1. INTRODUCTION
Thrombocytopenia is a common condition in several populations of hospitalized patients, including those with hematological and solid tumor cancer, 1 , 2 those with chronic liver disease, 3 and critically ill neonates 4 and adults, 5 and it has been associated with increased rates of bleeding, transfusion requirements, and mortality. 6 , 7 , 8 , 9 Prophylactic platelet transfusions are often recommended in patients with severe thrombocytopenia, but the supporting evidence is primarily derived from trials in hematological patients 10 , 11 , 12 and clinical practice varies considerably. 13 , 14 , 15 Prior to prophylactic platelet transfusion, the risk of bleeding and the beneficial effects of transfusion must be viewed in light of the potentially harmful effects, which, although rare, include serious and potentially life‐threatening reactions, such as anaphylaxis, transfusion‐transmitted infections, and transfusion‐related acute lung injury. 16 Harm from platelet transfusions has been observed in randomized clinical trials (RCTs) among preterm infants 17 and patients with intracerebral hemorrhage. 18 Therefore, we aimed to assess the benefits and harms of prophylactic platelet transfusions versus no prophylaxis on patient‐important outcomes in hospitalized patients with thrombocytopenia. We hypothesized that the evidence base for non‐hematological patients would be sparse and uncertain.
2. METHODS AND ANALYSES
2.1. Study design
This systematic review with meta‐analyses and trial sequential analyses (TSA) was registered in the International Prospective Register of Systematic Reviews (PROSPERO; CRD42021236014) and conducted in accordance with a published protocol. 19 We followed the recommendations by the Cochrane Collaboration, 20 the Grading of Recommendations Assessment, Development and Evaluation (GRADE) 21 approach and the Preferred Reporting Items for Systematic Review and Meta‐Analysis (PRISMA) statement (checklist available in Supplement S1). 22
2.2. Study selection
All RCTs and cluster RCTs comparing prophylactic platelet transfusion in any dose versus no prophylaxis or placebo in non‐bleeding hospitalized patients with thrombocytopenia (as defined in the trials) were eligible for inclusion without restriction regarding age, diagnoses, or settings. Cohort studies, case–control studies, reviews, quasi‐randomized trials, and cross‐over trials were excluded. We did not allow concomitant use of other interventions unless they were used in both allocation groups.
2.3. Outcomes
2.3.1. Primary outcome
All‐cause mortality at longest follow‐up.
2.3.2. Secondary outcomes
The proportion of participants with at least one episode of clinically important bleeding.
Days with clinically important bleeding.
The proportion of participants with at least one nosocomial infection.
The proportion of participants with at least one venous or arterial thromboembolic event.
The proportion of participants with at least one transfusion‐related adverse event.
Days alive without life support.
Length of hospital stay.
Health‐related quality of life.
Clinically important bleeding, nosocomial infection, venous or arterial thrombo‐embolic, and transfusion‐related adverse events were defined in the included trials. The unit of analysis was randomized patients, and all outcomes were assessed at the longest follow‐up. 19 Additional details are available in the protocol 19 and Supplement S3.
2.3.3. Process variables
We collected data on the number of units of platelets, red blood cells (RBC) and fresh frozen plasma (FFP) transfused per participant.
2.4. Search strategy
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, Embase, and Epistemonikos and searched for ongoing trials in the U.S. National Library of Medicine (ClinicalTrials.gov), EU Clinical Trials Register, and the World Health Organization (WHO) International Clinical Trials Registry. The searches were conducted without restrictions on language or publication status on March 29, 2021 and updated in PubMed on February 3, 2022 (Supplement S4).
2.5. Data collection and analysis
2.5.1. Study selection
All records were independently screened for eligibility by two authors (CTA, AG, PS, and NZ). Potential eligible articles were assessed in full text by two authors (CTA, AG, PS, and NZ). We resolved disagreements by discussion and consulted a third author (MHM and LR) if needed. We used Covidence (https://www.covidence.org; Veritas Health Innovation, Melbourne, Australia) to facilitate the study selection.
2.5.2. Data extraction and management
Two authors independently (CA and AG) extracted data from the included studies. We extracted data on trial characteristics, population characteristics, interventions, co‐interventions, outcomes, and process variables as specified above (Supplement S5). We contacted the corresponding authors at least twice for clarifications and unpublished or missing outcome data, if applicable. We resolved disagreements by discussion and involved a third author (MHM and LR) if needed.
2.5.3. Risk of bias in included trials
Two authors independently (CA and PS) assessed the risk of bias using the Risk of Bias 2.0 tool. 23 We assessed the risk of bias on the outcome level within all five domains: “bias arising from the randomization process,” “bias due to deviations from intended interventions,” “bias due to missing outcome data,” “bias in measurement of the outcome,” and “bias in selection of the reported result.” For each outcome in each RCT, the overall risk of bias judgment was judged as low risk of bias if all domains were judged to be low risk of bias, as some concerns if at least one domain was judged to be of some concerns, and as high risk of bias if at least one domain were judged to be high risk of bias. 20 , 23 Disagreements were resolved by consulting a third author (AG or MHM). We planned to base our primary conclusions on results from RCTs with an overall low risk of bias, 19 but this was only feasible for the primary outcome as no trials were judged to be low risk of bias for the secondary outcomes.
2.5.4. Measures of treatment effect
We calculated relative risks (RRs) with confidence intervals (CIs) for dichotomous outcomes and mean differences (MDs) with CIs for continuous outcomes. We contacted the corresponding authors for additional data if alternative statistical measures for continuous outcomes were reported. 19 We did not convert medians and interquartile range (IQR) to means and SDs, as correct conversion requires normally distributed data. Instead, we reported the results descriptively. Results are based on the analysis of intention‐to‐treat populations, and data was analyzed for superiority regardless of the original trial designs.
2.5.5. Adjusting for multiple testing
We adjusted thresholds for statistical significance using a compromise between no adjustment and a complete Bonferroni adjustment. 24 , 25 We divided the pre‐specified p‐value (.05) with the value half‐way between 1 (no adjustment) and the number of primary or secondary outcomes meta‐analyzed (Bonferroni adjustment). 24 , 25 As we were able to meta‐analyze the primary outcome and three secondary outcomes, the thresholds considered statistically significant were a p‐value of <.05 and <.025, respectively. For outcomes that were not meta‐analyzed, a p‐value of <.05 were considered statistically significant. The reported CIs and TSA‐adjusted CIs matched these significance thresholds.
2.5.6. Assessment of heterogeneity and subgroup analysis
We assessed statistical heterogeneity by the calculation of inconsistency (I 2), diversity (D 2) statistics, and the χ 2 test for subgroup differences and considered a p‐value of <.05 as statistically significant. 19 We report results from fixed effect models (FEM) if the I 2 = 0%. If I 2 > 0% we used both FEM and random effects models (REM) and based conclusions on the most conservative estimate (highest p‐value). 19 Results from both FEM and REM for the primary analyses are presented in Supplement S9.
We planned six subgroup analysis 19 :
Trials with overall low risk of bias versus trials with some concerns or high risk of bias.
Patients with hematological cancers versus patient with non‐hematological cancers versus patients without cancer.
Medical versus surgical versus mixed patient populations.
Patients undergoing invasive procedures versus patients not undergoing invasive procedures.
Neonates (including preterm infants) versus pediatric patients versus adult patients.
Intensive care unit (ICU) patients (including high‐dependency units) versus non‐ICU patients.
Additional details and the a priori hypothesized directions of subgroup effects are available in the protocol 19 and in Supplement S6.
Clinical heterogeneity was assessed using the Clinical Diversity in Meta‐analysis tool 26 and the credibility of the subgroup analyses were assessed using the Instrument for assessing the Credibility of Effect Modification Analyses (ICEMAN) tool. 27
2.5.7. Assessment of small trial bias
We planned to assess small trial bias but as all analyses included fewer than 10 RCTs, this was not feasible. 19
2.5.8. Trial sequential analysis
We conducted TSA to assess the risk of random errors due to repetitive testing in cumulative meta‐analyses. 28 In short, TSA estimates the required information size (RIS) needed for a conclusive meta‐analysis to detect or reject a predefined effect size. When data are sparse and/or statistical diversity is present, the TSA will adjust (expand) the CIs to account for the uncertainty around the overall effect estimate. 28 We report TSA‐adjusted CIs when feasible. We applied trial sequential monitoring boundaries according to 15% RR reduction for dichotomous outcomes and a MD of 1 day for continuous outcomes, an alpha of 5% and 2.5% for the primary and secondary outcomes, respectively, a beta of 10%, and a control event rate or variance suggested by the control groups in trials reporting on the outcome. 19 TSA is not feasible if the accrued information size was less than 5% of the RIS and in these circumstances, full TSAs were not presented.
