Abstract
The authors’ institution utilizes multi-staged induced membrane technique protocol based on post-debridement culture in treating patients with critical-sized bone defect in lower extremity due to infected nonunion or post-traumatic osteomyelitis. This study aimed to evaluate the success rate of this limb reconstruction method and which risk factors are associated with recurrence of infection. 140 patients were treated with multi-staged induced membrane technique from 2013 to 2018 and followed up more than 24 months after bone grafting. The primary success rate of limb reconstruction was 75% with a mean follow-up of 45.3 months. The mean Lower Extremity Functional Scale in success group improved from 12.1 ± 8.5 to 56.6 ± 9.9 after the treatment. There were 35 cases of recurrence of infection at a mean of 18.5 months after bone grafting. Independent risk factors for recurrence of infection were infected free flap, surprise positive culture, deviation from our surgical protocol, and elevated ESR before final bone graft procedure. In conclusion, this study showed that multi-staged induced membrane technique protocol based on post-debridement culture resulted in 75% success rate and revealed a number of risk factors for recurrence of infection.
Subject terms: Trauma, Outcomes research
Introduction
The induced membrane technique (IMT), which includes debridement and placement of polymethyl methacrylate (PMMA) cement during the first stage, followed by autogenous bone grafting once the osteoinductive membrane chamber has formed during the second stage, has been used to address critical-sized bone defects (CSBDs)1,2. Additionally, this technique has been applied and modified to treat chronic bone infections including the treatment of infected nonunions (IN) and post-traumatic osteomyelitis (PTOM)3,4.
Although several clinical studies on the biologically active membrane’s ability to consolidate grafted bone have yielded favorable results, high rates of recurrence of infection(ROI) have been reported following the staged reconstruction protocol5,6. With few large homogenous series using a standardized IMT protocol for the treatment of IN and PTOM, there is little evidence to support their effectiveness in treating these often challenging infections.
Our institution has treated recalcitrant IN and PTOM using a post-debridement culture (PDC)-based, multi-staged IMT protocol since 20137,8,9. In light of the above, we aimed to answer the following research questions: (1) What is the success rate of limb reconstruction in patients with CSBD of the lower extremity due to IN or PTOM using our PDC-based, multi-staged IMT protocol and (2) What risk factors are associated with ROI in this population?
Material and methods
Design and settings
We retrospectively analyzed a cohort of patients diagnosed with Fracture- related Infection (FRI) criteria of the lower extremity from January 2013 to 2018 at a trauma fellowship training university hospital10. The limb reconstruction team in hospital have been operated by two orthopaedic trauma fellowship-trained surgeons, one microsurgery trained plastic surgeon and one hand and microsurgery trained orthopaedic surgeon.
The patients had already undergone unsuccessful surgical treatment for infection of their IN or PTOM at least twice at an outside hospital and presented to our institution with clinical, laboratory, and radiographic findings all consistent with recalcitrant Fracture-related Infections11,12. Patients who underwent a PDC-based, multi-staged IMT protocol for the treatment of IN or PTOM and post-debridement CSBD reconstruction of the femur or tibia (critical sized bone defect was defined as ‘segmental defects of at least 2.5 cm in longitudinal axis or more than 50% of circumferential wedge bone defect’) were enrolled. We excluded patients with affected upper extremities, those who were treated using other modalities, including primary amputation and distraction osteogenesis.
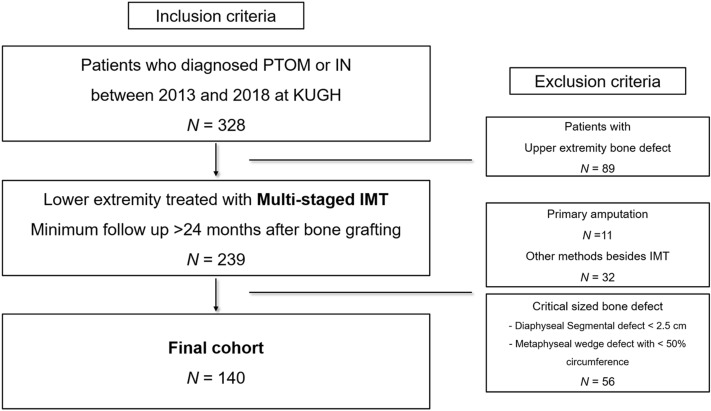
Among 239 patients who were treated by recalcitrant IN or PTOM in the lower extremity and followed up at least more than 24 months, 11 patients who were treated by primary amputation, 24 patients who were treated by distraction osteogenesis, 8 patients who did not want to reach final bone grafting then, had the defect filled with permanent cement and 56 patients whose defect before bone grafting was not matched with the radiologic criteria of critical size bone defect were excluded. A total of 140 patients were consisted of the final cohort (Fig. 1).
Figure 1.
Final cohort based on inclusion and exclusion criteria.
PDC based, multi-staged IMT protocol
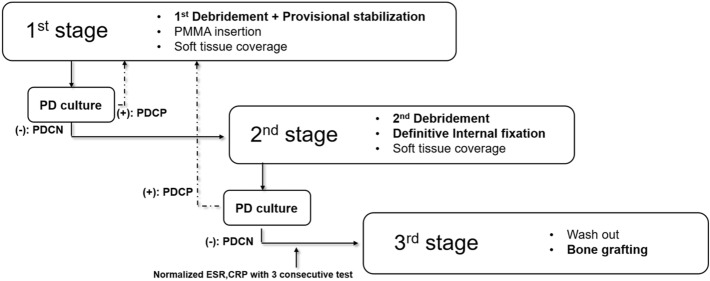
Our surgical protocol is based on the IMT, including multiple stages of reconstruction to address recalcitrant recurrent infections as per the aforementioned protocol7,8,9. The first stage involved thorough debridement and antibiotics loaded cement spacer insertion (vancomycin 4 g + , tobramycin 0.4 g per 40 g PMMA), followed by temporary stabilization using an external fixator, temporary plating, an antibiotic-loaded cement rod, or a combination thereof. The second stage included secondary debridement and conversion to the definitive fixation construct using an antibiotic-loaded PMMA cement spacer. In this stage, long bone realignment was achieved with nailing, plating, or a combination of methods. The final stage involved removing the cement spacer and bone grafting once the infection was eradicated. We confirmed the latter using serial serologic examination, including ESR and CRP. Clinical manifestations in the donor site were investigated via outpatient follow-up every 2–3 months. If soft tissue was infected or too fragile to cover the defect area, coverage with a fasciocutaneous or muscle flap was implemented at the first or second debridement stage before the final bone grafting (Fig. 2).
