Abstract
Objectives
Reactivation of HERV‐K(HML‐2) has been found in subsets of individuals with amyotrophic lateral sclerosis (ALS). This study examines the antibody response against HML‐2 in ALS and analyzes its clinical relevance.
Methods
Antibodies to HML‐2 envelope (env) were analyzed using a peptide array for epitope mapping and by a peptide enzyme‐linked immunosorbent assay (ELISA) in 242 healthy donors, and 243 ALS and 85 multiple sclerosis (MS) individuals. Extracellular levels of HML‐2 were analyzed by digital polymerase chain reaction (PCR).
Results
Antibodies in the sera of ALS individuals recognized more HML‐2 env peptides compared to healthy controls (p < 0.0001). ALS individuals had higher levels of HML‐2 than healthy donors (p = 0.02) and higher antibody levels against a select HML‐2 env peptide compared to healthy donors or individuals with multiple sclerosis (p < 0.0001). 55.14% of ALS compared to 21.16% of healthy donors and 13.10% of MS individuals had antibodies against the HML‐2 peptide (AUC = 0.769, p < 0.0001). Levels of extracellular HML‐2 DNA in serum (p = 0.02) and the number of HML‐2 env peptides recognized by ALS sera (p = 0.02) correlated with disease duration. Among ALS individuals, lower levels of HML‐2 antibodies were associated with a definite diagnosis per EL Escorial criteria (p = 0.03), and with a lower predicted (p = 0.02) and observed survival (p = 0.03).
Interpretation
There is a differential antibody response against specific epitopes of HML‐2 env in ALS and controls, suggesting epitope spreading, likely due to persistent antigenic exposure following reactivation of the viral genes. Low levels of antibodies to HML‐2 env in ALS are associated with poor prognosis and decreased survival probability. ANN NEUROL 2022;92:782–792
Introduction
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disease characterized by the loss of upper and lower motor neurons, which leads to rapidly progressive muscular weakness, paralysis, respiratory failure, and death. Factors that trigger the onset of the illness are poorly understood.
Human endogenous retroviruses (HERVs) are genomic sequences of retroviral origin that constitute around 8% of the human genome. HERVs are highly expressed in stem cells but get mostly silenced after cell differentiation. 1 The study of the involvement of HERVs in ALS pathogenesis started with the discovery of reverse transcriptase activity in the brain, blood, and cerebrospinal fluid of ALS individuals. 2 , 3 , 4 , 5 , 6 Several studies have associated HERV‐K (subtype HML‐2) with ALS, 7 , 8 although it might not be specific for this disease. 9 , 10 Reactivation of transposable elements has been found in 20% of ALS individuals by transcriptome stratification and this reactivation correlates with pathological TDP‐43 aggregation. 11 High levels of expression of HML‐2 have been found in autopsied brain samples from subsets of individuals with ALS. 12 , 13 In one study HML‐2 envelope (env) protein was detected in plasma‐derived extracellular vesicles with higher levels in advanced stages of the illness. 14 Expression of the env protein in neurons triggers neurodegeneration in vitro and motor neuron disease in a transgenic mouse model. 12 In an open label study, reduction of HML‐2 viral load in serum of individuals with ALS treated with anti‐retroviral therapy was associated with a slower clinical progression. 15 , 16 A conotoxin‐like protein (CTXLP) encoded by HML‐2 is expressed in the motor cortex of ALS individuals and has been linked with inflammation pathways and necroptosis. 17
Elevated levels of antibodies against specific epitopes of HML‐2 env were found in a cohort of Italian subjects with ALS when compared to normal controls and individuals with multiple sclerosis (MS). 18 The antibodies to HML‐2 env protein have a positive correlation with antibodies to TDP‐43. 19 HML‐2 env19–37 peptide also induced production of TNF‐α in CD8 + T cells indicating that this region stimulated both B and T cells. This study also defined another epitope HML‐2 env109–126 peptide that activated B cells in vitro. 20
The current study examines the antibody response against HML‐2 in ALS individuals and controls and analyzes its potential clinical relevance.
Subjects and Methods
Study Design
Case‐control study with retrospective data collection.
Samples
Serum samples from 243 ALS individuals from the Northeast ALS Consortium (NEALS) (99 females, 140 males, four unknown sex; mean age ± SD = 58.89 ± 10.61 years) and 242 sera of age‐ and sex‐matched healthy donors (HD) from the Blood Transfusion Centre of Sassari (84 females and 158 males; age = 53.29 ± 6.45 years) and 85 individuals with MS (54 females and 31 males; mean age ± SD = 57.35 ± 9.6 years). ALS individuals were classified into suspected, possible, probable, and definite ALS based on the EL Escorial criteria 21 or according to their calculated predicted survival by the ENCALS model 22 : very long survival (≥91 ± 1.84 months), moderate (from 25.3 ± 0.06 to 43.7 ± 0.21 months), and very short survival (≤17.7 ± 0.20 months). MS individuals were diagnosed according to McDonald's criteria 23 and only those treated with immunomodulators (interferon beta (n = 20), glatiramer acetate (n = 25) or teriflunomide (n = 13), or untreated (n = 27)) were included. All samples were stored at −80°C until used. This study was approved by the local ethics committees on human experimentation. All individuals provided written informed consent.
Epitope Mapping
We pre‐screened serum samples from 66 individuals with ALS and 46 HDs using an enzyme‐linked immunosorbent assay (ELISA) assay to detect antibodies against a recombinant HML‐2 envelope protein (MyBioSource; catalogue number MBS1391552_a0). We selected serum samples from 10 ALS and eight age‐ and sex‐matched controls that showed reactivity to the protein. Epitope mapping of the antibodies to HML‐2 env was performed by using peptide microarrays covering the complete sequence of the protein (Uniprot ID: Q69384) (PEPperCHIP Immunoassay, PepperPrint, Heidelberg, Germany). The elongated 699 amino acids antigen sequence was converted into 15 amino acids peptides with a peptide–peptide overlap of 14 amino acids, in duplicate. The assay was performed following manufacturer's instructions. To discriminate secondary antibody background, the microarray was pre‐stained with the secondary antibody and the background signal was subtracted from the final measurement. The peptide microarrays were incubated in washing buffer (phosphate‐buffered saline [PBS] with 0.05% Tween20, pH 7.4) for 15 min at room temperature (RT) and in blocking buffer (Rockland Blocking Buffer MB‐070) for 30 min at RT, followed by incubation with secondary antibody diluted 1 : 5000 in staining buffer (PBS with 0.05% Tween20 and 10% blocking buffer, pH 7.4) for 45 min. Then, microarrays were washed three times in washing buffer for 1 min each and in dipping buffer (1 mM Tris buffer, pH 7.4) three times until all visible contamination was removed. The microarrays were dried in a pressurized air stream from top to bottom and scanned (GenePix 4300A, Molecular Devices LLC, CA, USA).
