Abstract
Background
Burning mouth syndrome is an idiopathic condition characterized by burning pain in a normal-appearing oral mucosa lasting at least four to six months. In the case of secondary burning mouth syndrome is associated with local or systemic factors (such as thyroid disorders) that can cause these symptoms. The aim of this review was to study the relationship between thyroid disorders and burning mouth syndrome.
Material and Methods
The present study followed the PRISMA guidelines. An electronic search strategy was developed for PubMed/Medline, Scopus and Cochrane. The following combination of keywords and Boolean operators were used: Thyroid AND burning mouth; Thyroid AND burning mouth syndrome; Hypothyroidism AND burning mouth; Hypothyroidism AND burning mouth syndrome; Hyperthyroidism AND burning mouth; Hyperthyroidism AND burning mouth syndrome. The results were processed by existing free software in https://www.graphpad.com/. To evaluate the association of the categorical variables we used the Fisher test at a level of significance of p-value ≤ 0,05. As a primary summary measure the Odds Ratio (OR) has been used. To analyze the risk of bias the guidelines of the GRADE guide were used and the grade of evidence was analyzed by the guide of Joanna Briggs Institute: Levels of Evidence and Grades of Recommendations.
Results
After applying the inclusion and exclusion criteria, 5 studies were selected for review. The Chi-square was 10.92 and the Odds Ratio was 3.31 with respect to TSH values with p <0.0001 (Fisher's test). The population of patients with TSH alterations is increased in 80.49% and decreased in 19.51%.
Conclusions
It can be concluded that thyroid hormone abnormalities are a factor in secondary burning mouth syndrome; specially in patients with hypothyroidism.
Key words:Burning mouth syndrome, thyroid hormones, hypothyroidism.
Introduction
Burning mouth syndrome is an idiopathic condition characterized by burning pain in a normal-appearing oral mucosa lasting at least four to six months (1). It is relatively common in middle-aged and elderly women, and it is estimated that the prevalence can reach 18% in postmenopausal women (2). BMS is characterized by burning sensations in the tongue (particularly at the tip and lateral edges), lips, palate (2), and gums (3). In addition to the burning sensation, it can also be accompanied by changes in taste and xerostomia (2-5). Symptoms must be present for at least 4 or 6 months continuously (2), but periods without pain during the day are also reported (4). In fact, many patients have no pain at night, but it progressively increases during the day (6).
To catalog the BMS, two classifications have been proposed. The first classification is based on symptoms fluctuations during the day. In Type 1, patients get up without pain and it increases throughout the day. It is related to systemic diseases, nutritional deficiencies or diabetes mellitus. In Type 2, patients have continuous symptoms during the day and have difficulty falling asleep. Usually associated with psychological disorders. In Type 3 the symptoms are intermittent and present periods of the day without pain (7).
The second classification is based on the etiology of the symptoms. Primary or essential BMS is idiopathic, there are alterations in the central/peripheral neuropathological pathways, but no causes are identified. The secondary is characterized by the presence of local or systemic alterations that can explain the symptoms (8). Systemic factors that can cause secondary BMS include deficiencies (iron, zinc, vitamin B12, folate) (3,5,7,9,10), hormonal disturbances (diabetes, thyroid disorders, anemia), use of certain medications (benzodiazepines, neuroleptics, antihypertensives) (5,9,10). For this reason, these factors must be excluded in the diagnosis of primary BMS (9,10). Regarding the etiology, local factors include alterations such as poor fit of the prosthesis, parafunctional habits, galvanism (3,7), allergic reactions, or xerostomia (3,5,7). Numerous studies have shown the association between BMS and psychological factors, such as depression and anxiety (4,6,7).
