Abstract
Purpose:
To predict visual acuity (VA) 90 days after presentation for patients with microbial keratitis (MK) from data at the initial clinical ophthalmic encounter.
Methods:
Patients with MK were identified in the electronic health record between August 2012 and February 2021. Random forest (RF) models were used to predict 90-day VA <20/40 (visual impairment (VI)). Predictors evaluated included age, gender, initial VA, and information documented in notes at presentation. Model diagnostics are reported with 95% confidence intervals (CI) for area under the curve (AUC), misclassification rate, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
Results:
1791 patients were identified. Presenting LogMAR VA was on average 0.86 (Snellen equivalent and Standard Deviation = 20/144 ± 12.6 lines) in the affected or worse eye, and 43.6% with VI. VI at 90-day follow-up was present in the affected eye or worse eye for 26.9% of patients. The RF model for predicting 90-day VI had an AUC of 95% (CI: 93-97%) and a misclassification rate of 9% (8-13%). The percent sensitivity, specificity, PPV, and NPV were 86% (80-91%), 92% (89-95%), 81% (74-86%), and 95% (92-97%), respectively. Older age, worse presenting VA, and more mentions of “PKP” and “BCL” were associated with increased probability of 90-day VI, while more mentions of “quiet” were associated with decreased probability of 90-day VI.
Conclusion:
RF modeling yielded good sensitivity and specificity to predict VI at 90 days which could guide clinicians about the risk of poor vision outcomes for patients with MK.
Keywords: Microbial keratitis, electronic health record, risk-stratification
Introduction
Microbial keratitis (MK) has an estimated annual incidence of 1.5 to 2 million cases worldwide.1–3 MK is the result of an infectious organism invading the cornea. The consequences of the infection depend on the complex interaction of the patient, the organism, and the circumstances of the infection. Forms of MK can have similar appearances in the cornea, but morphologic features can be used to guide the diagnosis and grade the severity of the infection. Several morphologic and patient risk factors have been linked to outcomes of poor visual acuity (VA) or need for corneal transplantation.4–10
To date, clinicians do not have a guideline or strategy to determine MK severity. Currently, clinicians rely on prior experience and clinical risk factor data, such as contact lens history. An MK severity scoring system was proposed in 2007 but has not been widely adopted.11 And while cornea experts are better at gauging MK severity,12 they are not universally available.
The lack of a method to risk stratify MK leads most clinicians to manage MK severity with the same regimen: high-intensity, broad-spectrum antimicrobials. But microbial resistance is increasing, so these medications are less effective when used the same way for every patient. In addition, supplemental medications, such as topical corticosteroids and oral agents, have been shown to improve vision outcomes in severe MK cases.
Predicting MK outcomes for risk stratification is a method to guide clinicians at the initial management of keratitis. The main risk with MK is the potential for vision loss. In developed countries, vision loss worse than Snellen acuity of 20/40 often prompts an evaluation for optical corneal transplantation to restore vision. In other disciplines of medicine, risk stratification has improved clinician decision making for initial management of complex, heterogeneous diseases.13–19 Researchers have been able to develop prediction models to risk stratify patients thanks to the volume of data present in the electronic health record (EHR). This data is complex to extract precisely, but it represents “real world” cases.20,21 Risk stratification has already had impact within cornea domains, such as the identification of the risk for corneal ectasia22 and risk for vision-threatening anterior eye diseases.23
The purpose of this study is to evaluate whether EHR data can serve as a useful descriptor of current VA, and as a predictor of 90-day VA among patients with MK. Integration of an EHR-based risk stratification tool may be useful to clinicians in tailoring initial MK medications and therapies, and to clinical trialists in targeting interventions.
Methods
Patients with MK were identified in the University of Michigan EHR from August 2012 to February 2021. An MK diagnosis was determined by ICD-9 or ICD-10 codes with associated descriptions (Supplemental Table 1) that were linked to office visit encounters in the ophthalmology department. Laterality of infection was determined by ICD-10 diagnosis code when available (72%), or by natural language processing of the clinical encounters for terms associated with laterality when diagnosis codes were less specific. Detailed methods to determine laterality are described in Supplemental Table 2. Once laterality of the MK infection was established, distance VA was pulled from the EHR. To minimize missing data, we chose distance VA measures according to the following hierarchy: best corrected visual acuity (BCVA) by manifest refraction, BCVA with current eyeglasses, and uncorrected visual acuity. VA was collected for the affected eye(s) at the initial diagnosis visit and all follow-up visits through 120 days after diagnosis. VA at 90-days after diagnosis was chosen as that measured closest to 90 days after the initial encounter but within 30 days. For those MK patients who did not have a 90-day VA but their last VA prior to 60 days was 20/40 or better, we carried forward this last VA as their 90-day VA measure under the assumption that recovery of visual acuity had already occurred. For those MK patients who did not have a 90-day VA measure and their last measured VA prior to 60 days was worse than 20/40, we did not assign a 90-day VA measure as we were unsure of the amount of healing that may have occurred by day 90. VA at diagnosis and 90-day follow-up were categorized as visual impairment (VI) (VA<20/40) or ≥20/40 (“good’ VA) for prediction modeling. Demographic and clinical characteristics of the MK sample were summarized with descriptive statistics including means, standard deviations (SD), frequencies, and percentages.