Serum samples were diluted 1 : 200 in staining buffer and 200 μL were added to each array, which were incubated overnight at 4°C on an orbital shaker (85 rpm). Peptide microarrays were washed three times for 1 min in washing buffer and stained with the secondary antibody (1 : 5000 in staining buffer) for 45 min. Then, the microarrays were dipped three times in dipping buffer, dried in a pressurized air stream and scanned. The same process was performed with an anti‐ hemagglutinin control antibody. Finally, the scanned images were analyzed with MAPIX analyzer.
Analysis of the Results of the Peptide Array for Epitope Mapping
Since the total amount of antibodies against HML‐2 env was variable among individuals, to map the epitopes of HML‐2 env recognized by ALS and control sera, we first calculated the total OD of HML‐2 env in each sample as the sum of ODs of all HML‐2 env peptides (Total OD = ∑ODpeptides). Next, we calculated the percentage of the total OD that corresponded to each peptide in each sample (%ODpeptide = 100 × [ODpeptide/total OD]) and compared these percentages for each peptide between ALS and control samples by Wilcoxon signed‐rank test with correction for the number of comparisons (Fig 1A and Supporting Information Table S1). Next, we calculated the means of these percentages in ALS and control samples, generating a value for each peptide which was called epitope recognition score (ERS). The ERS for all peptides was compared between ALS and controls by Mann–Whitney test (Fig 1B). The median ERS of the controls was defined as a positivity threshold: peptides above this value were considered positive in eliciting a humoral response. Percentage of positive peptides was compared between ALS and controls by Fisher's exact test (Fig 1B). To search for the actual epitopes eliciting a differential response in ALS sera compared to controls', adjacent peptides were analyzed to find a common sequence between 4 and 12 amino acids 24 (Supporting Information Table S1).
FIGURE 1.
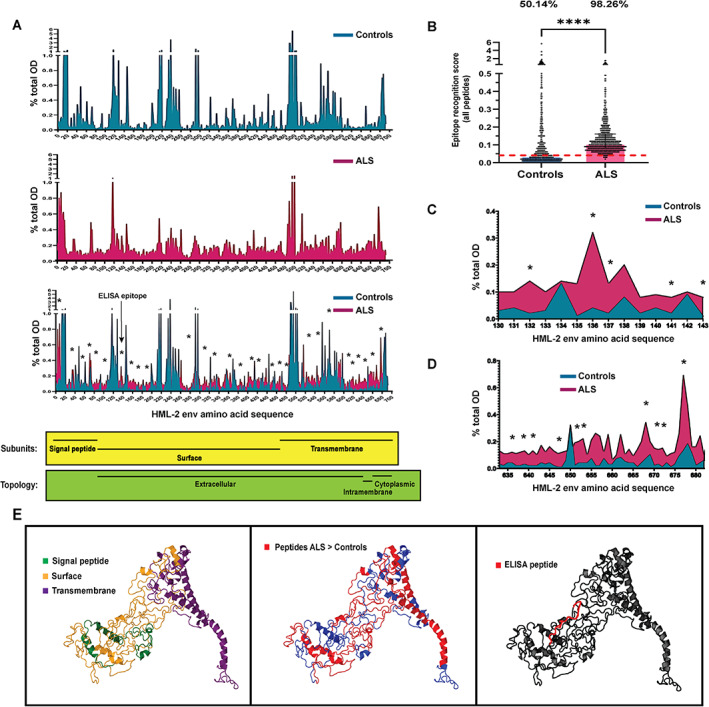
Differential recognition of HML‐2 env peptides in individuals with amyotrophic lateral sclerosis (ALS) and controls. (A) Analysis of the percentage of total optical density (OD) per peptide in controls (n = 8) (A, upper part), in individuals with ALS (n = 10) (B, center), and comparison of both groups (A, lower part) (Wilcoxon test; *p < 0.05), as determined by HML‐2 env peptide array. Location and topology of the protein areas according to the amino acid sequence (lower part). (B) Comparison of the values of epitope recognition score of all peptides covering the full sequence of HML‐2 env between ALS individuals and controls (Mann–Whitney; ****p < 0.0001), and comparison of percentage of peptides eliciting a humoral response. Red dashed line represents the cutoff used to consider a peptide as positive (median of controls) (Fisher's exact test; p < 0.0001). (C) Percentage of total OD in an area of the protein mapping the peptide used in the enzyme‐linked immunosorbent assay (ELISA) assay showing higher reactivity in ALS than in controls' sera (Wilcoxon test; *p < 0.05). (D) Percentage of total OD in a non‐extracellular part of the protein located in the transmembrane subunit, showing higher reactivity in ALS than in controls' sera (Wilcoxon test; *p < 0.05). (E) The 3D modeling of the HML‐2 env protein showing the location of the subunits (left), the peptides differentially recognized by ALS sera (center) and the peptide used in the ELISA assay (right). [Color figure can be viewed at www.annalsofneurology.org]
Analysis of HML‐2 Levels in Serum
HML‐2 was detected in serum by digital polymerase chain reaction as previously described. 15 Serum samples were centrifuged at 300g for 10 min to remove cells and debris. Total nucleic acids were extracted from 400 μL of the supernatants with an EZ1 Advance XL device (Qiagen) and the EZ1 Virus Mini Kit v2.0 (Qiagen), following manufacturer's instructions. Extracted nucleic acids were eluted in 60 μL of AVE buffer. Remaining magnetic beads were removed with a magnet. The digital PCR reaction was set in 96‐wells plates in duplicate in an AutoDG Droplet Digital PCR System (Bio‐Rad) with a set of primers and probe (FAM labeled) to detect HML‐2 env (forward primer: 5′ ATTTGGTGCCAGGAACTGAG 3′; reverse primer: 5′ GCTGTCTCTTCGGAGCTGTT 3′ and probe 5′ 6‐FAM‐AGGAGTTGCTGATGGCCTCG‐Iowa Black FQ 3′). To confirm the extracellular origin of HML‐2 in serum, a pre‐made assay of primers and probes targeting a cellular DNA (RPP30 gene, HEX‐tagged) was also included (Bio‐Rad). The master mix was composed of 12.5 μL of ddPCR Supermix (no dUTP) (Bio‐Rad), 1.25 μL of a mix of HML‐2 env primers (900 nm) and probe (250 nm) (Bio‐Rad), 1.25 μL of RPP30 assay (Bio‐Rad), 2.5 μL of nucleic acids and 7.5 μL of RNAse‐free water. After preparing the droplets, the PCR was conducted in a T100 Thermal cycler (Bio‐Rad) with the following cycling conditions: 95°C for 10 min, 40 cycles of 95°C for 30 s and 60°C for 1 min, and 95°C for 10 min. The number of copies was determined in a QX200 Digital PCR reader (Bio‐Rad). Results were expressed as a ratio of HML‐2 env copies/RPP30 copies.
