Abstract
The use of live microorganisms as an antigen delivery system is an effective means to elicit local immune responses and thus represents a promising strategy for mucosal vaccination. In this respect, lactic acid bacteria represent an original and attractive approach, as they are safe organisms that are used as food starters and probiotics. To determine whether an immune response could be elicited by intranasal delivery of recombinant lactobacilli, a Lactobacillus plantarum strain of human origin (NCIMB8826) was selected as the expression host. Cytoplasmic production of the 47-kDa fragment C of tetanus toxin (TTFC) was achieved at different levels depending on the plasmid construct. All recombinant strains proved to be immunogenic by the intranasal route in mice and able to elicit very high systemic immunoglobulin G (IgG1, IgG2b, and IgG2a) responses which correlated to the antigen dose. No significant differences in enzyme-linked immunosorbent assay IgG titers were observed when mice were immunized with live or mitomycin C-treated recombinant lactobacilli. Nevertheless, protection against the lethal effect of tetanus toxin was obtained only with the strains producing the highest dose of antigen and was greater following immunization with live bacteria. Significant TTFC-specific mucosal IgA responses were measured in bronchoalveolar lavage fluids, and antigen-specific T-cell responses were detected in cervical lymph nodes, both responses being higher in mice receiving a double dose of bacteria (at a 24-h interval) at each administration. These results demonstrate that recombinant lactobacilli can induce specific humoral (protective) and mucosal antibodies and cellular immune response against protective antigens upon nasal administration.
Most pathogens enter the body through mucosal surfaces, and the development of vaccines protective at such sites should be a very effective means of preventing a wide range of infectious diseases (4). Moreover, vaccines that can be administered by the oral or nasal route would not necessitate the professional health care infrastructure required for injectable preparations and may offer an inexpensive and convenient way of vaccination, limiting the risk for cross-contamination by needles. In addition, they are expected to induce less adverse effects (36). One approach for inducing efficient local immune responses relies on the development of live bacterial carriers. Attention in this area, has focused mainly on attenuated pathogenic vectors such as Salmonella, Bordetella, Vibrio, and Mycobacterium strains for the delivery of heterologous antigens to mucosal sites (1, 3, 10, 11, 19, 29). One of the drawbacks of such systems is the need to reduce the pathogenicity of the live carrier through the use of recombinant DNA techniques or classical genetics (33). This attenuation may impair their immunogenicity and raises questions about the safety of the final vector, especially when it is destined for immunodeficient individuals. Commensal bacteria, such as lactic acid bacteria, offer an original alternative as antigen delivery vehicles, as they are generally recognized as safe (17, 26 35). Moreover, their large-scale production is quite easy and inexpensive. The noncolonizing gram-positive bacterium Lactococcus lactis has been used successfully to induce secretory and protective systemic responses against tetanus toxin (TT) after intranasal or intragastric immunization (21, 28). In this respect, Lactobacillus strains which are able to persist in the intestinal tract for several days after administration may be particularly interesting for the mucosal presentation of antigens (18). In addition, specific members of this genus exert a probiotic, i.e., health-promoting, effect linked to their immunostimulation property and capacity to regulate the endogenous microflora (8, 13, 14, 15, 20, 21).
We have previously constructed recombinant Lactobacillus plantarum strains (NCIMB8826) producing different levels of the C fragment of TT (TTFC) intracellularly. These strains obtained by transformation with recombinant plasmids carrying the TTFC genetic determinant were shown to be immunogenic by the subcutaneous route (24; P. Chagnaud, M.-C. Geoffroy, C. Grangette, H. Müller-Alouf, N. Reveneau, D. Raze, and A. Mercenier, unpublished data). In the present study, we address the important issue as to whether these recombinant L. plantarum strains can be delivered by a mucosal (intranasal) route for induction of both mucosal and systemic immune responses against TTFC and of protection against the lethal effect of TT.
MATERIALS AND METHODS
Bacterial inocula.
Two recombinant L. plantarum NCIMB8826 TTFC-producing strains were used in this study. They were obtained by electroporation with a plasmid carrying the TTFC-encoding gene under the control of a constitutive (pldh) or inducible (pnisA) promoter, pMEC4 (erythromycin resistance) or pMEC46 (chloramphenicol resistance), respectively (24; Chagnaud et al., unpublished). A control strain harboring the nonexpressing plasmid pTG2247 (chloramphenicol resistance) was used as a negative control (7). Bacteria were grown in MRS broth (Difco, Detroit, Mich.) containing the appropriate antibiotic, erythromycin or chloramphenicol, at a final concentration of 5 or 10 μg per ml, respective. An overnight culture was used to inoculate fresh medium at a 1:20 dilution, and cells were grown to the exponential phase (optical density at 660 nm [OD660] of ≈2). For strain 8826(pMEC46), induction was performed by adding 20 ng of nisin (Sigma, St. Louis, Mo.) per ml 1 h after dilution and further incubation at 37°C for 5 h (24). The cells were then washed twice with sterile phosphate-buffered saline (PBS) and adjusted to 1011 CFU per ml. For chemical inactivation of bacteria, suspensions of 5 × 109 CFU per ml in MRS medium were treated with 200 μg of mitomycin C (MitC; Sigma) per ml for 90 mn at 37°C. The treated cells were washed twice in sterile PBS and resuspended at 1011 CFU per ml for immunization. An aliquot of serial dilutions was plated on selective MRS agar in order to calculate the efficiency of the treatment.
Heat-killed bacteria were prepared for T-cell in vitro stimulation by heating a cell suspension of wild-type L. plantarum NCIMB8826 (109 CFU per ml in PBS) for 1 h at 70°C. The absence of viable strains was checked by plating on MRS agar.
Immunoblotting assay.
