Abstract
Background
Neonatal hypothyroidism is often raised as a potential concern for the use of computed tomography pulmonary angiography (CTPA) in pregnant women with suspected pulmonary embolism (PE).
Objectives
To assess the incidence of neonatal hypothyroidism among newborns from mothers exposed to CTPA.
Patients/Methods
Pregnant women with clinically suspected PE were included in a multicenter, multinational prospective diagnostic management outcome study, based on pretest clinical probability assessment, high‐sensitivity D‐dimer testing, bilateral lower limb venous compression ultrasonography, and CTPA. Results of Guthrie tests were systematically collected for newborns of all women who required CTPA as part of the diagnostic strategy. A thyroid‐stimulating hormone (TSH) level above 15 U/ml was used to define hypothyroidism.
Results
Out of the 166 women included in the Swiss participating centers, 149 underwent a CTPA including 14 with twin pregnancies. Eight women suffered a pregnancy loss and results of the Guthrie test could not be retrieved for four newborns. All TSH levels were reported as being below 15 U/ml. The incidence of neonatal hypothyroidism was 0/151 (0.0%, 95% confidence interval: 0.0%–2.5%).
Conclusions
We did not identify any cases of neonatal hypothyroidism in our cohort of 149 pregnant women investigated for suspected PE using a CTPA. Along with previous literature data, this provides further reassuring data regarding the use of CTPA in this indication.
Keywords: diagnosis, Guthrie test, hypothyroidism, pregnancy, pulmonary embolism
Essentials.
Neonatal hypothyroidism is a potential complication of computed tomography pulmonary angiography (CTPA) use during pregnancy.
Analysis of this risk was done in a prospective management outcome study including pregnant women with suspected pulmonary embolism (PE).
Guthrie tests performed in newborns from mothers who had CTPA for suspected PE during pregnancy were collected.
No case of neonatal hypothyroidism in pregnant women investigated with CTPA for suspected PE was detected.
1. INTRODUCTION
Pulmonary embolism (PE) remains one of the most common causes of maternal death in developed countries. 1 , 2 Besides the fact that pregnancy is associated with an increased risk of venous thromboembolism (VTE), one of the potential explanations is that diagnosing PE is particularly challenging during pregnancy. Pregnant women often present symptoms and signs that can suggest PE, such as physiologic shortness of breath or tachycardia. 3 There is very limited evidence in the literature to guide clinicians on how to manage pregnant women with suspected PE. 4 , 5 Two prospective management outcome studies using computed tomography pulmonary angiography (CTPA) as an imaging test have suggested that this test is safe to rule out PE in this population. 6 , 7 There is an ongoing debate on whether lung ventilation/perfusion (V/Q) scan or CTPA should be preferred during pregnancy. In terms of fetal radiation, the dose received is lower with CT during early pregnancy and becomes equivalent by the end of pregnancy. 8 , 9 , 10 The dose to the mother's breast is significantly higher with CTPA throughout pregnancy. 11 , 12
Potential harmful effects related to intravenous iodinated contrast agents on fetal thyroid function have also been put forward as a reason to prefer the V/Q scan.
While reduced intakes of iodine is the major cause of hypothyroidism, exposure of the fetus or the neonate to excess iodine may also result in hypothyroidism. High iodine levels block thyroid hormone synthesis through inhibition of the organification process. This is known as the Wolff–Chaikoff effect. Also, excess iodine inhibits T4 and T3 secretion. These effects are thought to be present in the thyroid tissues for some weeks. In a normal thyroid gland, after some days, the excess iodine induces downregulation of the sodium–iodide symporter and decreased intra‐thyroid iodine concentration, leading to an escape from the Wolff–Chaikoff effect. However, the fetal thyroid gland is particularly sensitive to iodine overload because the ability to fully escape from the acute Wolff–Chaikoff effect does not mature until approximately 36 weeks. The risk of fetal hypothyroidism due to long‐term maternal ingestion of iodides has been well documented in the past. 13 , 14 , 15 , 16 A theoretical risk of contrast‐induced hypothyroidism in neonates exposed to iodinated contrast agents during the antenatal period exists but has never been assessed in a formal prospective study. 17
The CT‐PE Pregnancy Study was the first published prospective management outcome study that assessed the safety of a sequential diagnostic strategy in pregnant women with suspected PE. 6 This strategy was based on the assessment of clinical probability with the simplified Geneva score, D‐dimer measurement, compression ultrasonography of the lower limb veins, and CTPA. In Switzerland, a systematic screening of several neonatal conditions, including congenital hypothyroidism, is performed at birth by the measurement of capillary thyroid‐stimulating hormone (TSH) levels. To study the effect of iodinated contrast agents used for CTPA in pregnant women, we prospectively collected TSH measurements in babies born from mothers who underwent CTPA for a suspected PE in the CT‐PE Pregnancy Study.
