Abstract
Superior vena cava (SVC) syndrome is a rare disease induced by thrombosis and consequent occlusion of SVC, negatively affecting morbidity and mortality. The incidence of SVC syndrome from central venous catheters and pacemaker or defibrillator leads is increasing. Optimal treatment of pacemaker or defibrillator‐related SVC syndrome is not well defined. Lead extraction causes mechanical trauma to the vessel wall. In addition, subsequent device implantation on the contralateral side can be an added factor for venous occlusion. The use of leadless pacemakers could be an interesting option to reduce the risk of SVC restenosis after lead extraction. We report a clinical case of PM leads‐related SVC syndrome referred to our centers and treated with transvenous lead extraction, leadless pacemaker implantation and subsequent percutaneous angioplasty and stenting of the SVC and left innominate vein.
Keywords: cardiac implantable electronic devices, leadless pacemaker, percutaneous angioplasty, superior vena cava syndrome, transvenous lead extraction
Abbreviations
- CT
computed tomography
- ET
endovascular therapy
- ICD
Implantable cardioverter defibrillator
- PM
pacemaker
- PPI
Proton Pump Inhibitor
- PTA
percutaneous transluminal angioplasty
- RT
radiotherapy
- SVC
superior vena cava
1. INTRODUCTION
Superior vena cava (SVC) syndrome is a rare disease induced by thrombosis and consequent occlusion of the SVC, with subsequent negative effects on morbidity and mortality. Malignancy is considered the primary cause of this syndrome, however, there are increasing reports of SVC syndrome caused by central venous catheters and pacemaker or defibrillator leads. 1 , 2
The incidence of device‐related SVC syndrome has been increasing due to the increase in the number of pacemaker and defibrillator implantations. 3 In one study, 28% of all SVC syndromes were related to intravascular devices. 2 In patients with implantable cardiac devices, clinically silent venous thrombosis can occur in up to 30%; however, SVC obstruction is rare and seen in only 0.1%–3.3% of patients. 3
The stenosis at the SVC‐atrial junction occurs due to fibrin deposition on the surface of the pacing leads and incorporation into the intima followed by vessel wall inflammation, thrombus generation, fibrosis, and stenosis. 3 , 4 The primary driving mechanisms are chronic mechanical irritation and foreign body reaction. Lead extraction and subsequent reimplantation also causes mechanical trauma as an additional risk factor for venous occlusion. 4
1.1. Clinical presentation
Clinical presentation varies depending on the severity, location, and rapidity of onset of the obstruction and the establishment of collateral veins. The most common presenting symptoms include facial, neck and upper extremity swelling secondary to obstruction of blood flow in the SVC. In rare cases, there are other symptoms such as neurological symptoms (headache, blurry vision, decreased level of consciousness), laryngopharyngeal symptoms (tongue swelling, dyspnea), and facial symptoms (conjunctival/periorbital edema). Patients also typically describe worsening of their symptoms in the supine position. 5 Esophageal varices have also been reported. 6
In this article, we describe a clinical case referred to our centers with pacemaker lead‐related SVC syndrome. The patient underwent transvenous lead extraction and implantation of a leadless pacemaker with A‐V synchrony in the same session, followed by percutaneous angioplasty and stenting of the SVC and the left innominate vein in another session.
2. CLINICAL CASE
An 81‐year‐old female was referred to our center for PM lead extraction due to PM lead‐associated SVC syndrome. She had a history of paroxysmal atrial fibrillation (first diagnosis in 2015), sick‐sinus syndrome, multiple colic polyposis and previous therapy for H. Pylori. She had been receiving anticoagulant therapy with apixaban since 2015. Her medications on admission included flecainide 150 mg qd, bisoprolol 2.5 mg bid, furosemide 25 mg qd, apixaban 2.5 mg bid and proton pump inhibitor (PPI). Dual chamber Pacemaker (Medtronic Ensura DR) was implanted in January 2018 for symptomatic Tachycardia–Bradycardia syndrome with no periprocedural complications (only a small‐caliber left cephalic vein was noted on implant and subsequently a left subclavian puncture site was chosen). At implant, the patient weighed 45 Kg, with a height of 153 cm (BMI 19.22 Kg/m2).
