Abstract
Background
We have previously shown that wearable technology and machine learning techniques can accurately discriminate between progressive supranuclear palsy (PSP), Parkinson's disease, and healthy controls. To date these techniques have not been applied in longitudinal studies of disease progression in PSP.
Objectives
We aimed to establish whether data collected by a body‐worn inertial measurement unit (IMU) network could predict clinical rating scale scores in PSP and whether it could be used to track disease progression.
Methods
We studied gait and postural stability in 17 participants with PSP over five visits at 3‐month intervals. Participants performed a 2‐minute walk and an assessment of postural stability by standing for 30 seconds with their eyes closed, while wearing an array of six IMUs.
Results
Thirty‐two gait and posture features were identified, which progressed significantly with time. A simple linear regression model incorporating the three features with the clearest progression pattern was able to detect statistically significant progression 3 months in advance of the clinical scores. A more complex linear regression and a random forest approach did not improve on this.
Conclusions
The reduced variability of the models, in comparison to clinical rating scales, allows a significant change in disease status from baseline to be observed at an earlier stage. The current study sheds light on the individual features that are important in tracking disease progression. © 2022 The Authors. Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society
Keywords: clinical rating scales, gait and posture, inertial measurement units, kinematic features, machine learning, wearable sensors
Progressive supranuclear palsy (PSP) is an atypical parkinsonian disorder involving the aggregation of four‐repeat tau in neuronal/glial cells and the degeneration of brain regions, including parts of the brainstem, cerebellum, and basal ganglia. 1 It is typified by rapid progression and a short survival. 2 Characteristic clinical features of PSP include unintelligible speech, akinesia, bulbar impairments, cognitive dysfunction, supranuclear gaze palsy, and postural instability. 3 , 4 The impairment of these functions is measured using the Progressive Supranuclear Palsy Rating Scale (PSPRS) 5 and the Movement Disorder Society criteria (MDS‐PSP).6 Although developed for Parkinson's disease (PD), the Movement Disorders Society‐Unified Parkinson's Disease Rating Scale (MDS‐UPDRS) 7 , 8 may also be useful in evaluating the motor features of PSP. These clinical rating scales aim to track PSP natural history and progression.
Different clinical features appear at different stages as the disease progresses. For instance, postural instability, abnormal gait, and falls are commonly reported as the earliest symptoms by patients and carers. 9 , 10 In contrast, supranuclear gaze palsy, which is one of the cardinal features of PSP, can appear as late as 11 years after disease onset. 11 Diagnostic criteria that are based on clinical rating scales are often met late in the disease time line, several years after the appearance of the parkinsonian syndrome. 12 , 13 Considering the short disease time course, late diagnosis allows limited time to treat PSP with potential disease‐modifying therapeutics. These issues are compounded by the element of subjectivity introduced with rating scales that rely on an observer for disease scoring. This highlights the necessity of developing more objective tools to monitor disease progress.
Wearable technology is an increasingly popular method to monitor the progression of neurodegenerative diseases. 14 , 15 Body‐worn sensors, such as inertial measurement units (IMUs), have been used to measure a large number of kinematic features derived from walking 16 and postural sway when standing or initiating gait 17 , 18 in PD patients. The validity of wearable sensor outcomes is justified by the moderate/good correlation with the motor scores of the MDS‐UPDRS, Part III, in typical PD patients. 19 , 20 The ability of IMU‐derived data to classify PSP, PD patients, and healthy controls (HCs) has recently been demonstrated in a cross‐sectional analysis. 21
Wearable devices introduce the prospect of identifying objective biomarkers to measure the onset, progression, and severity of neurodegenerative conditions; accelerating clinical trials; and facilitating the discovery of new therapeutics. Although a few studies have utilized sensors to monitor motor features longitudinally in typical PD, 22 , 23 studies focused on PSP patients are virtually nonexistent. The current investigation utilizes a six‐sensor IMU array to explore the progression of the walking and swaying kinematic features in PSP participants at 3‐month intervals over a 1‐year period. Two hypotheses are stated: (1) that the walking and postural sway features extracted from serial measurements using the body‐worn sensors can reflect disease progression similar to the motor parts of the clinical rating scales; and if so (2) that the more precise and objective sensor measurements may contain less noise and thereby provide earlier markers of disease progression compared to the clinical rating scales. Our aim is to develop a progression model of PSP, complementary to the standard clinical rating scales.
