Abstract
Two bacterial products that have been demonstrated to function as mucosal adjuvants are cholera toxin (CT), produced by various strains of Vibrio cholerae, and the heat-labile enterotoxin (LT) produced by some enterotoxigenic strains of Escherichia coli. Although LT and CT have many features in common, they are clearly distinct molecules with biochemical and immunologic differences which make them unique. The goal of this study was to determine the basis for these biological differences by constructing and characterizing chimeric CT-LT molecules. Toxin gene fragments were subcloned to create two constructs, each expressing the enzymatically active A subunit of one toxin and the receptor binding B subunit of the other toxin. These hybrid toxins were purified, and the composition and assembly of CT A subunit (CT-A)-LT B subunit (LT-B) and LT A subunit (LT-A)-CT B subunit (CT-B) were confirmed. Hybrids were evaluated for enzymatic activity, as measured by the accumulation of cyclic AMP in Caco-2 cells, and the enterotoxicity of each toxin was assessed in a patent-mouse assay. The results demonstrated that LT-A–CT-B induces the accumulation of lower levels of cyclic AMP and has less enterotoxicity than either wild-type toxin or the other hybrid. Nonetheless, this hybrid retains adjuvant activity equivalent to or greater than that of either wild-type toxin or the other hybrid when used in conjunction with tetanus toxoid for intranasal immunization of BALB/c mice. Importantly, the ability of LT to induce a type 1 cytokine response was found to be a function of LT-A. Specifically, LT-A–CT-B was able to augment the levels of antigen-specific gamma interferon (IFN-γ) and interleukin 5 to levels comparable to those achieved with native LT, while CT-A–LT-B and native CT both produced lower levels of antigen-specific IFN-γ. Thus, these toxin hybrids possess unique biological characteristics and provide information about the basis for differences in the biological activities observed for CT and LT.
Infectious diseases remain one of the leading causes of death in adults and children worldwide. In 1998, for example, infectious diseases killed more than 13 million people and were responsible for 63% of deaths in children less than 4 years old (3). In addition to suffering and death, infectious diseases impose an enormous financial burden on society. Recently, a great deal of attention has been focused on mucosal vaccination as an alternative to parenteral immunization and on its associated costs and risks. Recent studies have shown that mucosal immunization via intranasal (IN), oral, or rectal routes can elicit both humoral and cell-mediated immune responses in both the mucosal and the systemic compartments.
Despite the attractiveness of mucosal vaccination, mucosally administered antigens are frequently not immunogenic. A number of strategies have been developed to facilitate and enhance the immune response obtained after mucosal immunization. Among these strategies are the use of attenuated mutants of bacteria (i.e., Salmonella spp.) as carriers of heterologous antigens, encapsulation of antigens into microspheres, gelatin capsules, different formulations of liposomes, adsorption onto nanoparticles, lipophilic immune stimulating complexes, and addition of bacterial products with known adjuvant properties. While a number of substances of bacterial origin have been tested as mucosal adjuvants (17, 21, 25), it is clear that the two bacterial proteins with the greatest potential to function as mucosal adjuvants are cholera toxin (CT), produced by various strains of Vibrio cholerae, and the heat-labile enterotoxin (LT) produced by some enterotoxigenic strains of Escherichia coli (7, 10, 18, 26, 27).
Although LT and CT have many features in common, they are clearly distinct molecules with biochemical and immunologic differences which make them unique (9). In addition, LT has an unusual affinity for carbohydrate-containing matrices (6, 8). LT binds not only to agarose in columns used for purification but also, more importantly, to other biological molecules containing galactose, including glycoproteins and lipopolysaccharides. This lectin-like binding property of LT results in a broader receptor population on mammalian cells for LT than for CT, which binds only to ganglioside GM1 (1, 8, 12). Moreover, LT and CT generally activate different subsets of T-helper cells. CT promotes CD4+ type 2 cytokine responses and help for immunoglobulin G1 (IgG1), IgE, and mucosal IgA, while LT induces CD4+ type 1 and type 2 cytokine responses and help for IgG1, IgG2a, IgG2b, and mucosal IgA (19, 26). This distinction between LT and CT may be important in terms of selecting a mucosal adjuvant for use with specific categories of pathogens, assuming that the type 2 bias reported for CT is also seen in humans. Possible sources for this bias include the availability of different receptors for LT and CT, differences in intracellular localizations based upon differences in endoplasmic reticulum signal sequences between CT and LT (5, 14, 15), and differences in the activation of intracellular signaling pathways.
The purpose of the present study was to construct and evaluate hybrid toxins, consisting of the A subunit of one toxin in conjunction with the B subunit of the other toxin, in order to provide information about the potential roles of the A and B subunits in making CT and LT unique. The hybrid toxins were purified, and the composition and assembly of CT A subunit (CT-A)-LT B subunit (LT-B) and LT A subunit (LT-A)-CT B subunit (CT-B) were demonstrated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and immunodiffusion with specific anti-A-subunit and anti-B-subunit antibodies. Hybrid toxins were evaluated for enzymatic activity, as measured by the accumulation of cyclic AMP (cAMP) in Caco-2 cells, and for enterotoxicity in a patent-mouse assay (4). Finally, hybrid toxins were evaluated for the ability to function as mucosal adjuvants for tetanus toxoid (TT).