Figure 2.
Author’s PDC based multi-staged induced membrane technique. The first stage involved thorough debridement and antibiotics loaded cement spacer insertion, followed by temporary stabilization using an external fixator, temporary plating, an antibiotic-loaded cement rod, or a combination thereof. The second stage included secondary debridement and conversion to the definitive fixation construct using an antibiotic-loaded PMMA cement spacer. The final stage involved removing the cement spacer and bone grafting once the infection was eradicated.
During the staged surgical protocol, we systematically assessed site-specific tissue cultures collected intraoperatively to identify the associated microorganisms. At each stage of the protocol (stages 1–3), tissue cultures were obtained. At least five intraoperative tissue cultures were taken, beginning at the center of the infected bony lesion and out to the periphery during the debridement stage of the procedure (defined as: center, intramedullary proximal, intramedullary distal, extramedullary proximal, and extramedullary distal). The first culture set was named the index culture. Copious irrigation with normal saline followed thorough debridement. Next all drapes and contaminated instruments were removed from the field and changed and post debridement cultures (PDC) were obtained in a similar fashion as the index cultures (5 cultures were obtained starting at the center of the lesion working to the periphery) (Fig. 3). With our staged protocol, negative PD cultures (PDCN) were required to proceed to the next stage. When the PD culture was identified as positive (PDCP), we repeated the previous stage with irrigation and debridement with additional cultures (both index and post debridement) obtained from the site. Whenever there was hardware, sonication fluid culture was taken to increase the detection rate of microorganism.
Figure 3.
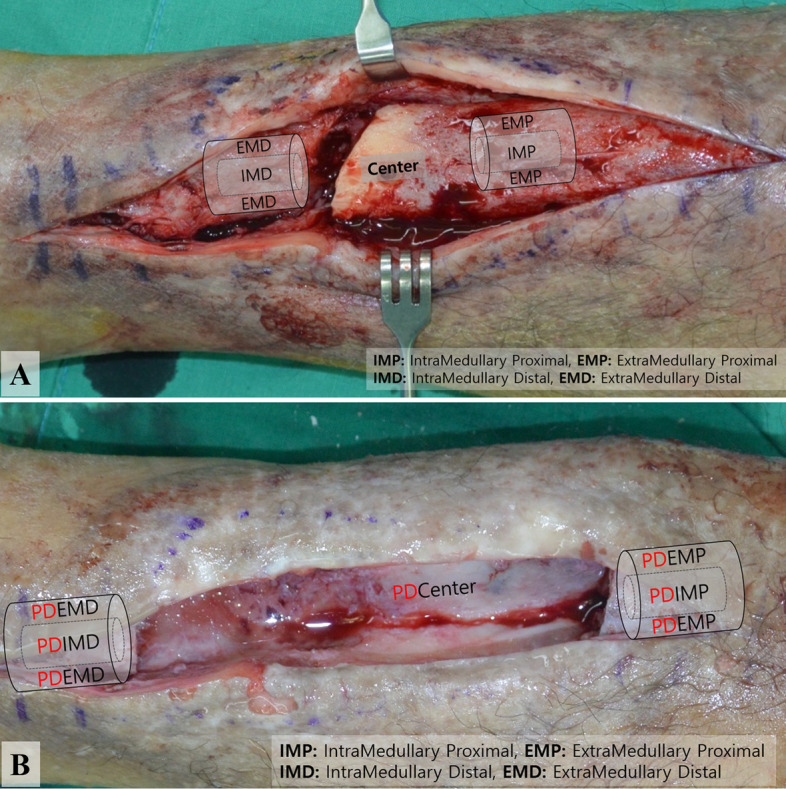
Intraoperative site-specific culture, including both index (A) and PD cultures (B).
General antibiotic protocol
All patients received at least 2 weeks of an antibiotic drug holiday prior to being engaged in the protocol. Once we identified the microorganisms from the initial debridement, targeted intravenous antibiotics were administered under the guidance of an infectious disease specialist. Intravenous antibiotics were administered for at least 6 weeks. “Surprise culture positive” cases were those in which a positive index culture result followed a negative post-debridement culture result from the previous stage13. In such cases, adjuvant targeted IV antibiotics were administered for at least 4 or 6 weeks from the time of last positive culture (Fig. 4).
Figure 4.
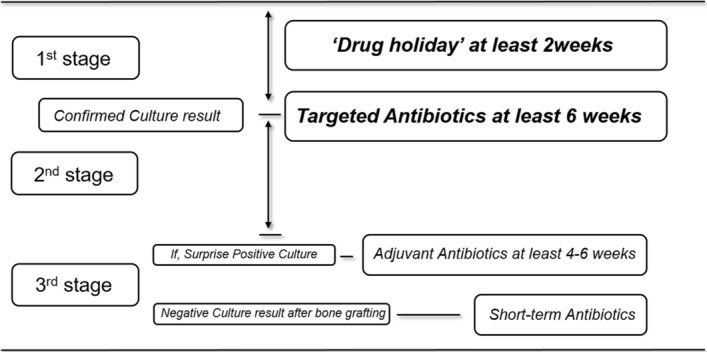
Antibiotics protocol during multi-staged IMT. All patients received at least 2 weeks of an antibiotic drug holiday prior to being engaged in the protocol. Once we identified the microorganisms from the initial debridement, targeted intravenous antibiotics were administered under the guidance of an infectious disease specialist. Intravenous antibiotics were administered for at least 6 weeks. “Surprise culture positive” cases were those in which a positive index culture result followed a negative post-debridement culture result from the previous stage. In such cases, adjuvant targeted IV antibiotics were administered for at least 4 or 6 weeks from the time of last positive culture.
