . Clinical study report HGS1006‐C1056. 2021. URL: https://www.gsk-studyregister.com/en/trial-details/?id=HGS1006-C1056.
- 45. GSK . Clinical study report HGS1006‐C1057. 2021. URL: https://www.gsk-studyregister.com/en/trial-details/?id=HGS1006-C1057.
- 46. GSK . Clinical study report 112341. 2021. URL: https://www.gsk-studyregister.com/en/trial-details/?id=112341.
- 47. GSK . Clinical study report 115471. 2022. URL: https://www.gsk-studyregister.com/en/trial-details/?id=115471.
- 48. GSK . Clinical study report 114054. 2021. URL: https://www.gsk-studyregister.com/en/trial-details/?id=114054.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form
Appendix S1 Supporting Information