After setting aside a one-third random sample of the data into a test set, two random forest models were fit on the remaining two-thirds of the data. The first predicted VI at 90 days from MK diagnosis using clinical chart information and VA at the time of diagnosis in the model. The second model predicted VI at time of diagnosis using clinical chart information. The purpose of this second model was to test viability of an algorithmic approach to predict 90-day VA. Factors included as model predictors included age, gender, VA at diagnosis (for the 90-day prediction model), and information documented in the clinical notes at presentation. The patient ocular history, technician comments, history of present illness, cornea, conjunctiva, anterior chamber, and pen light note sections were combined into a single narrative and processed using the R package clinspacy.24,25 The physician assessment and plan note sections were purposely excluded from processing as those would contain information, such as prescribed medications, that would affect visual acuity. The clinspacy package uses a medical language model to decompose the note into lemma, including indications of negation, family history, historical, and hypothetical contexts. For each note, aggregate counts of non-negated unique lemma were calculated. From the baseline notes (2990 MK patients identified), unique lemmas were identified with frequencies of 100 or larger (213 of 19762, 1.1%). Lemma that had a frequency of <100 mentions across all notes from all MK patients were removed. The remaining lemma and their counts were used as predictors in the random forest models. Descriptive information on lemma is presented in Supplemental Table 3.
The random forest models were comprised of 1000 trees, and each split of a tree selected an optimal split among a random subsample of predictors (lemma and patient age, gender, and baseline VA). Model diagnostics on the test dataset are reported with 95% confidence intervals (CI) for area under the curve (AUC), misclassification rate, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). Diagnostics are presented at a predicted probability cut point that maximized the F1 statistic, but performance plots are also presented to show the range of diagnostics for different probability cut points.26 Shapley summary plots27 were used to display variable importance and direction of effect. Models were fit using the VA of the affected eye for patients with unilateral infections and the worse eye for patients with bilateral infections. Worse eye was chosen because patients may need a corneal transplantation in an eye with worse final vision. As a sensitivity analysis, we refit the models using the better eye cases of bilateral infections. The final MK sample for analysis excluded those patients with missing data for clinical notes, demographics of interest, presenting VA, or those where their last VA measure was before 90 days and <20/40 and thus couldn’t be carried forward. Statistical analysis was performed using SAS version 9.4 (SAS Institute, Cary, NJ) and R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 1791 MK patients were identified in the EHR who had complete clinical notes at presentation, demographics, VA measurement at presentation, and VA measured at 90-day follow-up or a VA that could be carried forward at last measurement before 90 days (<20/40). Due to different severity of infections in eyes of bilateral MK patients and carrying forward only VAs ≥20/40, the sample size was slightly different when analyzing affected eye of unilateral patients with worse eye of bilateral patients (n=1787) versus affected eye of unilateral with better eye of bilateral patients (n=1791). For the 90-day VA measurement, 766 patients had the measurement at 90 ± 30-days follow-up and an additional 1021 or1025 patients had a VA of ≥20/40 for affected and worse or affected and better eye at last follow-up before 60 days to carry forward as their 90-day measure. Descriptive statistics of the 1791 sample are presented in Table 1. Patients were on average 48.0 years old at MK diagnosis (SD=20.3), 41.1% male, 85.4% White and 6.8% Black, and 3.0% Hispanic. Most patients had unilateral infections (n=1734, 96.8%) and a minority had bilateral infections (n=57, 3.2%). LogMAR VA at presentation was on average 0.86 (SD=1.26, median=0.28; Snellen equivalent mean=20/144, SD=12.6 lines, median=20/38) in the affected eye for unilateral infections or worse eye in patients with bilateral infections.
Table 1.
Demographic and baseline clinical characteristics of patients with microbial keratitis (MK) who had 90-day VA or a VA ≥20/40 to carry forward (n=1791).