MATERIALS AND METHODS
Construction of CT-LT hybrid toxins.
Restriction sites were introduced using a QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, Calif.). Mutator oligonucleotide primers (Gibco BRL, Grand Island, N.Y.) were designed to introduce restriction enzyme sites to facilitate screening of DNA products and subcloning of DNA fragments. Pfu DNA polymerase was used in a PCR to extend primers and replicate the plasmid template. After completion of each reaction, samples were treated with DpnI, which selectively digests the parental DNA template based on dam methylation. DpnI-digested DNA was transformed into E. coli XL-1 Blue supercompetent cells and then into E. coli JM83 (ara Δlac-proAB rpsL φ80δlacZΔM15) for expression and protein purification. Both hybrids were confirmed by DNA sequencing.
CT-A–LT-B. A plasmid containing the gene encoding wild-type CT, designated pCT2, was created by subcloning a 5.1-kb fragment from plasmid pBB6 (2), which contains the ctx genes from the classical Inaba V. cholerae strain 569B (a generous gift from J. B. Kaper, University of Maryland), into pUC19. PCR was performed with primers designed to amplify the ctxA gene and to introduce flanking EcoRI (5′-GGCTGTGGGTAGAATTCAAACGGGG-3′) and SstI (5′-GAGGAGCTCCATGTGCATATGCTG-3′) restriction endonuclease sites into the PCR product using pCT2 as a template. The PCR product was digested with EcoRI and SstI, which cleaved the restriction sites introduced at the ends of ctxA by the PCR primers, and then ligated into pUC18 to create pCTA3. A gene fragment encoding LT-B from pCS96, a pUC18-based plasmid which carries the genes for native LT from human enterotoxigenic E. coli isolate H10407, was introduced by restriction digestion with SstI and HindIII, both of which preexist in pCS96, to produce plasmid pCT-A/LT-B.
LT-A–CT-B. Beginning with unmodified pCT2 as a template, pCT2 was mutagenized to introduce SstI (5′-GAGGAGCTCCATGTGCATATGCTG-3′) and HindIII (5′-CTGATATTGATACACATAATAGAATTCGGG-3′) sites flanking ctxB. Restriction digestion with SstI and HindIII released the ctxB gene, which was then ligated into pUC18 to form pCTB5. pCS96 was digested with SstI to release a fragment containing the lta gene, which was then ligated into SstI-digested pCTB5, producing an LT-A–CT-B construct. Attempts at purifying CT from this plasmid containing the introduced SstI site, which changed a threonine to an alanine in the B subunit, proved problematic. The alanine was then changed back to the wild-type threonine in both pCT2 and pLT-A/CT-B.
Purification of toxins.
Purification of LT, CT, and the hybrid toxins was accomplished by galactose affinity chromatography by the method of Clements and Finkelstein (6). All toxins were purified from cultures grown overnight in 10-liter fermentors containing Evans' medium (11) supplemented with 0.5% glucose and 100 μg of ampicillin per ml. The cells were harvested by centrifugation, resuspended in TEAN (0.2 M NaCl, 0.05 M Tris, 0.001 M EDTA, 0.003 M NaN3 [pH 7.5]), and lysed by use of a French press. Cell lysates were dialyzed against TEAN overnight at 4°C, clarified by centrifugation, and subjected to chromatography on separate, dedicated, immobilized d-galactose columns (Pierce, Rockford, Ill.) Toxins were eluted with 0.3 M galactose (manufactured by Acros Organics and distributed by Fisher Scientific) in TEAN. Each purified toxin was characterized by SDS-PAGE and evaluated for biological activities as described below.
SDS-PAGE.
SDS–10% PAGE was performed by the technique of Laemmli (13). One microgram of each sample was heated at 100°C for 5 min and analyzed on a 10% acrylamide gel. After electrophoresis, protein bands were visualized by staining with 0.25% Coomassie brilliant blue in 40% methanol–7% acetic acid.
Immunodiffusion assays.
In order to differentiate between hybrid toxins, immunodiffusion assays were performed. Toxins and CT-A- and LT-B-specific antisera produced in our laboratory were added to an array of wells in a plate containing 1% Noble agar and 1% sodium azide as a preservative. Diffusion of the toxins and antibodies overnight at room temperature resulted in precipitin lines, which were then visually categorized as reactions of identity, partial identity, or nonidentity.
Patent-mouse enterotoxicity assay.
The patent-mouse assay was performed as previously described (20). Three female BALB/c mice per group were inoculated with 0.5 ml of buffer or with 5, 25, or 125 μg of native LT, native CT, or the different LT hybrids. Inoculations were made intragastrically with a blunt-tip feeding needle (Popper & Sons, Inc., New Hyde Park, N.Y.). Following inoculation, animals remained in their cages without food but with water ad libitum for 3 h and were then sacrificed by CO2 inhalation. The entire intestine from duodenum to anus was removed carefully from each mouse to retain any accumulated fluid; residual mesentery was eliminated prior to weighing. The carcass was weighed separately, and a gut-to-carcass (G/C) ratio was calculated for each animal.
Determination of intracellular cAMP.
