Abstract
Magnetoencephalography with optically pumped magnometers (OPM‐MEG) is an emerging and novel, cost‐effective wearable system that can simultaneously record neuronal activity with high temporal resolution ("when" neuronal activity occurs) and spatial resolution ("where" neuronal activity occurs). This paper will first outline recent methodological advances in OPM‐MEG compared to conventional superconducting quantum interference device (SQUID)‐MEG before discussing how OPM‐MEG can become a valuable and noninvasive clinical support tool in epilepsy surgery evaluation. Although OPM‐MEG and SQUID‐MEG share similar data features, OPM‐MEG is a wearable design that fits children and adults, and it is also robust to head motion within a magnetically shielded room. This means that OPM‐MEG can potentially extend the application of MEG into the neurobiology of severe childhood epilepsies with intellectual disabilities (e.g., epileptic encephalopathies) without sedation. It is worth noting that most OPM‐MEG sensors are heated, which may become an issue with large OPM sensor arrays (OPM‐MEG currently has fewer sensors than SQUID‐MEG). Future implementation of triaxial sensors may alleviate the need for large OPM sensor arrays. OPM‐MEG designs allowing both awake and sleep recording are essential for potential long‐term epilepsy monitoring.
Keywords: brain surgery, EEG, epilepsy, MEG, MRI, OPM‐MEG
Key Points.
OPM‐MEG can record neuronal activity at a good spatial and temporal resolution
OPM‐MEG is robust to head movement and can therefore be utilized in severe epilepsies
Most OPM‐MEG sensors are heated, which may become an issue with large sensor arrays
OPM‐MEG is wearable and relatively easy to use compared to conventional MEG
1. CLINICAL VALUE OF MEG
The clinical benefit of conventional superconducting quantum interference device (SQUID)–magnetoencephalography (MEG) is well known. 1 It is a technique that has the potential to identify and localize the origin of epileptiform activity in the brain, 2 using multichannel SQUID technology 3 that has been used to record epileptiform activity since the early 1990s. 4 , 5 Despite its clinical promise in epilepsy, the worldwide uptake of SQUID‐MEG equipment has not been as widespread as expected. The reason for this is not for lack of scientific/clinical validity, but rather its logistical limitations. MEG is a large piece of equipment that typically weighs half a ton, and it requires superconductive helium‐cooling at −269°C to record neuronal activity. 6 It also has a prefixed cylindrical headspace, where participants must keep their heads still during the scan.
Despite these issues, MEG has several strengths and complementarities in comparison to its most similar method, electroencephalography (EEG). MEG is a system that detects neuronal activity at a submillisecond temporal resolution, mainly from the magnetic field emanated by postsynaptic current flow from 10 000–50 000 cortical excitatory pyramidal neurons. 7 EEG detects the electric local field potentials that arise primarily from extracellular currents driven by the same postsynaptic potentials. 8 MEG is less susceptible to volume conduction compared to EEG, which means that it provides superior source localization of neuronal activity 9 and a higher theoretical spatial resolution limit. 10 Although MEG is theoretically insensitive to radial sources in a perfectly spherical conductor, these tend to occur at the cortical surface at the crests of gyri, and in practice, the limitation is somewhat mitigated by closely adjacent sources that, being near the surface, are relatively close to the MEG sensors. 11 Nevertheless, it has been observed in practice that there is a degree of complementarity in the sensitivity of MEG and EEG likely due to the depth and orientation differences of electromagnetic sources. 12 , 13 Despite the methodological advantages, SQUID‐MEG techniques suffer from several limitations that have hindered this system's widespread clinical and research uptake.
2. EMERGENCE OF WEARABLE OPTICALLY PUMPED MAGNETOMETER–MEG
Because conventional SQUID‐MEG systems are expensive and have some challenges in research and clinical settings, MEG with optically pumped magnetometers (OPM‐MEG) are an exciting development in human neuroscience. A series of recent publications have demonstrated the scientific promise of the new and improved OPM‐MEG. OPM‐MEG and SQUID‐MEG signals stem from magnetic fields in the brain, and have quantum physics origins. However, OPM‐MEG 14 overcomes several limitations of SQUID‐MEG. 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 OPM‐MEG is wearable, and participants have the freedom to move during the scans, at least 10 cm 15 and likely up to 1 m. 25 This is enabled by a system worn like a cap or a helmet that can be fitted to both children and adults. Also, OPM‐MEG does not require helium‐based superconductive cooling of the equipment, making it cost‐efficient to operate compared to low‐temperature MEG systems.
Like conventional SQUID‐MEG, OPM‐MEG systems measure the magnetic fields generated by current flow in assemblies of pyramidal neurons oriented perpendicular to the cortical surface below each sensor. This is typically achieved with two advanced physics components: (1) a shielded room that nullifies the earth's magnetic field and other magnetic interference, especially oscillating magnetic fields that can arise from nearby electrical wiring; and (2) OPM sensors that can detect tiny changes of the brain's magnetic field at the femtotesla scale (a tesla to the factor of 10−15).
