Abstract
Background
Cystic fibrosis (CF) is an inherited recessive disorder characterized by recurrent and persistent pulmonary infections, resulting in lung function deterioration and early mortality.
Methods
A cross-sectional study was conducted on the bacterial profile and antibiotic resistance pattern of 103 respiratory specimens from CF patients with signs of pulmonary exacerbation. Antibiotic susceptibility testing and biofilm formation of Staphylococcus aureus and Pseudomonas aeruginosa isolates were performed by the Kirby–Bauer disc diffusion method and microtiter plate assay, respectively. Molecular typing of S. aureus and P. aeruginosa isolates was carried out by spa typing and repetitive extragenic palindromic element PCR.
Results
In a total of 129 isolates, the most prevalent organisms were S. aureus (55.3%) and P. aeruginosa (41.7%). Other less prevalent bacterial isolates include coagulase-negative staphylococci, Escherichia coli, klebsiella spp., Enterobacter spp., and Achromobacter xylosoxidans. The highest rate of resistance for S. aureus was observed to azithromycin and erythromycin (80%), ciprofloxacin (52.3%), clindamycin (44.6%) and tetracycline (43%). Twenty percent of S. aureus isolates were methicillin-resistant S. aureus (MRSA) and 47.6% were MDR S. aureus. For P. aeruginosa isolates the highest resistance was to cefepime (38.3%) and levofloxacin (33.3%) and 20% showed MDR phenotype.
Conclusion
Our study demonstrated a significant decline in the prevalence of P. aeruginosa infections in comparison to previous studies. We found S. aureus to be more prevalent in younger patients, whereas mucoid P. aeruginosa showed a shift in prevalence toward older ages. Molecular typing methods showed great diversity between isolates.
1. Introduction
Cystic fibrosis (CF) is an inherited genetic disorder which affects 70,000 to 100,000 people around the world [1, 2]. It is caused by a mutation in the transmembrane conductance regulator (CFTR) gene [3]. A defective CFTR results in thickened and viscous mucus secretions in the respiratory tract that cannot be easily cleared by the mucociliary clearance system [1, 4]. The accumulated mucus creates an appropriate niche for bacterial colonization and the development of persistent pulmonary bacterial infections [2, 5]. This malicious cycle of chronic infection, inflammation, and tissue destruction results in a progressive decline in lung function, which is the primary cause of morbidity and mortality in patients with CF [6]. In addition to chronic lung infections, CF patients also experience recurrent episodes of acute decline in pulmonary function called “pulmonary exacerbation” (PE). The general presentations of a common PE are increased cough, change in sputum, shortness of breath, fever, decreased appetite, weight loss, and decrease in spirometric parameters [7]. PEs are often associated with the acquisition of new organisms, a change in the bacterial density, or the expansion of pre-existing strains [8]. Within the very first months of their life, CF patients develop airway infections. Staphylococcus aureus is the most dominant bacteria during childhood, but gradually, Gram-negative bacteria, especially Pseudomonas aeruginosa, become more dominant [9]. Other prevalent bacteria recovered by conventional culture techniques that are supposed to play a role in pulmonary infections in CF patients are Burkholderia cepacia complex species, Haemophilus influenzae, Stenotrophomonas maltophilia, and Achromobacter xylosoxidans [9, 10]. With the increasing survival of CF patients and more advanced methods for nontuberculous mycobacteria identification, the prevalence of NTM isolation from CF sputum samples is reported more frequently. Mycobacterium avium complex and Mycobacterium abscessus are the most common isolates [11, 12].
Epidemiological studies by molecular typing methods are of great importance to determining the genetic diversity of the pathogens, determining the prevalent molecular types of the isolated bacteria, tracking the spread of infections, and assessing the potential risk of person-to-person transmission of infection. Staphylococcal protein A typing and repetitive extragenic palindromic element PCR typing methods are among the well-established typing techniques used in the study of molecular evolution and outbreak investigations of S. aureus and P. aeruginosa, respectively [13–16].
Considering the vital impact of PEs on patient health, better understanding the microbiological signals associated with PEs will help to find new therapeutic strategies or predictive biomarkers to reduce the frequency and/or severity of PEs [17]. Given the importance of microbiological surveillance of CF patients, this study aims to investigate the frequency, antimicrobial susceptibility pattern, and molecular typing of bacterial pathogens isolated from CF patients during the PE phase who were admitted to The Cystic Fibrosis Center at Children's Medical Center University Hospital, Tehran, Iran, during a period of one year.
2. Material and Methods
2.1. Patients and Medical Records
Between March 2018 and February 2019, all confirmed CF patients (sweat test and clinical presentations) with signs of pulmonary exacerbation according to Goss and Burns criteria [18], referred to the cystic fibrosis center at Children's medical center University Hospital, Tehran, Iran, were included in the study. Patients who had multiple referrals over the sampling period were also included. Spontaneous sputum samples were obtained from patients involved during the study period. The patient was asked to rinse her/his mouth, cough, and expectorate the sputum into a sterile container. Sputum samples with high saliva contamination were excluded. When it was not possible to obtain spontaneous sputum samples, an oropharyngeal (OP) swab was used as a sample.
2.2. Ethics Statement
The study was approved by the Ethics Committee of Tehran University of Medical Sciences, Tehran, Iran. (code: IR.TUMS.MEDICINE.REC.1397.192). All participants included in the study and/or parents (in the case of children) were provided with oral consent before enrollment in the study. All sputum specimens were produced voluntarily. All the patients' information was kept confidential.
2.3. Bacterial Identification
Homogenized sputum or throat swab samples were inoculated on the following culture media for primary screening: general media (blood agar and chocolate agar) and selective/differential media (MacConkey agar, mannitol salt agar, Burkholderia cepacia selective agar (BCSA), and stenotrophomonas maltophilia agar). Chocolate agar plates were incubated in 5% CO2 atmosphere. Plates were incubated at 37°C for 48 hours [19]. BCSA plates were incubated for a further 5 days at room temperature. Suspected colonies were isolated and subcultured. The isolates were identified to the species level using standard biochemical methods [20]. For the detection of mycobacteria, sputum samples were processed by the modified Petroff's method [21] and inoculated on LJ (Löwenstein-Jensen) medium. All slopes were observed for any signs of growth daily for the first week and then at weekly intervals for 8 weeks. The absence of growth at the end of 8 weeks was regarded as a negative culture.
S. aureus and P. aeruginosa as the two most prevalent pathogens of CF patient's respiratory tract, were investigated in more details to illustrate a more precise picture of their phenotypic and molecular features.