2. METHODS
2.1. Study population
We used data from a multicenter, multinational prospective diagnostic management outcome study. We screened outpatient pregnant women presenting at one of the participating centers with a clinical suspicion of PE, as defined as acute onset of new or worsening shortness of breath or chest pain without another obvious cause. Exclusion criteria were: age below 18 years, allergy to iodinated contrast agent, impaired renal function (defined by a creatinine clearance below 30 ml/min as per the Cockcroft–Gault formula), diagnosis made prior to presentation, indication for or already on full dose anticoagulation, and inaccessibility for follow‐up. The study was performed in two countries (France and Switzerland) and 11 centers actively included patients. The study was approved by the ethics committee according to legislation at each study site. A written informed consent was obtained from all participating women.
2.2. Diagnostic work‐up
The pretest probability (PTP) of PE was determined using the revised Geneva score. A D‐dimer test was performed in all women, using a highly sensitive D‐dimer assay (Vidas® D‐dimer assay, bioMérieux). PE was deemed excluded in women with a non–high PTP and a negative D‐dimer test (i.e., D‐dimer < 500 μg/L). Women with a high PTP, and those with a non–high PTP and a positive D‐dimer test, underwent bilateral compression ultrasonography (CUS). When a proximal deep vein thrombosis (DVT; popliteal vein and/or above) was found, PE was considered confirmed without further testing. Women with a negative CUS underwent CTPA. The protocol for CTPA consisted of an evaluation of the pulmonary arteries up to and including the subsegmental vessels. Only multidetector CT machines were used. The acquisition parameters for CTPA were: injection of a total volume of 100 ml of non‐ionic contrast material (iodine concentration, 300–350 mg/ml) with a power injector at 3–5 ml per second; imaging 9–20 s after initiation of the contrast‐material injection; scanning performed at 1.0–1.3 mm per section with a pitch of 1.25–1.75, 120 KV, and 115–260 mA; and reconstruction of images at 0.6–0.8 mm intervals. The complete diagnostic algorithm is depicted in Figure 1.
FIGURE 1.
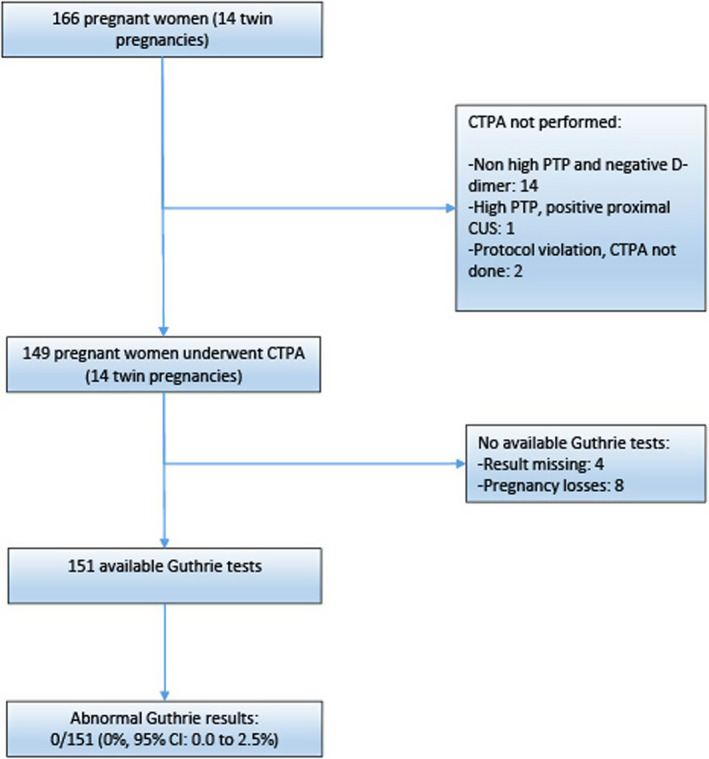
Study flowchart. CI, confidence interval; CTPA, computed tomography pulmonary angiography; CUS, compression ultrasonography; PTP, pretest probability
2.3. Follow‐up
Patients with a negative diagnostic work‐up were considered as not having PE and thus left without anticoagulant treatment and underwent clinical follow‐up for 3 months. They were instructed to contact the study team in case of any new or worsening symptoms. An independent adjudication committee (three members) reviewed all the suspected VTE events, blinded from the initial diagnostic work‐up.