Currently available commercial OPM‐MEG systems utilize a shielded room with degaussing coils shown capable of reducing the interference from the earth's magnetic field to ~1.5 nT 26 (down from almost 50 000 nT where the room was located 27 ). The need for attenuation more than an order of magnitude greater than that required for SQUID‐MEG is a potential disadvantage of wearable OPM‐MEG systems. 26 However, recent advances utilizing active field suppression facilitate the operation of OPM‐MEG in magnetically shielded rooms designed for SQUID‐MEG. 28 Although current commercial OPM‐MEG solutions require a shielded room to reduce external interferences, novel magnetically silent gradiometers may alleviate the need for a shielded room in the future. 29 These gradiometers have an unshielded sensitivity on the femtotesla scale (specifically, ~16 fT/cm/Hz1/2), sufficient to detect neuronal activity in naturalistic settings including outdoors. See Tierney et al. 14 for a detailed review of the physics of OPMs, and also Limes et al. 29 for a description of a magnetically silent sensor that exploits measurement of free‐precession frequency rather than photodiode voltage.
The general idea behind OPM is to project a polarized light of suitable frequency (e.g., laser) through a high‐pressure vapor contained in a glass cell, to establish a magnetically sensitive state in the vapor. This occurs via the transfer of angular momentum from the light to the vapor (a quantum effect known as optical pumping). Once the optically pumped state is complete, light is no longer absorbed and passes through the vapor unattenuated to a photodiode (detection mechanism) causing a voltage change. The pumped vapor is highly polarized, a state that is very sensitive to changes in the external magnetic environment. The vapor inside the OPM sensors is approximately 150°C, and although the sensors themselves are close to body temperature, it is essential to consider proper heat dissipation mechanisms in the helmet/cap design. 26 , 30 Newer metastable helium‐4 OPMs can be operated at room temperature, 31 which is a promising approach to alleviating heat issues of OPM‐MEG.
In a seminal paper published in 2018, Boto et al. 15 used 13 OPM sensors over the sensorimotor cortex while subjects were conducting a motor task, and demonstrated that OPM‐MEG elicits similar results to SQUID‐MEG. Additionally, OPM‐MEG obtained biologically meaningful results even if participants (deliberately) produced significant head movement. In 2020 and 2021, another series of papers were published, now with whole‐brain OPM‐MEG coverage achieved with 50 OPM sensors. 32
With whole‐brain coverage, it was established that OPM‐MEG has a finite millimeter spatial resolution, 26 likely <5 mm, and functional connectivity is similar between OPM‐MEG and SQUID‐MEG systems. 16 A comparison was also conducted within a wearable design between OPM sensors placed on a helmet (as seen in Figure 1C) and on a flexible cap akin to modern EEG systems. 26 The helmet design performed better than a cap design, because OPM sensors remain in the same position in relation to the skull during the scans. Sensors in a flexible cap, on the other hand, have a propensity to misalign in relation to the scalp. Despite a lower OPM‐MEG signal‐to‐noise ratio with the helmet design (due to sensors being closer to the scalp in a flexible cap design), there is less coregistration error for source localization using a helmet. This is because the position of the sensors is always known in relation to the head in a helmet design, and accurate head movement modeling is possible. 26
FIGURE 1.
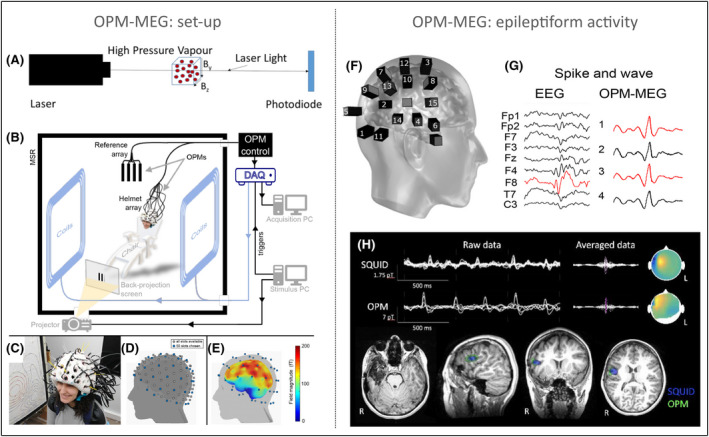
Optically pumped magnetometer (OPM)–magnetoencephalography (MEG) system. (A) Fundamental principles of OPMs. (B) An overview of the OPM‐MEG system including the shielded room, sensors, and equipment. (C) OPM‐MEG helmet system and (D) its available sensors. (E) Average field magnitude of OPM‐MEG. (F) OPM sensors in one epilepsy subject and (G) a comparison between electroencephalographic (EEG) and OPM‐MEG epileptiform activity (note signals are not spatially aligned). (H) OPM‐MEG and superconducting quantum interference device (SQUID)–MEG epileptiform activity and source localization. DAQ, digital aquisition system; MSR, magnetically shielded room. Images reproduced/amended under open‐access CC‐BY 4.0 licence from the following publications: A, 14 B–E, 16 F–G, 19 . H is reproduced with permission from Figure 2B in Feys et al. 39 [Correction added on 14 September 2022, after first online publication: In the preceding sentence, the text “with permission” was added.]