2.4. Antimicrobial Susceptibility Testing
Antimicrobial susceptibility tests were performed by Kirby-Bauer's disc diffusion method according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI, 2019) [22]. To determine the susceptibility pattern of S. aureus isolates the following antibiotics were tested: cefoxitin (FOX: 30 μg), rifampin (RP: 5 μg), gentamicin (GEN: 10), ciprofloxacin (CIP: 5), TMP/SMX (TS: 1.25/23.75 μg), clindamycin (C: 2 μg), linezolid (LZD: 30 μg), tetracycline (T: 30 μg), azithromycin (ATH: 15 μg) and erythromycin (E: 15 μg). For P. aeruginosa isolates the following antibiotics were tested: imipenem (IMI: 10 μg), ceftazidime (CAZ: 30 μg), meropenem (MEM: 10 μg), piperacillin-tazobactam (PTZ: 100/10 μg), cefepime (CPM: 30 μg), aztreonam (ATM: 30 μg), gentamicin (GM: 10 μg), tobramycin (TN: 10 μg), amikacin (AK: 30 μg), ciprofloxacin (CIP: 5 μg), and levofloxacin (LEV: 5 μg). All the antibiotic discs were purchased from MAST Company (Mast Group Ltd, UK). E. coli ATCC 25922 was used as the control strain [23]. Detection of methicillin-resistant Staphylococcus aureus (MRSA) isolates was performed by 30 μg cefoxitin antibiotic discs (Mast Group Ltd, UK) according to recommendations of CLSI 2019 [22]. The molecular confirmation of MRSA was accomplished by amplification of the mecA gene. Resistance to at least one agent in three or more antimicrobial classes was considered as multidrug resistance (MDR) [24].
2.5. Biofilm Formation Assay for S.aureus and P. aeruginosa Isolates
Biofilm formation was evaluated according to the method of O'Toole and Kolter [25] with some modifications. Briefly, 100 μL of each bacterial suspension, adjusted to McFarland standard 0.5 was inoculated into each well of flat-bottomed 96-well polystyrene microtiter plate (SPL Plastic Labware, Korea). After overnight incubation at 37°C the medium was removed and washed twice with 0.9% NaCl. The formed biofilms were fixed by methanol and stained with 1% (w/v) crystal violet solution; then 33% glacial acetic acid (Merck, Germany) was added to the wells. After 10 minutes the absorbance of solubilized crystal violet was measured at 550 nm. The experiments were performed in triplicate. Uninoculated medium was considered a negative control sample. According to the results of microtiter plate test, biofilm producer isolates were characterized as follows based on the optical density: nonbiofilm producers (OD test < ODc), weak biofilm producers (ODc < OD < 2 × ODc), moderate biofilm producers (2 × ODc < OD < 4 × ODc), and strong biofilm producers (4 × ODc < OD) [26].
2.6. Spa Typing for S. aureus Isolates
The Spa typing was performed according to Harmsen et al. [27]. Briefly, the polymorphic X region of the spa gene was amplified by specific primers. The PCR reaction was performed in 12.5 μL final volumes containing 5 μL of PCR Master Mix (BioFACT, South Korea), 4.5 μL of distilled water, 0.25 μL of each primer, and 2.5 μL of extracted DNA. The PCR amplification conditions were as follows: the initial denaturation at 94°C for five minutes, and the next 35 cycles consisting of a denaturation step at 94°C for 30 seconds, annealing at 58°C for 30 seconds, extension at 72°C for 30 seconds, and a final extension step at 72°C for ten minutes. The spa gene PCR products were sequenced at Pishgam Biotech Company (Tehran, Iran). Isolates were assigned to particular spa types according to the guidelines described by the spa typing website (https://www.spaserver.ridom.de) [23].
2.7. Repetitive Extragenic Palindromic Element PCR (Rep-PCR) Genotyping for P.aeruginosa Isolates
The molecular typing of P. aeruginosa isolates was performed by rep-PCR as previously described with some modifications [16]. DNA amplification was performed in a final volume of 25 μl containing 16 μl of 2X Multi-Star PCR Master Mix (BioFACT, South Korea), 1 μl of each primer (rep-F: 5′-ICGICTTATCIGGCCTAC-3′ and rep-R: 5′-IIIICGICGICATCIGGC-3′), 5 μl of distilled water, and 2 μl of the template DNA. The cycling conditions were as follows: initial denaturation for 2 minutes at 95°C, followed by 35 cycles for 1 minute at 95°C, 1 minute at 42°C, 4 minutes at 72°C, and a final extension for 16 minutes at 72°C. The rep-PCR products were loaded on a 1.5% (w/vol) agarose gel and were analyzed by gel electrophoresis at 80 V for 2 h. A 1 kilobase DNA ladder (Thermo Fisher scientific, USA) was used as a molecular size standard. To monitor the reproducibility of the method, a P. aeruginosa ATCC 27853 reference strain was used as a control in each PCR reaction. The rep-PCR fingerprints of P. aeruginosa isolates were analyzed using GelCompar II software, version 4.0 (Applied Maths, Belgium), on the basis of the number and weight of band differences. The relatedness among isolates was deduced as previously described: linked isolates (similarity above 95%) and different (similarity less than 95%) [28].
2.8. Statistical Analysis
Data were analyzed in SPSS software (version 23; IBM Corp, USA). Nominal variables have been described with frequencies and percentages. Continuous variables were described as the mean ± SD. In addition, a binary logistic regression analysis was utilized to establish the association between the outcome variable (presence or absence of different bacteria) and the explanatory variable (age).
A P value less than 0.05 was considered statistically significant.
3. Results
3.1. Study Population
In this descriptive cross-sectional study, 103 samples from 85 CF patients between 8 months and 30 years of age with pulmonary exacerbation signs were collected. Nine patients had two referrals, and four patients had three referrals over the sampling period that were eligible for inclusion in our study. A summary of basic patient demographics and types of samples included in the study are provided in Table 1. Most cases (28.2%) were in age group of 2 to 5 years and the least cases (5.9%) were in 20 to 30 years age group. The most common symptoms observed in patients were increased coughing (74.7%) and reduced FEV1 (59.2%).
Table 1.
Patients' demographic data and clinical presentations.
| Patients (N = 85) | Number (%) |
|---|---|
| Mean age∗ | 9.41 ± 6.33 |
| Age range | |
| <2 | 8 (9.5) |
| 2–5 | 24 (28.2) |
| 6–10 | 22 (25.9) |
| 11–15 | 15 (17.6) |
| 16–20 | 11 (12.9) |
| 21–30 | 5 (5.9) |
| Gender | |
| Male | 40 (46.6) |
| Female | 45 (53.4) |
| Number of samples | 103 |
| Types of samples | |
| Sputum | 22 (21.4) |
| Throat swab | 80 (77.7) |
| BAL | 1 (1.0) |
| Signs of pulmonary exacerbation | |
| Fever (oral temperature > 38°) | 2 (1.9) |
| More frequent coughing | 77 (74.7) |
| Increased sputum volume | 55 (53.3) |
| Loss of appetite | 27 (26.2) |
| Weight loss of at least 1 kg | 36 (34.9) |
| Symptoms of upper respiratory tract infection | 56 (54.3) |
| Decreased FEV1% | 61 (59.2) |
| Nail clubbing | 26 (25.2) |
∗ presented as mean ± SD.
