Abstract
Autologous chondrocyte implantation (ACI) is a promising approach to repair cartilage defects; however, the cartilage trauma-induced inflammatory environment compromises its clinical outcomes. Cell-derived decellularized extracellular matrix (DECM) has been used as a culture substrate for mesenchymal stem cells (MSCs) to improve the cell proliferation and lineage-specific differentiation. In this study, DECM deposited by synovium-derived MSCs was used as an in vitro expansion system for rabbit articular chondrocytes and the response of DECM-expanded chondrocytes to pro-inflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) was evaluated. Compared with those grown on tissue culture polystyrene (TCPS), the proliferation rate was significantly improved in DECM-expanded chondrocytes. TCPS- and DECM-expanded chondrocytes were isolated and induced to redifferentiation in a high-density pellet culture. DECM-expanded chondrocytes exerted a stronger resistance to 1 ng/mL of IL-1β than TCPS-expanded cells, but the production of cartilage matrix in both groups was inhibited by 5 ng/mL of IL-1β. When exposed to 1 or 5 ng/mL of TNF-α, DECM-expanded chondrocytes showed higher levels of cartilage matrix synthesis than TCPS-expanded cells. In addition, the gene expression of IL-1β- or TNF-α-induced matrix degrading enzymes (MMP3, MMP9, MMP13, and ADAMTS5) was significantly lower in DECM-expanded chondrocytes than TCPS-expanded cells. Furthermore, we found that SIRT1 inhibition by nicotinamide completely counteracted the protective effect of DECM on chondrocytes in the presence of IL-1β or TNF-α, indicating that the SIRT1 signaling pathway was involved in the DECM-mediated enhancement of anti-inflammatory properties of chondrocytes. Taken together, this work suggests that stem cell-derived DECM is a superior culture substrate for in vitro chondrocyte expansion by improving proliferation and enhancing the anti-inflammatory properties of chondrocytes. DECM-expanded chondrocytes with enhanced anti-inflammatory properties hold great potential in clinically ACI-based cartilage repair.
Keywords: extracellular matrix, decellularized, chondrocytes, IL-1β, TNF-α, SIRT1
1. Introduction
Articular cartilage has a limited capacity for intrinsic repair due to its avascular and aneural structure. Chondrocytes, the only cell type residing in cartilage, play a major role in maintaining cartilage homeostasis through the regulation of extracellular matrix (ECM) synthesis, such as type II, IX, and XI collagens, proteoglycans, and hyaluronan [1]. Aggrecan is the major proteoglycan in articular cartilage that is responsible for resisting compressive forces, owing to their high negative charges, while type II collagen (COL II) forms a highly organized fibrillar network that provides strong tensile strength [2]. However, cartilage is susceptible to damages through trauma and/or disease of the joint, and if left untreated, traumatic injury can lead to an irreversible ECM degradation and ultimately post-traumatic osteoarthritis (OA); this causes clinical symptoms of joint pain and eventually joint disability [3].
Autologous chondrocyte implantation (ACI) is a promising technique for improving the repair of cartilage traumatic lesions with favorable mid- to long-term clinical outcomes [4]. Autologous chondrocytes are enzymatically isolated from a small biopsy of articular cartilage that is taken from a non-load-bearing joint cartilage area. To obtain sufficient cell numbers, monolayer expansion of articular chondrocytes in vitro is necessary. However, long expansion time and multiple passaging result in the loss of chondrocyte-specific phenotypes and lead to a “dedifferentiation” process, in which chondrocytes change from their typical round shape into a flat fibroblast-like phenotype [5]. Meanwhile, in long-term cultures, a portion of articular chondrocytes can undergo hypertrophic transformation by synthesizing type X collagen (COL X) and matrix metalloproteinase 13 (MMP13) [6]. Dedifferentiated chondrocytes show decreased expression of chondrogenic markers, such as COL II and aggrecan, and increased expression of fibroblastic markers, such as type I collagen (COL I) and versican. They also show a reduced ability to produce hyaline cartilage-specific ECM in vitro and in vivo [7]. Additionally, the repair of cartilage defects by ACI is compromised by trauma-induced pro-inflammatory cytokines, such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), from activated chondrocytes and synovial fibroblasts. Accumulated evidence suggests that these two cytokines disturb cartilage homeostasis and contribute to the pathogenesis of post-traumatic OA by directly inhibiting cartilage-specific ECM synthesis [8–10]. More importantly, these pro-inflammatory cytokines up-regulate the expression of proteolytic enzymes in chondrocytes and synovial cells, such as matrix metalloproteinases (MMPs) and aggrecanases, both of which are responsible for matrix degradation [11]. Therefore, new anti-inflammatory strategies are urgently needed to improve the quality of in vitro cultured chondrocytes used in ACI and to protect neo-cartilage from pro-inflammatory cytokines [12].
Recently, decellularized cell-derived extracellular matrix (DECM) has attracted attention in cartilage tissue engineering because it greatly promotes the chondrogenic differentiation capacity of mesenchymal stem cells (MSCs) [13]. Intra-articular injection of DECM-expanded MSCs successfully repaired partial-thickness cartilage defects in mini pigs [14]. The cell-derived ECM is composed of net-like lattices and small bundles of collagen fibers, and the decellularization process preserved the fibrillar microstructure and protein components (e.g. COL I, COL III, fibronectin, and laminin) [15]. In addition, in vitro expansion of articular chondrocytes using stem cell-derived DECM has been reported to delay the replicative senescence [16] and attenuate chondrocyte dedifferentiation by maintaining the ratio of COL II/COL I [17]. However, the effects of DECM expansion on the anti-inflammatory properties of chondrocytes have not been explored in detail and the mechanisms underlying the potential chondroprotective actions have not been covered.
Silent information regulator type 1 (SIRT1), a prominent member of the nicotinamide adenine dinucleotide (NAD)+-dependent enzyme family, is a class III protein deacetylase that regulates a variety of cellular functions ranging from cell survival to energy homeostasis by deacetylation of target substrates. It has been reported that SIRT protein levels and activity were significantly reduced in chondrocytes derived from OA cartilage compared to those derived from normal cartilage [18]. Activation of SIRT1 not only protects chondrocytes from apoptosis [19], but also supports the chondrogenic differentiation of MSCs [20]. Additionally, the anti-inflammatory action of SIRT1 on human articular chondrocytes has been investigated by Moon et al., who demonstrated that SIRT1 activation inhibited inflammatory responses in TNF-α-treated chondrocytes through inactivation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway [21]. However, there have been no reports on the roles of DECM in the regulation of SIRT1 expression to date, and it is not known whether DECM mediates protection of chondrocytes from pro-inflammatory cytokines through SIRT1 activation.
The purpose of this study was to investigate the promotive effects of DECM expansion on the anti-inflammatory properties of chondrocytes and to explore the protective role of SIRT1 in chondrocyte redifferentiation in the presence of pro-inflammatory cytokines. Cell-deposited ECM was produced by rabbit synovium-derived MSCs (SMSCs) and, after decellularization, chondrocytes were cultured on the DECM substrate and conventional tissue culture polystyrene (TCPS) substrate in vitro. Passage 2 of chondrocytes were induced to redifferentiation in the form of high-density pellets in the presence of IL-1β or TNF-α. The levels of cartilage-specific marker genes and matrix degrading enzymes were evaluated and the underlying mechanisms involving the SIRT1 signaling pathway were investigated.
2. Materials and Methods
2.1. Isolation of rabbit articular chondrocytes and SMSCs
New Zealand white rabbits (6–8 weeks old; weight, 1.5–2.0 kg) were provided by the Laboratory Animal Center of Soochow University (Suzhou, China). Articular chondrocytes were isolated from the knee joints of rabbits as described previously [16] with slight modifications. Cartilage tissues were aseptically harvested from the femoral condyle, minced to 1-mm3 pieces, and digested with 2 mg/ml type II collagenase (Thermo Fisher Scientific, Waltham, MA, USA) for 4 h at 37°C. After filtering through a nylon mesh (70 μm pore size), chondrocytes were seeded in 75-cm2 cell culture plates (Costar, Tewksbury, MA, USA) in Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific), 100 U/mL penicillin, and 100 μg/mL streptomycin (Thermo Fisher Scientific) at 37°C in a 5% CO2 incubator. After reaching 90% confluence, chondrocytes were collected by treating with 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA, Thermo Fisher Scientific, Cat 25200056) for the following experiments.
SMSCs were isolated as described previously [22]. Briefly, synovial membrane tissues were harvested aseptically from the rabbit knees, minced meticulously, and digested with 2 mg/ml type II collagenase solution under shaking for 4 h at 37°C. After filtering through a 70 μm nylon mesh, the released cells were resuspended in alpha minimum essential medium (α-MEM; Thermo Fisher Scientific) supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin and plated at 2,000 cells/cm2 in 150-cm2 culture plates (Costar) at 37°C in a humidified 5% CO2 incubator. The medium was changed after two days to remove the non-adherent cells and, when reaching 90% confluence, SMSCs were harvested for the following experiments.
2.2. Preparation of cell-derived DECM
Cell culture plates were pretreated with 0.2% gelatin (Sigma-Aldrich, St. Louis, MO, USA) for 1 h at 37°C and SMSCs at passage 3 (P3) were seeded onto the pretreated surface at 5,000 cells/cm2. After the cells reached 90% confluence, a final concentration of 100 μM L-ascorbic acid (Sigma-Aldrich) was added to the media for an additional eight days to increase the production of ECM [23]. SMSCs were removed using phosphate buffered saline (PBS) containing 0.5% Triton X-100 (Sigma-Aldrich) and 20 mM NH4OH (pH = 7.4; Sigma-Aldrich) for 5 min at 37°C and the cellular residue was removed by incubating with 100 U/mL DNase I for 1 h at 37°C. The DECM-containing plates were stored at 4°C in PBS containing 100 U/mL penicillin and 100 μg/mL streptomycin until use within one month.