Determination of HML‐2 Antibodies by Peptide ELISA
Peptide HML‐2‐env‐su (VWVPGPTDDRCPAKPEEEG) (Uniprot ID: O42043) 18 was used for the ELISA assay. This peptide aligns to amino acids 131–149 of the sequence used for epitope mapping (Uniprot ID: Q69384). The peptide was synthesized at >95% purity (LifeTein, South Plainfield, NJ 07080, USA) and dissolved in dimethyl sulfoxide (DMSO). Indirect ELISA was performed to detect antibodies (Abs) against that peptide, as previously described. 18 Alignment of the peptide sequence with the different HML‐2 env sequences encoded in the human genome is provided in Table 1.
TABLE 1.
List of HERV‐K (HML‐2) Sequences Encoded in the Human Genome Aligning with the Peptide Used in the ELISA Assay
| Uniprot ID | Name | Locus | Length |
|---|---|---|---|
| Q9UQG0 | Endogenous retrovirus group K member 11 | 3q27.2 | 969 aa |
| O42043 | Endogenous retrovirus group K member 18 | 1q23.3 | 560 aa |
| Q9HDB8 | Endogenous retrovirus group K member 5 | 3q12.3 | 245 |
| P61566 | Endogenous retrovirus group K member 24 | 22q11.21 | 588 aa |
| P63135 | Endogenous retrovirus group K member 7 | 1q22 | 1,459 aa |
| Q902F8 | Endogenous retrovirus group K member 8 | 8p23.1 | 699 aa |
| Q69384 | Endogenous retrovirus group K member 6 | 7p22.1 | 699 aa |
| Q9UKH3 | Endogenous retrovirus group K member 9 | 6q14.1 | 698 aa |
| P61570 | Endogenous retrovirus group K member 25 | 11q22.1 | 661 aa |
| P61567 | Endogenous retrovirus group K member 7 | 1q22 | 588 aa |
| O71037 | Endogenous retrovirus group K member 19 | 19q11 | 699 aa |
| P10266 | Endogenous retrovirus group K member 10 | 5q33.3 | 1,014 aa |
| Q902F9 | Endogenous retrovirus group K member 113 | 19p13.11 | 699 aa |
| P61565 | Endogenous retrovirus group K member 21 | 12q14.1 | 698 aa |
| P61568 | Putative endogenous retrovirus group K member 11 | 1p13.3 | 191 aa |
ELISA = enzyme‐linked immunosorbent assay.
The 96 wells‐plates (Nunc) were coated with 10 μg/ml of peptide diluted in 0.05 M carbonate– bicarbonate buffer, pH 9.5 (Sigma), overnight at 4°C. Plates were then blocked with 5% skimmed milk (Sigma) for 1 hour at room temperature and washed twice with PBS containing 0.05% Tween‐20 (PBS‐T). Then, serum samples were diluted 1 : 100 in PBS‐T and 100 μL were added per well. Plates were incubated for 2 hours at room temperature. After five washes in PBS‐T, plates were incubated with alkaline phosphatase‐conjugated goat anti‐human immunoglobulin G polyclonal antibody (1 : 1000; Sigma) for 1 hour at room temperature. Plates were washed again five times in PBS‐T and incubated with para‐nitrophenylphosphate (Sigma) for 3–6 min at 37°C in the dark. Absorbance was read at 405 nm of wavelength on a microplate reader (Molecular Devices). Each sample was analyzed in triplicate. Mean values of negative controls (wells coated with the peptides and incubated with the secondary antibody alone) were subtracted from all samples. Positive control sera were also included in all experiments. Results are expressed as means of triplicates of optical density (OD) values.
Determination of Total IgG and IgM in Serum by ELISA
Levels of total immunoglobulin (Ig)G were measured in serum samples with Human ELISA Kits (Invitrogen) following manufacturer's instructions.
3D Modeling of HML‐2 Envelope Protein
Tertiary structure of the HML‐2 env protein was determined by Protein Homology/ analogy Recognition Engine version 2.0 (Phyre2), 25 using the intensive mode, which performs complete modeling of the entire protein using multiple templates and ab initio techniques. The 3D figures of the proteins were created with Jmol. 26
Statistical Analysis
GraphPad Prism 8.2.0 software was used for the statistical analysis. Wilcoxon signed‐rank test with correction for the number of comparisons was used to compare the percentages of optical density (OD) that corresponded to each peptide between ALS and control samples. Unpaired t‐test and analysis of variance (ANOVA) (with Sidak's post‐hoc analysis) were used to compare continuous variables between two groups and three or more groups, respectively. The non‐parametric test Mann–Whitney and Kruskal–Wallis (with Dunn's post‐hoc analysis) tests were used when the variables were not parametric or homoscedastic (ERS for all peptides, levels of HML‐2, antibodies against HML‐2 and total IgGs, HML‐2 ratio and HML‐2 antibodies [OD] among El Escorial ALS diagnosis groups). Differences in the percentage of peptides eliciting a humoral response in ALS and controls' sera were analyzed by the Fisher's exact test and in the percentage of individuals that were positive for HML‐2 env antibodies by ELISA were evaluated by chi‐squared exact test. The area under the curve (AUC) was calculated to analyze the sensitivity and specificity of the ELISA antibody test. Spearman's r correlation was used to analyze the correlation between two continuous variables (HML‐ 2 ratio, disease duration and number of epitopes) and to analyze the correlation between a continuous (HML‐2 ratio and HML‐2 env antibodies [OD]) and an ordinal variable with four categories or more (El Escorial ALS diagnosis). Since patients with very long disease duration are likely not representative of the cohort only ALS individuals with a disease duration <5 years were included in the correlation analyses. Log‐rank Mantel‐Cox test was used to analyze the probability of survival according to the levels of antibodies (patients were grouped in tertiles). A p‐value <0.05 was considered statistically significant. In the figures error bars represent median ± interquartile range.