Caco-2 cells (ATCC HTB-37) were obtained from the American Type Culture Collection. Cells were seeded in six-well cluster plates (Corning Costar, Cambridge, Mass.) and grown to near confluence. Prior to the addition of toxins, cells were incubated in minimal essential medium (Gibco BRL) containing 1% fetal bovine serum and 1 mM 3-isobutyl-1-methylxanthine (Sigma, St. Louis, Mo.) for 45 min at 37°C in 5% CO2. One microgram of each toxin was activated with 2 ng of trypsin in TEAN at 37°C for 45 min and then added to duplicate wells of cells. At 1, 2, 3, and 4 h after toxin addition, cells were washed twice with cold phosphate-buffered saline (PBS). Intracellular cAMP was extracted by adding 0.4 ml of 0.1 N HCl to each well and incubating the cells at room temperature for 20 min. cAMP was detected using an enzyme-linked immunosorbent assay (ELISA)-based low-pH cAMP kit (R&D Systems, Minneapolis, Minn.).
Stability assay.
The stability of the A- and B-subunit interactions in hybrid toxins was determined using 96-well plates coated with type III gangliosides (Sigma) as described by Rodighiero et al. (22). After the toxins were allowed to bind to the ganglioside-coated plates, the plates were washed and probed with goat anti-CT-A antibody, which recognizes both CT-A and LT-A, or with goat anti-LT-B antibody, which recognizes both LT-B and CT-B, followed by rabbit anti-goat IgG conjugated with alkaline phosphatase (Sigma). Reactions were developed with p-nitrophenyl phosphate and stopped with 3 N NaOH, and the absorbance at 405 nm was determined spectrophotometrically. Stability was determined by incubation with buffers of various stringencies (PBS or PBS with 0.5% SDS) before washing and addition of the anti-CT-A or anti-LT-B antibody, secondary antibody, and substrate.
Determination of adjuvanticity.
The procedures used for the determination of adjuvanticity were the same as those previously described by Cheng et al. (4). TT for immunization was obtained from Aventis Pasteur (Swiftwater, Pa.). Groups consisting of five BALB/c mice each were immunized intranasally once per week for 3 weeks. Intranasal inoculations consisted of 8 μl of the antigen preparation (10 μg of TT with 5 μg of toxin) introduced into one nostril while the mice were under light Metofane anesthesia (Pitman-Moore, Mundelein, Ill.). One week after the final immunization, animals were sacrificed, serum was obtained by cardiac puncture, and spleens were collected for in vitro antigen restimulation assays.
Antibody assay.
Reagents and antisera for the ELISA were obtained from Sigma unless indicated otherwise. Serum samples were serially diluted in PBS–0.05% Tween 20 and added to microplates precoated with 1 μg of TT per well. Anti-TT IgG levels were determined with rabbit antiserum against mouse IgG conjugated to alkaline phosphatase. Reactions were stopped with 3 N NaOH, and the absorbance at 405 nm was determined spectrophotometrically.
In vitro antigen restimulation assays.
BALB/c mice immunized as described above were sacrificed, and the spleens were removed and pooled for each group. Spleens were homogenized through a cell dissociation sieve (Sigma), pelleted, and resuspended in plain RPMI 1640. Mononuclear cells were purified by density gradient centrifugation using Histopaque-1119 (Sigma), washed, and resuspended in complete RPMI 1640 containing 10% fetal bovine serum, 100 μg of streptomycin sulfate, 0.25 μg of amphotericin B, and 100 U of penicillin G sodium (Gibco BRL) per ml. Purified mononuclear cells were enumerated and tested for viability by trypan blue exclusion. Mononuclear cells (107 per well) were added to wells containing 106 peritoneal macrophages elicited from naive BALB/c mice by injection with Freund's incomplete adjuvant (Difco). Macrophages were incubated with 10 μg of TT for 1 h prior to the addition of mononuclear cells. After 1, 3, 5, and 7 days of culturing, supernatants were collected and stored at –20°C until assayed. Cytokines in the culture supernatants were detected by use of murine cytokine ELISA kits (PharMingen).
Statistical analysis.
The standard error of the mean (SEM) was calculated for enterotoxicity and for serum anti-TT IgG antibody responses, and the means for different groups were compared by the Student t test. Statistical significance was considered to be a P value of ≤0.05.
RESULTS
Construction and physical characterization of hybrid toxins.
Hybrid toxins were constructed and compared with native LT and native CT for enterotoxicity, cAMP activity, and adjuvanticity, as defined by both antibody and T-cell responses against a coadministered antigen. Hybrid toxins were constructed by site-directed mutagenesis and purified by galactose affinity chromatography, a reflection of the ability of these molecules to bind to galactose residues in their natural ganglioside receptors. To avoid cross-contamination with different toxins, each mutant was purified using a separate, dedicated column. Purified native toxins and hybrids were then examined by SDS-PAGE.
As shown in Fig. 1, native CT (lane 1) and native LT (lane 2) dissociated into ca. 28-kDa A subunits and ca. 12-kDa B monomers, with LT-A migrating at an apparent molecular mass slightly smaller than that of CT-A. As indicated by this SDS-PAGE analysis, the A subunit of CT-A–LT-B (Fig. 1, lane 3) corresponded to the A subunit of native CT (lane 1), while the A subunit of LT-A–CT-B (lane 4) corresponded to the A subunit of native LT (lane 2). Figure 2 also demonstrates that the relative proportions of the A and B subunits in the hybrids were the same as those seen in the native toxins. The A- and B-subunit compositions of the hybrids were further confirmed by immunodiffusion against polyclonal antisera specific for CT-A and LT-B (data not shown).