Ten microliters (109 CFU) of each inoculum was resuspended in 1 ml of 10 mM Tris (pH 8) buffer containing lysozyme (1 mg/ml; Sigma). After 30 min of incubation at 37°C, the cells were washed once in 10 mM Tris-HCl (pH 8) and lysed by addition of 100 μl of lysis buffer (10% glycerol, 2% Sodium dodecyl sulfate, 375 mM Tris-HCl [pH 7.6]). An aliquot (10 μl) of each cell extract was submitted to sodium dodecyl sulfate-polyacrylamide gel electrophoresis in a 10% gel as described by Laemmli (12). After transfer to nitrocellulose membranes by electroblotting, the specific proteins were detected with a rabbit anti-TTFC polyclonal serum (kindly provided by Innogenetics N.V., Ghent, Belgium) and revealed by alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G (IgG; Promega, Madison, Wis.).
Immunizations.
Eight-week-old female C57BL/6 N Crl BR mice (Charles River Laboratories, St. Aubin-les-Elbeuf, France) were immunized intranasally. Initially, groups of eight mice were anesthetized by intraperitoneal injection of 200 μl of a 5% sodium pentobarbital solution (Sanofi, Libourne, France) or 150 μl of a cocktail (as described in reference 2) containing 20% Imalgene 1000 (Merial, Lyon, France), 0.5 mg of Valium (Roche, Neuilly-sur-Seine, France), and 62.5 μg of atropine (Aguettant Laboratory, Lyon, France) per ml. Ten microliters (109 CFU) of the bacterial suspension was then instilled into one nostril of each mouse. Control groups received 10 μl of buffer alone (PBS) or 10 μg of purified recombinant TTFC (Boehringer Mannheim, Mannheim, Germany) mixed with 1 μg of cholera toxin B subunit (CTB) as the mucosal adjuvant (Sigma). Antigens were given at 3-week intervals by one (single dose) or two consecutive (double dose at 24-h interval) intranasal administrations. Ten days after each administration (priming and two boosts), serum samples were collected and stored at −20°C until use. Bronchoalveolar lavage fluids (BALF) were obtained 10 days after the final boost by three consecutive lavages of the lungs with a total volume of 400 μl of PBS containing complete protease inhibitor cocktail (Boehringer Mannheim). After 10 min of centrifugation at 4,000 × g (at 4°C), the supernatants were collected and stored at −20°C until analysis.
ELISA for detection of antigen-specific serum or mucosal antibody responses.
Purified antigen (TTFC; Boehringer Mannheim) was coated overnight at 4°C on enzyme-linked immunosorbent assay (ELISA) plates (Immulon I; Dynatech, McLean, Va.) at 200 ng per 100 μl in 0.1 M carbonate-bicarbonate buffer (pH 9.5). After 1 h of saturation with PBS containing 3% bovine serum albumin (BSA; Sigma), samples were tested using twofold serial dilutions from a 1:50 dilution (for primary antisera) or a 1:2 dilution (for BALF) in PBS containing 1% BSA. After overnight incubation at 4°C, the plates were washed three times in PBS containing 0.1% Tween, and secondary anti-mouse biotinylated conjugate was applied in PBS containing 1% BSA and 0.1% Tween 20 at a 1:20,000 or 1:2,000 dilution for anti-mouse IgG (and IgG subtypes) or anti-mouse IgA (Southern Biotechnology Associates, Inc., Birmingham, Ala.), respectively. After 1 h of incubation at room temperature, horseradish peroxidase-conjugated streptavidine (Amersham, Buckinghamshire, United Kingdom) was applied at a 1:2,000 dilution in PBS containing 0.1% Tween for 30 min. After intensive washes (eight times), 1 mg of o-phenylenediamine substrate (Sigma) per ml in 0.2 M Na2HPO4–0.1 M citrate buffer (pH 5.5) containing 0.2% H2O2 was added and incubated for 30 min at 37°C. The reaction was stopped by addition of 50 μl of 2 N HCl, and the OD490 was measured with an Elx800GUV automated microplate reader (Bio-Tek Instruments, Inc., Vinooski, Vt.). Endpoint titers were calculated as the reciprocal of the dilution producing the same OD490 as 3 times the background, using the KC4 program (Kineticalc for Windows; Bio-Tek Instruments).
As the concentration of total IgA in BALF is not constant, the amount of TTFC-specific IgA was normalized to the total IgA concentration in each sample. Total IgA levels were determined by ELISA, as described above, with microplates coated with 100 μl of anti-mouse IgA α chain specific (Sigma) at 5 μg per ml. The concentration of each sample was calculated from a standard curve of mouse myeloma IgAk (Sigma) with twofold serial dilutions from 0.1 μg per ml. Endpoint titers were calculated as described above for IgG. Results are expressed as specific activity calculated by dividing the endpoint titer by the level of total IgA concentration for each serum.
Statistical analysis.
The results are expressed as the mean ± standard error of the mean (SEM). Statistical significance was evaluated by Mann-Whitney U test. Differences were considered significant at P < 0.05.
TTFC-specific T-cell response.
Cervical lymph nodes (CLN) were aseptically removed (10 days after the last boost) and pooled for each group of animals; single-cell suspensions were prepared by mechanical dissociation using a homogenizer tube and passage through a nylon cell strainer (Becton Dickinson, Franklin Lakes, N.J.). After two washes, the cells were resuspended in complete medium RPMI 1640 (Gibco-BRL, Paisley, Scotland) containing 10% fetal calf serum (Boehringer Mannheim), 2 mM glutamine, 8 μg of gentamicin/ml, and 0.05 mM 2-mercaptoethanol. The cells were cultured at 37°C at a density of 2.5 × 106 cells per ml, in the presence or absence of purified TTFC (different final concentrations) or heat-killed L. plantarum (107 CFU/ml), for 4 days. To measure antigen-specific T-cell proliferative responses, [methyl-3H]thymidine (2.5 μCi/ml) was added to the culture 16 h before harvesting. The stimulation index (experimental/control) was determined in duplicate cultures.
Direct protection assay: TT challenge of mice.
Immunized and control mice were challenged 13 days after the last boost (day 54) by subcutaneous inoculation of a standard challenge solution of TT. The number of 50% lethal doses (LD50) administered in the experiment was concomitantly determined by injection of dilutions of the challenge solution in three groups of three age-matched naive mice and by calculation of the LD50 by the method of Reed and Muench (27). The animals were observed each day. Mice showing no signs of paralysis 96 h after the challenge were considered fully protected; those surviving with signs were considered partially protected. Unprotected mice died at the latest on the second day after the challenge.