Results
We performed epitope mapping of the antibodies against HML‐2 detected in the serum of 10 individuals with ALS and eight controls using an HML‐2 peptide array covering the full sequence of the protein. Multiple regions of the protein elicited a higher antibody response in ALS than in controls (Wilcoxon corrected p < 0.05) (Fig 1A and Supporting Information Table S1). In fact, statistical significance was only achieved for peptides that were recognized more by ALS than controls' sera.
We observed that while HML‐2 env antibodies in control sera reacted against only specific peptides of the HML‐2 sequence, ALS individuals' antibodies reacted against most regions of the HML‐2 env. The median of the ERS was 0.04% (0.02%–0.11%) in controls and 0.11% (0.08%–0.16%) in ALS (Mann–Whitney's test; p < 0.0001). Considering the median of the controls as a positivity threshold, 50.14% of peptides elicited a humoral response in controls and 98.26% in ALS sera (Fisher exact test; p < 0.0001) (Fig 1B). Interestingly, one of the regions showing higher reactivity in ALS sera shared sequence with a peptide previously identified in an Italian cohort as being more antigenic in ALS 18 (Fig 1C). By 3D modeling with Phyre2 software 25 we localized the peptides on the HML‐2 env subunits. There seems to be an accumulation of peptides recognized by sera of ALS individuals in the transmembrane subunit of the protein, particularly in areas of the protein that are not extracellular (Fig 1D,E, left and center). However, the epitope previously studied in ALS 18 is located in the surface subunit (Fig 1E, left and right).
To define the actual epitopes (motifs from 4 to 12 amino acids), adjacent peptides eliciting a higher antibody response in ALS were analyzed to search for common sequences. The 35 distinct epitopes were identified, including the epitope (AKPE) that was located within the peptide used previously 18 (Supporting Information Table S1).
We analyzed sera of 243 ALS, 242 HDs and 85 individuals with MS by ELISA to detect antibodies against an HML‐2 env peptide (VWVPGPTDDRCPAKPEEEG), which had shown higher reactivity in ALS by ELISA in a small Italian cohort of 21 ALS individuals, 18 and which aligned to a region that had showed higher reactivity in ALS individuals by our epitope mapping. The levels of antibodies were higher in ALS (mean OD ± SEM = 0.667 ± 0.028) than in HDs (0.340 ± 0.021) (Mann–Whitney; p < 0.0001) and in MS (0.258 ± 0.027) (Fig 2A). A cutoff value of 0.478 for the optical density provided the best sensitivity and specificity. Using this cutoff, 55.14% of ALS individuals and only 21.16% of HDs and 13.10% of MS individuals showed a serological immune response against the HML‐2 peptide (AUC = 0.769, p < 0.0001, Fisher' exact test p < 0.0001) (Fig 2A,B). To determine whether there was a difference in HML‐2, we also analyzed the levels of extracellular HML‐2 DNA (as a ratio HML‐2 copies/RPP30 copies) in the serum of a subgroup of individuals for whom enough sample was available. ALS individuals (n = 60) had a higher HML‐2 ratio (mean ratio ± SEM = 14.75 ± 3.79) compared to HDs (n = 27) (13.11 ± 3.54), although the difference was not as prominent as with the antibodies, probably in part due to the smaller sample size (Mann–Whitney; p = 0.02) (Fig 2C). There was a positive weak correlation between the disease duration and the HML‐2 ratio (Spearman's r = 0.34; p = 0.02) (Fig 2D) when analyzed for ALS individuals with disease duration <5 years.
FIGURE 2.
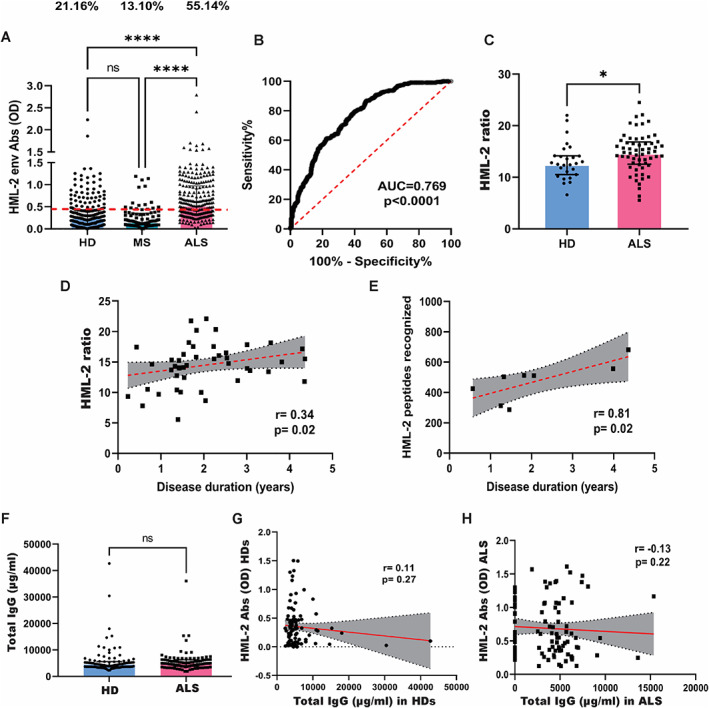
HML‐2 levels and antibodies against a select HML‐2 env peptide. (A) Comparison of levels of antibodies to a select HML‐2 peptide determined by enzyme‐linked immunosorbent assay (ELISA) in healthy donors (HD) (n = 242), individuals with amyotrophic lateral sclerosis (ALS) (n = 243) and in multiple sclerosis (MS) (n = 85) (Kruskal–Wallis test with Dunn post‐hoc test; ****p < 0.0001) and comparison of percentage of positive individuals. Red dashed line represents the threshold used to assess the samples' positivity (Fisher exact test; p < 0.0001). (B) ROC curve between sensitivity and specificity of the HML‐2 antibody ELISA test for every possible cutoff. The area under the ROC curve (AUC) is a measure of the diagnostic test accuracy (AUC = 0.769; p < 0.0001). (C) HML‐2 ratio (HML‐2 env copies/RPP30 copies) in individuals with ALS (n = 60) and HDs (n = 27) as determined by digital PCR (Mann–Whitney; *p = 0.02). (D) Correlation between disease duration and HML‐2 ratio in ALS patients (with duration <5 years) (n = 54; Spearman's r = 0.34; p = 0.02). (E) Correlation between the number of peptides recognized and the disease duration in individuals with ALS (with duration <5 years) (n = 8; Spearman's r = 0.81; p = 0.02). (F) Levels of total IgG antibodies as determined by ELISA in HDs (n = 100) and individuals with ALS (n = 100) (Mann–Whitney; p = 0.19). (G) Correlation between concentration of total IgG antibodies and levels of HML‐2 antibodies in the serum of HDs (n = 99; Spearman r = 0.11; p = 0.27). (H) Correlation between concentration of total IgG antibodies and levels of HML‐2 antibodies in the serum of individuals with ALS (n = 99; Spearman r = −0.13; p = 0.22). [Color figure can be viewed at www.annalsofneurology.org]
In most immune responses, the initial epitopes that are recognized by the adaptive immune system are limited. After repeated exposure to an antigen, the T and B cell responses expand to include other epitopes of that antigen. This process is known as intramolecular epitope spreading. 25 A strong positive correlation between the disease duration and the number of peptides eliciting an immune response (Spearman's r = 0.81; p = 0.02) (Fig 2E) could be found in individuals with a disease duration <5 years, suggesting epitope spreading as the cause of multiplicity of epitope recognition of HML‐2 env in ALS.