Study variables
Patient demographics, intraoperative findings, and clinical follow-up data were collected prospectively and analyzed retrospectively. Patient demographics, including age, sex, body mass index, and medical comorbidities, were documented.
The severity of infection, including duration and details regarding previous infection control surgeries, were analyzed by medical chart review and previous radiographs. The location of infection was subdivided using AO classification14. The type of bone infection was classified using the Cierny-Mader classification15.
During the staged operations, microorganisms grown from intraoperative cultures were documented. Patients were divided into culture-negative, monomicrobial, and polymicrobial infections. Anaerobic microorganisms, gram-negative microorganisms, and pseudomonal infections were documented as independent variables.
We analyzed the bone grafting environment, including the size (< 50 mm, 50-100 mm or > 100 mm) and type of bone defect (segmental type or wedged type); condition of the surrounding soft tissue and associated major vessels; number of debridement procedures before the final bone grafting; membrane duration; and fixation construct type. Details regarding the bone grafting, including the properties of the graft material (pure autogenous bone grafting or mixed grafting with demineralized bone matrix (DBM)), method of grafting, addition of bone morphogenetic protein 2, and operation time, were analyzed. Serologic markers, including Erythrocyte Sedimentation Rate (ESR) and C-Reactive Protein (CRP) prior to bone grafting, were documented.
Adherence to the surgical protocol was analyzed as a variable. Cases which diverted from the standardized protocol were termed “deviation of protocol.” These included instances where (1) protocol stages were combined or overlapped, (2) occasions where we did not revert back to a previous stage after positive cultures (e.g. PDCP from a previous stage) and (3) PDCP at second-stage treated with debridement and implant retention (where normal protocol calls for removal of previously placed implants in a PDCP). These three reasons for deviation of the protocol were all examined.
Outcome measures
The primary endpoint of this study was the success rate of limb reconstruction after our staged IMT for the treatment of IN and PTOM. We defined a successful reconstruction as no ROI at minimum 2-year follow-up after the final bone grafting with bony consolidation of the CSDB. The bony consolidation was evaluated with consecutive CT scans and determined by one independent observer who has completed an orthopaedic trauma fellowship. In addition, the functional outcomes before and after the treatment were evaluated at first visit and last follow up using the lower extremity functional scale (LEFS)16,17.
ROI was diagnosed with Fracture- related Infection (FRI) criteria10. When ROI was suspected based on clinical manifestations, such as erythema, swelling, pain, and elevated laboratory values including serum white blood cell count, and inflammatory markers, the final diagnosis was confirmed by intraoperative culture results during subsequent infection control surgery. The secondary endpoint of this study was to identify the risk factors associated infection recurrence.
Statistical analysis
Statistical analysis was performed using the SPSS software package (SPSS, Chicago, IL, USA). We performed a descriptive analysis of the epidemiological and morphological factors of the cohort. Survival analysis was performed using Kaplan–Meier curves, and ROI was defined as the end-point. To determine the risk factors associated with ROI, multivariate Cox regression analysis was performed using the variables with p values < 0.05, as determined using two-tailed tests.
Ethical approval
All experimental protocols were approved by Korea University Guro Hospital Institutional Review Board. All procedures and methods performed in studies involving human participants were in accordance with the ethical standards of the institution or practice at which the studies were conducted. (IRB number: 2021GR0003) Informed consent was obtained from all individual participants included in the study.
Results
One hundred fifteen male and 25 female patients with a mean age of 52.3 (range, 20–87) years, with a total of 43 femurs and 97 tibias were included in the study. The mean number of previous infection control surgeries at other hospitals prior to presenting to our institution was 3.5 (range, 2–18), while the mean infection duration before introduction of our protocol was 49.3 months (range, 2–239 months).
Eighty-one patients had monomicrobial infections, 39 patients had polymicrobial, and 20 patients had culture-negative infections. The mean length of the defects was 90.1 mm (range 30.3–219.0 mm). There were 110 segmental defects and 30 wedge type defects. Among the 70 flap-covered defects, 23 were performed at other hospitals and were found to have residual infection during the multi-staged IMT protocol. We used the term “infected previous free flap” for analysis.
Prior to bone grafting, the mean number of times patients underwent debridement and fixation surgery was 2.5 (range 1–7). The mean duration of the membrane at bone grafting was 129.5 days (range 42–375 days). The defects were stabilized using intramedullary nails in 69 patients, plating in 34 patients, and combined plate/nail fixation in 37 patients. Pure autogenous bone grafting alone was used in 70 patients, while mixed bone grafting with DBM (ALLOMIX®, CGBIO) was performed in other 70 patients. There was a deviation in protocol in 41 patients. This included combining stages of the protocol in 10 patients, 22 patients where the decision was made not to return to a previous stage in the protocol after a PDCP from a previous stage, and 9 patients who had retention of implants after PDCP. An elevated ESR at the time of bone grafting was observed in 84 patients (Tables 1, 2).
Table 1.