On the topic of source localization, it is possible to use pre‐existing magnetic resonance imaging (MRI) templates (e.g., normalized Montreal Neurological Institute templates) for OPM‐MEG source localization akin to SQUID‐MEG. In clinical settings, it is preferable to acquire high‐quality subject‐specific MRI to enhance the spatial sensitivity of anatomical coregistration and head modeling for source localization with OPM‐MEG. Several source localization modeling techniques exist, such as Beamformer, 33 which has been shown to achieve high spatial resolution in OPM‐MEG. 26 Advances in sensor technology also can improve source localization in OPM‐MEG compared to SQUID‐MEG. For example, triaxial OPM sensors could provide better source localization than radial OPM sensors and SQUID‐MEG. A theoretical study has shown that triaxial OPM sensors may reduce the need for a high number of sensors in OPM‐MEG systems, as 50 triaxial sensors showed lower measurement error on source localization than 150 radial sensors. Note that 50 triaxial sensors each record three orthogonal estimates from the brain's magnetic field, which is equivalent to 150 channels. 17 Using (fewer) triaxial channels will also likely make it easier for heat emanating from OPMs to dissipate from the cap/helmet.
From a methodological perspective, one of the main areas in need of development is interference suppression in OPM‐MEG. Because the OPM‐MEG signal emanating from neuronal magnetic fields is (incredibly) small, it is imperative to employ appropriate methods and processing tools that minimize the influence of external (and often confounding) signals. Despite the interference suppression provided with the shielded room, the OPM‐MEG signal includes low‐frequency movement artifacts (<6 Hz) that need to be corrected, in addition to other artifacts from urban traffic noise and vibrations (0–40 Hz), line noise (e.g., 50 Hz), and high‐frequency interference from equipment inside the shielded room (~120 Hz). 34 For example, a promising approach in hardware is the “magnetically silent” sensor recently reported in Limes et al. 29 Hardware and data processing solutions need to be validated to ensure an acceptable signal‐to‐noise ratio can be obtained in practical use.
As an interim summary, the first wave of OPM‐MEG research suggests that this novel technology has data quality comparable to conventional SQUID‐MEG. However, OPM‐MEG is more cost‐efficient and is easier to use across ages and clinical populations, and people can move around during the scans. Research has shown that movement associated with head motion (while sitting and standing up 25 ) and stretching, drinking, and ball games 15 is tolerated by OPM‐MEG. These advantages provide an opportunity for more widespread use of MEG technologies. In the remainder of the paper, we will highlight how OPM‐MEG can become a clinical support tool in epilepsy.
3. OPM‐MEG FOR LOCALIZING THE ONSET, AND NETWORK DYNAMICS, OF EPILEPTIFORM ACTIVITY
Approximately one third of people with pharmacoresistant focal epilepsy are "MRI‐negative," meaning no clear‐cut epileptogenic lesion is observed on structural MRI scans. 35 Research has shown that patients are two to three times more likely to be seizure‐free after surgery if a lesion is detected on histopathology or structural MRI, 36 and pharmacoresistant MRI‐negative extratemporal lobe epilepsies are particularly clinically challenging and are rarely operated on (only 3% of MRI‐negative epilepsy cases went to surgery in a previous report 37 ). Novel functional neurophysiological investigations, such as OPM‐MEG, are promising alternatives that could increase the chances of surgical success in MRI‐negative focal epilepsy. 38
Preliminary OPM‐MEG studies in focal epilepsy include two case reports 19 , 23 and a case series of five children with focal epilepsy. 39 Compared to SQUID‐MEG, OPM‐MEG showed more accurate detection of epileptiform activity. OPM‐MEG interictal epileptiform activity also demonstrated greater amplitude and signal‐to‐noise ratio compared to EEG (see Figure 1G) 19 and SQUID‐MEG (see Figure 1H). 39 The anatomical localization of epileptiform activity was similar between OPM‐MEG and SQUID‐MEG, despite fewer sensors used with OPM‐MEG (32 OPM‐MEG sensors vs. 102 SQUID‐MEG sensors). These findings suggest that it is feasible to use OPM‐MEG in a clinical setting, with children as subjects, and with improved data quality because sensors are closer to the scalp in wearable OPM‐MEG systems.
Focal cortical dysplasia is a common type of refractory epilepsy and is often associated with onset in childhood or adolescence. 40 Evidence suggests that lower age at surgery is related to good long‐term surgical outcomes in focal cortical dysplasia, 41 but detecting the seizure focus with conventional neuroimaging techniques can be hindered by compliance in children due to the need to stay still throughout the scans. Wearable OPM‐MEG systems can aid the presurgical process in people with epilepsy by identifying the temporal characteristics of epileptiform activity (e.g., the morphology and timing of epileptiform activity) in the same way as EEG. 42 MEG (including OPM‐MEG) and high‐density EEG also provide an additional capacity to capture millimeter‐resolution source reconstruction in the brain, by modeling where brain epileptiform activity originates. Whether MEG or EEG is better in this regard can depend on the location and morphology of the affected region in an individual; a comparison of EEG and SQUID‐MEG 13 found that superior localization is typically obtained from whichever modality detects the earliest abnormal activity. We are tempted to speculate that the improved sensitivity of OPM‐MEG compared to SQUID‐MEG could increase the proportion of cases in which MEG can detect the earliest relevant signal change.
Another area where OPM‐MEG can enhance our understanding of focal epilepsy is modeling the spatiotemporal spread of interictal and ictal epileptiform activity, permitting a systems view of regions comprising the epileptiform networks. 43 , 44 , 45 , 46 Network analyses of seizure spread can provide valuable information about multiple regions involved in seizure networks 42 and may be helpful to guide the planning of locations to target with surgically implanted intracranial electrodes for intraoperative electrophysiological recordings.