2.3. Cell culture of chondrocytes on DECM
Passage 1 chondrocytes were seeded at 3,000 cells/cm2 on two substrates, TCPS and DECM, in DMEM supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified 5% CO2 incubator. The medium was changed every three days. Cell morphology of chondrocytes cultured on TCPS or DECM was assessed using an Olympus IX51 microscope (Olympus Corporation, Tokyo, Japan).
2.4. Cell proliferation analysis
Cell proliferation was evaluated using a Cell Counting Kit-8 assay (CCK-8; Beyotime Institute of Biotechnology, Haimen, China). Chondrocytes were seeded on TCPS or DECM in 24-well tissue culture plates at a density of 1,000 cells/cm2. At days 1, 3, 5, and 7, the CCK-8 solution was added in each well and the cells were incubated at 37°C for 1 h. An absorbance of 450 nm was determined using a microplate spectrophotometer (BioTek, Winooski, VT, USA).
2.5. Redifferentiation of chondrocytes in a pellet culture system
TCPS- and DECM-expanded chondrocytes at passage 2 were isolated and 0.3 × 106 of cells were centrifuged at 500 g for 5 min in a 15-mL polypropylene tube to form a pellet [16]. After an overnight incubation (day 0), the pellets were cultured in a serum-free chondrogenic medium [high-glucose DMEM (Thermo Fisher Scientific), 100 μM L-ascorbic acid, 40 μg/mL proline (Sigma-Aldrich), 100 nM dexamethasone (Sigma-Aldrich), 100 U/mL penicillin, 100 μg/mL streptomycin, and 1 × ITS Solution (Insulin, Transferrin, Selenium Solution, from Thermo Fisher Scientific) with supplementation of 10 ng/mL transforming growth factor beta 1 (TGFβ1, PeproTech Inc., Rocky Hill, NJ, USA). The pellets were cultured at 37°C in a humidified 5% CO2 incubator and the medium was changed every three days.
2.6. Treatments with pro-inflammatory cytokines and nicotinamide (NAM)
To evaluate the anti-inflammatory properties of TCPS- and DECM-expanded chondrocytes, the pellets were cultured in a serum-free chondrogenic medium for 21 days. Two dosages of IL-1β and TNF-α, 1 ng/mL and 5 ng/mL, were applied to induce an inflammatory microenvironment throughout chondrocyte redifferentiation. Pellet samples were collected at the end of redifferentiation (day 21).
To evaluate the matrix degradation in TCPS- and DECM-expanded chondrocytes, pellets were first cultured in a serum-free chondrogenic medium for 14 days and two dosages of IL-1β and TNF-α, 1 ng/mL and 5 ng/mL, were applied during late stage (day 15 – 21) redifferentiation. Pellet samples were collected at the end of redifferentiation (day 21).
To investigate the role of SIRT1 in mediating the anti-inflammatory responses, pellets from both the TCPS and DECM groups were cultured for 21 days in a serum-free chondrogenic medium supplemented with NAM (10 mM) in the presence of IL-1β (1 ng/mL) or TNF-α (1 ng/mL). Pellet samples were collected at the end of redifferentiation (day 21).
2.7. Histology and immunohistochemistry (IHC)
Representative chondrocyte pellets (n = 3) were fixed in 4% paraformaldehyde at 4°C overnight, dehydrated in a gradient ethanol series, embedded in paraffin blocks, and cut into 5-μm-thick sections. Safranin O staining (S.O.; Sigma-Aldrich) and immunohistochemistry were performed to assess the deposition of sulfated glycosaminoglycans (GAGs) and COL II, respectively. Consecutive sections were immunolabeled with a primary antibody against COL II (II-II6B3; 1:100; Developmental Studies Hybridoma Bank, Iowa City, IA, USA) overnight at 4°C. The next day, after a 1 h incubation with the secondary antibody of biotinylated horse anti-mouse IgG (1:10,000; Vector Laboratories, Burlingame, CA, USA), the 3,3’-Diaminobenzidine method was used to reveal COL II deposition using a VECTASTAIN Universal Quick Kit (Vector Laboratories) according to the manufacturer’s instructions.
2.8. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from chondrocyte pellets (n = 4) using TRIzol® reagent (Thermo Fisher Scientific) and cDNA was synthesized from 1 μg of total RNA using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). Quantitative PCR was performed using the iTap™ Universal SYBR® Green Supermix kit (Bio-Rad, Hercules, CA, USA) on a CFX96™ Real-Time PCR System (Bio-Rad). Chondrogenic marker genes, including aggrecan (ACAN), COL II (COL2A1), and SRY (sex determining region Y)-box 9 (SOX9), were evaluated. Transcript levels of matrix metalloproteinase 3 (MMP3), MMP9, MMP13, and ADAM metallopeptidase with thrombospondin type 1 motif 5 (ADAMTS5) were also evaluated. The relative gene expression was normalized to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression and was presented as the fold change using the 2−ΔΔCt method. Primer sequences for target genes are listed in Table 1.
Table 1.
Primers used for RT-qPCR
| Gene | Forward Primer sequence(5’-3’) | Reverse Primer sequence(5’-3’) |
|---|---|---|
|
| ||
| GAPDH | ACTTTGTGAAGCTCATTTCCTGGTA | GTGGTTTGAGGGCTCTTACTCCTT |
| COL2A1 | AGCCACCCTCGGACTCT | TTTCCTGCCTCTGCCTG |
| ACAN | ATGGCTTCCACCAGTGCG | CGGATGCCGTAGGTTCTCA |
| SOX9 | AAGCTCTGGAGACTTCTGAACG | CGTTCTTCACCGACTTCCTCC |
| MMP3 | TTTTGGCCATCTCTTCCTTCA | TGTGGATGCCTCTGGGTATC |
| MMP9 | GCCTCCAGCCACCACCACAC | CGCCGAACAGCAGCACCTTG |
| ADAMTS5 | GCGGATGTGTGCAAGCTGACC | AGTAGCCCATGCCATGCAGGA |
| MMP13 | TTCGGCTTAGAGGTGACAGG | ACTCTTGCCGGTGTAGGTGT |
| SIRT1 | GCGGGAATCCAAAGGATAAT | CTGTTGCAAAGGAACCATGA |
2.9. Western blot assay
Total protein was isolated from chondrocytes by incubation in RIPA lysis buffer (Beyotime, Cat P0013K) supplemented with Pierce™ Protease Inhibitor Mini Tablets (Thermo Fisher Scientific, Cat A32955). Protein concentrations were quantified using a BCA protein assay kit (Beyotime). A 20 μg aliquot of protein was denatured, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and then transferred onto a piece of nitrocellulose membrane (Beyotime). Nonspecific bindings were blocked by incubation in a blocking buffer (Beyotime) for 30 min, and the membrane was incubated in properly diluted primary antibodies against SIRT1, SOX9, and GAPDH at 4°C overnight. Next, the membrane was incubated in horseradish peroxidase-conjugated secondary antibodies at room temperature for 1 h. Blots were detected using SuperSignal West Pico Substrate (Thermo Fisher Scientific) and X-OMAT BT Film (Beyotime). The protein intensity was analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
2.10. Statistical analysis
All results were reported as means ± standard error of mean (S.E.M.). Statistical differences were determined using the two-tailed Student’s t-test for comparisons between two groups and one-way Analysis of Variance (ANOVA) with a Tukey’s post hoc test for multiple group comparisons. All analyses were performed using the SPSS 13.0 statistical software (SPSS Inc., Chicago, IL, USA). Significance was indicated by a p-value < 0.05 (*) or < 0.01 (**).
3. Results
3.1. Evaluation of cell-derived DECM on chondrocyte proliferation
Chondrocytes cultured on TCPS showed a flattened and enlarged morphology. In contrast, when chondrocytes were grown on DECM, the cells exhibited a polygonal cell shape and small cell size (Fig. 1A). The proliferative rate of DECM-cultured chondrocytes was significantly higher than that of TCPS-cultured cells. On days 3, 5, and 7, DECM-expanded chondrocytes yielded a 1.0-, 2.9-, and 1.5-fold increase in cell proliferation, respectively, compared with TCPS expansion (Fig. 1B).
Fig. 1.

The effect of DECM expansion on cell proliferation of chondrocytes. Passage 1 chondrocytes were seeded at 3,000 cells/cm2 on the standard TCPS or DECM substrates. (A) The cell morphology of chondrocytes cultured on TCPS or DECM was observed over a 7-day culture. Scale bar = 50 μm. (B) Chondrocyte proliferation on TCPS and DECM was evaluated by CCK-8 assays at separate time points of days 1, 3, 5, and 7. Values are presented as the mean ± S.E.M. of eight independent experiments (n = 8). Statistically significant differences are indicated by * p < 0.05 or ** p < 0.01 between the indicated groups.
3.2. Comparison of redifferentiation of TCPS- and DECM-expanded chondrocytes
Chondrocytes expanded on TCPS and DECM were induced toward redifferentiation in high-density pellets for 21 days. On day 0, there was no detectable staining for sulfated GAGs or COL II in the chondrocyte pellets of either TCPS or DECM groups. After day 7, both the TCPS and DECM groups showed positive and intense staining of sulfated GAGs (S.O. staining) and COL II (IHC). The results showed that, at different time points, the depositions of GAGs and COL II in the DECM group were comparable to those in the TCPS group (Fig. 2A). On day 7, 14, and 21, the transcript levels of COL2A1 in DECM-expanded chondrocyte pellets were 38.7%, 41.1%, and 34.4% higher than those in the TCPS group (Fig. 2B). Similarly, on day 21, the gene expression of ACAN was significantly increased by 30.6% (Fig. 2C) and the mRNA level of SOX9 was up-regulated by 40.0% (Fig. 2D) in the DECM group compared with the TCPS group.
Fig. 2.