3.2. Prevalence of Microbial Isolates
A total of 120 bacteria and 9 yeasts were isolated from 103 respiratory samples. The most prevalent isolated species were S. aureus and P. aeruginosa. Other less prevalent bacterial isolates include coagulase-negative staphylococci, Escherichia coli, klebsiella spp., Enterobacter spp., and Achromobacter xylosoxidans. In 14.5% (15/103) of the samples, no pathogenic bacteria were isolated. The distribution pattern of each species within different age groups is presented in Table 2.
Table 2.
Distribution of microorganisms in different age groups.
| Microorganisms | Age groups (years) (number)/number (%) | ||||||
|---|---|---|---|---|---|---|---|
| <2 (15) | 2–5 (24) | 6–10 (24) | 11–15 (32) | 16–20 (25) | 21–30 (9) | Total number = 129 | |
| Staphylococcus aureus | 5 (8.7) | 14 (24.6) | 12 (21.2) | 16 (28.1) | 7 (12.2) | 3 (5.2) | 57 (55.3) |
| Pseudomonas aeruginosa | 2 (4.8) | 5 (11.8) | 9 (20.4) | 11 (25.7) | 13 (30.4) | 3 (6.9) | 43 (41.7) |
| Coagulase negative staphylococci | 3 (25.0) | 3 (25.0) | 2 (16.7) | 1 (8.3) | 3 (25.0) | 0 | 12 (11.6) |
| Yeast | 3 (33.4) | 0 | 1 (11.1) | 2 (22.2) | 1 (11.1) | 2 (22.2) | 9 (8.7) |
| Escherichia coli | 0 | 1 (25.0) | 0 | 2 (50.0) | 0 | 1 (25.0) | 4 (3.8) |
| Klebsiella spp. | 2 (100.0) | 0 | 0 | 0 | 0 | 0 | 2 (1.9) |
| Enterobacter spp. | 0 | 1 (100.0) | 0 | 0 | 0 | 0 | 1 (0.9) |
| Achromobacter xylosoxidans | 0 | 0 | 0 | 0 | 1 (100.0) | 0 | 1 (0.9) |
The prevalence of mono-microbial and polymicrobial infections was 52.4% (54/103) and 33.0% (34/103), respectively. S. aureus and P. aeruginosa coinfections were detected in 22.3% (23/103) of the samples that were mostly from patients in the 6 to 10 age group. Other double, triple, and quadruple coinfection patterns that were detected from patients are presented by age group in Figure 1.
Figure 1.
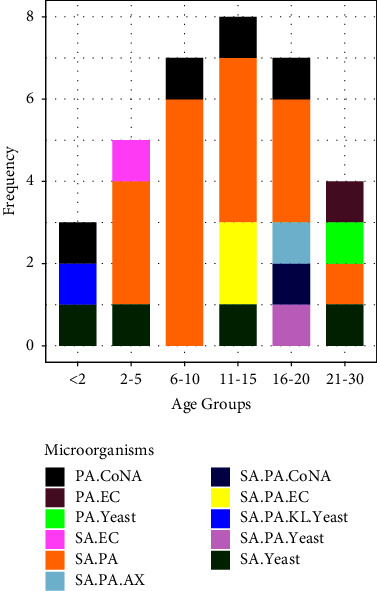
Distribution of coinfection patterns within different age groups. SA, Staphylococcus aureus; PA, Pseudomonas aeruginosa; KL, klebsiella spp; EC, Escherichia coli; E.spp, Enterobacter spp; CoNA, coagulase negative staphylococci; AX, Achromobacter xylosoxidans.
3.3. Antibiotic Susceptibility, Biofilm Formation, and Typing
3.3.1. Staphylococcus aureus
The most prevalent pathogenic bacterial species was S. aureus 55.3% (57/103). Seven patients (patient numbers 11, 12 in both two referrals, 26, 28, 53, and 60) were infected with more than one phenotype of S. aureus with either different antibiotic susceptibility pattern and/or different colony morphology and biofilm formation status (supplementary Table 1). Thus, in total, 65 morphotypes of S. aureus were assessed for antibiotic susceptibility, biofilm formation status, and molecular typing. Of the 65 S. aureus isolates tested for susceptibility to 10 antibiotics, the highest rate of resistance was observed to azithromycin and erythromycin 80% (52/65) followed by ciprofloxacin 52.3% (34/65), clindamycin 44.6% (29/65), tetracycline 43% (28/65), TMP/SMX 24.6% (16/65) and cefoxitin 24.6% (16/65). The lowest resistance rates were for gentamicin 10.7% (7/65) and rifampin 4.6% (3/65), and no isolate was resistant to linezolid (Figures 2(a) and 2(c)). Among the 16 cefoxitin-resistant S. aureus, 13 isolates were found to be positive for the mecA gene and none of them were found to carry the mecC gene. The overall prevalence of MRSA and MDR S. aureus was 20% (13/65) and 47.6% (31/65), respectively. Four out of 13 of the patients that were positive for MRSA had a P. aeruginosa coinfection, one for the E. coli and two for the yeast. The highest and lowest prevalences of MDR S. aureus were observed in 6 to 10 and 21 to 30 age groups, respectively. The distribution of MDR and non-MDR strains in different age groups is depicted in Figure 2(b). A history of antibiotic use at the time of sampling in patients with MDR S. aureus can be found in Table 2 of the supplementary files.
Figure 2.
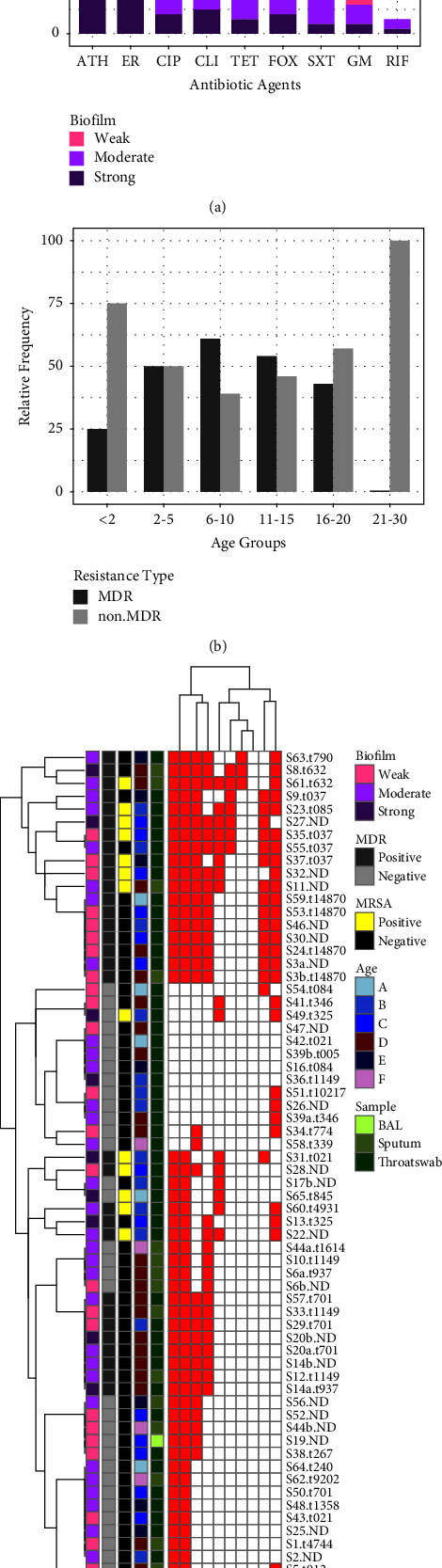
Population structure of S. aureus isolates. (a) Relative frequency of resistance to antibiotics in S. aureus isolates and biofilm formation status. (b) Relative frequency of MDR and non-MDRS. aureus isolates vs. age group. (c) Overview of antimicrobial-resistance profiles and other characteristics of S. aureus isolates. ATH, azithromycin; ER, erythromycin; FOX, cefoxitin; RIF, rifampin; GM, gentamicin; CIP, ciprofloxacin; SXT, trimethoprim-sulfamethoxazole; CLI, clindamycin; TET, tetracycline; age group is represented as A < 2, B 2–5, C 6–10, D 11–15, E 16–20, F 20–30 years of age; spa types are shown in front of isolate code; S, S. aureus; a and b letters represent the different morphotypes of the same bacteria isolated from the same patient; red squares are representative of resistance and white squares represent susceptibility to the tested antibiotic.