Patients and Methods
Participants
Participants were recruited through the Oxford Quantification in Parkinsonism (OxQUIP) study, a large observational study of neurophysiological biomarkers in parkinsonian patients conducted at the John Radcliffe Hospital, Oxford. This study was approved by the research ethics committee and the Health Research Authority (REC 16/SW/0262). All PSP participants had received a diagnosis of possible or probable PSP, according to the MDS PSP criteria 6 by a consultant neurologist. A total of 27 PSP participants were recruited and were provided written information on the study. Informed consent was obtained before their participation. Participants completed validated clinical rating scales at the beginning of the study, including the PSPRS and the MDS‐UPDRS, Part III.
Participants in the OxQUIP study were expected to visit the lab once every 3 months for up to 2 years. However, several participants in the PSP cohort withdrew before the end of the study period and/or missed some study visits. The PSP cohort from the OxQUIP study has recently been published in detail in a separate paper. 24 For the present analysis, we examined data from the first year only, that is, from visit 1 (baseline) to visit 5 (12 months). Participants with two or more consecutive missed visits in this period were excluded. Absent data for single missed visits were replaced with measurements from the previous visit (ie, last observation carried forward). This resulted in 17 participants being included in the subsequent analysis (Fig. 1A). Demographics of participants included in the analysis are summarized in Table 1. PSP subtypes for this study included only PSP‐RS and PSP‐P.
FIG 1.

Experimental setup. (A) Schematic diagram demonstrating the criteria required for participants to be included in subsequent statistical analysis. (B) Schematic diagram displaying the positions of six IMUs on the participant: the sternum, lumbar region, and each wrist and foot. (C) Schematic diagram demonstrating the pipelines for feature selection. Abbreviations: APA, anticipatory postural adjustment; IMU, inertial measurement unit; RMSE, root mean squared error. [Color figure can be viewed at wileyonlinelibrary.com]
TABLE 1.
Demographics of PSP participants
| PSP demographics (n = 17) | Mean (range) |
|---|---|
| Age at symptom onset (y) | 63 (51–73) |
| Gender, male/female | 9:8 |
| Time since onset of symptoms (y) | 1.6 (0–6) |
| On antiparkinsonian medication | 9/17 |
| Phenotype (PSP‐RS:PSP‐P) | 11:6 |
| PSPRS—visit 1 | 37.1 (19–58) |
| MDS‐UPDRS, Part III—visit 1 | 43.9 (21–72) |
| MMSE—visit 1 | 26.2 (20–30) |
Demographics, clinical characteristics, and cognitive scores of PSP participants.
Abbreviations: PSP, progressive supranuclear palsy; PSPRS, Progressive Supranuclear Palsy Rating Scale; MDS‐UPDRS, Movement Disorders Society‐Unified Parkinson's Disease Rating Scale; MMSE, Mini‐Mental State Examination.
Sensor Array and Tasks
Kinematic data were obtained from six IMU devices (Opal, APDM, Portland, OR, USA), located on the left and right wrists/feet, the sternum, and the lumbar region of the participant (Fig. 1B). Each device provides triaxial gyroscope, accelerometer, and magnetometer signals sampled at a frequency of 128 Hz. These devices were wirelessly connected to the APDM MobilityLab System (MobilityLab, APDM) for offline extraction of the kinematic parameters specific to tasks performed by the participants.
All motor and clinical tests were performed at each clinical visit unless participants' disease was too severe to allow task completion. Patients performed a 2‐minute walk in a quiet, uncarpeted 15‐meter corridor, making turns when necessary. To measure postural sway, participants were instructed to stand as still as possible for 30 seconds with their eyes closed. A footplate was utilized to ensure the distance, and angle between the feet was standardized for all participants, before the postural sway trial initiation (refer to Supplementary Material, part 3, for exemplar video regarding the sensor configuration and tasks). Participants were also scored using the MDS‐UPDRS, Part III, and PSPRS at each visit by an experienced MDS‐trained member of the OxQUIP team.
Two experienced clinical researchers were present during all clinics for safety reasons. During the postural sway testing, one researcher stood behind the patient, safeguarding against backward falling, whereas the other researcher stood in front of the patient, ensuring eye closure during the entire postural sway trial. During the 2‐minute walk, one researcher followed closely behind the patient, moving out of the way to the side during turns.
For the MDS‐UPDRS, Part III, our analysis omitted items 3.1 and 3.2 (speech and facial expression) because these functions are less related to walking and standing. For the PSPRS, we defined “PSPRS‐motor” as the “limbs” and “gait/midline” parts of the PSPRS (sum of items 18–28).