2.4. Study analysis
Although neonatal screening was performed in both countries, results were not systematically collected in the French participating centers, so this analysis is restricted to babies born from women included in the Swiss participating centers. Results of Guthrie tests were obtained at follow‐up or when data were not available at follow‐up, through the National Central Swiss Laboratory (Zürich, Switzerland). Results are provided as negative when the TSH measurements were below 15 U/ml. The actual TSH value is given in case of abnormal results. Women using medications that could have an influence on the babies’ thyroid function (levothyroxine, amiodarone) were excluded.
The analysis was performed using babies as a unit. We estimated the proportion of newborns with an abnormal TSH result using exact proportions along with the 95% confidence interval (CI).
3. RESULTS
Between August 2008 and July 2016, 395 pregnant women were included in the study. Of them, 166 were included in the Swiss participating centers. The characteristics of patients are depicted in Table 1.
TABLE 1.
Characteristics of included patients
| Characteristics | Patients (n = 149) |
|---|---|
| Female sex, n (%) | 149, 100 |
| Age in years, median (IQR) | 33 (29–36) |
| Trimester of pregnancy | |
| First, n (%) | 17 (11.4) |
| Second, n (%) | 60 (40.3) |
| Third, n (%) | 72 (48.3) |
| BMI (kg/m2) | 25.1 (6.0) |
| Personal history of VTE, n (%) | 11 (7.4) |
| Familial history of VTE, n (%) | 15 (10.1) |
| Active malignancy, n (%) | 0 (0.0) |
| Surgery within 1 month, n (%) | 2 (1.0) |
| Bedridden for >72 h during the last 4 weeks, n (%) | 15 (10.1) |
| Travel >6 h, n (%) | 5 (3.4) |
| Chest pain, n (%) | 95 (63.8) |
| Dyspnea, n (%) | 108 (72.5) |
| Syncope, lipothymia, n (%) | 19 (12.8) |
| Hemoptysis, n (%) | 5 (3.4) |
| Clinical signs or symptoms of DVT, n (%) | 14 (9.4) |
| Heart rate, bpm, mean (SD) | 90 (17) |
| O2 saturation, %, mean (SD) | 98.4 (1.6) |
Abbreviations: BMI, body mass index; DVT, deep vein thrombosis; IQR, interquartile range; SD, standard deviation; VTE, venous thromboembolism.
There were 23 (13.9%) women included during the first trimester of their pregnancy, 69 (41.6%) during the second, and 74 (44.6%) during the third trimester.
CTPA was not performed in 17 women: 14 women with a non‐high PTP and negative D‐dimer, 1 with a high PTP and proximal DVT on CUS, and 2 due to protocol violations. None of the included mothers were using drugs having a potential impact on the thyroid function of their babies. Therefore, 149 women, including 14 with twin pregnancies, were included in the analysis. The results of the Guthrie test could not be retrieved for four newborns, and there were eight pregnancy losses. Altogether, 151 Guthrie results were available. The study flow chart is depicted in Figure 1.
All Guthrie levels were reported as being below 15 U/ml. The proportion of newborns with neonatal hypothyroidism was: 0/151 (0.0%, 95% CI: 0.0%–2.5%).
4. DISCUSSION
Among women included in the CT‐PE Pregnancy Study, 6 we did not identify any case of neonatal hypothyroidism in newborns of mothers who underwent a CTPA during the pregnancy for a suspected PE.