The effect of DECM expansion on the redifferentiation of chondrocytes. TCPS- or DECM-expanded chondrocytes were induced to redifferentiation in a pellet culture system for 21 days. (A) Safranin O (S.O.) was used to stain sulfated GAGs and immunohistochemistry staining (IHC) was used to detect COL II. Scale bar = 500 μm. (B-D) RT-qPCR was used to measure the mRNA levels of chondrogenic marker genes, COL2A1 (B), ACAN (C), and SOX9 (D) between TCPS- and DECM-expanded chondrocytes. Values are presented as the mean ± S.E.M. of four independent experiments (n = 4) in RT-qPCR experiments. Statistically significant differences are indicated by * p < 0.05 or ** p < 0.01 between the indicated groups.
3.3. DECM expansion enhances the anti-inflammatory properties of chondrocytes
To investigate the effect of DECM expansion on the anti-inflammatory properties of chondrocytes, pellets in the TCPS and DECM groups were induced to redifferentiation in the presence of IL-1β or TNF-α. Two dosages of IL-1β, 1 ng/mL (representing a low concentration) and 5 ng/mL (representing a high concentration), were added in the chondrogenic medium for 21 days (Fig. 3A). Continuous exposure to IL-1β from day 0 to day 21 resulted in a significant decrease in cartilage-specific matrix synthesis. In particular, the deposition of sulfated GAGs and COL II was barely detectable in the TCPS group with the treatment of IL-1β. DECM expansion partially preserved the redifferentiation potential of chondrocytes in the presence of IL-1β, even at 5 ng/mL, though staining intensity of sulfated GAGs and COL II was much weaker than the untreated pellets (Fig. 3B). In the presence of 1 ng/mL IL-1β, the mRNA level of COL2A1 in the DECM group was 6.8- and 3.4-fold higher than the TCPS group, in the presence of 1 and 5 ng/mL IL-1β, respectively (Fig. 3C). The transcript levels of ACAN (Fig. 3D) and SOX9 (Fig. 3E) showed similar tendencies in DECM-expanded chondrocytes. When exposed to 1 ng/mL IL-1β, the gene expression of ACAN was 6.9-fold higher (Fig. 3D) and the level of SOX9 was 5.9-fold higher (Fig. 3E) in the DECM group than the TCPS group.
Fig. 3.
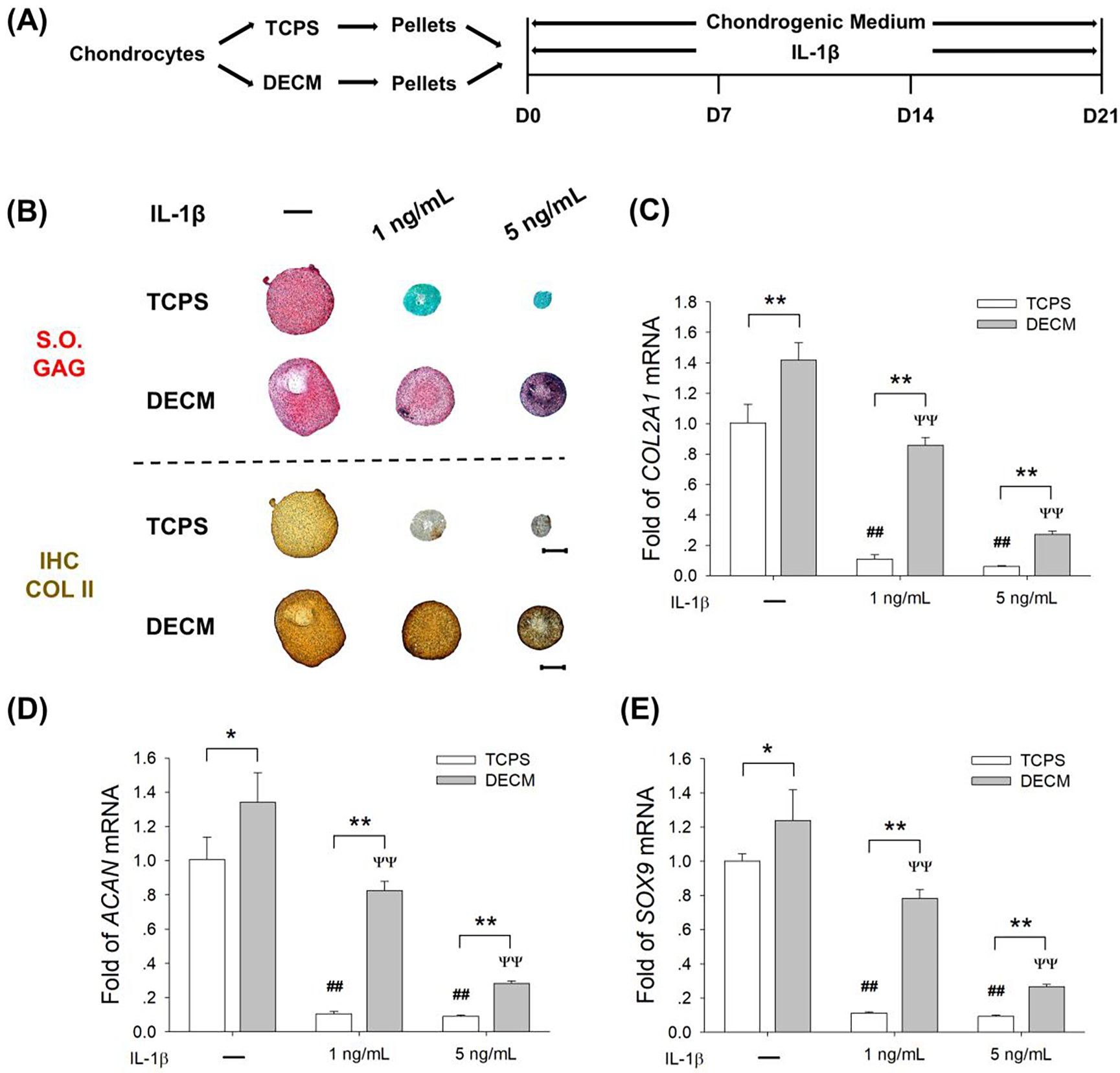
DECM expansion partly preserved the redifferentiation potential of chondrocytes in an IL-1β-induced inflammatory environment. (A) TCPS- or DECM-expanded chondrocytes were induced to redifferentiation in the presence of 1 ng/mL or 5 ng/mL of IL-1β for 21 days. (B) Safranin O (S.O.) was used to stain sulfated GAGs and immunohistochemistry staining (IHC) was used to detect COL II. Scale bar = 500 μm. (C-E) The mRNA levels of COL2A1 (C), ACAN (D), and SOX9 (E) were evaluated by RT-qPCR. Values are presented as the mean ± S.E.M. of four independent experiments (n = 4) in RT-qPCR experiments. Statistically significant differences are indicated by * p < 0.05 or ** p < 0.01 between the indicated groups; # p < 0.05 or ## p < 0.01 versus TCPS-expanded pellets without IL-1β treatment; Ψ p < 0.05 or ΨΨ p < 0.01 versus DECM-expanded pellets without IL-1β treatment.
Chondrocyte pellets in the TCPS and DECM groups were treated with two dosages of TNF-α, 1 ng/mL and 5 ng/mL, for a 21-day redifferentiation induction (Fig. 4A). The continuous exposure to TNF-α resulted in a significant decrease in the deposition of cartilage-specific matrix in TCPS-expanded chondrocytes, but DECM-expanded chondrocytes yielded pellets with a stronger resistance to TNF-α stimulation (Fig. 4B). In the TCPS group, treatments with 1 ng/mL and 5 ng/mL of TNF-α down-regulated the mRNA levels of COL2A1 by 35.2% and 53.2%, respectively. In the DECM group, however, the gene expression of COL2A1 was not decreased by 1 ng/mL of TNF-α and was down-regulated by 27.3% with treatment of 5 ng/mL of TNF-α (Fig. 4C). After a continuous exposure to 5 ng/mL TNF-α, the transcript level of ACAN was 1.5-fold higher (Fig. 4D) and the level of SOX9 was 68.8% (Fig. 4E) higher in the DECM group than the TCPS group.
Fig. 4.