To confirm that the differences found in the antibody response between ALS and controls were specific to HML‐2 and not associated to a general imbalance of immunoglobulins in the disease, we analyzed the levels of total IgGs in serum samples and found that there were no significant differences between individuals with ALS (mean ± SEM = 5,585 ± 383.5 μg/ml) and controls (5,805 ± 228.9 μg/ml) (Mann–Whitney; p = 0.19) (Fig 2F). Moreover, there was no correlation between the levels of total IgGs and the levels of antibodies to HML‐2 neither in controls (Spearman' r = 0.11; p = 0.3) (Fig 2G) nor in individuals with ALS (Spearman's r = −0.13; p = 0.2) (Fig 2H).
When we classified the ALS individuals according to EL Escorial criteria, a positive weak correlation between the HML‐2 ratio and the degree of certainty of ALS diagnosis was revealed (Spearman's r = 0.24; p = 0.03) (Fig 3A). Interestingly, although the levels of HML‐2 antibodies were higher in all categories of ALS compared to controls (Kruskal–Wallis test; p < 0.0001), a weak negative correlation with the degree of certainty of ALS diagnosis was found in ALS patients (Spearman's r = −0.15; p = 0.02) (Fig 3B). In fact, patients with a non‐definite diagnosis of ALS (suspected, possible, or probable combined) showed higher levels than patients with definite ALS (unpaired t‐test; p = 0.03) (Fig. 3C). Since the degree of certainty of the diagnosis is based on the degree of spread of the disease (definite ALS diagnosis requires upper and lower motor signs in at least three regions), this observation suggests that antibody levels decrease in later stages of the disease. To further determine if the antibody levels were associated with disease survival, we grouped the individuals with ALS into three categories according to the ENCALS prediction model of survival. 22 We found that individuals who were predicted to have a very long survival (≥91 ± 1.84 months) had higher levels of HML‐2 env antibodies than those predicted to have a moderate (from 25.3 ± 0.06 to 43.7 ± 0.21 months) or very short survival (≤17.7 ± 0.20 months) (ANOVA with Sidak' post‐hoc; p = 0.03 and p = 0.02, respectively) (Fig. 3D). Moreover, when we divided the ALS individuals into tertiles according to the levels of HML‐2 env antibodies, participants with the lowest levels of antibodies had lower observed survival (Tertile 1 median survival = 36 months) compared to those with higher levels of antibodies (Tertile 2 median survival = 51 months; Tertile 3 median survival = 50 months) (Log‐rank Mantel‐Cox test; p = 0.03) (Fig 3E). There were no differences in HML‐2 env antibody levels between males and females, between racial groups categorized as Whites, Blacks, and Asians, and there was no correlation with age at which the sample was obtained. There was also no correlation between the antibody titers and ALSFRS‐R score at time of sample collection neither with the progression rate (48‐ALSFRS‐R/ disease duration). There was also no difference in antibody titers between patients when classified by site of onset of disease (limb or bulbar onset or onset at multiple sites).
FIGURE 3.
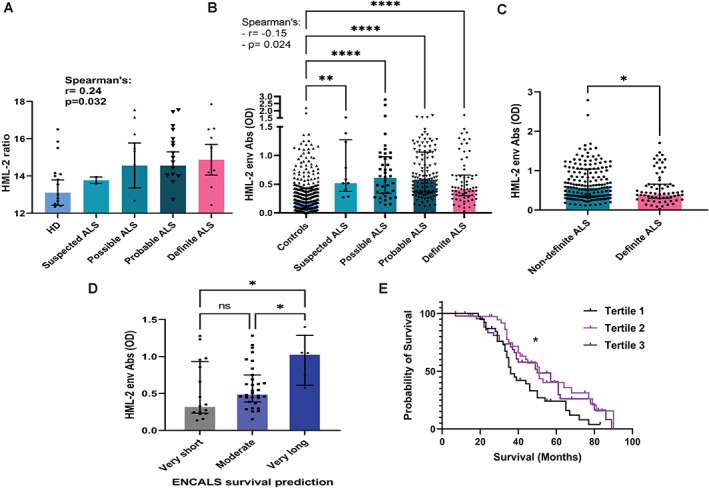
Correlation of HML‐2 ratio and antibodies with severity of amyotrophic lateral sclerosis (ALS). (A) HML‐2 ratio in individuals with ALS classified by El Escorial criteria (suspected: n = 2; possible: n = 13; probable: n = 29 and definite: n = 11) and in healthy donors (HD) (n = 27) as measured by digital polymerase chain reaction (PCR). Correlation analysis by Spearman's r test (r = 0.24; p = 0.03). (B) Levels of HML‐2 env antibodies in individuals with ALS classified by El Escorial criteria (suspected: n = 10; possible: n = 39; probable: n = 112 and definite: n = 67) and in controls (n = 327; 242 HD and 85 multiple sclerosis [MS]), as determined by peptide enzyme‐linked immunosorbent assay (ELISA). Comparison between groups (Kruskal–Wallis test with Dunn post‐hoc analysis). Correlation analysis by Spearman r test (r = −0.15; p = 0.02). (C) Levels of HML‐2 env antibodies in individuals with non‐definite and definite ALS according to El Escorial criteria (unpaired t‐test; p = 0.03). (D) Levels of HML‐2 env antibodies in ALS individuals grouped by predicted survival according to the ENCALS model (analysis of variance [ANOVA] with Sidak post‐hoc test; p = 0.02 and p = 0.03). (E) Analysis of observed survival in individuals with ALS according to the levels of antibodies to a select HML‐2 env peptide. HML‐2 antibodies optical density (ODs): Tertile 1 (n = 78); OD: 0.026–0.380; Tertile 2 (n = 78); OD: 0.381–0.749; Tertile 3 (n = 79); OD: 0.750–2.79. Log‐rank Mantel–Cox test; p = 0.03. [Color figure can be viewed at www.annalsofneurology.org]
Discussion
Our results show that individuals with ALS have higher levels of HML‐2 and antibodies against this endogenous retrovirus in serum than controls. But, interestingly, a diminished humoral response against HML‐2 was noted in patients in later stages of the disease when three or more anatomical regions were involved. Lower antibody levels were also associated with a lower predicted and observed survival. We have also found higher levels of extracellular HML‐2 DNA in the serum of ALS individuals, as shown in a previous study. 