| Continuous Variable | Mean (SD) | Min, Max | Median (IQR) |
|---|---|---|---|
| Age (years) | 48.0 (20.3) | 3.6, 96.0 | 48.4 (31.1, 63.8) |
| LogMAR VA - worse eye | 0.86 (1.26) | −0.12, 8.30 | 0.28 (0, 1.10) |
| LogMAR VA - better eye | 0.85 (1.25) | −0.12, 8.30 | 0.22 (0, 1.10) |
| Categorical Variables | n (%) | ||
| Sex | |||
| Female | 1055 (58.9) | ||
| Male | 736 (41.1) | ||
| Race | |||
| White | 1520 (85.4) | ||
| Black | 122 (6.8) | ||
| Asian | 82 (4.6) | ||
| American Indian | 9 (0.5) | ||
| Pacific Islander | 2 (0.1) | ||
| Other | 46 (2.6) | ||
| Ethnicity | |||
| Hispanic | 54 (3.0) | ||
| Non-Hispanic | 1737 (97.0) | ||
| Affected eye | |||
| Unilateral | 1734 (96.8) | ||
| OD | 848 | ||
| OS | 886 | ||
| Bilateral | 57 (3.2) | ||
SD, Standard Deviation; Min, Minimum; Max, Maximum; IQR, Interquartile Range; VA, Visual Acuity; OD, Right; OS, Left
Visual impairment in the affected eye for unilateral infections or worse eye for patients with bilateral infections was present in 26.9% of MK patients. The random forest model for predicting 90-day VI had an AUC on the test data of 95% (95% CI, 93% to 97%) and an overall misclassification rate of 9% (95% CI, 7% to 12%) (Table 2). The sensitivity of the model to correctly classify 90-day VI in the test data was 86% (95% CI, 80% to 91%). The specificity, PPV, and NPV were 92% (95% CI, 89% to 95%), 81% (95% CI, 74% to 86%), and 95% (95%CI, 92% to 97%), respectively. These diagnostics are presented at a probability cut point of 0.43 that maximized the F1 statistic, but a performance plot (Figure 1A) presents diagnostics over the range of probability thresholds. The most important variables for prediction included presenting VA, age of the patient, and counts of the lemma for “quiet,” “PKP,” (i.e., penetrating keratoplasty) and “BCL” (i.e., bandage contact lens) (Figure 1B, top 15 variables shown). Specifically, worse presenting VA, older age, and more mentions of “PKP,” and “BCL” were associated with increased probability of 90-day VI, whereas more mentions of “quiet” were associated with decreased probability of 90-day VI. In the sensitivity analysis, the random forest model for predicting 90-day VI in the affected eye for unilateral infections or better eye in patients with bilateral infections showed similar diagnostics on test data (Table 2).
Table 2.
Random forest model diagnostics for predicting 90-day visual impairment (VI)
| Visual Impairment at 90 days | ||
|---|---|---|
| Model Diagnostic | Affected or Worse Eye* | Affected or Better Eye* |
| Estimate (95% CI) | Estimate (95% CI) | |
| Misclassification | 0.09 (0.07, 0.12) | 0.11 (0.08, 0.13) |
| AUC | 0.95 (0.93, 0.97) | 0.95 (0.94, 0.97) |
| Sensitivity | 0.86 (0.80, 0.91) | 0.91 (0.86, 0.95) |
| Specificity | 0.92 (0.89, 0.95) | 0.89 (0.86, 0.91) |
| PPV | 0.81 (0.74, 0.86) | 0.73 (0.65, 0.79) |
| NPV | 0.95 (0.92, 0.97) | 0.97 (0.95, 0.98) |
CI, Confidence Interval; AUC, Area Under the Curve; PPV, Positive Predictive Value; NPV, Negative Predictive Value
In patients with unilateral infections the affected eye was analyzed, in patients with bilateral infection analyses were performed separately by including either the better or worse eye
Figure 1.
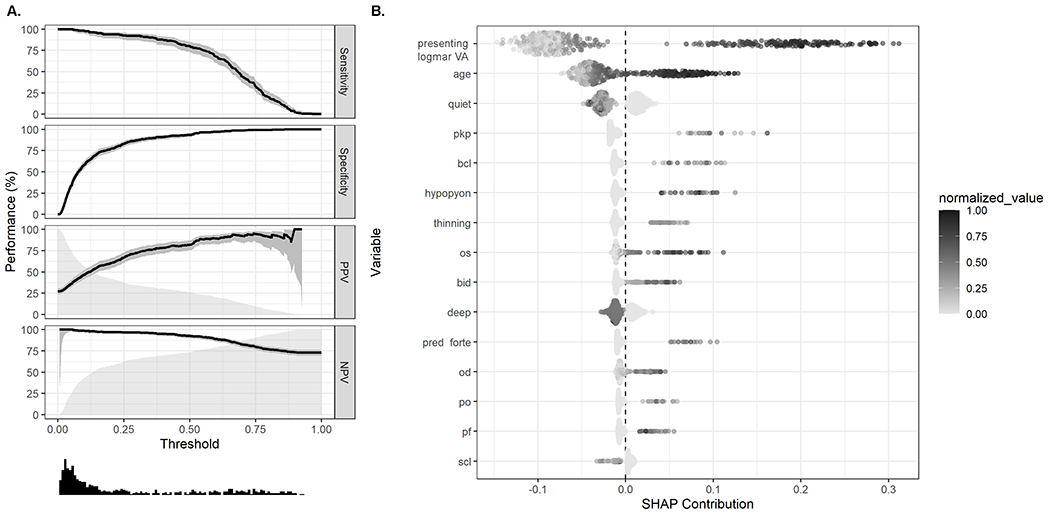
A. Performance plot showing diagnostics for random forest model predictions of 90-day visual acuity of <20/40 for the affected eye of unilateral infections or better eye for patients with bilateral infections. Performance statistics (sensitivity, specificity, positive predictive value [PPV], negative predictive value [NPV]) are plotted with 95% confidence intervals (shaded areas around lines) and a histogram (bottom) displays the distribution of predicted values. B. Shapley plot displaying variable importance and effect from random forest models predicting 90-day visual acuity <20/40 for the affected eye of unilateral infections or better eye for patients with bilateral infections. All variables were normalized to be on the same scale (0-1) for display purposes. SHAP contribution (SHapley Additive exPlanations) refers to the contribution each variable from each observation has on to prediction. VA, visual acuity; PKP, penetrating keratoplasty; BCL, bandage contact lens; OS, left eye; BID, twice daily; OD, left eye; PO, by mouth; PF, pred forte SCL, soft contact lens.