Results of the microtiter plate assay for biofilm production of S. aureus isolates showed that all 65 S. aureus isolates were biofilm producers in which 8 isolates (12.3%) were strong biofilm producers, 32 (49.2%) moderate, and 25 (38.4%) were weak biofilm producers. The biofilm formation status of resistant strains is illustrated in Figure 2(a). No relation between biofilm formation status and antibiotic resistance was evident.
As presented in Figure 2(c), S. aureus isolates fall into three clusters according to their respective resistance patterns. Isolates from the first and third clusters demonstrate similarities in the relative frequencies of resistance combinations and types of samples from which they have been isolated. MRSA isolates mostly fit into first and third cluster and MDR isolates mostly grouped into the first and to a lesser extent to the third cluster. The second cluster mostly included isolates with low relative frequencies of resistance most of them were methicillin susceptible and were mostly isolated from throat swabs.
Spa typing of S. aureus isolates revealed that the isolates came from a variety of genotypes and are not from a CF-specific clade. While most spa types were unique, t037 was found in 4 patients (all were MDR and grouped in the first cluster), t701 (all were MDR/MSSA and grouped in the second cluster), and t021 were found in 3 patients and t1149, t14870, t325, and t084 in 2 patients. The detailed result for each isolate is shown in Figure 2(c) and supplementary Table 1.
3.3.2. Pseudomonas aeruginosa
The second most prevalent species was P. aeruginosa which was positive in 41.7% (43/103) of the samples. There was a significant positive correlation between increased patients age and P. aeruginosa infection (p > 0.00); however, we failed to find any correlation between age and other bacteria. Among the P. aeruginosa isolates, 71.6% (43/60) showed a mucoid phenotype and were mostly isolated from patients older than 16 years of age (Figures 3(c) and 3(d)). Fourteen patients (patient numbers 4, 6, 10, 14, 19, 20, 23, 30, 43, 85, 86, 96, and 98) were infected with two or three morphotypes of P. aeruginosa with a difference in being mucoid/nonmucoid, pigment production, and/or different antibiotic susceptibility pattern (supplementary Table 3). Accordingly, 60 morphotypes of P. aeruginosa were evaluated for further investigations.
Figure 3.
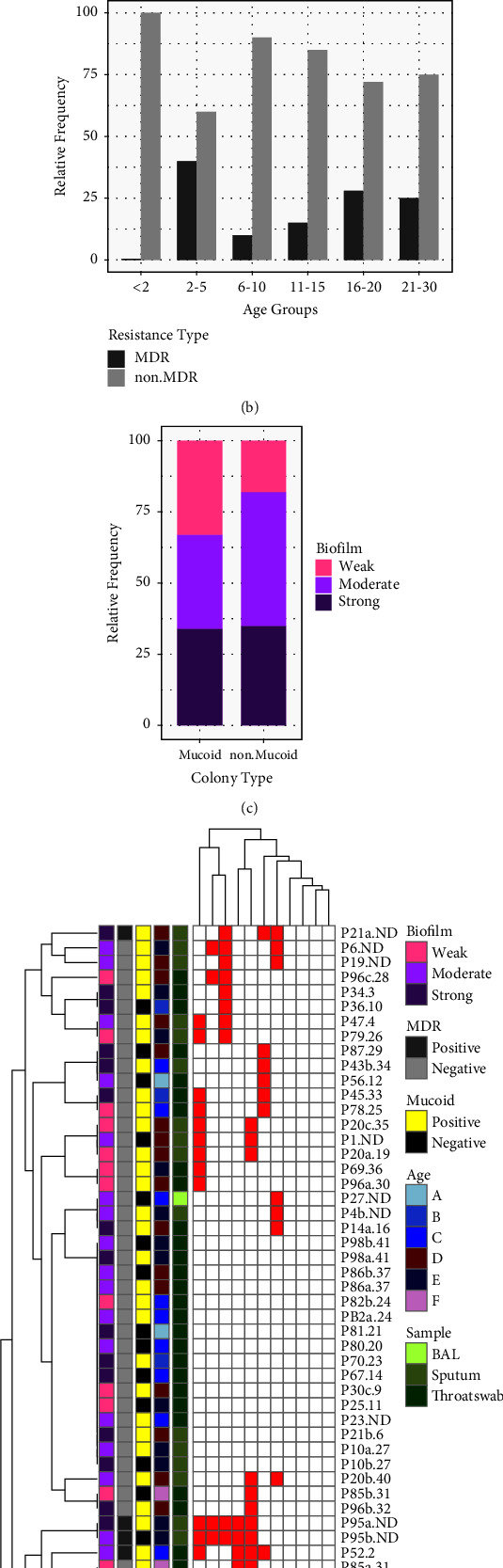
Population structure of P. aeruginosa isolates. (a) Relative frequency of resistance to antibiotics in P. aeruginosa isolates and biofilm formation status. (b) Relative frequency of MDR and non-MDRP. aeruginosa isolates vs. age group. (c) Relative frequency of mucoid/non mucoid phenotype of P. aeruginosa and biofilm formation status. (d) Overview of antimicrobial-resistance profiles and other characteristics of P. aeruginosa isolates. GM, gentamicin; CIP, ciprofloxacin; IMI, imipenem; CAZ, ceftazidime; MEM, meropenem; PTZ, piperacillin-tazobactam; CPM, cefepime; ATM, aztreonam; TN, tobramycin; AK, amikacin; LEV, levofloxacin; age group is represented as A < 2, B 2–5, C 6–10, D 11–15, E 16–20, F 20–30 years of age; (P) P. aeruginosa; a, b and c letters represent the different morphotypes of the same bacteria isolated from the same patient; red squares are representative of resistance and white squares represent susceptibility to the tested antibiotic.