2.5.9. Data synthesis and software
Analyses were conducted using R version 4.1.0 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria) with the “meta” package (version 5.1.0), and TSA was performed using the Copenhagen Trial Unit's TSA Software version 0.9.5.10b (available from http://www.ctu.dk/tsa).
2.5.10. Sensitivity analysis
We conducted a sensitivity analysis on the primary and secondary outcomes. 19 We performed analyses that included all bleeding episodes (i.e., not restricted to clinically important bleedings) and analyses restricted to long‐term all‐cause mortality, defined as mortality beyond 90 days. The impact of missing outcome data was assessed by performing best‐worst and worst‐best (BW/WB) case analyses, 24 and the information from zero‐event trials was accounted for by performing empirical continuity corrections. 29
2.5.11. Grade assessment
The overall certainty of evidence was rated independently by two authors (CA and AG) using the GRADE methodology 21 and disagreements were settled by discussion. We rated the certainty for each outcome as high, moderate, low, or very low based on assessments of risk of bias, inconsistency, indirectness, imprecision, and small trial bias.
3. RESULTS
3.1. Results of the search
We screened 15,696 records, assessed 66 records in full text and included nine trials in the qualitative synthesis 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 ; one trial did not report any relevant outcome data 34 and one trial was ongoing. 37 Hence, seven RCTs 30 , 31 , 32 , 33 , 35 , 36 , 38 enrolling a total of 1642 participants were included in the quantitative analysis (Figure 1). 39
FIGURE 1.
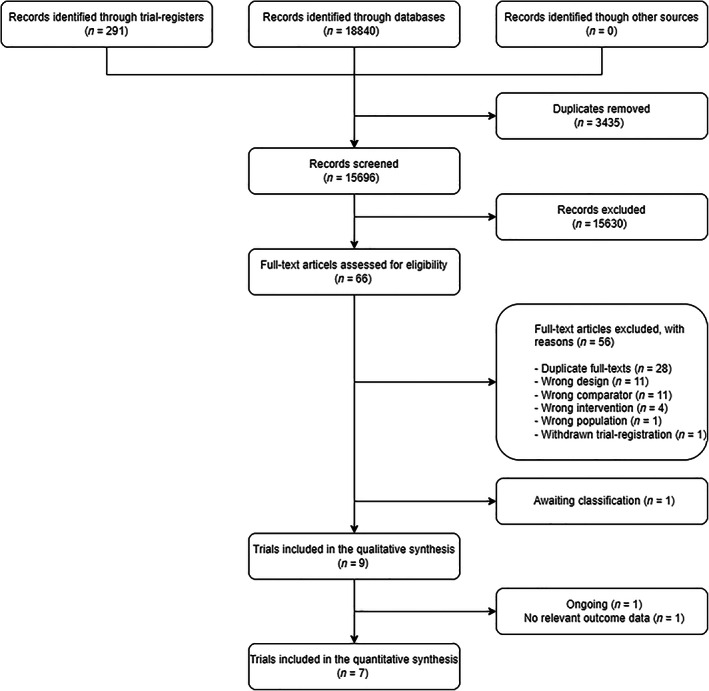
Flowchart of the trial selection process. One ongoing trial is awaiting classification due to insufficient information on the trial population.
3.2. Characteristics of included trials
The trials were published from 1980 until 2017. 30 , 31 , 32 , 33 , 34 , 35 , 36 , 38 Five trials were published as full reports, 30 , 32 , 33 , 36 , 38 one as a letter to the editor, 35 one as an abstract, 34 and one remained unpublished. 31 The smallest trial randomized 12 patients 34 whereas the largest randomized 600 patients. 36 Two trials were conducted at multiple centres 36 , 38 and all trials were conducted in hospitalized patients with hematological malignancies 31 , 33 , 34 , 35 , 36 , 38 or dengue fever. 30 , 32 One trial included pediatric (hematological) patients only, 33 and one was a non‐inferiority trial. 36 Trial characteristics are presented in Table 1 and further detailed in the Supplement S7.
TABLE 1.
Trial characteristics of the included trials
| Author, year | Country centers setting | Inclusion criteria | Exclusion criteria | Randomized, n (P/no P) | Trial outcomes | Follow‐up |
|---|---|---|---|---|---|---|
| Assir et al., 2013 |
Pakistan 1 Dengue HDU |
Adults ≥ 14 years, dengue fever or dengue hemorrhagic fever, platelet counts < 30 × 109/L, no or mild bleeding (WHO grade 1 or 2) | Other causes of thrombocytopenia, chronic ailments, history of platelet transfusion, severe bleeding (WHO grade 3 or 4) | 48 a (21/24) |
Primary:
Secondary:
|
72 h for the primary outcomes. Follow‐up for secondary outcomes unclear. |
| Grossman et al., 1980 |
Canada 1 Hospital ward |
Patients with amegakaryocytic thrombocytopenia, platelet counts less than 50 × 109/L | Refractory to platelet transfusions, not candidate for aggressive therapy, thrombocytopenia not expected to last for more than 7 days | 100 (49/51) |
Primary: Not specified
Secondary:
|
Unclear. Patients were followed throughout their initial hospital stay and all subsequent admissions, (mean length of follow‐up was 42.7 days in the P‐group and 41.6 days in the no P‐group). |
| Lye et al., 2017 |
Singapore, Malaysia 5 Hospital ward b |
Patients ≥ 21 years, probable or confirmed dengue (WHO 1997 or 2009 criteria), platelet count ≤20 × 109/L | Platelet count > 20 × 109/L, signs of clinical bleeding, pregnancy, lactating women, history of severe reactions to blood product transfusion, prior platelet transfusion within the same illness episode, patients likely to die within 48 h, history of peptic ulcer within 3 months, anticoagulants' use within 4 weeks, chronic liver disease, chronic renal failure or dialysis, active hematological or autoimmune disease, non‐availability of platelet supply from local blood bank, consent unobtainable | 372 (188/184) |
Primary:
Secondary:
|
21 days |
| Murphy et al., 1982 |
USA 1 Hospital ward b |
Children with previously untreated acute leukemia | Not reported | 56 (35/21) |
Primary: Not specified
Secondary:
|
Unclear. Patients were followed from study entry until death or study closure (mean length of follow‐up was 19.9 months in the P‐group and 20.4 in the no P‐group). |
| Sintnicolaas et al., 1981 |
Netherlands 1 b Hospital ward b |
Patients with acute leukemia and severe thrombocytopenia | Not reported | 12 c (?/?) |
Primary: Not specified
Secondary:
|
Unclear |
| Solomon et al., 1978 |
USA 1 b Hospital ward b |
Patients with previously untreated non‐lymphoblastic acute leukemia | Promyelocytic leukemia | 31 (19/12) |
Primary: Not specified
Secondary:
|
Within 1 month of chemotherapy course |
| Stanworth et al., 2013 |
United Kingdom, Australia 14 Hospital ward |
Patients ≥ 16 years, undergoing chemotherapy or stem‐cell transplantation to treat a hematological cancer, platelet count < 50 × 109/L or expected to be so for at least 5 days, able to comply with treatment and monitoring | Previous WHO grade 3 or 4 bleeding, WHO grade 2 bleeding during current admission, inherited hemostatic or thrombotic disorder, requirement for therapeutic doses of anticoagulant agents, acute promyelocytic leukemia, known HLA antibodies, pregnancy, prior randomization into the trial | 600 (299/301) |
Primary:
Secondary:
|
30 days |
| Wandt et al., 2012 |
Germany 8 Hospital ward |
Patients 16–80 years, undergoing intensive chemotherapy for acute myeloid leukemia or autologous haemopoietic stem‐cell transplantation for hematological cancers | Refractory to platelet transfusions, previous major bleeding, plasmatic coagulopathy, pulmonary or cerebral lesions (stem‐cell transplantations only) | 396 (197/199) |
Primary:
Secondary:
|
Unclear. The study was completed when the platelet count was self‐sustaining at more than 20 × 109 per L for 2 days or a maximum of 30 days, at hospital discharge, when treatment failure was diagnosed, at death, or at study withdrawal, which ever occurred first. |
| NCT03713489 (Ongoing) |
China 1 Unclear |
Patients 18–60 years, diagnosed with acute‐on‐chronic liver failure and chronic hepatitis B infection and ADP inhibition of ≥70% |
Other causes of chronic live disease than chronic hepatitis B infection, previous decompensation, intracranial hemorrhage, use of anti‐platelet or anticoagulants therapy within 4 weeks, esophageal variceal bleeding within 1 week, platelet transfusion within 1 week, malignant disease, pregnancy or breastfeeding, severe chronic extra‐hepatic disease. Considered not suitable for inclusion by researchers. |
Estimated enrolment: 20 |
Primary:
Secondary:
|
28 days for the primary outcome. Unclear for the secondary outcome. |
| van de Weerdt et al. (Ongoing) |
Netherlands 11 Hospital ward/ICU |
Adult (≥18 years) hematologic or ICU patients with thrombocytopenia (10–50 × 109/L) scheduled for emergency or elective insertion or replacement of a central line and an expectation of the inserted line to be in situ for at least 24 h | Patients with a non‐correctable INR < 1.5, history of congenital or acquired coagulation factor deficiency or bleeding diathesis, treatment with double platelet‐aggregation inhibitors or therapeutic unfractionated heparin not discontinued at least 1 h prior to insertion | Planned enrolment: 392 (196/196) |
Primary:
Secondary:
|
28 days |
Abbreviations: ADP, adenosine diphosphate; CVC, central venous catheter; HDU, high dependency unit; HEME, hemorrhage measurement; HLA, human leukocyte antigen; ICU, intensive care unit; INR, international normalized ratio; P, prophylaxis; RBC, red blood cells; WHO, World Health Organization.
