Abstract
In this case report, we preserved human livers for up to 13 days under normothermic conditions using a modified commercial perfusion system. Two whole livers were split into two left lateral segment grafts and two extended right grafts without interruption to blood flow and then perfused on separate machines. Not only does this provide the basis for a meaningful study of liver function in the long term, but this could also facilitate the development of a model of ex situ liver regeneration.

Keywords: ex situ machine perfusion, long‐term perfusion, split livers
Ex situ machine perfusion technology has provided the opportunity for assessment and resuscitation of grafts prior to implant but the focus of this technology has so far been on short‐term perfusion (≤24 h). 1 , 2 Long‐term ex situ machine perfusion for days‐to‐weeks raises the possibility of meaningful liver repair and regeneration. 3 Although this has been reported up to 7–11 days using subnormothermic conditions (34°C), 4 , 5 long‐term perfusions of grafts beyond this time and using true normothermic conditions (36°C) 6 have never been reported. Here, we report the longest ever ex situ perfusion of human livers.
A 30‐year‐old liver was declined for transplantation at our center through the donation after circulatory death (DCD) pathway due to a prolonged time between the withdrawal of cardiorespiratory support and cessation of circulation (31 min). Similarly, a 44‐year‐old DCD pathway liver was declined due to simultaneous offers at our center and the inability to perform simultaneous transplants. The functional warm ischemia time (WIT) (systolic blood pressure ≤ 50 mm Hg to cold flush) was 29 and 14 min, respectively. The asystolic WIT (cessation of circulation to cold flush) was 4 and 7 min, respectively. The livers were procured in the usual fashion and reperfused ex situ after a cold ischemia time (cold flush to ex situ reperfusion) of 449 and 274 min, respectively.
We modified a commercial organ perfusion system (LiverAssist, Xvivo, Gronigen, Netherlands) by adding long‐term oxygenators (Quadrox‐iD Pediatric, Macquet, Getinge Group, Rastatt, Germany) for long‐term perfusion and a gas‐blender (Low Flow 2003 Series, Bio‐Med Devices, Connecticut, USA) for mixing of compressed air and oxygen (Figure 1). The system utilizes a pressure‐controlled dual‐pump circuit which was set at 60 mm Hg and 8 mm Hg for the hepatic artery and portal vein respectively. A dialysis filter (Polyflux, Baxter, Illinois, USA) was added in parallel for filtration of water‐soluble toxins and regulation of perfusate volume (Figure 1). The perfusate was prepared by mixing 4 units of human‐packed red cells, 2 units of fresh frozen plasma, 200 ml of 20% albumin, 1 L of normal saline, and 10–20 ml of sodium bicarbonate (8.4%). Perfusion was maintained at normothermia (36°C) without perfusate exchange and anticoagulated using intermittent enoxaparin (100 mg twice daily). Cephazolin (1 g daily) was used for antibiotic prophylaxis. Nutrition was maintained with amino acids (10–20 ml/h, Synthamin 17, Baxter Healthcare, Illinois, USA), lipids (0.1 ml/h, Clinoleic 20%, Baxter Healthcare, Illinois, USA), taurocholic acid (7.7 mg/h) and methylprednisolone (21 mg/h). Glucose was maintained between 5–15 mmol/L by titration of glucose (10%), glucagon (20 μg/ml), and insulin (Actrapid 2 IU/ml) infusions. Oxygenation and acid–base balance was maintained to a pO2 of 100–200 mm Hg and a pH of 7.3–7.45 by adjustment of oxygen‐compressed air ratios. Hemoglobin was maintained between 55–65 g/L by adjustment of dialysis filtration.
FIGURE 1.