FIG. 1.
SDS-PAGE analysis of purified native and hybrid toxins. Native LT, native CT, CT-A–LT-B and LT-A–CT-B dissociated into ca. 28-kDa A subunits and ca. 12-kDa B monomers. Lane 1, native CT; lane 2, native LT; lane 3, CT-A–LT-B; lane 4, LT-A–CT-B.
FIG. 2.
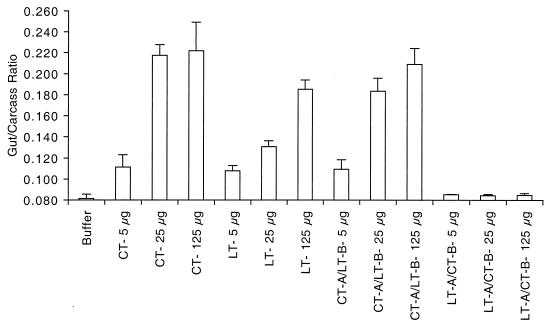
Patent-mouse assay for evaluation of enterotoxicity. Groups of BALB/c mice were orally inoculated with native LT, native CT, CT-A–LT-B, or LT-A–CT-B at 5, 25, or 125 μg. Following a 3-h interval, the G/C ratio for each animal was determined. The G/C ratio is defined as the intestinal weight divided by the remaining carcass weight. There were three animals per group, and the means and SEMs for each data point are shown.
Patent-mouse intestinal fluid accumulation caused by LT, CT, and hybrid toxins.
CT is generally regarded to be a more potent enterotoxin than LT for the induction of secretory diarrhea. In one human trial, 25 μg of CT was shown to elicit a full 20-liter cholera purge in volunteers (16). Our own studies have shown that 25 μg of native LT administered orally in conjunction with a whole-cell B-subunit cholera vaccine can elicit up to 6 liters of fluid (unpublished observation). These findings are consistent with the differential toxicities of CT and LT in a variety of in vivo and in vitro models.
The patent-mouse assay provides a measure of net secretion in a nonligated intestine, and the activity of this assay is defined as the mean G/C ratio for each group of mice (4). Native LT, native CT, and the hybrid toxins were evaluated for the ability to induce net fluid secretion in this model, and the results are shown in Fig. 2. The negative control group, which was given buffer alone, had baseline levels of fluid accumulation (G/C ratio = 0.083). In contrast, both native LT and native CT induced fluid accumulation in a dose-dependent fashion over a range of 5 to 125 μg, with CT being more active than LT in this assay. Based on our previous findings and those of others, we anticipated that LT-A–CT-B and CT-A–LT-B would have undiminished enterotoxicity compared to native LT and native CT, since the A subunits of the hybrid toxins were unaltered. As shown in Fig. 2, CT-A–LT-B had intermediate enterotoxicity, between that of LT and CT (G/C ratios at 25 μg: LT, 0.131; CT, 0.218; CT-A–LT-B, 0.184). Surprisingly, LT-A–CT-B had no enterotoxicity in the patent-mouse assay at any level examined. The G/C ratio for LT-A–CT-B at 125 μg (0.084) was not statistically different from that obtained with buffer alone (P = 0.25). Repeat experiments (data not shown) produced exactly the same outcomes. These findings suggest that there is something unique about the association of LT-A with CT-B. The reduced enterotoxicity observed in the patent-mouse assay could result from altered receptor binding by CT-B when associated with LT-A, instability of the LT-A–CT-B complex, or loss of or reduction in enzymatic activity.
Receptor binding and stability.
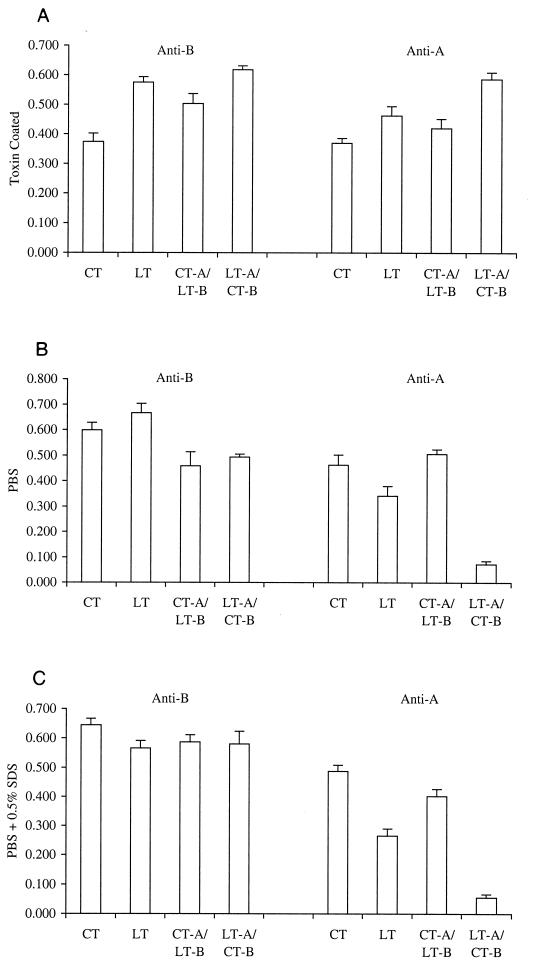
The ability of the B subunits of the hybrid toxins to bind to gangliosides and the stability of the A- and B-subunit interactions in hybrid toxins were examined using 96-well plates coated with type III gangliosides as described by Rodighiero et al. (22). After the toxins were allowed to bind to the ganglioside-coated plates, the plates were washed and then incubated with either PBS or PBS containing 0.5% SDS before being probed with goat anti-CT-A antibody, which recognizes both CT-A and LT-A, or with goat anti-LT-B antibody, which recognizes both LT-B and CT-B. Control plates were coated directly with toxin. As shown in Fig. 3, on toxin-coated plates, both A and B subunits of native LT, native CT, CT-A–LT-B, and LT-A–CT-B were recognized to approximately the same extents by the polyclonal antisera against CT-A and LT-B. The data shown are the means and SEMs for eight independent determinations.