Regarding the pathogenesis of BMS, it is multifactorial, including neuropathic mechanisms at different levels of neuraxis that help explain the pathophysiology of BMS. Recent studies have shown that various peripheral neuropathic mechanisms contribute to the pathophysiology of primary BMS (2). In a study by Lauria et al (11) it was observed that patients with BMS presented a lower density of nerve fibers in the lateral border of the tongue than in patients without BMS, in addition to morphological changes are indicative of neuronal degeneration. Therefore, they concluded that BMS is a trigeminal small fiber sensory neuropathy. Other authors, such as Albuquerque et al (12) advocate the involvement of central neuropathic mechanisms. In their study, they demonstrate qualitative and quantitative alterations in central activation patterns. Using MRI, they observed less brain activity in patients with BMS.
The thyroid gland secretes triiodothyronine (T3) and thyroxine (T4) hormones that play an important role in tissue development and metabolism, as well as in the regulation of numerous functions and processes of the nervous system (13). Additionally, thyroid hormones have been shown to play a role in the maturation and specialization of taste buds (14). Therefore, the deficiency of these hormones can play a role in dysgeusia (15) and in peripheral/central neuropathies (13,15).
The objective of this review is to study the relationship between thyroid disorders and BMS.
Material and Methods
The present study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (16). A detailed protocol was prepared before starting the review and registered on Prospero (CRD42021248348)
- Hypothesis
Null Hypothesis (H0): Patients with thyroid disorders are not at increased risk of BMS
Alternative Hypothesis (H1): Patients with thyroid disorders are at increased risk of developing BMS
- Focused Question
We propose to review the existing literature asking the following question: Is the any association between thyroid alterations and the occurrence of burning mouth syndrome?
PECO Question: P (Population): Patients with BMS; E (Event): Thyroid disorders; C (Control): Patients without BMS; O (Outcome): Patients with thyroid disorders present higher prevalence of BMS.
- Eligibility Criteria
Inclusion criteria: case series, cross-sectional studies, cohort studies, case control studies, clinical trials, patients with thyroid disorders, human studies.
Exclusion criteria: Case studies, reviews; No restrictions were made for the year of publication, patients with previous thyroid pathology or other alterations/deficiencies; animal studies.
- Search strategy
An electronic search strategy was developed for PubMed/Medline, Scopus and Cochrane. A partial grey literature search was also performed. The following combination of keywords and Boolean operators were used: Thyroid [Mesh] AND burning mouth [tw]; Thyroid [Mesh] AND burning mouth syndrome [Mesh]; Hypothyroidism [Mesh] AND burning mouth [tw]; Hypothyroidism [Mesh] AND burning mouth syndrome [tw]; Hyperthyroidism AND burning mouth [tw]; Hyperthyroidism AND burning mouth syndrome [tw]. The lists of references of included studies were also hand-searched to identify additional relevant studies.
- Study selection
The last search was performed on March 5th, 2022. Two researchers (S.E.M and J.V.R) independently screened the title and abstract of every article identified in the search in order to establish its eligibility. A Coen’s kappa coefficient for each database was calculated to determine the reliability between researchers. Afterward, the full text of the selected articles was assessed for a definitive inclusion in the systematic review. A third reviewer (J.L.L.) resolved any discrepancies and agreed upon with S.E.M and J.V.R.
- Data Extraction y Statistical analysis
Data were extracted by the authors (S.E.M and J.V.R) and entered into a data collection form (Microsoft® Excel version 16.53) in which the data of Author(s), year of publication, number of patients, type of thyroid abnormalities (TSH, Anti-TPO, FT3, FT4, ultrasound abnormalities, TGA, GPCA, TMA).
These results were processed by the free software available at https://www.graphpad.com/. To evaluate the association of the categorical variables, we will use the Fisher test at a significance level of p-value ≤ 0.05. As a primary summary measure, the Odds Ratio (OR) will be used.