In October of the same year the patient started complaining of upper left arm and neck swelling as well as facial fullness. She had no medical history of previous thrombotic events, trauma or any intravascular procedure other than the pacemaker insertion were reported. A doppler ultrasound showed a partial deep vein thrombosis of the left axillary vein and the proximal left brachial vein. Since the thrombotic event happened on oral anticoagulation therapy, she was referred to a thrombosis center. Subsequent hematologic investigations showed adequate levels of anti‐Xa activity, a complete blood count and coagulation assay (including D‐dimer) showed no abnormalities with negative thrombophilia screening (ATIII activity 83%, Protein C 94%, Protein S 84%; negative lupus anticoagulant, anticardiolipin, antiglycoprotein I, normal levels of homocysteine, B12 and folate; no Factor II or Factor V mutation).
She also underwent a neck and chest CT scan, a complete abdominal ultrasound, mammography and breast ultrasound with no evidence of neoplastic lesions. Colonoscopy showed multiple colonic polyps, with subsequent biopsy showing no histopathological evidence of malignancy. Thoracic outlet syndrome was also ruled out. Apixaban was switched to dabigatran 110 mg bid (a direct thrombin inhibitor) and she was prescribed local elastic compressive therapy with gradual symptomatic improvement at the following outpatient visits. Follow‐up ultrasound scans (March 2019 and January 2020) revealed a near total recanalization of the left jugular, axillary and subclavian veins with minor post‐thrombotic changes. Two months later, the patient developed a drug reaction in the form of rash, so she was switched back to apixaban 5 mg bid.
In February 2020 she presented to the emergency services for progressive facial, periorbital and tongue swelling as well as dyspnea. The symptoms worsened in the supine position. Physical examination showed edema of upper body and neck, the presence of collateral superficial veins and bilateral jugular vein distension. A chest CT scan showed a fibrotic degeneration of the left innominate vein in the proximity of the PM leads and severe stenosis of the cavoatrial junction with evidence of suprajugular compensatory left to right collateral circulation. On the 5th of March 2020 the patient underwent percutaneous transluminal angioplasty (PTA) of the SVC with significant clinical improvement. A CT scan in June 2020 confirmed the complete left innominate vein fibrotic retraction with a regular caliber of SVC.
A follow‐up CT scan in February 2021 highlighted a new severe stenosis of SVC and the patient was referred again to our center. On admission the patient presented with signs and symptoms of SVC syndrome. A bilateral vein angiography documented a complete occlusion of the left innominate vein at its junction with the SVC, with left to right collateral circulation. The right venogram confirmed the severe SVC stenosis at the cavoatrial junction level (Figure 1)
FIGURE 1.
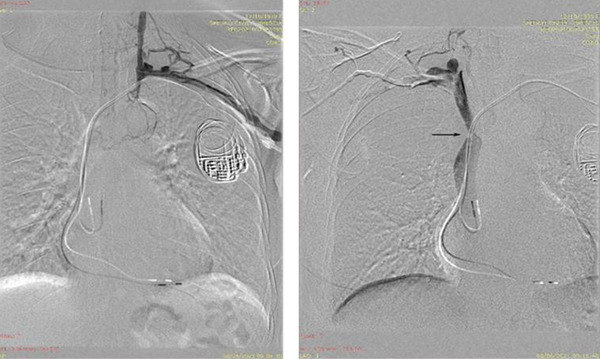
Contrast venography of the left subclavian vein (left) and the right subclavian vein (right). Note the complete obstruction of the left innominate vein in the proximity of the PM leads. Left to right collateral is present. The arrow highlights the SVC stenosis [Colour figure can be viewed at wileyonlinelibrary.com]
Device interrogation revealed atrial pacing of 7% and ventricular pacing of 9%. after consulting the patient and her family, considering the relapsing SVC stenosis, the concurrent signs and symptoms of SVC syndrome, the temporal correlation with transvenous PM lead implantation and the need for pacing we chose to implant a leadless pacemaker to avoid the risk of intravascular leads. A sequential approach was chosen: the patient first underwent a complete extraction of the PM generator and leads via progressive manual traction without periprocedural complications. In the same session, a right femoral vein access was obtained, and a Medtronic Micra AV system was implanted through the dedicated catheter delivery system on the right ventricular apical septum (Figure 2). The implantation was completed without complications and with adequate postimplant electrical parameters. Four days later, the patient was transferred to the referring hospital to complete the treatment plan with a PTA plus stenting of the SVC and the left innominate vein. The procedure was completed successfully (Figure 3) and the patient was discharged 2 days later. At 3 months follow‐up the patient was asymptomatic.