The highest resistance rate was for cefepime 38.3% (23/60) and levofloxacin 33.3% (20/60), and the lowest resistance was for ceftazidime 6.6% (4/60), imipenem 5.0% (3/60), and tobramycin 3.3% (2/60) (Figure 3(a)). Prevalence of MDR P. aeruginosa was 20% (12/60) which is represented by age group in Figure 3(b). The highest and lowest prevalences of MDR P. aeruginosa were observed in the 2 to 5 and less than two age groups, respectively. Additional data on antibiotic consumption at the time of sampling in patients with MDR P. aeruginosa can be found in Table 4 of the supplementary files.
A microtiter plate assay for P. aeruginosa isolates demonstrated that 59/60 (98.3%) were biofilm producers, from which 23 (39.0%) produced strong biofilm, 19 (32.2%) moderate biofilm, and 17 (28.8%) weak biofilms. The biofilm formation status in resistant strains and mucoid/nonmucoid isolates is illustrated in Figures 3(a) and 3(c) respectively.
Using a similarity cut-off of 95%, rep-PCR typing allowed the differentiation of 53 P. aeruginosa isolates into 41 types (supplementary Figure 1). Rep-types are presented in numbers for each isolate in Figure 3(d) and supplementary Table 3.
As it is evident in Figure 3(d), P. aeruginosa isolates fall into four clusters. Isolates within the first cluster show a high level of resistance to amikacin, are more likely to express a mucoid phenotype, and are isolated mostly from adolescent patients. Isolates in the second cluster showed the lowest individual and resistance combination levels of all populations. They are mostly isolated from throat swabs, and the relative prevalence of the mucoid phenotype is the lowest in this group. A relatively high level of resistance is apparent in the third and fourth cluster. MDR P. aeruginosa isolates with a prevalence of 20% are grouped in these two clusters.
4. Discussion
Cystic fibrosis patients are predisposed to bacterial colonization and infections throughout their life. This study provides an overview of the bacterial profile and their antibiotic resistance pattern from the CF patients visiting the main referral children hospital in Iran. The most prevalent isolate in our study was S. aureus 55.3% (57/103) which is roughly comparable with Ukraine (40.8%) [29], but more than studies conducted in India (15.7%) [30], Canada (24%) [31], and Iran on 2012 (9.3%) [32] and Germany (63.3%) [33]. In the latest report from Iran on 2021, the prevalence of S. aureus was 15.6% [34]. This inconsistency may be due to dissimilar demographic composition, different identification methods, and longer incubation times in our study which improve the chance of slow-growing strains isolation. In 2008, 50.9% of CF patients included in the cystic fibrosis foundation (CFF) patient registry had positive S. aureus cultures and the most affected groups were children between 6 to 10 and adolescents of 11 to 17 years of age which is identical to our study [35, 36]. Several reports have suggested that chronic MRSA infection is associated with a high decline rate of lung function, failure to recover lung function after a pulmonary exacerbation and decreased survival [37, 38]. The prevalence of MRSA in our study was 20% which is similar to Chmiel et al. who reported the prevalence of MRSA infection at 25% in the USA, as compared to 11% in Canada and Europe [39]. MRSA infections have been increasingly reported among populations with CF worldwide [40]. According to the annual reports of CFF Patient Registry on 2020, the highest prevalence of MRSA occurs in individuals between the ages of 10 and 30, whereas MSSA peaks among those younger than 15. The pattern was different in our study, in which MSSA was predominant in 11 to 15 age group and MRSA peaked in younger patients, mostly in 2 to 5-year-old patients (Figure 2(c)). The high frequency of MRSA in younger children in our study may probably be caused by a circulating MRSA clone in the community. Another possible explanation may be the fact that in countries where care protocols have improved, MRSA colonization has shifted toward older ages which reflects the critical role of health care policies and appropriate treatment in improving the CF population health.
The results of spa typing showed a great diversity across S. aureus isolates infecting individuals with CF (Figure 2(c) and supplementary Table 1). In this study, spa t037 was the most frequent lineage, which is different from results reported from the USA and Argentina, where spa t002 was more prevalent in pediatric CF patients [41, 42]. To the best of our knowledge, there is no information on the pattern of common spa types of S. aureus isolates from cystic fibrosis patients in Iran, and this is the first report. Based on the analysis, we conclude that the S. aureus clinical isolates surveyed here are not from clonal lineages that transmit between CF patients but instead are from multiple lineages. The majority of patients that were coinfected with two or more phenotypically different S. aureus isolates, harbored the same spa type except for patient numbers 11 and 53 that were colonized with two different spa types (supplementary Table 1). Patient number 13 had two visits with an interval of 8 month in both of which S. aureus t632 was isolated that may imply the stability of initial strain (supplementary Table 1).
The second-most prevalent isolate was P. aeruginosa 41.7% (43/103). With more rapid detection and development of antipseudomonal antibiotics among the pediatric population, new P. aeruginosa acquisitions can be more effectively eradicated [43]. Annual report of the CFF Patient Registry on 2020 demonstrated the percentage of individuals with a positive culture for P. aeruginosa has continued to decline over time, with the largest decrease observed among individuals younger than 18 years (44.5 percent had a positive culture in 2000 compared with 18.1 percent in 2020) [44, 45]. In our study the prevalence varied significantly by age, from 4.8% in patients under 2 years of age to 30.4% in 16 to 20-year-old patients (p > 0.00). In a study, conducted at Mofid Children's Hospital in Iran from 2004 to 2010, the main infecting pathogen was P. aeruginosa (38.8%) [32] which is not identical to our results. A recent study from Iran reported P. aeruginosa as the most common bacterial isolate from CF patients with a prevalence of 55.5% [34]. The most important underlying reason for this discrepancy may be the time of sampling and the different population age composition.
As shown in Figure 3(d), the overall resistance rate is low among P. aeruginosa isolates. Isolates within the first cluster which show a high level of resistance to amikacin are mostly isolated from adolescent patients. Higher amikacin resistance in this group in comparison to others may have been caused by a higher treatment frequency with this antibiotic for chronic infections with P. aeruginosa. A relative high level of resistance is apparent in the third and fourth cluster, where there are more adults that are likely to have been received more antibiotics throughout their life span. In our study, ceftazidime and imipenem were the most effective antibiotics against P. aeruginosa isolates, and the least effective antibiotics were cefepime and levofloxacin (Figure 3(a) and supplementary Table 3). A study in Iran showed the most effective antibiotics against P. aeruginosa isolated from CF patients included rifampin, vancomycin, imipenem, ciprofloxacin, ofloxacin, and ceftazidime and the less effective antibiotics were penicillin, ampicillin, cephalothin, and cefixime, respectively [32]. The latest report from Iran on P. aeruginosa isolates from CF patients showed the highest resistance rate was observed for gentamicin, followed by amikacin, imipenem, and ceftazidime, and the lowest resistance rates were observed in piperacillin-tazobactam [34].
