Abstract
Liver resection (LR) is considered the treatment of choice for resectable neuroendocrine liver metastases (NELM), while liver transplantation (LT) is currently reserved for highly selected unresectable patients. We retrospectively analyzed data from consecutive patients undergoing either curative resection or transplantation for liver‐only NELM meeting Milan criteria at a single center between 1984 and 2019. Patients who fit Milan criteria were 48 in the transplantation group and 56 in the resection group. After a median follow‐up of 158 months for the transplantation group and 126 for the resection group, the 10‐year survival rate was 93% for transplantation and 75% for resection (p = .007). The 10‐year disease‐free survival rate was 52% for transplantation and 18% for resection (p < .001). Transplantation was associated with improved survival at univariate analysis. The median disease‐free interval between surgery and recurrence was 78 months for transplantation vs. 24 months for resection (p < .001). The transplantation group had more multisite recurrences (12/25, 48% vs. 5/42, 12% in the resection group, p = .001), while most recurrences in the resection group were intra‐hepatic (37/42, 88%, versus 2/25, 8% in the transplantation group). In conclusion, LT was associated with improved survival outcomes in NELM meeting the Milan criteria compared with LR.
Keywords: cancer/malignancy/neoplasia: metastatic disease; classification systems: Milan criteria, clinical decision‐making; clinical research/practice; hematology/oncology; liver disease: malignant, patient survival; liver transplantation/hepatology
Short abstract
Liver transplantation for neuroendocrine liver metastases within Milan Criteria is associated with improved patient outcomes compared to liver resection.
Abbreviations
- DFS
disease‐free survival
- LR
liver resection
- LT
liver transplantation
- NELM
neuroendocrine liver metastases
- OS
overall survival
1. INTRODUCTION
Neuroendocrine tumors (NETs) represent rare but increasingly prevalent neoplasms with heterogeneous clinical behavior. Gastro‐entero‐pancreatic (GEP)‐NETs frequently metastasize to the liver with up to 77% of patients developing neuroendocrine liver metastases (NELM) during the course of the disease. 1 The presence of NELM is a well‐recognized negative prognostic factor for long‐term survival. 2
The optimal management of patients with NELM is still controversial. Although no randomized controlled trials are available comparing surgery versus non‐surgical therapies, resection of the primary tumor and its metastases, when possible, remains the only curative treatment in patients with GEP‐NETs. 3 Surgical resection with curative intent is associated with excellent long‐term outcomes, but is feasible only in a small proportion of cases. 4 In carefully selected patients with liver‐only metastases, liver transplantation (LT) represents the most effective radical cure. 1 , 2 , 3 , 4 , 5 Excellent long‐term outcomes have been reported after LT under restrictive criteria, despite a significant incidence of NET long‐term recurrence. 6 The only currently available comparative study demonstrates a survival benefit of nearly 4 years at 10 years from the intervention, in favor of LT versus non‐transplant strategies 7 : based on this evidence, NET patients within Milan criteria can be listed for transplantation as MELD exceptions. 8 , 9
The current donor shortage and the heterogeneity in the results among different centers limit the application of LT for NELM. In addition, some retrospective studies reporting similar survival outcomes among patients within Milan criteria who underwent surgical resection compared to LT, might suggest resection as the first option for patients with resectable NELM. 10 Despite survival after liver resection (LR) is satisfactory, the recurrence rate of NELM undergoing LR is high and can approach 90%, with the large majority of recurrences occurring in the liver. 11
As data comparing long‐term outcomes after LR versus LT in the specific setting of patients with metastatic NET meeting Milan criteria (Milan‐in) are scanty, we sought to investigate the possible differences in the long‐term outcomes of a selected cohort of patients with Milan‐in NELM undergoing LR or LT at a tertiary referral center.
2. MATERIALS AND METHODS
2.1. Study design and study population
This is a retrospective analysis of a prospectively and consecutively collected series of patients with NELM who were assigned, after multidisciplinary board discussion, to surgery with curative intent (either LR or LT) at a single Institution (Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy) between 1984 and 2019. The present analysis was approved by the Institutional ethical and scientific review board and was conducted in accordance with the Helsinki Declaration as revised in 2013.
Only patients meeting Milan criteria for LT8 at the pre‐surgical staging were included in the analysis. These are as follows: (1) confirmed histology of low‐grade (G1‐G2) NET; (2) primary tumor drained by the portal system and resected prior to LT consideration; (3) metastatic diffusion to <50% of the total liver volume; (4) exclusion of extra‐hepatic disease; (5) stable disease for at least 6 months prior to LT consideration; and (6) age < 60 years (considered as a relative criteria). Other inclusion criteria were: (a) LT or LR for NELM performed as primary indication with curative intent; (b) complete clinical, radiological, and pathological data before and after surgery; and (c) at least 6 months of follow‐up after surgery.