Indirect protection assay: determination of TT neutralizing antibodies in mouse sera.
The toxin neutralization test was performed on the pooled sera of each group of immunized and control mice according to the specifications of the European Pharmacopoeia, with slight modifications. In brief, mixtures containing serial twofold dilutions of a pool and a definite amount of TT (L+/4,000 level) were injected subcutaneously in two OF1 mice (obtained from the animal facility, Institut Pasteur de Bruxelles) per mixture. A series of dilutions of a standard antitoxin solution mixed with the same toxin amount was included in each experiment. The neutralizing antibody content is expressed, in international units (IU) per milliliter, as a range that includes the actual value. Due to the small volumes of sera that were available, the detection limit was 0.0025 IU per ml of pooled sera. The protective level of tetanus antitoxin is usually considered to be 0.01 IU/ml (16).
RESULTS
TTFC production by recombinant strains.
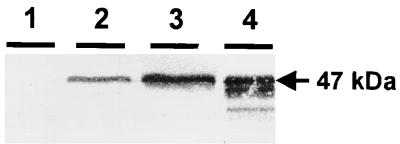
To estimate the TTFC amount contained in the different inocula, cell extracts corresponding to 1/10 of each bacterial dose (108 CFU) were analyzed by immunoblotting. As shown in Fig. 1, a specific signal at the expected molecular mass (47 kDa) was detected for both types of TTFC-producing strains. TTFC production was notably higher in strain 8826(pMEC46) (inducible pnisA) than in 8826(pMEC4) (constitutive pldh). No specific signal was detected from the control strain cell extracts [8826(pTG2247)]. These results confirmed previous analysis (24; Chagnaud et al., unpublished) and were reproducible for each preparation.
FIG. 1.
Immunoblot analysis of recombinant L. plantarum cell extracts (equivalent to 108 CFU) of 8826(pTG2247) (control strain) (lane 1), 8826(pMEC4) (lane 2), 8826(pMEC46) (lane 3), and of 50 ng of purified TTFC (lane 4).
Role of anesthesia procedure.
Two different anesthetic protocols were evaluated. When mice were anesthetized with pentobarbital, not only they were not completely relaxed and therefore sneezed (loss of material), but a large part of the inoculum was swallowed. This was checked (data not shown) by an instillation assay of bacteria mixed with trypan blue, followed by a postmortem examination which showed that most of the inoculum was located in the stomach (blue colored), with only a small portion reaching the lung (data not shown). Similar experiments performed with the anesthetic cocktail (2) showed that all of the inoculum reached the lungs and bronchi and that no liquid was swallowed (absence of blue color in the stomach). We verified that there were larger variations in the individual immune responses and less reproducible levels of anti-TTFC serum IgG with the pentobarbital treatment than with the anesthetic cocktail (results not shown). Therefore, the latter protocol was used in all subsequent immunization experiments.
Induction of serum IgG antibody responses.
Two protocols differing in the number of bacterial doses received at each administration were compared in the same experiment. In the first, mice received a single dose of live recombinant L. plantarum strains; in the second, they were immunized on 2 consecutive days for each administration. In each case, two boosts were performed after the priming.
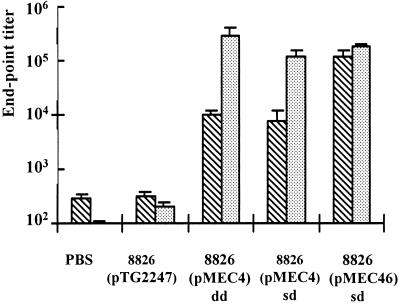
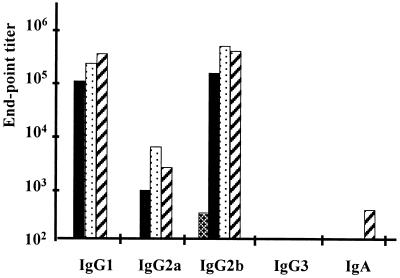
First, the two expression systems leading to low [8826(pMEC4)] or high [induced 8826(pMEC46)] doses of cytoplasmic TTFC were compared to each other. As shown in Fig. 2, immunization with a single dose of L. plantarum 8826(pMEC46) resulted in a very high ELISA anti-TTFC serum IgG response, which was significantly (P < 0.05) higher after the first boost than the IgG levels induced with single dose of L. plantarum 8826(pMEC4), IgG endpoint titers reaching 122,902 ± 36,025 and 7,957 ± 3,847, respectively. Nevertheless, after two boosts, both live vectors were able to elicit very high and significant (P < 0.05) responses (serum IgG titers of >105) in comparison with the nonexpressing control strain [8826(pTG2247)] or buffer alone. With the double-dose protocol, the response obtained with the 8826(pMEC4) strain was significantly higher than with a single-dose protocol (P < 0.05), leading to endpoint titers of 297,953 ± 102,190 and 118,554 ± 30,506, respectively, after the second boost. Isotypes of the anti-TTFC antibodies induced by each immunization scheme were principally IgG1 and IgG2b and to a lesser extent IgG2a, suggesting a mixed Th1-Th2 response (Fig. 3). While no IgG3 could be detected, a low level of IgA was observed in sera of mice immunized with 8826(pMEC4) following a double-dose protocol.
FIG. 2.
Anti-TTFC serum IgG titers following intranasal
immunization with recombinant L. plantarum NCIMB8826.
Individual sera were collected 10 days after the first
( ) or
second
(
) or
second
( ) boost
from groups of eight mice intranasally immunized with buffer alone
(PBS), with 109 CFU of control nonexpressing strain
8826(pTG2247), or with 109 CFU of TTFC-producing strain
8826(pMEC4) (constitutive promoter) or 8826(pMEC46) (inducible
promoter) in a single dose (sd) or double dose (dd) at each
administration. Bars represent the mean ELISA IgG titer ± SEM in
each group.