- Quality Assessment of Risk of Bias
To analyze the risk of bias, the guidelines of the guide “Grading of Recommendations, Assessment, Development and Evaluation” (GRADE) (17) and the Joanna Briggs Institute (JBI) Levels of Evidence and Grades of Recommendations, accessible at: https://jbi.global/sites/default/files/2019-05/JBI%20Levels%20of%20Evidence%20Supporting%20Documents-v2.pdf. [date 06-10-2022], referenced among other scientific works by that of Zhang et al (18), were used to analyze the degree of evidence.
Results
- Results search strategy
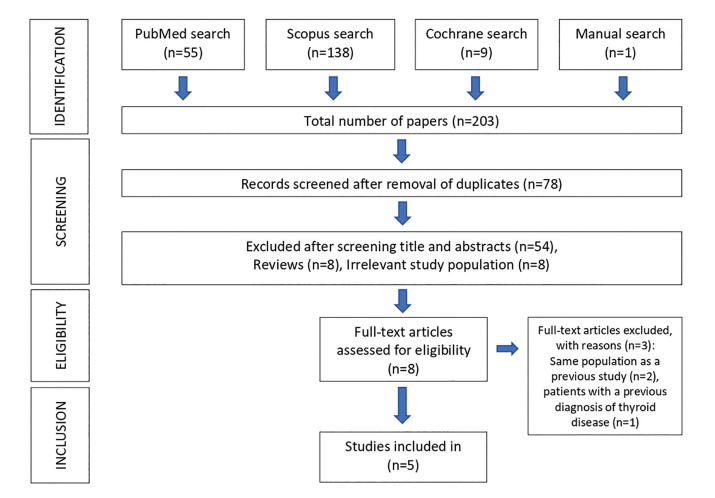
A total of 203 articles were obtained using our search strategy. 125 articles were eliminated because they were repeated, of these 70 studies were excluded and 8 articles were selected for full text evaluation; 2 were excluded because the population was the same as that of another previous study and 1 study was excluded because the patients had a previous diagnosis of thyroid disease; so we finally selected 6 articles for the synthesis. The Cohen Kappa coefficient was 0.886 for the Pubmed search, 0.743 for Scopus, and 1 for the Cochrane search. Finally, 5 articles were chosen for the review plus 1 article that was chosen by manual search (3,15,19-21): 2 cross-sectional studies (3,15), 1 non-randomized clinical trial (20) and 2 case series (19,21) (Fig. 1).
Figure 1.
Flow diagram of literature search and selection criteria adapted from PRISMA.
- Study Results
The total population included 2,607 patients, of whom 1,992 had BMS versus 615 in the control group. Regarding gender, 3 studies inform us of the gender of the participants (15,19,21), the study by Femiano et al (20) does not inform us of the sex of the control group and the study by Cho et al (3) does not give us data regarding sex; finally, we found 1681 (76.13%) women versus 527 (23.87%) men. Making a distinction between the BMS group and the control group, in the BMS group we found 1,317 (76.75%) women versus 399 men (2.25%). In the control group, there are 364 (73.98%) women versus 128 (26.02%) men.
The mean age of the participants was 57.6±5.21, calculated in a population of 2,208 patients from 3 studies (15,19,21) plus the BMS group from the study by Femiano et al (20), the study by Cho et al. (3) did not report the age of the participants. Comparing the study group to the control group, in the BMS group (n=1666) the mean age of the participants was 62.98 years calculated in 3 studies (19-21) and that of the control group (n=442) was 57.5 years only reported by the study by Chiang et al (19) (Table 1).
