Abstract
Objective:
Correlations between cognitive ability and psychopathology are well-recognized, but prior research has been limited by focusing on 1) individuals with intellectual disability, 2) single-diagnosis psychiatric populations, or 3) few measures of psychopathology. Here, we quantify relationships between full-scale IQ and multiple dimensions of psychopathology in a diverse care-seeking population, with a novel focus on differential coupling between psychopathology dimensions as a function of IQ.
Method:
Seventy dimensional measures of psychopathology, plus IQ and demographic data, were collated for 2,752 children and adolescents from the Healthy Brain Network dataset. We first examined univariate associations between IQ and psychopathology, and then characterized how the correlational architecture of psychopathology differs between groups at extremes of the IQ distribution.
Results:
Associations with IQ vary in magnitude between different domains of psychopathology: IQ shows the strongest negative correlations with attentional and social impairments, but is largely unrelated to affective symptoms and psychopathy. Lower IQ is associated with stronger coupling between internalizing problems and aggression, repetitive behaviors, and hyperactivity/inattentiveness.
Conclusion:
Our analyses reveal that variation in general cognitive ability is not only associated with significant and selective shifts in severity of psychopathology, but also in the coupling between different dimensions of psychopathology. These findings have relevance for the clinical assessment of mental health in populations with varying IQ, and may also inform ongoing efforts to improve the measurement of psychopathology and understand how relationships between cognition and behavior are reflected in brain organization.
Keywords: cognition, psychopathology, development, Healthy Brain Network (HBN), comorbidity
Lay summary:
A lay summary of the manuscript of no more than four sentences that will be published as part of the Journal’s Table of Contents, if accepted. Lay summaries are short accounts of manuscripts that are targeted for general audiences. They should include information about the study (where the data comes from), what the authors found (key results, ideally with at least one specific statistic), and 1–2 key implications (especially non-prevention or treatment-related implications). Lay language summaries are required prior to the acceptance of a manuscript.
“Using behavioral questionnaire and IQ data collected from the Healthy Brain Network database, we examined how cognitive ability is related to both the severity and co-occurrence of psychiatric symptoms in youth aged 6–17 years. We found that IQ shows the strongest negative relationship with attentional and social impairments, but is largely unrelated to anxiety, mood, and psychopathy. Lower IQ is associated with stronger co-occurrence of internalizing symptoms and hyperactivity/inattentiveness. These findings may help improve clinical assessment and measurement of mental health symptoms in populations with varying IQ and inform ongoing efforts to understand how relationships between cognition and behavior are reflected in brain organization.”
Introduction
There is a long history of research on the relationship between variation in cognitive ability and variation in psychopathology 1–4, with a general consensus that cognitive impairment is associated with increased risk for mental health difficulties 5–7. However, most studies to date have adopted one of the following three study designs, each with its own limitations.
Comparing groups with and without intellectual disability (ID).
Many studies have explored associations between general cognitive ability and mental health by comparing the prevalence of psychiatric disorders in individuals with and without ID (IQ < 70). Studies adopting this design have typically focused on categorical diagnostic outcomes 8,9 or a few dimensional measures of psychopathology 10, and there is currently little consensus as to which specific aspects of psychopathology are most elevated in groups with ID. Furthermore, a focus on the presence versus absence of ID excludes youth with an IQ in the “borderline” range (IQ 71–85), despite the evidence that this group is also at elevated risk for psychopathology relative to the general population 11. A focus on ID also overlooks youth with unusually high IQ, despite some reports that have linked high IQ in childhood with increased risk of certain psychiatric concerns, especially bipolar disorder and mania, in adulthood 12–14.
Correlates of cognitive ability within a selected psychiatric diagnosis.
An important complementary literature explores the relationship between cognitive ability and variables such as symptom severity, overall functioning, or treatment outcomes in a group of participants who all share the same psychiatric diagnosis. For example, variation in IQ has been associated with variation in symptom profiles and adaptive functioning in several developmentally-emergent psychiatric diagnoses including autism spectrum disorder (ASD 15,16), attention-deficit hyperactivity disorder (ADHD 17), tic disorders 18, and obsessive-compulsive disorder (OCD 19). However, focusing on individual diagnostic groups overlooks the often dimensional and comorbid nature of mental illness in the general population 20,21.
Linking cognitive ability to broad dimensions of psychopathology in the general population.
Finally, some studies do not constrain their sample on either IQ score or a specific psychiatric diagnosis, but instead examine relationships between dimensional measures of cognitive ability and psychopathology in the general population. However, to date, such studies have tended to consider sparse summary measures of psychopathology that collapse diverse symptom profiles into two or three broad dimensions, such as internalizing versus externalizing 5; or other broad groupings 22,23. Furthermore, the variation in psychopathology within the general population is limited relative to that found in care-seeking populations 24. Therefore, we still lack a fine-grained analysis that independently quantifies links between cognitive ability and symptom severity for several different dimensional measures of psychopathology in childhood.
The recent availability of large clinical datasets with rich phenotypic information allows for a systematic reappraisal of IQ-psychopathology relationships in childhood and adolescence that could address the limitations enumerated above. Specifically, transformative resources such as the Healthy Brain Network (HBN) dataset 25 provide fine-grained dimensional measures of multiple aspects of psychopathology in large care-seeking populations with accompanying measures of IQ that enable both categorical and continuous estimation of the relationship between IQ and psychopathology. The availability of many dimensional symptom scales for many study participants also permits the investigation of entirely new questions regarding relationships between IQ and psychopathology. In particular, although dependence between different domains of psychopathology is well established—as demonstrated by patterns of symptom score correlations and comorbid diagnoses across individuals 26,27—it remains unknown whether cognitive ability modulates the coupling between different measures of psychopathology. Several prior observations hint that such a phenomenon might exist. First, correlations between psychiatric outcomes are already known to be related to non-psychiatric outcomes (e.g., neurological, metabolic, or musculoskeletal disorders)—highlighting the limitations of considering psychiatric versus cognitive symptoms independently 28. Second, recent work on symptom networks in psychiatry posits that correlations between scores on different symptoms may partly reflect causal influences of one symptom on another 29, and differences in cognitive ability can similarly shape how psychiatric symptoms are experienced and expressed 30–33. Third, from a psychometric perspective, the strength of agreement between parent and child ratings of some dimensions of psychopathology appears to be greater in older versus younger children 34. If this varying rater agreement reflects developmental variation in cognitive ability (i.e., age-related differences in cognition), then variations in cognitive ability between individuals (i.e., trait-related differences in cognition) may also be expected to influence the degree of agreement between parent and child ratings of psychopathology. Fourth, variation in cognitive ability has been linked to variation in the degree of structural and functional coupling between different brain systems 35–37, and may therefore modulate coupling between the behaviors that these brain systems underpin.
