Abstract
Iron deficiency (ID) is common in patients with cardiovascular disease. Up to 60% of patients with coronary artery disease, and an even higher proportion of those with heart failure (HF) or pulmonary hypertension have ID; the evidence for cerebrovascular disease, aortic stenosis and atrial fibrillation is less robust. The prevalence of ID increases with the severity of cardiac and renal dysfunction and is probably more common amongst women. Insufficient dietary iron, reduced iron absorption due to increases in hepcidin secondary to the low-grade inflammation associated with atherosclerosis and congestion or reduced gastric acidity, and increased blood loss due to anti-thrombotic therapy or gastro-intestinal or renal disease may all cause ID. For older people in the general population and patients with HF with reduced ejection fraction (HFrEF), both anaemia and ID are associated with a poor prognosis; each may confer independent risk. There is growing evidence that ID is an important therapeutic target for patients with HFrEF, even if they do not have anaemia. Whether this is also true for other HF phenotypes or patients with cardiovascular disease in general is currently unknown. Randomized trials showed that intravenous ferric carboxymaltose improved symptoms, health-related quality of life and exercise capacity and reduced hospitalizations for worsening HF in patients with HFrEF and mildly reduced ejection fraction (<50%). Since ID is easy to treat and is effective for patients with HFrEF, such patients should be investigated for possible ID. This recommendation may extend to other populations in the light of evidence from future trials.
Keywords: Iron deficiency, Anaemia, Heart failure, Coronary artery disease, Pulmonary hypertension, Cerebrovascular disease
Graphical Abstract
Graphical Abstract.
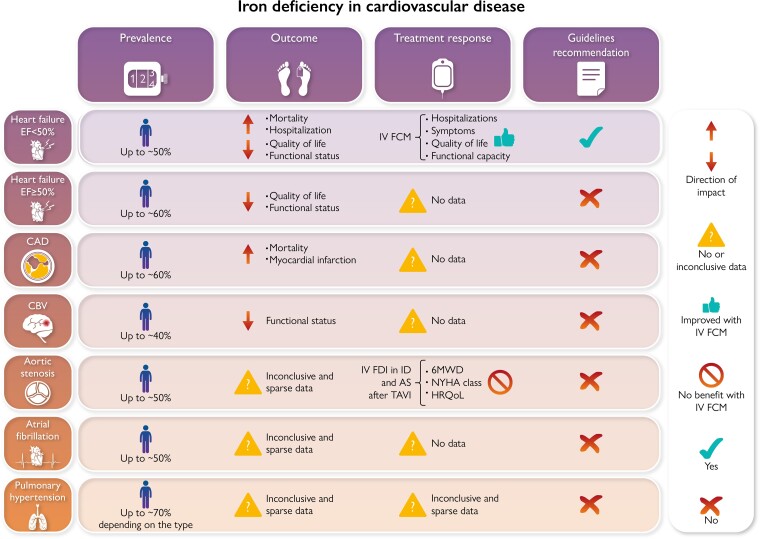
Iron deficiency in cardiovascular disease. CAD, coronary artery disease; CBV, cerebrovascular disease; 6MWD, 6 min walking distance; HRQoL, health-related quality of life; NYHA, New York Heart Association; NT-proBNP, N-terminal pro-B-type natriuretic peptide; RV, right ventricular; FDI, ferric derisomaltose.
Permission information
Permissions for reproducing Figure 1 and 2 have been received.
Figure 1.
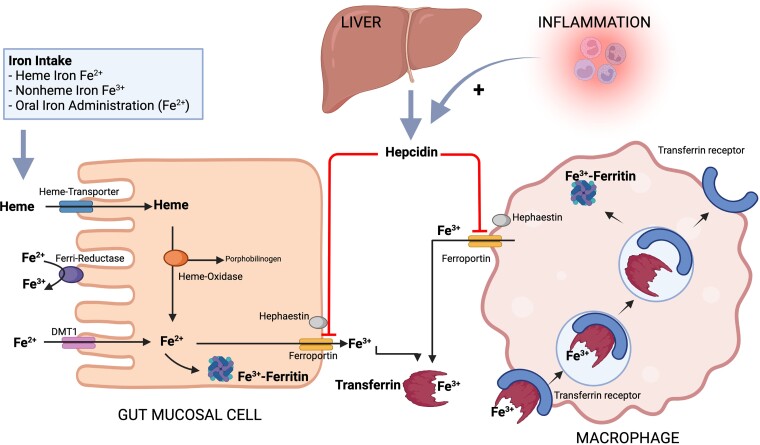
Iron homeostasis. Heme-bound iron is transported from the gut lumen into the mucose cell through the heme carrier protein 1 (HCP1) and here Fe2 + is released by the activity of the heme-oxidase. Additionally, in the gut lumen Fe3 + is converted to Fe2 + by the ferri-reductase or through reduction by dietary ascorbate and then transported into the mucose cell through the divalent metal transporter 1 (DMT1). Fe2 + can be then converted again to Fe3 + and stored as ferritin, or exported to the bloodstream through ferroportin where it is converted to Fe3 + by hephaestin and binds to transferrin whose role is to transport iron where there is metabolic need. Macrophages contribute to iron homeostasis by taking up transferrin-bound iron by receptor-mediated endocytosis. In the macrophages iron can be stored as ferritin and released when needed through ferroportin. Inflammation leads to an increased release of hepcidin from the liver which downregulates ferroportin and therefore the transport of dietary intake from the inside of the mucosa cells in the small intestine to the bloodstream and also the release of recycled iron from macrophages in the spleen and the liver, potentially leading to iron deficiency. Created with BioRender.com Adapted from Nat Rev Cardiol 2015; 12: 659–669.5
Figure 2.
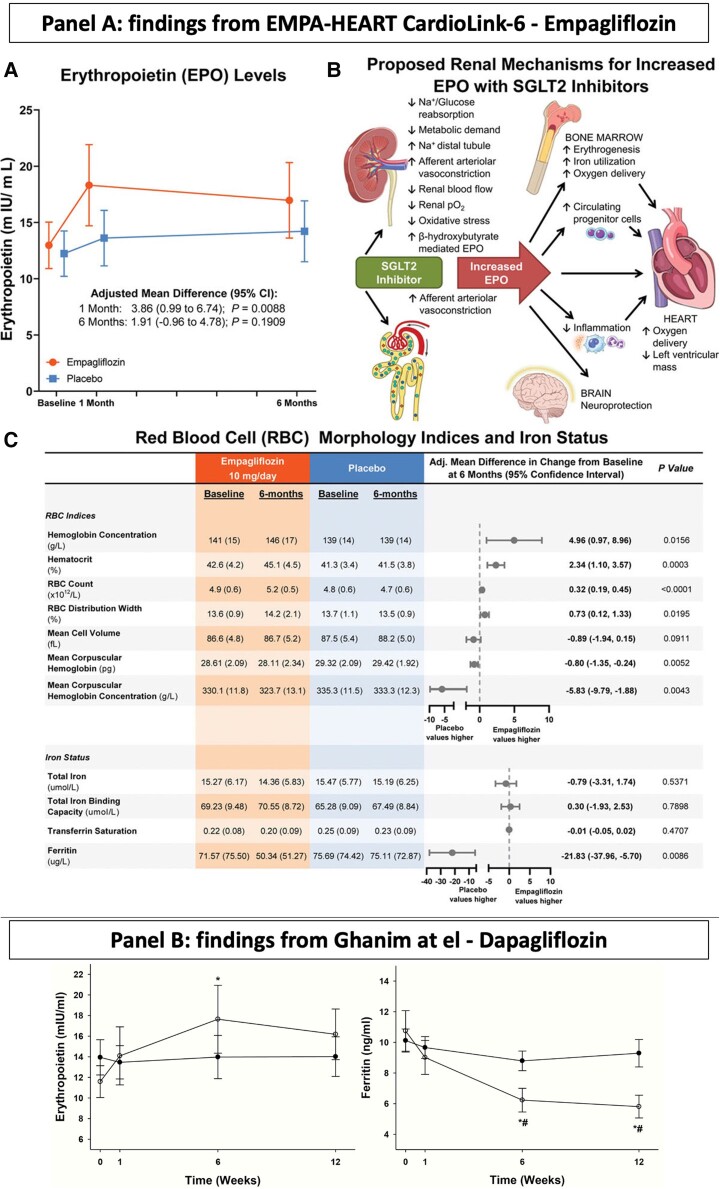
Effect of empagliflozin (panel A) and dapagliflozin (panel B) on erythropoietin levels and iron status. Panel A (A) EPO levels in patients receiving empagliflozin 10 mg daily or placebo. (B) RBC indices, hemoglobin and hematocrit values, and iron status at baseline and after 6 months of empagliflozin or placebo. (C) Potential renal mechanisms for increased EPO with sodium-glucose cotransporter 2 (SGLT2) inhibition. Reprint permissions from Mazer et al.14Panel B: Plasma levels of erythropoietin and ferritin before and following 12 weeks of treatment with placebo or 10 mg daily of dapagliflozin. * = P < 0.05 compared to baseline in dapagliflozin group using ANOVA and paired t-test and #=P < 0.05 compared to placebo using two-way ANOVA and t-test. Adapted from J Clin Endocrinol Metab. 2020; 105:e1056-e1063.15
Introduction
Iron is needed in all organ systems for various metabolic processes, e.g. erythropoiesis, mitochondrial function, oxygen transport, myocardial and skeletal muscle metabolism, immune and nervous systems, inflammatory response, lipid metabolism and many others.
Iron deficiency (ID) is extremely common,1 and recent trials have shown that it is an important therapeutic target in patients with heart failure (HF) with reduced ejection fraction (HFrEF). However, ID appears common in a broad range of cardiovascular (CV) diseases.
The aim of this review is to summarize the available data from epidemiological, clinical and interventional studies on ID in CV diseases (Graphical Abstract).