) boost
from groups of eight mice intranasally immunized with buffer alone
(PBS), with 109 CFU of control nonexpressing strain
8826(pTG2247), or with 109 CFU of TTFC-producing strain
8826(pMEC4) (constitutive promoter) or 8826(pMEC46) (inducible
promoter) in a single dose (sd) or double dose (dd) at each
administration. Bars represent the mean ELISA IgG titer ± SEM in
each group.
FIG. 3.
Serum anti-TTFC antibody isotype titers after intranasal
immunization with a single dose (109 CFU) of 8826(pTG2247)
(▩), 8826(pMEC4) (■), or 8826(pMEC46)
( ) or a
double dose (109 CFU twice at 24 h interval) of
8826(pMEC4)
(
) or a
double dose (109 CFU twice at 24 h interval) of
8826(pMEC4)
( ).
Isotypic responses were analyzed by ELISA on pooled sera of each group
collected 10 days after the last boost.
).
Isotypic responses were analyzed by ELISA on pooled sera of each group
collected 10 days after the last boost.
Induction of local antibody and cellular responses.
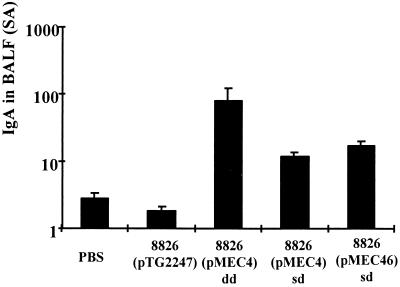
The mucosal IgA response in BALF was determined 10 days after the second boost. Nasal administration of the recombinant strains led to a significant (P < 0.05) anti-TTFC IgA response in BALF in comparison with the control strain [8826(pTG2247)] or buffer alone (Fig. 4). In the single-dose protocol, the level of the response was not significantly different (P > 0.05) between 8826(pMEC4) and 8826(pMEC46). Nevertheless, local IgA levels were significantly higher (P < 0.05) in mice that received a double dose of the 8826(pMEC4) strain.
FIG. 4.
Anti-TTFC IgA levels in BALF from groups of eight mice immunized intranasally with a single dose (sd) or double dose (dd) of nonexpressing recombinant L. plantarum 8826(pTG2247) or TTFC-producing L. plantarum 8826 (pMEC4) (constitutive promoter, low TTFC dose) or 8826(pMEC46) (inducible promoter, high TTFC dose) or with buffer alone (PBS) as a control. Individual BALF samples were collected 10 days after the last boost, and specific IgA response was normalized according to the level of total IgA and expressed in specific activity (SA). Bars represent the mean IgA level ± SEM in each group.
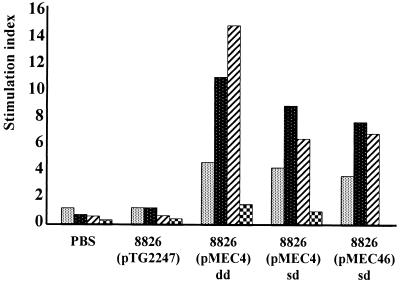
T cells from CLN of mice immunized with the recombinant strains, when restimulated in vitro with increasing doses of TTFC, elicited clear specific dose-dependent proliferative responses. The levels of these proliferative responses were similar for the two recombinant strains [stimulation indices of 7.70 and 8.93 for 8826(pMEC4) and 8826(pMEC46), respectively] but was higher when mice received two successive doses of the 8826(pMEC4) strain (SI of 14.82). No proliferative response was elicited against the vector itself upon in vitro restimulation with 107 CFU of heat-killed wild-type L. plantarum per ml (Fig. 5).
FIG. 5.
Antigen-specific T-cell proliferative responses in CLN
of mice immunized intranasally with PBS or with a single dose (sd) or
double dose (dd) of 8826(pTG2247), 8826(pMEC4), or 8826(pMEC46). T
cells were isolated from CLN 10 days after the second boost, pooled by
group of mice, and cultured for 4 days at a density of 5 ×
106 cells/ml in the presence or absence of purified TTFC at
5( ),
10(
),
10( ), or
50(
), or
50( )
μg/ml or in the presence of heat-killed L. plantarum
(107 CFU/ml)
(
)
μg/ml or in the presence of heat-killed L. plantarum
(107 CFU/ml)
( ).
).
TT neutralization capacity of serum antibodies.
Because mice were sacrificed for local and cellular response analysis, the protective effect of the circulating antibodies was assessed by an indirect method, i.e., the ability of elicited antibodies to neutralize TT and to protect naive mice against its lethal effect. As shown in Table 1, all recombinant strains elicited detectable neutralizing antitoxin antibodies, but only for L. plantarum 8826(pMEC46) was the level higher than the protective limit (>0.01 IU/ml) and obtained as early as the first boost. In this case, a neutralizing titer 10 times higher than the protective limit was measured after the second boost (0.08 to 0.16 IU/ml).
TABLE 1.
TT neutralizing activity in serum antibodies elicited by recombinant L. plantarum strains
| Immunization protocola | Neutralizing antibody
level (IU/ml)b
|
|
|---|---|---|
| 1st boost | 2nd boost | |
| PBS | ND | <0.0025 |
| 8826(pTG2247), sd | ND | <0.0025 |
| 8826(pMEC4), sd | <0.0025 | 0.0025–0.005 |
| 8826(pMEC4), dd | <0.0025 | 0.005–0.01 |
| 8826(pMEC46), sd | 0.04–0.08 | 0.08–0.16 |
sd, single-dose protocol; dd, double-dose protocol. ND, not determined.
Sera from intranasally immunized mice were collected and pooled 10 days after the first and second boosts and used in the TT neutralization assay; protection against tetanus requires a minimum neutralizing antibody titer of 0.01 IU/ml.
Responses elicited by live or MitC-treated recombinant L. plantarum strains.