In these early years of OPM‐MEG development, no studies have yet tested whether high‐frequency epileptiform oscillations can be identified with this technology. Epileptiform high‐frequency brain oscillations (ripples = 80–200 Hz, fast ripples = 200–500 Hz) have emerged as a localizing marker that can help define the abnormal epileptogenic area. 47 Although SQUID‐MEG has been used to detect high‐frequency oscillations in people with refractory epilepsy in the past, 48 it remains unknown how well OPM‐MEG will operate in the high‐frequency domain.
4. OPM‐MEG IN SEVERE EPILEPSY
In addition to being a presurgical tool in refractory focal epilepsy, OPM‐MEG has the potential to significantly improve research in the most severe of developmental and epileptic encephalopathies. Severe epilepsy is often associated with developmental delay and intellectual disabilities and has been challenging to study with noninvasive technologies due to significant head motion that may cause artifacts on functional MRI (fMRI) and EEG. 49 Initial evidence suggests that OPM‐MEG systems are robust to head motion. On this point, Boto et al. 15 first showed comparable results in an experiment with minimal motion versus motion up to 10 cm, nodding of the head, stretching, and drinking as well as playing a ball game. Other studies also suggest that moving up to 1 m can be tolerated with OPM‐MEG, 25 and with significantly fewer signal artifacts than EEG. 18 This research implies that it is possible to conduct experiments on people who are susceptible to excessive head motion. This presents a unique opportunity to study further the neurobiological underpinnings of severe epilepsies such as Dravet syndrome, 50 progressive myoclonus epilepsy, 51 and Lennox–Gastaut syndrome, 52 all associated with persistent movement potentially without sedation. This would help us to understand and monitor brain changes associated with treatment response, including antiseizure medication 53 and cannabinoid treatment, 54 as well as the development of novel targets, and efficacy, of deep brain stimulation. 55 , 56 Although OPM‐MEG is robust to head motion, the OPM sensors should not move in relation to the head (i.e., the head and sensors need to be aligned throughout the scan). This means that an OPM‐MEG helmet design is likely the preferable choice of sensor placement in severe epilepsies.
5. COMPARISON BETWEEN MEG TECHNOLOGIES AND NONINVASIVE IMAGING IN EPILEPSY
With high temporal/spatial resolution, MEG technologies are a promising development for the clinical neurosciences, including as a potential additional tool in epilepsy alongside existing functional imaging such as nuclear medicine (e.g., positron emission tomography [PET] and single photon emission computed tomography [SPECT] 57 ) and hybrid imaging (e.g., simultaneous EEG‐fMRI 58 ). Despite the clinical utility of existing methods, 59 they each have intrinsic methodological limitations that can hamper neurobiological interpretation. For example, although fMRI is sensitive to blood oxygenation changes from small brain regions (i.e., excellent spatial resolution), it captures hemodynamic response activity over several seconds, which is much slower than a neuronal activity (i.e., poor temporal resolution). On the other hand, scalp EEG captures neuronal activity at a millisecond scale (i.e., excellent temporal resolution), but because sensors are placed on the skull, volume conduction hinders accurate source localization, meaning that it is challenging to model where the neuronal activity originates in the brain (i.e., poor spatial resolution).
There is emerging evidence of patient benefit of simultaneously acquired EEG and fMRI data for surgical planning in focal epilepsy. 58 , 60 , 61 , 62 Simultaneous EEG‐fMRI analysis typically estimates the hemodynamic activity in the brain that correlates with the timing of epileptogenic activity simultaneously recorded with EEG. The aims of EEG‐fMRI (gives a spatial solution that can be more regional) and MEG (dynamics at high temporal resolution) are similar in epilepsy: to localize spatial regions and networks associated with epileptiform activity. Both EEG‐fMRI and MEG technologies need enough epileptiform activity to be detectable at the scalp, which is more difficult if the epileptiform activity stems from subcortical areas or mesial temporal lobes. Additionally, these brain areas also have more spatial distortion on fMRI 63 and lower field magnitude signals on MEG. 16 Thus, these techniques are more useful in focal epilepsies with a neocortical seizure onset, rather than temporal lobe epilepsy.
6. ENVISAGING THE FUTURE WITH OPM‐MEG
An important point is that, in the absence of a clear structural lesion on MRI, obtaining multiple lines of evidence from different imaging and neurophysiological modalities is likely to be clinically beneficial. 64 OPM‐MEG is not yet approved for clinical use, but it is feasible that the future hospital experience for people with refractory epilepsy includes a suite of noninvasive presurgical investigations including MEG/EEG, fMRI/MRI, and PET/SPECT. Given the user‐friendly aspects of OPM‐MEG, we envisage that tertiary hospitals may include a setup with a magnetically shielded room that can fit a hospital bed, to enable continuous OPM‐MEG monitoring. A practical enhancement to consider for OPM‐MEG design in this context is suitability for long‐term monitoring during both wakefulness and sleep (e.g., as recommended by the American Clinical MEG Society). 65
At the time of writing, the average up‐front cost of OPM‐MEG is around USD 1.4 million (this figure can be higher/lower depending on the number of OPM sensors and size of the shielded room), which is significantly lower than SQUID‐MEG, which has an up‐front cost of approximately USD 3.5 million (companies that offer OPM‐MEG systems include Cerca Magnetics, https://www.cercamagnetics.com/; FieldLine, https://fieldlineinc.com/; and Mag4Health, https://www.mag4health.com/). The ongoing operating expenditure cost of OPM‐MEG is thought to be approximately USD 70 000/year, compared to USD 200 000/year for SQUID‐MEG including the cost of helium. Currently, magnetically shielded OPM‐MEG rooms are available in sizes from 1.3 × 1.3 m to 4 × 3 m (see, e.g., https://magneticshields.co.uk). We hope that rapid advances in the field will allow less onerous room‐shielding, reducing cost and potentially making larger rooms economically viable. The ease‐of‐use and methodological advantages of OPM‐MEG may lead to broader uptake of these systems in epilepsy clinics and hospitals, as it may provide additional clinical evidence about the source and spread of epileptiform activity, in childhood and adult epilepsy.