Definition of ID
ID can be characterized by (i) depleted iron stores linked with a decrease in the total body iron supply due to insufficient nutritional iron intake, impaired absorption or chronic blood loss, i.e. absolute ID; and/or (ii) reduced circulating iron, which can be linked to a persistent inflammatory state as in many CV diseases, i.e. functional ID.2 Inflammation leads to an increased release of hepcidin, a liver-expressed type II acute phase protein and a major player in iron homeostasis.3 Hepcidin regulates the degradation of ferroportin, a transmembrane protein which transports iron absorbed after dietary intake from the inside of the mucosal cells in the small intestine to the bloodstream, and also mediates the release of recycled iron from macrophages in the spleen and the liver4 (Figure 1). Therefore, ID observed with CV diseases may be due to the increased levels of hepcidin linked with a chronic inflammatory status, which leads to decreased iron absorption and mobilization from the reticuloendothelial system, i.e. functional ID. In severe HF, beyond the above-explained role of inflammation, a contributing cause of ID might be the reduction in iron absorption in the bowel due to an HF-related generalized oedema.6,7 In a population of 15 patients with HFrEF and 15 with HF with preserved ejection fraction (HFpEF), the increase in plasma iron 2 h after supplementation with oral ferroglycin sulphate complex was almost two-fold higher compared with 12 control subjects without HF and ID, and no difference was observed across the ejection fraction (EF) spectrum,8 which might lead to question a major role for limited absorption as key mechanisms for ID in HF. Alternatively, it might be hypothesised that ferroglycin, by having a good iron availability, could bypass the hepcidin block on iron absorption.
The definition of ID which is usually used in the CV field comes from the large randomized controlled trials in HF, comprising a serum ferritin concentration <100 ng/mL, or a ferritin concentration 100–299 ng/mL in combination with a transferrin saturation (TSAT) <20%. These cut-offs have been borrowed from the nephrology field where they were suggested to have good performance in terms of sensitivity and specificity for a diagnosis of ID but also as targets for iron therapy.9 Recent studies raise the question of whether this definition accurately reflects the presence of ID as assessed by bone marrow histology, the ‘gold-standard’ investigation for ID. Therefore, it might lead to consider as ID patients who do not have ID, or to deny an ID diagnosis to patients who are iron-deficient. However, the positive results of intravenous (IV) iron trials in HF might suggest that a valuable proportion of patients diagnosed with ID by using the current definition is genuinely iron-deficient.
In HF patients, serum concentrations of transferrin receptor (sTfR), which mediates the endocytosis of transferrin-iron complexes into cells, outperforms serum ferritin concentrations or TSAT for predicting bone marrow iron depletion.10 Additionally, TSAT ≤19.8% and serum iron ≤13 μmoL/L had a 94% sensitivity and a 84 and 88% specificity, respectively, for predicting ID in the bone marrow, whereas the common definition of ID had only 82 and 72%, respectively.11 Serum ferritin may be a poor guide to ID in patients with HF or atherosclerotic heart disease. Indeed, both conditions are associated with chronic inflammation, which increases hepcidin secretion, thus reducing iron absorption but provoking release of ferritin from cells (akin to troponin release from damaged cardiac myocytes), and therefore uncoupling ferritin from its usual relationship with ID.12 However, it should also be recognized that the clinical trials showing the benefits of iron have included serum ferritin as an inclusion criteria; we should be cautious before abandoning serum ferritin for selecting patients for IV iron treatment.13 Of interest, the EMPA-HEART14 trial that enrolled stable patients with type 2 diabetes and coronary artery disease (CAD) reported that the rise in haemoglobin following treatment with empagliflozin was associated with an increase in erythropoietin and a reduction in serum ferritin but no change in TSAT. In a single centre randomized controlled trial enrolling 52 patients with obesity and type 2 diabetes, after 12-week treatment dapagliflozin increased haemoglobin and erythropoietin (only at week 6) with a parallel reduction in ferritin and TSAT compared with placebo.15 Whether the decline in ferritin reflects reduced iron availability due to increased red cell production or the anti-inflammatory properties of SGLT2i is uncertain (Figure 2).14
Acute and chronic heart failure
Prevalence, clinical phenotype, and outcome
About half of patients with HF has ID,16–18 with specific prevalence estimates for ID in chronic HF ranging between 47 and 68% depending on the definition used for ID.19 A slightly higher prevalence has been in general observed in HFpEF vs. HF with mildly reduced EF (HFmrEF) vs. HFrEF.20,21 In some cohorts the prevalence of ID was similar regardless of anaemia,22 whereas in some others it was higher in patients with anaemia.19,23 Patients with more severe HF are more likely to have ID and anaemia. It is likely that this is a ‘vicious cycle’, with worsening HF exacerbating ID that, in turn, drives the further progression of HF.19,21,24,25
In a regional HF cohort of 7160 patients from Hull (UK) higher ln-transformed ferritin concentration, but not serum iron or TSAT, was independently associated with higher 5-year mortality.19 In a smaller cohort of ∼1500 HF patients with chronic HF, ID was independently associated with a 42% increased risk of all-cause death.24 In an analysis of the Swedish HF registry, ID was independently associated with the risk of recurrent all-cause hospitalizations but not of mortality.21 In a multi-ethnic Asian population, a TSAT <20% was independently associated with lower peak oxygen consumption and higher mortality.26
There are limited data on the natural history of ID and the trajectories of ID biomarkers over time. In the AFFIRM-AHF trial, after 6 weeks from baseline ferritin increased in average by ∼20 ng/mL and TSAT by 5%.27 In an ambulatory cohort of 906 chronic HF patients from Hull, within 1-year follow-up 30% of patients reported new-onset ID defined as serum iron ≤13 μmol/L, while ID resolved in 44%. Compared with iron repletion, persistent and incident ID were associated with 80 and 40% increased risk of all-cause death, respectively.28 In a multicentre study from New Zealand and Singapore enrolling 1563 patients with HF, when ID was defined according to a TSAT <20%, 20% of HF patients developed ID by 6 months, whereas of those with ID at baseline 53% had persistent ID and in 47% there was ID resolution. Persistent ID was associated with higher risk of death/HF hospitalization compared with ID resolution or no ID.23 These findings on risk of incident ID in HF lead to suggest a pragmatic approach of regularly re-checking haemoglobin and ID markers once/twice a year. Interestingly, in these studies a minority of patients received iron therapy, and therefore ID resolution was spontaneous and probably explained by an improved HF status linked with optimized treatment.
Consequences of ID in patients with chronic heart failure across the ejection fraction spectrum
In patients with chronic HFmrEF and HFrEF, the prevalence of ID has been observed to range 37–62% depending on the definition applied,11,29–31 and to be higher in patients with concomitant anaemia.10,30 In BIOSTAT-CHF including mainly patients with HF and EF ≤45%, 62% had ID defined as a TSAT <20% and of these 34% had a deficit in iron utilization (serum ferritin concentrations >128 ng/mL) while 66% had low iron stores (serum ferritin concentrations ≤128 ng/mL).31 In HFmrEF/HFrEF ID has been shown to be associated with lower peak oxygen consumption, higher ratios of ventilation to carbon dioxide production, higher mortality and risk of HF hospitalization and heart transplantation independently of anaemia.29,30 Notably, in a study enrolling 42 patients with HF and EF ≤45% undergoing coronary artery bypass grafting, 40% had bone marrow ID, and a TSAT ≤19.8% or serum iron concentrations ≤13 μmoL/L, but not ferritin, predicted mortality.11
In chronic HFpEF patients, a meta-analysis of 15 HFpEF studies reported a 59% prevalence of ID and ID was associated with lower oxygen consumption at peak exercise (VO2max), worse dyspnoea class or 6 min walking distance (6MWD) and worse health-related quality of life (HRQoL), but with no impact on risk of death or hospitalization.32
There are few studies aiming to compare prevalence and outcome associated with ID in HF across the EF spectrum. In a French cohort of ∼2800 HF patients, 38% were tested for ID and 34% of these were diagnosed with ID.20 Testing in HFpEF, HFmrEF and HFrEF was 23, 24 and 53%, respectively, whereas ID was diagnosed in 36, 30 and 31%, respectively.20 ID diagnostic testing was more likely performed during hospitalizations than outpatient visits.20 Among HFrEF patients diagnosed with ID, 49% received iron supplementation, with 80% treated with IV iron.20 In the Swedish HF registry, testing for ID was only 27% (29% in HFrEF, 24% in HFmrEF and 21% in HFpEF), and those who were tested were more likely to have anaemia, HFrEF, more severe HF and receive more specialized follow-up.21 Prevalence of ID was overall 49%, 56% in HFpEF, 50% in HFmrEF and 48% in HFrEF. 20% had concomitant anaemia and ID, 29% ID without anaemia, 15% anaemia without ID, and 36% neither ID nor anaemia. Iron supplementation with ferric carboxymaltose (FCM) was 19% in the overall ID population and 24% in HFrEF. Prevalence of ID was similar in HFrEF and HFpEF, i.e. 58%, in a longitudinal multicentre study in New Zealand and Singapore, and with higher co-prevalence of anaemia in HFpEF (56%) vs. HFrEF (39%). A TSAT <20% independently predicted all-cause mortality and risk of death/HF hospitalization in HFrEF (EF <50%) but not in HFpEF. In ∼1200 patients with HF across the EF spectrum, overall prevalence was 53% (64% in HFpEF, 61% in HFmrEF, and 50% in HFrEF) and ID was associated with lower VO2max regardless of EF.33 In the French CARENFER study, overall ID prevalence was 49.6%, 57.5% in HFpEF, 47.4% in HFmrEF and 44.3% in HFrEF.25
Acute decompensated HF
In patients with acute decompensated HF, ID was observed in 54 and 56% of patients with HFrEF and HFpEF, respectively, but only in HFrEF it was independently associated with longer hospital stay.34 In another cohort of ∼600 patients hospitalized for HF, depleted iron stores but not low circulating iron was associated with all-cause mortality or HF hospitalization.35 In ∼850 patients hospitalized for decompensation of chronic HF, ID was observed in 69% of men and 75% of women, and in non-anaemic patients it was higher in women vs. men (79 vs. 57%).36 In 165 patients with acute HF, concomitant low hepcidin and high sTfR highlighting severe ID found in 37% of the population was associated with more severe HF and low haemoglobin, and with 5% in-hospital mortality.37
Treatments (Table 1, Figure 3)
Table 1.