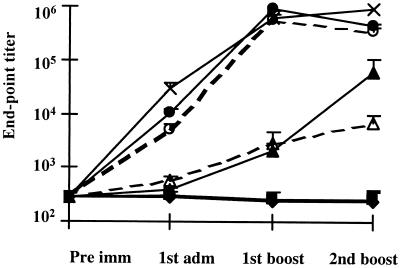
We further studied the effect of bacterial viability on the immune response after intranasal immunization. Both recombinant L. plantarum 8826(pMEC4) and 8826(pMEC46) were administered live (109 CFU) or after inactivation with MitC treatment. More than fourfold loss of viability was obtained after MitC treatment, the administration of 109 total cells thus corresponding only to 0.5 × 105 viable cells (CFU). No difference in antigen level production was observed after inactivation, as analyzed by Western blotting analysis (data not shown). As shown in Fig. 6, chemically inactivated recombinant L. plantarum elicited systemic IgG response, as measured by ELISA, in the same magnitude as that induced with live vectors. The difference between the response elicited by inactivated and live 8826(pMEC4) was not significant (P > 0.05) except after the second boost, when the live vector was 10 times more immunogenic. For 8826(pMEC46), no significant differences were found between live and inactivated strains (P > 0.05). The levels of IgG antibody response were very similar to those obtained in the previous experiments with both vectors. Strain 8826(pMEC46) induced a higher systemic IgG response (P < 0.05) than strain 8826(pMEC4), (43,6014 ± 33,928 and 61,595 ± 48,162, respectively) after the second boost. The level of the response induced by strain 8826(pMEC46) was not significantly (P > 0.05) different from that elicited by intranasal administration of 10 μg of purified TTFC in the presence of 1 μg of (CTB) as the mucosal adjuvant (Fig. 6).
FIG. 6.
Anti-TTFC serum IgG titers following intranasal
immunization with recombinant L. plantarum NCIMB8826.
Individual sera were collected 10 days after the priming and the first
and second boosts from eight mice immunized intranasally with PBS
(—⧫—) with 109 CFU of live 8826 (pTG2247)
( ), live
(
), live
( ) or
MitC-treated
(
) or
MitC-treated
( )
8826(pMEC4) or live
(
)
8826(pMEC4) or live
( ) or
MitC-treated (--○--) 8826(pMEC46), or with 10 μg of purified TTFC
in the presence of 1 μg of CTB (—×—). Bars represent the mean
ELISA IgG level ± SEM in each group.
) or
MitC-treated (--○--) 8826(pMEC46), or with 10 μg of purified TTFC
in the presence of 1 μg of CTB (—×—). Bars represent the mean
ELISA IgG level ± SEM in each group.
In this experiment, the protection induced by recombinant L. plantarum immunization was evaluated both by a direct lethal challenge with TT (17 LD50) and by measuring the levels of neutralizing antibodies for comparison. As shown in Table 2, only those mice immunized with live and MitC-inactivated L. plantarum 8826(pMEC46) and with purified TTFC adjuvanted with CTB were fully protected (except for one mouse vaccinated with inactivated bacteria that was only partially protected). The few surviving mice immunized with live or MitC-inactivated 8826(pMEC4) were only partially protected (two of eight and one of seven, respectively). Moreover, when the protection was indirectly evaluated by the in vivo neutralization test, both live and inactivated 8826(pMEC46) organisms could induce neutralizing titers higher than the protective limit (>0.01 IU/ml); nevertheless, after the second boost, the titer of the pooled sera was 10-fold higher with live vector (0.16 to 0.32 IU/ml) than with the MitC-treated strain (0.02 to 0.04 IU/ml). The highest neutralizing antibody titers were obtained with a mixture of purified TTFC (10 μg) and CTB. As the ELISA IgG antibody titers were quite similar for the three protected groups, we further analyzed the isotypic response. No differences were observed between live and inactivated recombinant strains, for which the same high IgG1 and IgG2b and moderate IgG2a antibody responses were obtained (data not shown). No qualitative difference was obtained for the group of mice immunized with purified TTFC in the presence of CTB; here also the isotypic response was dominated by a high IgG1 and IgG2b response whereas the IgG2a response was limited (data not shown).
TABLE 2.
Protection of mice against TTa
| Immunization protocol | No. of survivors/ no. of challenged mice | Neutralizing tetanus antibody levels in pooled sera
(IU/ml)
|
|
|---|---|---|---|
| 1st boost | 2nd boost | ||
| Live 8826(pTG2247) | 0/8 | NDe | <0.0025 |
| Live 8826(pMEC4) | 2b/8 | ND | <0.0025 |
| MitC-treated 8826(pMEC4) | 1c/7 | ND | <0.0025 |
| Live 8826(pMEC46) | 7/7 | 0.04–0.08 | 0.16–0.32 |
| MitC-treated 8826(pMEC46) | 8/8d | 0.005–0.01 | 0.02–0.04 |
| TTFC + CTB | 7/7 | 0.64–1.28 | 2.56–5.12 |
Mice were immunized intranasally with a single dose (109 CFU) of live or MitC-treated recombinant L. plantarum strains or with 10 μg of purified TTFC mixed with 1 μg of CTB on days 0, 21, and 42 before being challenged subcutaneously by a lethal dose of TT (17 LD50) on day 54. Animals developing no signs of paralysis after 4 days were considered fully protected. Neutralization tests for protective antibodies were performed on pools of sera collected after the first boost (day 31) and the second boost (day 51).
Mice only partially protected, surviving with mild or moderate signs of tetanus.
mouse surviving with severe signs of tetanus.
one of the mice was only partially protected (mild signs of tetanus).
ND, not determined.
DISCUSSION
The main goal of this investigation was to ascertain whether mucosal immunization with recombinant lactobacilli would stimulate systemic and local antibody responses and exhibit a protective ability. To this end, intranasal administration was explored using as a model antigen the 47-kDa TTFC produced intracellularly by recombinant L. plantarum NCIMB8826 strains. Two main parameters were examined: effect of antigen dose, and viability of the vaccine vector. Prior to conducting these studies, we adjusted the anesthetic protocol to ensure minimal individual variation and high reproducibility of the immune responses induced after intranasal immunization. The procedure described in this report fulfilled these needs better than the pentobarbital treatment.