The primary endpoint of this study was overall survival (OS). Secondary endpoints were disease‐free survival (DFS) and patterns of recurrence in resected and transplanted patients.
2.2. Diagnostic workup and therapeutic strategy
At baseline, patients underwent a thorough staging including total body contrast‐enhanced computed tomography (CT) scan, somatostatin receptor scintigraphy (Octreoscan) or positron emission tomography Gallium68 (PET‐Ga68) to exclude extra‐hepatic disease, full clinical examination, and laboratory tests including serum Chromogranin A (CgA) and specific peptides for functioning neoplasms.
As per Institutional policy, the treatment strategy was discussed within the multidisciplinary NET and liver tumor board for every patient. LR was offered to patients with resectable disease. In case of unresectable disease, patients were considered for LT in case of liver‐only metastatic spread, involving up to 50% of the liver, and no contraindications to transplantation. The primary tumor had to be removed before any LT consideration.
The definition of unresectable disease was the following:
Impossibility to achieve an R0 resection due to technical obstacles, or necessity of complex parenchyma‐regenerating procedures with a predicted risk of perioperative mortality >3% to achieve R0
Impossibility to guarantee sufficient post‐resection remnant liver volume.
LR was performed in patients with normal liver function and fit for surgery. A thorough assessment of remnant liver function was performed and this included indocyanine green retention test, liver scintigraphy, transient elastography, and full laboratory tests. LR included both anatomical resections and metastasectomies. Major resections were defined as right hepatectomy and left hepatectomy with our without associated wedge resections, and sectoriectomy with associated wedge resections. Minor resections were defined as wedge resection or segmentectomy. No routine lymphadenectomy was performed in case of resection, while patients undergoing LT also received lymphadenectomy of the hepatic hilum.
All transplants were performed with grafts from deceased donors. Exploratory laparotomy was performed and, if negative for extrahepatic dissemination, LT was carried out after total hepatectomy and lymphadenectomy including stations 12b, 12p 12a, eight, and nine. Vascular anastomoses were performed according to standard techniques.
After curative surgery, the patients were followed‐up with chest and abdomen CT scan, full blood tests including CgA and clinical examination every 6 months at least for the first 5 years, then annually thereafter. PET‐Ga68 was generally performed on an annual basis. Recurrence was defined as the presence of an histologically confirmed lesion, or of a persistently enlarging suspicious deposit at imaging associated with sustained increase of CgA or metabolically positive PET‐Ga.68
2.3. Data collection and terminology
Baseline patients' characteristics and data on the primary neoplasm (i.e. site, grading, functioning vs. non‐functioning status, pathology variables including the T and N status according to the WHO grading system for NETs of the GEP system 12 ), LT (i.e., timing, complications, type and levels of immunosuppression) or LR (i.e., timing, type of intervention, complications), pre‐surgery therapies (i.e., type of surgery on the primary tumor, loco‐regional treatments, somatostatin analogs [SSAs], peptide receptor radionuclide therapy‐PRRT, targeted therapies, chemotherapy), post‐surgery recurrence (i.e., time from LT or LR, site, and pattern of recurrence), treatments at recurrence, and long‐term outcomes (including OS and DFS) were collected.
2.4. Statistical analysis
All continuous variables were reported as mean ± standard deviation or as median and range, depending on the data distribution. Distribution of continuous variables was assessed with the Shapiro–Wilk's test. Categorical variables were reported as number of cases and percentages. Continuous variables were analyzed with the student's t‐test or Mann–Whitney test, as appropriate, while categorical variables were analyzed using Fisher's exact test.
OS was calculated as the interval between the date of surgery and the date of death for any reason, with censoring at the date of last follow‐up in alive patients. DFS was calculated as the interval between surgery and the date on which tumor recurrence was recorded at any site, with censoring at the date of death or last follow‐up in recurrence‐free patients. Proportional hazard assumption was verified by Schonfeld residual analysis, and survival curves were obtained with the Kaplan–Meier method and compared with the log‐rank test. Median follow‐up time was computed with the reverse Kaplan–Meier method. Post‐recurrence survival was calculated with the Kaplan–Meier method.
A univariate regression analysis of the entire cohort was conducted to assess preoperative and operative factors independently associated with OS and DFS; a univariate analysis including only patients who underwent LT was also carried out.
We also performed a subgroup analysis including patients older than 60 years.
All analyses were two‐sided, and statistical significance was defined as a p < .05. Statistical analyses were performed with the IBM SPSS Advanced Statistics 27.0 package.