Eighty‐seven patients were randomized but 39 patients had bleeding (WHO grade 1 or 2) at randomization and were ineligible for this review.
Assumption; not clearly reported.
Allocation to intervention groups not reported.
3.3. Interventions
All trials assessed prophylactic platelet transfusion compared with no prophylaxis; however, in two trials, prophylactic platelet transfusions were also administered in the no prophylaxis group under specific circumstances with a perceived high risk of bleeding. 35 , 38 Two trials administered prophylactic platelets in both groups prior to invasive procedures or surgery 31 , 36 and most trials administered platelets in both treatment groups when bleeding occurred. 31 , 32 , 35 , 36 , 38 One trial used a platelet count ≤30 × 109/L as a threshold for prophylactic transfusion, 30 five trials used ≤20 × 109/L 31 , 32 , 33 , 34 , 35 and two trials used ≤10 × 109/L. 36 , 38 Duration of the intervention, type of platelets, number of units administered per transfusion and platelets per unit varied between trials (Table 2).
TABLE 2.
Interventions and definitions and assessments of bleeding in the included trials
| Author, year | Intervention (prophylaxis group) | Control (no prophylaxis group) | Bleeding scale | Definitions of bleeding | Assessment of bleeding |
|---|---|---|---|---|---|
| Assir et al., 2013 | Platelet transfusion with 1 SD platelet unit (≥5 × 1011 platelets/unit) at study entry | No platelet transfusion | WHO Bleeding scale |
Clinically important bleeding: “Severe bleeding” defined as WHO grade 3 and 4 Any bleeding: “New onset bleeding” defined as WHO grade 1–4 |
Assessor: Not reported Assessment: Patients were assessed for WHO bleeding ever 12 h |
| Grossman et al., 1980 | Platelet transfusion with 1 unit of either SD (4.8 × 1011 platelets/unit) or RD (4.8–6.4 × 1011/unit) platelets to maintain platelet count above 20 × 109/L through all hospital admissions | Platelet transfusions were given for clinically significant bleeding and prior to invasive procedures | Study specific |
Clinically important bleeding: “Severe bleeding” Any bleeding: “Mild bleeds” defined as bleeds not requiring active intervention |
Assessor: The clinical team performed the assessment Assessment: Daily clinical assessment for signs of bleeding. Fundoscopic examination twice daily when the platelet count was ≤20 × 109/L. |
| Lye et al., 2017 |
Supportive care plus 4 units RD (platelets/unit not reported) platelets each day the platelet count was ≤20 × 109/L up until day 7 or discharge If bleeding occurred, platelet transfusions were given at the clinician's discretion in both groups |
Supportive care | None |
Clinically important bleeding: Defined according to the WHO 2009 dengue guidelines: gum, nose, hemoptysis, hematuria, hematemesis, melaena, melaena or hematemesis‐not controlled by procedure, menorrhagia, menorrhagia or intermenstrual bleeding‐not controlled by progesterone, intermenstrual, hematoma, menses, others Definition of any bleeding: Not reported |
Assessor: Not reported Assessment: Daily clinical assessment from day 1 until day 7 or discharge and at day 21 (+/−3) |
| Murphy et al., 1982 | Platelet transfusion with RD (4 units/m2 body surface, platelets/unit not reported) platelets when platelet count was <20 × 109/L irrespective of clinical events. The goal was to maintain a platelet count above 20 × 109/L throughout the patient's course. | Platelet transfusion were given for serious bleeding episodes | None |
Definition of clinically important bleeding: Serious bleeding episodes (bleeds) was defined as nasal or oral bleeding requiring packing, gross gastrointestinal bleeding, gross genitourinary bleeding, any central nervous system bleeding, or bleeding requiring red blood cell transfusion. Uncomplicated dermal bleeding was not included. Definition of any bleeding: Not reported |
Assessor: Not reported Assessment: Not reported |
| Sintnicolaas et al., 1981 | Platelet transfusion (units/transfusion not reported) with either RD or SD platelets (4 × 1011 platelets/unit) were given to maintain a platelet count above 20 × 109/L. Duration unclear. | Platelet transfusion were given for hemorrhage only | None |
Clinically important bleeding: Not reported Any bleeding: Not reported |
Assessor: Not reported Assessment: Not reported |
| Solomon et al., 1978 | Platelet transfusion with RD platelet (units/transfusion and dose not reported) whenever platelet count was <20 × 109/L and when clinically significant bleeding occurred throughout the patient's course | Platelet transfusion were given when clinically significant bleeding occurred or when a platelet count of <20 × 109/L was preceded by a decline of 50% in the platelet count during the preceding 24 h | Bleeding was not assessed |
Clinically important bleeding: NA Any bleeding: NA |
Assessor: NA Assessment: NA |
| Stanworth et al., 2013 |
Platelet transfusion with 1 unit of primarily RD (>240 × 109/L platelets/unit) platelets when platelet count was <10 × 109/L and continued daily until the platelet count is greater than 10 × 109/L for 30 days Both groups were transfused with platelets if bleeding of WHO grade 2 or more occurred and prior to invasive procedures or surgery |
Platelets transfusions were not prophylactically given irrespective of platelet counts | Modified WHO Bleeding scale |
Clinically important bleeding: “Clinical bleeding” was defined as bleeding of a modified WHO grade 2 or higher Any bleeding: Not reported |
Assessor: Local research nurse for inpatients and self‐assessment for discharged patients (unblinded) Assessment: Daily standardized bleeding assessment forms were completed each day that the patient was in hospital. Self‐assessed bleeding diaries were completed for patients who were discharged. |
| Wandt et al., 2012 |
Platelet transfusion with 1 unit SD (200‐400 × 109 platelets/unit) or RD (>200 × 109/unit) platelets was given when platelet count was ≤10 × 109/L If bleeding continued despite one platelet transfusion, further transfusions was given at the discretion of the treating clinician in both groups |
Stable patients were only given platelet transfusion when clinically relevant bleeding occurred. Unstable patients with platelet count was ≤10 × × 109/L were given prophylactic platelet transfusions. | Modified WHO Bleeding scale |
Clinically important bleeding: “Clinically relevant bleeding” was defined as a modified WHO grade 2 or higher Any bleeding: Not reported |
Assessor: A physician or experienced nurse (unblinded). Two investigators masked to treatment strategy later transformed the bedside bleeding report into modified WHO categories. Assessment: Clinical bleeding assessments was performed twice daily |
| NCT03713489 (ongoing) | Platelet transfusion with 1 unit of SD (platelets/unit not reported) platelets 3 times for the first week after enrolment, then 2 times a week in the following 3 weeks | Standard care | Bleeding not planned to be assessed |
Clinically important bleeding: NA Any bleeding: NA |
Assessor: NA Assessment: NA |
| van de Weerdt et al., (ongoing) |
Platelet transfusion with 1 unit of RD (platelets/unit not reported) platelets prior to placement of central catheters The proceduralist can administer rescue platelets at clinical indication in both arms in case of procedure related bleeding |
No platelet transfusion | Modified WHO Bleeding scale |
Clinically important bleeding: Modified WHO grade 2–4 Any bleeding: Not reported |
Assessor: Not reported Assessment: Clinical bleeding will be assessed at 1 h and 24 h post‐procedural. Clinical photos taken at 1 h and 24 h will be used to evaluate size of hematoma in a blinded fashion. |
Abbreviations: NA, not applicable; RD, pooled random donor platelets/buffy coat; SD, single donor apheresis platelets; WHO, World Health Organization.