MK patients had VI at presentation in the affected eye for unilateral infections or worse eye for patients with bilateral infections for 43.6% of the sample. The random forest model for predicting presenting VI had an AUC on the test data of 84% (95% CI, 81% to 98%) and an overall misclassification rate of 20% (95% CI, 17% to 23%) (Table 3). The sensitivity to classify baseline VI in the test data was 70% (95% CI, 65% to 76%). The specificity, PPV, and NPV were 89% (95% CI, 85% to 92%), 84% (95% CI, 79% to 88%), and 78% (95% CI, 74% to 82%), respectively. These diagnostics are presented at a probability cut point of 0.48 that maximized the F1 statistic, but a performance plot (Figure 2A) presents diagnostics over the range of probability thresholds. The most important variables for prediction included age of the patient and counts of the lemmas for “quiet,” “hypopyon,” “BID,” and “deep” (Figure 2B). Specifically, older age, more mentions of “hypopyon,” and “BID” were associated with increased probability of presenting VI, whereas more mentions of “quiet” and “deep” were associated with decreased probability of presenting with VI. The random forest model for predicting presenting VI in the affected eye for unilateral infections or better eye for patients with bilateral infections showed similar diagnostics on test data (Table 3).
Table 3.
Random forest model diagnostics for predicting presenting visual impairment (VI)
| Baseline Visual Impairment | ||
|---|---|---|
| Model Diagnostic | Affected or Worse Eye* | Affected or Better Eye* |
| Estimate (95% CI) | Estimate (95% CI) | |
| Misclassification | 0.20 (0.17, 0.23) | 0.17 (0.14, 0.20) |
| AUC | 0.84 (0.81, 0.88) | 0.88 (0.85, 0.91) |
| Sensitivity | 0.70 (0.65, 0.76) | 0.77 (0.72, 0.82) |
| Specificity | 0.89 (0.85, 0.92) | 0.88 (0.84, 0.91) |
| PPV | 0.84 (0.79, 0.88) | 0.83 (0.78, 0.88) |
| NPV | 0.78 (0.74, 0.82) | 0.83 (0.79, 0.87) |
CI, Confidence Interval; AUC, Area Under the Curve; PPV, Positive Predictive Value; NPV, Negative Predictive Value
In patients with unilateral infections the affected eye was analyzed, in patients with bilateral infection analyses were performed separately by including either the better or worse eye
Figure 2.
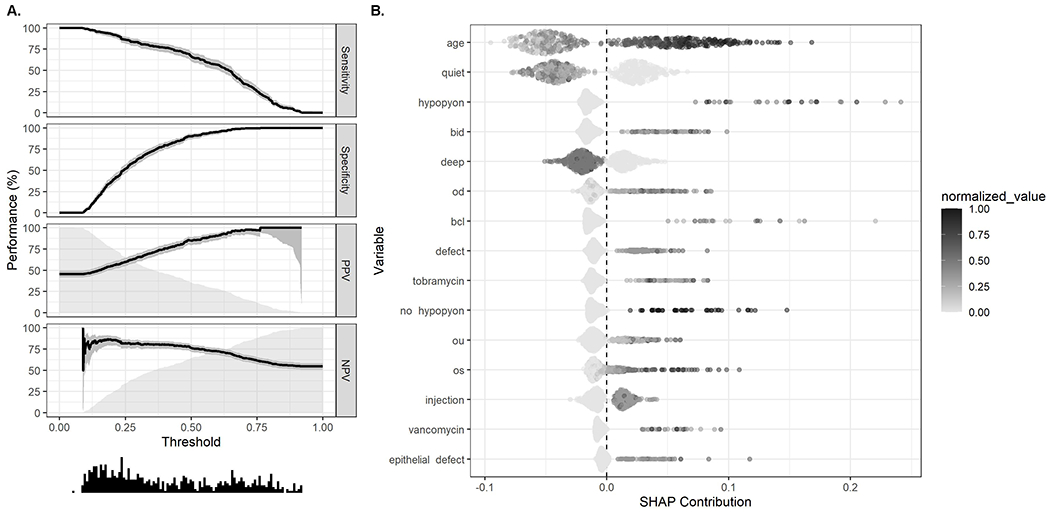
A. Performance plot showing diagnostics for random forest model predictions of presenting visual acuity of <20/40 for the affected eye of unilateral infections or better eye for patients with bilateral infections. Performance statistics (sensitivity, specificity, positive predictive value [PPV], negative predictive value [NPV]) are plotted with 95% confidence intervals (shaded areas around lines) and a histogram (bottom) displays the distribution of predicted values. B. Shapley plot displaying variable importance and effect from random forest models predicting presenting visual acuity of <20/40 for the affected eye of unilateral infections or better eye for patients with bilateral infections. All variables were normalized to be on the same scale (0-1) for display purposes. SHAP contribution (SHapley Additive exPlanations) refers to the contribution each variable from each observation has on prediction. BID, twice daily; OD, right eye; BCL, bandage contact lens; OU, bilateral; OS, left eye.