Motivated by the above considerations, we harnessed the HBN dataset to (i) characterize the relationship between IQ and symptom severity for 70 different dimensional measures of psychopathology in children and adolescents, and (ii) determine if variation in IQ also modulates the strength of correlations between these dimensional measures of psychopathology.
Method
Participants
We conducted all analyses using data from the Healthy Brain Network (HBN), a large-scale psychiatric and neuroimaging database, available for download here: http://fcon_1000.projects.nitrc.org/indi/cmi_healthy_brain_network/. The first nine releases of the database, described in this study, include phenotypic data from 3,625 participants. All HBN procedures were approved by the Chesapeake Institutional Review Board. Written informed consent was obtained from legal guardians of all participants.
The HBN’s community-referred recruitment model is designed to encourage participation of families who have concerns about psychiatric symptoms or learning problems in their children 25. As such, the HBN represents a naturalistic and representatively heterogeneous sample of care-seeking individuals that is enriched for psychopathology compared to the general population. For more information about the inclusion and exclusion criteria and the study design for the HBN, see25.
For this study, we selected only those participants whose IQ was assessed using the Wechsler Intelligence Scale for Children, Fifth Edition (WISC-V 38), intended for use in youth aged 6–17 years, and for whom the WISC was coded as “complete.” Our final sample contained 2,752 participants (ages 5.59–16.99 years, mean = 10.20 years, SD = 2.76 years; n = 971 female, 35.27%). This subsample of the HBN with complete WISC data differs from the total HBN sample in age (younger, t = −5.18, df = 1035.2, p < 0.011), socio-economic status (higher SES, t = 4.69, df = 1140.6, p < 0.001), and self-reported race/ethnicity (χ2 = 71.82, df = 7, p < 0.001), but not in proportion of male and female participants (χ2 = 0.56, df = 1, p = 0.456).
Measures
General cognitive ability was assessed using the full-scale IQ composite score (henceforth “IQ”) of the Wechsler Intelligence Scale for Children (WISC). Eight behavioral questionnaire instruments assessing diverse domains of psychopathology were selected as response variables: the Child Behavior Checklist (CBCL), Conner’s 3 Self-Report (CSR), Inventory of Callous-Unemotional Traits (ICU), Repetitive Behavior Scale (RBS), Screen for Child Anxiety-Related Disorders (SCARED), Strengths and Difficulties Questionnaire (SDQ), Social Responsiveness Scale (SRS), and Youth Self-Report (YSR). These eight instruments yielded a total of 70 subscales, in which a higher score indicates greater severity of that symptom domain. See Table 1 for further information on the measures used. Age- and sex-normed t-scores for the subscales were used when available; when not available, raw scores were used. Sensitivity analyses confirmed that results were stable when controlling for age and sex effects (see Sensitivity Analyses for further information). All questionnaires were completed by participants and/or their parents using the NextGen electronic medical record system 25.
Table 1:
Instruments Included in the Study
| Color in figures | Instrument (Abbreviation) | Age range in HBN sample | Domain of psychopathology | Subscales (Abbreviations) | Reporter | Raw or scaled? | Citation |
|---|---|---|---|---|---|---|---|

|
Child Behavior Checklist 6–18 (CBCL) | 6–18 years | Multi-domain | Aggressive behavior (Aggression), Anxious depression (Anxious Dep), Attention problems (Attention), Rule-breaking behavior (Rule Breaking), Somatic complaints (Somatic), Social problems (Social), Thought problems (Thought), Withdrawn depression (Withdrawn Dep), Externalizing total (Externalizing), Internalizing total (Internalizing), Total composite score (Total) | Parent | Scaled | 39 |

|
Conner’s 3 Self-Report (CSR) | 8+ years | Attention-deficit/hyperactivity disorder (ADHD) | Inattentiveness (Inattention), Hyperactivity/impulsivity (Hyperactivity), Learning problems (Learning), Aggression, Peer/family relations (Relationships) | Youth | Scaled | 40 |

|
Inventory of Callous-Unemotional Traits, Self-Report (ICU-SR) | 7–18 years | Callous and unemotional traits typical of certain antisocial and aggressive youth | Callousness, Uncaring, Unemotional, Total composite score (Total Psychopathy) | Youth | Raw | 41 |

|
Inventory of Callous-Unemotional Traits, Parent-Report (ICU-PR) | 5+ years | Callous and unemotional traits typical of certain antisocial and aggressive youth | Callousness, Uncaring, Unemotional, Total composite score (Total Psychopathy) | Parent | Raw | 41 |

|
Repetitive Behavior Scale (RBS) | 5–21 years | Restricted and repetitive behaviors typical of individuals with Autism Spectrum Disorders | Compulsive behavior, Self-injurious behavior (Self-injury), Stereotyped behavior, Restricted interests, Ritualized behavior, Total composite score (Total Repetitive) | Parent | Raw | 42 |

|
Screen for Child Anxiety-Related Disorders, Self-Report (SCARED-SR) | 8–18 years | Anxiety | Generalized anxiety (Generalized Anx), Panic disorder (Panic), Social anxiety (Social Anx), School avoidance, Separation anxiety (Separation Anx), Total composite score (Total Anx) | Youth | Raw | 43 |

|
Screen for Child Anxiety-Related Disorders, Parent-Report (SCARED-PR) | 8–18 years | Anxiety | Generalized anxiety (Generalized Anx), Panic disorder (Panic), Social anxiety (Social Anx), School avoidance, Separation anxiety (Separation Anx), Total composite score (Total Anx) | Parent | Raw | 43 |

|
Strengths and Difficulties Questionnaire (SDQ) | 5+ years | Multi-domain | Conduct problems (Conduct), Emotional problems (Emotional), Generating impact (Total), Hyperactivity (Hyperactivity), Peer problems, Prosociality (Prosocial (reverse))*, Externalizing, Internalizing, Total difficulties (Total) *Note: Prosociality has been reverse-scored (Prosocial (reverse)) for consistency with other subscales, in which a higher score indicates greater impairment in that symptom domain. Therefore, a higher Prosocial (reverse) score encodes a relative absence of prosocial behaviors. |
Parent | Raw | 44 |

|
Social Responsiveness Scale, Second Edition (SRS) | 5+ years | Social impairments typical of individuals with ASD | Social awareness, Social cognition, Social communication, Social motivation, Restricted interests and repetitive behavior (Repetitive Behaviors), Total composite score (Total Social); plus 2 DSM-compatible subscales: Restricted interests and repetitive behavior (Repetitive Behaviors), Social communication and interaction (Social Comm/Interaction) | Parent | Scaled | 45 |

|
Youth Self-Report (YSR) | 11–18 years | Multi-domain | Aggressive behavior (Aggression), Anxious depression (Anxious Dep), Attention problems (Attention), Rule-breaking behavior (Rule Breaking), Somatic complaints (Somatic), Social problems (Social), Thought problems (Thought), Withdrawn depression (Withdrawn Dep), Externalizing total (Externalizing), Internalizing total (Internalizing), Total composite score (Total) | Youth | Scaled | 39 |
Note: “Reporter” indicates whether the instrument was completed by the parent or the youth. “Raw or scaled?” indicates whether the publisher of that instrument provides age- and sex-normed scaled scores based on population norms.