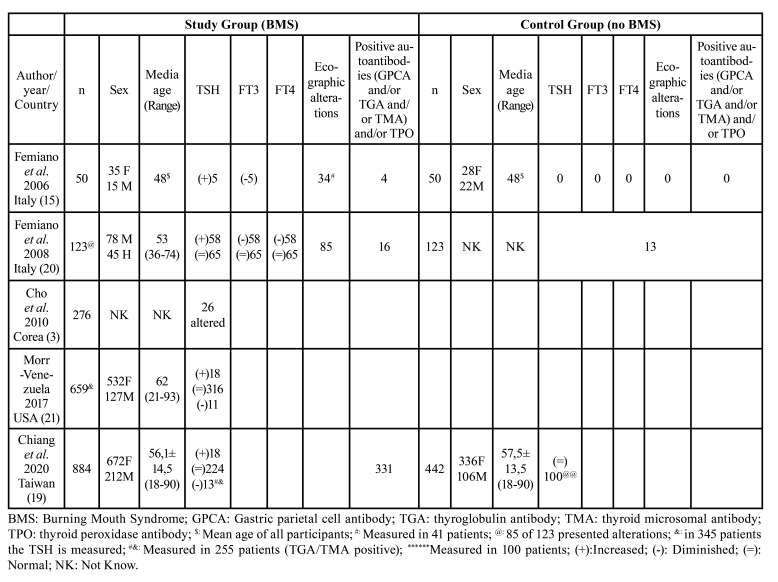
Table 1. Study characteristics, analytic and ecographic alterations.
Thyroid abnormalities were evaluated by blood tests and ultrasound tests.
In the BMS group, the TSH hormone is evaluated by all the studies (3,15,19-21) (n=1049), although Chiang et al (19) only evaluated it in 255 patients. It is observed that 149 patients (14.2%) present alterations in this hormone. In the study by Cho et al (3) they only inform us that 26 patients present alterations in the hormone, but they do not specify whether there is an increase or decrease in it. In other studies (15,19-21), of a population of 123 patients with TSH abnormalities, 80.49% (n=99) had an increased level and 19.51% (n=24) had had a decreased level.
In the non-BMS group, the TSH hormone is evaluated by 3 studies (15,19,20), although the study by Chiang et al (19) only studied it in 100 patients out of 442; and the studies of (3,21) do not have a control group. The study population is 273 patients, of which 95.24% (n=260) do not have TSH abnormalities and 4.7% (n=13) have TSH abnormalities. Although they do not inform us of the type of alteration that there is.
The Chi-square was 10.92; therefore, the null hypothesis is rejected, and the Odds Ratio was calculated with respect to the TSH values; this was 3.31, so thyroid abnormalities are a risk factor for BMS; therefore, patients with thyroid abnormalities are 3.31 times more likely to have SBM than a patient without thyroid abnormalities and p <0.0001 (Fisher's test).
As for the other thyroid hormones, FT3 and FT4, ultrasound abnormalities or positivity for antithyroid antibodies, we do not have homogeneous data, so a quantitative analysis has not been possible. Although the article by Femiano et al (15) gave altered FT3, ultrasound and antibody values for the SBM group and found no alterations in the control group. The study by Feminano et al (20) found a higher proportion of alterations (FT3, FT4, ultrasound and antibodies) in patients in the SBM group than in those in the control group. And finally Chiang et al (19) focuses on the positivity of antithyroid antibodies, which in the SBM group are positive in 37.44% and in the control group they are positive in 17% (Table 1).
- Risk of bias
Studies were classified according to the GRADE and JBI system. According to the GRADE system; one study has a moderate methodological quality (20), two low (19,21) and the other two very low (3,15). Based on the JBI system, an article has a level 2.c. (20), two articles 4.b. (3,15) and the other two 4.c. (19,21).
Discussion
This systematic review attempts to assess the relationship between thyroid abnormalities and burning mouth syndrome.
The literature estimates the prevalence of BMS between 0.7 and 5.1% (4), but in a review by Ariyawardana et al (22) on the definitions and criteria used in randomized clinical trials observed that only 19% of the trials used thyroid abnormalities as an exclusion criteria. Therefore, the data we have on primary/secondary BMS may be biased (22). The association of thyroid disorders and BMS is controversial. Since some authors are of the opinion that these alterations seem to have little influence on BMS and that the distinction between primary and secondary BMS is purely theoretical, a well as agreeing that BMS should be diagnosed only by the presence of symptoms excluding comorbidities. This theory is supported by the multimorbidity found in aging people, which increases in frequency due to the increase in life expectancy. It is alleged that the trials only focus on the target disease, excluding the rest of the pathologies, which makes it difficult to extrapolate the results to the real population (23).