3. RESULTS
3.1. Characteristics of the two cohorts
During the study period, 53 patients underwent LT and 96 patients underwent LR for NELM. Overall, 104 (70%) patients fit into Milan criteria. Patients who fit into Milan criteria were 48 (91%) for the LT group (four of the excluded patients had H3 disease, namely with tumor burden >50% of the liver, one had a G3 tumor) and 56 (58%) for the LR group (37 of the excluded patients were older than 60 years, seven had G3 tumors). The distribution of patients over the study period is shown in Figure 1.
FIGURE 1.
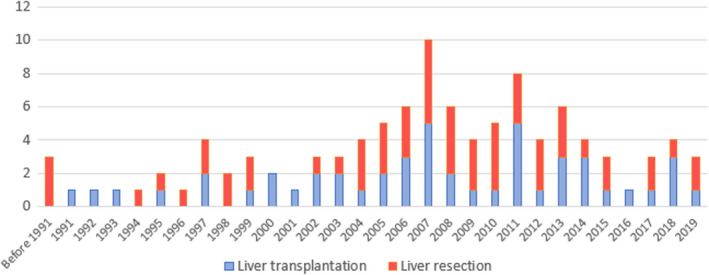
Patient distribution over the study period.
Demographic and preoperative variables are depicted in Table 1. Patients who underwent LT were significantly younger at diagnosis. The two cohorts had similar rates of synchronous liver metastases. In case of metachronous metastases, patients who underwent LT had a significantly higher N+ primary tumor and longer disease‐free interval between resection of the primary tumor and development of liver metastases with respect to the LR cohort.
TABLE 1.
Demographics and primary tumor characteristics
| Liver transplantation (n = 48) | Liver resection (n = 56) | p value | |
|---|---|---|---|
| Age at diagnosis | 44 (15–60) | 48 (24–60) | .007 |
| Sex | .069 | ||
| Female | 18 (37.5%) | 31 (55%) | |
| Male | 30 (62.5%) | 25 (45%) | |
| BMI | 23.5 (19–33) | 24 (20–32) | .587 |
| Primary tumor site | .872 | ||
| Small bowel | 31 (65%) | 33 (63%) | |
| Pancreas | 14 (29%) | 16 (31%) | |
| Duodenum | 2 (4%) | 1 (2%) | |
| Stomach | 1 (2%) | 2 (4%) | |
| Primary tumor N+ | 19/24 (79%) | 8/44 (18%) | <.001 |
| SSA treatment after primary tumor resection | 26 (54%) | 27 (48%) | .545 |
| Synchronous metastases | 40 (83%) | 45 (80%) | .695 |
| Time between primary tumor and metachronous liver metastases (months) | 95 (1–180) | 23 (2–132) | .003 |
Note: Data are number (percentage) or median (range) as appropriate.
Abbreviations: BMI, body mass index; N+, positive lymph nodes; SSA, somatostatin analogs.
Table 2 shows the characteristics of the two groups at the time of liver surgery. The median MELD at transplant in the presented cohort was seven (IQR 5–7). Patients who underwent LT were significantly younger at liver surgery than patients who underwent LR. The LT group had more patients with metastatic liver involvement >25%. Overall, 50 (89%) patients in the LR group received an R0 resection, while 6 (11%) were R1. At postoperative pathology, the lesions of the LT group had higher levels of Ki67 than the LR group.
TABLE 2.
Operative characteristics
| Liver transplantation (n = 48) | Liver resection (n = 56) | p value | |
|---|---|---|---|
| Age at liver surgery | 48 (15–60) | 50 (24–60) | .052 |
| Months between primary tumor resection and liver surgery | 38.5 (6–131) | 6.5 (0–131) | <.001 |
| CgA at liver surgery | 72 (15–2045) | 125 (7–6050) | .102 |
| Liver involvement | <.001 | ||
| <25% | 20 (42%) | 44 (79%) | |
| 25–50% | 21 (44%) | 12 (21%) | |
| >50% | 7 (15%) | 0 | |
| Extent of liver resection | – | ||
| Major resection | 20 (36%) | ||
| Minor resection | 36 (64%) | ||
| Radical surgery (R0) | – | 50 (89%) | |
| Grading | .187 | ||
| G1 | 26 (54%) | 37 (66%) | |
| G2 | 20 (42%) | 19 (34%) | |
| G3 | 2 (4%) | 0 | |
| Ki67 | 3 (0.2–30) | 2 (0.01–22) | .061 |
| N+ at liver hilum | 16 (33%) | – | |
| Immunosuppressive regimen | – | ||
| Cyclosporine | 6 (12.5%) | ||
| Tacrolimus | 40 (83%) | ||
| Tacrolimus + MMF | 2 (4%) |
Note: Data are number (percentage) or median (range) as appropriate.
Abbreviations: CgA, chromogranin A; MMF, mycophenolate mofetil; N+, positive lymph nodes.