3.4. Assessment of bleeding, bleeding scales and definitions of clinically important bleeding
Two trials reported bleeding as the primary outcome. 32 , 36 Three trials explicitly described who performed the assessment of bleeding outcomes 31 , 36 , 38 and the method of bleeding assessment was described in five trials. 30 , 31 , 32 , 36 , 38 Newer trials typically used the WHO scale in the original 30 or a modified version 36 , 38 to grade bleeding severity whereas older studies either used a study specific bleeding scale 31 or none at all. 33 , 34 We used the trials' definitions of clinically important bleeding, which varied substantially (Table 2).
3.5. Risk of bias in included trials
An overview of risk of bias for all outcomes are provided in Table 3, with supportive comments available in Supplement S8. For the primary outcome, one trial was judged as overall low risk of bias. The domains “bias due to deviations from intended interventions” and “bias in selection of the reported results” were generally of concern in the remaining trials. For the remaining outcomes, all trials were judged to be of “some concerns” or “high risk of bias” with the domain “bias in measurement of the outcome” representing concerns for subjective outcomes.
TABLE 3.
Risk of bias in the included trials
| Risk of bias domain (assessment for the effect of assignment to intervention) | Overall risk of bias | |||||
|---|---|---|---|---|---|---|
| Outcome and study | 1. Randomization process | 2. Deviations from intended interventions | 3. Missing outcome data | 4. Measurement of the outcome | 5. Selection of the reported result | |
| All‐cause mortality | ||||||
| Lye et al., 2017 | Low | Low | Some concerns | Low | Low | Some concerns |
| Murphy et al., 1982 | Some concerns | Some concerns | Low | Low | Some concerns | Some concerns |
| Solomon et al., 1978 | Some concerns | Some concerns | Low | Low | Some concerns | Some concerns |
| Stanworth et al., 2013 | Low | Low | Low | Low | Low | Low |
| Wandt et al., 2013 | Low | Some concerns | Low | Low | Some concerns | Some concerns |
| Clinically important bleeding | ||||||
| Grossman et al., 1980 | High | Low | Low | High | Some concerns | High |
| Lye et al., 2017 | Low | Low | Some concerns | High | Low | High |
| Murphy et al., 1982 | Some concerns | Some concerns | Low | High | Some concerns | High |
| Stanworth et al., 2013 | Low | Low | Low | Some concerns | Low | Some concerns |
| Wandt et al., 2012 | Low | Some concerns | Low | Some concerns | Some concerns | Some concerns |
| Days with clinically important bleeding | ||||||
| Murphy et al., 1980 | Some concerns | Some concerns | Low | High | Some concerns | High |
| Stanworth et al., 2013 | Low | Low | Low | Some concerns | Low | Some concerns |
| Nosocomial infection | ||||||
| Stanworth et al., 2013 | Low | Low | Low | Some concerns | Low | Some concerns |
| Transfusion related adverse events | ||||||
| Lye et al., 2017 | Low | Some concerns | Some concerns | Some concerns | Low | Some concerns |
| Stanworth et al., 2013 | Low | Low | Low | Some concerns | Low | Some concerns |
| Wandt et al., 2012 | Low | Some concerns | Low | Some concerns | Some concerns | Some concerns |
| Length of hospital stay | ||||||
| Lye et al., 2017 | Low | Some concerns | Some concerns | Some concerns | Low | Some concerns |
| Stanworth et al., 2013 | Low | Low | Low | Some concerns | Low | Some concerns |
| Wandt et al., 2012 | Low | Some concerns | Low | Some concerns | Some concerns | Some concerns |
| Any bleeding (sensitivity analysis) | ||||||
| Assir et al. 2013 | Some concerns | Some concerns | Low | High | Some concerns | High |
| Grossman et al., 1980 | High | Low | Low | High | Some concerns | High |
| Lye et al., 2017 | Low | Low | Some concerns | High | Low | High |
| Murphy et al., 1982 | Some concerns | Some concerns | Low | High | Some concerns | High |
| Stanworth et al., 2013 | Low | Low | Low | Some concerns | Low | Some concerns |
| Wandt et al., 2012 | Low | Some concerns | Low | Some concerns | Some concerns | Some concerns |
| Days with any bleeding (sensitivity analysis) a | ||||||
| Murphy et al., 1980 | Some concerns | Some concerns | Low | High | Some concerns | High |
| Stanworth et al., 2013 | Low | Low | Low | Some concerns | Low | Some concerns |
| Long term all‐cause mortality (>90 days) (Sensitivity analysis) | ||||||
| Murphy et al., 1982 | Some concerns | Some concerns | Low | Low | Some concerns | Some concerns |
Wandt et al. (2012) did not report days with clinically important bleeding with individual patients as the unit of analysis and hence the study was not included in risk of bias assessment for that outcome.
3.6. Primary outcome
3.6.1. All‐cause mortality at longest follow‐up in overall low risk of bias trials
One low risk of bias trial reported data on all‐cause mortality at the longest follow‐up (n = 598) and showed uncertain results (RR 0.81; 95% CI 0.22 to 2.97, p = .75). 36 TSA could not be performed as only 0.6% of the RIS of 102,293 patients had been accrued. The certainty of evidence was low (Table 4). The sensitivity analysis was consistent with the primary analysis (Supplement S13).
TABLE 4.