FIG. 3.
Stability testing of toxins. Native LT, native CT, CT-A–LT-B, and LT-A–CT-B were treated with buffers of various stringencies to evaluate the strength of the interaction between the A and B subunits. (A) Plates were coated with toxin and probed with goat anti-CT-A antibody, which recognizes both CT-A and LT-A, or with goat anti-LT-B antibody, which recognizes both LT-B and CT-B. (B and C) Plates were coated with gangliosides prior to binding of toxins and treatment with PBS (B) or PBS containing 0.5% SDS (C). Detection of A and B subunits with specific antisera shows the extent of the association between subunits after treatment with SDS. Data are reported as means and SEMs.
These findings are consistent with those of densitometry scans of SDS-polyacrylamide gels, which demonstrated that the ratio of A to B subunits was indistinguishable between native and hybrid toxins (data not shown). Interestingly, when the toxin preparations were first bound to ganglioside-coated plates, B-subunit complexes remained bound even when incubated in the presence of 0.5% SDS. This result was also obtained in the study of Rodighiero et al. (22). However, in contrast to the findings of Rodighiero et al. (22), we found that there was a significant reduction in the recognition of the A subunit by anti-A-subunit antibodies in the LT-A–CT-B hybrid following ganglioside binding, even in the absence of SDS. In the studies by Rodighiero et al. (22), a CT-A1–LT-A2–CT-B mutant was recognized by anti-A-subunit antibodies in the absence of SDS but lost stability in the presence of 0.5% SDS.
Taken together, our findings suggest that the LT-A–CT-B hybrid may have reduced enterotoxicity because of instability of the A- and B-subunit interactions. Alternatively, binding to ganglioside may mask determinants in the A subunit recognized by the cross-reactive polyclonal anti-A-subunit antiserum and this assay may reveal only an apparent reduction in stability.
Induction of cAMP by LT, CT, and hybrid toxins.
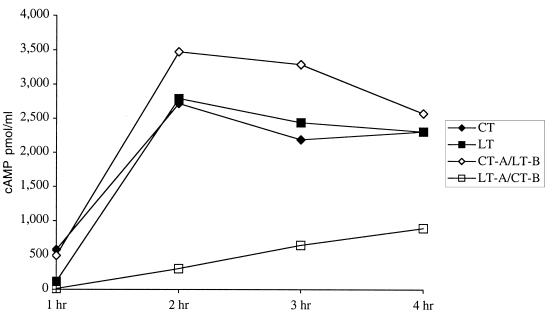
In order to evaluate the enzymatic activity of the hybrid toxins, native LT, native CT, CT-A–LT-B, and LT-A–CT-B were examined for the ability to induce the production of cAMP in cultured Caco-2 cells. The ability to induce the production of cAMP is thought to be a prerequisite for the enterotoxicity of LT. As shown in Fig. 4, despite having an unaltered, intact A subunit, the hybrid molecule LT-A–CT-B induced significantly less cAMP in this assay than did native LT, native CT, or the other hybrid molecule, CT-A–LT-B. The observation that LT-A–CT-B induces lower levels of cAMP than does CT-A–LT-B is important because it confirms that there is something unique about this specific combination of A and B subunits and that the reduction in the toxicity and enzymatic activity of the hybrid is not a generalized phenomenon.
FIG. 4.
Accumulation of cAMP in Caco-2 cells. The enzymatic activity of native LT, native CT, CT-A–LT-B, and LT-A–CT-B was assessed by measurement of intracellular cAMP accumulation in Caco-2 cells over a 4-h period. Cells were incubated with 1 μg of trypsin-cleaved toxin, washed, and lysed. cAMP levels in cell lysates were measured by an ELISA.
Adjuvant activity following intranasal immunization.