3.2. Long‐term outcomes
Long‐term outcomes are shown in Table 3. The median follow‐up was 158 months (95% CI 131–184) for the LT group and 126 (95% CI 104–147) for the LR group. During the study period, nine deaths occurred in the LT group, of which three were cancer‐related, and 19 in the LR group, of whom 17 were cancer‐related. Of the six non cancer‐related deaths in the LT group, three occurred due to occurrence of another solid neoplasia, one due to Klebsiella pneumoniae‐related sepsis, one due to a lymphoproliferative disease, and one due to cardiovascular event. The majority of the deaths (17/19, 89%) in the LR group was due to disease progression in the liver and subsequent liver failure.
TABLE 3.
Long‐term outcomes
| Liver transplantation (n = 48) | Liver resection (n = 56) | p value | |
|---|---|---|---|
| Median follow‐up (months) | 158 (131–184) | 126 (104–147) | .538 |
| Overall survival rate | 39/48 | 35/56 | |
| Cancer‐related death | 3/9 | 17/19 | |
| Overall recurrence rate | 25/48 | 42/56 | |
| Time between liver surgery and recurrence (months) | 78 (13–204) | 24 (2–119) | <.001 |
| Site of first recurrence | |||
| Locoregional LNs | 7 (28%) | 2 (5%) | .007 |
| Distant LNs | 16 (64%) | 6 (14%) | <.001 |
| Bone | 9 (36%) | 3 (7%) | .003 |
| Liver | 2 (8%) | 37 (88%) | <.001 |
| Peritoneum | 5 (20%) | 1 (2%) | .015 |
| Lung | 1 (4%) | 1 (2%) | .706 |
| Other | 5 (20%) | 2 (5%) | – |
| Multisite recurrence | 12/25 (48%) | 5/42 (12%) | .001 |
Note: Data are number (percentage) or median (range) as appropriate. Median follow‐up is expressed as median (95% confidence interval).
Abbreviation: LN, lymph node.
Recurrences were 25 for the LT group (52%) and 42 for the LR group (75%).
Figure 2 shows the Kaplan–Meier curves comparing OS (Figure 1A), DFS (Figure 2B), and cancer‐related survival between the LT group and the LR group. The 3‐year, 5‐year, and 10‐year survival rates were 98%, 95.5%, and 93% for LT and 92%, 90%, and 75% for LR, respectively (p = .007). The 3‐year, 5‐year, and 10‐year DFS rates were 84%, 75%, and 52% for LT and 49%, 33%, and 18% for LR, respectively (p < .001).
FIGURE 2.
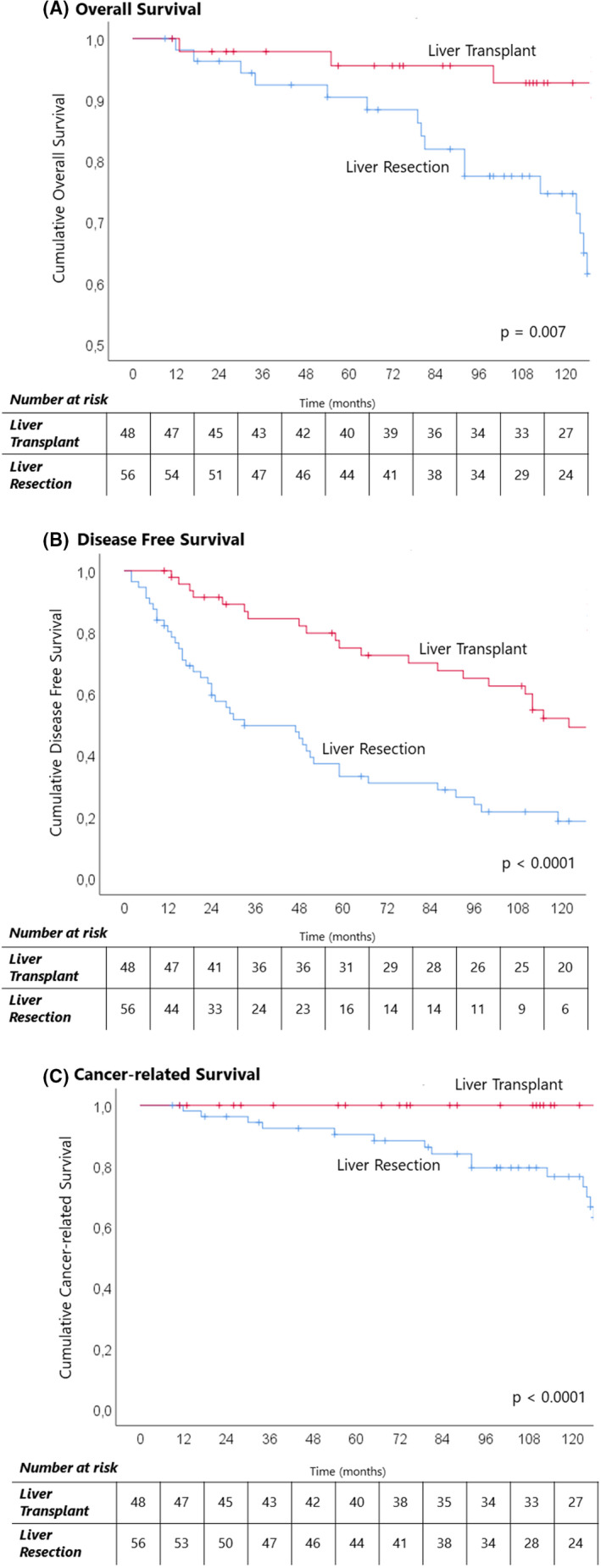
Kaplan–Meier curves of long‐term outcomes for Milan‐in patients who underwent liver transplantation and liver resection for NELM. (A) Overall survival; (B) disease‐free survival; (C) cancer‐related survival.