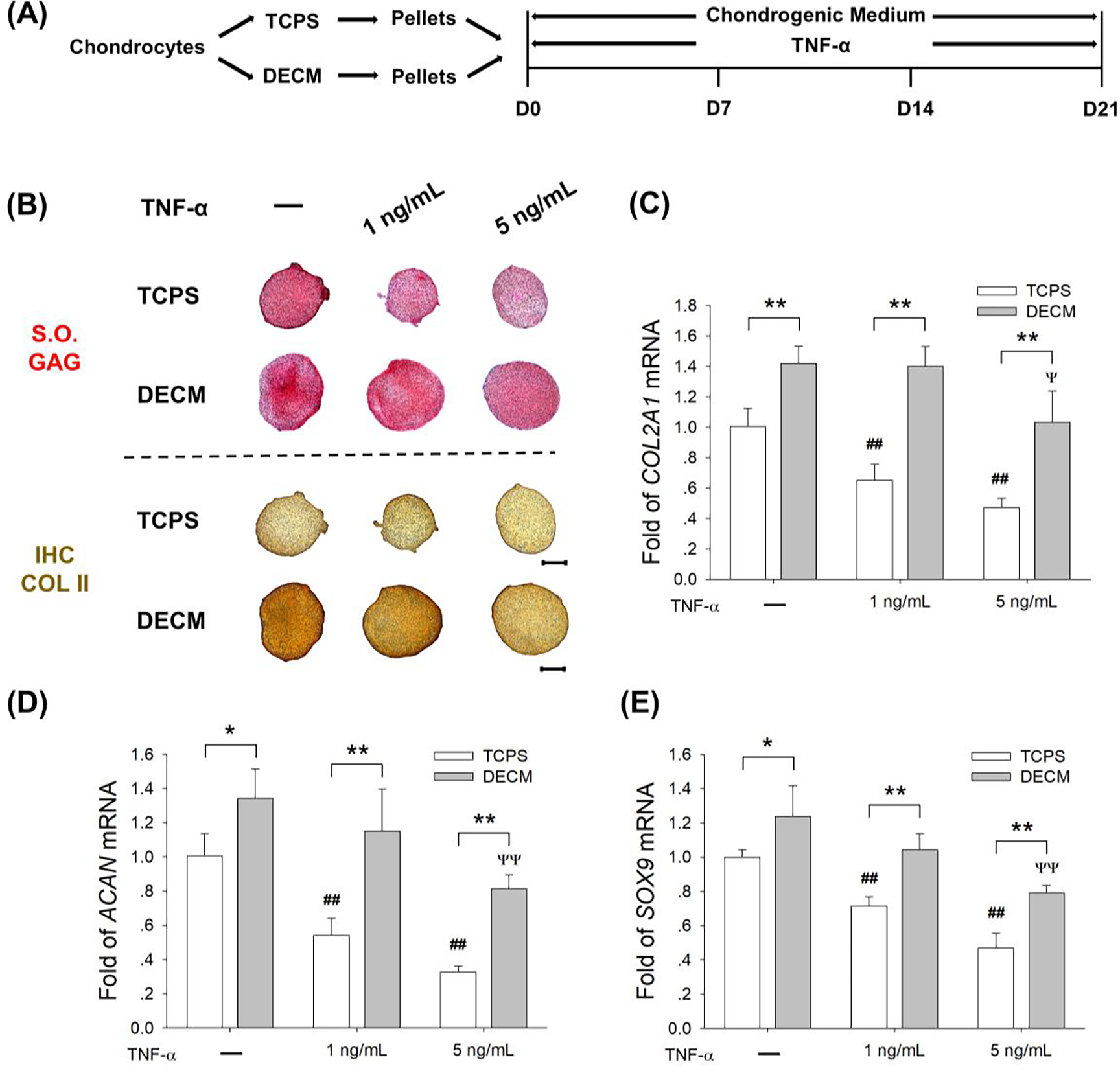
DECM expansion of chondrocytes improved their resistance to TNF-α-induced inflammatory stress. (A) TCPS- or DECM-expanded chondrocytes were induced to redifferentiation in the presence of 1 ng/mL or 5 ng/mL of TNF-α for 21 days. (B) Safranin O (S.O.) was used to stain sulfated GAGs and immunohistochemistry staining (IHC) was used to detect COL II. Scale bar = 500 μm. (C-E) The mRNA levels of COL2A1 (C), ACAN (D), and SOX9 (E) were evaluated by RT-qPCR. Values are presented as the mean ± S.E.M. of four independent experiments (n = 4) in RT-qPCR experiments. Statistically significant differences are indicated by * p < 0.05 or ** p < 0.01 between the indicated groups; # p < 0.05 or ## p < 0.01 versus TCPS-expanded pellets without TNF-α treatment; Ψ p < 0.05 or ΨΨ p < 0.01 versus DECM-expanded pellets without TNF-α treatment.
3.4. DECM-expanded chondrocytes show suppressed levels of matrix degrading enzymes
To investigate the effect of DECM expansion on inflammation-induced matrix degradation during chondrocyte redifferentiation, TCPS- and DECM-expanded chondrocytes were first incubated in chondrogenic medium for 14 days and subsequently exposed to IL-1β (1 ng/mL or 5 ng/mL) for an additional 7 days (Fig. 5A). Sulfated GAGs and COL II in TCPS-expanded chondrocytes decreased in a dose-dependent manner, but the cartilage-specific matrix in DECM-expanded chondrocytes was intensively stained with or without IL-1β treatment (Fig. 5B). In the presence of 5 ng/mL IL-1β, DECM-expanded chondrocytes showed higher transcript levels of COL2A1 by 2.2-fold (Suppl Fig. 1A), ACAN by 1.3-fold (Suppl Fig. 1B), and SOX9 by 1.1-fold (Suppl Fig. 1C), compared with the TCPS group. More importantly, the mRNA levels of MMP3 in the DECM group were 79.5% (treated with 1 ng/mL of IL-1β) and 78.5% (treated with 5 ng/mL of IL-1β) lower than the TCPS group (Fig. 5C). Similarly, after exposure to 5 ng/mL of IL-1β, gene expression in DECM-expanded chondrocytes was significantly lower than TCPS-expanded cells by 72.4% (MMP9 mRNA, Fig. 5D) and 71.9% (ADAMTS5 mRNA, Fig. 5E). The treatment with 5 ng/mL of IL-1β resulted in a 292.8- and 23.9-fold increase in the mRNA levels of MMP13 in TCPS- and DECM-expanded cells, respectively. However, the DECM group was 93.9% lower than the TCPS group (Fig. 5F).
Fig. 5.

DECM expansion suppressed the expression of cartilage matrix degrading enzymes in chondrocytes in an IL-1β-induced inflammatory environment. (A) TCPS- or DECM-expanded chondrocytes were induced to redifferentiation for 14 days and then exposed to 1 ng/mL or 5 ng/mL of IL-1β for additional 7 days. (B) Safranin O (S.O.) was used to stain sulfated GAGs and immunohistochemistry staining (IHC) was used to detect COL II. Scale bar = 500 μm. (C-F) The mRNA levels of MMP3 (C), MMP9 (D), ADAMTS5 (E), and MMP13 (F) were evaluated by RT-qPCR. Values are presented as the mean ± S.E.M. of four independent experiments (n = 4) in RT-qPCR experiments. Statistically significant differences are indicated by * p < 0.05 or ** p < 0.01 between the indicated groups; # p < 0.05 or ## p < 0.01 versus TCPS-expanded pellets without IL-1β treatment; Ψ p < 0.05 or ΨΨ p < 0.01 versus DECM-expanded pellets without IL-1β treatment.
Chondrocytes pellets in the TCPS and DECM groups were induced to redifferentiation for 14 days and then exposed to TNF-α (1 ng/mL or 5 ng/mL) for an additional 7 days (Fig. 6A). The TNF-α treatment suppressed the deposition of cartilage-specific matrix (sulfate GAGs and COL II) in TCPS-expanded chondrocyte pellets, but in the DECM group, TNF-α-treated pellets showed a strongly positive stain for S.O. and immunohistochemistry staining (Fig. 6B). Treatment with TNF-α at a low concentration (1 ng/mL) did not affect the mRNA levels of COL2A1 (Suppl Fig. 2A) or ACAN (Suppl Fig. 2B) in DECM-expanded chondrocytes. Although 5 ng/mL of TNF-α suppressed the transcript levels of cartilage-specific marker genes in the DECM group, the gene expression was still 85.8% (COL2A1, Suppl Fig. 2A), 1.8-fold (ACAN, Suppl Fig. 2B), and 1.1-fold (SOX9, Suppl Fig. 2C) higher than the TCPS group. With regard to cartilage degrading enzymes, DECM-expanded chondrocyte pellets showed lower levels of MMP3 by 81.0%, MMP9 by 53.5%, ADAMTS5 by 54.8%, and MMP13 by 97.6% compared with the TCPS group in the presence of 5 ng/mL of TNF-α.
Fig. 6.
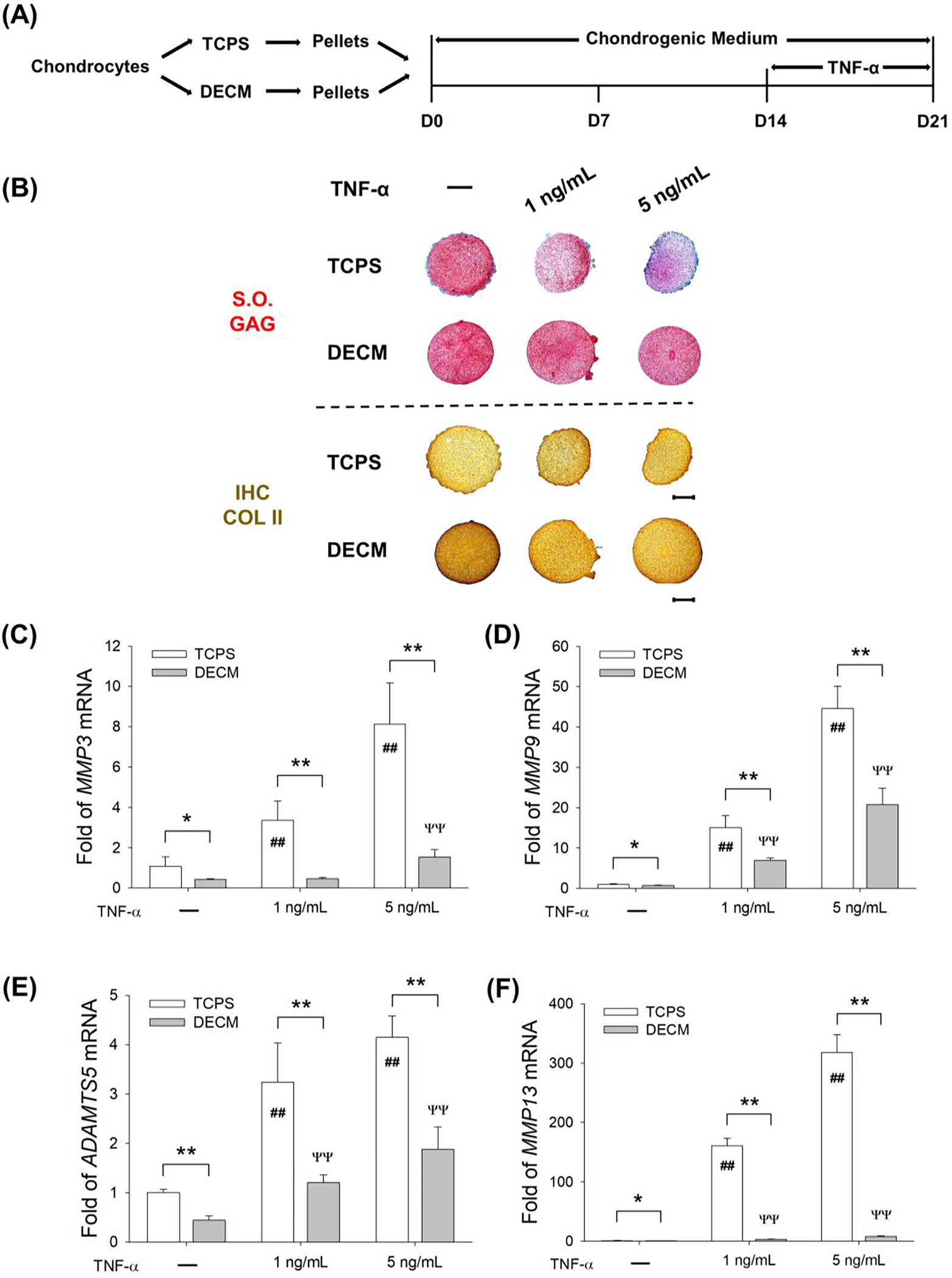
DECM expansion suppressed the expression of cartilage matrix degrading enzymes in chondrocytes in a TNF-α-induced inflammatory environment. (A) TCPS- or DECM-expanded chondrocytes were induced to redifferentiation for 14 days and then exposed to 1 ng/mL or 5 ng/mL of TNF-α for additional 7 days. (B) Safranin O (S.O.) was used to stain sulfated GAGs and immunohistochemistry staining (IHC) was used to detect COL II. Scale bar = 500 μm. (C-F) The mRNA levels of MMP3 (C), MMP9 (D), ADAMTS5 (E), and MMP13 (F) were evaluated by RT-qPCR. Values are presented as the mean ± S.E.M. of four independent experiments (n = 4) in RT-qPCR experiments. Statistically significant differences are indicated by * p < 0.05 or ** p < 0.01 between the indicated groups; # p < 0.05 or ## p < 0.01 versus TCPS-expanded pellets without TNF-α treatment; Ψ p < 0.05 or ΨΨ p < 0.01 versus DECM-expanded pellets without TNF-α treatment.