A multi-center study conducted in the United Kingdom, Belgium, and Germany on the antimicrobial susceptibility of P. aeruginosa isolates showed high resistance levels of 54% for penicillins (ticarcillin, piperacillin, and piperacillin-tazobactam), 59% for ceftazidime, 46% for amikacin, 27% for ciprofloxacin, 20% for carbapenems, and 16% for tobramycin. Resistance levels were notably much higher than that which have been reported before [46–48]. High levels of resistance were mainly attributed to the epidemic clone the Liverpool Epidemic Strain (LES) which was prevalent in the UK, and four MDR sequence type 958 (ST958) isolates that were found to be spread over the three countries [49]. Differing antibiotic treatment strategies, demographics, genetic characteristics of the population, laboratory methods, and hygiene practice may justify the difference observed between studies.
By rep-PCR typing, P. aeruginosa isolates were divided into 41 types with a 95% similarity cut-off level (supplementary Figure 1) [28]. Analysis of rep-PCR results indicate that the majority of our patients are infected with different lineages (Figure 3(d) and supplementary Table 3). The great diversity may be associated with microevolutionary events in the airway environment of CF patients [50]. The surveillance of the different morphotypes of P. aeruginosa isolated from individual CF patients to recognize when new morphotypes that may be more resistant to antimicrobial agents emerge is of great concern. As for patients' number 6, 7, 10, and 14, who had multiple referrals, different morphotypes with a higher resistance rate were isolated (supplementary Table 3).
S. aureus and P. aeruginosa are the two most commonly recognized bacterial pathogens associated with chronic lung infections in patients with CF [51]. A number of studies have shown that coinfection is associated with diminished lung function and more rapid pulmonary decline [2, 17, 52]. In our study, 22.3% (23/103) of the cases were coinfected with S. aureus and P. aeruginosa (Figure 1). The global rates of S. aureus/P. aeruginosa coinfection around the world range from 8.6% to 60% with an average of 28.3% which is highest for patients in their mid-twenties [41].
Almost all P. aeruginosa and S. aureus (more than 98%) were biofilm producers (Figures 2(a), 2(c), 3(a), 3(c) and 3(d)) which exemplify the important role of biofilm mode of growth as a key factor facilitating persistence of infection in the CF respiratory tract. A report from Iran showed that 76% and 67% of P. aeruginosa and S. aureus isolates from CF patients were biofilm producers, respectively [26]. As it is evident in Figure 3(c), contrary to expectations, nonmucoid showed a greater ability to form more strong biofilm structures than mucoid isolates. This may be due to the inadequacy of the microtiter plate method for measuring the ability of P. aeruginosa from CF patients to form biofilms. As previously mentioned, P. aeruginosa strains in a CF lung are more likely to form aggregate structures known as microcolonies by connecting to each other and to the mucin rather than attaching to a surface [53].
In spite of significant advances in treatment procedures focusing on CFTR potentiator drugs, respiratory infections remain an important cause of lung disease [39]. Thus, monitoring of such infections and the changing trend of infectious agents that may play a role in pulmonary exacerbation is vitally important in CF patients. The limitations of our study are the small number of patients from a single CF center and the unavailability of clinical histories and previous culture results. Lack of corroboration of culture methods with molecular approaches is another limitation which could help to have a better scheme of the bacterial profile during exacerbation. Furthermore, anaerobic bacteria, viruses, and fungi which may play a crucial role in proceeding exacerbation of respiratory parameters were not assessed in our study.
5. Conclusion
This study provides a picture of the bacterial profile from the respiratory tract of CF patients.
S. aureus and P. aeruginosa were the most common isolated bacteria during pulmonary exacerbation episodes. Our study demonstrated a significant decline in the prevalence of P. aeruginosa infections in comparison to previous studies. We found S. aureus to be more prevalent in younger patients, whereas mucoid P. aeruginosa was the dominant species in adults.
The polymicrobial nature of airway infections in CF patients makes it problematic to isolate fastidious and slow-growing microorganisms such as H. influenzae and B. cepacia complex, which may be hampered by more dominant species. It is suggested to use molecular techniques to detect these less frequent and fastidious organisms. The current study highlights the importance of epidemiological surveillance of CF pulmonary exacerbations. Early detection and prompt eradication treatment at the very onset of infection may effectively prevent the establishment of chronic infection and reduce the risk of morbidity, mortality, recurrent pulmonary exacerbation, and hospitalization.
Acknowledgments
This research has been supported by the Tehran University of Medical Sciences & Health Services (grant no. 97-01-30-38032).
Data Availability
The data used to support the findings of this study are included within the article and the supplementary information files.
Ethical Approval
The study was approved by the Ethics Committee of Tehran University of Medical Sciences, Tehran, Iran. (code: IR.TUMS.MEDICINE.REC.1397.192).
Consent
All participants included in the study and/or parents (in case of children) were provided with informed consent before enrollment in the study. All sputum specimens were produced voluntarily.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors' Contributions
Soroor Erfanimanesh carried out all laboratory experiment, collected data and drafted the manuscript. Mohammad Reza Modaresi and Mohammad Mehdi Feizabadi participated in the design of the study and advised in all parts of the study. Shahnaz Halimi, Reza Bigverdi and Vajiheh Sadat Nikbin analyzed data and performed the quality control of data. Fereshteh Jabalameli and Mohammad Emaneini supervised all parts of the study. The authors read and approved the final manuscript.
Supplementary Materials
The following supporting information can be downloaded. Table S1: characteristics of Staphylococcus aureus isolates; Table S2: history of antibiotic usage by patients with MDR S. aureus. Table S3: characteristics of Pseudomonas aeruginosa isolates. Table S4: history of antibiotic usage by patients with MDR P. aeruginosa. Figure S1: rep-PCR dendrogram of P. aeruginosa isolates.