FIGURE 2.
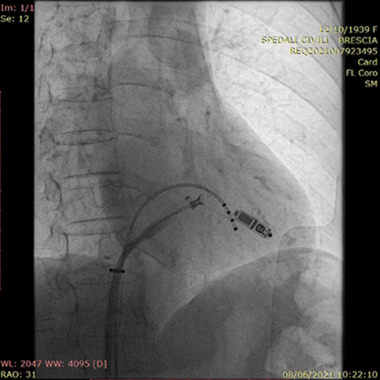
Successful implantation of the MICRA AV leadless device. The pacemaker was implanted in the right ventricle apical septum via right transfemoral percutaneous route. A temporary PM is in place [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
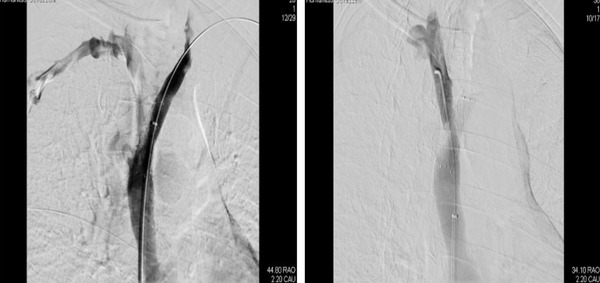
Contrasts venography after stent implantation showing a patent SVC and left innominate vein (left) and right innominate vein (right)
3. DISCUSSION
SVC syndrome is an uncommon but serious complication associated with chronic transvenous leads. In the case of lead‐induced SVC obstruction, lead extraction can be necessary. Device reimplantation should be evaluated on a case‐by‐case basis, considering that device reimplantation is an additional risk factor for venous occlusion, apart from the mechanical trauma cause by the transvenous extraction procedure. 4 In this setting, avoiding a new transvenous lead placement was preferred in order to prevent reocclusion of the venous system. A leadless pacemaker provided an option to avoid the recurrence of pacemaker‐induced SVC syndrome after transvenous extraction of the device. In the past, the major limitation of leadless pacing was that it provided only VVI mode stimulation, but now the possibility of VDD mode stimulation is an interesting option to preserve atrio‐ventricular synchronization.
Treatment options for SVC syndrome include chemotherapy, radiotherapy (RT), surgical bypass, endovascular therapy (ET) such as angioplasty, stenting, and catheter‐based thrombus removal (Table 1). 7 , 8 Recently, ET has been used as the first‐line therapy in the majority of patients with SVC syndrome, particularly those presenting with life‐threatening symptoms. 7 , 9 , 10 , 11 , 12
TABLE 1.