Regarding age and gender, our review coincides with that published in the literature. In a recent review and meta-analysis on prevalence, a higher prevalence was found in women over 50 years of age (24).
The study by Femiano et al (20) proposes the term Burning mouth in hypothyroidism (BMHT) to differentiate them from cases of BMS, in which the burning sensation in the mouth corresponds to a symptomatic expression of hypothyroidism. In the current absence of evaluation of thyroid function and ultrasound, patients with true or subclinical hypothyroidism or only with ultrasound thyroid abnormality without thyroid abnormality are erroneously considered patients with BMS. Therefore, it is necessary to establish a protocol that includes thyroid function tests and ultrasound to differentiate patients with BMHT from real BMS (20).
The study by Talattof et al (25) studied the presence of BMS in patients with Hashimoto's thyroiditis. In which it was concluded that the levels of TSH, Anti-TPO, Anti-TG, Free T3 in these patients were related to the presence and severity of BMS. Treatment of this pathology can help prevent and treat these symptoms.
The symptoms of BMS in the literature relate them to hypothyroidism (20,25). This coincides with our results, in which it is observed that in 80% of the cases of TSH alteration, it is increased, which may indicate insufficient production of thyroid hormone, and therefore hypothyroidism.
Some patients, in addition to the sensation of burning, also present alterations in taste (26), this happens more frequently when there are thyroid alterations (20), in which, due to latent or subclinical hypothyroidism, they are more predisposed to dysgeusia or ghost tastes (15). Thyroid hormones are related to the maturation and specialization of the taste buds (14). Braud et al (27) in their study showed altered taste sensitivity in BMS patients, with increased thresholds of fungiform and foliate taste buds. The taste buds are surrounded by a collection of pain neurons from the trigeminal nerve (6). With reduced taste, loss of inhibition of pain afferent fibers occurs. Therefore, the origin of dysgeusia is due to the imbalance between somatosensory and gustatory afferent stimuli (28). In addition to an increased sensitivity of the trigeminal nerves (25).
The main limitation of our review was the heterogeneity of the data from the included studies, which did not allow us to perform a meta-analysis. In addition, some of the included studies did not have a control group (reference) and generally have poor methodological quality, which can lead to biased results.
Conclusions
After carrying out this systematic review, we can conclude that alterations in thyroid hormones are a factor in secondary burning mouth syndrome; especially in patients with hypothyroidism. Although it should be corroborated with studies of higher methodological quality in which all the analytical aspects in relation to thyroid alterations were evaluated.
Acknowledgments
Authors contributions Conceptualization: J.L-L. and S.E-M; Investigation: J.L-L, S.E-M, J.R-U; Methodology: J.L-L, S.E-M, and M.P-S; Data curation: S.E-M, M.P-SZ; Validation: J.L-L, M.P-S; A.B-C; and E.J-S; Writing-original draft preparation: S.E-M; and J.V-H; Writing-review and editing: S.E-M, J.L-L; M.P-S; A.B-C; E.J-S; Supervision: J.L-L, A.B-C; All authors have read and agreed to the final draft.
Conflicts of interest The authors declare no conflict of interest.
Funding This study did not have any funding sources.