Data Reduction and Analysis
The software used in the current study (MobilityLab) automatically outputs more than 150 features for gait and postural sway tasks. A printed list of the exported features can be found elsewhere.21 All analysis was performed using custom software written in Python (v3.8).
The software automatically detects and excludes periods of freezing from the analysis. We confirmed its correctness by visually inspecting the accelerometer data for freezing episodes.
Stride length features were normalized with the individual participant's height (expressed in meters). Because the symptoms of PSP tend to be symmetrical (unlike PD), left‐ and right‐limb‐specific features were averaged, and asymmetry features extracted from the sensor array were discarded. Features relating to anticipatory postural adjustments were also not used here. This left 88 IMU features. These 88 features were each averaged across subjects per visit, and only those features for which the group mean significantly regressed across visits (P < 0.05), signifying progression over time, were used for further analysis. This step provided 32 features, a list of which is provided in the Supplementary Material (part 1).
Three different automated feature selection pipelines were then utilized to model each of MDS‐UPDRS, Part III, and PSPRS‐motor.
Pipeline A: UPDRS LRA and PSPRS LRA
The aim of the first pipeline was to measure disease progression across visits. Only the three features that showed the most evident group‐average progression, based on p‐value of the linear regression (LR) of group means across visits, were included. These were used to predict the MDS‐UPDRS, Part III, and the PSPRS‐motor.
Pipeline B: UPDRS LRB and PSPRS LRB
The aim of the second pipeline was to improve the accuracy of the estimates of individual clinical rating scores. A forward feature selection process was performed to determine the set of features that minimized the difference between the predicted and the actual clinical score values. The data sets were initially split into training and test subsets, in five iterations. In each iteration, data from one of the visits served as the test subset, whereas data from the four remaining visits were used as the training subset (thus visits 1, 2, 3, and 4 training with visit 5 test; then visits 1, 2, 3, and 5 training with visit 4 test; etc.). Both training (four visits) and test (one visit) subsets were standardized with respect to the mean and standard deviation (SD) of the training subset within each iteration. Simple LR was used to test which array of kinematic features can better predict the clinical scores (MDS‐UPDRS, Part III, and PSPRS‐motor) in each visit. Each of the five predictive models (corresponding to one of the five visits being selected as the test subset) was initialized with the three features with the most significant progression, as with the first pipeline. Subsequently, the algorithm explored the list of the 29 other significantly progressing features and added the one that reduced the test error (root mean square error [RMSE]) by the greatest amount. This process was repeated until the test error could not be further reduced by adding more features. Effectively, this step provided a list of feature predictors for each visit or model. Those features that were present in at least four of the five models were selected as important predictors for the linear model predicting each clinical score (MDS‐UPDRS, Part III, and PSPRS‐motor). For a graphical representation showing the number of features selected in each visit, see Supplementary Material (part 2).
Pipeline C: UPDRS RF and PSPRS RF
For the third pipeline, a random forest (RF) regressor was used on all 32 significantly progressing features to predict each score in turn. No prior feature selection process was utilized because RF algorithms work well with high‐dimensional feature sets.
Model Validation
A fivefold repeated cross‐validation procedure was utilized to assess the performance of each of the six models (UPDRS LRA , UPDRS LRB , UPDRS RF , PSPRS LRA , PSPRS LRB , and PSPRS RF ). The entire data set, for all 17 participants tested across five visits (ie, 85 instances), was randomly split into five subsets. The LR and RF models were trained on four subsets (80% of the full data set), whereas the single remaining subset (20%) served as the validation set. This process was performed five times for each instance to be included once in the validation set.
Statistical Analysis of Individual Features
Addressing the second hypothesis of the current study, we tested whether the predicted clinical scores could serve as potential biomarkers of disease progression. We particularly aimed to determine whether the model‐predicted clinical scores provide an earlier signal of disease progression than the actual clinical scores. For this reason, one‐way analysis of variance was performed on all models for both clinical scores, with time (V1–V5) used as a repeated factor. Where a significant effect of time was found, pairwise comparisons were performed for each consecutive visit against baseline (V1). A false discovery rate (FDR) method was used to account for the effect of multiple comparisons; the two‐stage step‐up method of Benjamini, Kreiger, and Yukutieli with a 1% FDR was implemented.