Advantages and disadvantages in different types of treatment in SVC syndrome 7
| Advantages | Disadvantages | |
|---|---|---|
| Radiation | Can be useful if caused by tumor |
Delayed symptom relief Potential complications: SVC perforation, induction of fibrosis in blood vessels, inhibition of collateral development |
| Endovascular therapy |
Minimally‐invasive High success rates Immediate relief of symptoms Can be combined with other Treatment options |
Lower durability than surgery Potential complications: stent migration, reocclusion, cardiac tamponade |
| Surgery |
Durable High success rate |
Invasive procedure‐related morbidity Potential complications: mediastinal hematoma, pulmonary embolism, deep vein thrombosis |
| Chemotherapy | Can be useful if caused by tumor |
Delayed symptom relief Potential complications: anemia, neutropenia, coagulopathy, infection, gastrointestinal disturbances |
Regarding SVC syndrome induced by pacemaker leads, balloon angioplasty with or without thrombolytic therapy, as well as mechanical thrombectomy, have been shown to provide adequate results. 13 , 14 , 15 Endovascular stenting of the occluded vessels without lead extraction has also proved efficient. Three cases who underwent endovascular stenting over the pacemaker leads showed no evidence of pacemaker malfunction and no recurrence of symptoms at a mean follow‐up of 18.6 months. 16
Lead extraction is, later on, met with the challenge of pacemaker reimplantation. FU et al. reported venous reocclusion in three out of six patients with lead‐induced SVC syndrome who underwent lead extraction and endovascular stenting followed by conventional device reimplantation. 17 Insulation breach of the pacemaker leads by the endovascular stents has also been described. 18 As an alternative to conventional pacemakers, epicardial pacemaker leads were used in a patient who underwent surgery for the extraction of four infected leads as well as SVC reconstruction. 19 Another alternative is the implantation of a conventional pacemaker through the azygous vein through minimally invasive thoracotomy. 20 Moreover, a leadless pacemaker was implanted under direct vision in a case with SVC syndrome that underwent surgical reconstruction of the SVC. 21
4. CONCLUSION
In this case, we chose the combination of transvenous lead extraction and leadless VDD pacemaker implantation to reduce the possibility of SVC syndrome recurrence and allow endovascular treatment (ET) by venous balloon angioplasty of the SVC.
The combination of these procedures is feasible and may represent an effective alternative to the treatment of PM lead‐related SVC syndrome.
DISCLOSURES
All authors declare no conflicts of interest.
Curnis A, Milidoni A, Arabia G, et al. Leadless pacemakers as a new alternative for pacemaker lead‐related superior vena cava syndrome: A case report. Pacing Clin Electrophysiol. 2022;45:1051–1055. 10.1111/pace.14520
Consent of publication is not required as information is anonymized and the submission does not include images that may identify the person.
All authors have read and approved the paper.
The article is the original work of all the authors listed
The article has not been published elsewhere and is not under consideration elsewhere
REFERENCES
- 1. Wilson LD, Detterbeck FC, Yahalom J. Superior vena cava syndrome with malignant causes. N Engl J Med. 2007;356(18):1862‐9. 10.1056/nejmcp067190. Published online. [DOI] [PubMed] [Google Scholar]
- 2. Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore). Published online 2006;85(1):37‐42. 10.1097/01.md.0000198474.99876.f0 [DOI] [PubMed] [Google Scholar]
- 3. Chee CE, Bjarnason H, Prasad A, Superior vena cava syndrome: an increasingly frequent complication of cardiac procedures. Nat Clin Pract Cardiovasc Med. Published online 2007;4(4):226‐30. 10.1038/ncpcardio0850 [DOI] [PubMed] [Google Scholar]
- 4. Sfyroeras GS, Antonopoulos CN, Mantas G, et al. A review of open and endovascular treatment of superior vena cava syndrome of benign aetiology. Eur J Vasc Endovasc Surg. 2017;53(2):238‐254. 10.1016/j.ejvs.2016.11.013 Published online 2017 [DOI] [PubMed] [Google Scholar]