3.5. SIRT mediated the anti-inflammatory properties of DECM-expanded chondrocytes
To investigate the underlying molecular mechanisms involved in the DECM-enhanced anti-inflammatory properties of chondrocytes, we evaluated the mRNA and protein levels of SIRT1. RT-qPCR data showed that, compared to the TCPS group, DECM-expanded chondrocytes showed a significant increase in the mRNA levels of SIRT1 by 1.1-fold in chondrogenic medium, 79.7% with IL-1β treatment (1 ng/mL), and 1.5-fold with TNF-α treatment (1 ng/mL) (Fig. 7A). The Western blot assay confirmed that DECM-expanded chondrocytes showed a higher protein level of SIRT1 compared with the CTRL group (Fig. 7B), which were 73.6% (in the presence of IL-1β) and 57.0% higher (in the presence of TNF-α) in the DECM group than the TCPS group (Fig. 7C). In addition, the protein levels of SOX9 in DECM-expanded chondrocytes were by 29.6% (in chondrogenic medium), 57.1% (in the presence of IL-1β), and 48.5% higher (in the presence of TNF-α) compared with the TCPS group (Fig. 7D).
Fig. 7.
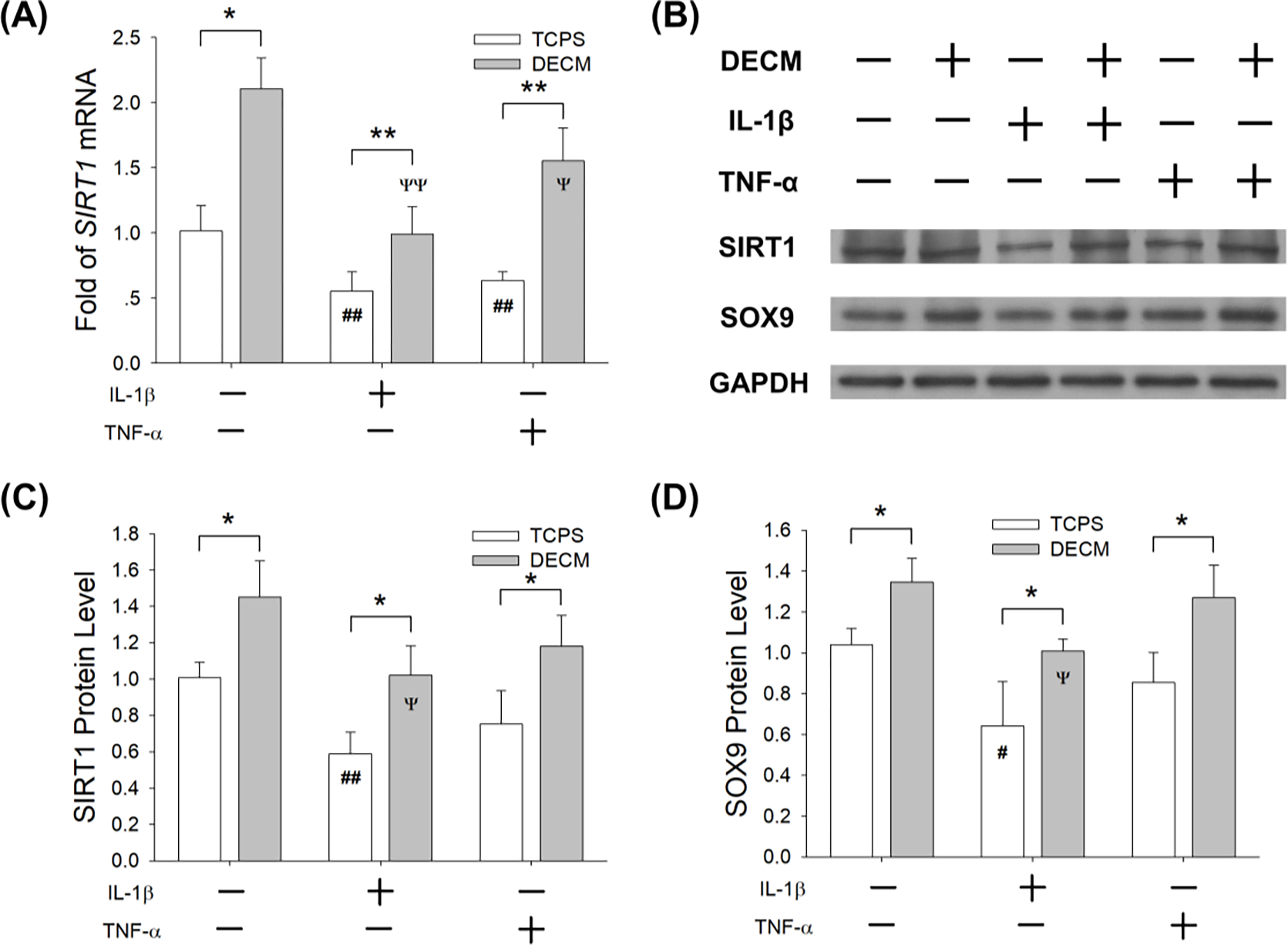
DECM expansion enhanced the anti-inflammatory properties of chondrocytes through the up-regulation of SIRT1. TCPS- or DECM-expanded chondrocytes were induced to redifferentiation in the presence of IL-1β (1 ng/mL) or TNF-α (1 ng/mL). (A) The mRNA levels of SIRT1 in chondrocytes were quantified using RT-qPCR. Values are presented as the mean ± S.E.M. of four independent experiments (n = 4) in RT-qPCR experiments. (B) The protein levels of SIRT1 and SOX9 in chondrocytes were determined using Western blot assays. (C) Quantification of SIRT1 protein levels in TCPS- and DECM-expanded chondrocytes. (D) Quantification of SOX9 protein levels in TCPS- and DECM-expanded chondrocytes. Values are presented as the mean ± S.E.M. of three independent experiments (n = 3) in Western blot assays. Statistically significant differences are indicated by * p < 0.05 or ** p < 0.01 between the indicated groups; # p < 0.05 or ## p < 0.01 versus TCPS-expanded untreated cells; Ψ p < 0.05 or ΨΨ p < 0.01 versus DECM-expanded untreated cells.
To confirm the role of SIRT1 in the DECM-mediated enhancement of anti-inflammatory properties, DECM-expanded chondrocytes were induced to redifferentiation with the treatment of NAM (10 mM) to inhibit SIRT1 activity, in the presence of IL-1β or TNF-α (1 ng/mL). NAM treatment remarkably suppressed the deposition of sulfated GAGs and COL II in IL-1β or TNF-α-stimulated pellets (Fig. 8A). The transcript levels of COL2A1 were significantly down-regulated by 70.5% (in the presence of IL-1β) and 59.5% (in the presence of TNF-α) by NAM treatment (Fig. 8B). Co-exposure to IL-1β and NAM significantly decreased gene expression by 71.9% (ACAN, Fig. 8C) and 51.7% (SOX9, Fig. 8D) compared with the IL-1β group. Similarly, treatments with TNF-α and NAM down-regulated the gene expression by 57.2% (ACAN) and 37.5% (SOX9) compared with the TNF-α group. Furthermore, the mRNA levels of SIRT1 were down-regulated by 52.2% (in the presence of IL-1β) and 65.7% (in the presence of TNF-α) by NAM treatment (Fig. 8E).The protein levels of SIRT1 were decreased by 52.8% (in the presence of IL-1β) and 52.1% (in the presence of TNF-α) (Fig. 8F & Suppl Fig. 3A) by NAM treatment.
Fig. 8.