These instruments were selected primarily because they all report subscale scores—typically, the sum of the responses to Likert-scale items that aim to measure a similar behavioral or emotional construct. Subscales are advantageous for three reasons. First, they provide standardized dimensional measures of psychopathology at a level of resolution above that of single item-level data, but more specific than total composite scores. Second, measuring psychopathology in a continuous, rather than categorical (present versus absent), manner is more accurate to the presentation of mental illnesses. Third, the subscale level of resolution captures similar constructs through different rating scales (e.g., CBCL Social Problems and SDQ Peer Problems) and different informants (e.g., CBCL Rule-Breaking Behavior and YSR Rule-Breaking Behavior), providing an opportunity to detect convergent validity for associations with IQ, or for coupling of symptom correlations by IQ.
Subscale-specific associations with IQ
We implemented a “tertile split” strategy as our primary analysis for quantifying the sensitivity of behavioral subscale scores to variation in IQ. A key motivation for using a tertile split approach as our primary analytic approach is that—unlike regression analyses—it is also suitable for characterization of coupling differences (see below). Therefore, the tertile split approach offers a consistent analytic tool that can be used across both sets of analyses that we perform.
To implement the tertile split, we first stratified participants according to their IQ tertile, and focused on comparing psychopathology scores between participants in the top 33% of the IQ distribution and participants in the bottom 33% of the IQ distribution. Upper-lower tertile differences for each subscale score were estimated as Cohen’s d values by dividing the difference in mean score by the pooled standard deviation in the two tertiles. Statistical significance of these score differences was determined using a permutation procedure that resampled participants. Specifically, we randomly sampled a first 33% and then second 33% of the total sample without replacement, and then calculated the difference in mean score on each subscale between these two randomized groups. This process was iterated 10,000 times to generate a null distribution of 10,000 differences in mean scores per subscale. Observed differences that fell below the 2.5th percentile or above the 97.5th percentile of the null distribution were considered significant at p < 0.05. We then corrected for multiple comparisons using Bonferroni correction across all 70 subscales (p = 0.0007).
The tertile split procedure described above provides a simple and intuitive comparison between high and low IQ groups relative to the IQ distribution observed in the naturalistic HBN sample. However, given that IQ is a continuous variable, and that categorizing a continuous variable can be associated with power loss, we complemented the tertile split approach with a continuous method using Pearson correlations between IQ and each subscale in the entire sample of participants, using pairwise deletion on missing data where necessary. This alternative correlational approach was adopted after first verifying that relationships between IQ and psychopathology were almost universally linear, rather than quadratic, in nature (i.e., six out of 70 subscales had significant quadratic terms, but none of these showed an elevation in scores with increasing IQ into the high-IQ tertile upon further analysis; see Supplement 1 and Figure S1, available online, for details).
IQ modulation of symptom coupling
We next applied the tertile split approach to test whether IQ modulates the strength of the correlation between symptom domains across individuals. Unlike the tertile-split approach, alternative regression-based approaches for testing IQ modulation of psychopathology coupling suffer from asymmetry. Specifically—using depression and hyperactivity as an example—the β3 value of ‘depressioni ~ β0 + β1(IQi) + β2(hyperactivityi) + β3(IQi * hyperactivityi)’ is not necessarily the same as the β3 of ‘hyperactivityi ~ β0 + β1(IQi) + β2(depressioni) + β3(IQi * depressioni)’ because different residuals are being minimized in each version 46.
To implement the tertile split approach, we calculated the association between each pair of behavioral subscales (a total of 2,415 unique edges) in the upper and lower IQ tertiles separately (Figure 1A–B). All associations were computed as Pearson’s correlation coefficients using the cor() function in the stats package in R Studio (with use = “pairwise.complete.obs”) and then Fisher z-transformed using the fisherz() function from the psych package. For further discussion of the treatment of missing data, see Supplement 2, available online.
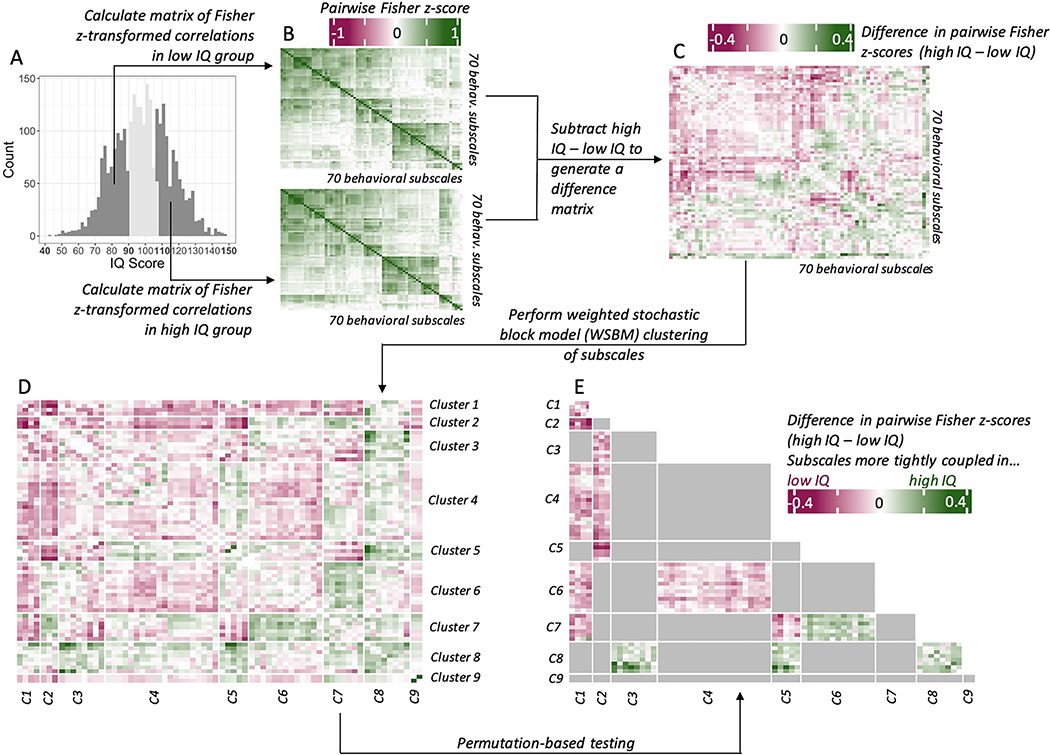
Figure 1: Workflow for Coupling-Change Analyses.