Discussion:
In this study of 1791 MK patients, random forest model to predict visual impairment at 90-days yielded a sensitivity and specificity of 86% and 92%, respectively. The model’s AUC on the test data was 95% (95% CI, 93% to 97%). The model included patient characteristics and features of the keratitis and excluded the physician’s assessment and plan text. The prediction of visual acuity would be expected to improve if the model included physician notes and culture results, for example. For this analysis, it was important to not include information reflect the physicians’ expertise (such as clinical notes) and thus would vary by clinician and could influence the accuracy of the prediction. Culture results also were not included as they would be known not at the time of the first patient encounter. However, future models that incorporate capture longitudinal information certainly could include these important clinical prediction factors. All information was readily accessible within electronic health records. In our study, 43.6% of patients had VI at presentation, and 26.9% of patients had VI at 90 days after presentation, showing good distribution of the key outcome (i.e., final visual acuity) and key predictor (i.e., initial visual acuity).
A second model, to predict VA at clinical presentation and test viability of an algorithmic approach to predict 90-day VA, had a sensitivity and specificity of 70% and 89%, respectively. While a model to predict current VA may be less practically useful, as an ophthalmic clinician can certainly check a VA at the time of clinical presentation, it was an important step in identifying if our method for algorithm development had face validity and was feasible. The good performance of this model indicates feasibility of analyzing presenting data from the electronic health record (that excluded the physician’s assessment and plan). Predicting presenting VA may have applications in telemedicine settings with remote non-ophthalmic technicians.
The baseline visual acuity of MK patients was similar to presenting VA in previous MK studies performed at tertiary care centers.7,28 Our model identified specific patient and keratitis features that predicted visual impairment (Snellen <20/40 in this context) at 90 days. Patient age was one of these factors. Prior research indicates that older patients tend to present with more central and severe ulcers and have poorer visual outcomes.4,29–31 Unsurprisingly, presenting VA was a key factor in the algorithm predicting 90-day VA, similar to other studies.4,7,32 Unique lemma identified within clinical notes that predicted worse VA included “BCL” and “PKP.” Use of bandage contact lenses (BCL) is not a surprising association for sicker eyes. The mention of penetrating keratoplasty (i.e., PKP) unsurprisingly predicts poorer visual outcomes as many patients who acquire MK already have a sick eye (and have an existing PKP). Hypopyon at presentation was the 6th lemma identified (Figure 1B); has been associated with clinical perforation or need for penetrating keratoplasty (PKP) in fungal keratitis.8 Hypopyon is a marker of severe intraocular inflammation. Severe inflammation also has been shown to predict worse visual outcomes,6,9,11 while more mentions of lemmas “deep” and “quiet” at presentation were associated with decreased probability of VI, indicated little to no intraocular inflammation.
Many MK patients, 66%, did not return for a 90-day follow-up visit. Loss to follow-up in other MK studies ranges from 11 to 30%.4,6,10,33 In this retrospective study, our higher percentage may have been due to some patients having adequately treated infections where 90-day follow-up was not needed. This data requires further exploration outside the scope of this work.
This model relied on data available at ophthalmic clinical practices via the EHR and is the basis of a tool to aid clinicians. EHR-based models are widespread in medicine and are being used to predict and inform sepsis management,34 renal function deterioration,35 ward cardiac arrest,36 prostate cancer treatment,37,38 and hospital utilization.39 But prediction modeling is less commonly used in ophthalmology despite our widespread adoption of EHRs and our widespread use of images. In studies of corneal conditions, clinicians likely use the risk prediction calculator for corneal ectasia most frequently.22 For corneal ulcers, a team of investigators created a “1-2-3 rule” from a cohort of 41 eyes, which uses morphologic findings to predict sight-threatening ulcers.11 The 1-2-3 rule had a very high sensitivity of 100%, but only moderate specificity of 57%. The 1-2-3 rule is simple and thus easy to use, but the rule was likely not tested on a large enough population to know if it is generalizable. Our current study’s algorithm has an AUC of 95%, but its current state is as an algorithm and lacks the simplicity of a “tool” ready for mainstream adoption and integration with electronic health records. For future use, the algorithm will need to be further tested and evaluated for clinical usability. The disconnect between a complex algorithm with high validity and a simple rule that is easier to implement has the potential to be overcome when algorithms can be embedded within her systems.40
Predicting VA or other outcomes is mainly useful when it can be used to guide clinicians to risk-stratify patients and tailor treatments. Tailoring is especially important in MK given the variety of treatment options possible for patients. MK can be managed with various antimicrobial agents – compounded or non-compounded and medications for bacteria, fungi, viral, or other organisms. MK management can include the use of corticosteroids and other supportive topical and oral agents. But there are only so many drops on the eye that can be tolerated by a patient and by their eye. Additionally, the financial burden of MK therapy is substantial.10,41,42 The wide use of medications is compounded by issues of increasing rates of antimicrobial resistance worldwide. Within the context of MK, antimicrobial resistance contributes to prolonged corneal healing time and increased risk of corneal perforation.43,44 Ideally, an algorithm that predicts vision could be modified to a clinical tool that aids clinicians to stratify risk for poor outcomes. This will help clinicians tailor treatment, consider different diagnostic or therapeutic procedures, or refer patients to an expert quickly.