Design and results of the randomized controlled trials on iron deficiency in heart failure enrolling >100 patients
| IRONOUT HF38 | FAIR-HF39 | CONFIRM-HF40 | EFFECT-HF41 (2017) | AFFIRM-AHF27 | |
|---|---|---|---|---|---|
| Patients (n) | 225 | 459 | 301 | 172 | 1108 |
| Duration (weeks) | 16 | 24 | 52 | 24 | 52 |
| Women | 36% | 53% | 47% | 25% | 45% |
| Age, median (years) | 63 | 67 | 69 | 63 | 71 |
| Treatment vs. control | Oral IP vs. Placebo | IV FCM vs. Placebo | IV FCM vs. Placebo | IV FCM vs. SoC | IV FCM vs. Placebo |
| Key inclusion criteria | |||||
| Acute or Chronic | Chronic | Chronic | Chronic | Chronic | Acute |
| NYHA class | II-IV | II-III | II-III | II-III | I-IV |
| LVEF | ≤40% | ≤45% | ≤45% | ≤45% | <50% |
| Hb (g/dL) | ♀9−13.5;♂9–15 | 9.5–13.5 | <15 | ≤15 | 8–15 |
| ID definition | Current ESC HF Guidelinesa | Current ESC HF Guidelinesa | Current ESC HF Guidelinesa | Current ESC HF Guidelinesa | Current ESC HF Guidelinesa |
| Primary outcome | = Peak VO2 | ↑ PGA ↓ NYHA |
↑ 6MWD | ↑ Peak VO2 | = Recurrent HF hosp or CV death |
| Key secondary outcomes | = 6MWD | ↑ 6MWD | ↓ NYHA | = VE/VCO2 | ↓ Recurrent CV hosp or CV death |
| = VE/VCO2 | ↑ EQ-5D | ↑ PGA | ↓ NYHA | = CV death | |
| = NT-proBNP | ↑ KCCQ | ↓ Fatigue score | ↑ PGA | ↓ Total HF hosp | |
| = KCCQ-CSS | ↑ KCCQ | = NT-proBNP/BNP | ↓ Time-to-first HF hosp or CV death | ||
| = EQ-5D | ↓ Days lost due to HF hosp or CV death | ||||
| Time to: = All-cause death = CV death = HF death | |||||
| Risk of first/repeated: = All-cause hosp = CV hosp ↓ HF hosp |
6MWD, 6 min walking distance; IP, iron polysaccharide; FCM, ferric carboxymaltose; IV, intravenous; LVEF, left ventricular ejection fraction; Hb, haemoglobin; HF, heart failure; PGA, patient global assessment; NYHA, New York Heart Association; KCCQ, Kansas City Cardiomyopathy Questionnaire; CSS, clinical summary score; BNP, B-type natriuretic peptide; NT-proBNP, N-terminal pro-B-type natriuretic peptide; VE/VCO2, ventilation/carbon dioxide production; TSAT, transferrin saturation; EQ-5D, European Quality of Life Five Dimension; MLHFQ, Minnesota Living with Heart Failure Questionnaire; CV, cardiovascular; hosp: hospitalization; ESC, European Society of Cardiology.
↑, increased by the intervention vs. control; ↓, decreased by the intervention vs. control; =, no difference with the intervention vs. control.
Ferritin < 100 ng/mL OR ferritin 100–299 ng/mL AND TSAT <20%.
Figure 3.
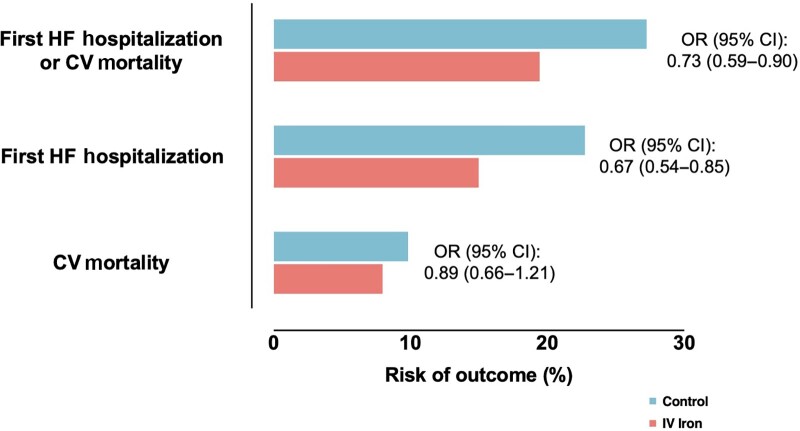
Risk of cardiovascular outcomes with intravenous iron vs. placebo. Data from Graham et al.42 pooling data from seven randomized controlled trials on intravenous iron supplementation in HF with iron deficiency. HF, heart failure; CV, cardiovascular; OR, odds ratio; CI, confidence interval.
Pioneering research on iron supplementation which has provided the background for investigation in HF and CV diseases comes from the nephrology field, where oral and IV iron therapies are used in patients with non-dialysis and dialysis-dependent chronic kidney disease and ID anaemia.9 Anaemia is the most common extraintestinal manifestation of bowel disease and since its major cause is ID, IV iron is often the therapy of choice being oral iron often not tolerated or ineffective.43 The current section aims to provide an overview on ID treatment in patients with HF.
Oral iron supplementation
Use of oral iron supplements has several limitations, such as the limited amount of iron absorbed in the gut, the metallic taste, and high percentage of side effects such as nausea, diarrhoea, constipation, flatulence, and constipation.2 Current evidence from randomized controlled trials does not support the use of oral iron for ID in patients with HF. In the IRONOUT HF trial, randomizing 225 patients with HFrEF and ID, oral iron polysaccharide or placebo for 16 weeks led to similar changes in peak VO2.38 Potential explanations for these neutral findings could be, beyond the lack of efficacy, (i) the use of an inappropriate primary outcome which has been neither improved by the treatment with IV iron; (ii) the enrolment of a proportion of non-iron-deficient patients given the median ferritin and TSAT levels of 75 ng/mL and 19%, respectively, at baseline; (iii) too short treatment.44 Additionally, a proof-of-concept non-randomized study, showing a significant increase in haemoglobin, serum iron and ferritin concentrations, together with an improvement in 6MWD and Kansas City Cardiomyopathy Questionnaire (KCCQ) at 3 and 6-month follow-up would suggest not to dismiss a potential use for oral iron in this setting.45
Intravenous iron supplementation in chronic HF
The evidence for using IV iron in HF patients with ID is much stronger than other routes of application. In small cohort of 16 anaemic patients with HFrEF, after 12 days of treatment with 1 g of IV iron sucrose there was a significant increase in haemoglobin levels, an improvement in Minnesota Living with Heart Failure Questionnaire (MLHFQ) score, 6MWD and New York Heart Association (NYHA) class.46
A randomized controlled trial enrolling 40 patients with chronic HFrEF (EF ≤35%), chronic renal failure, ID anaemia defined as haemoglobin <12.5 g/dL, TSAT <20% and serum ferritin concentration <100 ng/mL, to IV iron sucrose or placebo showed better creatinine clearance, lower N-terminal pro-B-type natriuretic peptide (NT-proBNP), lower C-reactive protein levels, higher EF, higher MLHFQ score and 6MWD with IV iron supplementation.47
The FERRIC-HF trial randomized 35 patients with chronic HF and ID to IV iron sucrose or no treatment in a single blind design. This was a proof-of-concept study that for the first time also included patients without anaemia (50%). A trend toward statistically significance for improved peak VO2 was observed.48 Of note, this trial was the first study to use the guideline recommended definition of ID, based on serum ferritin <100 ng/mL or serum ferritin 100–299 ng/mL with TSAT <20%.
The latter study provided the rationale for the subsequent FAIR-HF trial, which recruited 459 patients with HF and EF ≤40% if NYHA Class II or EF ≤45% if NYHA Class III. ID was defined as in FERRIC-HF, and haemoglobin levels of included patients were 9.5–13.5 g/dL. Patients were randomized 2:1 to FCM or placebo in an elaborate double-blind setting. At Week 24, compared with placebo, FCM improved patient global assessment, NYHA class, 6MWD, and HRQoL, without any difference in mortality and adverse/serious events, regardless of anaemia.39,49,50 Notably in patients without anaemia, these benefits were seen despite no change in haemoglobin. Treatment with FCM improved renal function at Week 24, and no interaction between treatment effect and baseline renal function was observed.51 An early reduction in calculated plasma volume status and weight was observed with FCM, suggesting that at least parts of its benefits might be mediated by decongestive effects.52
In the CONFIRM-HF trial, 304 patients with symptomatic HF with EF ≤45% and ID were randomized 1:1 to FCM or placebo for 52 weeks. At week 24 FCM use led to improved 6MWD, NYHA class, patient global assessment, quality of life and fatigue score. FCM reduced the risk of HF hospitalizations by 61%, and there was no difference in incidence of adverse events across the trial arms.40
In a meta-analysis of four randomized controlled trials, treatment with FCM vs. placebo led to a 41% lower risk of recurrent CV hospitalizations or CV mortality, 47% lower risk of recurrent HF hospitalizations or CV mortality, 40% lower risk of recurrent CV hospitalizations or all-cause mortality, with similar results when time-to-first-event analyses were carried out, and without any increased risk of adverse event associated with FCM.53
In the EFFECT-HF trial, randomizing 172 patients with EF ≤45% and mild to moderate symptoms despite optimal HF therapy, FCM preserved peak VO2 which instead decreased in the control group, and significantly improved patients’ global assessment and NYHA class vs. standard of care.41
In the FERWON-NEPHRO trial enrolling patients with non-dialysis-dependent chronic kidney disease, a single infusion of 1000 mg of ferric derisomaltose vs. up to five doses of iron sucrose was associated with a 41% lower risk of CV adverse events in both patients with and without HF.54
ID has been observed to be a negative predictor of effective cardiac resynchronization therapy (CRT) therapy in terms of reverse cardiac remodelling and clinical response.55 Consistently, in the IRON-CRT trial, randomizing 75 patients with ID and HFrEF (EF <45%) at least 6 months after CRT implantation, FCM vs. standard of care showed a greater improvement in EF and left ventricular end-systolic volume, as well as an improvement in the cardiac contractility index slope during incremental biventricular pacing, functional status, exercise capacity and peak VO2 with FCM.56
Intravenous iron supplementation following acute decompensated HF
In 50 patients hospitalized for acute decompensated HF from the PRACTICE-ASIA-HF trial randomized to FCM or placebo following HF stabilization, no difference across trial arms was observed for changes in 6MWD at week 12, as well as for quality of life.57
The AFFIRM-AHF randomized 1132 patients with ID after a hospitalization for decompensated HF with EF <50% to FCM vs. placebo for up to 24 weeks. There was no statistically significant difference in risk of the primary outcome, i.e. a composite of total hospitalizations for HF or CV death, across the study groups, but total HF hospitalizations and the composite of first HF hospitalization or CV death were significantly reduced by FCM by 26 and 20%, respectively. Also length of hospital stay was lower with FCM vs. placebo.27 In a pre-specified sensitivity analysis censoring patients in each country on the date when the first COVID-19 patient was reported, FCM significantly reduced the risk of the primary outcome by 25%, of total CV hospitalization or CV death by 23%, and total HF by 30%.27 Treatment with FCM was associated with an early beneficial effect on HRQoL measured by KCCQ-12 at week 4 which lasted till week 24.58
Following meta-analyses pooling the data of the above-mentioned trials together with data from AFFIRM-AHF showed a reduction of the composite of first HF hospitalization or CV death, recurrent HF hospitalizations and recurrent HF hospitalizations, without any effect on all-cause or CV mortality.13,42
Key upcoming randomized controlled trials in the field are reported in Table 2.