Grading of Recommendations Assessment, Development and Evaluation evidence profile
| Certainty assessment | No. of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Prophylactic platelet transfusion | No prophylaxis | Relative (95% CI) | Absolute (95% CI) | ||
| All‐cause mortality (low risk of bias trials only) a | ||||||||||||
| 1 | Randomized trials | Not serious | Not serious | Not serious | Very serious b | None | 4/298 (1.3%) | 5/300 (1.7%) | RR 0.81 (0.22 to 2.97) | 3 fewer per 1.000 (from 13 fewer to 33 more) |
⊕⊕◯◯ Low |
Critical |
| All‐cause mortality (all trials) a | ||||||||||||
| 5 | Randomized trials | Serious c | Not serious d | Not serious e | Very serious f | None | 27/714 (3.8%) | 21/684 (3.1%) | RR 1.00 (0.59 to 1.69) | 0 fewer per 1.000 (from 13 fewer to 21 more) |
⊕◯◯◯ Very low |
Critical |
| Proportion of participants with at least one episode of clinically important bleeding a | ||||||||||||
| 5 | Randomized trials | Serious g | Serious h | Not serious i | Serious j | None | 218/647 (33.7%) | 284/629 (45.2%) | RR 0.70 (0.53 to 0.92) | 135 fewer per 1.000 (from 212 fewer to 36 fewer) |
⊕◯◯◯ Very low |
Important |
| Days with clinically important bleeding a | ||||||||||||
| 2 | Randomized trials | Serious k | Serious l | Not serious m | Not serious n | None | Stanworth 2013 reported a MD (95% CI) in number of days with clinically important bleeding of 0.5 (0.1–0.9) days fewer in the prophylaxis group versus the no prophylaxis group. Murphy 1982 reported a mean of 1.9 days with clinically important bleeding in the prophylaxis group versus 2.2 days in the no prophylaxis group. |
⊕⊕◯◯ Low |
Important | |||
| Proportion of participants with at least one nosocomial infection a | ||||||||||||
| 1 | Randomized trials | Serious o | Not serious | Not serious p | Very serious q | None | 14/466 (3.0%) | 16/453 (3.5%) | RR 0.89 (0.40 to 1.97) | 4 fewer per 1.000 (from 21 fewer to 34 more) |
⊕◯◯◯ Very low |
Important |
| Proportion of participants with at least one venous or arterial thromboembolism a —not reported | ||||||||||||
| — | — | — | — | — | — | — | — | — | — | — | — | Important |
| Proportion of participants with at least one transfusion‐related adverse event a | ||||||||||||
| 3 | Randomized trials | Serious r | Serious s | Not serious t | Very serious u | None | 35/660 (5.3%) | 27/650 (4.2%) | RR 2.54 (0.27 to 23.61) | 64 more per 1.000 (from 30 fewer to 939 more) |
⊕◯◯◯ Very low |
Important |
| Days alive without the use of life support a —not reported | ||||||||||||
| — | — | — | — | — | — | — | — | — | — | — | — | Important |
| Length of hospital stay a | ||||||||||||
| 3 | Randomized trials | Serious v | Serious w | Not serious e | Not serious x | None | We were able to meta‐analyze data from Lye 2017 and Wandt 2012 which showed a MD (97.5% CI) in length of hospital stay of 0.23 days fewer (0.60 fewer to 0.13 more) in the prophylaxis group versus the no prophylaxis group. Stanworth 2013 reported the median (IQR) days to be 12 (9–18) versus 12 (9–18) in the prophylaxis group (n = 298) and no prophylaxis group (n = 300) respectively. |
⊕⊕◯◯ Low |
Important | |||
| Quality of life a —not reported | ||||||||||||
| — | — | — | — | — | — | — | — | — | — | — | — | Important |
Abbreviations: CI, confidence interval; IQR, interquartile range; MD, mean difference; RR, relative risks.
All outcomes were assessed at longest follow‐up.
Only one trial was included. There were very few events and CI around effect estimate includes substantial benefit and harm. Trial sequential analysis (TSA) could not be performed as the accrued information size (AIS) of 598 patients only corresponds to 0.59% of the required information size (RIS) of 102,293 patients. Thus, we rated down two levels.
Effect estimates in trials at overall low risk of bias versus trials at some concerns or high risk of bias does not seem to differ. However, there are very few events and CI a broad making judgment difficult. Only one trial was at overall low risk of bias and trials at some concerns or high risk of bias contributed with 84% of the weight in the meta‐analysis. Thus, we rated down one level.
We detected no statistical heterogeneity, I 2 = 0% (p = .91), D 2 = 0%. The Clinical Diversity in Meta‐analyses (CDIM) score was 11 corresponding to “low” clinical diversity. Thus, we did not to rate down for inconsistency.
Only trials conducted in the hematological and infectious disease ward setting contributed to the meta‐analysis. Results may not be applicable to other hospitalized patient populations (i.e., surgical patients, critically ill patients, neonates), but as the all trials reported on the defined patient population, we did not to rate down for indirectness.
The meta‐analysis included very few events and the CI around both the relative and absolute effect estimates include substantial benefit and harm. TSA could not be performed as the AIS of 1398 patients only corresponds to 2.0% of the RIS of 52,950 patients. Thus, we rated down two levels.
All trials were at some concerns or high for risk of bias. We chose to rate down one level as there is no information from low risk of bias trials and as it is thus impossible to estimate the effects of risk of bias.
We detected substantial statistical heterogeneity; I 2 = 59% (p = .04), D 2 = 72%. The CDIM score was 17 corresponding to moderate clinical heterogeneity. However, all effect estimates favored prophylaxis. Thus, we rated down one level.
Only trials conducted in the hematological and infectious disease ward setting contributed to the meta‐analysis. Results may not be applicable to other hospitalized patient populations (i.e., surgical patients, critically ill patients, neonates), but as all trials reported on the defined patient population, we did not to rate down for indirectness.
TSA highlighted that the AIS of 1276 patients corresponds to 13.4% of the RIS of 9546 patients. The TSA adjusted CI: 0.26 to 1.87 includes both substantial benefit and harm corresponding to 334 fewer to 388 more per 1000 patients. As the D 2 = 72% is high, the TSA adjusted CIs are broadened when using a random effects model. As we already rated down one level for inconsistency, we chose to only rate down one level for imprecision.
The trials was at some concerns or high risk of bias. We chose to rate down one level as there is no information from low risk of bias trials and as it is thus impossible to estimate the effects of risk of bias.
The CDIM score was 15 corresponding to moderate clinical diversity. Considering this and the sparsity of data, we chose to rate down one level.
The trials only reported on hematological patients for this outcome and results may not be applicable to other hospitalized patient populations (i.e., surgical patients, critically ill patients, children, neonates), but as the trials reported on the defined patient population, we chose not to rate down for indirectness.
TSA (assuming alfa of 5% and a beta of 0.1 and a clinically relevant difference in means of 1 day) was redundant as more than 100% of the RIS of 261 had been obtained. Thus, we did not rate down.
The trial was at some concerns for risk of bias. We chose to rate down one level as there is no information from low risk of bias trials and as it is thus impossible to estimate the effects of risk of bias.
The trial reported on hematological patients for this outcome and results may not be applicable to other hospitalized patient populations (i.e., surgical patients, critically ill patients, children, neonates), but as the trials reported on the defined patient population, we chose not to rate down for indirectness.
TSA (assuming alfa of 5% and a beta of 0.1 and a clinically relevant risk reduction or increase of 15%) could not be performed as only 1.23% of the RIS of 48,730 patients had been accrued Therefore, we rate down two levels.
All trials were at some concerns for risk of bias. We chose to rate down one level as there is no information from low risk of bias trials and as it is thus impossible to estimate the effects of risk of bias.
We detected substantial statistical heterogeneity; I 2 = 60% (p = .08), D 2 = 94%. CDIM tool yielded a score of 13 corresponding to moderate clinical diversity. We chose to rate down one level.
Only trials conducted in the hematological and infectious disease ward setting contributed to the meta‐analysis. Results may not be applicable to other hospitalized patient populations (i.e., surgical patients, critically ill patients, neonates), but as all trials reported on the defined patient population, we did not to rate down for indirectness.
This analysis included very few events and CI around both the relative and absolute estimates of effect include substantial benefit and harm. TSA could not be performed as the AIS of 1310 patients only corresponds to 0.2% of the RIS of 73,050 patients. We chose to rate down two levels.
All trials were at some concerns for risk of bias. We chose to rate down one level as there is no information from low risk of bias trials and as it is thus impossible to estimate the effects of risk of bias.
We observed no statistical heterogeneity I 2 = 0%, D 2 = 0% in the meta‐analysis which did not include the data from Stanworth 2013 because of different statistical summary data used (median, IQR). However, the CDIM tool yielded a score of 12 corresponding to moderate diversity. We chose to rate down one level.
TSA could not be performed as the RIS of 238 patients had already been accumulated within any of the two included trials.
3.6.2. All‐cause mortality at longest follow‐up in all trials
Five trials reported data on all‐cause mortality at longest follow‐up (n = 1398). 32 , 33 , 35 , 36 , 38 Conventional meta‐analysis showed uncertain results (FEM, RR of 0.99; 95% CI 0.58 to 1.68, p = .97) (Figure 2). TSA could not be performed as only 2.0% of the RIS of 52,950 patients had been accrued. We observed no statistical heterogeneity (I 2 = 0%; p = .91, D 2 = 0%) and low clinical diversity (Supplement S10). The certainty of evidence was very low (Table 4).
FIGURE 2.