The median disease‐free interval between liver surgery and recurrence was longer for the LT group (78 months, vs 24 months for the LR group, p < .001).
The two groups exhibited different recurrence patterns, as shown in Figure 3. Patients in the LT group had more multisite recurrences (12/25, 48% vs 5/42, 12%, p = .001). Most of the recurrences in the LR group involved the liver (37/42, 88%, with 31 cases involving only the liver, versus 2/25, 8% in the LT group, all multisite). Patients who received LT tended to recur in the lymph nodes, either loco‐regional (7/25, 28%, vs 2/42, 5% in the LR group, p = .007) or distant (16/25 cases, 64%, vs 6/42, 14% in the LR group, p < .001), in the bone (9/25, 36%, vs 3/42, 7% in the LR group, p = .003), and in the peritoneum (5/25, 20%, vs 1/42, 2% in the LR group, p = .015). Other sites of recurrence for the LT group were ovaries in two cases, pleura in two cases, and lung, pancreas, breast, and sphenoid process in one case each. Other sites of recurrence for the LR group were one case in the skin and one in the ovary.
FIGURE 3.
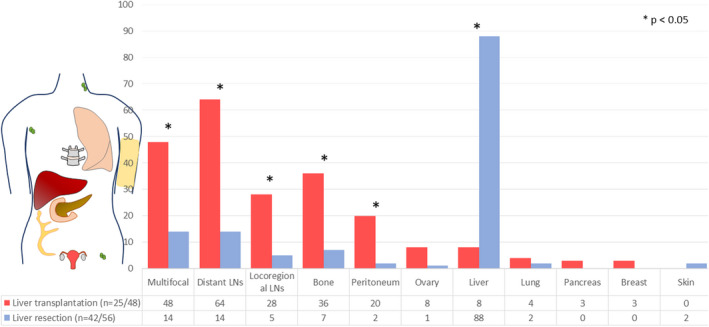
Different patterns of first recurrence in liver transplantation and liver resection for NELM. The numbers are percentages of patients.
Post‐recurrence survival was similar between the two groups (p = .276), with a 3‐year and 5‐year survival of 95% and 72% for LT and 83% and 69% for LR.
At univariate analysis, the only factor independently associated with OS and DFS was LT, as shown in Table 4. Univariate analysis including only patients who underwent LT was also performed: nodal involvement at final pathology was independently associated with worsened OS (HR 5.7, 95%CI 1.3–24, p = .004) and DFS (HR 2.6, 95%CI 1.1–5.9, p = .22), while liver involvement >50% and G3 tumor were not.
TABLE 4.
Univariate analysis of factors independently associated with OS and DFS
| Factor | Overall survival | Disease‐free survival | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | |
| Liver transplantation | 0.35 (0.16–0.78) | .010 | 0.36 (0.21–0.59) | <.0001 |
Abbreviation: CI, confidence interval.
3.3. Analysis including patients older than 60 years
Out of our entire cohort of patients undergoing surgery for NELM, a subgroup analysis including Milan‐in patients older than 60 years was also performed: included patients were 48 for the LT group and 89 for the LR group. The Kaplan–Meier curves reported in Figure 4A for OS and Figure 4B for DFS, respectively, confirmed survival outcomes significantly in favor of LT.
FIGURE 4.

Kaplan–Meier curves of long‐term outcomes for Milan‐in patients who underwent liver transplantation and liver resection for NELM, including patients older than 60 years. (A) Overall survival; (B) disease‐free survival.