27 Together, these results support the possibility that reactivation of HML‐2 contributes to ALS pathogenesis and that a humoral response against it would be protective for the patients, or conversely, a loss of antibodies is associated with a poor prognosis. Alternatively, it may indicate a B cell activation early in the disease with loss of function later in the illness. In addition, we have found that the sera from subjects with ALS react differently to the envelope protein of HML‐2 since more regions of the protein are antigenic in ALS compared to controls. The levels of HML‐2 in serum correlate with disease duration, indicating that at least some individuals with ALS might be continuously exposed to HML‐2 antigens. Moreover, we found that the number of HML‐2 peptides recognized by ALS sera correlated with disease duration. These observations suggest that the increase in epitope recognition of HML‐2 in ALS might be due to epitope spreading. Epitope spreading is the diversification of the specificity of the immune response from the initial response that is directed to dominant epitopes on a self or foreign protein, to subdominant or cryptic epitopes on the same protein (intramolecular spreading) or other proteins (intermolecular spreading). 28 Epitope spreading can initiate as a result of tissue damage, since more antigens would be released. In our study, this possibility is suggested by the fact that several HML‐2 env peptides specifically recognized by individuals with ALS are found in intramembrane and cytosolic portions of the protein. These epitopes are not naturally exposed to antibody recognition but, because of neural death, proteins are released to the extracellular media and those normally cryptic sequences get exposed to immune cells and might elicit immune responses. In a study on experimental autoimmune encephalomyelitis (EAE), an animal model of MS, immunization of mice with the immunodominant PLP epitope PLP139–151 led to reactivity to that epitope in 3 days. Immediately before the first relapse, T‐cell reactivity against PLP178–191 was detected (intramolecular epitope spreading), and during the second relapse responses against MBP84–104 were also identified (intermolecular epitope spreading). The development of these responses correlated with the extent of myelin destruction. 28
HML‐2 env is the product of a human gene thus, antibodies against HML‐2 env would be considered autoantibodies. While the pathogenic role of autoantibodies is well established, numerous studies have also shown that autoimmunity is protective in many situations. For example, IgM anti–double‐stranded DNA (anti‐dsDNA) antibodies negatively correlate with glomerulonephritis severity in systemic lupus erythematosus (SLE) 29 , 30 and the administration of IgM anti‐dsDNA prevented the development of nephritis in a SLE mouse model. 31 Similarly, autoantibodies anti‐phospholipid (aPA), which cross‐react with LDL and oxLDL, reduces plaque formation in atherosclerosis‐prone mice. 32
Increasing evidence suggests that the immune system has a beneficial role in the progression of ALS. It has been suggested that, similar to the situation in cancer immunology, onset of clinical symptoms in neurodegenerative diseases might reflect immune tolerance towards neurotoxic self‐antigens and loss of immune surveillance. Fighting off neurodegenerative conditions might require breaking peripheral immune tolerance to central nervous system (CNS) self‐antigens to boost protective autoimmunity. 33 Immune response in peripheral axons delays disease progression in the ALS model fast progressor SOD1G93A mice, which have downregulation of the pro‐inflammatory chemokine MCP‐1/CCL‐2. 34
Moreover, protective autoimmunity seems to be a physiological response to CNS trauma. 35 Although not proven, ALS has been proposed to be associated in some people with repetitive CNS trauma, as rates of ALS have been reported to be higher among military veterans and collision‐sport athletes. 36 , 37 Traumatic brain injury is also associated with an increased risk of other neurodegenerative diseases including Alzheimer' disease, Parkinson disease, and chronic traumatic encephalopathy. 38 A hypothesis that needs to be tested is that HML‐2 expression might contribute to the higher risk of neurodegeneration after brain trauma. Neural stem cells migrate to sites of trauma in the CNS to repair brain tissue 39 and HML‐2 env is highly expressed in neural stem cells but is silenced in neurons and differentiated cells. 1 Moreover, HML‐2 env is neurotoxic, 12 so degeneration in neurons adjacent to contusion areas could occur if expression of HML‐2 in differentiating neural stem cells does not get silenced quickly or if this process occurs too often due to repetitive trauma exposure. Autoantibodies against HML‐2 env could protect against neurodegeneration and ALS development in individuals with increased expression of HML‐2 subsequent to brain injury.
Strengths of this study include a large sample size and extensive follow‐up of the individuals with ALS. This allowed for the analysis of the predicted ENCALS survival and the observed survival according to the levels of antibodies. However, information on the date of onset was not available for all patients. The observation that there is an association between the levels of HML‐2 env antibodies and the ENCALS model of predicted survival but not with the progression rate can be explained because the ENCALS score is based on several prognostic factors in addition to the progression rate, such as bulbar versus nonbulbar onset, age at onset, definite versus probable or possible ALS, diagnostic delay, forced vital capacity, frontotemporal dementia and presence of a C9orf72 repeat expansion, which may influence antibody levels. Importantly ENCALS is a strong predictive model of survival with a c‐statistic or AUC of 0.78 (95% CI 0.77–0.80; 95% prediction interval 0.74–0.82). 22
Another limitation of the study was that serum sample was only available to analyze the levels of HML‐2 in a subgroup of the ALS participants with data on HML‐2 antibodies. In addition, this is a cross‐sectional study; longitudinal studies to determine the evolution of HML‐2 and HML‐2 antibody levels in ALS and controls are warranted. A limitation of the peptide array used for epitope mapping is that it analyzes only linear epitopes; thus, it remains unknown whether conformational epitopes would follow a similar pattern of differential recognition in ALS.