Table 2.
Upcoming randomized controlled trials on iron deficiency in heart failure
| Trial | Key selection criteria | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Iron prep | Placebo | NYHA | LVEF | Acute or chronic | Hb (g/dL) | Definition of ID | Other key selection criteria | Primary outcome | |
| FAIR-HF2 (NCT03036462), Phase 4, double-blind | 1200 | IV FCM | Yes | II-IV | ≤45% | Chronic | 9.5–14.0 | Current ESC HF Guidelinesa | — | Composite of recurrent HF hosp and CV death |
| IRONMAN (NCT02642562), Phase 4, unblind | 1160 | IV FDI | No | II-IV and recent HF hosp OR raised NPs | ≤45% | Chronic | ♀9–13 ♂9–14 | Ferritin <100 OR TSAT <20% | — | Composite of CV mortality of HF Hosp (first and recurrent) |
| FAIR-HFpEF (NCT03074591), Phase 2 | 200 | IV FCM | Yes | II-III | ≥45% | Chronic | 9–14 | Current ESC HF Guidelinesa | • HF hosp within 12 months OR raised NPs • Diastolic dysfunction • Diuretic use • 6MWD <450 m | Δ 6MWD |
| IRONMET-HFpEF (NCT04945707), Phase 4, double-blind | 70 | IV FDI | Yes | II-IV | ≥50% | Chronic | ♀9.0–13.5 ♂9.0–14.0 | Current ESC HF Guidelinesa | Raised NPs OR PCWP ≥15 mmHg OR PCWP/CO ≥ 2.0 mmHg/L/min | Δ peak VO2 |
| NCT04063033, Phase 4 | 200 | IV FG | No | — | — | Acute | 8–14 | Current ESC HF Guidelinesa | • Raised NPs • Signs of congestion • IV diuretics | 6MWD at follow-up |
| HEART-FID (NCT03037931), Phase 3 | 3068 | IV FCM | Yes | II-IV | ≤40% | Chronic | ♀9.0–13.5 ♂9.0–15.0 | Current ESC HF Guidelinesa | • HF hosp within 12 months OR raised NPs | Hierarchical composite of death and HF hosp and Δ 6MWD |
EF, ejection fraction; FCM, ferric carboxymaltose; FDI, ferric derisomaltose; 6MWD, 6 min walking distance; ID, iron deficiency; NYHA, New York Heart Association; NP, natriuretic peptide; ESC, European Society of Cardiology; TSAT, transferrin saturation; PCWP, pulmonary capillary wedge pressure; CO, cardiac output; CV, cardiovascular; HF, heart failure; VO2, oxygen consumption.
Ferritin < 100 ng/mL OR ferritin 100–299 ng/mL AND TSAT <20%.
Current recommendations: efficacy and safety
Based on the prognostic relevance of ID in HF patients, the 2021 European Society of Cardiology (ESC) guidelines on HF provide a Class I, level of evidence C, recommendation for periodical screening for ID and anaemia with a full blood count, serum ferritin concentration, and TSAT.59 They also provide a Class IIa, level of evidence A, recommendation for FCM use in symptomatic HF patients with EF <45% for alleviating patients’ symptoms, and improve exercise capacity and quality of life; a Class IIa, level of evidence B, recommendation for FCM in patients with HF recently hospitalized for HF and EF <50% to reduce the risk of HF hospitalization.59 The current guideline recommendations mention only FCM for the treatment of ID in HF since no large randomized trial has delivered yet evidence showing benefit with other IV iron treatments in patients with HF and ID regardless of anaemia. The ongoing IRONMAN (NCT02642562) trial will highlight whether the beneficial effects observed with FCM in HF are extended to ferric derisomaltose. Regarding tolerability and safety, hypersensitivity and anaphylactic reactions have been observed in general more frequently with iron dextran vs. non-dextran IV treatments,60 although the dextran ferric derisomaltose seems to be at least as well tolerated as the non-dextran FCM and iron sucrose. In the FERWON programme, the incidence of serious or severe hypersensitivity reactions was rare, i.e. 0.3% with ferric derisomaltose vs. 0.2% with iron sucrose.61 In the PHOSPHARE trials the incidence of serious or severe hypersensitivity reactions was similarly low with ferric derisomaltose (0.8%) and FCM (1.7%).60 Incidence of hypophosphataemia has been shown to be higher with FCM compared with ferric derisomaltose, which is mediated by the increased levels of fibroblast growth factor (FGF)-23 reducing proximal tubular reabsorption of filtered phosphate and suppressing circulating concentrations of 1,25-dihydroxyvitamin D, leading also to reduced dietary calcium absorption.62 However, in patients with chronic kidney disease, those with HFrEF had only a transient increase in phosphates, with normalized levels after 28 days following FCM infusion, likely due to a lower increase in intact FGF-23 and assumed Klotho deficiency in HFrEF, which leads the tubular phosphate reabsorption system to be less sensitive to FGF-23.63
Summary
ID in HF is common regardless of EF, i.e. up to 60%, more likely observed in women, older patients, and with increasing disease severity and symptoms. The association with clinical events varies based on the specific ID definition, but may be more closely related to low serum iron and TSAT than to low ferritin, which may be confounded by increases in inflammatory markers with advancing HF. However, ID is associated with an increased risk of hospitalizations across the EF spectrum and all-cause mortality in HFrEF/HFmrEF, even in the absence of anaemia. Current evidence from randomized controlled trials does not support the use of oral iron for ID in patients with HF, but highlights that IV FCM reduces hospitalizations, improves quality of life, symptoms and functional capacity in ID patients with stable HFrEF and in those hospitalized for decompensated HF with an EF <50%.
Coronary artery disease
Iron status and risk of coronary artery disease
In the 1980 s, Sullivan suggested that iron could increase the incidence of heart disease by a variety of mechanisms.64 This hypothesis was supported by the Kuopio Ischaemic Heart Disease Risk Factor Study (KIHD) enrolling ∼2000 men without symptomatic CAD in 1984–1989. This study showed a 2.2-fold higher risk of myocardial infarction with higher serum ferritin concentrations (≥200 ng/mL) in patients with low density lipoprotein (LDL) cholesterol ≥5 mmoL/L but not in those with lower levels, suggesting an interaction between ferritin and the atherogenic effects of LDL cholesterol.65 In a subsequent study, a higher TfR/ferritin ratio was measured among ∼100 male patients with vs. ∼100 males without history of myocardial infarction, suggesting a link between higher body stores of iron and risk of myocardial infarction.66 The Nutrition Canada Survey, which enrolled ∼10 000 patients without heart disease in 1970–1972, found that higher serum iron concentrations were associated with a 2-fold increased risk of fatal acute myocardial infarction, and the magnitude of the association was greater when cholesterol levels were higher.67 However, high serum ferritin, as noted above, may reflect inflammation rather than iron repletion. Inflammation may lead to, and be caused by, atherosclerosis.
Later studies questioned the above findings. A meta-analysis of 17 registry-based, prospective, and case control studies up to 2014 could not show any statistically significant association between high serum ferritin concentrations, as well as total iron binding capacity, serum iron concentrations and risk of ischaemic heart disease, while highlighting a potential prognostic benefit associated with higher TSAT.68 Consistently, a Mendelian randomized study demonstrated a protective effect on CAD for higher serum iron concentrations, TSAT and also for higher serum ferritin concentrations.69 In the prospective, community-based, cohort PREVEND study, that enrolled ∼6400 individuals, a higher total annual incidence of new CV events (a composite of ischaemic heart disease, stroke and peripheral artery disease) and all-cause death was observed together with increased serum concentrations of ferritin and hepcidin, but the association disappeared after adjusting for other factors.70 Consistently, in the EPIC-Heidelberg Study, serum ferritin concentrations were associated with most CV risk factors, and although an association between serum ferritin concentrations and increased risk of myocardial infarction was observed, it was no longer statistically significant after adjustments.71 In ∼12 000 individuals from three European population-based cohorts, ID defined as serum ferritin <100 ng/mL or ferritin 100–299 ng/mL and TSAT <20% was associated with a 24% increased risk of CAD, 26% increased risk of CV mortality and 12% increased risk of all-cause death, with 5.4% of all deaths, 11.7% of all CV deaths, and 10.7% of all the CAD events attributable to ID.72
One possible explanation for the iron-heart disease hypothesis was that iron increased oxidative stress.73 Serum ferritin concentrations have not been shown to be independently associated with increased oxidation of LDL cholesterol.74 However, ferritin concentrations are very much confounded by acute or chronic inflammation, which clusters with most CV risk factors.75 Finally, the often-observed U-shaped relationship between serum ferritin concentrations and CV outcomes is not consistent with the iron-heart disease hypothesis, which would be rather supported by a linear association between iron stores, oxidative stress and harm.76
ID in patients with CAD
In patients with CAD, the prevalence of ID was 63% when defined as depleted extracellular iron stores and ≤10% of erythroblasts containing iron in bone marrow aspirates. Only 32% of patients diagnosed with ID also had anaemia.77 In patients admitted for acute coronary syndrome, ID prevalence has ranged between 29–56% depending on the cohorts’ characteristics,78–80 with a meta-analysis estimating an overall prevalence of 43%.81 In 836 patients with acute coronary syndrome, ID predicted a 50% increased risk of non-fatal myocardial infarction and CV death after extensive adjustments including anaemia.79 In the AtheroGene study, which enrolled ∼3400 patients with angiographically documented CAD, higher concentrations of sTfR were strongly associated with the risk of myocardial infarction or CV death independently of haemoglobin, CV risk factors, C-reactive protein, surrogates of cardiac function and extent of myocardial necrosis.82 When ID was defined according to serum ferritin concentrations and TSAT, this was associated with a 50% increased risk of CV death and myocardial infarction.79 In a study of 55 patients undergoing coronary balloon angioplasty after acute myocardial infarction, serum iron concentration was an independent predictor of left ventricular systolic function 6 months after revascularization, even after adjusting for traditional prognostic factors, whereas haemoglobin was not.83 Consistently, in 141 patients with a first anterior ST-elevation myocardial infarction (STEMI) treated by percutaneous coronary intervention (PCI), ID was associated with larger infarct sizes, higher likelihood of adverse left ventricular remodelling and more extensive microvascular obstruction.78 Conversely, in a different cohort of 420 STEMI patients undergoing primary PCI, those with ID had greater increases in troponin but, surprisingly, no difference in infarct size measured by cardiac magnetic resonance, and reported lower in-hospital mortality/Killip class ≥3 compared with patients without ID.80
In 39 STEMI patients, those receiving (n = 17) IV ultrasmall superparamagnetic iron-oxide (USPIO)-based iron administration within 4 days following an acute myocardial infarction had a much smaller infarct size, with a decrease in both endocardial extent and transmural infarction, and a smaller left ventricular end-systolic volume.84
Summary
These data highlight a high prevalence of ID in CAD, i.e. up to 60%. The overall body of evidence suggests that ID is associated with higher risk of ischaemic heart events and CV mortality in people with and without CAD, and adverse remodelling after a myocardial infarction. Evidence supporting iron supplementation for treating ID regardless of anaemia in patients with CAD is currently missing.