Results
Thirty‐two features were found to progress significantly over time, that is, from V1 to V5 (P < 0.05). Figure 2 shows the three best progressing features: (1) mean turn velocity, (2) SD of stride length, and (3) mean toe‐off angle. These three features served exclusively as predictors in pipeline A. RMSE was utilized to compute the test error between the actual clinical scores and the predicted values. For the MDS‐UPDRS, Part III, model predictions, the UPDRS LRA test error (RMSE) was 12.75 (SD = 1.07). For the PSPRS‐motor predictions, the PSPRS LRA test error (RMSE) was 3.81 (SD = 0.30).
FIG 2.
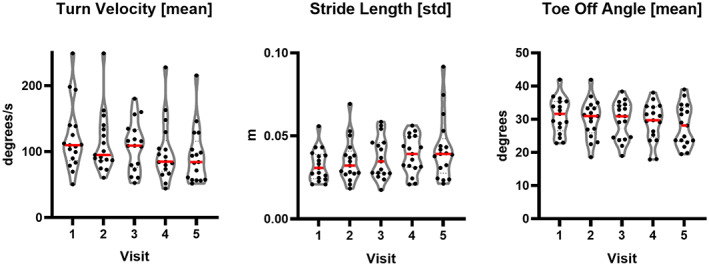
. Progressing kinematic features. Violin plots demonstrating the progression of the features utilized to predict MDS‐UPDRS, Part III, and PSPRS‐motor across five visits. [Color figure can be viewed at wileyonlinelibrary.com]
The forward feature selection process in pipeline B was designed to minimize the error between the predicted and actual values of MDS‐UPDRS, Part III, and PSPRS‐motor. This process added three more features to the MDS‐UPDRS, Part III, model, to form UPDRS LRB , resulting in a smaller and less‐variable RMSE than UPDRS LRA (mean = 11.86, SD = 0.48). Similarly, a single extra feature was added to the PSPRS‐motor model to form PSPRS LRB , resulting in a slightly smaller RMSE than PSPRS LRA (mean = 3.74, SD = 0.29).
In pipeline C, the RF regressor UPDRS RF further reduced the RMSE to 10.29 but with a higher variability (SD = 1.74). Similarly, the RF regressor for PSPRS‐motor (PSPRS RF ) further reduced RMSE to 3.29 but with a slightly higher variability (SD = 0.34).
Figure 3 shows the results for the actual and predicted progressions of the clinical scores. Repeated measures analysis applied to the actual and predicted clinical motor scores revealed significant progression (increase) over time for the actual MDS‐UPDRS, Part III (F = 6.94, P = 0.001), UPDRS LRA, (F = 7.23, P < 0.001), UPDRS LRB (F = 3.29, P = 0.029), and UPDRS RF (F = 3.88, P = 0.016). Similarly, repeated measures revealed significant progression across visits for the actual PSPRS‐motor (F = 4.25, P = 0.001), PSPRS LRA (F = 6.19, P = 0.001), PSPRS LRB (F = 6.72, P < 0.001), and PSPRS RF (F = 5.75, P = 0.002). Furthermore, pairwise comparisons demonstrated that when compared to baseline, all actual and predicted clinical scores were significantly higher at V5 (P < 0.05), but only UPDRS LRA (P < 0.001), PSPRS LRA (P < 0.001), and PSPRS LRB (P < 0.001) were significantly higher at V4, compared to V1.
FIG 3.