Note: (A) IQ distribution with lower and upper tertiles highlighted. (B) Pairwise Fisher z-transformed correlation matrices in low IQ group and high IQ groups. (C) Difference matrix calculated as the pairwise Fisher z-score in the high IQ tertile, minus the pairwise Fisher z-score in the low IQ tertile. (D) Difference matrix after weighted stochastic block model (WSBM) clustering, with each edge grouped into a block. (E) Thresholded WSBM difference matrix, showing only those WSBM blocks that were significant after permutation-based testing.
The correlations in the lower tertile were subtracted from those in the upper tertile to quantify differences in coupling between measures of psychopathology as a function of IQ. This procedure generated a difference matrix in which a positive difference in Fisher z-score (Δz) indicates that two subscales were more tightly coupled in the high IQ group, and a negative Δz indicates that two subscales were more tightly coupled in the low IQ group (Figure 1C). To test for psychopathology subscales with similar coupling differences, we then clustered the Δz matrix using weighted stochastic block modeling (WSBM)—a generative clustering algorithm that is capable of finding non-assortative (e.g., “hub and spoke”) network structures 47. We implemented WSBM using the BM_gaussian() function (with default settings) from the blockmodels package in R 48, which identified an optimal number of clusters using independent completed likelihood criteria 49 and generated an assignment of subscales to clusters under this optimum (Figure 1D). We named each cluster according to the groups of subscales within it; a list of cluster assignments and names can be found in Table S1, available online. We calculated the observed average Δz values for each block by taking the mean of all unique edges in each block (excluding diagonal edges within diagonal blocks, which have a Δz of zero).
To determine whether the block-wise Δz values were significant, we again used a permutation procedure similar to the one described above. For each iteration, we sampled a first 33% and a second 33% of the total sample without replacement, computed the 2,415 pairwise Fisher z-scores in each group, and subtracted one matrix from the other to produce a matrix of random Δz values for each pair of subscales. This process was repeated 10,000 times. We then imposed the WSBM block structure from the observed data onto each of these 10,000 null Δz matrices such that, for each observed block-average Δz value, there was a corresponding null distribution of 10,000 block-average Δz values. Observed block-average Δz values that fell below the 2.5th percentile or above the 97.5th percentile of the null distribution were considered significant at p < 0.05 (Figure 1E). We then corrected for multiple comparisons across all 45 unique blocks using Bonferroni’s method (p = 0.001).
We also pursued two complementary individual edge-wise analyses to provide a granular accompaniment to the above block-wise analyses: ranking all unique edges by Δz value, and screening for any edges that showed a Δz value which survived Bonferroni correction across all 2,415 edges (p = 0.00002).
Sensitivity analyses
We performed two sensitivity analyses. First, we tested for robustness of IQ effects to variation in socio-economic status (SES) and self-reported race/ethnicity by regressing SES (measured by the Barratt Simplified Measure of Social Status total score 50), and race/ethnicity out of IQ using a linear model. Second, given that some measures reported age- and sex-normed t-scores and others did not, we compared findings derived from the heterogeneously-normed measures to those derived from measures that we independently normed for age and sex. Specifically, we regressed age and sex out of the raw scores on all subscales using a generalized additive model (GAM) in the mgcv package in R 51. We then calculated the correlation coefficient between the vectors of IQ-subscale correlations, Cohen’s d values, and Δz values resulting from non-residualized analyses and each of the analyses controlling for SES, race/ethnicity, age, and sex.
All code is available for download at https://github.com/bridgetmahony/IQpsychHBN.
Results
Descriptive statistics
Participant characteristics are reported in Table 2. Full-scale IQ scores ranged between 42–147 (mean = 98.21, SD = 16.92). The upper tertile (n = 936) had a range of 106–147 (mean = 116.46, SD = 8.64), and the lower tertile (n = 937) had a range of 42–91 (mean = 79.59, SD = 8.40). The proportions of male and female participants in the upper (36.40% female) and lower (34.90% female) IQ tertiles were not significantly different (χ2 = 0.42, df = 1, p = 0.519). Participants in the upper tertile were on average younger (mean = 9.92 years, SD = 2.81 years) and of a higher SES (mean Barratt Total SES = 54.57, SD = 11.57) than those in the lower tertile (mean = 10.57 years, SD = 2.72 years, p < 0.001; mean Barratt Total SES = 43.33, SD = 16.17, p < 0.001). There was a significantly different proportion of self-reported racial/ethnic groups in the upper vs. the lower IQ tertile (χ2 = 235.98, p < 0.001), an effect that was attenuated slightly when controlling for the effect of SES on IQ (χ2 = 129.39, p < 0.001). Given that HBN is not a representative sample for the population, race was not studied as a variable of interest, though we did establish that our findings hold when controlling for race and SES variables (see Methods and Results sections) and also further considered the implications of observed demographic associations with the IQ tertile groups in our Discussion.