This paper has focused on the immediate clinical benefits OPM‐MEG may have in epilepsy, but it is worth bearing in mind how this novel system can change the landscape for a range of psychiatric and neurological disorders. Previous research has demonstrated that SQUID‐MEG is a useful tool in several brain conditions, such as dementia. 66 A case in point is recent findings showing that low‐frequency MEG activity is a promising marker of amyloid‐beta deposition and cognitive function in Alzheimer disease. 67 Dementia is associated with progressive damage to the brain that occurs before any symptoms become obvious, 68 and techniques such as OPM‐MEG may change how we diagnose/monitor preclinical stages of dementia or its response to therapy.
Another neurological area of interest is a traumatic brain injury. Even "mild" traumatic brain injuries, often called concussions, can lead to life‐changing difficulties. 69 Yet, we still cannot answer the most basic questions: Will I recover quickly? Will my brain injury result in long‐term problems? A recent systematic review suggests that low‐frequency (delta) activity measured with SQUID‐MEG is a promising biomarker of traumatic brain injury, 70 and its validation in mild brain injury is dependent on prospective and large studies using optimal methodological approaches. If these biomarkers are validated, OPM‐MEG has the potential to "see" previously invisible traumatic brain injuries, enabling us to predict when people are likely to recover.
As we are still early in the development of OPM‐MEG, more research is needed to understand its full potential in epilepsy. Nevertheless, we anticipate that clinical and basic research using OPM‐MEG will grow over the next few years due to its lower cost and practical improvements over SQUID‐MEG (e.g., allowing for movement within a magnetically shielded room). Symbiotic collaborations of physics, engineering, neuroscience and medicine will become imperative to continue improving OPM‐MEG's clinical capability, such as optimization of design to allow comfortable monitoring during both wakefulness and sleep, while tackling OPM‐MEG heat dissipation issues, potentially by retaining a low number of triaxial sensors.
AUTHOR CONTRIBUTIONS
Mangor Pedersen conceived the study, conducted the literature review, and wrote the first draft of the manuscript. David F. Abbott provided physics and electrophysiology expertise and wrote significant portions of the manuscript. Graeme D. Jackson provided epilepsy and electrophysiology expertise and wrote significant portions of the manuscript.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENTS
The Florey Institute of Neuroscience and Mental Health acknowledges the strong support from an Operational Infrastructure Support Grant. We also acknowledge the facilities, and the scientific and technical National Collaborative Research Infrastructure Strategy capability at the Florey node, and the Victorian Biomedical Imaging Capability. The Australian Epilepsy Project received funding from the Australian Government under the Medical Research Future Fund (Frontier Health and Medical Research Program, grant RFRHPSI000008). M.P. is supported by an Emerging Researcher Fellowship from the Health Research Council of New Zealand. D.F.A. is supported by fellowship funding from the Australian National Imaging Facility. We also wish to thank the two anonymous reviewers for their critical reading of the manuscript and their constructive suggestions, which helped improve the final manuscript. Open access publishing facilitated by Auckland University of Technology, as part of the Wiley ‐ Auckland University of Technology agreement via the Council of Australian University Librarians.