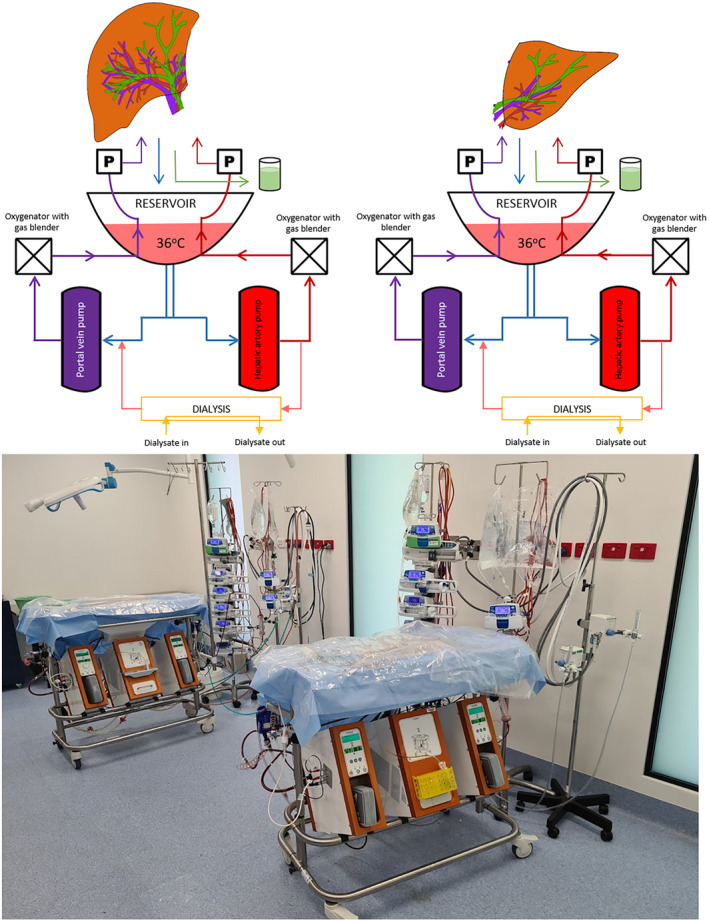
Schematic and system set up for the modified commercial liver perfusion system. We modified a LiverAssist (Xvivo, Gronigen, Netherlands) by adding long‐term oxygenators (Quadrox‐iD Pediatric, Macquet, Getinge Group, Rastatt, Germany), a gas‐blender (Low Flow 2003 Series, Bio‐Med Devices, Connecticut, USA) and a dialysis filter (Polyflux, Baxter, Illinois, USA). The system utilizes a dual‐pump pressure‐controlled circuit with a thermoregulator and an open venous reservoir. [Color figure can be viewed at wileyonlinelibrary.com]
The whole livers were surgically split during continuous normothermic perfusion into extended right grafts (ERG, segments I, IV‐VIII) and left lateral segment grafts (LLSG, segments II and III), as we have previously described, 7 and the ERGs were transferred to a second liver perfusion system. This model of liver splitting and long‐term perfusion was chosen for its potential in studying therapeutics with a matched control and to facilitate the study of liver injury and regeneration. Supportive perfusion under physiological conditions continued until the grafts were clearly non‐viable with an exponentially rising lactate, cessation of bile production, or unresponsive hypoglycemia. For the first liver, the whole liver was split after 15.5 h of resuscitation and the splitting took 110 min. The resulting LLSG survived for a total of 107 h, and the ERG for 288 h (12 days). For the second liver, the whole liver was split after 21.5 h of resuscitation and the splitting took 106 min. The LLSG and ERG from this liver both survived for 328 h (13 days).
Preserved liver metabolic activity was evidenced by lactate clearance, production of bile, maintenance of bile pH, stable liver biochemistry, production of coagulation factors, and stable deposition of ATP in both grafts (Figure 2). As grafts became non‐viable, we noted an increase in vascular resistance, unresponsive hypoglycemia (with an increased glucose requirement), rising bilirubin, and worsening perfusate acidosis (Figures 2 and 3). The liver architecture was preserved in liver biopsies taken throughout perfusion with minimal evidence of hepatocyte detachment, bile duct injury, or coagulative necrosis (Figure 4). Perfusate cultures taken at 96 h after splitting were negative but a mixed growth of gram‐negative and gram‐positive bacteria was evident in perfusate cultures at 306 h after splitting for the two grafts that survived to 13 days.
FIGURE 2.