In this study, we risk-stratified MK based on VA rather than the eventual need for corneal transplantation because receipt of a transplant depends not only on medical need but also on social determinants of health (e.g., health insurance). Predicting the need for transplantation could result in an unfair algorithm because it would assign risk based on social determinants of health in addition to medical factors. A prediction model worth exploring in future work would be to develop an algorithm that could predict final VA on a continuous scale or predict the receipt of urgent procedures such as corneal gluing or urgent therapeutic corneal transplantation.
There are limitations to our study. The model is limited to what is documented in clinical notes and exams. We have previously shown that symptoms and morphology can be under- documented in the EHR.45 Incorporating information from slit-lamp photographs that quantify keratitis features46 into modeling and risk stratification tools is a future goal of the research. In addition, this model was created based on the health records of patients at a tertiary ophthalmologic center. The model will be tested and updated with data from different settings and unique patient populations to improve generalizability.47,48 Additionally, our model analyzed 90-day VA but was limited to those with 90-day follow-up or those who had VAs ≥20/40 that we could carry forward, thus reducing generalizability. Follow-up studies will investigate differences between patients who do and do not return for follow-up, including differences in patient demographics, clinical characteristics, and socioeconomic neighborhood-level factors. Lastly, processing of the clinical notes is imperfect. Although we used a natural language processing application that filters out negated terms, we still see that “hypopyon” and its negated state (“no hypopyon”) were both identified as lemma. Each show increased probability of presenting VI with more mentions (Figure 2B), although the range of mentions for “hypopyon” was larger than “no hypopyon” (0-4 versus 0-2, respectively; Supplemental Table 3). Further, abbreviations of words were sometimes recognized as separate lemma (ex: Pred Forte and PF).
Prediction modeling from a large cohort of MK patients is accurate and useful for the identification of patients at risk of poor VA outcomes. The ability of clinicians to identify factors associated with worse VA outcomes in MK patients will contribute to early intervention and better outcomes. This algorithm is a first step towards the creation of an automated decision- support tool that could be incorporated into clinical practice.
Supplementary Material
Supplemental Table 1: The diagnosis codes used to identify patients with microbial keratitis in the electronic health record.
Supplemental Table 2: The methodology to interpret the eye laterality of microbial keratitis infection.
Supplemental Table 3: Descriptive statistics for the lemma identified in clinical notes from microbial keratitis patients at presentation. 2990 microbial keratitis patients were identified, but 2908 had notes at presentation that returned at least 1 lemma. Lemma that had at least 100 mentions across the notes of these patients are presented (n=213).
Acknowledgements:
This project was supported in part by a gift by Ms. Susan Lane.
Financial Support:
National Eye Institute (R01EY031033 [MAW]), Research to Prevent Blindness, Career Advancement Award (MAW). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures:
Unrelated to submitted work. Blue Cross Blue Shield of Michigan (KS, grant funding), Teva Pharmaceuticals (KS, grant funding), Flatiron Health (KS, scientific advisory board).