In this study, we compare the immunogenicity of isogenic antigen-producing strains where the heterologous gene was cloned under the control of one of two different promoters (constitutive or inducible), leading to doses of antigen that were estimated to vary from 1 to 10% of the intracellular protein content. Even if after one boost the strain producing a high amount of TTFC (single-dose protocol) was more immunogenic than the strain synthesizing less antigen (single or double-dose protocol), the levels of circulating IgG after the second boost were very high and quite similar for both constructions when measured by ELISA. The endpoint titers reached levels above 105. The protective response against TT correlated to the level of antigen production, indicating that immunization with higher amounts of antigen could have induced IgG with higher avidity. Indeed, induction of a sufficient amount (>0.01 IU/ml) of antibodies to neutralize TT in vivo, i.e., high-avidity circulating IgG directed against protective epitopes, is a prerequisite for protection against tetanus. The neutralization test used in this work measures such antibodies. On the contrary, sandwich ELISA takes into account antibodies directed against both protective and nonprotective epitopes, whatever their avidity. The discrepancy between the results obtained with these methods is well established, especially at low neutralizing antibody concentrations and in the early phases of primary immunization. This problem can generally be overcome by using competition or inhibition variants of the ELISA (6, 32). However, sandwich ELISA remains widely used in studies using TTFC as a model antigen for evaluation of new vaccine strategies, as it is rapid and easy to perform, relies on readily available reagents, and, interestingly, needs very low serum volumes. Our results emphasize once more that protection cannot be deduced solely from titers of circulating anti-TTFC IgG, as determined by sandwich ELISA, and that it is necessary to apply methods able to reflect the neutralizing potency of elicited antibodies when evaluating the potential of the delivery system under study. In the case of tetanus, these methods include in vivo challenge with TT, in vivo neutralization test, or in vitro assays using appropriate standards and validation by comparison with neutralization test results.
Different mucosal presentations of antigen have been recently designed for protection against TT, including oral or intranasal administrations of TTFC with cholera toxin (5, 9) or recombinant live vectors expressing TTFC, e.g., Salmonella (1, 3, 34). Recent work on protection against TT with DNA-based vaccines (30) has shown that they induce preferentially cytotoxic T-cell responses dominated by a T helper type 1 propensity and a lower antibody response and then are less efficient than a polypeptide subunit vaccine which induces a protective Th2 response. Mucosal administration (intranasal or oral) able to initiate a protective antibody response should thus be advantageous for vaccination against tetanus with respect to safety, low cost, and ease of administration (4).
In the case of lactobacilli, the isotypic response obtained after intranasal administration of recombinant strains showed a high IgG2b and IgG1 response associated with a moderate level of IgG2a response. This suggests that recombinant Lactobacillus strains are able to activate both Th1 and Th2 T helper cell subsets. Similar results have been obtained after intranasal and intragastric immunization of mice with recombinant L. lactis producing TTFC, which also conferred a protective immune response (22, 28). In the case of L. plantarum, immune responses against the vector itself were not detectable (i.e., absence of proliferative responses in cervical lymph nodes after in vitro restimulation with heat-inactivated bacteria), while for L. lactis low serum antibody responses directed against the bacteria were detected (28). This contrasts with the Th1 (IgG2a isotype) profile elicited by oral administration of recombinant Salmonella as well as the higher innate antigenicity of other recombinant bacterial vectors, e.g., Mycobacterium bovis BCG, Bordetella, and Salmonella (3, 10, 19). The nature of the immune response elicited by a given bacterial vector with respect to Th1 versus Th2 and humoral versus cellular immunity can differ considerably and should thus be taken into account during selection of the bacterial vector to be used.
Shaw et al, (31) recently described successful mucosal immunization with recombinant lactobacilli expressing TTFC intracellularly or at the cell surface, which were able to induce systemic IgG responses reaching endpoint titers of 104 or 102 after intranasal or intragastric administration, respectively, to mice. However, protection was not evaluated in this study. Local IgA responses were detectable after priming and boosting with three successive bacterial doses (days 1 to 3). With the system we describe, significant local IgA responses were measured in BALF, and antigen-specific T-cell proliferative responses were elicited by both types of recombinant lactobacilli [8826(pMEC4) and 8826(pMEC46)]. The levels were higher when mice received two consecutive doses at each administration, but responses were readily detectable after a single dose. Our results support the hypothesis (31) that the absolute levels of TTFC produced by the lactobacilli is probably a key factor in their immunogenicity, as illustrated by the IgG titers induced by the isogenic pair of L. plantarum strains producing low [8826(pMEC4)] or high [8826(pMEC46)] amounts of the same antigen.
In addition, we evaluated the impact of the viability of the bacterial vector on the induction of protective immune responses. Comparable serum IgG responses (as measured by ELISA) were obtained with live and inactivated recombinant lactobacilli, as also observed for TTFC-producing L. lactis strains administered by the nasal route (28). In the latter case, this effect was not unexpected, as this nonpersisting bacterium might be considered a live antigen-loaded particle. However, no comparison of protection was conducted with live and dead L. lactis (28). In our case the level of protective response was 10-fold higher with the live 8826(pMEC46) strain. Possibly different cytokine induction patterns were initiated by live and MitC-treated recombinant bacteria. It was recently reported that in vitro adhesion of probiotic strains to intestinal cell lines was affected differently depending on the bacterial killing or inactivation procedure (23). If applicable to the in vivo situation, this observation may, at least partly, explain why live and dead lactobacilli differ in their immunogenicity or immunomodulation capacity. Nevertheless, no differences in isotypic response were observed after administration of live and inactivated Lactobacillus vectors.