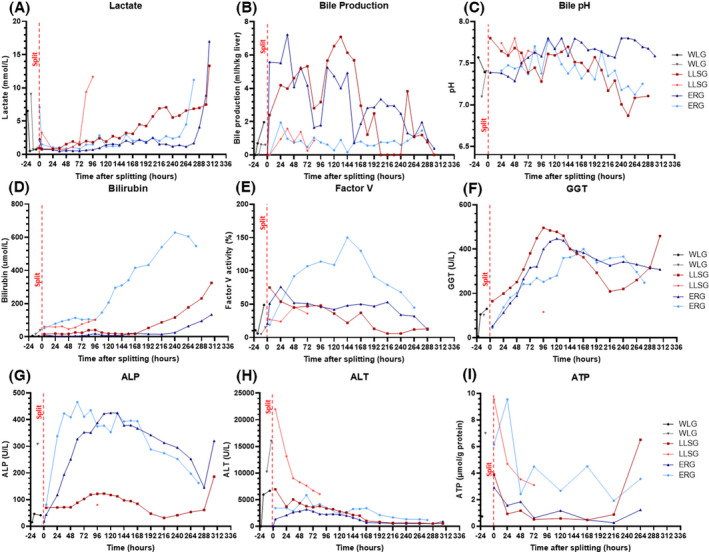
Evidence of preserved metabolic activity during long‐term ex situ machine perfusion of human livers. (A) Lactate from packed red blood cells was cleared rapidly by all grafts, and lactate clearance was maintained until graft failure at 107 h, 288 h, 328, and 328 h for the 4 grafts respectively. (B) Bile production adjusted to the weight of each partial graft was constant with a deterioration in the rate of production towards the point of graft failure. (C) Bile pH remained high (>7.45) for the majority of perfusion for both partial grafts but decreased towards the point of graft failure. (D) Perfusate bilirubin levels remained stable during perfusion before rising exponentially towards the point of graft failure. (E) Factor V levels rose during the corresponding period of stable liver function and decreased towards the point of graft failure. (F and G) Perfusate gamma‐glutamyl transferase (GGT) and alkaline phosphatase (ALP) levels rose in the first 72 h and 48 h of perfusion respectively before plateauing. There was a second peak toward the end of the perfusion. (H) Perfusate levels of alanine aminotransferase (ALT) peaked in the first 24 h of perfusion and stabilized during the long‐term phase. (I) Tissue adenosine triphosphate (ATP) demonstrated an initial fall followed by a plateau in all grafts during long‐term perfusion. [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
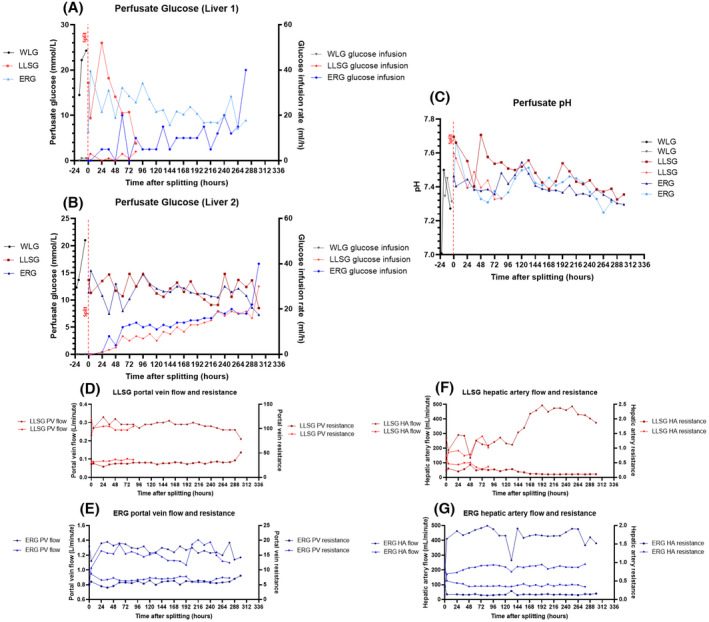
Evidence of worsening liver function at the end of perfusion during long‐term ex situ machine perfusion of human livers. (A and B) For all livers (A: Liver 1, B: Liver 2) and all partial grafts, perfusate glucose levels remained stable during long‐term perfusion sustained on infusions of glucose and glucagon. Towards the end of perfusion, there was a trend towards hypoglycemia with a corresponding increasing glucose requirement until the liver failed. (C) Perfusate pH was neutral or alkalotic throughout perfusion until the end of perfusion where acidosis predominated and was unresponsive to treatment. (D and E) Portal vein flow and corresponding resistance in the left lateral segment grafts (LLSG) (D) and extended right grafts (ERG) (E) remained stable until the end of perfusion where there was an increase in vascular resistance and a corresponding decrease in portal vein flow. (F and G) Hepatic artery flow and resistance in the left lateral segment grafts (F) and extended right grafts (G) remained stable during perfusion. There was an increase in hepatic artery flow in the left lateral segment graft towards the end of the perfusion. [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 4.