4. DISCUSSION
Although the best treatment strategy for NELM is often challenging, surgery with radical intent remains the only potential curative treatment. In such respect, randomized controlled trials comparing surgery versus non‐surgical therapies are lacking, while large cohort studies have demonstrated that LR is associated with long‐term survival as high as 60–80% at 5 years among eligible patients. 3 As curative LR is not always feasible, LT represents an option for patients fulfilling specific restrictive criteria, among which the Milan criteria are associated with a 5‐year OS up to 90%. 13
In the current study, patients with a tumor burden meeting the Milan criteria showed longer OS and DFS after LT compared to patients who underwent LR, suggesting that LT should be preferred over LR in patients with limited, liver‐only NELM. This is in line with the only available prospective study on this topic which reported that, in well‐selected patients, LT is associated with a significant survival benefit when compared to non‐transplant strategies, 7 with the survival gain increasing with time and being maximized 10 years after LT.
Conversely, a recent retrospective study including 238 patients undergoing LR, of whom 12% met the Milan criteria for LT, reported promising 5‐ and 10‐year OS of 83.3% and 71.4%, respectively. Among patients meeting Milan criteria in conjunction with other favorable clinic‐pathological characteristics (i.e., G1 patients, patients undergoing minor LR, patients with 1–2 NELM and/or with tumor size <3 cm) an excellent 5‐year OS > 90% was achieved. 10 As these results of LR were comparable with those reported in the literature for patients undergoing LT for NELM within Milan criteria, it could be argued that resection with radical intent should be the first option for patients with resectable NELM, while LT should be reserved to patients with unresectable NELM.
In our series, approximately one‐third of patients with NELM had unresectable disease within Milan criteria: in our experience, NELM often present as ubiquitous granular lesions (“wax drops”) that, although involving <50% of hepatic volume, are not amenable to surgical resection.
According to the Italian organ allocation policies, transplant candidates with NELM are considered as third‐class priority (P3) MELD exceptions, 14 with priority increasing with time on the waiting list up to a maximum of 29 points. Overall, LT for NELM is offered quite infrequently with respect to other therapies 15 and, in the large majority of the presented transplants, donors of marginal/poor quality were used to compensate the shortage of available organs and the scarcity of high‐quality evidence (i.e., randomized trials) in favor of LT for NELM. Indeed, the ENETS guidelines recommend LT only as “an option in highly selected patients with carcinoid syndrome or other functional NET and extended liver disease, refractory to multiple systemic treatments including SSA, interferon (IFN)‐alpha, loco‐regional therapies and PRRT.” 1 As highlighted by the National Cancer Institute NET Clinical Trials Planning Meeting, 16 it is unlikely that randomized controlled trials in the setting of LT for NELM will ever be conducted. Nevertheless, the reported 97% survival at 5 years after LT in patients meeting Milan criteria 7 remains a comparator against which any other treatment available for NELM should be confronted.
Rather than being considered as palliative treatment in patients with unresectable NELM, the present study suggests that any patient with disease presentation meeting the Milan criteria may achieve a significant survival advantage if offered transplantation instead of resection.
The risk of recurrence represents an issue after either LR or LT for NELM with different timing and patterns of presentation. Post‐transplant recurrence mostly occurs either at distant lymph‐nodal stations, at distant organs or at multiple sites, whereas after resection the majority of recurrences are found within the liver. 17 Our current results confirm these findings, with 37 out of 42 (88%) recurrences in the LR group involving the liver, compared to only two out of 25 (8%) in the LT group (p < .001), which were all multisite. As previously reported, 6 post‐LT recurrence usually occurs in 30%–50% of the cases. However, excellent long‐term survival has been observed even after recurrence, especially when that occurs more than 24 months after transplant. In those cases, aggressive surgical treatment, if possible, might lead to a new chance of cure in a large proportion of patients.
Conversely, recurrence following LR of NELM can approach 50–95%, 11 with repeated LR for recurrent NELM reported to be feasible and associated with good long‐term survival outcomes in well‐selected patients. 18 In our study, the LT group had a median time to recurrence of 6.5 years, compared to 2 years for the LR group.
Whether the significant predominance of recurrence through the lymphatic route after LT with respect to LR was related to an indirect selection by the Milan criteria of tumors with a different biology is a matter of debate. Notably, while tumor grading and extrahepatic disease are included in the Milan criteria, the lymph‐nodal status of the primary tumor is not. Whether LT selection criteria should be further restricted on the basis of lymph nodal status, however, is questionable, considering the significant superiority of LT with respect to any other therapy available for NELM within Milan.
A potential limitation of the presented results in favor of LT for NELM within Milan criteria is that the two cohorts were not perfectly balanced at baseline, with the LT group consisting of younger patients with more aggressive tumor biology (i.e., showing higher levels of Ki67 at pathology) and a higher rate of lymph‐nodal involvement of the primary tumor. This could imply a possible selection bias for young patients with NELM: because of their longer life expectance, they might be offered transplantation more frequently than older patients despite adverse prognostic factors, such as lymph‐nodal status and tumor replication rate.