Table 2:
Participant Demographics
| n | 2752 |
|
| |
| Sex (F (%)) | 970 (35.20) |
|
| |
| Age (mean (SD)) | 10.20 (2.76) |
|
| |
| IQ (mean (SD)) | 98.21 (16.92) |
|
| |
| Barratt Total SES (mean (SD)) | 49.16 (14.57) |
|
| |
| Race/Ethnicity (%) | |
| American Indian/Alaskan Native | 1 (< 0.01) |
| Asian | 80 (3.3) |
| Black | 366 (15.0) |
| Hispanic | 268 (11.0) |
| Indian | 9 (0.4) |
| Native American Indian | 4 (0.2) |
| Native Hawaiian/Other Pacific Islander | 3 (0.1) |
| Other race | 38 (1.6) |
| Two or more races | 402 (16.5) |
| Unknown | 11 (0.5) |
| White | 1258 (51.6) |
|
| |
| Not reported | 312 (11.3) |
Subscale-specific associations between IQ and psychopathology
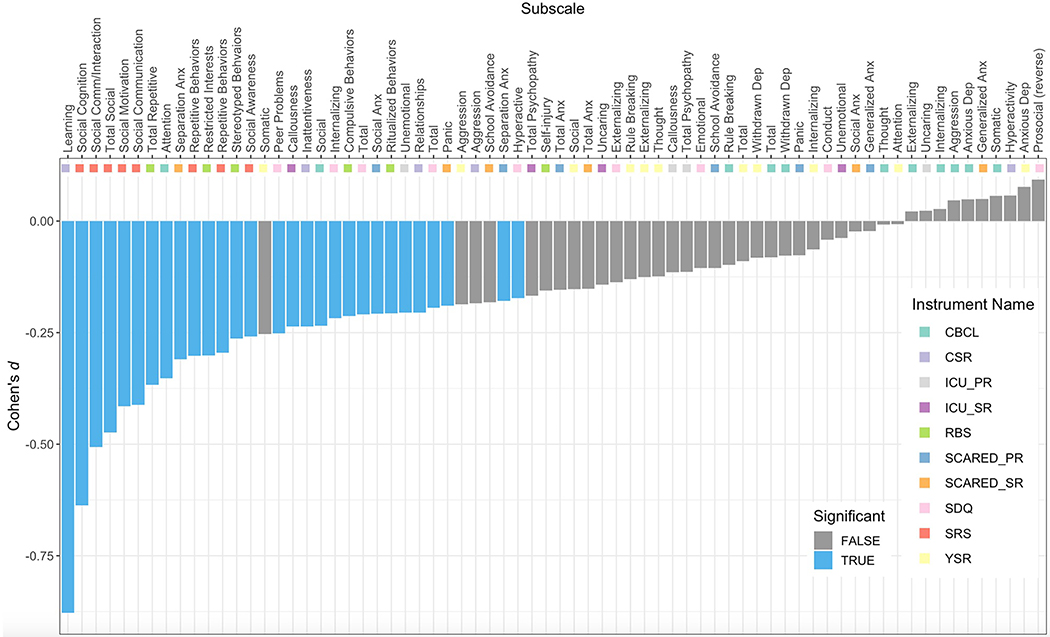
The difference in mean subscale scores in the upper versus lower IQ tertiles was nominally significant (p < 0.05) against the null distribution for 44 of 70 behavioral subscales, 29 of which also survived Bonferroni correction for multiple comparisons (Figure 2; Table S2, available online). Notably, all subscales for which the difference in means was significant after correction for multiple comparisons had negative Cohen’s d values, indicating greater symptom severity with lower IQ. Those subscales with the most negative Cohen’s d values measured learning problems (CSR Learning Problems, d = −0.88, Bonferroni-corrected p value (BF p) < 0.001), social impairments (all SRS subscales (ex: SRS Total, d = −0.47, BF p < 0.001); CBCL Social Problems, d = −0.24, BF p < 0.001), repetitive behaviors (most RBS subscales (ex: RBS Total, d = −0.37, BF p < 0.001)), and attentional impairments (CBCL Attention Problems, d = −0.35, BF p < 0.001; CSR Inattentiveness, d = −0.24, BF p < 0.001). Symptoms of depression, aggressive behavior, and conduct problems showed no significant relationship to IQ.
Figure 2: Diverse Relationships Between IQ and 70 Subscale Measures of Psychopathology.
Note: Bar plot showing Cohen’s d score difference (y-axis) between lower and upper IQ tertiles in the HBN for all subscale measures of psychopathology (x-axis). Positive Cohen’s d indicates greater symptom severity among the upper tertile, while negative d indicates greater symptom severity among the lower tertile. Blue bars denote the 29 subscales that showed statistically significant non-zero Cohen’s d values after 10,000 permutations and Bonferroni correction across subscales. Multi-colored tiles provide information on which instrument the subscale came from. Notable associations in decreasing order of magnitude were: learning problems, social abilities, repetitive behaviors, and attentional impairments. Severity of psychopathy and general anxiety symptoms were notably uncorrelated with IQ. CBCL = Child Behavior Checklist; CSR = Conner’s 3 Self-Report; ICU_PR = Inventory of Callous-Unemotional Traits, Parent-Report; ICU_SR = Inventory of Callous-Unemotional Traits, Self-Report; RBS = Repetitive Behavior Scale; SCARED_PR = Screen for Child Anxiety-Related Disorders, Parent-Report; SCARED_SR = Screen for Child Anxiety-Related Disorders, Self-Report; SDQ = Strengths and Difficulties Questionnaire; SRS = Social Responsiveness Scale; YSR = Youth Self-Report.
Interestingly, relationships with IQ could vary among different measures from the same overarching domain of psychopathology. For example, several measures of specific anxiety symptoms were significantly associated with IQ (SCARED Parent-Report Social Anxiety, d = −0.21, BF p < 0.001; SCARED Parent-Report Separation Anxiety, d = −0.18, BF p = 0.014; SCARED Self-Report Panic Disorder, d = −0.19, BF p = 0.014), whereas measures of more general anxiety symptoms were not (CBCL Anxious Depression, d = 0.05, BF p > 1.000; YSR Anxious Depression, d = 0.08, BF p > 1.000; SCARED Generalized Anxiety (Parent-Report: d = −0.02, BF p > 1.000; Self-Report: d = 0.05, BF p > 1.000); and SCARED Total Composite score (Parent-Report: d = −0.15, BF p = 0.098; Self-Report: d = −0.15, BF p = 0.217)). Similarly, some measures of psychopathy were significantly related to IQ (ICU Self-Report Callousness, d = −0.24, BF p < 0.001; and ICU Parent-Report Unemotionality, d = −0.21, BF p < 0.001) whereas others were not—showing both subdomain and rater effects. Rater effects were also seen for measures of hyperactivity, which were significantly associated with IQ when based on parent-report (SDQ Hyperactivity, d = −0.17, BF p < 0.001), but not self-report (CSR Hyperactivity, d = 0.06, BF p > 1.000).
There was no evidence that a higher IQ score was associated with significantly greater symptom severity in any domain of psychopathology after Bonferroni correction (Figure 2), although a few subscales trended towards a higher mean score in the high IQ group (e.g., SDQ Prosociality (reverse-scored), d = 0.09, BF p > 1.000; YSR Anxious Depression, d = 0.08, BF p > 1.000; SCARED SR Generalized Anxiety, d = 0.05, BF p > 1.000; and more).