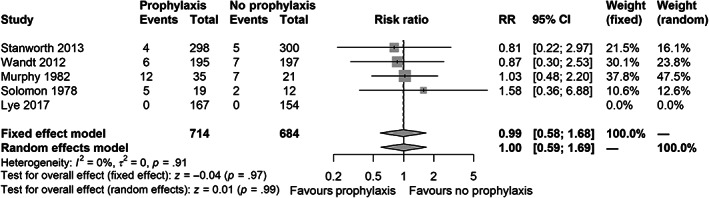
Forest plot for the primary outcome all‐cause mortality at longest follow‐up in all trials
Subgroup analyses did not indicate heterogeneity of the treatment effect (Supplement S11), but the credibility was rated as very low as the analysis of effect modification was based solely on a between‐trial comparison and the number of trials was very low (Supplement S12). Sensitivity analyses for long‐term all‐cause mortality (>90 days) and empirical continuity correction were consistent with the primary analysis, but BW/WB case scenarios showed conflicting results (Supplement S13).
3.7. Secondary outcomes
3.7.1. Clinically important bleeding
Five trials reported data on clinically important bleeding (n = 1276). 31 , 32 , 33 , 36 , 38 The conventional meta‐analysis indicated that prophylactic platelet transfusion may reduce the proportion of patients with at least one episode of clinically important bleeding (REM, RR 0.70, 97.5% CI 0.53 to 0.92, p < .01) (Figure 3), but the TSA showed that only 13.4% of the RIS of 9546 patients had been accrued (TSA adjusted CI 0.26 to 1.87) (Figure 4). We detected significant statistical heterogeneity (I 2 = 59%; p = .04, D 2 = 72%), partly explained by the moderate clinical diversity (Supplement S10). Despite this, all individual trial point estimates favored the prophylaxis group. The certainty of evidence was very low (Table 4). Data from a subgroup of patients with acute myeloid leukemia (n = 190) from one of the larger trials were not included, as they were reported per treatment cycle received, but showed fewer clinically important bleeding episodes in the prophylactic group (n = 96, 245 cycles) as compared to the therapeutic group (n = 94, 198 cycles); 24% (95% CI 18% to 30%) versus 51% (95% CI 43% to 59%). 38
FIGURE 3.
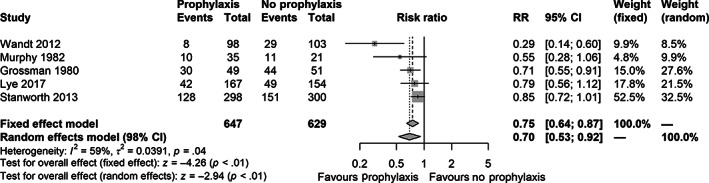
Forest plot for the secondary outcome clinically important bleeding (as defined in the included trials) at longest follow‐up. Data from Wandt 2012 were reported per treatment cycle and only data for a subgroup of patients who had undergone autologous transplantation was extractable.
FIGURE 4.
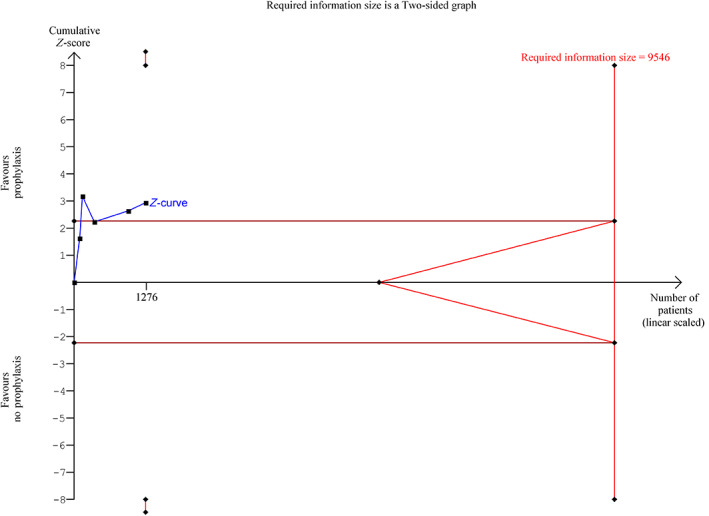
Trial sequential analyses (TSA) plot for the secondary outcome clinically important bleeding using a control event rate of 45.2% (from the included trials), a diversity D 2 of 72%, an alpha of 2.5%, a beta of 0.1 and a relative risk (RR) reduction of 15%. The meta‐analytic RR was 0.70 with a TSA adjusted confidence interval of 0.26 to 1.87. The required information size was not reached (13.7% acquired) and the trial monitoring boundaries or futility boundaries were not crossed. Hence, the TSA highlights that the accrued information size was insufficient to reject or confirm a 15% reduction in RR. [Color figure can be viewed at wileyonlinelibrary.com]
Sensitivity analyses were consistent with the primary analysis, but the results were no longer statistically significant for all bleeding episodes (i.e., not restricted to clinically important bleedings) and in BW/WB case scenarios (Supplement S13).
3.7.2. Days with clinically important bleeding
Two trials reported data on this outcome (n = 654) but the available summary data did not allow meta‐analysis. 33 , 36 A smaller trial reported the mean number of days with clinically important bleeding to be 1.9 days in the prophylactic group versus 2.2 days in the no prophylaxis group 33 (n = 56) and a larger trial (n = 598) indicated that prophylactic platelet transfusions slightly reduced the number of days with clinically important bleeding (MD 0.5, 95% CI 0.1 to 0.9, p = .01). The TSA was redundant as more than 100% of the RIS of 261 had been accrued. We detected moderate clinical diversity (Supplement 10). The certainty of evidence was low (Table 4). Sensitivity analysis provided similar results (Supplement S13). A third trial 38 (n = 396) reported on the percentage of days with clinically important bleeding out of the total number of days on which a morning platelet count was available and found that the prophylactic strategy generally reduced the percentage of days with bleeding, with a larger effect on days with platelet counts <10 × 109/L. As data was not reported with individual patients as the unit of analysis, the trial was not included in the meta‐analysis, CDIM‐evaluation, nor the GRADE evidence profile for this outcome.
3.7.3. Nosocomial infection
One trial reported data on infections (n = 598) as part of serious adverse events, and we assumed that these infections were nosocomial. 36 The author did not have further details readily available. We found inconclusive results (RR of 0.94, 95% CI 0.46 to 1.91, p = .86) and TSA could not be performed as only 1.2% of the RIS of 48,730 patients had been accrued. The certainty of evidence was low (Table 4). Sensitivity analysis provided similar results (Supplement S13).
3.7.4. Transfusion‐related adverse events
Three trials reported data on this outcome (n = 1310). 32 , 36 , 38 The conventional meta‐analysis showed inconclusive results (REM, RR 2.54, 97.5% CI 0.27 to 23.61, p = .35) (Supplement S9). TSA could not be performed as only 0.2% of the RIS of 73,050 patients had been accrued. There were considerable differences in the reported event rates between studies, and we detected statistical heterogeneity (I 2 = 60%; p = .08, D 2 = 94%) and moderate clinical diversity (Supplement S10). The certainty of evidence was very low (Table 4).
The empirical continuity correction agreed with the primary analysis, but the BW/WB case scenarios showed conflicting results (Supplement S13).
3.7.5. Length of hospital stay
Three trials reported data on this outcome (n = 1310) 32 , 36 , 38 but the statistical summary measures differed and only data from two trials were pooled. 32 , 38 These pooled results in the conventional meta‐analysis suggested that the effect of the intervention may be of little to no clinical importance (FEM, MD −0.23, 97.5% CI −0.60 to 0.13, p = .16) (Supplement S9). The third study reported a median (IQR) length of stay of 12 (9–18) in both groups with no impact from prophylactic platelet transfusion. 36 We did not detect statistical heterogeneity (I 2 = 0%; p = .33, D 2 = 0%,) but found moderate clinical diversity (Supplement S10). The certainty of evidence was low (Table 4).
BW/WB case scenarios showed conflicting results (Supplement S13).
3.7.6. Other secondary outcomes
No trials reported data on venous or arterial thromboembolic events, days alive without life support, or health‐related quality of life.