Cerebrovascular disease
Data on the link between iron status and risk of cerebrovascular disease are sparse and conflicting. In the Prospect-EPIC cohort, serum ferritin ≥137 ng/mL was associated with a 45% higher risk of strokes, and in particular with a 2.2-fold higher risk of ischaemic stroke, whereas other markers of iron status were not.85 A Mendelian randomized study showed increased risk of stroke, especially cardio-embolic events, with higher serum concentrations of iron and ferritin or TSAT but with lower serum transferrin concentrations.86 Conversely, in a population-based health survey enrolling people aged ≥65 years old, lower serum concentrations of iron predicted the risk of stroke.87 In a nested case–control study in Sweden, the highest quartile of TSAT predicted decreased risk of stroke in men, while the highest quartile of total iron binding capacity predicted increased risk in both sexes.88 These apparently discordant findings might reflect an U-shaped relationship between iron status and risk of stroke, which leads to different results when iron status markers are categorized according to different cut-offs. Consistently, in the NHANES I study, an analysis of ∼5.000 women and men aged 45–74 years who were free of stroke at baseline showed an U-shaped association between TSAT and risk of incident stroke, with an almost 2-fold higher risk associated with a TSAT >44% or <20% vs. 30–36% in women; however, no association was observed in men.89
In 746 patients with ischaemic or haemorrhagic stroke, prevalence of ID was 45%. ID was associated with lower functional capacity, inflammation and anaemia. Rehabilitation led to an improvement of functional capacity regardless of ID.90
Summary
The evidence about iron status and risk of stroke is limited and conflicting. There may be a U-shaped association between iron status and risk of stroke. Prevalence of ID might be ∼45%. Data on the prognostic significance of ID, and accordingly, on the prognostic impact of treating ID in patients with cerebrovascular disease are missing. ID might be associated with worse functional status. Currently there is no indication to treat ID in patients with cerebrovascular diseases independently of anaemia.
Aortic stenosis
In a study analysing 464 patients referred for aortic valve replacement, 53% had ID and 20% anaemia. Although patients with ID had an overall worse clinical profile, there was no association between ID, sTfR, or hepcidin and the risk of major adverse CV events or mortality regardless of the treatment approach for aortic stenosis.91 A lack of association between ID and death/HF hospitalization was also observed in patients undergoing transcatheter aortic valve implantation (TAVI), although anaemia predicted prognosis and ID was reported in 79% of patients with anaemia.92 Conversely, in another study enrolling 495 patients referred to TAVI, ID was present in 54% of the entire cohort and was independently associated with a 64% higher risk of all-cause mortality and unplanned HF hospitalization during the first year after TAVI. In a sample of 56 ferropenic patients, treatment with IV iron before TAVI was associated with an improvement in symptoms at 30 days.93 The IIISAS randomized 149 patients with severe aortic stenosis evaluated for TAVI and ID (defined as ferritin <100 µg/L, or 100–299 µg/L with a TSAT <20%) to IV ferric derisomaltose or placebo ∼3 months before TAVI, and showed iron stores restoration in 76 vs. 13%, but no difference across the study arms in baseline-adjusted 6MWD which was the primary outcome of the trial, or in NYHA class or HRQoL 3 months after TAVI.94 These results might suggest that although ID might be a prognostic marker in patients with severe aortic stenosis, it only limitedly contribute to determine the occurrence of outcomes, and therefore its treatment in the context of a multidimensional frailty status does not affect prognosis.
Therefore, based on the available data there is no indication to treat ID in patients with aortic stenosis diseases independently of anaemia.
Atrial fibrillation
Evidence on epidemiology and prognostic role of ID in atrial fibrillation (AF) is very limited.95 In the National Inpatient Sample database, 2.5% of patients with a primary AF hospitalization had ID, which was associated with higher rates of acute myocardial infarction, use of vasopressors and mechanical ventilation, longer hospital stay and acute kidney injury, but not of mortality or cardiogenic shock.96 In 127 patients with AF and without HF, 47.6% had ID defined according to the ESC HF guidelines definition, whereas prevalence was 20% in an age- and gender-matched control population. Prevalence reached ∼50% in patients with permanent vs. 30% in those with persistent AF, and permanent but not persistent vs. paroxysmal AF was independently associated with ID.97 Based on the above-mentioned benefits in terms of improvement in functional status and quality of life which have been linked with FCM use in patients with HF, and being both functional status and quality of life impaired in patients with AF, the IRON-AF iron aims to randomize at least 84 patients with AF and ID to assess the effect of iron repletion with FCM vs. placebo in terms of change in peak VO2 from baseline to Week 12 as measured by cardiopulmonary exercise test, with quality of life, 6MWD, NYHA class, and AF disease burden scores as secondary outcomes.98 Currently, there is no indication to treat ID in patients with AF independently of anaemia.
Pulmonary hypertension
ID is highly prevalent in precapillary pulmonary hypertension (PH) with estimates up to 75%.99 Prevalence has been observed to be highest in connective tissue disease-related pulmonary arterial hypertension (PAH) (70%) vs. idiopathic and congenital heart disease-related PAH (∼38–40%) vs. in chronic thromboembolism (20%).100,101 In patients with systemic sclerosis, ID prevalence was higher whether there was concomitant PH (46.1 vs. 16.4%) and was linked with more impaired 6MWD, maximal achieved work at ergometry and higher mortality.102 In idiopathic PAH, ID is associated with worse NYHA class and functional capacity, higher mean pulmonary arterial pressure and lower cardiac index, independently of the anaemia status.103–105 Additionally, sTfR concentrations >28.1 nmol/L (2.4 mg/L) have been shown to independently predict mortality.104 In PH caused by chronic lung disease, 53% of patients had ID which was associated with higher mean pulmonary arterial pressure and lower pulmonary arterial compliance.106 In patients with chronic obstructive pulmonary disease without anaemia, ID was associated with a modest increase in systolic pulmonary artery pressure and limited diffusion capacity.107 Patients with ID have also shown an abnormal response to hypoxia consisting of an exaggerated hypoxic PH which was reversed by iron supplementation.108,109
In PAH, ID might be a consequence of polycythaemia due to hypoxaemia or of reduced insufficient iron absorption. Levels of hepcidin have been shown to be higher in idiopathic PAH than in healthy controls, which might be explained by various factors including BMPR2 (bone morphogenetic protein receptor type 2) mutation/downstream pathway dysfunction and enhanced inflammatory response.104,110 At the same time, the intracellular iron overload which parallels the circulating ID leads to mitochondrial dysfunction and increases oxidative stress, which in turn contributes to the progression of PH by inducing pulmonary vascular remodelling.110,111
In the Jackson Heart Study enrolling 2800 individuals, ID was not associated with PH, and there was no association between ferritin or serum iron concentration and PH.112 In small samples of patients with PAH and ID, IV iron supplementation resulted in a better functional capacity and quality of life.113,114 In two trials randomizing ∼60 patients with PAH and ID to FCM (in Europe) and iron dextran (in China) vs. placebo, both treatments were well tolerated, improved iron status but did not affect exercise capacity, cardiopulmonary hemodynamics or NT-proBNP at 12 weeks.115 In the ORION-PH study enrolling 22 patients with different forms of PH and ID anaemia, oral iron supplementation with ferric maltol improved haemoglobin and iron status, as well as 6MWD and NT-proBNP, decreased right ventricular dimensions while improving fractional area change at week 12.116
Based on the current data, currently there is no indication to treat ID in patients with PH independently of anaemia.
Conclusions
Research investigating the link between ID and CV disease has led to identify a role for ID as therapeutic target in patients with HFrEF/HFmrEF. ID is endemic within HF, however there are signals suggesting that systematic screening for ID is still not optimally performed in HF clinical practice. Consequences are a late or even a missed chance of identifying those patients who would benefit from IV iron supplementation, which has been shown to improve symptoms, functional capacity, quality of life and prevent hospitalizations in HFrEF/HFmrEF populations.
Although there could be a potential role for ID in other CV disease, such as CAD, valvular heart disease, cerebrovascular disease, AF and PH, evidence is fragmentary, often conflicting, and the underlying pathophysiological mechanisms often unknown. Since ID can be easily treated, future research should aim for a full characterization of patients with CV diseases for ID, to identify those phenotypes who are more likely to benefit from iron supplementation.
Contributor Information
Gianluigi Savarese, Division of Cardiology, Department of Medicine, Karolinska Institutet, Stockholm, Sweden; Heart and Vascular Theme, Karolinska University Hospital, Stockholm, Sweden.
Stephan von Haehling, Department of Cardiology and Pneumology, University of Göttingen Medical Center, Göttingen, Germany; German Center for Cardiovascular Research (DZHK), partner site Göttingen, Göttingen, Germany.