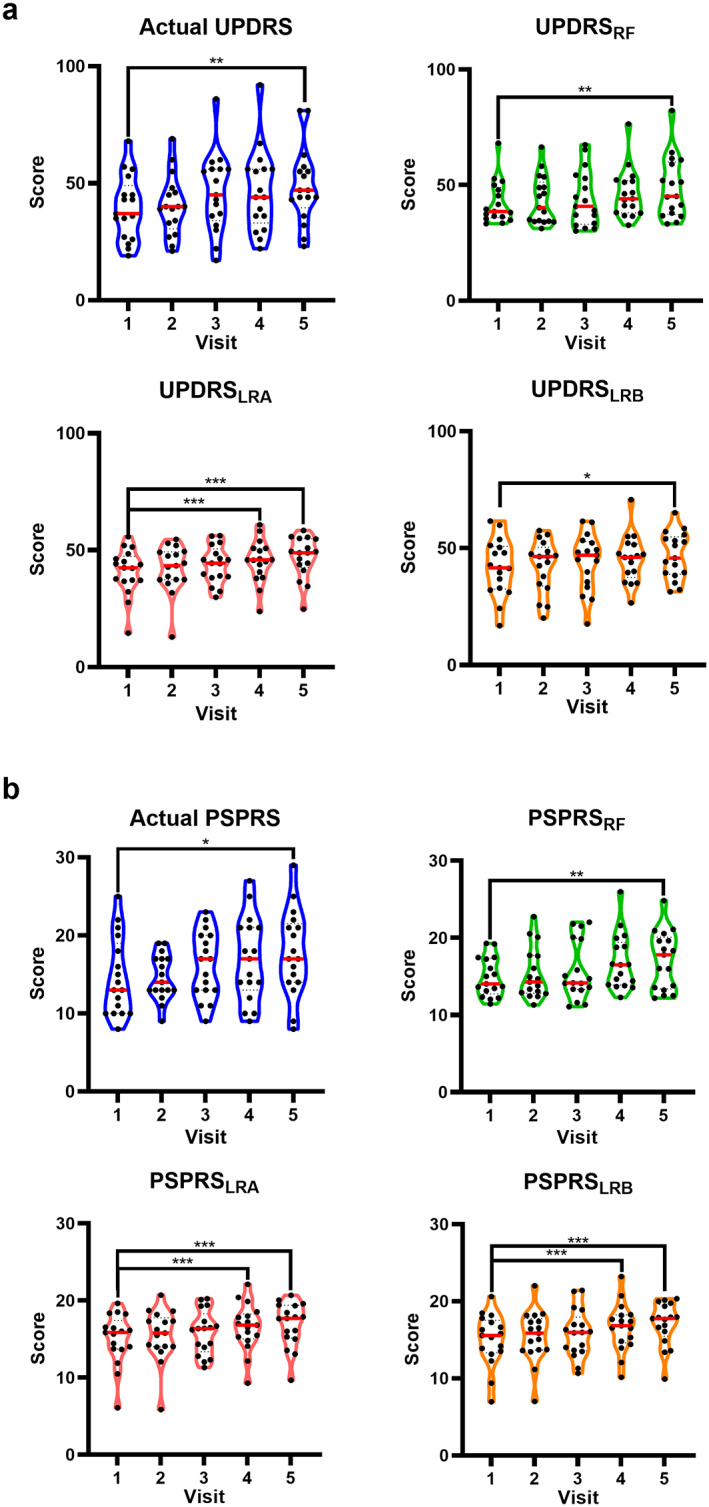
Actual and predicted clinical motor scores. Violin plots demonstrating the progression of actual and predicted MDS‐UPDRS, Part III (a), and PSPRS‐motor (b). Significant pairwise comparisons compared to baseline highlighted by *P ≤ 0.05*, P ≤ 0.01**, and P ≤ 0.001***. Red solid line represents the group mean. Abbreviations: LR, linear regression; PSPRS, Progressive Supranuclear Palsy Rating Scale; RF, random forest; UPDRS, Unified Parkinson's Disease Rating Scale. [Color figure can be viewed at wileyonlinelibrary.com]
Figure 4 shows the linear fit of the actual and predicted clinical scores plotted over the five visits (V1–V5) for each participant. The residual sum of squares (RSS) was calculated for each participant separately as an indicator of the linear model's unexplained variance. The predicted MDS‐UPDRS, Part III, scores resulted in smaller unexplained variance for most of the participants (mean RSS: UPDRS LRA = 36.7, UPDRS LRB = 50.7, and UPDRS RF = 80.9) compared to the actual values (mean RSS = 90.4). Similarly, the predicted PSPRS‐motor part showed less‐unexplained variance (mean RSS: PSPRS LRA = 4.37, PSPRS LRB = 4.65, and PSPRS RF = 7.59) compared to the actual values (mean RSS = 16.11).
FIG 4.

Individual regression plots for actual and predicted MDS‐UPDRS, Part III/PSPRS‐motor. Regression plots of individual participants calculated from the actual and predicted (A) MDS‐UPDRS, Part III, and (B) PSPRS‐motor. Blue = actual, red = UPDRS LRA /PSPRS LRA , orange = UPDRS LRB /PSPRS LRB , and green = UPDRS RF /PSPRS RF . Abbreviations: LR, linear regression; MDS‐UPDRS, Movement Disorder Society‐Unified Parkinson's Disease Rating Scale; PSPRS, Progressive Supranuclear Palsy Rating Scale; RF, random forest; RSS, residual sum of squares. [Color figure can be viewed at wileyonlinelibrary.com]
Discussion
This study aimed to build on earlier work showing the ability to classify PSP versus PD versus HC, by modeling PSP progression using data derived from measurements of gait and postural sway. To our knowledge, this is the first study to model the progression of the disease based on kinematic data.