3.7.7. Process variables
Two studies reported the number of platelet units and red blood cell units transfused per participant (n = 989). 36 , 38 Conventional meta‐analysis indicated that patients in the prophylaxis group received more platelet units as compared with the no prophylaxis group (REM, MD 1.00, 95% CI 0.53 to 1.47, p < .01, I 2 = 57%) and possibly fewer red blood cell units (FEM, MD −0.25, 95% CI −0.58 to 0.07, p = .13, I 2 = 0%) (Supplement S14).
4. DISCUSSION
This systematic review identified seven RCTs with relevant outcome data comparing prophylactic platelet transfusion to no prophylaxis in 1642 hospitalized patients with thrombocytopenia. 30 , 31 , 32 , 33 , 35 , 36 , 38 We found uncertain results for all‐cause mortality at longest follow‐up between patients allocated to prophylactic platelet transfusion versus no prophylaxis when analyzing low risk of bias trials only and when incorporating data from all trials. The uncertainty about the effect is substantial since the conventional CIs were wide and included both clinically relevant harm and benefit and less than 5% of the RIS had been accrued. The overall certainty of evidence was low or very low, respectively, indicating that the present evidence is insufficient to draw conclusions on the effect of prophylactic platelet transfusions on all‐cause mortality.
The primary argument for the use of prophylactic platelet transfusions in patients with severe thrombocytopenia is to prevent bleeding. 10 , 11 , 12 We found that prophylactic platelet transfusion may reduce the proportion of patients with at least one episode of clinically important bleeding. However, less than 15% of the RIS has been accrued, the TSA‐adjusted CI was wide and included clinically relevant benefit and harm, and the overall certainty of evidence was very low. We observed substantial statistical heterogeneity, partly due to clinical heterogeneity, as the included trials were conducted over a period of 37 years and had substantial differences in patient populations, interventions, timing of outcome measurement, and in definitions, assessments, and grading of clinically important bleeding. Despite the different pathophysiology and etiology of bleeding in patients with hematological malignancy and dengue fever, 40 , 41 all point estimates favored the prophylaxis group, indicating a similar effect of prophylactic platelet transfusion. Only two trials reported on the number of days with clinically important bleeding per participant, which was found to be slightly lower in the prophylaxis group. Taken together, the evidence suggests that prophylactic platelet transfusion may reduce the risk of clinically important bleeding, but uncertainty remains.
For the remaining secondary outcomes, we found substantial uncertainty around effect estimates for the proportions of patients experiencing at least one nosocomial infection or transfusion‐related adverse event, and the evidence was too sparse to draw any meaningful conclusions. For the length of hospital stay, we found the difference between the allocation groups to be of little or no clinical importance, but the certainty of evidence was low. No trials reported on venous or arterial thrombotic events, days alive without life support or health‐related quality of life.
Our findings concur with a previous systematic review in patients with hematological disorders treated with myelosuppressive chemotherapy or stem cell transplantation that also found high levels of heterogeneity in the timing of outcome measurements and in the definitions and assessment of bleeding and uncertainty around the effect on mortality and adverse events. 42 As our review had a wider scope, but still ended up including many of the same trials, it adds to the uncertainty around the effect of prophylactic platelet transfusions in patients outside the hematological setting.
This review has several strengths. We conducted the review in accordance with the protocol 19 and followed the recommendations by the Cochrane Collaboration 20 including independent selection of studies, data extraction, and assessment of risk bias using the ROB 2.0 tool. 23 We used TSA to estimate the RIS and calculate TSA‐adjusted CIs where feasible. 28 We used the GRADE 21 approach to grade the overall certainty of evidence and we evaluated clinical diversity and subgroup credibility using the CDIM tool 26 and the ICEMAN tool, 27 respectively.
This review also has important limitations. First, due to the wide scope of the review, the included trials were heterogenous with respect to the populations, duration, and dose of the intervention, timing of outcome measurements and definitions, assessments, and grading of bleeding outcomes, which makes direct comparison uncertain. However, as we found only low to moderate clinical diversity for all outcomes and as the evidence base was sparse and event rates rare, conducting meta‐analysis to answer the research questions seems justified. Further, we found no indications of very serious inconsistency in the results. Second, the number of patients and event rates were generally low, which led to imprecise results and increased risk of type two errors. In particular, omitting patients with acute myeloid leukemia from one of the larger trials due to the data format might have underestimated the effect of prophylactic platelet transfusions on clinically important bleeding as these patients in general are at increased risk of bleeding due to a longer duration of profound thrombocytopenia. 38 Third, newer trials defined clinically important bleeding as WHO grade ≥2, 30 , 36 , 38 which has been challenged as WHO grade 2 bleeding may not be considered clinically important. 43 Fourth, most outcomes were at risk of bias, which may decrease the overall validity of our results. Fifth, there was insufficient evidence to assess harm from prophylactic platelet transfusions; and sixth, the included patient populations were restricted to hematology‐oncology patients and dengue fever patients, limiting the generalizability to other hospitalized patient populations with different reasons for thrombocytopenia, such as neonates, surgical patients, and ICU patients.
In conclusion, prophylactic platelet transfusion may reduce clinically important bleeding in hospitalized patients with hematological malignancy or dengue fever, but the evidence is very uncertain, and the generalizability to other patient populations is unclear. The effects on mortality and adverse events are uncertain and data from non‐hematological settings are sparse. To move forward, RCTs are warranted to test the benefits and harms of platelet transfusion in diverse hospitalized populations with thrombocytopenia.
FUNDING INFORMATION
The primary and last author received funding from the Research Council of Rigshospitalet, Copenhagen, Denmark. The primary author also received funding from the Ehrenreich Foundation. The funding parties were not involved in the conduct of this review.
CONFLICT OF INTEREST
The Department of Intensive Care at Rigshospitalet (CA, AG, PS, MHM, AP, and LR) has received funding for other projects from the Novo Nordisk Foundation, Pfizer, Sygeforsikringen “danmark” and Fresenius Kabi and conducts contract research for AM‐Pharma. FP has received personal fees from Gilead and an institutional grant from Alexion for other projects. KP and NZ have no conflict of interests.
Supporting information
Appendix S1. Supporting information.
ACKNOWLEDGMENTS
We would like to sincerely thank Dr. David C. Lye, Dr. Gemma L. Crighton and Dr. Larry Grossman for providing additional information upon request.