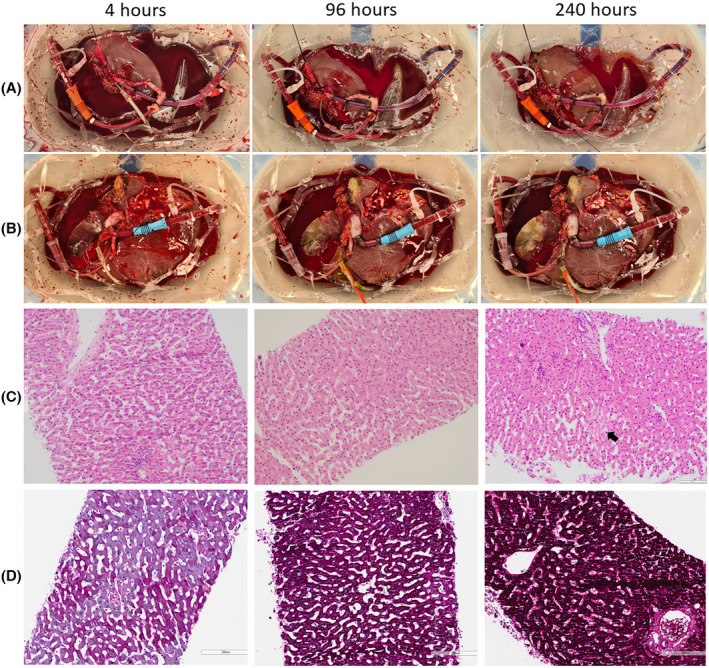
Macroscopic and microscopic evidence of preserved architectural integrity during long‐term ex situ machine perfusion of human livers. Macroscopic images of left lateral segment grafts (A), and extended right grafts (B) at 4, 96, and 240 h after splitting. Hematoxylin and eosin‐stained sections of core biopsies (C) were taken at 4, 96, and 240 h after splitting (200× magnification). Occasional patchy areas of necrosis are seen (arrow) but the liver architecture was preserved in all sections from 4 h to 240 h after splitting (scale bar 100 μm). Periodic‐acid Schiff‐stained sections of core biopsies (D) were taken at 4, 96, and 240 h after splitting (200× magnification, scale bar 200 μm). Glycogen deposition increases within hepatocytes over time demonstrating energy storage during perfusion. [Color figure can be viewed at wileyonlinelibrary.com]
To our knowledge, this is the longest ever reported ex situ perfusion of a liver under normothermic conditions. After surviving a surgical split, the partial grafts demonstrated preserved metabolic function for up to 13 days, 1 week longer than previous reports, which also used subnormothermic conditions (34°C). 4 , 5 , 6 We believe that the key modifications facilitating this extended survival were the dialysis filter for toxin removal and the air‐oxygen gas‐blender for intensive regulation of acid–base balance. This was also all achieved using a modified‐commercial system, making this an easily reproducible and repeatable model.
Currently available markers of liver function are by no means perfect. Unfortunately, livers in this study were not currently considered suitable for clinical use at our center and, therefore, evaluation of preserved function by transplantation was not possible. All 4 grafts, however, met the viability criteria for transplantation proposed by the VITTAL clinical trial (lactate ≤ 2.5 mmol/L, two or more of: bile production, pH ≥ 7.30, glucose metabolism, hepatic arterial flow ≥ 150 ml/min, and portal vein flow ≥ 500 ml/min, or homogeneous perfusion) 8 immediately prior to splitting and at 24 h after splitting (Table 1).
TABLE 1.
Viability assessment of whole livers prior to splitting and partial livers 24 h after splitting
| Whole liver viable a prior to splitting | Details | Partial liver viable a at 24 h after splitting | Details | |
|---|---|---|---|---|
| Liver 1 | Yes |
Lactate 0.93 mmol/L Bile production pH 7.313 |
LLSG: Yes |
Lactate 0.63 mmol/L Bile production pH 7.395 |
| ERG: Yes |
Lactate 1.03 mmol/L Bile production pH 7.394 |
|||
| Liver 2 | Yes |
Lactate 0.74 mmol/L Bile production pH 7.303 |
LLSG: Yes |
Lactate 0.83 mmol/L Bile production pH 7.552 |
| ERG: Yes |
Lactate 0.69 mmol/L Bile production pH 7.445 |
Abbreviations: ERG, extended right graft; LLSG, left lateral segment graft.