Footnotes
Conflict of Interest: The authors have no proprietary or commercial interest in any of the materials discussed in this article
References:
- 1.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
- 2.Keratitis Sharma S.. Biosci Rep. 2001;21:419–444. [DOI] [PubMed] [Google Scholar]
- 3.Brown L, Leck AK, Gichangi M, et al. The global incidence and diagnosis of fungal keratitis. Lancet Infect Dis. 2021;21:e49–e57. [DOI] [PubMed] [Google Scholar]
- 4.Venkatesh Prajna N, Krishnan T, Mascarenhas J, et al. Predictors of outcome in fungal keratitis. Eye. 2012;26:1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaefer F, Bruttin O, Zografos L, et al. Bacterial keratitis: a prospective clinical and microbiological study. Br J Ophthalmol. 2001;85:842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourcier T, Thomas F, Borderie V, et al. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol. 2003;87:834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoddenbach JG, Boekhoorn SS, Wubbels R, et al. Clinical presentation and morbidity of contact lens–associated microbial keratitis: a retrospective study. Graefe’s Arch Clin Exp Ophthalmol. 2014;252:299–306. [DOI] [PubMed] [Google Scholar]
- 8.Prajna N, Krishnan T, Rajaraman R, et al. Predictors of Corneal Perforation or Need for Therapeutic Keratoplasty in Severe Fungal Keratitis: A Secondary Analysis of the Mycotic Ulcer Treatment Trial II. JAMA Ophthalmol. 2017;135:987–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keay L, Edwards K, Naduvilath T, et al. Factors Affecting the Morbidity of Contact Lens–Related Microbial Keratitis: A Population Study. Invest Ophthalmol Vis Sci. 2006;47:4302–4308. [DOI] [PubMed] [Google Scholar]
- 10.Keay L, Edwards K, Naduvilath T, et al. Microbial Keratitis: Predisposing Factors and Morbidity. Ophthalmology. 2006;113:109–116. [DOI] [PubMed] [Google Scholar]
- 11.Vital MC, Belloso M, Prager TC, et al. Classifying the Severity of Corneal Ulcers by Using the “1, 2, 3” Rule. Cornea. 2007;26:16–20. [DOI] [PubMed] [Google Scholar]
- 12.McLeod SD, LaBree LD, Tayyanipour R, et al. The importance of initial management in the treatment of severe infectious corneal ulcers. Ophthalmology. 1995;102:1943–1948. [DOI] [PubMed] [Google Scholar]
- 13.Huang Z, Dong W, Duan H, et al. A Regularized Deep Learning Approach for Clinical Risk Prediction of Acute Coronary Syndrome Using Electronic Health Records. IEEE Trans Biomed Eng. 2018;65:956–968. [DOI] [PubMed] [Google Scholar]
- 14.Buchlak QD, Kowalczyk M, Leveque J-C, et al. Risk stratification in deep brain stimulation surgery: Development of an algorithm to predict patient discharge disposition with 91.9% accuracy. J Clin Neurosci. 2018;57:26–32. [DOI] [PubMed] [Google Scholar]
- 15.Zeiberg D, Prahlad T, Nallamothu BK, et al. Machine learning for patient risk stratification for acute respiratory distress syndrome. Mortazavi B, ed. PLoS One. 2019;14:e0214465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendu ML, Schneider LI, Aizer AA, et al. Implementation of a CKD Checklist for Primary Care Providers. Clin J Am Soc Nephrol. 2014;9:1526–1535. d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gluckman TJ, Kovacs RJ, Stone NJ, et al. The ASCVD Risk Estimator App. J Am Coll Cardiol. 2016;67:350–352. [DOI] [PubMed] [Google Scholar]
- 18.Lloyd-Jones DM, Huffman MD, Karmali KN, et al. Estimating Longitudinal Risks and Benefits From Cardiovascular Preventive Therapies Among Medicare Patients: The Million Hearts Longitudinal ASCVD Risk Assessment Tool: A Special Report From the American Heart Association and American College of Cardiolog. Circulation. 2017;135:e793–e813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaul DR, Valesano AL, Petrie JG, et al. Donor to recipient transmission of SARS-CoV-2 by lung transplantation despite negative donor upper respiratory tract testing. Am J Transplant. 2021;00:ajt.16532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stein JD, Rahman M, Andrews C, et al. Evaluation of an Algorithm for Identifying Ocular Conditions in Electronic Health Record Data. JAMA Ophthalmol. 2019;137:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maganti N, Tan H, Niziol LM, et al. Natural Language Processing to Quantify Microbial Keratitis Measurements. Ophthalmology. 2019;126:1722–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Randleman JB, Woodward MA, Lynn MJ, et al. Risk Assessment for Ectasia after Corneal Refractive Surgery. Ophthalmology. 2008;115:37–50. [DOI] [PubMed] [Google Scholar]
- 23.Woodward MA, Valikodath NG, Newman-Casey PA, et al. Eye Symptom Questionnaire to Evaluate Anterior Eye Health. Eye Contact Lens Sci Clin Pract. 2018;44:384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh K, Kompa B, Beam A, et al. clinspacy: Clinical Natural Language Processing using “spaCy”, “scispaCy”, and “medspaCy.” https://cran.r-project.org/web/packages/clinspacy/index.html. Accessed January 18, 2022.
- 25.Beam AL, Kompa B, Schmaltz A, et al. Clinical Concept Embeddings Learned from Massive Sources of Multimodal Medical Data. April 2018. https://arxiv.org/abs/1804.01486. Accessed January 18, 2022. [PMC free article] [PubMed]
- 26.Singh K, Meyer S, Sequeira K, et al. ML4LHS/runway: Visualizing Prediction Model Performance. https://github.com/ML4LHS/runway. Accessed January 24, 2022.
- 27.Lundberg S, Lee S-I. A Unified Approach to Interpreting Model Predictions. May 2017. https://arxiv.org/abs/1705.07874. Accessed January 18, 2022.