Pedersen M, Abbott DF & Jackson GD. Wearable OPM‐MEG: A changing landscape for epilepsy. Epilepsia. 2022;63:2745–2753. 10.1111/epi.17368
REFERENCES
- 1. Cohen D, Edelsack EA, Zimmerman JE. Magnetocardiograms taken inside a shielded room with a superconducting point‐contact magnetometer. Appl Phys Lett. 1970;16:278–80. [Google Scholar]
- 2. Tovar‐Spinoza ZS, Ochi A, Rutka JT, Go C, Otsubo H. The role of magnetoencephalography in epilepsy surgery. Neurosurg Focus. 2008;25(3):E16. [DOI] [PubMed] [Google Scholar]
- 3. Jaklevic RC, Lambe J, Silver AH, Mercereau JE. Quantum interference effects in Josephson tunneling. Phys Rev Lett. 1964;12(7):159–60. [Google Scholar]
- 4. Nakasatp N, Levesque MF, Barth DS, Baumgartner C, Rogers RL, Sutherling WW. Comparisons of MEG, EEG, and ECoG source localization in neocortical partial epilepsy in humans. Electroencephalogr Clin Neurophysiol. 1994;91(3):171–8. [DOI] [PubMed] [Google Scholar]
- 5. Barkley GL, Baumgartner C. MEG and EEG in epilepsy. J Clin Neurophysiol. 2003;20(3):163–78. [DOI] [PubMed] [Google Scholar]
- 6. Hironaga N, Takei Y, Mitsudo T, Kimura T, Hirano Y. Prospects for future methodological development and application of magnetoencephalography devices in psychiatry. Front Psychiatry. 2020;11:863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murakami S, Okada Y. Contributions of principal neocortical neurons to magnetoencephalography and electroencephalography signals. J Physiol. 2006;575(Pt 3):925–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lopes da Silva F. EEG and MEG: relevance to neuroscience. Neuron. 2013;80(5):1112–28. [DOI] [PubMed] [Google Scholar]
- 9. Tamilia E, AlHilani M, Tanaka N, Tsuboyama M, Peters JM, Grant PE, et al. Assessing the localization accuracy and clinical utility of electric and magnetic source imaging in children with epilepsy. Clin Neurophysiol. 2019;130(4):491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baillet S. Magnetoencephalography for brain electrophysiology and imaging. Nat Neurosci. 2017;20(3):327–39. [DOI] [PubMed] [Google Scholar]
- 11. Hillebrand A, Barnes GR. A quantitative assessment of the sensitivity of whole‐head MEG to activity in the adult human cortex. Neuroimage. 2002;16(3 Pt 1):638–50. [DOI] [PubMed] [Google Scholar]
- 12. Ahlfors SP, Han J, Belliveau JW, Hämäläinen MS. Sensitivity of MEG and EEG to source orientation. Brain Topogr. 2010;23(3):227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Plummer C, Vogrin SJ, Woods WP, Murphy MA, Cook MJ, Liley DTJ. Interictal and ictal source localization for epilepsy surgery using high‐density EEG with MEG: a prospective long‐term study. Brain J Neurol. 2019;142(4):932–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tierney TM, Holmes N, Mellor S, López JD, Roberts G, Hill RM, et al. Optically pumped magnetometers: from quantum origins to multi‐channel magnetoencephalography. Neuroimage. 2019;199:598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boto E, Holmes N, Leggett J, Roberts G, Shah V, Meyer SS, et al. Moving magnetoencephalography towards real‐world applications with a wearable system. Nature. 2018;555(7698):657–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boto E, Hill RM, Rea M, Holmes N, Seedat ZA, Leggett J, et al. Measuring functional connectivity with wearable MEG. Neuroimage. 2021;230:117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brookes MJ, Boto E, Rea M, Shah V, Osborne J, Holmes N, et al. Theoretical advantages of a triaxial optically pumped magnetometer magnetoencephalography system. Neuroimage. 2021;236:118025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boto E, Seedat ZA, Holmes N, Leggett J, Hill RM, Roberts G, et al. Wearable neuroimaging: combining and contrasting magnetoencephalography and electroencephalography. Neuroimage. 2019;201:116099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vivekananda U, Mellor S, Tierney TM, Holmes N, Boto E, Leggett J, et al. Optically pumped magnetoencephalography in epilepsy. Ann Clin Transl Neurol. 2020;7(3):397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Roberts G, Holmes N, Alexander N, Boto E, Leggett J, Hill RM, et al. Towards OPM‐MEG in a virtual reality environment. Neuroimage. 2019;199:408–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wittevrongel B, Holmes N, Boto E, Hill R, Rea M, Libert A, et al. Practical real‐time MEG‐based neural interfacing with optically pumped magnetometers. BMC Biol. 2021;19(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tierney TM, Mellor S, O'Neill GC, Holmes N, Boto E, Roberts G, et al. Pragmatic spatial sampling for wearable MEG arrays. Sci Rep. 2020;10(1):21609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mellor S, Vivekananda U, O'Neill GC, Tierney TM, Doig D, Seymour RA, et al. First experiences of whole‐head OP‐MEG recordings from a patient with epilepsy. medRxiv. 2021;2021.09.28.21264047. 10.1101/2021.09.28.21264047v1 [DOI] [Google Scholar]