The time interval between resection of the primary tumor and liver surgery was significantly longer for patients undergoing LT. This reflects the policy of very aggressive surgical treatment in case of resectable liver metastases, while a cautious attitude was maintained in patients with unresectable NELM eligible to transplantation. Transplant candidates undergo a thorough staging process, which adds a test of time to screen out patients with rapidly progressive disease. In addition, while the percentage of metachronous liver metastases was similar between the two groups, those in the LT group tended to grow at a slower pace. Whether those factors could have led to the selection of candidates with a more favorable biology in the LT group is difficult to assess. It should be noted that the LT group achieved better outcomes despite a higher incidence of N+ primary tumors and worse pathologic features (higher tumor burden and more G2‐G3 tumors).
Regarding cancer‐related death, most of the deaths observed in the LR group were due to progressive disease within the liver evolving into liver failure. As previously noticed, LR could be considered a suboptimal loco‐regional treatment of NELM, as tumor is almost always left behind 19 regardless of the type of resection. Although LT seems to shift the problem of tumor control from inside the liver to extra‐hepatic locations, the demonstrated long‐term survival benefit of the transplant vs. non‐transplant options supports LT as a potential curative treatment. Patients undergoing LT should, however, be managed with integrated multidisciplinary peri‐transplant strategies.
Besides technical complexity and risk of morbidity, LT requires life‐long immunosuppressive therapy, which might predispose to long‐term complications including infection, de‐novo tumors or cardiovascular diseases. This is highlighted in the current series, with 6/9 patients dying of causes that were not cancer related, but, likely, due to LT sequelae.
The current study is a single‐center experience spanning more than three decades, during which changes in indications, classifications, and organ allocation policies have influenced the results. Due to the retrospective nature of the study and the long period of time considered, an intention‐to‐treat analysis taking into consideration dropouts from the transplant waiting list could not be performed. The two groups were retrospectively selected from historical cohorts and selection bias cannot be excluded. The presented series included a little more than a hundred patients, thus a relatively small sized cohort. This however represents to date the largest mono‐centric experience on patients with NELM within Milan criteria undergoing surgical treatment with curative intent.
The retrospective nature of our data prevents practice‐changing conclusions due to the high risk of selection bias. All conclusions should be confirmed by future studies: in particular, the impact of tumor biology on long‐term surgical outcomes and the association of neoadjuvant and adjuvant treatments with curative surgery require further investigation. However, the presented experience showed the efficacy and benefit of LT in patients with diffuse, bilateral, and non‐resectable intra‐hepatic NELM involving a limited portion of the liver (<50%). This puts into question whether it is recommendable to proceed with complex resections such as multi‐stage hepatectomies or ALPPS in those patients with NELM meeting the Milan criteria who could be marginally served by LRs at high risk of complications and recurrence. Our results could encourage clinicians to consider upfront LT in resectable patients with liver‐only diffuse, bilateral, non‐G3 NELM. Given the survival outcomes after LT and the high percentage of liver‐only recurrence after resection, LT could also be taken into consideration as salvage treatment in resected patients with recurrences within Milan criteria. As demonstrated in previous analyses, 7 the indication to LT should be tailored on the presumed transplant benefit achieved at long‐term intervals with respect to non‐transplant therapies. In the current conditions of limited donor availability, patients with NELM within Milan criteria should be considered for LT if young (i.e., with an expected survival of enough duration, to see the long‐term benefit of LT) and in case of recurring liver‐only metastases after resection (i.e., with limited expected survival and therefore eligible to salvage LT).
In summary, in the current study, LT was associated with better long‐term outcomes than LR in patients with NELM within Milan criteria. LT could be considered as the first treatment option for selected patients with NELM, provided restrictive clinical and biological selection criteria are applied and transplant listing for this indication is accepted.
FUNDING INFORMATION
No specific funding was assigned to the project.
CONFLICT OF INTEREST
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
ACKNOWLEDGMENT
Open access funding provided by BIBLIOSAN.
Maspero M, Rossi RE, Sposito C, Coppa J, Citterio D, Mazzaferro V. Long‐term outcomes of resection versus transplantation for neuroendocrine liver metastases meeting the Milan criteria. Am J Transplant. 2022;22:2598‐2607. doi: 10.1111/ajt.17156
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, VM, upon reasonable request.