- 28.McClintic SM, Prajna NV, Srinivasan M, et al. Visual Outcomes in Treated Bacterial Keratitis: Four Years of Prospective Follow-up. Invest Ophthalmol Vis Sci. 2014;55:2935–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parmar P, Salman A, Kalavathy CM, et al. Microbial keratitis at extremes of age. Cornea. 2006;25:153–158. [DOI] [PubMed] [Google Scholar]
- 30.Butler TKH, Spencer NA, Chan CCK, et al. Infective keratitis in older patients: a 4 year review, 1998–2002. Br J Ophthalmol. 2005;89:591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khoo P, Cabrera-Aguas MP, Nguyen V, et al. Microbial keratitis in Sydney, Australia: risk factors, patient outcomes, and seasonal variation. Graefe’s Arch Clin Exp Ophthalmol = Albr von Graefes Arch fur Klin und Exp Ophthalmol. 2020;258:1745–1755. [DOI] [PubMed] [Google Scholar]
- 32.Tananuvat N, Punyakhum O, Ausayakhun S, et al. Etiology and clinical outcomes of microbial keratitis at a tertiary eye-care center in northern Thailand. J Med Assoc Thai. 2012;95 Suppl 4:S8–17. [PubMed] [Google Scholar]
- 33.Khor W-B, Prajna VN, Garg P, et al. The Asia Cornea Society Infectious Keratitis Study: A Prospective Multicenter Study of Infectious Keratitis in Asia. Am J Ophthalmol. 2018;195:161–170. [DOI] [PubMed] [Google Scholar]
- 34.Singh K, Choudhry NK, Krumme AA, et al. A concept-wide association study to identify potential risk factors for nonadherence among prevalent users of antihypertensives. Pharmacoepidemiol Drug Saf. 2019;28:1299–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao J, Singh K. Integrating risk prediction models into chronic kidney disease care. Curr Opin Nephrol Hypertens. 2020;29:339–345. [DOI] [PubMed] [Google Scholar]
- 36.Churpek MM, Yuen TC, Winslow C, et al. Multicenter development and validation of a risk stratification tool for ward patients. Am J Respir Crit Care Med. 2014;190:649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Auffenberg GB, Merdan S, Miller DC, et al. Evaluation of Prostate Cancer Risk Calculators for Shared Decision Making Across Diverse Urology Practices in Michigan. Urology. 2017;104:137–142. [DOI] [PubMed] [Google Scholar]
- 38.Auffenberg GB, Ghani KR, Ramani S, et al. askMUSIC: Leveraging a Clinical Registry to Develop a New Machine Learning Model to Inform Patients of Prostate Cancer Treatments Chosen by Similar Men. Eur Urol. 2019;75:901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brannon E, Wang T, Lapedis J, et al. Towards a Learning Health System to Reduce Emergency Department Visits at a Population Level. AMIA . Annu Symp proceedings AMIA Symp. 2018;2018:295–304. [PMC free article] [PubMed] [Google Scholar]
- 40.Bright TJ, Wong A, Dhurjati R, et al. Effect of clinical decision-support systems: A systematic review. Ann Intern Med. 2012;157:29–43. [DOI] [PubMed] [Google Scholar]
- 41.Ballouz D, Maganti N, Tuohy M, et al. Medication Burden for Patients With Bacterial Keratitis. Cornea. 2019;38:933–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ashfaq H, Maganti N, Ballouz D, et al. Procedures, Visits, and Procedure Costs in the Management of Microbial Keratitis. Cornea. 2021;40:472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaye S, Tuft S, Neal T, et al. Bacterial susceptibility to topical antimicrobials and clinical outcome in bacterial keratitis. Invest Ophthalmol Vis Sci. 2010;51:362–368. [DOI] [PubMed] [Google Scholar]
- 44.Lalitha P, Prajna NV, Oldenburg CE, et al. Organism, minimum inhibitory concentration, and outcome in a fungal corneal ulcer clinical trial. Cornea. 2012;31:662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valikodath NG, Newman-Casey PA, Lee PP, et al. Agreement of Ocular Symptom Reporting Between Patient-Reported Outcomes and Medical Records. JAMA Ophthalmol. 2017;135:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kriegel MF, Loo J, Farsiu S, et al. Measurement Reliability for Keratitis Morphology. Cornea. 2020;39:1503–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gianfrancesco MA, Tamang S, Yazdany J, et al. Potential Biases in Machine Learning Algorithms Using Electronic Health Record Data. JAMA Intern Med. 2018;178:1544–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X, Pérez-Stable EJ, Bourne PE, et al. Big Data Science: Opportunities and Challenges to Address Minority Health and Health Disparities in the 21st Century. Ethn Dis. 2017;27:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: The diagnosis codes used to identify patients with microbial keratitis in the electronic health record.
Supplemental Table 2: The methodology to interpret the eye laterality of microbial keratitis infection.
Supplemental Table 3: Descriptive statistics for the lemma identified in clinical notes from microbial keratitis patients at presentation. 2990 microbial keratitis patients were identified, but 2908 had notes at presentation that returned at least 1 lemma. Lemma that had at least 100 mentions across the notes of these patients are presented (n=213).