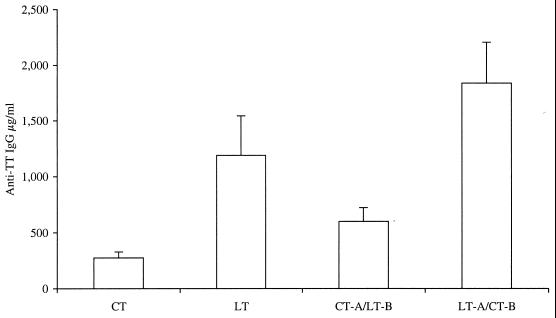
A major purpose of this study was to determine the role of the different subunits of CT and LT in the adjuvanticity of these molecules, with special emphasis on the type 2 bias reported for CT. It was also of interest to determine if the reduced enterotoxicity and enzymatic activity of the LT-A–CT-B hybrid altered the adjuvant effect. As shown in Fig. 5, the serum anti-TT IgG responses produced with LT (P = 0.0167), CT-A–LT-B (P = 0.0207), and LT-A–CT-B (P = 0.0015) were significantly greater than the responses produced with CT. LT-A–CT-B was as effective as native LT (P = 0.1205) and more effective than native CT (P = 0.0015) or CT-A–LT-B (P = 0.0065) in enhancing antigen-specific antibody responses in immunized animals, despite the significantly reduced enterotoxicity and enzymatic activity of LT-A–CT-B compared to native LT, native CT, or CT-A–LT-B. No detectable serum anti-TT response was observed when TT was administered without a mucosal adjuvant (data not shown).
FIG. 5.
Comparison of native LT, native CT, CT-A–LT-B, and LT-A–CT-B for the ability to induce serum anti-TT IgG following IN immunization. BALB/c mice were immunized IN with 10 μg of TT alone or in conjunction with 5 μg of native LT, native CT, CT-A–LT-B, or LT-A–CT-B three times at weekly intervals. Serum was obtained by cardiac puncture 1 week after the last immunization and examined for TT-specific IgG by an ELISA. Data shown are the means and SEMs for serum IgG levels for each group (n = 5). Results are representative of two independent experiments.
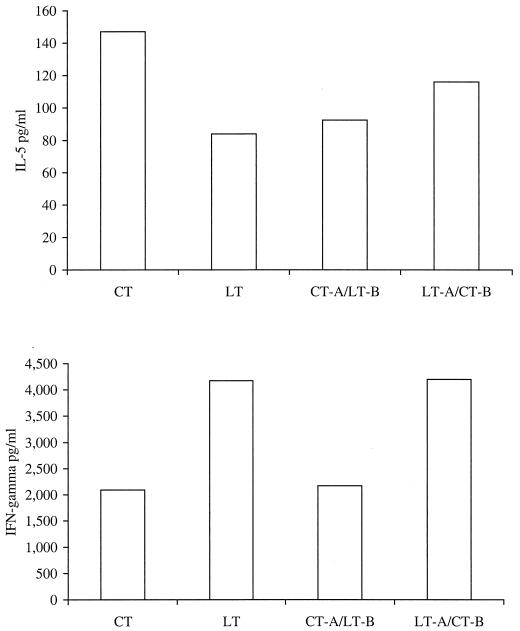
In addition to examining antibody responses, we also examined LT, CT, and the hybrid toxins for the ability to enhance the production of antigen-specific type 1 and type 2 cytokine responses in splenic mononuclear cells in in vitro antigen restimulation assays. Levels of gamma interferon (IFN-γ) and interleukin 5 (IL-5) in supernatants collected after 1, 3, 5, and 7 days of culturing were determined by a cytokine ELISA. As shown in Fig. 6, LT and LT-A–CT-B produced higher levels of antigen-specific IFN-γ in immunized animals than did either CT or CT-A–LT-B, suggesting that a type 1 cytokine response is associated with LT-A. Levels of IL-5 were highest in the group immunized with TT (the antigen) and CT, and slightly lower levels were observed in the other three groups. Day 5 results were representative and are shown.
FIG. 6.
Comparison of native LT, native CT, CT-A–LT-B, and LT-A–CT-B for the ability to induce antigen-specific cytokine responses in in vitro antigen restimulation assays. Splenocytes harvested from animals immunized for this experiment were cultured with naive macrophages and 10 μg of TT per well. Supernatants were collected and analyzed for the presence of IL-5 and IFN-γ by an ELISA on days 1, 3, 5, and 7. Antigen-specific cytokine levels are shown for day 5 culture supernatants. Results are representative of two independent experiments.
DISCUSSION
The purpose of the current study was to construct and evaluate hybrid toxins, consisting of the A subunit of one toxin in conjunction with the B subunit of the other toxin, in order to provide information about the potential roles of the A and B subunits in making CT and LT unique. The most striking finding of these studies was that LT-A–CT-B has significantly reduced enterotoxicity and enzymatic activity compared to either native LT, native CT, or CT-A–LT-B and yet retains the adjuvant properties of native LT for the induction of humoral and cellular immune responses to a coadministered antigen. The toxicity findings are in contrast to the findings of Takeda et al. (24), who found that hybrid toxin molecules constructed through dissociation chromatography had toxicity equivalent to that of the parent proteins from which the A subunits were generated.