Demographic, location and severity of infection, Microorganism, Environment of bone grafting, Details of bone grafting, and surgical procedure in 140 patients.
| Variables | N or mean | Range | SD | SE | 95% C.I |
|---|---|---|---|---|---|
| Patient demographics | |||||
| Age | 52.3 yrs | 20–87 | 15.1 | 1.32 | 49.94–55.15 |
| Sex | Female: 25 | ||||
| Male: 115 | |||||
| ASA classification | I: 47 | ||||
| II: 70 | |||||
| III: 19 | |||||
| IV: 4 | |||||
| Previous smoking history | Smoker: 64 | ||||
| Non-Smoker: 76 | |||||
| Diabetes | 23 pts | ||||
| BMI | 25.6 kg/m2 | 19.3–38.8 | 3.6 | 0.3 | 24.99–26.17 |
| Normal < 25 | 68 | ||||
| 25 < Overweight < 30 | 58 | ||||
| Obesity > 30 | 14 | ||||
| Location and severity of infection | |||||
| Location of infection | Femur: 43 cases | ||||
| 32:23 | |||||
| 33:14 | |||||
| 33 with knee joint involvement: 6 | |||||
| Tibia : 97 cases | |||||
| 41:21 | |||||
| 42:43 | |||||
| 43:19 | |||||
| 43 with ankle joint involvement : 14 | |||||
| Duration of infection | 49.3 months | 3–239 | 27.2 | 14.4 | 44.8–53.8 |
| < 3 month: | 24 | ||||
| 3–24 month | 68 | ||||
| > 24 month | 48 | ||||
| No. of previous infection control surgery | 3.5 times | 2-18 | 2.68 | 0.22 | 3.15–4.04 |
| C-M classification | III: 28 | ||||
| IV: 112 | |||||
| No. of microorganism | Culture negative : 20 | ||||
| Monomicrobial : 81 | |||||
| Polymirobial: 39 | |||||
| Presence of P.aeruginosa | 23 | ||||
| Presence of anerobes | 26 | ||||
| Presence of Gram negative organism | 42 | ||||
| Bone grafting environment | |||||
| Type of defect | Segmental :110 | ||||
| Wedged : 30 | |||||
| Length of defect | 90.1 mm | 30.3–219.0 | 41.2 | 3.48 | 83.13–96.63 |
| < 50 mm | 25 | ||||
| 50-100 mm | 67 | ||||
| > 100 mm | 48 | ||||
| Soft tissue coverage | Flap coverage: 70 | ||||
| None: 70 | |||||
| Infected previous flap | 23 | ||||
| Single vessled limb | 29 | ||||
| No. of stages before Bone grafting | 2.5 | 1-7 | 1 | 0.1 | 2.34–2.69 |
| Duration of induced membrane | 129.5 days | 42–375 | 57.3 | 4.84 | 120.45–139.22 |
| < 90 days | 32 | ||||
| 90–120 days | 38 | ||||
| > 120 days | 70 | ||||
| Fixation construct | Nailing : 69 | ||||
| Plating : 34 | |||||
| Combined : 37 | |||||
| Details of bone grafting | |||||
| Property of bone grafts | Pure autogenous bone graft:70 | ||||
| Mixed graft with DBM:70 | |||||
| Percentage of DBM (%) | 35.3 | 10.3–50.0 | 11.4 | 2.84 | 32.63–37.97 |
| Addition of BMP-2 | 14 cases | ||||
| Circumferential Bone grafting | 55 cases | ||||
| Mean operation time | 215 min | 62–445 | 71.5 | 6.04 | 203.48–225.72 |
| Surgical procedure | |||||
| Deviation of protocol | 41 | ||||
| Surprise Positive Culture | 29 | ||||
| Abnormal ESR before BG | 84 | ||||
| Abnormal CRP before BG | 16 | ||||
S.D.: Standard Deviation, S.E.: Standard Error, C.I.: Confidence Interval, BMI: Body Mass Index, ASA classification: American Society of Anesthesiologists physical status classification, DBM: Demineralized Bone Matrix.
Table 2.
Isolated microorganisms.
| Microorganism | |
|---|---|
| Single Microorganism | 81 (57.9%) |
| MRSA | 25 |
| MSSA | 13 |
| MRCN | 13 |
| Pseudomonas aeruginosa | 11 |
| Enterobacter cloacae | 4 |
| Enterococcus faecalis | 4 |
| MSSE | 2 |
| Staphylococcus lugdunensis | 2 |
| Serratia marcescnes | 1 |
| E. coli | 1 |
| Burkholderia arboris | 1 |
| Enterobacter hormaechei | 1 |
| Corynebacterium striatum | 1 |
| Staphylococcus caprae | 1 |
| Bacteriodes fragilis | 1 |
| Poly-Microorganism | 39 (27.9%) |
| 2 | 25 |
| 3 | 12 |
| > 4 | 2 |
| Culture negative infection | 20 (14.3%) |
MRSA: methicillin-resistant staphylococcus aureus, MSSA: methicillin-susceptible staphylococcus aureus, MRCN: methicillin- resistant coagulase negative streptococcus epidermidis, MSSE: methicillin-susceptible streptococcus epidermidis.
Primary outcome
A total of 140 patients had follow-up of at least 2 years after bone grafting. The primary success rate at 2 years was 75% (105/140). The mean infection-free interval was 45.3 months (range 24–84). The primary success rate of tibia and femur was 71.1% (69/97) and 83.7% (36/43), respectively. After excluding all “deviated protocol” cases, the success rate of our multi-staged IMT was 82.8% (82/99), (Tibia: 80.6% (54/67), Femur: 87.5% (28/32), respectively). The mean LEFS score in success group before and after the treatment were 12.1 ± 8.5 and 56.6 ± 9.9, respectively. Infection recurrence after final bone grafting occurred in 35 patients. The mean time of recurrence was 18.5 months (range 1–49 months) after final bone grafting. (Fig. 5).
Figure 5.
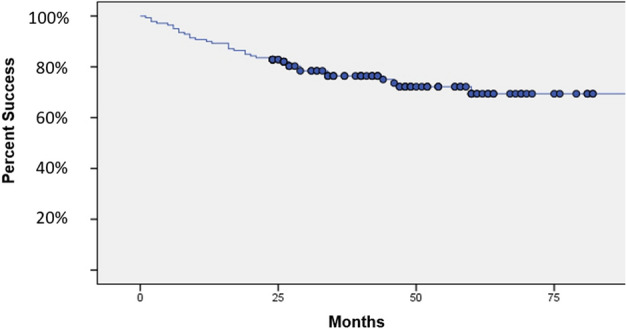
Kaplan–Meier curve showing success rate of the PDC based, multi-staged IMT with the end point for recurrence of infection. The success rate of multi-staged IMT at 24 months after bone grafting was approximately 80%.