Congenital hypothyroidism, which can be definitive or transient, has an incidence of 1 in 2000 to 1 in 4000 newborns in the United States, and is the most common and treatable cause of mental retardation. 18 Thyroid function during the intra‐uterine period is essential for the neurologic development of the fetus as highlighted by the fact that the fetal gland begins to produce thyroxine under the influence of TSH between 10 and 12 weeks of gestation. 19 Congenital hypothyroidism may be a result of athyreosis, thyroid dysgenesis, or defects in pathways for biosynthesis of thyroid hormones. Transient congenital hypothyroidism may be due to placental transfer of maternal antibodies, or excessive iodine exposure. As iodine crosses the placenta, a potential adverse effect on the fetal thyroid gland from maternal intravenous administration of iodinated contrast agent resulting in neonatal hypothyroidism has been postulated. 20 Indeed, some reports of transient congenital hypothyroidism have been described, for example after amniofetography with iodinated contrast agents. 15 On the other hand, some small series did not report hypothyroidism in babies born from mothers receiving iodinated contrast medium during pregnancy. 17 Therefore, the issue is still highly debated and has been put forward to avoid CTPA during pregnancy and use a V/Q scan instead. 21
Depending on the generation of the CTPA and the protocol used, 75–150 ml of iodinated contrast medium is administered intravenously. The iodine content of iodinated contrast media varies from 320 to 350 mg/ml and is much higher than the recommended daily allowance of 150 μg for adults and 220–290 μg for pregnant and lactating women. 22
A V/Q scan has been the cornerstone for PE diagnosis for more than three decades and is often proposed in pregnant women with suspected PE, starting with a perfusion scan and adding a ventilation phase only in case of perfusion abnormality. 23 However, this kind of strategy has never been validated in a properly conducted prospective study. Moreover, the availability of the test is limited to tertiary care centers.
CTPA has become the reference standard test for PE diagnosis outside pregnancy 24 , 25 and has recently also been used in pregnant women with suspected PE. 6 , 7 Indeed, the sequential use of an assessment of PTP, D‐dimer measurement, and CTPA has been tested in two management prospective studies, which confirmed the safety of this diagnostic approach. 6 , 7
Therefore, our prospective data suggesting no detected case of neonatal hypothyroidism in our cohort could further reassure clinicians on the safety of CTPA for PE diagnosis during pregnancy. Of course, chest maternal radiation associated with the use of CTPA is significantly higher than with a V/Q scan, and there is an ongoing debate regarding the long‐term risk of such irradiation. However, the risk–benefit balance of using CTPA in pregnant women seems acceptable as misdiagnosing PE during pregnancy may have immediate dramatic consequences for the mother and her baby.
Our study has strengths and limitations. This was a prospective study and we had a priori decided to systematically collect newborn outcomes. We were able to retrieve results through a centralized laboratory that performs tests for all newborns in Switzerland. This ensures the use of the same pre‐analytics variables and the same assay for all newborns. Also, all included pregnant women underwent a standardized diagnostic algorithm, using a similar CTPA protocol and the same contrast agent throughout the conduct of the study. 6
Despite being the largest prospective study on PE diagnosis in pregnancy collecting newborn outcomes, our sample size remains limited. We were only able to exhaustively collect all Guthrie test results for women included in Switzerland. This limited us to providing very narrow estimates of the risk. However, our results are in line with those of a retrospective study including 344 women who underwent CTPA for suspected PE. In this study, all newborns had a normal T4 at birth, and only one newborn had a transient abnormal TSH, which normalized after few days. 17
In summary, we did not identify any case of neonatal hypothyroidism in our cohort of 149 pregnant women investigated for suspected PE using a CTPA. Along with previous literature data, this provides further reassuring data regarding the use of CTPA in this indication.
AUTHOR CONTRIBUTIONS
Marc Righini, Helia Robert‐Ebadi, Grégoire Le Gal: designed research, performed research, collected data, analyzed data, and wrote the paper. Alessio Cremonesi, Antoine Elias, Olivier Sanchez, Emmanuelle Le Moigne, Jeannot Schmidt, Catherine Le Gall, Jacques Cornuz, Drahomir Aujesky, Pierre‐Marie Roy, Céline Chauleur, Frédéric Rouyer, Pierre‐Alexandre Poletti, Caroline Moreau: performed research, collected data, analyzed data, and wrote the paper.