Anthon CT, Granholm A, Sivapalan P, Zellweger N, Pène F, Puxty K, et al. Prophylactic platelet transfusions versus no prophylaxis in hospitalized patients with thrombocytopenia: A systematic review with meta‐analysis. Transfusion. 2022;62(10):2117–2136. 10.1111/trf.17064
Funding information Ehrenreich Foundation; Research Council of Rigshospitalet
REFERENCES
- 1. Ten Berg MJ, Van Den Bemt PMLA, Shantakumar S, Bennett D, Voest EE, Huisman A, et al. Thrombocytopenia in adult cancer patients receiving cytotoxic chemotherapy: results from a retrospective hospital‐based cohort study. Drug Saf. 2011;34(12):1151–60. [DOI] [PubMed] [Google Scholar]
- 2. Shaw JL, Nielson CM, Park JK, Marongiu A, Soff GA. The incidence of thrombocytopenia in adult patients receiving chemotherapy for solid tumors or hematologic malignancies. Eur J Haematol. 2021;106(5):662–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Afdhal N, McHutchison J, Brown R, Jacobson I, Manns M, Poordad F, et al. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48:1000–7. [DOI] [PubMed] [Google Scholar]
- 4. Sparger K, Deschmann E, Sola‐Visner M. Platelet transfusions in the neonatal intensive care unit. Clin Perinatol. 2015;42:613–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hui P, Cook DJ, Lim W, Fraser GA, Arnold DM. The frequency and clinical significance of thrombocytopenia complicating critical illness: a systematic review. Chest. 2011;139(2):271–8. [DOI] [PubMed] [Google Scholar]
- 6. Webert KE, Cook RJ, Sigouin CS, Rebulla P, Heddle NM. The risk of bleeding in thrombocytopenic patients with acute myeloid leukemia. Haematologica. 2006;91(11):1530–7. [PubMed] [Google Scholar]
- 7. Jonsson AB, Rygård SL, Hildebrandt T, Perner A, Møller MH, Russell L. Thrombocytopenia in intensive care unit patients: a scoping review. Acta Anaesthesiol Scand. 2021;65(1):2–14. [DOI] [PubMed] [Google Scholar]
- 8. Pendry K, Davies T. An audit of use an wastage in the north west England and North Wales: where have all the platelets gone? Blood Transplant Matters. 2011;34:17–9. [Google Scholar]
- 9. Kaur A, Sethi GK, Goyal RK, Kaur A, Kaur R, Dhir SK, et al. Thrombocytopenia in Paediatric ICU: incidence, transfusion requirement and role as prognostic indicator. J Clin Diagn Res. 2015;9(12):SC05–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Estcourt LJ, Birchall J, Allard S, Bassey SJ, Hersey P, Kerr JP, et al. Guidelines for the use of platelet transfusions. Br J Haematol. 2017;176:365–94. [DOI] [PubMed] [Google Scholar]
- 11. Kaufman RM, Djulbegovic B, Gernsheimer T, Kleinman S, Tinmouth AT, Capocelli KE, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2015;162:205–13. [DOI] [PubMed] [Google Scholar]
- 12. Vlaar AP, Oczkowski S, de Bruin S, Wijnberge M, Antonelli M, Aubron C, et al. Transfusion strategies in non‐bleeding critically ill adults: a clinical practice guideline from the European Society of Intensive Care Medicine. Intensive Care Med. 2020;46(4):673–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van de Weerdt EK, Peters AL, Goudswaard EJ, Binnekade JM, van Lienden KP, Biemond BJ, et al. The practice of platelet transfusion prior to central venous catheterization in presence of coagulopathy: a national survey among clinicians. Vox Sang. 2017;112(4):343–51. [DOI] [PubMed] [Google Scholar]
- 14. de Bruin S, Scheeren TWL, Bakker J, van Bruggen R, Vlaar APJ. Transfusion practice in the non‐bleeding critically ill: an international online survey‐the TRACE survey. Crit Care. 2019;23(1):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cremer M, Sola‐Visner M, Roll S, Josephson CD, Yilmaz Z, Bührer C, et al. Platelet transfusions in neonates: practices in the United States vary significantly from those in Austria, Germany, and Switzerland. Transfusion. 2011;51(12):2634–41. [DOI] [PubMed] [Google Scholar]
- 16. Gilliss BM, Looney MR, Gropper MA. Reducing noninfectious risks of blood transfusion. Anesthesiology. 2011;115:635–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Curley A, Stanworth SJ, Willoughby K, Fustolo‐Gunnink SF, Venkatesh V, Hudson C, et al. Randomized trial of platelet‐transfusion thresholds in neonates. N Engl J Med. 2019;380(3):242–51. [DOI] [PubMed] [Google Scholar]
- 18. Baharoglu MI, Cordonnier C, Salman RAS, de Gans K, Koopman MM, Brand A, et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open‐label, phase 3 trial. Lancet. 2016;387(10038):2605–13. [DOI] [PubMed] [Google Scholar]
- 19. Anthon CT, Sivapalan P, Granholm A, Pène F, Puxty K, Perner A, et al. Prophylactic platelet transfusions in hospitalised patients with thrombocytopenia – protocol for a systematic review with meta‐analysis. Acta Anaesthesiol Scand. 2021;65:988–94. [DOI] [PubMed] [Google Scholar]
- 20. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/hanbook. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Granholm A, Alhazzani W, Møller MH. Use of the GRADE approach in systematic reviews and guidelines. Br J Anaesth. 2019;123:554–9. [DOI] [PubMed] [Google Scholar]
- 22. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 24. Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta‐analytic methods. BMC Med Res Methodol. 2014;14(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jakobsen JC, Wetterslev J, Lange T, Gluud C. Viewpoint: taking into account risks of random errors when analysing multiple outcomes in systematic reviews. Cochrane Database Syst Rev. 2016;3:ED000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barbateskovic M, Koster TM, Eck RJ, Maagaard M, Afshari A, Blokzijl F, et al. A new tool to assess Clinical Diversity In Meta‐analyses (CDIM) of interventions. J Clin Epidemiol. 2021;135:29–41. [DOI] [PubMed] [Google Scholar]
- 27. Schandelmaier S, Briel M, Varadhan R, Schmid CH, Devasenapathy N, Hayward RA, et al. Development of the instrument to assess the credibility of effect modification analyses (ICEMAN) in randomized controlled trials and meta‐analyses. CMAJ. 2020;192(32):E901–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. J Clin Epidemiol. 2008;61(1):64–75. [DOI] [PubMed] [Google Scholar]
- 29. White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ. 2011;342:d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Assir MZK, Kamran U, Ahmad HI, Bashir S, Mansoor H, Anees SB, et al. Effectiveness of platelet transfusion in dengue fever: a randomized controlled trial. Transfus Med Hemother. 2013;40(5):362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grossman L, Benny WB, Buskard NA, Carter WH, Growe GH, Mangal A, et al. A Randomized trial of therapeutic vs. prophylactic platelet transfusions, with a comparison of multiple, random donor to selectively mismatched single donor platelets. 1980. (Unpublished).
- 32. Lye DC, Archuleta S, Syed‐Omar SF, Low JG, Oh HM, Wei Y, et al. Prophylactic platelet transfusion plus supportive care versus supportive care alone in adults with dengue and thrombocytopenia: a multicentre, open‐label, randomised, superiority trial. Lancet. 2017;389(10079):1611–8. [DOI] [PubMed] [Google Scholar]
- 33. Murphy S, Litwin S, Herring L, Koch P, Remischovsky J, Donaldson MH, et al. Indications for platelet transfusion in children with acute leukemia. Am J Hematol. 1982;12(4):347–56. [DOI] [PubMed] [Google Scholar]
- 34. Sintnicolaas K, van de Velden K, Sizoo W, Haije WG, Abels J, Lowenberg B. Comparison of “prophylactic” and “therapeutic” single‐donor platelet transfusions in patients with acute leukemia. Br J Haematol. 1981;50:684. [Google Scholar]
- 35. Solomon J, Bofenkamp T, Fahey J, Chillar RK, Beutler E. Platelet prophylaxis in acute non‐lymphoblastic leukemia. Lancet. 1978;1:267. [DOI] [PubMed] [Google Scholar]
- 36. Stanworth SJ, Estcourt LJ, Powter G, Kahan BC, Dyer C, Choo L, et al. A no‐prophylaxis platelet‐transfusion strategy for hematologic cancers. N Engl J Med. 2013;368(19):1771–80. [DOI] [PubMed] [Google Scholar]
- 37. van de Weerdt EK, Biemond BJ, Zeerleder SS, van Lienden KP, Binnekade JM, Vlaar APJ, et al. Prophylactic platelet transfusion prior to central venous catheter placement in patients with thrombocytopenia: study protocol for a randomised controlled trial. Trials. 2018;19(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wandt H, Schaefer‐Eckart K, Wendelin K, Pilz B, Wilhelm M, Thalheimer M, et al. Therapeutic platelet transfusion versus routine prophylactic transfusion in patients with haematological malignancies: an open‐label, multicentre, randomised study. Lancet. 2012;380(9850):1309–16. [DOI] [PubMed] [Google Scholar]
- 39. Chen J, Qi T. Platelet transfusion in HBV‐related acute‐on chronic liver failure. Ongoing.
- 40. Franchini M, Frattini F, Crestani S, Bonfanti C. Bleeding complications in patients with hematologic malignancies. Semin Thromb Hemost. 2013;39(1):94–100. [DOI] [PubMed] [Google Scholar]
- 41. Srichaikul T, Nimmannitya S. Haematology in dengue and dengue haemorrhagic fever. Baillieres Best Pract Res Clin Haematol. 2000;13(2):261–76. [DOI] [PubMed] [Google Scholar]
- 42. Crighton GL, Estcourt LJ, Wood EM, Trivella M, Doree C, Stanworth S. A therapeutic‐only versus prophylactic platelet transfusion strategy for preventing bleeding in patients with haematological disorders after myelosuppressive chemotherapy or stem cell transplantation. Cochrane Database Syst Rev. 2015;2015:CD010981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heddle NM, Arnold DM, Webert KE. Time to rethink clinically important outcomes in platelet transfusion trials. Transfusion. 2011;51(2):430–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information.