Secondary outcome—which factors determined infection recurrence?
Univariate cox regression analysis showed that the following variables were associated with ROI: “Type of soft tissue coverage,” “Number of stages before bone grafting,” “Infected previous free flap,” “Surprise positive culture,” “Deviation of protocol,” and “Elevated ESR before bone grafting”. Multivariate cox regression analysis identified the following variables as independent risk factors associated with ROI: “Infected previous free flap,” “Surprise positive culture,” “Deviation of protocol,” and “Elevated ESR before bone grafting” (Table 3).
Table 3.
Risk factor analysis of Recurrence of Infection.
| Variables | Univariate cox regression | Multivariate cox regression | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Type of soft tissue | 2.113 (0.986–4.531) | 0.0445 | 1.537 (0.629–3.757) | 0.345 |
| Number of stages before BG | 1.437 (1.116–1.85) | 0.005 | 1.083 (0.845–1.387) | 0.5284 |
| Infected previous free flap | 2.38 (1.19–4.761) | 0.0142 | 3.14 (1.393–7.077) | 0.0058* |
| Surprise culture positive | 4.736 (2.359–9.507) | 0.0001 | 3.608 (1.733–7.514) | 0.0006* |
| Deviation of protocol | 3.341 (1.703–6.553) | 0.0004 | 2.585 (1.295–5.163) | 0.0071* |
| Elevated ESR before BG | 2.525 (1.142–5.581) | 0.0221 | 2.24 (1.007–4.98) | 0.0479* |
*Significant at p value < 0.05.
BG: bone grafting.
Discussion
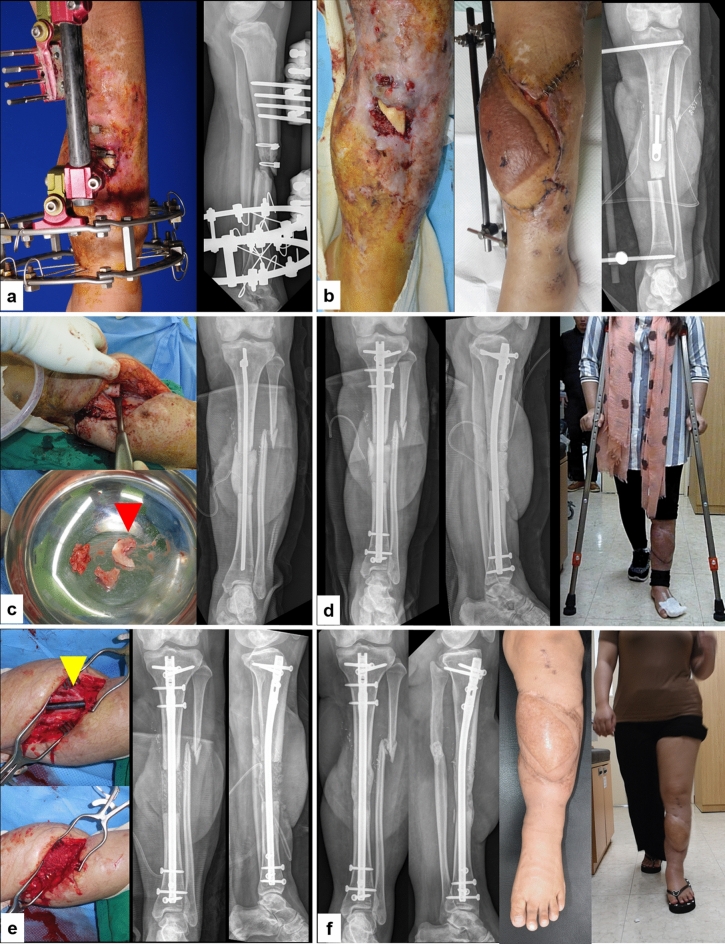
In our study, the success rate of limb reconstruction of CSBD in patients with IN and PTOM is slightly lower than in previous reports. Xie et al. reported an overall cure rate of 87.74% after 16 months of follow-up among 424 cases of osteomyelitis of the extremities treated with IMT18. One meta-analysis reported a primary success rate of 82% when the Masquelet technique was used in the treatment of 427 cases including the treatment of tumor reconstruction, infection (osteomyelitis and infected nonunions), aseptic nonunions and post-traumatic defects across 17 studies19. These slight differences in success rates can be attributed to several causes, including differences in the bone defects and anatomical sites of treatment, such as the upper extremity and in non-long bone infections; the severity of the infection; and follow-up duration. Our study evaluated cases of recalcitrant long bone infections of the lower extremity with a mean duration of 49 months before presentation to our institution, with a Cierny-Mader classification of III or IV that underwent at least two failed treatments at outside hospitals. All reasons why our infection eradication outcomes differed from those reported in previous case series using the IMT. However, our success rate of the 99 cases that did not deviate from our protocol, demonstrated similar rates (82.8%) from previous studies (Fig. 6).
Figure 6.
(a) A 24-year-old female patient sustained a recalcitrant infected nonunion of the left tibia. (b) According to the Multi-staged IMT protocol, we performed thorough debridement, placed an antibiotic-loaded PMMA spacer, and provided temporary bony stabilization. Soft tissue defects were covered using an anterolateral thigh flap (stage 1). (c) As PDCP had been identified at EMP and IMP (pseudomonas and MRSA), targeted debridement at proximal area of bone defect (red arrow head, sequestrum) and temporary fixation using antibiotics loaded PMMA coated intramedullary rod was performed (repetitive stage1). (d) After confirming all PDCN at previous stage, definitive fixation using IM nail was performed. An antibiotic-loaded PMMA spacer was reinserted into the bone defect for staged bone grafting to confirm that the infection had been eradicated (stage 2). Prompt partial weight bearing exercise was allowed after definitive fixation. (e) The 6-cm sized segmental bone defect was filled with autogenous bone (45 g) and DBM (15 g) in induced membrane chamber (yellow arrow head) at 16 weeks after stage 2 (stage 3). (f) Two years follow up radiographs show complete consolidation of the grafted bone without recurrent infection. The injured limb recovered to full function.