CONFLICTS OF INTEREST
This manuscript represents original work, and it is not under consideration for publication elsewhere. It has never been neither submitted nor published in another scientific journal. All authors meet criteria for authorship and none of the authors have any conflict of interest. All had access to all data in the study, read and approved the final manuscript, and held responsibility for the decision to submit it for publication. The original trial was registered at clinicaltrials.gov (NCT 00740454).
ACKNOWLEDGMENTS
The study was supported by a grant from the Swiss National Foundation for scientific research (FNS32003B‐120760) by a grant from the Groupe d'Etude de la Thrombose de Bretagne Occidentale (GETBO) and by an International Society on Thrombosis and Haemostasis Presidential Grant (2017). GLG holds an Early Researcher Award from the Province of Ontario, a ‘CP has heart’ cardiovascular clinician scientist award from the Heart and Stroke Foundation of Ontario, and the Chair on Diagnosis of Venous Thromboembolism from the Department of Medicine, Faculty of Medicine, Ottawa. We would like to thank all the residents and physicians from the emergency departments and vascular medicine units of all participating centers. We would also like to thank all study nurses, secretaries, and clinical research technicians for their invaluable help. We extend our thanks to the staff of the Clinical Research Center, Geneva University Hospital, and in particular to Khaled Mostaguir (clinical research associate, no compensation), who developed the electronic eCRF for the study; to the coordinating center in Brest for French study sites: Isabelle Pichon (multicenter coordinator), Elise Poulhazan, Céline Dolou, Nabahats Ibrir, Florence Morvan, Véronique Kouassi, Floriane Masson. And last but not least, we would like to express our gratitude to the patients who made the study possible by accepting the invitation to participate to the trial. Open access funding provided by Universite de Geneve. [Correction added on 30 Nov 2022, after first online publication: CAUL funding statement has been added.]
Righini M, Robert‐Ebadi H, Cremonesi A, et al. Risk of neonatal hypothyroidism in newborns from mothers exposed to CTPA during pregnancy: Ancillary data from a prospective outcome study. J Thromb Haemost. 2022;20:2550‐2555. doi: 10.1111/jth.15843
Manuscript handled by: Saskia Middeldorp
Final decision: Saskia Middeldorp, 03 August 2022
REFERENCES
- 1. Cantwell R, Clutton‐Brock T, Cooper G, et al. Saving Mothers' Lives: reviewing maternal deaths to make motherhood safer: 2006–2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011;118(Suppl 1):1‐203. [DOI] [PubMed] [Google Scholar]
- 2. Liu S, Rouleau J, Joseph KS, et al. Epidemiology of pregnancy‐associated venous thromboembolism: a population‐based study in Canada. J Obstet Gynaecol Can. 2009;31:611‐620. [DOI] [PubMed] [Google Scholar]
- 3. Lee SY, Chien DK, Huang CH, Shih SC, Lee WC, Chang WH. Dyspnea in pregnancy. Taiwan J Obstet Gynecol. 2017;56:432‐436. [DOI] [PubMed] [Google Scholar]
- 4. van Mens TE, Scheres LJ, de Jong PG, Leeflang MM, Nijkeuter M, Middeldorp S. Imaging for the exclusion of pulmonary embolism in pregnancy. Cochrane Database Syst Rev. 2017;1:CD011053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wan T, Skeith L, Karovitch A, Rodger M, Le Gal G. Guidance for the diagnosis of pulmonary embolism during pregnancy: Consensus and controversies. Thromb Res. 2017;157:23‐28. [DOI] [PubMed] [Google Scholar]
- 6. Righini M, Robert‐Ebadi H, Elias A, et al. Diagnosis of pulmonary embolism during pregnancy: a multicenter prospective management outcome study. Ann Intern Med. 2018;169:766‐773. [DOI] [PubMed] [Google Scholar]