We utilized a machine learning approach to perform longitudinal monitoring of PSP across five visits with reference to the clinical rating scales. The models are capable of both accurately reflecting the information obtained from clinical rating scales and predicting the progression of patients across visits. The reduced variability of the models, in comparison to the clinical rating scales, is shown by the lower RSS, and it is this reduction in noise that allows a significant change in disease status from baseline to be observed at an earlier stage. Strongly significant differences from baseline were apparent 3 months earlier in both UPDRS LRA and PSPRS LRA than in the actual scores. Using this technology alongside standard clinical rating scales in trials may allow the efficacy of potential therapeutics for PSP to be verified at an earlier point.
For tracking progression, the simplest models based solely on the features that most linearly regressed with time (UPDRS LRA and PSPRS LRA ) performed the best. Adding more features (UPDRS LRB and PSPRS LRB ) produced a slightly closer estimate of individual actual scores but resulted in poorer performance in tracking the MDS‐UPDRS, Part III, and made little difference in tracking PSPRS‐motor. The small number of features in our models suggests that it may be feasible to use fewer sensors to monitor patients. The higher variability observed with the RF regressor indicates dependence on how the data are partitioned into the various subsets for each iteration, suggesting that there is not sufficient data to make the RF results robust. We intend to revisit this in the future with an expanded cohort of participants.
The predicted scores tracked the actual scores well in most patients but with some exceptions. In patients 1 and 4 (see Fig. 4A), the actual MDS‐UPDRS, Part III, trends down, whereas true improvement in the condition is almost never observed even with treatment; this suggests variability in the MDS‐UPDRS, Part III, scores. The estimates provide a credible progression, which might suggest that the gait parameters provide better estimates than the human rater. A different pattern is observed in participants 9, 10, and 12: these feature a progression in MDS‐UPDRS, Part III, yet the model lines are flat. It seems unlikely that the progression of MDS‐UPDRS, Part III, in all of these patients is not genuine. It is probable that, for some individuals, the gait parameters did not track the progression of MDS‐UPDRS, Part III. However, at the group level (which is what will matter for trials), the model still outperforms the MDS‐UPDRS, Part III, in terms of time to significant change from baseline; thus, presumably the reduction in noise outweighs any reduction in signal.
The current study sheds light on the individual features that are important in tracking disease progression. The progressive decrease in toe‐off angle is an indicator of decreased plantar flexion, leading to smaller propulsive torques when walking, which results in smaller steps and decreased walking speed. 25 Increases in stride length variability have been previously interpreted as indicators of less‐controlled walking, with increased proneness to falls for people with neurodegenerative diseases. 26 Further, turning velocity progressively decreased across visits. This finding is in agreement with previous work, showing that higher clinical scoring in turns is associated with increased number of falls in PSP. 27
The absence of any postural sway variables in the model feature sets is notable but does not imply that postural sway was unrelated to PSP progression. In fact, several postural sway variables were present in the initial set of 32 features that were found to significantly progress (see Supplementary Material), indicating that postural sway was related to PSP progression. The reason these features do not appear in the feature sets in the models is simply that (1) they were not among the group of three with the tightest correlation with time that were selected for pipeline 1, and (2) they did not provide sufficient additional independent information for the algorithm to select them for pipeline 2. That is, they do progress, but their progression will have been captured through their correlation with the other variables that are already included in the model. The practical result is that if a sufficiently detailed gait recording is performed, an additional postural sway test may not be necessary.
The IMU software used in the current study generated a very large number of features. As a first step in our modeling, it was necessary to reduce the dimensionality of the data. The array of kinematic features was reduced by first excluding all those features that did not progress significantly with time at the group level. This first step removed 6 of the 10 features identified in our previous study 21 as being key to the differential diagnosis of PSP from PD patients and HCs. Of the remaining 4, none were in the group of three features that regressed most clearly with time (turn velocity, toe‐off angle, and variability of stride length), and thus none were included in the best‐performing progression models (UPDRS LRA and PSPRS LRA ). This demonstrates that the features that are the most useful for diagnosis are not necessarily the most useful for monitoring progression.