Javed Butler, Department of Medicine, University of Mississippi School of Medicine, Jackson, MS, USA; Baylor Scott and White Research Institute, Dallas TX, USA.
John G F Cleland, Robertson Centre for Biostatistics and Clinical Trials, Institute of Health & Wellebing, University of Glasgow, Glasgow, UK; National Heart & Lung Institute, Imperial College London, London, UK.
Piotr Ponikowski, Department of Heart Diseases, Wroclaw Medical University, Wrocław, Poland; Centre for Heart Diseases, University Hospital, Wroclaw, Poland.
Stefan D Anker, Department of Cardiology (CVK) and Berlin Institute of Health Centre for Regenerative Therapies, German Centre for Cardiovascular Research (DZHK) partner site Berlin; Charité Universitätsmedizin Berlin, Germany.
Data availability
‘No new data were generated or analysed in support of this research’
References
- 1. Stoltzfus RJ. Iron deficiency: global prevalence and consequences. Food Nutr Bull 2003;24:S99–S103. [DOI] [PubMed] [Google Scholar]
- 2. von Haehling S, Ebner N, Evertz R, Ponikowski P, Anker SD. Iron deficiency in heart failure: an overview. JACC Heart Fail 2019;7:36–46. [DOI] [PubMed] [Google Scholar]
- 3. Nemeth E, Ganz T. Hepcidin-Ferroportin interaction controls systemic iron homeostasis. Int J Mol Sci 2021;22:6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li Y, Huang X, Wang J, Huang R, Wan D. Regulation of iron homeostasis and related diseases. Mediators Inflamm 2020;2020:6062094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. von Haehling S, Jankowska E, van Veldhuisen D, Ponikowski P, Anker SD. Iron deficiency and cardiovascular disease. Nat Rev Cardiol 2015;12:659–669. [DOI] [PubMed] [Google Scholar]
- 6. Naito Y, Tsujino T, Fujimori Y, Sawada H, Akahori H, Hirotani S, et al. Impaired expression of duodenal iron transporters in dahl salt-sensitive heart failure rats. J Hypertens 2011;29:741–748. [DOI] [PubMed] [Google Scholar]
- 7. Markousis-Mavrogenis G, Tromp J, Ouwerkerk W, Devalaraja M, Anker SD, Cleland JG, et al. The clinical significance of interleukin-6 in heart failure: results from the BIOSTAT-CHF study. Eur J Heart Fail 2019;21:965–973. [DOI] [PubMed] [Google Scholar]
- 8. Cabrera CC, Ekstrom M, Linde C, Persson H, Hage C, Eriksson MJ, et al. Increased iron absorption in patients with chronic heart failure and iron deficiency. J Card Fail 2020;26:440–443. [DOI] [PubMed] [Google Scholar]
- 9. Babitt JL, Eisenga MF, Haase VH, Kshirsagar AV, Levin A, Locatelli F, et al. Controversies in optimal anemia management: conclusions from a kidney disease: improving global outcomes (KDIGO) conference. Kidney Int 2021;99:1280–1295. [DOI] [PubMed] [Google Scholar]
- 10. Sierpinski R, Josiak K, Suchocki T, Wojtas-Polc K, Mazur G, Butrym A, et al. High soluble transferrin receptor in patients with heart failure: a measure of iron deficiency and a strong predictor of mortality. Eur J Heart Fail 2021;23:919–932. [DOI] [PubMed] [Google Scholar]
- 11. Beverborg N G, Klip IT, Meijers WC, Voors AA, Vegter EL, van der Wal HH, et al. Definition of iron deficiency based on the gold standard of bone marrow iron staining in heart failure patients. Circ Heart Fail 2018;11:e004519. [DOI] [PubMed] [Google Scholar]
- 12. Pellicori P, Zhang J, Cuthbert J, Urbinati A, Shah P, Kazmi S, et al. High-sensitivity C-reactive protein in chronic heart failure: patient characteristics, phenotypes, and mode of death. Cardiovasc Res 2020;116:91–100. [DOI] [PubMed] [Google Scholar]
- 13. Khan MS, Usman MS, von Haehling S, Doehner W, Stewart Coats AJ. Ferric carboxymaltose for the treatment of iron-deficient heart failure patients: a systematic review and meta-analysis. ESC Heart Fail 2020;7:3392–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mazer CD, Hare GMT, Connelly PW, Gilbert RE, Shehata N, Quan A, et al. Effect of empagliflozin on erythropoietin levels, iron stores, and red blood cell morphology in patients with type 2 diabetes Mellitus and coronary artery disease. Circulation 2020;141:704–707. [DOI] [PubMed] [Google Scholar]
- 15. Ghanim H, Abuaysheh S, Hejna J, Green K, Batra M, Makdissi A, et al. Dapagliflozin suppresses hepcidin and increases erythropoiesis. J Clin Endocrinol Metab 2020;105:dgaa057. [DOI] [PubMed] [Google Scholar]
- 16. von Haehling S, Gremmler U, Krumm M, Mibach F, Schön N, Taggeselle J, et al. Prevalence and clinical impact of iron deficiency and anaemia among outpatients with chronic heart failure: the PrEP registry. Clin Res Cardiol 2017;106:436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bekfani T, Pellicori P, Morris D, Ebner N, Valentova M, Sandek A, et al. Iron deficiency in patients with heart failure with preserved ejection fraction and its association with reduced exercise capacity, muscle strength and quality of life. Clin Res Cardiol 2019;108:203–211. [DOI] [PubMed] [Google Scholar]
- 18. Sindone AP, Haikerwal D, Audehm RG, Neville AM, Lim K, Parsons RW, et al. Clinical characteristics of people with heart failure in Australian general practice: results from a retrospective cohort study. ESC Heart Fail 2021;8:4497–4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Masini G, Graham FJ, Pellicori P, Cleland JGF, Cuthbert JJ, Kazmi S, et al. Criteria for iron deficiency in patients with heart failure. J Am Coll Cardiol 2022;79:341–351. [DOI] [PubMed] [Google Scholar]
- 20. Pezel T, Audureau E, Mansourati J, Baudry G, Ben Driss A, Durup F, et al. Diagnosis and treatment of iron deficiency in heart failure: oFICSel study by the French heart failure working group. ESC Heart Fail 2021;8:1509–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Becher PM, Schrage B, Benson L, Fudim M, Corovic Cabrera C, Dahlström U, et al. Phenotyping heart failure patients for iron deficiency and use of intravenous iron therapy: data from the Swedish heart failure registry. Eur J Heart Fail 2021;23:1844–1854. [DOI] [PubMed] [Google Scholar]
- 22. Chobufo MD, Rahman E, Gayam V, Bei Foryoung J, Agbor VN, Farah F, et al. Prevalence and association of iron deficiency with anemia among patients with heart failure in the USA: NHANES 2017–2018. J Community Hosp Intern Med Perspect 2021;11:124–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fitzsimons S, Yeo TJ, Ling LH, Sim D, Leong KTG, Yeo PSD, et al. Impact of change in iron status over time on clinical outcomes in heart failure according to ejection fraction phenotype. ESC Heart Fail 2021;8:4572–4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klip IT, Comin-Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013;165:575–582.e3. [DOI] [PubMed] [Google Scholar]
- 25. Cohen-Solal A, Philip JL, Picard F, Delarche N, Taldir G, Gzara H, et al. Iron deficiency in heart failure patients: the French CARENFER prospective study. ESC Heart Fail 2022;9:874–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yeo TJ, Yeo PS, Ching-Chiew Wong R, Ong HY, Leong KTG, Jaufeerally F, et al. Iron deficiency in a multi-ethnic Asian population with and without heart failure: prevalence, clinical correlates, functional significance and prognosis. Eur J Heart Fail 2014;16:1125–1132. [DOI] [PubMed] [Google Scholar]
- 27. Ponikowski P, Kirwan BA, Anker SD, McDonagh Th, Dorobantu M, Drozdz J, et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double-blind, randomised, controlled trial. Lancet 2020;396:1895–1904. [DOI] [PubMed] [Google Scholar]
- 28. Graham FJ, Masini G, Pellicori P, Cleland JGF, Greenlaw N, Friday J, et al. Natural history and prognostic significance of iron deficiency and anaemia in ambulatory patients with chronic heart failure. Eur J Heart Fail 2022;24:807–817. [DOI] [PubMed] [Google Scholar]
- 29. Okonko DO, Mandal AK, Missouris CG, Poole-Wilson PA. Disordered iron homeostasis in chronic heart failure: prevalence, predictors, and relation to anemia, exercise capacity, and survival. J Am Coll Cardiol 2011;58:1241–1251. [DOI] [PubMed] [Google Scholar]
- 30. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, et al. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J 2010;31:1872–1880. [DOI] [PubMed] [Google Scholar]
- 31. Grote Beverborg N, van der Wal HH, Klip IT, Anker SD, Cleland J, Dickstein K, et al. Differences in clinical profile and outcomes of low iron storage vs defective iron utilization in patients with heart failure: results from the DEFINE-HF and BIOSTAT-CHF studies. JAMA Cardiol 2019;4:696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Beale AL, Warren JL, Roberts N, Meyer P, Townsend NP, Kaye D. Iron deficiency in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Open Heart 2019;6:e001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martens P, Nijst P, Verbrugge FH, Smeets K, Dupont M, Mullens W. Impact of iron deficiency on exercise capacity and outcome in heart failure with reduced, mid-range and preserved ejection fraction. Acta Cardiologica 2018;73:115–123. [DOI] [PubMed] [Google Scholar]
- 34. Beale A, Carballo D, Stirnemann J, Garin N, Agoritsas T, Serratrice J, et al. Iron deficiency in acute decompensated heart failure. J Clin Med 2019;8:1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nakano H, Nagai T, Sundaram V, Nakai M, Nishimura K, Honda Y, et al. Impact of iron deficiency on long-term clinical outcomes of hospitalized patients with heart failure. Int J Cardiol 2018;261:114–118. [DOI] [PubMed] [Google Scholar]
- 36. Cohen-Solal A, Damy T, Terbah M, Kerebel S, Baguet JP, Hanon O, et al. High prevalence of iron deficiency in patients with acute decompensated heart failure. Eur J Heart Fail 2014;16:984–991. [DOI] [PubMed] [Google Scholar]
- 37. Jankowska EA, Kasztura M, Sokolski M, Bronisz M, Nawrocka S, Ole kowska-Florek W, et al. Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur Heart J 2014;35:2468–2476. [DOI] [PubMed] [Google Scholar]