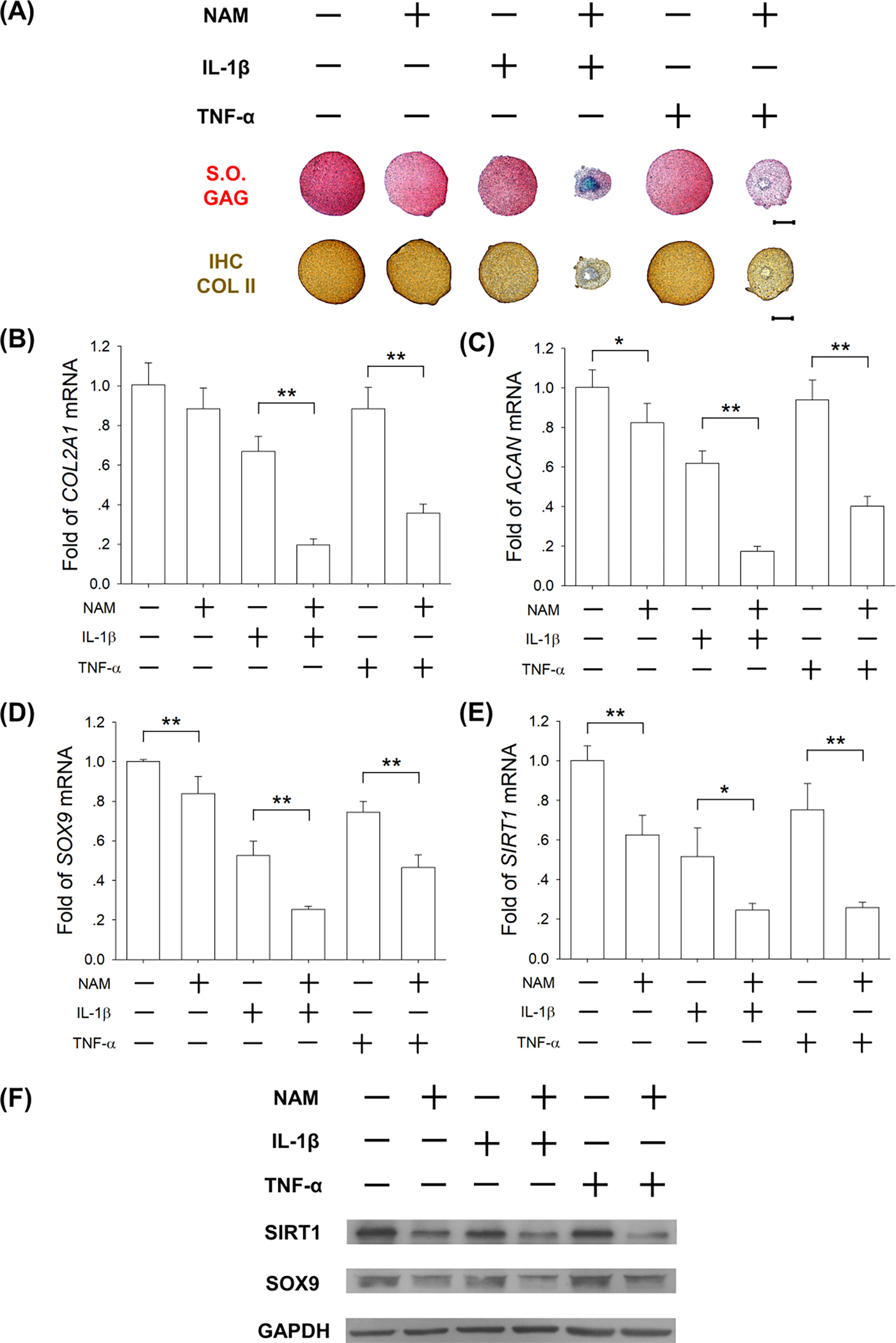
Inhibition of SIRT1 abrogated the DECM-enhanced anti-inflammatory properties of chondrocytes. DECM-expanded chondrocytes were induced to redifferentiation and treated with 10 mM of NAM in the presence of 1 ng/mL of IL-1β and TNF-α for 21 days. (A) Safranin O (S.O.) was used to stain sulfated GAGs and immunohistochemistry staining (IHC) was used to detect COL II. Scale bar = 500 μm. (B-E) The mRNA levels of COL2A1 (B), ACAN (C), SOX9 (D), and SIRT1 (E) were evaluated by RT-qPCR. Values are presented as the mean ± S.E.M. of four independent experiments (n = 4) in RT-qPCR experiments. (F) The protein levels of SIRT1 and SOX9 in chondrocytes were determined using Western blot assays. Statistically significant differences are indicated by * p < 0.05 or ** p < 0.01 between the indicated groups.
4. Discussion
Decellularization of native tissue matrix has been demonstrated to be a novel method to provide bioimplant scaffolds for cartilage repair. By applying a new chemical process, human cartilage tissues can be decellularized and sterilized, and these decellularized matrices can be used as chondro-inductive materials for cartilage replacement [24]. Compared with tissue-derived DECM, cell-derived DECM has recently gained a lot of interest as an instructive culture platform for in vitro MSC expansion, which does not have the issues of limited organ availability, disease transmission, donor-site morbidity, or immune reactions. More interestingly, DECM-expanded MSCs showed a superior lineage-specific differentiation potential than those cultured on traditional culture substrates (i.e., TCPS or coatings of individual matrix proteins on plastic surfaces), because native ECM provides a complex of structural, physical, biochemical signals that cannot be substituted for simple substrates [25].
When culturing on the DECM, articular chondrocytes maintained their original round morphology and the different stiffness between the DECM and TCPS might be responsible for the alternation of chondrocyte phenotype. It has been reported that the actin cytoskeleton of the adherent chondrocytes cultured on stiff gels was re-organized [26]. The cytoskeletal tension increased as the substrate stiffness decreased and chondrocytes on soft substrates showed high levels of COL II and aggrecan via inactivation of the RhoA/ROCK pathway [27]. When inducing to redifferentiation, both TCPS- and DECM-expanded chondrocytes were incubated in serum-free chondrogenic differentiation medium to avoid the interference of FBS [28] and to better stimulate the synthesis of cartilage-specific ECM in the passaged chondrocytes [16]. In this study, we observed that both the TCPS and DECM groups exhibited similar levels of GAG and COL II, possibly due to the early passage (passage 1) of the expanded chondrocytes. Cha et al. reported that culturing on naturally occurring cell-derived DECM significantly enhanced the redifferentiation of chondrocytes in late passages (passage 4) [29]. In addition, SMSCs were stimulated to produce the cell-derived matrix, because SMSCs were native joint-resident MSCs that are not only crucial for the development of joint cavity during embryogenesis but also the primary drivers of cartilage repair following joint injury in adulthood [30]. SMSCs have shown higher potential for chondrogenesis compared with bone marrow-derived (BMMSCs) and adipose-derived MSCs (AMSCs) [31]. A previous study from our laboratory suggested that expansion of MSCs on SMSC-derived DECM enhanced the capacity of cartilage-specific matrix synthesis but decreased the expression of hypertrophic markers such as COL X [32]. A direct comparison of SMSC-derived DECM with BMMSC- and AMSC-derived DECM is planned in our future studies.
Joint cartilage injury, which is frequently caused by mechanical overloading, results in an inflammatory response [33]. Elevated levels of pro-inflammatory cytokines, such as IL-1β and TNF-α, not only inhibit the migration of chondrogenic progenitor cells from non-fibrillated cartilage into traumatized cartilage [34], but also impair the transduction of the Smad2/3 signaling pathway in injured cartilage [35]. In this study, IL-1β or TNF-α was added in redifferentiation medium at two different concentrations to create an inflammatory environment. DECM-expanded chondrocytes exerted a strong resistance to pro-inflammatory cytokines at a low concentration; however, when exposed to 5 ng/mL IL-1β, the redifferentiation was significantly inhibited in both the DECM- and TCPS-expanded chondrocytes. These results suggested that culturing on DECM successfully increased the chondrocytes’ tolerance against a moderate inflammatory stress but was ineffective against high concentrations of pro-inflammatory cytokines. Additionally, we observed that the IL-1β treatments resulted in a greater reduction in GAG deposition and cartilage matrix-related gene expression than the TNF-α treatments. Consistent with our results, a previous study demonstrated that IL-1β was more potent than TNF-α in inhibiting MSC chondrogenesis [36]. Capsoni et al. demonstrated that in human chondrocytes IL-1β stimulated higher levels of pro-inflammatory cytokines (IL-6 and IL-8) and matrix degrading enzymes (MMP3 and MMP13) than TNF-α at the same concentration, which might be responsible for the more severe effects of IL-1β on inhibiting chondrocyte redifferentiation [37]. van Vulpen et al. confirmed that IL-1β is crucial in the development of blood-induced cartilage damage, as the cartilage was protected from the addition of IL-1β monoclonal antibody, whereas addition of a TNF-α monoclonal antibody had no effect on cartilage damage [38]. Even in the presence of 5 ng/mL TNF-α, DECM-cultured chondrocytes largely preserved their redifferentiation potential, while the redifferentiation of TCPS-cultured cells was greatly suppressed. Therefore, chondrocyte expansion on DECM in vitro might be a potent strategy for repairing cartilage defects in a TNF-α-induced inflammatory environment.
The underlying mechanisms by which DECM enhanced the anti-inflammatory properties of chondrocytes involved the up-regulation of SIRT1. We found that both the mRNA and protein levels of SIRT1 were significantly increased in DECM-expanded chondrocytes. Consistent with our results, Liu et al. observed an increased level of SIRT1 in DECM-cultured human umbilical cord-derived MSCs [39]. The role of SIRT1 in protecting chondrocytes from the inflammatory environment of OA has been demonstrated in previous studies. Activation of SIRT1 was proven to effectively enhance chondrocyte survival in the presence of TNF-α, while suppression of SIRT1 by siRNA resulted in chondrocyte apoptosis [40]. Bar Oz et al. demonstrated that activation of SIRT1 promoted SOX9 nuclear translocation by deacetylation and thus increased the expression of ACAN and COL2A1 in OA chondrocytes [41]. The mechanism by which SIRT1 governed cartilage matrix gene expression involved forming a protein complex with the histone methyltransferase Set7/9 and binding to the promotor site of COL2A1 to increase its gene transactivation [42]. Furthermore, enhancement of antioxidant capacity by DECM might contribute to the increased resistance to pro-inflammatory cytokines in chondrocytes. DECM-enhanced SIRT1 in chondrocytes may promote the nuclear entry of Forkhead box type O transcription factor 3a (FoxO3a), which stimulates the expression of antioxidant enzymes, such as superoxide dismutase 2 (SOD2) and catalase [43].