In conclusion, the results obtained so far (reference 31 and this study) demonstrate that lactobacilli are capable of delivering antigen to the mucosal and systemic immune systems following intranasal immunization. The development of lactic acid bacteria as vaccine delivery vehicles involves the use of various strains which are able to colonize different mucosal sites such as the gastrointestinal, urinary, and genital tracts and could thus be used specifically to vaccinate against pathogens at their site of entry. While both gut colonizers and noncolonizers seem to work equally well by the systemic and nasal routes, the importance of colonization or adhesion in oral administration remains an open question. We therefore plan to examine the potency of our recombinant L. plantarum strains as oral vaccines, with a special focus on the effect of persistence time in the gastrointestinal tract.
ACKNOWLEDGMENTS
This work was supported by EU grant BIO4-CT96-0542 and funds from the Institut Pasteur de Lille, Institut Pasteur de Bruxelles, and FEDER.
We are grateful to E. Van Nerom and F. Tweepenninckx for skillful help with TT protection experiments and to D. Guittard for help with graphic design. We thank J. Alouf, J. Content, and C. Locht for critical reading of the manuscript and E. Dei Cas for advice on the anesthetic protocol. We very much appreciated the stimulating discussions with J. Delcour, P. Hols, C. Rush, and J. M. Wells. Rabbit anti-TTFC antibodies were kindly supplied by E. Sablon, Innogenetics N. V., Ghent, Belgium.
REFERENCES
- 1.Chatfield S N, Charles I G, Makoff A J, Oxer M D, Dougan G, Pickard D, Slater D, Fairweather F. Use of the nirB promoter to direct the stable expression of heterologous antigens in Salmonellaoral vaccine strains: development of a single-dose oral tetanus toxin vaccine. Biotechnology. 1992;10:888–892. doi: 10.1038/nbt0892-888. [DOI] [PubMed] [Google Scholar]
- 2.Dei-Cas E, Brun-Pascaud M, Bille-Hansen V, Allaert A, Aliouat E. Animal models of pneumocystosis. FEMS Immunol Med Microbiol. 1998;22:163–168. doi: 10.1111/j.1574-695X.1998.tb01201.x. [DOI] [PubMed] [Google Scholar]
- 3.Dunstan S J, Simmons C P, Strugnell R A. Comparison of the abilities of different attenuated Salmonella typhimuriumstrains to elicit humoral immune responses against a heterologous antigen. Behring Inst Mitt. 1998;98:732–740. doi: 10.1128/iai.66.2.732-740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox J. Taking multiple paths towards mucosal vaccines immunity. ASM News. 1997;63:413–414. [Google Scholar]
- 5.Harokopakis E, Hajishengallis G, Greenway T E, Russell M W, Michalek S M. Mucosal immunogenicity of a recombinant Salmonella typhimurium-cloned heterologous antigen in the absence or presence of coexpressed cholera toxin A2 and B subunits. Infect Immun. 1997;65:1445–1454. doi: 10.1128/iai.65.4.1445-1454.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendricksen C F M, van den Gun J W, Nagel J, Kreeftenberg J G. The toxin binding inhibition test as a reliable in vitro alternative to the toxin neutralisation test in mice for the estimation of tetanus antitoxin in human sera. J Biol Stand. 1988;16:287–297. doi: 10.1016/0092-1157(88)90017-0. [DOI] [PubMed] [Google Scholar]
- 7.Hols P, Slos P, Dutot P, Reymund J, Chabot P, Delplace B, Delcour J, Mercenier A. Efficient secretion of the model antigen M6-gp41E in Lactobacillus plantarumNCIMB 8826. Microbiology. 1997;143:2733–2741. doi: 10.1099/00221287-143-8-2733. [DOI] [PubMed] [Google Scholar]
- 8.Holzapfel W H, Haberer P, Snel J, Schillinger U, Huis in't Veld J H J. Overview of gut flora and probiotics. Int J Food Microbiol. 1998;41:85–101. doi: 10.1016/s0168-1605(98)00044-0. [DOI] [PubMed] [Google Scholar]
- 9.Isaka M, Yasuda Y, Kozuka S, Miura Y, Taniguchi T, Matano K, Goto N, Tochikubo K. Systemic and mucosal immune responses of mice to aluminium-adsorbed or aluminium-non-adsorbed tetanus toxoid administered intranasally with recombinant cholera toxin B subunit. Vaccine. 1998;16:1620–1626. doi: 10.1016/s0264-410x(98)00066-8. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs W R, Snapper S B, Lugosi L, Bloom B R. Development of BCG as recombinant vaccine delivery vehicle. Curr Top Microbiol Immunol. 1990;155:153–160. doi: 10.1007/978-3-642-74983-4_11. [DOI] [PubMed] [Google Scholar]
- 11.Kremer L, Dupré L, Riveau G, Capron A, Locht C. Systemic and mucosal immune responses after intranasal administration of recombinant Mycobacterium bovis bacillus Calmette-Guérin expressing gluthathione S-transferase from Schistosoma haematobium. Infect Immun. 1998;66:5669–5676. doi: 10.1128/iai.66.12.5669-5676.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laemmli U K. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 13.Maassen C B M, Van Holten J C A M, Balk F, Heijne den Bak-Glashouwer M-J, Leer R, Laman J D, Boersma W J A, Claassen E. Orally administrated Lactobacillusstrains differentially affect the direction and efficacity of the immune response. Vet Q. 1998;20:S81–S83. [PubMed] [Google Scholar]
- 14.Marteau P, Rambaud J C. Potential of using lactic acid bacteria for therapy and immunomodulation in man. FEMS Microbiol Rev. 1993;12:207–220. doi: 10.1111/j.1574-6976.1993.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 15.Matsuzaki T. Immunomodulation by treatment with Lactobacillus casei strain Shirota. Int J Food Microbiol. 1998;41:133–140. doi: 10.1016/s0168-1605(98)00046-4. [DOI] [PubMed] [Google Scholar]