In addition to calculating Cohen’s d effect sizes for the difference in mean scores on each behavioral subscale in the high and low IQ groups, we calculated Pearson’s correlations (r) for the relationship between IQ and each of the 70 behavioral variables (see Table S2, available online). The cross-scale correlation between Cohen’s d and r values was r = 0.99, indicating very high agreement between these two approaches. All subscales that were significantly different between the high and low groups were also significantly correlated with IQ. Of the forty-seven behavioral subscales that were significantly correlated with IQ (p < 0.05), four failed to show statistically-significant differences between high and low IQ groups after Bonferroni correction for multiple comparisons (SCARED SR School Avoidance, SCARED PR Total Anxiety, and YSR Somatic Complaints). There were an additional four subscales that were significant at p < 0.05 in the correlational analyses, but not in Cohen’s d (YSR Social Problems, SCARED PR Panic Disorder, and SDQ Prosocial (reverse-scored)).
Results were stable when controlling for the effect of SES and self-reported race/ethnicity on IQ, and when controlling for the effects of age and sex on behavioral raw scores in sensitivity analyses (see Supplement 3, Figure S2, and Figure S3, available online). Additionally, analyses on the presence of clinician-consensus psychiatric diagnoses among participants in the upper vs. the lower IQ tertiles were consistent with results from our dimensional analyses (see Supplement 4 and Table S3, available online).
IQ and coupling between different domains of psychopathology
In both the lower and upper IQ tertiles, behavioral subscales were generally positively correlated with one another (Figure 1B; Figure S4, available online). Therefore, Δz values reported in the coupling change analyses can be understood to generally indicate differences in the strength of the positive association between two variables in the upper versus lower tertiles, rather than differences in the strength of the negative association. There were broad similarities between IQ groups in the correlational structure between scales, although we observed a trend towards a more distinct block-diagonal structure in the high IQ group (see Supplement 5 and Figure S5, available online). We also note that these differences in coupling cannot necessarily be assumed to be reflective of differences in clustering structure. While questions about the underlying structure of psychopathology are beyond the scope of this paper, in Supplement 6 and Figure S6, available online, we conduct analyses that examine how cluster assignments of each subscale change in relation to IQ across the entire IQ range, a complementary perspective that does not focus on continuous measures in changes of edge strength.
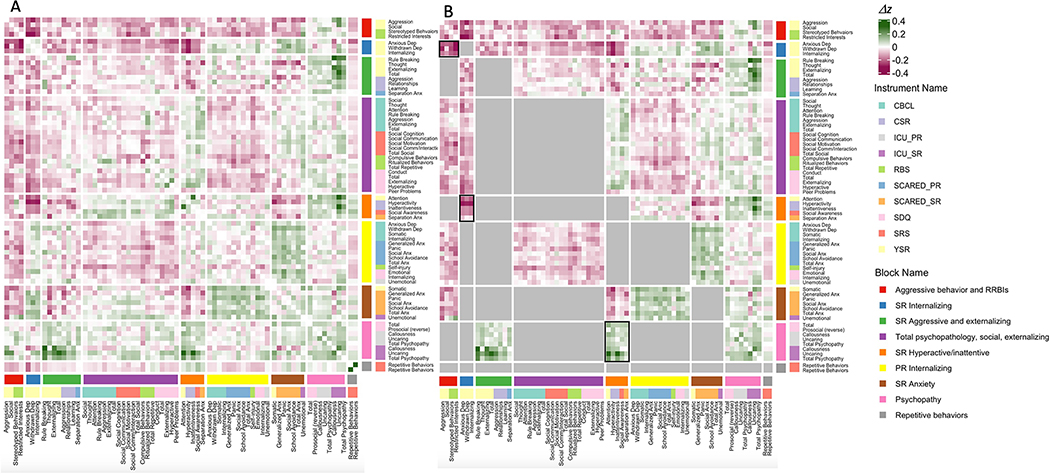
Subtracting the Fisher z-score matrix in the low IQ group from that in the high IQ group (Figure 1B) quantified the relationship between IQ and the strength of coupling between different measures of psychopathology (edge-level metrics, Supplemental 8, available online). Clustering this Δz matrix with WSBM identified a nine-cluster solution as being optimal, and arranging Δz values according to cluster assignments provided a mesoscale view of coordinated shifts in the coupling between measures of psychopathology as a function of IQ (Figure 3A). Dimensions of psychopathology were more clearly clustered by domain, rather than instrument—indicating that IQ modulation of symptom coupling showed coherence across different measurement instruments (e.g., co-clustering for diverse measures of psychopathy or anxiety). Permutation testing (Methods) identified statistically significant shifts in coupling at the nominal p < 0.05 level for 14 out of 45 unique blocks, of which three survived Bonferroni correction for multiple comparisons (p ≤ 0.001) across 45 block pairs (Figure 3B; Table S4, available online).
Figure 3: Characterizing Differences in Coupling Between Measures of Psychopathology as a Function of IQ.
Note: (A) Difference matrix (upper IQ tertile minus lower IQ tertile) organized by WSBM blocks. (B) WSBM-organized difference matrix where lower triangle shows color only for those unique blocks with nominally significant changes in coupling after permutation testing (p < 0.05). Blocks with a black outline were also significant after further Bonferroni correction for multiple comparisons across all 45 unique blocks. CBCL = Child Behavior Checklist; CSR = Conner’s 3 Self-Report; ICU_PR = Inventory of Callous-Unemotional Traits, Parent-Report; ICU_SR = Inventory of Callous-Unemotional Traits, Self-Report; PR = parent-report; RBS = Repetitive Behavior Scale; RRBIs = restricted, repetitive behaviors and interests; SCARED_PR = Screen for Child Anxiety-Related Disorders, Parent-Report; SCARED_SR = Screen for Child Anxiety-Related Disorders, Self-Report; SDQ = Strengths and Difficulties Questionnaire; SR = self-report; SRS = Social Responsiveness Scale; YSR = Youth Self-Report.
At a nominal p < 0.05 level, ten blocks showed increases in coupling among the low IQ versus the high IQ group, whereas four showed decreases in coupling—indicating that IQ is associated with a complex mix of both strengthening and weakening effects on coupling between different measures of psychopathology. The ten domains showing greater coupling in the lower as compared to the higher IQ group generally involved a strengthening of correlations among measures of internalizing, behavioral dyscontrol, and hyperactive/inattentive problems. This phenomenon was well-captured by the two blocks that showed statistically significant coupling differences after Bonferroni correction for multiple comparisons: lower-IQ youth who endorsed internalizing problems were significantly more likely to show aggression, social problems, and repetitive behaviors (Δz = −0.21, BF p < 0.001), and to self-report problems with inattentiveness and hyperactivity, than were high-IQ youth (Δz = −0.17, BF p < 0.001). The four blocks that were more tightly coupled in the higher IQ group were (i) within the domain of antisociality/psychopathy; and (ii) between domains of self-report anxiety and parent-report internalizing problems; between self-report externalizing/aggression and antisociality/psychopathology; and between self-report hyperactivity/inattentiveness and antisociality/psychopathology. The latter of these blocks survived Bonferroni correction for multiple comparisons, indicating that high-IQ youth with higher levels of hyperactivity/inattentiveness were more likely to also have higher levels of antisociality/psychopathology than lower-IQ youth with high hyperactivity/inattentiveness (Δz = 0.10, BF p = 0.005).