References
- 1.Périer JM, Boucher Y. History of burning mouth syndrome (1800-1950): A review. Oral Dis. 2019;25:425–38. doi: 10.1111/odi.12860. [DOI] [PubMed] [Google Scholar]
- 2.Sun A, Wu K, Wang Y, Lin H, Chen H, Chiang C. Burning mouth syndrome: a review and update. J Oral Pathol Med. 2013;42:649–55. doi: 10.1111/jop.12101. [DOI] [PubMed] [Google Scholar]
- 3.Cho GS, Han MW, Lee B, Roh JL, Choi SH, Cho KJ. Zinc deficiency may be a cause of burning mouth syndrome as zinc replacement therapy has therapeutic effects. J Oral Pathol Med. 2010;39:722–7. doi: 10.1111/j.1600-0714.2010.00914.x. [DOI] [PubMed] [Google Scholar]
- 4.Chimenos-Küstner E, de Luca-Monasterios F, Schemel-Suárez M, Rodríguez de Rivera-Campillo ME, Pérez-Pérez AM, López-López J. Burning mouth syndrome and associated factors: A case-control retrospective study. Med Clin. 2017;148:153–7. doi: 10.1016/j.medcli.2016.09.046. [DOI] [PubMed] [Google Scholar]
- 5.Bender SD. Burning Mouth Syndrome. Dent Clin North Am. 2018;62:585–96. doi: 10.1016/j.cden.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Grushka M, Epstein JB, Gorsky M. Burning Mouth Syndrome. Am Fam Physician. 2002;15:615–20. [PubMed] [Google Scholar]
- 7.López-Jornet P, Camacho-Alonso F, Andujar-Mateos P, Sánchez-Siles M, Gómez-Garcia F. Burning mouth syndrome: an update. Med Oral Patol Oral Cir Bucal. 2010;15:562–8. doi: 10.4317/medoral.15.e562. [DOI] [PubMed] [Google Scholar]
- 8.Scala A, Checchi L, Montevecchi M, Marini I, Giamberardino MA. Update on burning mouth syndrome: overview and patient management. Crit Rev Oral Biol Med. 2003;14:275–91. doi: 10.1177/154411130301400405. [DOI] [PubMed] [Google Scholar]
- 9.Russo M, Crafa P, Guglielmetti S, Franzoni L, Fiore W, Di Mario F. Burning Mouth Syndrome Etiology: A Narrative Review. J Gastrointestin Liver Dis. 2022;31:223–8. doi: 10.15403/jgld-4245. [DOI] [PubMed] [Google Scholar]
- 10.Carreño-Hernández I, Cassol-Spanemberg J, Rodríguez de Rivera-Campillo E, Estrugo-Devesa A, López-López J. Is Burning Mouth Syndrome a Neuropathic Pain Disorder? A Systematic Review. J Oral Facial Pain Headache. 2021;35:218–29. doi: 10.11607/ofph.2861. [DOI] [PubMed] [Google Scholar]
- 11.Lauria G, Majorana A, Borgna M, Lombardi R, Penza P, Padovani A. Trigeminal small-fiber sensory neuropathy causes burning mouth syndrome. Pain. 2005;115:332–7. doi: 10.1016/j.pain.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 12.Albuquerque RJC, de Leeuw R, Carlson CR, Okeson JP, Miller CS, Andersen AH. Cerebral activation during thermal stimulation of patients who have burning mouth disorder: an fMRI study. Pain. 2006;122:223–34. doi: 10.1016/j.pain.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 13.Gupta N, Arora M, Sharma R, Arora KS. Peripheral and Central Nervous System Involvement in Recently Diagnosed Cases of Hypothyroidism: An Electrophysiological Study. Ann Med Health Sci Res. 2016;6:261–6. doi: 10.4103/amhsr.amhsr_39_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brosvic GM, Doty RL, Rowe MM, Harron A, Kolodiy N. Influences of hypothyroidism on the taste detection performance of rats: a signal detection analysis. Behav Neurosci. 1992;106:992–8. doi: 10.1037//0735-7044.106.6.992. [DOI] [PubMed] [Google Scholar]