In two recently published studies, genetically derived hybrid toxins were used to explore the differential toxicities of CT and LT (22, 23). In those studies, hybrid toxins were constructed in which the A1 fragment of one toxin was substituted for that of the other (CT-A1:LT-A2RDEL/LT-B; LT-A1:CT-A2KDEL/CT-B), as were hybrids in which the putative endoplasmic reticulum retention signal was altered (CT-A1:CT-A2RDEL/CT-B; CT-A1:CT-A2RDEL/LT-B). Those investigators suggested that the differential toxicities of CT and LT are a consequence of the stability of the AB5 complex, with CT being more stable than LT as a function of the CT-A2–CT-B interaction. Consequently, one possible basis for the reduced enterotoxicity and enzymatic activity of LT-A–CT-B observed in our study is instability of the AB5 complex as a consequence of LT-A2 interacting with CT-B. The ganglioside ELISA stability assay (Fig. 3) suggests that this possibility may indeed be the case, since there was significantly less anti-A-subunit antibody reactivity against LT-A–CT-B than against either native toxin or CT-A–LT-B. On the other hand, SDS-PAGE and densitometry data, as well as data from ELISAs in which toxin was directly coated onto microtiter plates, indicated that the LT-A–CT-B complex was stable in vitro and that LT-A and LT-B were in the correct proportions when produced by the organism and purified by affinity chromatography.
It has been established that translocation of the A subunits of CT and LT occurs from an endosomal compartment after endocytosis (5, 14, 15). Reduced in vivo stability of LT-A–CT-B could affect trafficking through the endocytic pathway, with differential compartmentalization within the cell and activation of differential intracellular signaling. It is not possible to measure in vivo stability by any of the assays used in this study.
The reduced enterotoxicity observed in the patent-mouse assay most likely results from the reduction in enzymatic activity, as reflected by the Caco-2 cell assay. Indeed, we have recently shown that enterotoxicity in this assay is directly correlated with the ability of enterotoxins to induce cAMP (4), and the findings of the present study are consistent with those observations. With respect to adjuvanticity, despite the significant reduction in enterotoxicity and enzymatic activity, LT-A–CT-B retains the ability to function as a mucosal adjuvant for a coadministered antigen. These data suggest that the intracellular compartments involved in enterotoxicity may be different from those involved in adjuvanticity. This question is currently under investigation in our laboratory. Based upon the increased induction of IFN-γ by LT and LT-A–CT-B compared to CT and CT-A–LT-B, we conclude that the ability of LT to induce a type 1 cytokine response is a function of LT-A. Furthermore, the level of cAMP induced is not directly correlated with the type 1 response, suggesting that LT-A may have some other function or may compartmentalize differently than does CT-A. Finally, since a type 2 cytokine response is generally regarded as the default response to inert antigens, perhaps research should focus not on why CT has a type 2 bias but rather on what attribute LT-A has that allows it to induce a type 1 response.
ACKNOWLEDGMENT
This investigation was supported by Public Health Service grant AI42777 from the National Institutes of Health.
REFERENCES
- 1.Angstrom J, Teneberg S, Karlsson K A. Delineation and comparison of ganglioside-binding epitopes for the toxins of Vibrio cholerae, Escherichia coli, and Clostridium tetani: evidence for overlapping epitopes. Proc Natl Acad Sci USA. 1994;91:11859–11863. doi: 10.1073/pnas.91.25.11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baudry B, Fasano A, Ketley J, Kaper J B. Cloning of a gene (zot) encoding a new toxin produced by Vibrio cholerae. Infect Immun. 1992;60:428–434. doi: 10.1128/iai.60.2.428-434.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brundtland G H. W. H. O. report on infectious diseases. Wkly Epidemiol Rec. 1999;74:279. [PubMed] [Google Scholar]
- 4.Cheng E, Cardenas-Freytag L, Clements J D. The role of cAMP in mucosal adjuvanticity of Escherichia coli heat-labile enterotoxin (LT) Vaccine. 1999;18:38–49. doi: 10.1016/s0264-410x(99)00168-1. [DOI] [PubMed] [Google Scholar]
- 5.Cieplak W, Jr, Messer R J, Konkel M E, Grant C C R. Role of potential endoplasmic reticulum retention sequence (RDEL) and the Golgi complex in the cytotonic activity of Escherichia coli heat-labile enterotoxin. Mol Microbiol. 1995;16:789–800. doi: 10.1111/j.1365-2958.1995.tb02440.x. [DOI] [PubMed] [Google Scholar]
- 6.Clements J D, Finkelstein R A. Isolation and characterization of homogeneous heat-labile enterotoxins with high specific activity from Escherichia coli cultures. Infect Immun. 1979;24:760–769. doi: 10.1128/iai.24.3.760-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clements J D, Hartzog N M, Lyon F L. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine. 1988;6:269–277. doi: 10.1016/0264-410x(88)90223-x. [DOI] [PubMed] [Google Scholar]