- 38. Lewis GD, Malhotra R, Hernandez AF, McNulty SE, Smith A, Felker GM, et al. Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency: the IRONOUT HF randomized clinical trial. JAMA 2017;317:1958–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009;361:2436–2448. [DOI] [PubMed] [Google Scholar]
- 40. Ponikowski P, van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, Mareev V, et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiencydagger. Eur Heart J 2015;36:657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Veldhuisen DJ, Ponikowski P, van der Meer P, Metra M, Böhm M, Doletsky A, et al. Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation 2017;136:1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Graham FJ, Pellicori P, Ford I, Petrie MC, Kalra PR, Cleland JGF. Intravenous iron for heart failure with evidence of iron deficiency: a meta-analysis of randomised trials. Clin Res Cardiol 2021;110:1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dignass AU, Gasche C, Bettenworth D, Birgegård G, Danese S, Gisbert JP, et al. European Consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis 2015;9:211–222. [DOI] [PubMed] [Google Scholar]
- 44. Ghafourian K, Chang HC, Ardehali H. Intravenous iron therapy in heart failure: a different perspective. Eur J Heart Fail 2019;21:703–714. [DOI] [PubMed] [Google Scholar]
- 45. Karavidas A, Troganis E, Lazaros G, Balta D, Karavidas IN, Polyzogopoulou E, et al. Oral sucrosomial iron improves exercise capacity and quality of life in heart failure with reduced ejection fraction and iron deficiency: a non-randomized, open-label, proof-of-concept study. Eur J Heart Fail 2021;23:593–597. [DOI] [PubMed] [Google Scholar]
- 46. Bolger AP, Bartlett FR, Penston HS, O’Leary J, Pollock N, Kaprielian R, et al. Intravenous iron alone for the treatment of anemia in patients with chronic heart failure. J Am Coll Cardiol 2006;48:1225–1227. [DOI] [PubMed] [Google Scholar]
- 47. Toblli JE, Lombrana A, Duarte P, Di Gennaro F. Intravenous iron reduces NT-pro-brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol 2007;50:1657–1665. [DOI] [PubMed] [Google Scholar]
- 48. Okonko DO, Grzeslo A, Witkowski T, Mandal AKJ, Slater RM, Roughton M, et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: a randomized, controlled, observer-blinded trial. J Am Coll Cardiol 2008;51:103–112. [DOI] [PubMed] [Google Scholar]
- 49. Filippatos G, Farmakis D, Colet JC, Dickstein K, Lüscher TF, Willenheimer R, et al. Intravenous ferric carboxymaltose in iron-deficient chronic heart failure patients with and without anaemia: a subanalysis of the FAIR-HF trial. Eur J Heart Fail 2013;15:1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Comin-Colet J, Lainscak M, Dickstein K, Filippatos GS, Johnson P, Lüscher TF, et al. The effect of intravenous ferric carboxymaltose on health-related quality of life in patients with chronic heart failure and iron deficiency: a subanalysis of the FAIR-HF study. Eur Heart J 2013;34:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ponikowski P, Filippatos G, Colet JC, Willenheimer R, Dickstein K, Lüscher T, et al. The impact of intravenous ferric carboxymaltose on renal function: an analysis of the FAIR-HF study. Eur J Heart Fail 2015;17:329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Okonko DO, Jouhra F, Abu-Own H, Filippatos G, Colet JC, Suki C, et al. Effect of ferric carboxymaltose on calculated plasma volume status and clinical congestion: a FAIR-HF substudy. ESC Heart Fail 2019;6:621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Anker SD, Kirwan BA, van Veldhuisen DJ, Filippatos G, Comin-Colet J, Ruschitzka F, et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Fail 2018;20:125–133. [DOI] [PubMed] [Google Scholar]
- 54. Ambrosy AP, von Haehling S, Kalra PR, Court E, Bhandari S, McDonagh T, et al. Safety and efficacy of intravenous ferric derisomaltose compared to iron sucrose for iron deficiency Anemia in patients with chronic kidney disease with and without heart failure. Am J Cardiol 2021;152:138–145. [DOI] [PubMed] [Google Scholar]
- 55. Lacour P, Dang PL, Morris DA, Parwani AS, Doehner W, Schuessler F, et al. The effect of iron deficiency on cardiac resynchronization therapy: results from the RIDE-CRT study. ESC Heart Fail 2020;7:1072–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Martens P, Dupont M, Dauw J, Nijst P, Herbots L, Dendale P, et al. The effect of intravenous ferric carboxymaltose on cardiac reverse remodelling following cardiac resynchronization therapy-the IRON-CRT trial. Eur Heart J 2021;42:4905–4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yeo TJ, Yeo PSD, Hadi FA, Cushway T, Lee KY, Yin FF, et al. Single-dose intravenous iron in southeast Asian heart failure patients: a pilot randomized placebo-controlled study (PRACTICE-ASIA-HF). ESC Heart Fail 2018;5:344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jankowska EA, Kirwan BA, Kosiborod M, Butler J, Anker SD, McDonagh T, et al. The effect of intravenous ferric carboxymaltose on health-related quality of life in iron-deficient patients with acute heart failure: the results of the AFFIRM-AHF study. Euro Heart J 2021;42:3011–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach As, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 60. Wang C, Graham DJ, Kane RC, Xie D, Wernecke M, Levenson M, et al. Comparative risk of anaphylactic reactions associated with intravenous iron products. JAMA 2015;314:2062–2068. [DOI] [PubMed] [Google Scholar]
- 61. Wolf M, Auerbach M, Kalra PA, Glaspy J, Thomsen LL, Bhandari S. Safety of ferric derisomaltose and iron sucrose in patients with iron deficiency anemia: the FERWON-IDA/NEPHRO trials. Am J Hematol 2021;96:E11–E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wolf M, Rubin J, Achebe M, Econs MJ, Peacock M, Imel EA, et al. Effects of iron isomaltoside vs ferric carboxymaltose on hypophosphatemia in iron-deficiency Anemia: two randomized clinical trials. JAMA 2020;323:432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stohr R, Sandstede L, Heine GH, Marx N, Brandenburg V. High-Dose ferric carboxymaltose in patients with HFrEF induces significant hypophosphatemia. J Am Coll Cardiol 2018;71:2270–2271. [DOI] [PubMed] [Google Scholar]
- 64. Sullivan JL. Iron and the sex difference in heart disease risk. Lancet 1981;1:1293–1294. [DOI] [PubMed] [Google Scholar]
- 65. Salonen JT, Nyyssonen K, Korpela H, Tuomilehto J, Seppänen R, Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation 1992;86:803–811. [DOI] [PubMed] [Google Scholar]
- 66. Tuomainen TP, Punnonen K, Nyyssonen K, Salonen JT. Association between body iron stores and the risk of acute myocardial infarction in men. Circulation 1998;97:1461–1466. [DOI] [PubMed] [Google Scholar]
- 67. Morrison HI, Semenciw RM, Mao Y, Wigle DT. Serum iron and risk of fatal acute myocardial infarction. Epidemiology 1994;5:243–246. [DOI] [PubMed] [Google Scholar]
- 68. De S D, Krishna S, Jethwa A. Iron status and its association with coronary heart disease: systematic review and meta-analysis of prospective studies. Atherosclerosis 2015;238:296–303. [DOI] [PubMed] [Google Scholar]
- 69. Gill D, Del Greco MF, Walker AP, Srai SKS, Laffan MA, Minelli C. The effect of iron Status on risk of coronary artery disease: a Mendelian randomization study-brief report. Arterioscler Thromb Vasc Biol 2017;37:1788–1792. [DOI] [PubMed] [Google Scholar]
- 70. Klip IT, Voors AA, Swinkels DW, Bakker SJL, Kootstra-Ros JE, et al. Serum ferritin and risk for new-onset heart failure and cardiovascular events in the community. Eur J Heart Fail 2017;19:348–356. [DOI] [PubMed] [Google Scholar]
- 71. Pacheco DA Q, Sookthai D, Wittenbecher C, Graf ME, Schübel R, Johnson T, et al. Red meat consumption and risk of cardiovascular diseases-is increased iron load a possible link? Am J Clin Nutr 2018;107:113–119. [DOI] [PubMed] [Google Scholar]
- 72. Schrage B, Rubsamen N, Ojeda FM, Thorand B, Peters A, Koenig W, et al. Association of iron deficiency with incident cardiovascular diseases and mortality in the general population. ESC Heart Fail 2021;8:4584–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Galaris D, Pantopoulos K. Oxidative stress and iron homeostasis: mechanistic and health aspects. Crit Rev Clin Lab Sci 2008;45:1–23. [DOI] [PubMed] [Google Scholar]
- 74. Iribarren C, Sempos CT, Eckfeldt JH, Folsom AR. Lack of association between ferritin level and measures of LDL oxidation: the ARIC study. Atherosclerosis risk in communities. Atherosclerosis 1998;139:189–195. [DOI] [PubMed] [Google Scholar]
- 75. Williams MJ, Poulton R, Williams S. Relationship of serum ferritin with cardiovascular risk factors and inflammation in young men and women. Atherosclerosis 2002;165:179–184. [DOI] [PubMed] [Google Scholar]
- 76. Ponikowska B, Suchocki T, Paleczny B, Olesinska M, Powierza S, Borodulin-Nadzieja L, et al. Iron status and survival in diabetic patients with coronary artery disease. Diabetes Care 2013;36:4147–4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jankowska EA, Wojtas K, Kasztura M, Mazur G, Butrym A, Kalicinska E, et al. Bone marrow iron depletion is common in patients with coronary artery disease. Int J Cardiol 2015;182:517–522. [DOI] [PubMed] [Google Scholar]
- 78. Inserte J, Barrabes JA, Aluja D, Otaegui I, Bañeras J, Castellote L, et al. Implications of iron deficiency in STEMI patients and in a murine model of myocardial infarction. JACC Basic Transl Sci 2021;6:567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zeller T, Waldeyer C, Ojeda F, Schnabel R, Schäfer S, Altay A, et al. Adverse outcome prediction of iron deficiency in patients with acute coronary syndrome. Biomolecules 2018;8:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cosentino N, Campodonico J, Pontone G, Guglielmo M, Trinei M, Sandri MT, et al. Iron deficiency in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Int J Cardiol 2020;300:14–19. [DOI] [PubMed] [Google Scholar]