The main limitation of this study was its low sample number. The criteria used resulted in the exclusion of 10 participants from the original 27, so that the models included data from only 17 participants. The study included only the more common subtypes, PSP‐RS and PSP‐P, but given the low sample number we did not attempt subgroup analysis. Substantial additional recruitment would be required to capture a representative sample of all subtypes 6 ; even then it would be unlikely to capture sufficient rarer phenotypes, for example, PSP‐SL or PSP‐CBS.
Our findings may be most relevant to the brainstem‐predominant variants (PSP‐RS, PSP‐P, PSP‐PGF) rather than cortical variants that often develop later gait/mobility impairments. It is possible that patients with more advanced disease might not be able to complete gait testing, and this may limit the applicability of these findings in late‐stage disease. Use of physical therapy was not included in the analysis, but we intend to do so in future work due to its potential to alter posturography results.
Conclusion
Data from wearable IMU arrays coupled with mathematical modeling can be used to track progression of PSP, complementing established clinical rating scales. In this study, the reduced variability in the modeled data allowed a progression signal to be discerned 3 months earlier than would otherwise be expected.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Statistical analysis: A. Design, B. Execution, C. Review and critique; (3) Manuscript: A. Writing of the first draft, B. Review and critique.
C.S.: 2A, 2B, 2C, 3A,3B
N.C.: 2A, 2B, 2C, 3A, 3B
Z.S.: 1B, 1C, 3B
M.V.: 2C, 3B
L.T.: 2C, 3B
J.J.F.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
C.A.A.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
Ethical Compliance
The measurements were obtained during the OxQUIP, a longitudinal study conducted at the John Radcliffe Hospital in Oxford, UK. The study was approved by the research ethics committee and the Health Research Authority (REC 16/SW/0262). The aim of the OxQUIP study was to develop novel methods of objective disease progression measurement. This is achieved by repeated administration of a battery of tests, according to a structured protocol, at 3‐month intervals. All participants were provided written information on the study, and informed consent was obtained before participation. We confirm that we have read the journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Conflict of Interest
JJF and CAA were supported by the National Institute for Health Research Oxford Biomedical Research Centre. JJF has received consulting fees from Abbott and Medtronic, unrelated to this study. JJF and CAA have received research grant support from UCB Pharma and MSD Laboratories. LT has no financial disclosures or conflicts of interest related to the research in this article. He was a was a Director of Sensyne Health until June 2022. He is a Director of Oxehealth Ltd, in which he has a minor shareholding. Other authors report no conflicts of interest.
Supporting information
APPENDIX S1. Supporting Information
APPENDIX S2. Supporting Information
APPENDIX S3. Supporting Information
Acknowledgments
We thank the participants and their families for their endless support with our research work.
Relevant conflicts of interest/financial disclosures: None.
Funding agencies: This study was funded by a grant from UCB Biopharma SRL. J.J.F. was supported by the National Institute for Health Research Oxford Biomedical Research Centre.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Williams DR, Lees AJ. Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet Neurol 2009;8(3):270–279. [DOI] [PubMed] [Google Scholar]
- 2. Golbe LI, Ohman‐Strickland P, Beisser EB, Elghoul FT. A convenient prognostic tool and staging system for progressive Supranuclear palsy. Mov Disord Clin Pract 2020;7(6):664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Litvan I, Kong M. Rate of decline in progressive supranuclear palsy. Mov Disord 2014;29(4):463–468. [DOI] [PubMed] [Google Scholar]
- 4. Brittain C, McCarthy A, Irizarry MC, et al. Severity dependent distribution of impairments in PSP and CBS: interactive visualizations. Parkinsonism Relat Disord 2019;60:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Golbe LI, Ohman‐Strickland PA. A clinical rating scale for progressive supranuclear palsy. Brain 2007;130(Pt 6):1552–1565. [DOI] [PubMed] [Google Scholar]
- 6. Hoglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord 2017;32(6):853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goetz CG, Fahn S, Martinez‐Martin P, et al. Movement Disorder Society‐sponsored revision of the unified Parkinson's disease rating scale (MDS‐UPDRS): process, format, and clinimetric testing plan. Mov Disord 2007;22(1):41–47. [DOI] [PubMed] [Google Scholar]