To investigate whether DECM culturing could prevent pro-inflammatory cytokine-induced matrix degradation, chondrocyte pellets were first incubated in chondrogenic medium for 14 days and then treated with different concentrations of IL-1β or TNF-α. The mRNA levels of matrix degrading enzymes were significantly lower in DECM-expanded chondrocytes than those cultured on TCPS. For the first time, we demonstrated that DECM expansion significantly suppressed cartilage-specific matrix degradation induced by IL-1β or TNF-α during the redifferentiation of chondrocytes. We speculate that up-regulated levels of SIRT1 might contribute to the suppression of matrix degrading enzymes. Matsushita et al. showed that overexpression of SIRT1 in human chondrocytes significantly inhibited the IL-1β-induced cartilage-degrading enzymes, such as MMP-1, 2, 9, and 13, while inhibition of SIRT1 by siRNA increased the expression of these genes [44]. An in vivo study further confirmed that the intra-articular injection of resveratrol, a SIRT1 activator, prevented the progression of cartilage destruction in a mouse model of OA by down-regulating catabolic factors such as MMP-13 [45]. Additionally, NF-κB is an important regulator in IL-1β or TNF-α-induced inflammation and plays a critical role in the degradative cartilage process. Stem cell-derived DECM has been shown to suppress the osteoclast differentiation of bone marrow monocytes by inhibiting NF-κB nuclear translocation, which is induced by receptor activator of nuclear factor-κB ligand (RANKL) [46]. Whether the DECM-mediated anti-inflammatory effect on cytokine-stimulated chondrocytes is through inhibition of NF-κB will be elucidated in our future studies.
There are still some limitations we would like to point out regarding our investigation. First, we demonstrated that DECM expansion improved the anti-inflammatory properties of chondrocytes, but it is unknown whether they can effectively repair cartilage lesions, particularly in an inflammatory environment. Our future work will continue to investigate the therapeutic effects of DECM-expanded chondrocytes on repairing cartilage defects in an OA animal model. Second, patients with cartilage defects in clinical settings are usually middle-aged, which has a significant impact on the reparative ability of chondrocytes. Li et al. compared two types of DECMs, which were deposited by fetal and adult MSCs, and demonstrated that young DECM was superior to aged DECM in promoting cell proliferation and chondrogenic differentiation [47]. The different effects of young and aged DECM on the anti-inflammatory functions of articular chondrocytes will be investigated in our future studies. Third, synthetic scaffolds have been widely used for cartilage repair, but the lack of native ECM components fails to provide bioactive cell-ECM interaction signals. Decoration of synthetic polymeric materials by MSC-deposited DECM has been proven to effectively support the osteogenic differentiation of human MSCs [48]. Therefore, our future work will investigate the effects of DECM-scaffold composite on repairing cartilage defects.
5. Conclusions
In summary, we demonstrated that culturing on SMSC-derived DECM improved the proliferation capacity, redifferentiation potential, and anti-inflammatory properties of articular chondrocytes. In an IL-1β or TNF-α-induced inflammatory environment, DECM-expanded chondrocytes maintained the synthesis of cartilage-specific matrix and suppressed the expression of matrix degrading enzymes. The DECM-mediated anti-inflammatory effects on chondrocytes were achieved through the SIRT1 signaling pathway. The cell-derived DECM holds great potential in providing high-quality chondrocytes with superior anti-inflammatory properties for clinical ACI application and cartilage tissue engineering. Future studies are necessary to investigate the therapeutic effects of DECM-expanded chondrocyte on repairing cartilage defects in an OA inflammatory joint and to examine the reparative potential of the combination of DECM with polymeric scaffolds.
Supplementary Material
Highlights.
DECM expansion improves the proliferation and matrix synthesis of chondrocytes
DECM expansion enhances the anti-inflammatory properties of chondrocytes
Cytokine-induced matrix degradation is suppressed in DECM-expanded chondrocytes
SIRT1 contributes to the enhanced anti-inflammatory properties of chondrocytes
The anti-inflammatory DECM promotes autologous chondrocyte implantation
Statement of Significance.
Decellularized extracellular matrix (DECM) deposited by synovium-derived mesenchymal stem cells was used for in vitro expansion of rabbit articular chondrocytes. Both the proliferation and matrix synthesis potentials of chondrocytes were improved by DECM expansion. More importantly, DECM-expanded chondrocytes were more resistant to TNF-α-induced inflammatory stress, but had an unchanged redifferentiation potential in the presence of IL-1β. SIRT1 played a rival role in the DECM-mediated enhancement of anti-inflammatory properties of chondrocytes. DECM holds great potential in providing high-quality chondrocytes for autologous implantation in clinical settings by improving resistance to the joint inflammatory environment.
Acknowledgments
The authors are grateful to Suzanne Danley (West Virginia University, USA) and Angela Carley Chen (University of Waterloo, Canada) for carefully reviewing and editing the manuscript. This work was supported by the National Natural Science Foundation of China [31771063, 31570978, 81601899, 81871789, 81702146]; the Natural Science Foundation of Jiangsu Province (BK20180052); the National Institutes of Health (NIH) [AR067747-01A1] and an Established Investigator Grant from the Musculoskeletal Transplant Foundation (MTF) to M.P.; and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Footnotes
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- [1].Schulze-Tanzil G, Activation and dedifferentiation of chondrocytes: implications in cartilage injury and repair, Ann. Anat 191 (4) (2009) 325–338. [DOI] [PubMed] [Google Scholar]
- [2].Dudhia J, Aggrecan, aging and assembly in articular cartilage, Cell. Mol. Life Sci 62 (19–20) (2005) 2241–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Becerra J, Andrades JA, Guerado E, Zamora-Navas P, Lopez-Puertas JM, Reddi AH, Articular cartilage: structure and regeneration, Tissue Eng. Part B Rev 16 (6) (2010) 617–627. [DOI] [PubMed] [Google Scholar]
- [4].Schuette HB, Kraeutler MJ, McCarty EC, Matrix-Assisted Autologous Chondrocyte Transplantation in the Knee: A Systematic Review of Mid- to Long-Term Clinical Outcomes, Orthop. J. Sports Med. 5 (6) (2017) 2325967117709250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schnabel M, Marlovits S, Eckhoff G, Fichtel I, Gotzen L, Vecsei V, Schlegel J, Dedifferentiation-associated changes in morphology and gene expression in primary human articular chondrocytes in cell culture, Osteoarthritis Cartilage 10 (1) (2002) 62–70. [DOI] [PubMed] [Google Scholar]
- [6].Pacifici M, Golden EB, Adams SL, Shapiro IM, Cell hypertrophy and type X collagen synthesis in cultured articular chondrocytes, Exp. Cell Res. 192 (1) (1991) 266–270. [DOI] [PubMed] [Google Scholar]
- [7].Darling EM, Athanasiou KA, Rapid phenotypic changes in passaged articular chondrocyte subpopulations, J. Orthop. Res. 23 (2) (2005) 425–432. [DOI] [PubMed] [Google Scholar]
- [8].Blasioli DJ, Kaplan DL, The roles of catabolic factors in the development of osteoarthritis, Tissue Eng. Part B Rev. 20 (4) (2014) 355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ossendorff R, Grad S, Stoddart MJ, Alini M, Schmal H, Sudkamp N, Salzmann GM, Autologous Chondrocyte Implantation in Osteoarthritic Surroundings: TNFalpha and Its Inhibition by Adalimumab in a Knee-Specific Bioreactor, Am. J. Sports Med 46 (2) (2018) 431–440. [DOI] [PubMed] [Google Scholar]
- [10].Furman BD, Mangiapani DS, Zeitler E, Bailey KN, Horne PH, Huebner JL, Kraus VB, Guilak F, Olson SA, Targeting pro-inflammatory cytokines following joint injury: acute intra-articular inhibition of interleukin-1 following knee injury prevents post-traumatic arthritis, Arthritis. Res. Ther 16 (3) (2014) R134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sun HB, Mechanical loading, cartilage degradation, and arthritis, Ann. N. Y. Acad. Sci. 1211 (2010) 37–50. [DOI] [PubMed] [Google Scholar]
- [12].Zhang Y, Pizzute T, Pei M, Anti-inflammatory strategies in cartilage repair, Tissue Eng. Part B Rev. 20 (6) (2014) 655–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pei M, Li JT, Shoukry M, Zhang Y, A review of decellularized stem cell matrix: a novel cell expansion system for cartilage tissue engineering, Eur. Cell. Mater 22 (2011) 333–343. [DOI] [PubMed] [Google Scholar]
- [14].Wang M, Sampson ER, Jin H, Li J, Ke QH, Im HJ, Chen D, MMP13 is a critical target gene during the progression of osteoarthritis, Arthritis. Res. Ther 15 (1) (2013) R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].He F, Liu X, Xiong K, Chen S, Zhou L, Cui W, Pan G, Luo ZP, Pei M, Gong Y, Extracellular matrix modulates the biological effects of melatonin in mesenchymal stem cells, J. Endocrinol. 223 (2) (2014) 167–180. [DOI] [PubMed] [Google Scholar]