- 81. Reinhold J, Papadopoulou C, Baral R, Vassiliou VS. Iron deficiency for prognosis in acute coronary syndrome—a systematic review and meta-analysis. Int J Cardiol 2021;328:46–54. [DOI] [PubMed] [Google Scholar]
- 82. Weidmann H, Bannasch JH, Waldeyer C, Shrivastava A, Appelbaum S, Ojeda-Echevarria FM, et al. Iron metabolism contributes to prognosis in coronary artery disease: prognostic value of the soluble transferrin receptor within the AtheroGene study. J Am Heart Assoc 2020;9:e015480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Huang CH, Chang CC, Kuo CL, Huang CS, Chiu TW, Lin CS, et al. Serum iron concentration, but not hemoglobin, correlates with TIMI risk score and 6-month left ventricular performance after primary angioplasty for acute myocardial infarction. PLoS One 2014;9:e104495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Florian A, Ludwig A, Rosch S, Yildiz H, Klumpp S, Sechtem U, et al. Positive effect of intravenous iron-oxide administration on left ventricular remodelling in patients with acute ST-elevation myocardial infarction—a cardiovascular magnetic resonance (CMR) study. Int J Cardiol 2014;173:184–189. [DOI] [PubMed] [Google Scholar]
- 85. van der AD, Grobbee DE, Roest M, Marx JJM, Voorbij HA, van der Schouw YT. Serum ferritin is a risk factor for stroke in postmenopausal women. Stroke 2005;36:1637–1641. [DOI] [PubMed] [Google Scholar]
- 86. Gill D, Monori G, Tzoulaki I, Dehghan A. Iron Status and risk of stroke. Stroke 2018;49:2815–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Marniemi J, Alanen E, Impivaara O, Seppänen R, Hakala P, Rajala T, et al. Dietary and serum vitamins and minerals as predictors of myocardial infarction and stroke in elderly subjects. Nutr Metab Cardiovasc Dis 2005;15:188–197. [DOI] [PubMed] [Google Scholar]
- 88. Ekblom K, Hultdin J, Stegmayr B, Johansson I, Van Guelpen B, Hallmans G, et al. Iron stores and HFE genotypes are not related to increased risk of ischemic stroke. A prospective nested case-referent study. Cerebrovasc Dis 2007;24:405–411. [DOI] [PubMed] [Google Scholar]
- 89. Gillum RF, Sempos CT, Makuc DM, Looker AC, Chien CY, Ingram DD. Serum transferrin saturation, stroke incidence, and mortality in women and men. The NHANES I epidemiologic followup study. National health and nutrition examination survey. Am J Epidemiol 1996;144:59–68. [DOI] [PubMed] [Google Scholar]
- 90. Doehner W, Scherbakov N, Schellenberg T, Jankowska EA, Scheitz JF, Haehling S, et al. Iron deficiency is related to low functional outcome in patients at early rehabilitation after acute stroke. J Cachexia Sarcopenia Muscle 2022;13:1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kvaslerud AB, Hussain AI, Auensen A, Ueland T, Michelsen AE, Pettersen KI, et al. Prevalence and prognostic implication of iron deficiency and anaemia in patients with severe aortic stenosis. Open Heart 2018;5:e000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rheude T, Pellegrini C, Michel J, Trenkwalder T, Mayr NP, Kessler T, et al. Prognostic impact of anemia and iron-deficiency anemia in a contemporary cohort of patients undergoing transcatheter aortic valve implantation. Int J Cardiol 2017;244:93–99. [DOI] [PubMed] [Google Scholar]
- 93. Rheude T, Pellegrini C, Lessmann L, Wiebe J, Mayr NP, Michel J, et al. Prevalence and clinical impact of iron deficiency in patients with severe aortic stenosis referred for transcatheter aortic valve implantation. Am J Cardiol 2019;124:1442–1448. [DOI] [PubMed] [Google Scholar]
- 94. Kvaslerud AB, Bardan S, Andresen K, Kløve SF, Fagerland MW, Edvardsen T, et al. Intravenous iron supplement for iron deficiency in patients with severe aortic stenosis scheduled for transcatheter aortic valve implantation: results of the IIISAS randomised trial. Eur J Heart Fail 2022;24:1269–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hanna-Rivero N, Tu SJ, Elliott AD, Pitman BM, Gallagher C, Lau DH, et al. Anemia and iron deficiency in patients with atrial fibrillation. BMC Cardiovasc Disord 2022;22:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Minhas AMK, Sagheer S, Shekhar R, Sheikh AB, Nazir S, Ullah W, et al. Trends and inpatient outcomes of primary atrial fibrillation hospitalizations with underlying iron deficiency Anemia: an analysis of the national inpatient sample database from 2004 -2018. Curr Probl Cardiol 2021;47:101001. [DOI] [PubMed] [Google Scholar]
- 97. Keskin M, Ural D, Altay S, Argan O, Börklü EB, Kozan Ö. Iron deficiency and hematinic deficiencies in atrial fibrillation: a new insight into comorbidities. Turk Kardiyol Dern Ars 2018;46:103–110. [DOI] [PubMed] [Google Scholar]
- 98. Tu SJ, Elliott AD, Hanna-Rivero N, Gallagher C, Mishima RS, Lyrtzis E, et al. Rationale and design of the IRON-AF study: a double-blind, randomised, placebo-controlled study to assess the effect of intravenous ferric carboxymaltose in patients with atrial fibrillation and iron deficiency. BMJ Open 2021;11:e047642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sonnweber T, Nairz M, Theurl I, Petzer V, Tymoszuk P, Haschka D, et al. The crucial impact of iron deficiency definition for the course of precapillary pulmonary hypertension. PLoS One 2018;13:e0203396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Yu X, Luo Q, Liu Z, Zhao Z, Zhao Q, An C, et al. Prevalence of iron deficiency in different subtypes of pulmonary hypertension. Heart Lung 2018;47:308–313. [DOI] [PubMed] [Google Scholar]
- 101. Yu X, Zhang Y, Luo Q, Liu Z, Zhao Z, Zhao Q, et al. Iron deficiency in pulmonary arterial hypertension associated with congenital heart disease. Scand Cardiovasc J 2018;52:378–382. [DOI] [PubMed] [Google Scholar]
- 102. Ruiter G, Lanser IJ, de Man FS, van der Laarse WJ, Wharton J, Wilkins MR, et al. Iron deficiency in systemic sclerosis patients with and without pulmonary hypertension. Rheumatology (Oxford) 2014;53:285–292. [DOI] [PubMed] [Google Scholar]
- 103. Ruiter G, Lankhorst S, Boonstra A, Postmus PE, Zweegman S, Westerhof N, et al. Iron deficiency is common in idiopathic pulmonary arterial hypertension. Eur Respir J 2011;37:1386–1391. [DOI] [PubMed] [Google Scholar]
- 104. Rhodes CJ, Howard LS, Busbridge M, Ashby D, Kondili Ea, Gibbs JSR, et al. Iron deficiency and raised hepcidin in idiopathic pulmonary arterial hypertension: clinical prevalence, outcomes, and mechanistic insights. J Am Coll Cardiol 2011;58:300–309. [DOI] [PubMed] [Google Scholar]
- 105. van Empel VP, Lee J, Williams TJ, Kaye DM. Iron deficiency in patients with idiopathic pulmonary arterial hypertension. Heart Lung Circ 2014;23:287–292. [DOI] [PubMed] [Google Scholar]
- 106. Tatah J, Keen JL, Prisco SZ, Pritzker M, Thenappan T, Prins KW. Iron deficiency is associated with more severe pulmonary vascular disease in pulmonary hypertension caused by chronic lung disease. Chest 2022;161:232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Plesner LL, Schoos MM, Dalsgaard M, Goetze JP, Kjøller E, Vestbo J, et al. Iron deficiency in COPD associates with increased pulmonary artery pressure estimated by echocardiography. Heart Lung Circ 2017;26:101–104. [DOI] [PubMed] [Google Scholar]
- 108. Frise MC, Cheng HY, Nickol AH, Curtis MK, Pollard KA, Roberts DJ, et al. Clinical iron deficiency disturbs Normal human responses to hypoxia. J Clin Invest 2016;126:2139–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Smith TG, Talbot NP, Privat C, Rivera-Ch M, Nickol AH, Ratcliffe PJ, et al. Effects of iron supplementation and depletion on hypoxic pulmonary hypertension: two randomized controlled trials. JAMA 2009;302:1444–1450. [DOI] [PubMed] [Google Scholar]
- 110. Zou HX, Qiu BQ, Lai SQ, Zhou XL, Gong CW, Wang LJ, et al. Iron metabolism and idiopathic pulmonary arterial hypertension: new insights from bioinformatic analysis. Biomed Res Int 2021;2021:5669412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Decker I, Ghosh S, Comhair SA, Farha S, Wilson Tang WH, Park M, et al. High levels of zinc-protoporphyrin identify iron metabolic abnormalities in pulmonary arterial hypertension. Clin Transl Sci 2011;4:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Jankowich M, Elston B, Evans SK, Wu WC, Choudhary G. Relationship of iron deficiency and Serum ferritin levels with pulmonary hypertension: the Jackson heart study. PLoS One 2016;11:e0167987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Viethen T, Gerhardt F, Dumitrescu D, Knoop-Busch S, ten Freyhaus H, Rudolph TK, et al. Ferric carboxymaltose improves exercise capacity and quality of life in patients with pulmonary arterial hypertension and iron deficiency: a pilot study. Int J Cardiol 2014;175:233–239. [DOI] [PubMed] [Google Scholar]
- 114. Ruiter G, Manders E, Happe CM, Schalij I, Groepenhoff H, Howard LS, et al. Intravenous iron therapy in patients with idiopathic pulmonary arterial hypertension and iron deficiency. Pulm Circ 2015;5:466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Howard L, He J, Watson GMJ, Huang L, Wharton J, Luo Q, et al. Supplementation with iron in pulmonary arterial hypertension. Two randomized crossover trials. Ann Am Thorac Soc 2021;18:981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Olsson KM, Fuge J, Brod T, Kamp JC, Schmitto J, Kempf T, et al. Oral iron supplementation with ferric maltol in patients with pulmonary hypertension. Eur Respir J 2020;56:2000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
‘No new data were generated or analysed in support of this research’