- 8. Hall DA, Forjaz MJ, Golbe LI, et al. Scales to assess clinical features of progressive Supranuclear palsy: MDS task force report. Mov Disord Clin Pract 2015;2(2):127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arena JE, Weigand SD, Whitwell JL, et al. Progressive supranuclear palsy: progression and survival. J Neurol 2016;263(2):380–389. [DOI] [PubMed] [Google Scholar]
- 10. Goetz CG, Leurgans S, Lang AE, Litvan I. Progression of gait, speech and swallowing deficits in progressive supranuclear palsy. Neurology 2003;60(6):917–922. [DOI] [PubMed] [Google Scholar]
- 11. Kurz C, Ebersbach G, Respondek G, Giese A, Arzberger T, Hoglinger GU. An autopsy‐confirmed case of progressive supranuclear palsy with predominant postural instability. Acta Neuropathol Commun 2016;4(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. dell'Aquila C, Zoccolella S, Cardinali V, et al. Predictors of survival in a series of clinically diagnosed progressive supranuclear palsy patients. Parkinsonism Relat Disord 2013;19(11):980–985. [DOI] [PubMed] [Google Scholar]
- 13. Respondek G, Roeber S, Kretzschmar H, et al. Accuracy of the National Institute for neurological disorders and stroke/Society for Progressive Supranuclear Palsy and neuroprotection and natural history in Parkinson plus syndromes criteria for the diagnosis of progressive supranuclear palsy. Mov Disord 2013;28(4):504–509. [DOI] [PubMed] [Google Scholar]
- 14. FitzGerald JJ, Lu Z, Jareonsettasin P, Antoniades CA. Quantifying motor impairment in movement disorders. Front Neurosci 2018;12:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Del Din S, Kirk C, Yarnall AJ, Rochester L, Hausdorff JM. Body‐worn sensors for remote monitoring of Parkinson's disease motor symptoms: vision, state of the art, and challenges ahead. J Parkinsons Dis 2021;11(s1):S35–S47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Raccagni C, Gassner H, Eschlboeck S, et al. Sensor‐based gait analysis in atypical parkinsonian disorders. Brain Behav 2018;8(6):e00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mancini M, Carlson‐Kuhta P, Zampieri C, Nutt JG, Chiari L, Horak FB. Postural sway as a marker of progression in Parkinson's disease: a pilot longitudinal study. Gait Posture 2012;36(3):471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mancini M, Zampieri C, Carlson‐Kuhta P, Chiari L, Horak FB. Anticipatory postural adjustments prior to step initiation are hypometric in untreated Parkinson's disease: an accelerometer‐based approach. Eur J Neurol 2009;16(9):1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodriguez‐Molinero A, Perez‐Lopez C, Sama A, et al. A kinematic sensor and algorithm to detect motor fluctuations in Parkinson disease: validation study under real conditions of use. JMIR Rehabil Assist Technol 2018;5(1):e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mancini M, Weiss A, Herman T, Hausdorff JM. Turn around freezing: community‐living turning behavior in people with Parkinson's disease. Front Neurol 2018;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Vos M, Prince J, Buchanan T, FitzGerald JJ, Antoniades CA. Discriminating progressive supranuclear palsy from Parkinson's disease using wearable technology and machine learning. Gait Posture 2020;77:257–263. [DOI] [PubMed] [Google Scholar]
- 22. Coates L, Shi J, Rochester L, Del Din S, Pantall A. Entropy of real‐world gait in Parkinson's disease determined from wearable sensors as a digital marker of altered ambulatory behavior. Sensors (Basel) 2020;20(9):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Di Lazzaro G, Ricci M, Saggio G, et al. Technology‐based therapy‐response and prognostic biomarkers in a prospective study of a de novo Parkinson's disease cohort. NPJ Parkinsons Dis 2021;7(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pereira MF, Buchanan T, Höglinger GU, et al. Longitudinal changes of early motor and cognitive symptoms in progressive supranuclear palsy: the OxQUIP study. BMJ Neurology Open 2022;4(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Judge JO, Davis RB 3rd, Ounpuu S. Step length reductions in advanced age: the role of ankle and hip kinetics. J Gerontol A Biol Sci Med Sci 1996;51(6):M303–M312. [DOI] [PubMed] [Google Scholar]
- 26. Nakamura T, Meguro K, Sasaki H. Relationship between falls and stride length variability in senile dementia of the Alzheimer type. Gerontology 1996;42(2):108–113. [DOI] [PubMed] [Google Scholar]
- 27. Bluett B, Litvan I, Cheng S, et al. Understanding falls in progressive supranuclear palsy. Parkinsonism Relat Disord 2017;35:75–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1. Supporting Information
APPENDIX S2. Supporting Information
APPENDIX S3. Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