References
- 1.Kelly J. Environmental scan of cystic fibrosis research worldwide. Journal of Cystic Fibrosis . 2017;16(3):367–370. doi: 10.1016/j.jcf.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Bernardy E. E., Petit R. A., Raghuram V., Alexander A. M., Read T. D., Goldberg J. B. Genotypic and phenotypic diversity of Staphylococcus aureus isolates from cystic fibrosis patient lung infections and their interactions with Pseudomonas aeruginosa. mBio . 2020;11(3):007355-20–e820. doi: 10.1128/mbio.00735-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burke D., Fouhy F., Harrison M. J., et al. The altered gut microbiota in adults with cystic fibrosis. BMC Microbiology . 2017;17(1):58–11. doi: 10.1186/s12866-017-0968-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bos A. C., Passé K. M., Mouton J. W., Janssens H. M., Tiddens H. A. The fate of inhaled antibiotics after deposition in cystic fibrosis: how to get drug to the bug? Journal of Cystic Fibrosis . 2017;16(1):13–23. doi: 10.1016/j.jcf.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Sherrard L. J., Tunney M. M., Elborn J. S. Antimicrobial resistance in the respiratory microbiota of people with cystic fibrosis. The Lancet . 2014;384(9944):703–713. doi: 10.1016/s0140-6736(14)61137-5. [DOI] [PubMed] [Google Scholar]
- 6.Jaganathan D., Bruscia E. M., Kopp B. T. Emerging concepts in defective macrophage phagocytosis in cystic fibrosis. International Journal of Molecular Sciences . 2022;23(14):p. 7750. doi: 10.3390/ijms23147750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walicka-Serzysko K., Postek M., Milczewska J., Sands D. Lung clearance index in children with cystic fibrosis during pulmonary exacerbation. Journal of Clinical Medicine . 2021;10(21):p. 4884. doi: 10.3390/jcm10214884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khanolkar R. A., Clark S. T., Wang P. W., et al. Ecological succession of polymicrobial communities in the cystic fibrosis airways. mSystems . 2020;5(6):e00809-20–e00820. doi: 10.1128/msystems.00809-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boutin S., Dalpke A. H. Acquisition and adaptation of the airway microbiota in the early life of cystic fibrosis patients. Molecular and cellular pediatrics . 2017;4(1):1–9. doi: 10.1186/s40348-016-0067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llorca Otero L., Girón Moreno R., Buendía Moreno B., Valenzuela C., Guiu Martínez A., Alarcón Cavero T. Achromobacter xylosoxidans infection in an adult cystic fibrosis unit in Madrid. Enfermedades Infecciosas y Microbiología Clínica . 2016;34(3):184–187. doi: 10.1016/j.eimc.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Cavalli Z., Reynaud Q., Bricca R., et al. High incidence of non-tuberculousmycobacteria-positive cultures among adolescent with cystic fibrosis. Journal of Cystic Fibrosis . 2017;16(5):579–584. doi: 10.1016/j.jcf.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 12.Wyrostkiewicz D., Opoka L., Filipczak D., et al. Nontuberculous mycobacterial lung disease in the patients with cystic fibrosis—a challenging diagnostic problem. Diagnostics . 2022;12(7):p. 1514. doi: 10.3390/diagnostics12071514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dendani Chadi Z., Dib L., Zeroual F., Benakhla A. Usefulness of molecular typing methods for epidemiological and evolutionary studies of Staphylococcus aureus isolated from bovine intramammary infections. Saudi Journal of Biological Sciences . 2022;29(8) doi: 10.1016/j.sjbs.2022.103338.103338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Hara F. P., Suaya J. A., Ray G. T., et al. Spa typing and multilocus sequence typing show comparable performance in a macroepidemiologic study of Staphylococcus aureus in the United States. Microbial Drug Resistance . 2016;22(1):88–96. doi: 10.1089/mdr.2014.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serrano I., De Vos D., Santos J. P., et al. Antimicrobial resistance and genomic rep-PCR fingerprints of Pseudomonas aeruginosa strains from animals on the background of the global population structure. BMC Veterinary Research . 2016;13(1):58–8. doi: 10.1186/s12917-017-0977-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salehi B., Goudarzi H., Nikmanesh B., Houri H., Alavi-Moghaddam M., Ghalavand Z. Emergence and characterization of nosocomial multidrug-resistant and extensively drug-resistantAcinetobacter baumannii isolates in Tehran, Iran. Journal of Infection and Chemotherapy . 2018;24(7):515–523. doi: 10.1016/j.jiac.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Layeghifard M., Li H., Wang P. W., et al. Microbiome networks and change-point analysis reveal key community changes associated with cystic fibrosis pulmonary exacerbations. Npj Biofilms and Microbiomes . 2019;5(1):4–12. doi: 10.1038/s41522-018-0077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goss C. H., Burns J. L. Exacerbations in cystic fibrosis . 1: epidemiology and pathogenesis. Thorax . 2007;62(4):360–367. doi: 10.1136/thx.2006.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goss C. H. Acute pulmonary exacerbations in cystic fibrosis. Seminars in Respiratory and Critical Care Medicine . 2019;40 doi: 10.1055/s-0039-1697975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahon C. R., Lehman D. C., Manuselis G. Textbook of Diagnostic Microbiology-E-Book . Oxford, UK: Elsevier Health Sciences; 2018. [Google Scholar]
- 21.Tripathi K., Tripathi P. C., Nema S., Shrivastava A. K., Dwiwedi K., Dhanvijay A. K. Modified Petroff’s method: an excellent simplified decontamination technique in comparison with Petroff’s method. International Journal of Recent Trends in Science and Technology . 2014;10(3):461–464. [Google Scholar]
- 22.Clinical and Laboratory Standards Institute (Clsi) “Performance Standards for Antimicrobial Susceptibility Testing,” CLSI Supplement M100–S29 . 29th. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2019. [Google Scholar]
- 23.Emaneini M., Khoramrooz S. S., Taherikalani M., Jabalameli F., Aligholi M. Molecular characterization of Staphylococcus aureus isolated from children with adenoid hypertrophy: emergence of new spa types t7685 and t7692. International Journal of Pediatric Otorhinolaryngology . 2011;75(11):1446–1449. doi: 10.1016/j.ijporl.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Magiorakos A.-P., Srinivasan A., Carey R. B., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clinical microbiology and infection . 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 25.O’Toole G. A. Microtiter dish biofilm formation assay. Journal of Visualized Experiments: Journal of Visualized Experiments . 2011;30(47):p. e2437. doi: 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emaneini M., Kalantar-Neyestanaki D., Jabalameli L., Hashemi M., Beigverdi R., Jabalameli F. Molecular analysis and antimicrobial resistance pattern of distinct strains of Pseudomonas aeruginosa isolated from cystic fibrosis patients in Iran. Iranian Journal of Microbiology . 2019;11(2):98–107. doi: 10.18502/ijm.v11i2.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harmsen D., Claus H., Witte W., et al. Typing of methicillin-resistantStaphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. Journal of Clinical Microbiology . 2003;41(12):5442–5448. doi: 10.1128/jcm.41.12.5442-5448.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maâtallah M., Bakhrouf A., Habeeb M. A., et al. Four genotyping schemes for phylogenetic analysis of Pseudomonas aeruginosa: comparison of their congruence with multi-locus sequence typing. PLoS One . 2013;8(12) doi: 10.1371/journal.pone.0082069.e82069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishchenko O., Koshova I., Borysova I., Stepanskyi D. Microbiological features of Staphylococcus aureus isolated from respiratory tract of children with cystic fibrosis. Wiadomosci Lekarskie . 2021;74(9):2094–2099. doi: 10.36740/wlek202109112. [DOI] [PubMed] [Google Scholar]