Viable according to the criteria proposed by the VITTAL clinical trial (lactate ≤ 2.5 mmol/L, and two or more of: bile production, pH ≥ 7.30, glucose metabolism, hepatic arterial flow ≥ 150 ml/min, and portal vein flow ≥ 500 ml/min, or homogeneous perfusion). 8
The successful ex situ perfusion of partial human livers for up to 13 days in this case series sets the new standard for long‐term perfusion. With ongoing developments in the evaluation of graft viability, 9 we believe that the value of this model is in its ability to provide the basis for a meaningful study of liver function ex situ in the long‐term. This may in turn permit the development of sophisticated viability protocols. Despite these grafts coming from young donors which could have contributed to their extended survival, questions remain about why these organs eventually failed. Possible causes could include failure to meet metabolic needs and overwhelming infection. Future work should aim to understand these reasons for organ failure with the goal of preservation for weeks to months to truly realize the potential of long‐term perfusion.
AUTHOR CONTRIBUTIONS
Ngee‐Soon Lau designed the study, performed the research, collected and analyzed the data, and drafted the manuscript. Mark Ly performed the research, collected and analyzed the data, and reviewed the manuscript. Claude Dennis collected and analyzed the data and reviewed the manuscript. Ken Liu and James Kench supervised the research, analyzed the data, and reviewed the manuscript. Michael Crawford and Carlo Pulitano supervised the research, designed the study, and reviewed the manuscript.
CONFLICT OF INTEREST
Nil.
ACKNOWLEDGMENTS
We acknowledge the technical support provided by S Chanda, C Wang, A Jacques, D Cheung, A Kulapvirat, F Mahboob, P Yousif, and G McCaughan. We would also like to thank DonateLife and all organ donors and their families for their support without which this research would not be possible. Financial support was provided by the Royal Prince Alfred Hospital Transplant Institute.
Contributor Information
Ngee‐Soon Lau, @soon_lau.
Carlo Pulitano, Email: carlo.pulitano@sydney.edu.au.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Vogel T, Brockmann JG, Quaglia A, Morovat A, Jassem W, Heaton ND, et al. The 24‐hour normothermic machine perfusion of discarded human liver grafts. Liver Transpl. 2017;23(2):207–20. [DOI] [PubMed] [Google Scholar]
- 2. Linares I, Selzner N, Selzner M. Machine preservation of the liver: what is the future holding? Curr Transplant Rep. 2018;5(1):82–92. [Google Scholar]
- 3. Lascaris B, de Meijer VE, Porte RJ. Normothermic liver machine perfusion as a dynamic platform for regenerative purposes: What does the future have in store for us? J Hepatol. 2022;S0168‐8278(22)00269‐0. 10.1016/j.jhep.2022.04.033 [DOI] [PubMed] [Google Scholar]
- 4. Eshmuminov D, Becker D, Bautista Borrego L, Hefti M, Schuler MJ, Hagedorn C, et al. An integrated perfusion machine preserves injured human livers for 1 week. Nat Biotechnol. 2020;38(2):189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mueller M, Hefti M, Eshmuminov D, Schuler MJ, Sousa da Silva RX, Petrowsky H, et al. Long‐term normothermic machine preservation of partial livers: first experience with 21 human hemi‐livers. Ann Surg. 2021;274(5):836–42. [DOI] [PubMed] [Google Scholar]
- 6. Karangwa SA, Dutkowski P, Fontes P, Friend PJ, Guarrera JV, Markmann JF, et al. Machine perfusion of donor livers for transplantation: a proposal for standardized nomenclature and reporting guidelines. Am J Transplant. 2016;16(10):2932–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lau NS, Ly M, Jacques A, Ewenson K, Mestrovic N, Almoflihi A, et al. Prolonged ex vivo normothermic perfusion of a split liver: an innovative approach to increase the number of available grafts. Transplant Direct. 2021;7(10):e763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mergental H, Laing RW, Kirkham AJ, Perera MTPR, Boteon YL, Attard J, et al. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat Commun. 2020;11(1):2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brüggenwirth IMA, de Meijer VE, Porte RJ, Martins PN. Viability criteria assessment during liver machine perfusion. Nat Biotechnol. 2020;38(11):1260–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