REFERENCES
- 1. Pavel M, O'Toole D, Costa F, et al. ENETS consensus guidelines update for the Management of Distant Metastatic Disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology. 2016;103(2):172‐185. doi: 10.1159/000443167 [DOI] [PubMed] [Google Scholar]
- 2. Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335‐1342. doi: 10.1001/jamaoncol.2017.0589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fairweather M, Swanson R, Wang J, et al. Management of neuroendocrine tumor liver metastases: long‐term outcomes and prognostic factors from a large prospective database. Ann Surg Oncol. 2017;24(8):2319‐2325. doi: 10.1245/s10434-017-5839-x [DOI] [PubMed] [Google Scholar]
- 4. Frilling A, Clift AK. Therapeutic strategies for neuroendocrine liver metastases. Cancer. 2015;121(8):1172‐1186. doi: 10.1002/cncr.28760 [DOI] [PubMed] [Google Scholar]
- 5. Sposito C, Droz Dit Busset M, Citterio D, Bongini M, Mazzaferro V. The place of liver transplantation in the treatment of hepatic metastases from neuroendocrine tumors: pros and cons. Rev Endocr Metab Disord. 2017;18(4):473‐483. doi: 10.1007/s11154-017-9439-7 [DOI] [PubMed] [Google Scholar]
- 6. Sposito C, Rossi RE, Monteleone M, et al. Postrecurrence survival after liver transplantation for liver metastases from neuroendocrine tumors. Transplantation. 2021;105:2579–2586. Retrieved from https://journals.lww.com/transplantjournal/Fulltext/9000/Postrecurrence_Survival_After_Liver.95264.aspx. [DOI] [PubMed] [Google Scholar]
- 7. Mazzaferro V, Sposito C, Coppa J, et al. The long‐term benefit of liver transplantation for hepatic metastases from neuroendocrine tumors. Am J Transplant. 2016;16(10):2892‐2902. doi: 10.1111/AJT.13831 [DOI] [PubMed] [Google Scholar]
- 8. Mazzaferro V, Pulvirenti A, Coppa J. Neuroendocrine tumors metastatic to the liver: how to select patients for liver transplantation? J Hepatol. 2007;47(4):460‐466. doi: 10.1016/j.jhep.2007.07.004 [DOI] [PubMed] [Google Scholar]
- 9. Kalra A, Wedd JP, Biggins SW. Changing prioritization for transplantation: MELD‐Na, hepatocellular carcinoma exceptions, and more. Curr Opin Organ Transplant. 2016;21(2):120‐126. doi: 10.1097/MOT.0000000000000281 [DOI] [PubMed] [Google Scholar]
- 10. Ruzzenente A, Bagante F, Bertuzzo F, et al. Liver resection for neuroendocrine tumor liver metastases within Milan criteria for liver transplantation. J Gastrointest Surg. 2019;23(1):93‐100. doi: 10.1007/s11605-018-3973-9 [DOI] [PubMed] [Google Scholar]
- 11. Cloyd JM, Ejaz A, Konda B, Makary MS, Pawlik TM. Neuroendocrine liver metastases: a contemporary review of treatment strategies. Hepatobiliary Surg Nutr. 2020;9(4):440‐451. doi: 10.21037/hbsn.2020.04.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182‐188. doi: 10.1111/HIS.13975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rossi RE, Burroughs AK, Caplin ME. Liver transplantation for unresectable neuroendocrine tumor liver metastases. Ann Surg Oncol. 2014;21(7):2398‐2405. doi: 10.1245/S10434-014-3523-Y [DOI] [PubMed] [Google Scholar]
- 14. Cillo U, Burra P, Mazzaferro V, et al. A multistep, consensus‐based approach to organ allocation in liver transplantation: toward a “blended principle model.” Am J Transplant. 2015;15(10):2552‐2561. doi: 10.1111/AJT.13408 [DOI] [PubMed] [Google Scholar]
- 15. Clift AK, Frilling A. Liver transplantation and multivisceral transplantation in the management of patients with advanced neuroendocrine tumours. World J Gastroenterol. 2018;24(20):2152‐2162. doi: 10.3748/wjg.v24.i20.2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kulke MH, Siu LL, Tepper JE, et al. Future directions in the treatment of neuroendocrine tumors: consensus report of the National Cancer Institute neuroendocrine tumor clinical trials planning meeting. J Clin Oncol. 2011;29(7):934‐943. doi: 10.1200/JCO.2010.33.2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saxena A, Chua TC, Perera M, Chu F, Morris DL. Surgical resection of hepatic metastases from neuroendocrine neoplasms: a systematic review. Surg Oncol. 2012;21(3):e131‐e141. doi: 10.1016/J.SURONC.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 18. Spolverato G, Bagante F, Aldrighetti L, et al. Management and outcomes of patients with recurrent neuroendocrine liver metastasis after curative surgery: an international multi‐institutional analysis. J Surg Oncol. 2017;116(3):298‐306. doi: 10.1002/jso.24670 [DOI] [PubMed] [Google Scholar]
- 19. Frilling A, Sotiropoulos GC, Li J, Kornasiewicz O, Plöckinger U. Multimodal management of neuroendocrine liver metastases. HPB (Oxford). 2010;12(6):361‐379. doi: 10.1111/J.1477-2574.2010.00175.X [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, VM, upon reasonable request.