In addition to examining block-level phenomena, we also identified those individual edges that were significant against the null distribution (Figure S7, Supplement 8, available online). Notably, the distribution of edges that were significantly more coupled in high IQ or low IQ groups was closely aligned with the results of our block-wise analysis: specifically, all ten blocks that showed significant increases in coupling in the low IQ group were significantly enriched for individual edges that were more tightly coupled in the low IQ group (p < 0.05, see Supplement 7, available online, for methods). Similarly, all four blocks that showed significant increases in coupling in the high IQ group were significantly enriched for individual edges that were more tightly coupled in the high IQ group (p < 0.05). Finally, the correlation between the Cohen’s d value for each subscale and the mean of the absolute value of all Δz values for a given subscale (i.e., the average across all 70 cells in a row) was r = 0.17 (Figure S8, available online). This quantifies the lack of redundancy between IQ’s association with symptom severity and IQ’s association with symptom coupling.
Results were stable when controlling for the effect of SES and self-reported race/ethnicity on IQ, and when controlling for the effects of age and sex on behavioral raw scores in sensitivity analyses, both at the edge level and the block level (see Supplement 3, available online).
Discussion
As detailed above, our study provides several new insights into the complex relationships between IQ and psychopathology in development by leveraging a large dataset derived from a care-seeking population.
First, we add new granularity to the long-established association between impairments in general cognitive ability and increased risk for psychopathology 7–9,11. Multiple analytic approaches highlighted social functioning, repetitive and stereotyped behaviors, learning and attentional impairments, and specific anxiety symptoms as being most strongly negatively associated with IQ, whereas general anxiety, depression, and aggression and conduct problems were largely unrelated to IQ variation. Of note, our study also highlights how measures of the same domain of psychopathology from different informants (i.e., parent versus child) can show distinct relationships with IQ. These findings help to prioritize certain domains of psychopathology for especially close investigation in clinical presentations involving cognitive impairment.
Second, our findings also build upon prior studies of the relationship between IQ and psychopathology by systematically screening all measured domains for examples of increasing psychopathology with greater IQ. We find no evidence of such relationships. However, it is important to note that our study focused on the pediatric age-range, meaning that we cannot rule out previously-reported positive associations between childhood IQ and later-emergent domains of psychopathology that are not well-captured in the HBN sample, such as bipolar and manic symptoms 12–14. Nevertheless, by considering a large care-seeking population we conclude that few measures of psychopathology are positively associated with IQ in a cross-sectional manner before adulthood.
Third, by extending analysis of cognition-psychopathology relationships to the level of symptom interactions, our study reveals how cognitive ability modulates the co-occurrence of psychiatric issues in youth. We detect a highly heterogeneous pattern of coupling change between pairs of scales, with lower versus higher IQ tending to strengthen some relationships and weaken others. Critically, we show that these changes in symptom co-occurrence as a function of IQ are unrelated to IQ effects on the severity of individual symptoms—highlighting how analyses of IQ relationships with behavior and behavioral coupling provide distinct information. The most prominent pattern detected by analysis of behavioral coupling was that symptoms of internalizing problems were more likely to be accompanied by symptoms of behavioral dyscontrol—in the form of aggression, stereotyped behaviors and restricted interests, hyperactivity, and inattention—in low-IQ as compared to high-IQ youth. This finding provides quantitative support for the common clinical practice in neurodevelopmental psychiatry of emphasizing changes in motor behavior as a potential indicator of emerging mood problems in groups with limited verbal communication—often in the context of cognitive impairment. Our cross-sectional observational study cannot establish causality, but these findings help to prioritize testable hypotheses regarding interactions between cognitive ability, attention, and mood. Coupling analysis also revealed that correlations between parent and child reports of anxiety symptoms are significantly stronger in high-IQ than low-IQ youth. This new finding echoes prior reports that, as a child’s age (and, therefore, cognitive capacity) increases, so too does the agreement between parent and youth reports of anxiety symptoms 34.
The findings reviewed above carry several implications for future work on measurement and mechanistic understanding of psychopathological variation in the context of cognitive ability. From a psychometrics perspective, the finding that IQ modulates coupling between measures of psychopathology could be interpreted as evidence for “measurement noninvariance” in measures of psychopathology 52. However, given that we lack gold-standard, objective measures against which to validate questionnaire-based measures of psychopathology, our findings are perhaps most immediately relevant for the real-world question of how to optimally use those measurement instruments that are currently available—especially as an adjunct to clinical assessments. For example, our coupling change analyses motivate particularly careful screening for co-occurring repetitive behavior and affective disorders in lower-IQ youth who present with aggressive, hyperactive, or inattentive symptomatology.
Our results also motivate and inform future research on potential mechanisms underlying observed relationships between psychopathology and IQ. Three (mutually compatible) theoretical frameworks for considering these mechanisms are as follows. First, under the “cognitive reserve” model, intact cognitive functioning may help individuals better cope with life stressors, reap greater benefit from available psychiatric treatments, and obtain jobs and social networks that support their mental health—all of which would result in decreased vulnerability, and increased resilience, to psychopathology among higher-IQ individuals. Second, our findings can also be considered in the context of network perspectives on psychiatric symptomatology 53 which focus on the relationships between pairs of behavioral variables and the network-level features that emerge from many such relationships. The edges in such networks can represent causal relationships between different behavioral measures (e.g., depression leading to inattention or vice versa), or collectively define sets of closely inter-related measures. In this context, changes in the correlational architecture of psychopathology as a function of IQ variation may reflect shifts in the causal relationships between or shared causes amongst different measures of behavior. Third, observed associations between IQ and psychopathology may reflect the degree to which the features of interest share a common neurobiological substrate 54. For example, variation in functional connectivity of a shared set of lateral temporal and superior parietal brain regions seems to be predictive of variation in both IQ and overall psychopathology 55, and it is possible that such overlaps are greater for those aspects of psychopathology that are closely related to intelligence (e.g., social and attentional functioning) than those that are not (e.g., affective symptoms and psychopathy). Testing this hypothesis would require direct estimation of regional or network-level overlaps between the neuroimaging correlates of IQ and psychopathology 56. Similar datasets could be used to test if differences in symptom coupling as a function of IQ variation reflect IQ modulation of functional or structural coupling between the distributed brain systems that are likely to underpin psychiatric symptomatology.