The findings presented here suggest the possibility of a protective role for HML‐2 antibodies against disease progression, but all the same it could be a biomarker with no causal effect. An attractive approach might be to test such antibodies as a therapeutic option in individuals with ALS. A monoclonal antibody against the envelope of another endogenous retrovirus, HERV‐W, has been developed to treat MS. 40 HERV‐W env is an early marker of immune activation that is elevated in MS, mostly during relapses. 41 Preincubation with a HERV‐W env monoclonal antibody reduced the nitrosative stress induced by HERV‐W env in oligodendrocytes and rescued myelin expression. 42 A pre‐clinical study conducted by our group to explore the therapeutic use of an HML‐2 env monoclonal antibody in ALS has shown the antibody can protect against neurodegeneration caused by HML‐2 env protein both in vitro and in vivo. 43 , 44 Moreover, antibodies against HML‐2 env have been found to positively correlate with antibodies against TDP‐43 in ALS individuals. 19 An intrabody developed against TDP‐43 has been shown to reduce cytoplasmic aggregation of TDP‐43 in an in vitro model of ALS. 45 Immunotherapy against HML‐2 env 46 and TDP‐43 could be promising options for the treatment of individuals with ALS. Alternatively, vaccination with specific HML‐2 peptides to induce an immune response against the retrovirus may be another therapeutic approach.
Author Contributions
Conception and design of the study: Marta Garcia‐Montojo, Leonardo A. Sechi, Elena Rita Simula, Avindra Nath. Acquisition and analysis of data: Marta Garcia‐Montojo, Elena Rita Simula, Saeed Fathi, Cynthia McMahan, Anubrata Ghosal, James D. Berry, Merit Cudkowicz, Abdel Elkahloun, Kory Johnson, Gina Norato, Leonardo A. Sechi, Peter Jensen, Tony James, Avindra Nath. Drafting a significant portion of the manuscript or figures: Marta Garcia‐Montojo, Elena Rita Simula, Leonardo A. Sechi, Avindra Nath.
Potential Conflicts of Interest
The authors report no conflicts of interest relevant to the manuscript.
Supporting information
TABLE S1 Analysis of epitope recognition score (ERS) of antibodies to 15 mer peptides derived from HML‐2 envelope in serum samples from amyotrophic lateral sclerosis (ALS) individuals and controls. Highlighted motifs are the common epitopes among the peptides differentially recognized in ALS samples.
Acknowledgments
We thank our collaborators from the Massachusetts General Hospital and the University of Sassari for the samples provided. We are grateful to all the study participants and the clinical staff who collaborated in the sample collection. We thank Caroline Anderson for her contributions to the development of the HML‐2 antibody ELISA assay. This study was supported by intramural funds from NINDS (NS003130) and the ALS Association (Grant ID: 20‐SI‐559).
References
- 1. Wang T, Medynets M, Johnson KR, et al. Regulation of stem cell function and neuronal differentiation by HERV‐K via mTOR pathway. Proc Natl Acad Sci USA 2020;117:17842–17853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andrews WD, Tuke PW, Al‐Chalabi A, et al. Detection of reverse transcriptase activity in the serum of patients with motor neurone disease. J Med Virol 2000. Aug;61:527–532. [DOI] [PubMed] [Google Scholar]
- 3. McCormick AL, Brown RH Jr, Cudkowicz ME, et al. Quantification of reverse transcriptase in ALS and elimination of a novel retroviral candidate. Neurology 2008. Jan 22;70:278–283. [DOI] [PubMed] [Google Scholar]
- 4. Steele AJ, Al‐Chalabi A, Ferrante K, et al. Detection of serum reverse transcriptase activity in patients with ALS and unaffected blood relatives. Neurology 2005. Feb 8;64:454–458. [DOI] [PubMed] [Google Scholar]
- 5. MacGowan DJ, Scelsa SN, Imperato TE, et al. A controlled study of reverse transcriptase in serum and CSF of HIV‐negative patients with ALS. Neurology 2007;68:1944–1946. [DOI] [PubMed] [Google Scholar]
- 6. Viola MV, Frazier M, White L, et al. RNA‐instructed DNA polymerase activity in a cytoplasmic particulate fraction in brains from Guamanian patients. J Exp Med 1975;142:483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garcia‐Montojo M, Doucet‐O'Hare T, Henderson L, Nath A. Human endogenous retrovirus‐K (HML‐2): a comprehensive review. Crit Rev Microbiol 2018;44:715–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Phan KHY, Fu Y, Dzamko N, et al. Pathological manifestation of human endogenous retrovirus K in frontotemporal dementia. Commun Med 2021;1:1–11. 10.1038/s43856-021-00060-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mayer J, Harz C, Sanchez L, et al. Transcriptional profiling of HERV‐K(HML‐2) in amyotrophic lateral sclerosis and potential implications for expression of HML‐2 proteins. Mol Neurodegener 2018;13:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garson JA, Usher L, Al‐Chalabi A, et al. Quantitative analysis of human endogenous retrovirus‐K transcripts in postmortem premotor cortex fails to confirm elevated expression of HERV‐K RNA in amyotrophic lateral sclerosis. Acta Neuropathol Commun 2019;7:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tam OH, Rozhkov NV, Shaw R, et al. Postmortem cortex samples identify distinct molecular subtypes of ALS: retrotransposon activation, oxidative stress, and activated glia. Cell Rep 2019;29:1164–77 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li W, Lee MH, Henderson L, et al. Human endogenous retrovirus‐K contributes to motor neuron disease. Sci Transl Med 2015;7:307ra153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Douville R, Liu J, Rothstein J, Nath A. Identification of active loci of a human endogenous retrovirus in neurons of patients with amyotrophic lateral sclerosis. Ann Neurol 2011;69:141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li Y, Chen Y, Zhang N, Fan D. Human endogenous retrovirus K (HERV‐K) env in neuronal extracellular vesicles: a new biomarker of motor neuron disease. Amyotroph Lateral Scler Frontotemporal Degener 2021;23:1–8. [DOI] [PubMed] [Google Scholar]
- 15. Gold J, Rowe DB, Kiernan MC, et al. Safety and tolerability of Triumeq in amyotrophic lateral sclerosis: the lighthouse trial. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20:595–604. [DOI] [PubMed] [Google Scholar]