- 15.Femiano F, Gombos F, Esposito V, Nunziata M, Scully C. Burning mouth syndrome (BMS): evaluation of thyroid and taste. Med Oral Patol Oral Cir Bucal. 2006;1:22–5. [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Int J Surg. 2010;3:36–41. [PMC free article] [PubMed] [Google Scholar]
- 17.Balshem H, Helfand M, Sch HJ, Oxman AD, Kunz R, Brozek J. GRADE guidelines: 3. Rating the quality of evidence. 2011;64:401–6. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Zhang NM, Vesty G, Zheng Z. Healthcare Professionals' Attitudes to Integration of Acupuncture in Western Medicine: A Mixed-Method Systematic Review. Pain Manag Nurs. 2021;22:684–93. doi: 10.1016/j.pmn.2021.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Chiang CP, Wu YC, Wu YH, Chang JY, Wang YP, Sun A. Gastric parietal cell and thyroid autoantibodies in patients with burning mouth syndrome. J Formos Med Assoc. 2020;119:1758–63. doi: 10.1016/j.jfma.2020.08.035. [DOI] [PubMed] [Google Scholar]
- 20.Femiano F, Lanza A, Buonaiuto C, Gombos F, Nunziata M, Cuccurullo L. Burning mouth syndrome and burning mouth in hypothyroidism: proposal for a diagnostic and therapeutic protocol. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:22–7. doi: 10.1016/j.tripleo.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 21.Morr Verenzuela CS, Davis MDP, Bruce AJ, Torgerson RR. Burning mouth syndrome: results of screening tests for vitamin and mineral deficiencies, thyroid hormone, and glucose levels-experience at Mayo Clinic over a decade. Int J Dermatol. 2017;56:952–56. doi: 10.1111/ijd.13634. [DOI] [PubMed] [Google Scholar]
- 22.Ariyawardana A, Chmieliauskaite M, Farag AM, Albuquerque R, Forssell H, Nasri-Heir C. CS. World Workshop on Oral Medicine VII: Burning mouth syndrome: A systematic review of disease definitions and diagnostic criteria utilized in randomized clinical trials. Oral Dis. 2019;25:141–56. doi: 10.1111/odi.13067. [DOI] [PubMed] [Google Scholar]
- 23.Suga T, Takenoshita M, Toyofuku A. Medical comorbidities of patients with burning mouth syndrome. Oral Dis. 2020;26:238–9. doi: 10.1111/odi.13186. [DOI] [PubMed] [Google Scholar]
- 24.Wu S, Zhang W, Yan J, Noma N, Young A, Yan Z. Worldwide prevalence estimates of burning mouth syndrome: A systematic review and meta-analysis. Oral Dis. 2022;28:1431–40. doi: 10.1111/odi.13868. [DOI] [PubMed] [Google Scholar]
- 25.Talattof Z, Dabbaghmanesh MH, Parvizi Y, Esnaashari N, Azad A. The Association between Burning Mouth Syndrome and Level of Thyroid Hormones in Hashimotos Thyroiditis in Public Hospitals in Shiraz, 2016. J Dent. 2019;20:42–7. doi: 10.30476/DENTJODS.2019.44562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolkka-Palomaa M, Jääskeläinen SK, Laine MA, Teerijoki-Oksa T, Sandell M, Forssell H. Pathophysiology of primary burning mouth syndrome with special focus on taste dysfunction: a review. Oral Dis. 2015;21:937–48. doi: 10.1111/odi.12345. [DOI] [PubMed] [Google Scholar]
- 27.Braud A, Descroix V, Ungeheuer MN, Rougeot C, Boucher Y. Taste function assessed by electrogustometry in burning mouth syndrome: a case-control study. Oral Dis. 2017;23:395–402. doi: 10.1111/odi.12630. [DOI] [PubMed] [Google Scholar]
- 28.Formaker BK, Frank ME. Taste function in patients with oral burning. Chem Senses. 2000;25:575–81. doi: 10.1093/chemse/25.5.575. [DOI] [PubMed] [Google Scholar]