Our results demonstrate how a PDC-based, multi-staged IMT is a promising treatment modality for recalcitrant IN or PTOM in the lower extremity by addressing residual infection, bony deformity, weight-bearing requirements, disuse osteopenia, soft tissue atrophy, and bone defects in a systemic stepwise fashion.
Our protocol emphasizes additional debridements and intraoperative cultures after these serial debridements. There is some debate as to how many additional debridements are needed to manage the infection. Although cases of false positive intraoperative cultures due to contamination have been reported20, the cultures and histological findings of the resected margins must be heavily considered when diagnosing residual osteomyelitis21,22. In our protocol, a PDCP indicated residual infection, thereby enabling targeted debridement for the next procedure. To reduce the likelihood of contamination, we changed all surgical drapes and instruments frequently.
Our treatment protocol is based on tissue cultures. Previous studies have reported low sensitivity of tissue culture results between 30 and 61%. To overcome the low detection rate of tissue culture, other methods such as PCR or inflammatory cell counts (PMN cell counts), next-generation sequencing are suggested in the literature. However, these methods do not have clear evidence yet or clinical application is limited10,23. In our protocol, to increase the detection rate of microorganisms, we tried to take tissue samples from multiple sites including PD cultures, implant sonication fluid cultures were taken, and these cultures were taken in each stage of the protocol.
Use of antibiotics plays a critical role in treating bone infections. As we mentioned, targeted antibiotics were administered for at least 6 weeks. Consultation with infectious disease specialists regarding the culture results, type of host, intraoperative findings is essential in selecting the appropriate antibiotics. Coadministration of rifampicin in the setting of retaining implant can be very effective against staphylococcal biofilm24. Also, longer duration upto 12 weeks with implant retention is suggested according to the recommendations from fracture-related infection expert group25.
Treatment of infected bone defects using induced membrane technique originally consisted of two stages as reported by Masquelet, et al. On the contrary, our protocol consists of three stages. By implementing definitive fixation (2nd Stage) and bone grafting (3rd Stage) as separate stages, we were able to confirm eradication of the infection at multiple time points prior to final bone grafting. Additionally, by creating a stable fixation construct at the 2nd stage of the protocol, early range of motion and partial weight bearing was permitted following stage 2, as the PMMA could help to bear the load of the body weight. Another potential benefit to having separate stages for fixation and bone grafting is the ability to perform complex deformity corrections in a staged manner to decrease the surgical time required when combining fixation and bone grafting. In spite of these advantages of three staged protocol, we suggest that reducing the stages or combining 2nd and 3rd stage should be considered when the PD cultures are negative to reduce the burden on the patients.
Although previous reports have demonstrated that patients who needed soft tissue reconstruction with free flap coverage were more likely to have postoperative complications18,26, a thorough surgical debridement of infected tissue following a systematic soft tissue reconstruction is critical to achieve favorable results2,27. Type of soft tissue coverage was not statistically significant as a solitary risk factor in the multivariate regression model. However, among the 23 infected previous flaps, the ROI increased to 56.5% (13/23), and this variable was proven to be a solitary risk factor of ROI in our cohort (HR: 3.14, p = 0.0058). Recurrence of infection after an infected previous free flap can attributed to several factors. All these patients had initial free flaps at outside hospitals and were treated initially by limited debridement without a systematic protocol. These 23 patients with infected previous free flaps had a high ROI (13/23) possibly due to less aggressive excisional debridement for fear of compromising the flap and flap blood supply.
Although the bioactivity of the membrane has been found to peak 6–8 weeks after PMMA cement insertion28–30, eradication of infection must be verified before bone grafting. It was suggested that clinical examination, trends in acute inflammatory markers (including ESR and CRP), and intraoperatively obtained specimens pertaining to culture and pathology should be considered before bone grafting31. In addition, the clinical value of ESR was found to be a sensitive, specific, and independent marker for osteomyelitis recurrence32,33. We also could prove that the case when bone grafting was performed with elevated ESR prone to recur.
Detecting indolent infection is imperative during aseptic nonunion repair and revision arthroplasty13,34. Olszewski et al. identified 83 positive surprise cultures among 453 nonunion cases with suspected indolent infection-related, reporting an 80% survival rate following either residual infection or nonunion treated with an additional 6–8 weeks of antibiotics13. In the study by Tsukayama et al., the proportion of positive intraoperative cultures among 275 cases of revision hip arthroplasty for suspected aseptic loosening was 11%, with a 90% success rate after 6 weeks of antibiotics35. In our cohort of 140 cases, we found that 29 cases (20.7%) of intraoperative “surprise positive culture” progressed to the next treatment stage. Although we treated indolent infection with 6 weeks of antibiotics, only 14 cases (48.3%) were successfully treated. Hence, a “surprise positive culture” during our staged IMT protocol was associated with a 3.6-fold increased risk of recurrence (p = 0.0006). Contrary to prior reports on indolent infections, additional debridement must be considered when such positive cultures are found during staged reconstruction, as antibiotic suppression therapy may be insufficient.
“Deviation of protocol” was defined as an alteration of the treatment protocol with combining protocol stages, retaining implants after PDCP, and not returning to a previous stage of the protocol after PDCP. A total of 41 cases were identified as Deviation of protocol in our cohort. These deviations in protocol were independent risk factors for ROI. Of these 41 cases, 18 resulted in ROI (43.9%). A deviation from our protocol resulted in a 2.6-fold higher risk of ROI (p = 0.0071). This demonstrates adhering to a systemized reconstruction protocol is crucial when treating recalcitrant chronic bone infections (Table 3).