- 8.Clements J D, Yancey R J, Finkelstein R A. Properties of homogeneous heat-labile enterotoxin from Escherichia coli. Infect Immun. 1980;24:91–97. doi: 10.1128/iai.29.1.91-97.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickinson B L, Clements J D. Use of Escherichia coli heat-labile enterotoxin as an oral adjuvant. In: Kiyono H, Ogra P L, McGhee J R, editors. Mucosal vaccines. San Diego, Calif: Academic Press, Inc.; 1996. pp. 73–87. [Google Scholar]
- 10.Elson C O. Cholera toxin and its subunits as potential oral adjuvants. Immunol Today. 1989;146:29–33. doi: 10.1007/978-3-642-74529-4_3. [DOI] [PubMed] [Google Scholar]
- 11.Evans D G, Evans D J, Jr, Gorbach S L. Identification of enterotoxigenic Escherichia coli and serum antitoxin activity by the vascular permeability factor assay. Infect Immun. 1973;8:731–735. doi: 10.1128/iai.8.5.731-735.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmgren J. Receptors for cholera toxin and Escherichia coli heat-labile enterotoxin revisited. Prog Brain Res. 1994;101:163–177. doi: 10.1016/s0079-6123(08)61947-0. [DOI] [PubMed] [Google Scholar]
- 13.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Lencer W I, Constable C, Moe S, Jobling M G, Webb H M, Ruston S, Madara J L, Hirst T R, Holmes R K. Targeting of cholera toxin and Escherichia coli heat-labile toxin in polarized epithelia: role of COOH-terminal KDEL. J Cell Biol. 1995;131:951–962. doi: 10.1083/jcb.131.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lencer W I, Moe S, Rufo P A, Madara J L. Transcytosis of cholera toxin subunits across model human intestinal epithelia. Proc Natl Acad Sci USA. 1995;92:10094–10098. doi: 10.1073/pnas.92.22.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levine M M, Kaper J B, Black R E, Clements M L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983;47:510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowell G H, Kaminski R W, VanCott T C, Slike B, Kersey K, Zawoznik E, Loomis-Price L, Smith G, Redfield R R, Amselem S, Birx D L. Proteosomes, emulsomes, and cholera toxin B improve nasal immunogenicity of human immunodeficiency virus gp160 in mice: induction of serum, intestinal, vaginal, and lung IgA and IgG. J Infect Dis. 1997;175:292–301. doi: 10.1093/infdis/175.2.292. [DOI] [PubMed] [Google Scholar]
- 18.Lycke N, Tsuji T, Holmgren J. The adjuvant effect of Vibrio cholerae and Escherichia coli heat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur J Immunol. 1992;22:2277–2281. doi: 10.1002/eji.1830220915. [DOI] [PubMed] [Google Scholar]
- 19.Marinaro M, Staats H F, Hiroi T, Jackson R J, Coste M, Boyaka P N, Okahashi N, Yamamoto M, Kiyono H, Bluethmann H, Fujihashi K, McGhee J R. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J Immunol. 1995;155:4621–4629. [PubMed] [Google Scholar]
- 20.Mason H S, Haq T A, Clements J D, Arntzen C J. Edible vaccine protects mice against Escherichia coli heat-labile enterotoxin (LT): potatoes expressing a synthetic LT-B gene. Vaccine. 1998;16:1336–1343. doi: 10.1016/s0264-410x(98)80020-0. [DOI] [PubMed] [Google Scholar]
- 21.Roberts M, Bacon A, Rappuoli R, Pizza M, Cropley I, Douce G, Dougan G, Marinaro M, McGhee J, Chatfield S. A mutant pertussis toxin molecule that lacks ADP-ribosyltransferase activity, PT-9K/129G, is an effective mucosal adjuvant for intranasally delivered protein. Infect Immun. 1995;63:2100–2108. doi: 10.1128/iai.63.6.2100-2108.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodighiero C, Aman A T, Kenny M J, Moss J, Lencer W I, Hirst T R. Structural basis for the differential toxicity of cholera toxin and Escherichia coli heat-labile enterotoxin. Construction of hybrid toxins identifies the A2-domain as the determinant of differential toxicity. J Biol Chem. 1999;274:3962–3969. doi: 10.1074/jbc.274.7.3962. [DOI] [PubMed] [Google Scholar]
- 23.Rodighiero C, Aman A T, Lencer W I, Hirst T R. Differential activity of cholera toxin and E. coli enterotoxin: construction and purification of mutant and hybrid derivatives. Biochem Soc Trans. 1998;26:S364. doi: 10.1042/bst026s364. [DOI] [PubMed] [Google Scholar]
- 24.Takeda Y, Honda T, Taga S, Miwatani T. In vitro formation of hybrid toxins between subunits of Escherichia coli heat-labile enterotoxin and those of cholera enterotoxin. Infect Immun. 1981;34:341–346. doi: 10.1128/iai.34.2.341-346.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van De Verg L, Hartman A, Bhattacharjee A, Tall B, Yuan L, Sasala K, Hadfield T, Zollinger W, Hoover D, Warren R. Outer membrane protein of Neisseria meningitidis as a mucosal adjuvant for lipopolysaccharide of Brucella melitensis in mouse and guinea pig intranasal immunization models. Infect Immun. 1996;64:5263–5268. doi: 10.1128/iai.64.12.5263-5268.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu-Amano J, Kiyono H, Jackson R J, Staats H F, Fujihashi K, Burrows P D, Elson C O, Pillai S, McGhee J R. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J Exp Med. 1993;178:1309–1320. doi: 10.1084/jem.178.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto M, Vancott J L, Okahashi N, Marinaro M, Kiyono H, Fujihashi K, Jackson R J, Chatfield S N, Bluethmann H, McGhee J R. The role of Th1 and Th2 cells for mucosal IgA responses. Ann N Y Acad Sci. 1996;778:64–71. doi: 10.1111/j.1749-6632.1996.tb21115.x. [DOI] [PubMed] [Google Scholar]