- 7. van der Pol LM, Tromeur C, Bistervels IM, et al. Pregnancy‐adapted YEARS algorithm for diagnosis of suspected pulmonary embolism. N Engl J Med. 2019;380:1139‐1149. [DOI] [PubMed] [Google Scholar]
- 8. Righini M, Le Gal G, Bounameaux H. Venous thromboembolism diagnosis: unresolved issues. Thromb Haemost. 2015;113:1184‐1192. [DOI] [PubMed] [Google Scholar]
- 9. Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64‐slice computed tomography coronary angiography. JAMA. 2007;298:317‐323. [DOI] [PubMed] [Google Scholar]
- 10. Chan WS. Diagnosis of venous thromboembolism in pregnancy. Thromb Res. 2018;163:221‐228. [DOI] [PubMed] [Google Scholar]
- 11. McLintock C, Brighton T, Chunilal S, et al. Recommendations for the diagnosis and treatment of deep venous thrombosis and pulmonary embolism in pregnancy and the postpartum period. Aust N Z J Obstet Gynaecol. 2012;52:14‐22. [DOI] [PubMed] [Google Scholar]
- 12. Groves AM, Yates SJ, Win T, et al. CT pulmonary angiography versus ventilation‐perfusion scintigraphy in pregnancy: implications from a UKsurvey of doctors' knowledge of radiation exposure. Radiology. 2006;240:765‐770. [DOI] [PubMed] [Google Scholar]
- 13. Atwell TD, Lteif AN, Brown DL, McCann M, Townsend JE, Leroy AJ. Neonatal thyroid function after administration of IV iodinated contrast agent to 21 pregnant patients. AJR Am J Roentgenol. 2008;191:268‐271. [DOI] [PubMed] [Google Scholar]
- 14. Etling N, Gehin‐Fouque F, Vielh JP, Gautray JP. The iodine content of amniotic fluid and placental transfer of iodinated drugs. Obstet Gynecol. 1979;53:376‐380. [PubMed] [Google Scholar]
- 15. Rodesch F, Camus M, Ermans AM, Dodion J, Delange F. Adverse effect of amniofetography on fetal thyroid function. Am J Obstet Gynecol. 1976;126:723‐726. [DOI] [PubMed] [Google Scholar]
- 16. Cosman BC, Schullinger JN, Bell JJ, Regan JA. Hypothyroidism caused by topical povidone‐iodine in a newborn with omphalocele. J Pediatr Surg. 1988;23:356‐358. [DOI] [PubMed] [Google Scholar]
- 17. Bourjeily G, Chalhoub M, Phornphutkul C, Alleyne TC, Woodfield CA, Chen KK. Neonatal thyroid function: effect of a single exposure to iodinated contrast medium in utero. Radiology. 2010;256:744‐750. [DOI] [PubMed] [Google Scholar]
- 18. Harris KB, Pass KA. Increase in congenital hypothyroidism in New York State and in the United States. Mol Genet Metab. 2007;91:268‐277. [DOI] [PubMed] [Google Scholar]
- 19. Lee SY, Rhee CM, Leung AM, Braverman LE, Brent GA, Pearce EN. A review: radiographic iodinated contrast media‐induced thyroid dysfunction. J Clin Endocrinol Metab. 2015;100:376‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raymond J, LaFranchi SH. Fetal and neonatal thyroid function: review and summary of significant new findings. Curr Opin Endocrinol Diabetes Obes. 2010;17:1‐7. [DOI] [PubMed] [Google Scholar]
- 21. Lim W, Le Gal G, Bates SM, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: diagnosis of venous thromboembolism. Blood Adv. 2018;2:3226‐3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rajaram S, Exley CE, Fairlie F, Matthews S. Effect of antenatal iodinated contrast agent on neonatal thyroid function. Br J Radiol. 2012;85:e238‐e242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chan WS, Ray JG, Murray S, Coady GE, Coates G, Ginsberg JS. Suspected pulmonary embolism in pregnancy: clinical presentation, results of lung scanning, and subsequent maternal and pediatric outcomes. Arch Intern Med. 2002;162:1170‐1175. [DOI] [PubMed] [Google Scholar]
- 24. Righini M, Le Gal G, Aujesky D, et al. Diagnosis of pulmonary embolism by multidetector CT alone or combined with venous ultrasonography of the leg: a randomised non‐inferiority trial. Lancet. 2008;371:1343‐1352. [DOI] [PubMed] [Google Scholar]
- 25. Konstantinides SV, Meyer G. The 2019 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2019;40:3453‐3455. [DOI] [PubMed] [Google Scholar]