The inherent limitations of any retrospective cohort study is also applied to the present study despite the data being collected prospectively. First, we did not consider cases in which patients underwent amputation preceding bone grafting for uncontrolled and disseminated infection. Second, while we identified four variables predicting ROI, it is likely that more exist. Third, it was difficult to conclude whether recurrence of infection was due to latent or new infection. Despite these limitations, this study includes a large cohort of patients with CSBD secondary to IN and PTOM, treated with a systematic, detailed protocol with clearly defined outcomes while elucidating factors which increase risk of infection recurrence and graft failure.
Conclusion
The PDC based, multi-staged IMT protocol yielded promising success rates in limb reconstruction for the treatment of recalcitrant IN and PTOM. The recurrence of infection was influenced by Infected previous free flap, Surprise positive culture, Deviation of protocol, and Elevated ESR before bone grafting.
Author contributions
J.W.C (Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Writing) W.T.K, J.K.K, S.H.J, S.S, H.K, W.S.S, E.J.L (Methodology, Data curation, Investigation) W.C, J.K.O (Conceptualization, Supervision, Validation, Writing-review and editing).
Funding
This research was supported by grants from the Technology Innovation Program (10077279) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea).
Data availability
All data generated or analysed during this study are included in this published article and uploaded as Supplementary material.
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wonseok Choi, Email: wscool@gmail.com.
Jong-Keon Oh, Email: jkoh@korea.ac.kr.
References
- 1.Masquelet AC. Induced membrane technique: pearls and pitfalls. J. Orthop. Trauma. 2017;31:S36–38. doi: 10.1097/BOT.0000000000000979. [DOI] [PubMed] [Google Scholar]
- 2.Masquelet AC, Kanakaris NK, Obert L, Stafford P, Giannoudis PV. Bone repair using the masquelet technique. J. Bone J. Surg. Am. 2019;101:1024–1036. doi: 10.2106/JBJS.18.00842. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Luo F, Huang K, Xie Z. Induced membrane technique for the treatment of bone defects due to post-traumatic osteomyelitis. Bone Joint Res. 2016;5:101–105. doi: 10.1302/2046-3758.53.2000487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu H, Shen J, Yu X, Fu J, Yu S, Sun D, et al. Two stage management of Cierny-Mader type IV chronic osteomyelitis of the long bones. Injury. 2017;48:511–518. doi: 10.1016/j.injury.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Giotikas D, Tarazi N, Spalding L, Nabergoj M, Krkovic M. Results of the induced membrane technique in the management of traumatic bone loss in the lower limb: A cohort study. J. Orthop. Trauma. 2019;33:131–136. doi: 10.1097/BOT.0000000000001384. [DOI] [PubMed] [Google Scholar]
- 6.Morris R, Hossain M, Evans A, Pallister I. Induced membrane technique for treating tibial defects gives mixed results. Bone Joint J. 2017;99:680–685. doi: 10.1302/0301-620X.99B5.BJJ-2016-0694.R2. [DOI] [PubMed] [Google Scholar]
- 7.Cho JW, Kent WT, Oh CW, Kim BS, Cho WT, Oh JK. Bone-graft resorption reduced by the induced membrane technique and factors affecting volumetric changes: An analysis of 120 serial computed tomographic scans in 40 patients. J. Bone Joint Surg. Am. 2020;102:1269–1278. doi: 10.2106/JBJS.19.00804. [DOI] [PubMed] [Google Scholar]
- 8.Cho JW, Kim J, Cho WT, Kent WT, Kim HJ, Oh JK. Antibiotic coated hinged threaded rods in the treatment of infected nonunions and intramedullary long bone infections. Injury. 2018;49:1912–1921. doi: 10.1016/j.injury.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Cho JW, Kim J, Cho WT, Kim JK, Song JH, Kim HJ, et al. Circumferential bone grafting around an absorbable gelatin sponge core reduced the amount of grafted bone in the induced membrane technique for critical-size defects of long bones. Injury. 2017;48:2292–2305. doi: 10.1016/j.injury.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Metsemakers WJ, Morgenstern M, McNally MA, Moriarty TF, McFadyen I, Scarborough M, et al. Fracture-related infection: A consensus on definition from an international expert group. Injury. 2018;49:505–510. doi: 10.1016/j.injury.2017.08.040. [DOI] [PubMed] [Google Scholar]
- 11.Luo TD, Nunez FA, Jr, Lomer AA, Nunez FA., Sr Management of recalcitrant osteomyelitis and segmental bone loss of the forearm with the Masquelet technique. J. Hand Surg. Eur. 2017;42:640–642. doi: 10.1177/1753193416650171. [DOI] [PubMed] [Google Scholar]
- 12.Ma CH, Chiu YC, Tu YK, Yen CY, Wu CH. Three-stage treatment protocol for recalcitrant distal femoral nonunion. Arch. Orthop. Trauma Surg. 2017;137:489–498. doi: 10.1007/s00402-017-2634-x. [DOI] [PubMed] [Google Scholar]
- 13.Olszewski D, Streubel PN, Stucken C, Ricci WM, Hoffmann MF, Jones CB, et al. Fate of patients with a “surprise” positive culture after nonunion surgery. J. Orthop. Trauma. 2016;30:e19–e23. doi: 10.1097/BOT.0000000000000417. [DOI] [PubMed] [Google Scholar]
- 14.Kellam JF, Meinberg EG, Agel J, Karam MD, Roberts CS, Wilber JH, et al. Introduction: fracture and dislocation classification compendium-2018: International comprehensive classification of fractures and dislocations committee. J. Orthop. Trauma. 2018;32:S1–S10. doi: 10.1097/BOT.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 15.Cierny Iii G, Mader JT, Penninck JJ. The classic: A clinical staging system for adult osteomyelitis. Clin. Orthop. Relat. Res. 2003;414:7–24. doi: 10.1097/01.blo.0000088564.81746.62. [DOI] [PubMed] [Google Scholar]