- 24. Mellor SJ, Tierney T, O'Neill G, Alexander N, Seymour R, Holmes N, et al. Magnetic field mapping and correction for moving OP‐MEG. IEEE Trans Biomed Eng. 2021;69:528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seymour RA, Alexander N, Mellor S, O'Neill GC, Tierney TM, Barnes GR, et al. Using OPMs to measure neural activity in standing, mobile participants. Neuroimage. 2021;244:118604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hill RM, Boto E, Rea M, Holmes N, Leggett J, Coles LA, et al. Multi‐channel whole‐head OPM‐MEG: helmet design and a comparison with a conventional system. Neuroimage. 2020;219:116995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alken P, Thébault E, Beggan CD, Amit H, Aubert J, Baerenzung J, et al. International geomagnetic reference field: the thirteenth generation. Earth Planets Space. 2021;73(1):49. [Google Scholar]
- 28. Iivanainen J, Zetter R, Grön M, Hakkarainen K, Parkkonen L. On‐scalp MEG system utilizing an actively shielded array of optically‐pumped magnetometers. Neuroimage. 2019;194:244–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Limes ME, Foley EL, Kornack TW, Caliga S, McBride S, Braun A, et al. Portable magnetometry for detection of biomagnetism in ambient environments. Phys Rev Appl. 2020;14(1):011002. [Google Scholar]
- 30. Hill RM, Devasagayam J, Holmes N, Boto E, Shah V, Osborne J, et al. Using OPM‐MEG in contrasting magnetic environments. Neuroimage. 2022;253:119084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fourcault W, Romain R, Gal GL, Bertrand F, Josselin V, Prado ML, et al. Helium‐4 magnetometers for room‐temperature biomedical imaging: toward collective operation and photon‐noise limited sensitivity. Opt Express. 2021;29(10):14467–75. [DOI] [PubMed] [Google Scholar]
- 32. Jas M, Jones SR, Hämäläinen MS. Whole‐head OPM‐MEG enables noninvasive assessment of functional connectivity. Trends Neurosci. 2021;44(7):510–2. [DOI] [PubMed] [Google Scholar]
- 33. Chen JC, Yao K, Hudson RE. Source localization and beamforming. IEEE Signal Process Mag. 2002;19(2):30–9. [Google Scholar]
- 34. Seymour RA, Alexander N, Mellor S, O'Neill GC, Tierney TM, Barnes GR, et al. Interference suppression techniques for OPM‐based MEG: opportunities and challenges. Neuroimage. 2022;247:118834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang ZI, Alexopoulos AV, Jones SE, Jaisani Z, Najm IM, Prayson RA. The pathology of magnetic‐resonance‐imaging‐negative epilepsy. Mod Pathol. 2013;26(8):1051–8. [DOI] [PubMed] [Google Scholar]
- 36. Téllez‐Zenteno JF, Hernández Ronquillo L, Moien‐Afshari F, Wiebe S. Surgical outcomes in lesional and non‐lesional epilepsy: a systematic review and meta‐analysis. Epilepsy Res. 2010;89(2–3):310–8. [DOI] [PubMed] [Google Scholar]
- 37. Baud MO, Perneger T, Rácz A, Pensel MC, Elger C, Rydenhag B, et al. European trends in epilepsy surgery. Neurology. 2018;91(2):e96–106. [DOI] [PubMed] [Google Scholar]
- 38. So EL, Lee RW. Epilepsy surgery in MRI‐negative epilepsies. Curr Opin Neurol. 2014;27(2):206–12. [DOI] [PubMed] [Google Scholar]
- 39. Feys O, Corvilain P, Aeby A, Sculier C, Holmes N, Brookes M, et al. On‐Scalp Optically Pumped Magnetometers versus Cryogenic Magnetoencephalography for Diagnostic Evaluation of Epilepsy in School‐aged Children. Radiology. August 2022;304(2):429–34. [DOI] [PubMed] [Google Scholar]
- 40. Bast T, Ramantani G, Seitz A, Rating D. Focal cortical dysplasia: prevalence, clinical presentation and epilepsy in children and adults. Acta Neurol Scand. 2006;113(2):72–81. [DOI] [PubMed] [Google Scholar]
- 41. Fauser S, Essang C, Altenmüller D‐M, Staack AM, Steinhoff BJ, Strobl K, et al. Long‐term seizure outcome in 211 patients with focal cortical dysplasia. Epilepsia. 2015;56(1):66–76. [DOI] [PubMed] [Google Scholar]
- 42. Walz JM, Pedersen M, Omidvarnia A, Semmelroch M, Jackson GD. Spatiotemporal mapping of epileptic spikes using simultaneous EEG‐functional MRI. Brain J Neurol. 2017;140(4):998–1010. [DOI] [PubMed] [Google Scholar]
- 43. Bernhardt BC, Hong S, Bernasconi A, Bernasconi N. Imaging structural and functional brain networks in temporal lobe epilepsy. Front Hum Neurosci. 2013;7:624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gotman J. Epileptic networks studied with EEG‐fMRI. Epilepsia. 2008;49(Suppl 3):42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pedersen M, Omidvarnia AH, Walz JM, Jackson GD. Increased segregation of brain networks in focal epilepsy: an fMRI graph theory finding. Neuroimage Clin. 2015;8:536–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia. 2002;43(3):219–27. [DOI] [PubMed] [Google Scholar]
- 47. Staba RJ, Wilson CL, Bragin A, Fried I, Engel J. Quantitative analysis of high‐frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol. 2002;88(4):1743–52. [DOI] [PubMed] [Google Scholar]
- 48. Velmurugan J, Nagarajan SS, Mariyappa N, Mundlamuri RC, Raghavendra K, Bharath RD, et al. Magnetoencephalography imaging of high frequency oscillations strengthens presurgical localization and outcome prediction. Brain. 2019;142(11):3514–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Spencer GS, Smith JA, Chowdhury MEH, Bowtell R, Mullinger KJ. Exploring the origins of EEG motion artefacts during simultaneous fMRI acquisition: implications for motion artefact correction. Neuroimage. 2018;173:188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Guerrini R, Striano P, Catarino C, Sisodiya SM. Neuroimaging and neuropathology of Dravet syndrome. Epilepsia. 2011;52(Suppl 2):30–4. [DOI] [PubMed] [Google Scholar]