- 30.Gautam V., Kaza P., Mathew J. L., Kaur V., Sharma M., Ray P. Review of a 7-year record of the bacteriological profile of airway secretions of children with cystic fibrosis in North India. Indian Journal of Medical Microbiology . 2019;37(2):203–209. doi: 10.4103/ijmm.ijmm_18_424. [DOI] [PubMed] [Google Scholar]
- 31.Wolter D. J., Onchiri F. M., Emerson J., et al. Prevalence and clinical associations of Staphylococcus aureussmall-colony variant respiratory infection in children with cystic fibrosis (SCVSA): a multicentre, observational study. The Lancet Respiratory Medicine . 2019;7(12):1027–1038. doi: 10.1016/s2213-2600(19)30365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khanbabaee G., Akbarizadeh M., Sayyari A., et al. A survey on pulmonary pathogens and their antibiotic susceptibility among cystic fibrosis patients. Brazilian Journal of Infectious Diseases . 2012;16(2):122–128. doi: 10.1590/s1413-86702012000200003. [DOI] [PubMed] [Google Scholar]
- 33.Schwerdt M., Neumann C., Schwartbeck B., et al. Staphylococcus aureus in the airways of cystic fibrosis patients-A retrospective long-term study. International Journal of Medical Microbiology . 2018;308(6):631–639. doi: 10.1016/j.ijmm.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Kodori M., Nikmanesh B., Hakimi H., Ghalavand Z. Antibiotic susceptibility and biofilm formation of bacterial isolates derived from pediatric patients with cystic fibrosis from Tehran, Iran. Archives of Razi Institute . 2021;76(2):397–406. doi: 10.22092/ari.2020.128554.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahlgren H. G., Benedetti A., Landry J. S., et al. Clinical outcomes associated with Staphylococcus aureus and Pseudomonas aeruginosa airway infections in adult cystic fibrosis patients. BMC Pulmonary Medicine . 2015;15(1):67–76. doi: 10.1186/s12890-015-0062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cystic Fibrosis Foundation Patient Registry. Annual Data Report . Bethesda, MD: Cystic Fibrosis Foundation; 2015. [Google Scholar]
- 37.Banjar H., Al-Qahtani H., Yasin W., et al. The first report of Methicillin-resistant Staphylococcus aureus (MRSA) in cystic fibrosis (CF) patients in Saudi Arabia. International Journal of Pediatrics and Adolescent Medicine . 2020;7(4):186–190. doi: 10.1016/j.ijpam.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilpin D., Hoffman L. R., Ceppe A., Muhlebach M. S. Phenotypic characteristics of incident and chronic MRSA isolates in cystic fibrosis. Journal of Cystic Fibrosis . 2021;20(4):692–698. doi: 10.1016/j.jcf.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chmiel J. F., Aksamit T. R., Chotirmall S. H., et al. Antibiotic management of lung infections in cystic fibrosis. I. The microbiome, methicillin-resistantStaphylococcus aureus, gram-negative bacteria, and multiple infections. Annals of the American Thoracic Society . 2014;11(7):1120–1129. doi: 10.1513/annalsats.201402-050as. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cafiso V., Bertuccio T., Spina D., et al. Methicillin resistance and vancomycin heteroresistance in Staphylococcus aureus in cystic fibrosis patients. European Journal of Clinical Microbiology & Infectious Diseases . 2010;29(10):1277–1285. doi: 10.1007/s10096-010-1000-5. [DOI] [PubMed] [Google Scholar]
- 41.Limoli D. H., Hoffman L. R. Help, hinder, hide and harm: what can we learn from the interactions between Pseudomonas aeruginosa and Staphylococcus aureus during respiratory infections? Thorax . 2019;74(7):684–692. doi: 10.1136/thoraxjnl-2018-212616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pena Amaya P., Haim M. S., Fernández S., et al. Molecular epidemiology of methicillin-resistantStaphylococcus aureus in cystic fibrosis patients from Argentina. Microbial Drug Resistance . 2018;24(5):613–620. doi: 10.1089/mdr.2017.0162. [DOI] [PubMed] [Google Scholar]
- 43.Crull M. R., Ramos K. J., Caldwell E., Mayer-Hamblett N., Aitken M. L., Goss C. H. Change in Pseudomonas aeruginosa prevalence in cystic fibrosis adults over time. BMC Pulmonary Medicine . 2016;16(1):176–177. doi: 10.1186/s12890-016-0333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crull M. R., Somayaji R., Ramos K. J., et al. Changing rates of chronic Pseudomonas aeruginosa infections in cystic fibrosis: a population-based cohort study. Clinical Infectious Diseases . 2018;67(7):1089–1095. doi: 10.1093/cid/ciy215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramsay K. A., Sandhu H., Geake J. B., et al. The changing prevalence of pulmonary infection in adults with cystic fibrosis: a longitudinal analysis. Journal of Cystic Fibrosis . 2017;16(1):70–77. doi: 10.1016/j.jcf.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 46.Riou M., Carbonnelle S., Avrain L., et al. In vivo development of antimicrobial resistance in Pseudomonas aeruginosa strains isolated from the lower respiratory tract of Intensive Care Unit patients with nosocomial pneumonia and receiving antipseudomonal therapy. International Journal of Antimicrobial Agents . 2010;36(6):513–522. doi: 10.1016/j.ijantimicag.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 47.Fihman V., Messika J., Hajage D., et al. Five-year trends for ventilator-associated pneumonia: correlation between microbiological findings and antimicrobial drug consumption. International Journal of Antimicrobial Agents . 2015;46(5):518–525. doi: 10.1016/j.ijantimicag.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 48.Micek S. T., Wunderink R. G., Kollef M. H., et al. An international multicenter retrospective study of Pseudomonas aeruginosa nosocomial pneumonia: impact of multidrug resistance. Critical Care . 2015;19(1):219–228. doi: 10.1186/s13054-015-0926-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mustafa M.-H., Chalhoub H., Denis O., et al. Antimicrobial susceptibility of Pseudomonas aeruginosa isolated from cystic fibrosis patients in Northern Europe. Antimicrobial Agents and Chemotherapy . 2016;60(11):6735–6741. doi: 10.1128/aac.01046-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodrigues D., Lima D., Cohen R., Marques E., Leão R. Molecular characterisation of methicillin-resistantStaphylococcus aureus from chronically colonised cystic fibrosis paediatric patients in Brazil. Epidemiology and Infection . 2020;26:p. 148. doi: 10.1017/S0950268820001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Camus L., Briaud P., Vandenesch F., Moreau K. How bacterial adaptation to cystic fibrosis environment shapes interactions between Pseudomonas aeruginosa and Staphylococcus aureus. Frontiers in Microbiology . 2021;12 doi: 10.3389/fmicb.2021.617784.617784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Briaud P., Bastien S., Camus L., et al. Impact of coexistence phenotype between Staphylococcus aureus and Pseudomonas aeruginosa isolates on clinical outcomes among cystic fibrosis patients. Frontiers in Cellular and Infection Microbiology . 2020;10:p. 266. doi: 10.3389/fcimb.2020.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haley C. L., Colmer-Hamood J. A., Hamood A. N. Characterization of biofilm-like structures formed by Pseudomonas aeruginosa in a synthetic mucus medium. BMC Microbiology . 2012;12(1):181–220. doi: 10.1186/1471-2180-12-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following supporting information can be downloaded. Table S1: characteristics of Staphylococcus aureus isolates; Table S2: history of antibiotic usage by patients with MDR S. aureus. Table S3: characteristics of Pseudomonas aeruginosa isolates. Table S4: history of antibiotic usage by patients with MDR P. aeruginosa. Figure S1: rep-PCR dendrogram of P. aeruginosa isolates.
Data Availability Statement
The data used to support the findings of this study are included within the article and the supplementary information files.