- 16.Pan SL, Liang HW, Hou WH, Yeh TS. Responsiveness of SF-36 and lower extremity functional scale for assessing outcomes in traumatic injuries of lower extremities. Injury. 2014;45:1759–1763. doi: 10.1016/j.injury.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 17.Dingemans SA, Kleipool SC, Mulders MAM, Winkelhagen J, Schep NWS, Goslings JC, et al. Normative data for the lower extremity functional scale (LEFS) Acta Orthop. 2017;88:422–426. doi: 10.1080/17453674.2017.1309886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Wang S, Fu J, Sun D, Shen J, Xie Z. Risk factors associated with recurrence of extremity osteomyelitis treated with the induced membrane technique. Injury. 2020;51:307–311. doi: 10.1016/j.injury.2019.11.026. [DOI] [PubMed] [Google Scholar]
- 19.Morelli I, Drago L, George DA, Gallazzi E, Scarponi S, Romanò CL. Masquelet technique: Myth or reality? A systematic review and meta-analysis. Injury. 2016;47:S68–S76. doi: 10.1016/S0020-1383(16)30842-7. [DOI] [PubMed] [Google Scholar]
- 20.Mijuskovic B, Kuehl R, Widmer AF, Jundt G, Frei R, Gürke L, et al. Culture of bone biopsy specimens overestimates rate of residual osteomyelitis after toe or forefoot amputation. J. Bone Joint Surg. Am. 2018;100:1448–1454. doi: 10.2106/JBJS.17.01152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atway S, Nerone VS, Springer KD, Woodruff DM. Rate of residual osteomyelitis after partial foot amputation in diabetic patients: A standardized method for evaluating bone margins with intraoperative culture. J. Foot Ankle Surg. 2012;51:749–752. doi: 10.1053/j.jfas.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Weiner RD, Viselli SJ, Fulkert KA, Accetta P. Histology versus microbiology for accuracy in identification of osteomyelitis in the diabetic foot. J. Foot. Ankle Surg. 2011;50:197–200. doi: 10.1053/j.jfas.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Boers SA, Jansen R, Hays JP. Understanding and overcoming the pitfalls and biases of next-generation sequencing (NGS) methods for use in the routine clinical microbiological diagnostic laboratory. Eur. J. Clin. Microbiol. Infect Dis. 2019;38:1059–1070. doi: 10.1007/s10096-019-03520-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiedrowski MR, Horswill AR. New approaches for treating staphylococcal biofilm infections. Ann. N. Y. Acad. Sci. 2011;1241:104–121. doi: 10.1111/j.1749-6632.2011.06281.x. [DOI] [PubMed] [Google Scholar]
- 25.Depypere M, Kuehl R, Metsemakers WJ, Senneville E, McNally MA, Obremskey WT, et al. Recommendations for systemic antimicrobial therapy in fracture-related infection: A consensus from an international expert group. J. Orthop. Trauma. 2020;34:30–41. doi: 10.1097/BOT.0000000000001626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petfield JL, Tribble DR, Potter BK, Lewandowski LR, Weintrob AC, Krauss M, et al. Is bone loss or devascularization associated with recurrence of osteomyelitis in wartime open tibia fractures? Clin. Orthop. Relat. Res. 2019;477:789–801. doi: 10.1097/CORR.0000000000000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tice AD, Hoaglund PA, Shoultz DA. Risk factors and treatment outcomes in osteomyelitis. J. Antimicrob. Chemother. 2003;51:1261–1268. doi: 10.1093/jac/dkg186. [DOI] [PubMed] [Google Scholar]
- 28.Aho OM, Lehenkari P, Ristiniemi J, Lehtonen S, Risteli J, Leskelä HV. The mechanism of action of induced membranes in bone repair. J. Bone Joint Surg. Am. 2013;95:597–604. doi: 10.2106/JBJS.L.00310. [DOI] [PubMed] [Google Scholar]
- 29.Cuthbert RJ, Churchman SM, Tan HB, McGonagle D, Jones E, Giannoudis PV. Induced periosteum a complex cellular scaffold for the treatment of large bone defects. Bone. 2013;57:484–492. doi: 10.1016/j.bone.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Tang Q, Tong M, Zheng G, Shen L, Shang P, Liu H. Masquelet’s induced membrane promotes the osteogenic differentiation of bone marrow mesenchymal stem cells by activating the Smad and MAPK pathways. AM. J. Transl. Res. 2018;10:1211–1219. [PMC free article] [PubMed] [Google Scholar]
- 31.Mauffrey C, Hake ME, Chadayammuri V, Masquelet AC. Reconstruction of long bone infections using the induced membrane technique: tips and tricks. J. Orthop. Trauma. 2016;30:e188–e193. doi: 10.1097/BOT.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 32.Lin Z, Vasudevan A, Tambyah PA. Use of erythrocyte sedimentation rate and C-reactive protein to predict osteomyelitis recurrence. J. Orthop. Surg. 2016;24:77–83. doi: 10.1177/230949901602400118. [DOI] [PubMed] [Google Scholar]
- 33.Rabjohn L, Roberts K, Troiano M, Schoenhaus H. Diagnostic and prognostic value of erythrocyte sedimentation rate in contiguous osteomyelitis of the foot and ankle. J. Foot Ankle Surg. 2007;46:230–237. doi: 10.1053/j.jfas.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Purudappa PP, Sharma OP, Priyavadana S, Sambandam S, Villafuerte JA. Unexpected positive intraoperative cultures (UPIC) in revision Hip and knee arthroplasty-A review of the literature. J. Orthop. 2020;17:1–6. doi: 10.1016/j.jor.2019.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg. Am. 1996;78:512–523. doi: 10.2106/00004623-199604000-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article and uploaded as Supplementary material.