- 5. Cheng S. Superior vena cava syndrome: a contemporary review of a historic disease. Cardiol Rev. 2009;17(1):16‐23. Published online 2009. 10.1097/CRD.0b013e318188033c [DOI] [PubMed] [Google Scholar]
- 6. Loudin M, Anderson S, Schlansky B. Bleeding “downhill” esophageal varices associated with benign superior vena cava obstruction: case report and literature review. BMC Gastroenterol. 2016; 16: 4‐8. 10.1186/s12876-016-0548-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Azizi AH, Shafi I, Shah N, et al. Superior vena cava syndrome. JACC Cardiovasc Interv. 2020; 13:2896‐2910. 10.1016/J.JCIN.2020.08.038 [DOI] [PubMed] [Google Scholar]
- 8. Rowell NP, Gleeson FV. Steroids, radiotherapy, chemotherapy and stents for superior vena caval obstruction in carcinoma of the bronchus: a systematic review. Clin Oncol. 2002;14:338‐351. 10.1053/clon.2002.0095 [DOI] [PubMed] [Google Scholar]
- 9. Barshes NR, Annambhotla S, El Sayed HF, et al, Percutaneous stenting of superior vena cava syndrome: treatment outcome in patients with benign and malignant etiology. Vascular. 2007;15:314‐321. 10.2310/6670.2007.00067 [DOI] [PubMed] [Google Scholar]
- 10. Breault S, Doenz F, Jouannic AM, Qanadli SD. Percutaneous endovascular management of chronic superior vena cava syndrome of benign causes: long‐term follow‐up. Eur Radiol. 2017; 27:97‐104. 10.1007/s00330-016-4354-y [DOI] [PubMed] [Google Scholar]
- 11. Kee ST, Kinoshita L, Razavi MK, Nyman URO, Semba CP, Dake MD. Superior vena cava syndrome: treatment with catheter‐directed thrombolysis and endovascular stent placement. Radiology. 1998;206:187‐193. 10.1148/radiology.206.1.9423671 [DOI] [PubMed] [Google Scholar]
- 12. Lanciego C, Pangua C, Chacón JI, et al. Endovascular stenting as the first step in the overall management of malignant superior vena cava syndrome. Am J Roentgenol. 2009;193:549‐558. 10.2214/AJR.08.1904 [DOI] [PubMed] [Google Scholar]
- 13. Chuang W, Juang J, Hsu K, Chiang F, Tseng C. Successful treatment of superior vena cava syndrome induced by transvenous permanent pacemaker implantation with balloon angioplasty. Acta Cardiologica Sinica. 2008;24:39. Mar 1. [Google Scholar]
- 14. Tan CW, Vijitbenjaronk P, Khuri B. Superior vena cava syndrome due to permanent transvenous pacemaker electrodes: successful treatment with combined thrombolysis and angioplasty: a case report. Angiology. 2000;51:963‐969. Nov. [DOI] [PubMed] [Google Scholar]
- 15. Tilahun Gebreyes A, Nath Pant H, Williams DM, Kuehl S, Be aware of wires in the veins: a case of superior vena cava syndrome in a patient with permanent pacemaker. J Community Hosp Intern Med Perspect. 2012;2:19159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Slonim SM, Semba CP, Sze DY, Dake MD. Placement of SVC stents over pacemaker wires for the treatment of SVC syndrome. J Vasc Interv Radiol. 2000;11:215‐219. [DOI] [PubMed] [Google Scholar]
- 17. FU HX, HUANG XM, Zhong L, et al, Outcome and management of pacemaker‐induced superior vena cava syndrome. Pacing Clin Electrophysiol. 2014;37:1470‐1476. [DOI] [PubMed] [Google Scholar]
- 18. Desai S, Ip J, Sista A, Truong Q, Legasto A, Cheung J. A novel complication and treatment strategy in pacemaker‐induced superior vena cava syndrome. J Am Coll Cardiol. 2017;69:2136. Mar 21. [Google Scholar]
- 19. Madkaiker AN, Krishna N, Jose R, et al. Superior vena cava syndrome caused by pacemaker leads. Ann Thorac Surg. 2016;101:2358‐2361. Jun 1. [DOI] [PubMed] [Google Scholar]
- 20. Goktekin O, Besoglu Y, Dogan SM, et al. Permanent pacemaker lead implantation via azygous vein in a patient with silent superior vena cava syndrome. Int J Cardiol. 2007;117:e4‐6. Apr 12. [DOI] [PubMed] [Google Scholar]
- 21. Hodges K, Tuohy S, Miletic K, Wilkoff BL, Soltesz EG, Superior vena cava reconstruction and implantation of a leadless pacemaker for management of pacemaker‐induced superior vena cava syndrome. HeartRhythm Case Rep. 2019;5:539. [DOI] [PMC free article] [PubMed] [Google Scholar]