- 16. Garcia‐Montojo M, Fathi S, Norato G, et al. Inhibition of HERV‐K (HML‐2) in amyotrophic lateral sclerosis patients on antiretroviral therapy. J Neurol Sci 2021;423:117358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Di Curzio D, Gurm M, Turnbull M, et al. Pro‐inflammatory signaling upregulates a neurotoxic Conotoxin‐like protein encrypted within human endogenous retrovirus‐K. Cells 2020;9:1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arru G, Mameli G, Deiana GA, et al. Humoral immunity response to human endogenous retroviruses K/W differentiates between amyotrophic lateral sclerosis and other neurological diseases. Eur J Neurol 2018;25:1076–e84. [DOI] [PubMed] [Google Scholar]
- 19. Simula ER, Arru G, Zarbo IR, et al. TDP‐43 and HERV‐K envelope‐specific immunogenic epitopes are recognized in ALS patients. Viruses 2021;13:2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arru G, Galleri G, Deiana GA, et al. HERV‐K modulates the immune response in ALS patients. Microorganisms 2021;9:1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brooks BR. El Escorial world Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on motor neuron diseases/amyotrophic lateral sclerosis of the world Federation of Neurology Research Group on neuromuscular diseases and the El Escorial "clinical limits of amyotrophic lateral sclerosis" workshop contributors. J Neurol Sci 1994;124:96–107. [DOI] [PubMed] [Google Scholar]
- 22. Westeneng HJ, Debray TPA, Visser AE, et al. Prognosis for patients with amyotrophic lateral sclerosis: development and validation of a personalised prediction model. Lancet Neurol 2018;17:423–433. [DOI] [PubMed] [Google Scholar]
- 23. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121–127. [DOI] [PubMed] [Google Scholar]
- 24. Buus S, Rockberg J, Forsstrom B, et al. High‐resolution mapping of linear antibody epitopes using ultrahigh‐density peptide microarrays. Mol Cell Proteomics 2012;11:1790–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kelley LA, Mezulis S, Yates CM, et al. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 2015;10:845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Willighagen E, Howard M. Fast and scriptable molecular graphics in web browsers without Java3D. Nat Preced 2007. 10.1038/npre.2007.50.1. [DOI] [Google Scholar]
- 27. Phan K, He Y, Fu Y, et al. Pathological manifestation of human endogenous retrovirus K in frontotemporal dementia. Commun Med 2021;1:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vanderlugt CL, Miller SD. Epitope spreading in immune‐mediated diseases: implications for immunotherapy. Nat Rev Immunol 2002. Feb;2:85–95. [DOI] [PubMed] [Google Scholar]
- 29. Hahn BH. Antibodies to DNA. N Engl J Med 1998;338:1359–1368. [DOI] [PubMed] [Google Scholar]
- 30. Shoenfeld Y, Toubi E. Protective autoantibodies: role in homeostasis, clinical importance, and therapeutic potential. Arthritis Rheum 2005;52:2599–2606. [DOI] [PubMed] [Google Scholar]
- 31. Conrad K, Bachmann MP, Matsuura E, Shoenfeld Y. From animal models to human genetics: research on the induction and pathogenicity of autoantibodies. Autoimmun Rev 2005;4:178–187. [DOI] [PubMed] [Google Scholar]
- 32. Nicolo D, Goldman BI, Monestier M. Reduction of atherosclerosis in low‐density lipoprotein receptor‐deficient mice by passive administration of antiphospholipid antibody. Arthritis Rheum 2003;48:2974–2978. [DOI] [PubMed] [Google Scholar]
- 33. Schwartz M, Baruch K. Breaking peripheral immune tolerance to CNS antigens in neurodegenerative diseases: boosting autoimmunity to fight‐off chronic neuroinflammation. J Autoimmun 2014;54:8–14. [DOI] [PubMed] [Google Scholar]
- 34. Nardo G, Trolese MC, de Vito G, et al. Immune response in peripheral axons delays disease progression in SOD1(G93A) mice. J Neuroinflammation 2016;13:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yoles E, Hauben E, Palgi O, et al. Protective autoimmunity is a physiological response to CNS trauma. J Neurosci 2001;21:3740–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stein TD, Alvarez VE, McKee AC. Chronic traumatic encephalopathy: a spectrum of neuropathological changes following repetitive brain trauma in athletes and military personnel. Alzheimers Res Ther 2014;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beard JD, Kamel F. Military service, deployments, and exposures in relation to amyotrophic lateral sclerosis etiology and survival. Epidemiol Rev 2015;37:55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Graham NS, Sharp DJ. Understanding neurodegeneration after traumatic brain injury: from mechanisms to clinical trials in dementia. J Neurol Neurosurg Psychiatry 2019;90:1221–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bye N, Turnley AM, Morganti‐Kossmann MC. Inflammatory regulators of redirected neural migration in the injured brain. Neurosignals 2012;20:132–146. [DOI] [PubMed] [Google Scholar]
- 40. Curtin F, Perron H, Kromminga A, et al. Preclinical and early clinical development of GNbAC1, a humanized IgG4 monoclonal antibody targeting endogenous retroviral MSRV‐Env protein. MAbs 2015;7:265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Garcia‐Montojo M, Rodriguez‐Martin E, Ramos‐Mozo P, et al. Syncytin‐1/HERV‐W envelope is an early activation marker of leukocytes and is upregulated in multiple sclerosis patients. Eur J Immunol 2020;50:685–694. [DOI] [PubMed] [Google Scholar]
- 42. Kremer D, Forster M, Schichel T, et al. The neutralizing antibody GNbAC1 abrogates HERV‐W envelope protein‐mediated oligodendroglial maturation blockade. Mult Scler 2015;21:1200–1203. [DOI] [PubMed] [Google Scholar]
- 43. Steiner J, Bachani M, Malik N, et al. Neurotoxic properties of human endogenous retrovirus‐K envelope protein and detection in cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Ann Neurol 2021;90:S213. [Google Scholar]
- 44. Steiner JBM, Malik N, DeMarino C, et al. HERV‐K envelope in spinal fluid of amyotrophic lateral sclerosis is toxic. Ann Neurol 2022. 10.1002/ana.26452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tamaki Y, Shodai A, Morimura T, et al. Elimination of TDP‐43 inclusions linked to amyotrophic lateral sclerosis by a misfolding‐specific intrabody with dual proteolytic signals. Sci Rep 2018;8:6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li W, Pandya D, Pasternack N, et al. Retroviral elements in pathophysiology and as therapeutic targets for amyotrophic lateral sclerosis. Neurotherapeutics 2022;1–17. 10.1007/s13311-022-01233-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Analysis of epitope recognition score (ERS) of antibodies to 15 mer peptides derived from HML‐2 envelope in serum samples from amyotrophic lateral sclerosis (ALS) individuals and controls. Highlighted motifs are the common epitopes among the peptides differentially recognized in ALS samples.