- 51. Jaksch M, Klopstock T, Kurlemann G, Dörner M, Hofmann S, Kleinle S, et al. Progressive myoclonus epilepsy and mitochondrial myopathy associated with mutations in the tRNAser(UCN) gene. Ann Neurol. 1998;44(4):635–40. [DOI] [PubMed] [Google Scholar]
- 52. Archer JS, Warren AEL, Stagnitti MR, Masterton RAJ, Abbott DF, Jackson GD. Lennox‐Gastaut syndrome and phenotype: secondary network epilepsies. Epilepsia. 2014;55(8):1245–54. [DOI] [PubMed] [Google Scholar]
- 53. Vigevano F, Arzimanoglou A, Plouin P, Specchio N. Therapeutic approach to epileptic encephalopathies. Epilepsia. 2013;54(s8):45–50. [DOI] [PubMed] [Google Scholar]
- 54. Ali S, Scheffer IE, Sadleir LG. Efficacy of cannabinoids in paediatric epilepsy. Dev Med Child Neurol. 2019;61(1):13–8. [DOI] [PubMed] [Google Scholar]
- 55. Gross RE, Fisher RS, Sperling MR, Giftakis JE, Stypulkowski PH, on behalf of the SANTÉ Study Group . Analysis of deep brain stimulation lead targeting in the stimulation of anterior nucleus of the thalamus for epilepsy clinical trial. Neurosurgery. 2021;89(3):406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dalic LJ, Warren AEL, Young JC, Thevathasan W, Roten A, Bulluss KJ, et al. Cortex leads the thalamic centromedian nucleus in generalized epileptic discharges in Lennox‐Gastaut syndrome. Epilepsia. 2020;61(10):2214–23. [DOI] [PubMed] [Google Scholar]
- 57. Won HJ, Chang K‐H, Cheon J‐E, Kim HD, Lee DS, Han MH, et al. Comparison of MR imaging with PET and ictal SPECT in 118 patients with intractable epilepsy. Am J Neuroradiol. 1999;20(4):593–9. [PMC free article] [PubMed] [Google Scholar]
- 58. Kowalczyk MA, Omidvarnia A, Abbott DF, Tailby C, Vaughan DN, Jackson GD. Clinical benefit of presurgical EEG‐fMRI in difficult‐to‐localize focal epilepsy: a single‐institution retrospective review. Epilepsia. 2020;61(1):49–60. [DOI] [PubMed] [Google Scholar]
- 59. Pittau F, Grouiller F, Spinelli L, Seeck M, Michel CM, Vulliemoz S. The role of functional neuroimaging in pre‐surgical epilepsy evaluation. Front Neurol. 2014;5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zijlmans M, Huiskamp G, Hersevoort M, Seppenwoolde J‐H, van Huffelen AC, Leijten FSS. EEG‐fMRI in the preoperative work‐up for epilepsy surgery. Brain. 2007;130(9):2343–53. [DOI] [PubMed] [Google Scholar]
- 61. Thornton R, Laufs H, Rodionov R, Cannadathu S, Carmichael DW, Vulliemoz S, et al. EEG correlated functional MRI and postoperative outcome in focal epilepsy. J Neurol Neurosurg Psychiatry. 2010;81(8):922–7. [DOI] [PubMed] [Google Scholar]
- 62. Koupparis A, von Ellenrieder N, Khoo HM, Zazubovits N, Nguyen DK, Hall JA, et al. Association of EEG‐fMRI responses and outcome after epilepsy surgery. Neurology. 2021;97(15):e1523–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jezzard P, Clare S. Sources of distortion in functional MRI data. Hum Brain Mapp. 1999;8(2–3):80–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kuzniecky RI, Bilir E, Gilliam F, Faught E, Palmer C, Morawetz R, et al. Multimodality MRI in mesial temporal sclerosis: relative sensitivity and specificity. Neurology. 1997;49(3):774–8. [DOI] [PubMed] [Google Scholar]
- 65. Bagić AI, Knowlton RC, Rose DF, Ebersole JS. ACMEGS Clinical Practice Guideline (CPG) Committee. American Clinical Magnetoencephalography Society clinical practice guideline 1: recording and analysis of spontaneous cerebral activity. J Clin Neurophysiol. 2011;28(4):348–54. [DOI] [PubMed] [Google Scholar]
- 66. Mandal PK, Banerjee A, Tripathi M, Sharma A. A comprehensive review of magnetoencephalography (MEG) studies for brain functionality in healthy aging and Alzheimer's disease (AD). Front Comput Neurosci. 2018;12:60. 10.3389/fncom.2018.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ranasinghe KG, Cha J, Iaccarino L, Hinkley LB, Beagle AJ, Pham J, et al. Neurophysiological signatures in Alzheimer's disease are distinctly associated with TAU, amyloid‐β accumulation, and cognitive decline. Sci Transl Med. 2020;12(534):eaaz4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Beason‐Held LL, Goh JO, An Y, Kraut MA, O'Brien RJ, Ferrucci L, et al. Changes in brain function occur years before the onset of cognitive impairment. J Neurosci. 2013;33(46):18008–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fadyl JK, Theadom A, Channon A, McPherson KM. Recovery and adaptation after traumatic brain injury in New Zealand: longitudinal qualitative findings over the first two years. Neuropsychol Rehabil. 2019;29(7):1095–112. [DOI] [PubMed] [Google Scholar]
- 70. Allen CM, Halsey L, Topcu G, Rier L, Gascoyne LE, Scadding JW, et al. Magnetoencephalography abnormalities in adult mild traumatic brain injury: a systematic review. Neuroimage Clin. 2021;31:102697. [DOI] [PMC free article] [PubMed] [Google Scholar]