- 16.McComb J A. The prophylactic dose of homologous tetanus antitoxin. N Engl J Med. 1964;270:175–178. doi: 10.1056/NEJM196401232700404. [DOI] [PubMed] [Google Scholar]
- 17.Medaglini D, Rush C M, Sestini P, Pozzi G. Commensal bacteria as vectors for mucosal vaccines against sexually transmitted diseases: vaginal colonization with recombinant streptococci induces local and systemic antibodies in mice. Vaccine. 1997;15:1330–1337. doi: 10.1016/s0264-410x(97)00026-1. [DOI] [PubMed] [Google Scholar]
- 18.Mercenier A, Dutot P, Kleinpeter P, Aguirre M, Paris P, Reymund J, Slos P. Development of lactic acid bacteria as live vectors for oral or local vaccines. Adv Food Sci. 1996;18:73–77. [Google Scholar]
- 19.Mielcarek N, Cornette J, Schacht A M, Pierce R J, Locht C, Capron A, Riveau G. Intranasal priming with recombinant Bordetella pertussisfor the induction of a systemic immune response against a heterologous antigen. Infect Immun. 1997;65:544–550. doi: 10.1128/iai.65.2.544-550.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miettinen M, Vuopio-Varkila J, Varkila K. Production of human tumor necrosis factor alpha, interleukin-6, and interleukin-10 is induced by lactic acid bacteria. Infect Immun. 1996;64:5403–5405. doi: 10.1128/iai.64.12.5403-5405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müller-Alouf H, Grangette C, Goudercourt D, Reveneau N, Mercenier A. Comparative cytokine inducing pattern of lactic acid bacteria used for mucosal vaccine development. Immunol Lett. 1999;69:33. [Google Scholar]
- 22.Norton P M, Wells J M, Brown H W G, Macpherson A M, Le Page R W F. Protection against tetanus toxin in mice nasally immunized with recombinant Lactococcus lactisexpressing tetanus toxin fragment C. Vaccine. 1997;15:616–619. doi: 10.1016/s0264-410x(96)00241-1. [DOI] [PubMed] [Google Scholar]
- 23.Ouwehand A C, Tölkkö S, Kulmala J, Salminen S, Salminen E. Adhesion of inactivated probiotic strains to intestinal mucus. Lett Appl Microbiol. 2000;31:82–86. doi: 10.1046/j.1472-765x.2000.00773.x. [DOI] [PubMed] [Google Scholar]
- 24.Pavan S, Hols P, Delcour J, Geoffroy M C, Grangette C, Kleerebezem M, Mercenier A. Adaptation of the nisin-controlled expression system in Lactobacillus plantarum: a tool to study in vivo biological effects. Appl Environ Microbiol. 2000;66:4427–4432. doi: 10.1128/aem.66.10.4427-4432.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poulain-Godefroy O, Gaubert S, Lafitte S, Capron A, Grzych J-M. Immunoglobulin A response in murine schistosomiasis: stimulatory role of egg antigens. Infect Immun. 1996;64:763–768. doi: 10.1128/iai.64.3.763-768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pouwels P H, Leer R J, Shaw M, Heijne den Bak-Glashhouver M-J, Tielen F D, Smit E, Martinez B, Jore J, Conway P L. Lactic acid bacteria as antigen delivery vehicles for oral immunization purposes. Int J Food Microbiol. 1998;41:155–167. doi: 10.1016/s0168-1605(98)00048-8. [DOI] [PubMed] [Google Scholar]
- 27.Reed L J, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 28.Robinson K, Chamberlain L M, Schofield K M, Wells J M, Le Page R W F. Oral vaccination of mice against tetanus with recombinant Lactococcus lactis. Nat Biotechnol. 1997;15:653–657. doi: 10.1038/nbt0797-653. [DOI] [PubMed] [Google Scholar]
- 29.Ryan E T, Butterton J R, Smith R N, Carroll P A, Crean T I, Calderwood S B. Protective immunity against Clostridium difficile toxin A induced by oral immunization with a live, attenuated Vibrio choleraevector strain. Infect Immun. 1997;65:2941–2949. doi: 10.1128/iai.65.7.2941-2949.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saikh K U, Sesno J, Brandler P, Ulrich R G. Are DNA-based vaccines useful for protection against secreted bacterial toxins? Tetanus toxin test case. Vaccine. 1998;16:1029–1038. doi: 10.1016/s0264-410x(97)00280-6. [DOI] [PubMed] [Google Scholar]
- 31.Shaw D M, Gaerthé B, Leer R J, Van Der Stap J G M M, Smittenaar C, Heijne den Bak-Glashouwer M-J, Thole J E R, Tielen F J, Pouwels P H, Havenith C E G. Engineering the microflora to vaccinate the mucosa: serum immunoglobulin G responses and activated draining cervical lymph nodes following mucosal application of tetanus toxin fragment C-expressing lactobacilli. Immunology. 2000;100:510–518. doi: 10.1046/j.1365-2567.2000.00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simonsen O, Schou C, Heron I. Modification of the ELISA for the estimation of tetanus antitoxin in human sera. J Biol Stand. 1987;15:143–157. doi: 10.1016/0092-1157(87)90037-0. [DOI] [PubMed] [Google Scholar]
- 33.Strugnell R, Simmons C, Wijburg O, Uren T, Drew D, Dunstan S. Bacterial vectors: why have they failed to deliver? Australas Biotechnol. 1998;8:89–90. [Google Scholar]
- 34.VanCott J L, Staats H F, Pascual D W, Roberts M, Chatfield S N, Yamamoto M, Coste M, Carter M, Kiyono H, McGhee J R. Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages, and derived cytokines following oral immunization with live recombinant Salmonella. J Immunol. 1996;156:1504–1514. [PubMed] [Google Scholar]
- 35.Wells J M, Robinson K, Chamberlain L M, Schofield K M, Le Page R W F. Lactic acid bacteria as vaccine delivery vehicles. Antonie Leeuwenhoek. 1996;70:317–330. doi: 10.1007/BF00395939. [DOI] [PubMed] [Google Scholar]
- 36.Wells J M, Pozzi G. An overview of gram-positive bacteria as vaccine vehicles for mucosal immunization. In: Pozzi G, Wells J M, editors. Gram positive bacteria as vaccine vehicles for mucosal immunization. Austin, Tex: Biotechnology Intelligence Unit, Landes; 1997. pp. 1–8. [Google Scholar]