- [16].Pei M, He F, Extracellular matrix deposited by synovium-derived stem cells delays replicative senescent chondrocyte dedifferentiation and enhances redifferentiation, J. Cell. Physiol. 227 (5) (2012) 2163–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mao Y, Block T, Singh-Varma A, Sheldrake A, Leeth R, Griffey S, Kohn J, Extracellular matrix derived from chondrocytes promotes rapid expansion of human primary chondrocytes in vitro with reduced dedifferentiation, Acta Biomater. 85 (2019) 75–83. [DOI] [PubMed] [Google Scholar]
- [18].Dvir-Ginzberg M, Gagarina V, Lee EJ, Hall DJ, Regulation of cartilage-specific gene expression in human chondrocytes by SirT1 and nicotinamide phosphoribosyltransferase, J. Biol. Chem. 283 (52) (2008) 36300–36310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Takayama K, Ishida K, Matsushita T, Fujita N, Hayashi S, Sasaki K, Tei K, Kubo S, Matsumoto T, Fujioka H, Kurosaka M, Kuroda R, SIRT1 regulation of apoptosis of human chondrocytes, Arthritis Rheum. 60 (9) (2009) 2731–2740. [DOI] [PubMed] [Google Scholar]
- [20].Shakibaei M, Buhrmann C, Mobasheri A, Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-kappaB ligand (RANKL) activation of NF-kappaB signaling and inhibit osteoclastogenesis in bone-derived cells, J. Biol. Chem. 286 (13) (2011) 11492–11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Moon MH, Jeong JK, Lee YJ, Seol JW, Jackson CJ, Park SY, SIRT1, a class III histone deacetylase, regulates TNF-alpha-induced inflammation in human chondrocytes, Osteoarthritis Cartilage 21 (3) (2013) 470–480. [DOI] [PubMed] [Google Scholar]
- [22].Pei M, He F, Vunjak-Novakovic G, Synovium-derived stem cell-based chondrogenesis, Differentiation 76 (10) (2008) 1044–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhou L, Chen X, Liu T, Zhu C, Si M, Jargstorf J, Li M, Pan G, Gong Y, Luo ZP, Yang H, Pei M, He F, SIRT1-dependent anti-senescence effects of cell-deposited matrix on human umbilical cord mesenchymal stem cells, J. Tissue Eng. Regen. Med 12 (2) (2018) e1008–e1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schwarz S, Koerber L, Elsaesser AF, Goldberg-Bockhorn E, Seitz AM, Durselen L, Ignatius A, Walther P, Breiter R, Rotter N, Decellularized cartilage matrix as a novel biomatrix for cartilage tissue-engineering applications, Tissue Eng. Part A 18 (21–22) (2012) 2195–2209. [DOI] [PubMed] [Google Scholar]
- [25].Zhang Y, Li J, Davis ME, Pei M, Delineation of in vitro chondrogenesis of human synovial stem cells following preconditioning using decellularized matrix, Acta Biomater. 20 (2015) 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sanz-Ramos P, Mora G, Vicente-Pascual M, Ochoa I, Alcaine C, Moreno R, Doblare M, Izal-Azcarate I, Response of sheep chondrocytes to changes in substrate stiffness from 2 to 20 Pa: effect of cell passaging, Connect. Tissue Res. 54 (3) (2013) 159–66. [DOI] [PubMed] [Google Scholar]
- [27].Zhang T, Gong T, Xie J, Lin S, Liu Y, Zhou T, Lin Y, Softening Substrates Promote Chondrocytes Phenotype via RhoA/ROCK Pathway, ACS Appl. Mater. Interfaces 8 (35) (2016) 22884–22891. [DOI] [PubMed] [Google Scholar]
- [28].Bianchi VJ, Weber JF, Waldman SD, Backstein D, Kandel RA, Formation of Hyaline Cartilage Tissue by Passaged Human Osteoarthritic Chondrocytes, Tissue Eng. Part A 23 (3–4) (2017) 156–165 [DOI] [PubMed] [Google Scholar]
- [29].Cha MH, Do SH, Park GR, Du P, Han KC, Han DK, Park K, Induction of re-differentiation of passaged rat chondrocytes using a naturally obtained extracellular matrix microenvironment, Tissue Eng. Part A 19 (7–8) (2013) 978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McGonagle D, Baboolal TG, Jones E, Native joint-resident mesenchymal stem cells for cartilage repair in osteoarthritis, Nat. Rev. Rheumatol 13 (12) (2017) 719–730. [DOI] [PubMed] [Google Scholar]
- [31].Sakaguchi Y, Sekiya I, Yagishita K, Muneta T, Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source, Arthritis Rheum. 52 (8) (2005) 2521–2529. [DOI] [PubMed] [Google Scholar]
- [32].He F, X Chen M Pei, Reconstruction of an in vitro tissue-specific microenvironment to rejuvenate synovium-derived stem cells for cartilage tissue engineering, Tissue Eng. Part A 15 (12) (2009) 3809–3821. [DOI] [PubMed] [Google Scholar]
- [33].Wang Y, Li Y, Khabut A, Chubinskaya S, Grodzinsky AJ, Onnerfjord P, Quantitative proteomics analysis of cartilage response to mechanical injury and cytokine treatment, Matrix Biol. 63 (2017) 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Joos H, Wildner A, Hogrefe C, Reichel H, Brenner RE, Interleukin-1 beta and tumor necrosis factor alpha inhibit migration activity of chondrogenic progenitor cells from non-fibrillated osteoarthritic cartilage, Arthritis Res. Ther 15 (5) (2013) R119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Madej W, Buma P, van der Kraan P, Inflammatory conditions partly impair the mechanically mediated activation of Smad2/3 signaling in articular cartilage, Arthritis research & therapy. 2016;18:146. Arthritis Res. Ther. 18 (2016) 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Liu X, Xu Y, Chen S, Tan Z, Xiong K, Li Y, Ye Y, Luo ZP, He F, Gong Y, Rescue of proinflammatory cytokine-inhibited chondrogenesis by the antiarthritic effect of melatonin in synovium mesenchymal stem cells via suppression of reactive oxygen species and matrix metalloproteinases, Free Radic. Biol. Med. 68 (2014) 234–246. [DOI] [PubMed] [Google Scholar]
- [37].Capsoni F, Ongari AM, Lonati C, Accetta R, Gatti S, Catania A, alpha-Melanocyte-stimulating-hormone (alpha-MSH) modulates human chondrocyte activation induced by proinflammatory cytokines, BMC Musculoskelet. Disord. 16 (2015) 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].van Vulpen LF, Schutgens RE, Coeleveld K, Alsema EC, Roosendaal G, Mastbergen SC, Lafeber FP, IL-1beta, in contrast to TNFalpha, is pivotal in blood-induced cartilage damage and is a potential target for therapy, Blood 126 (19) (2015) 2239–2246. [DOI] [PubMed] [Google Scholar]
- [39].Liu X, Zhou L, Chen X, Liu T, Pan G, Cui W, Li M, Luo Z, Pei M, Yang H, He F, Culturing on decellularized extracellular matrix enhances antioxidant properties of human umbilical cord-derived mesenchymal stem cells, Mater. Sci. Eng. C Mater. Biol. Appl 61 (2016) 437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gagarina V, Gabay O, Dvir-Ginzberg M, Lee EJ, Brady JK, Quon MJ, Hall DJ, SirT1 enhances survival of human osteoarthritic chondrocytes by repressing protein tyrosine phosphatase 1B and activating the insulin-like growth factor receptor pathway, Arthritis Rheum. 62 (5) (2010) 1383–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bar Oz M, Kumar A, Elayyan J, Reich E, Binyamin M, Kandel L, Liebergall M, Steinmeyer J, Lefebvre V, Dvir-Ginzberg M, Acetylation reduces SOX9 nuclear entry and ACAN gene transactivation in human chondrocytes, Aging Cell 15 (3) (2016) 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Oppenheimer H, Kumar A, Meir H, Schwartz I, Zini A, Haze A, Kandel L, Mattan Y, Liebergall M, Dvir-Ginzberg M, Set7/9 impacts COL2A1 expression through binding and repression of SirT1 histone deacetylation, J. Bone Miner. Res. 29 (2) (2014) 348–360. [DOI] [PubMed] [Google Scholar]
- [43].Guan XH, Liu XH, Hong X, Zhao N, Xiao YF, Wang LF, Tang L, Jiang K, Qian YS, Deng KY, Ji G, Fu M, Xin HB, CD38 Deficiency Protects the Heart from Ischemia/Reperfusion Injury through Activating SIRT1/FOXOs-Mediated Antioxidative Stress Pathway, Oxid. Med. Cell. Longev 2016 (2016) 7410257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Matsushita T, Sasaki H, Takayama K, Ishida K, Matsumoto T, Kubo S, Matsuzaki T, Nishida K, Kurosaka M, Kuroda R, The overexpression of SIRT1 inhibited osteoarthritic gene expression changes induced by interleukin-1beta in human chondrocytes, J. Orthop. Res. 31 (4) (2013) 531–537. [DOI] [PubMed] [Google Scholar]
- [45].Li W, Cai L, Zhang Y, Cui L, Shen G, Intra-articular resveratrol injection prevents osteoarthritis progression in a mouse model by activating SIRT1 and thereby silencing HIF-2alpha, J. Orthop. Res. 33 (7) (2015) 1061–1070. [DOI] [PubMed] [Google Scholar]
- [46].Li M, Chen X, Yan J, Zhou L, Wang Y, He F, Lin J, Zhu C, Pan G, Yu J, Pei M, Yang H, Liu T, Inhibition of osteoclastogenesis by stem cell-derived extracellular matrix through modulation of intracellular reactive oxygen species, Acta Biomater. 71 (2018) 118–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Li J, Hansen KC, Zhang Y, Dong C, Dinu CZ, Dzieciatkowska M, Pei M, Rejuvenation of chondrogenic potential in a young stem cell microenvironment, Biomaterials 35 (2) (2014) 642–653. [DOI] [PubMed] [Google Scholar]
- [48].Sadr N, Pippenger BE, Scherberich A, Wendt D, Mantero S, I Martin A Papadimitropoulos, Enhancing the biological performance of synthetic polymeric materials by decoration with engineered, decellularized extracellular matrix, Biomaterials 33 (20) (2012) 5085–5093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