Our findings should be considered in light of several caveats and study limitations. First, with regards to external validity of our findings: the HBN recruits participants on the basis of “perceived clinical concern.” This feature increases the likelihood that our findings generalize to “real world” clinical samples, but means that we cannot assume generalizability of findings to an unselected population. Additionally, given that the sample is primarily focused on individuals seeking help for mental health or learning needs (~90% are diagnosed with one or more mental health or learning disorders), it likely also captures complex patterns of service use driven by availability of services (e.g., less available in suburbs than in cities; less available to lower- and middle-income families than high). These limitations are likely representative of most clinically-focused studies in the literature, as epidemiologic designs are rarely feasible. Multisite clinical studies that sample diverse locations are one approach to mitigating such biases. Importantly, for the present work, we establish that our main findings hold after controlling for the measured demographic variables of SES and self-reported race, although we note that these variables are non-randomly distributed between IQ tertiles. The complex interrelationships between measures of SES, race, and IQ are well-documented, and we underline the dominant interpretation that observed associations between race/ethnicity and IQ reflect environmental factors 57.
Second, our results are only generalizable to the age range covered by the WISC, ages 6–17 years, and therefore future research is needed to determine whether our findings extend to preschool and young adult populations. Third, we focus here on how one global measure of cognition relates to many different dimensions of psychopathology, but we acknowledge that cognition itself is far from monolithic. An important goal for future studies will be to extend the analyses above to incorporate multiple cognitive and behavioral measures at the same time. Fourth, our study is observational and cross-sectional in design. Future work with longitudinal data could help to narrow hypotheses regarding the directions of causality that lead to the interrelationships we describe cross-sectionally. Fifth, there is the possibility that instruments used, both in the clinic and in this study, to quantify symptoms of psychopathology may be vulnerable to decreased validity in people with low cognitive ability. Future research may focus on 1) specifically demonstrating that currently-available instruments are robust to confounding factors of IQ, communication ability, and more, or 2) developing new instruments to address these confounds. Finally, as noted in the methods section, dichotomizing a continuous variable, such as IQ, can be inefficient and reduce statistical power. This approach had significant benefits for the present study, but future research should aim to develop new statistical approaches for modeling how, within a set of continuous variables, the relationship between any pair is modulated by others.
Notwithstanding the above caveats and limitations, our study details relationships between IQ and psychopathology with a greater granularity than was available to date, and also addresses the novel idea that IQ variation may also modulate the strength of coupling between different aspects of psychopathology. These insights suggest ways of improving mental health assessments in groups with cognitive impairment, and inform future research on the psychological and biological mechanisms that might underpin the complex interdependence of cognition and psychopathology.
Supplementary Material
Social media – Facebook.
“Study using Healthy Brain Network data from @ChildMindInstitute finds variation in general cognitive ability related to differential co-occurrence of psychiatric symptoms in youth, in addition to differences in symptom severity; e.g., lower IQ associated with stronger coupling between internalizing symptoms and hyperactivity/inattentiveness #psychiatry #openscience <link>”
Social media – Twitter.
“New study @JAACAP finds variation in general cognitive ability related to differential co-occurrence of psychiatric symptoms in youth, in addition to differences in symptom severity @childmindinst #openscience <link>”
Acknowledgments
We worked to ensure sex and gender balance in the recruitment of human participants. We worked to ensure sex balance in the selection of non-human subjects. One or more of the authors of this paper self-identifies as a member of one or more historically underrepresented racial and/or ethnic groups in science. One or more of the authors of this paper received support from a program designed to increase minority representation in science. We actively worked to promote sex and gender balance in our author group. We actively worked to promote inclusion of historically underrepresented racial and/or ethnic groups in science in our author group. While citing references scientifically relevant for this work, we also actively worked to promote sex and gender balance in our reference list. The author list of this paper includes contributors from the location and/or community where the research was conducted who participated in the data collection, design, analysis, and/or interpretation of the work. One or more of the authors of this paper self-identifies as a member of one or more historically underrepresented sexual and/or gender groups in science.
This article is part of a special series devoted to addressing bias, bigotry, racism, and mental health disparities through research, practice, and policy. The series is edited by Assistant Editor Eraka Bath, MD, Deputy Editor Wanjikũ F.M. Njoroge, Associate Editor Robert R. Althoff, MD, PhD, and Editor-in-Chief Douglas K. Novins, MD.
The authors have reported funding from the Intramural Research Program of the National Institute of Mental Health (NIH Annual Report Number: ZIAMH002949-06).
Dr. Shinohara served as the statistical expert for this research.
The authors thank the NIH Fellows Editorial Board for their assistance in editing and preparing this manuscript for publication. They also thank Helena Hansen, MD, PhD, of the University of California, Los Angeles, for her valuable input regarding demographic aspects of our sample.
Footnotes
Disclosure: Dr. Shinohara has received consulting income from Octave Bioscience and compensation for scientific reviewing from the American Medical Association, the National Institutes of Health, the Department of Defense, and the Emerson Collective. Drs. Rau, Liu, Lalonde, Alexander-Bloch, Satterthwaite, Bassett, Milham, Raznahan and Mss. Mahony and Tu have reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Bridget W. Mahony, National Institute of Mental Health, Bethesda, Maryland.
Danni Tu, Perelman School of Medicine, Philadelphia, Pennsylvania.
Srishti Rau, National Institute of Mental Health, Bethesda, Maryland; Children’s National Health System, Rockville, Maryland.
Siyuan Liu, National Institute of Mental Health, Bethesda, Maryland.
François M. Lalonde, National Institute of Mental Health, Bethesda, Maryland.
Aaron F. Alexander-Bloch, Perelman School of Medicine, Philadelphia, Pennsylvania.
Theodore D. Satterthwaite, Perelman School of Medicine, Philadelphia, Pennsylvania.
Russell T. Shinohara, Perelman School of Medicine, Philadelphia, Pennsylvania.
Dani S. Bassett, University of Pennsylvania, Philadelphia, and the Santa Fe Institute, New Mexico; Perelman School of Medicine, Philadelphia, Pennsylvania.
Michael Peter Milham, Child Mind Institute, New York, New York.
Armin Raznahan, National Institute of Mental Health, Bethesda, Maryland.
References
- 1.Shaffer D, Schonfeld I, O’Connor PA, et al. Neurological soft signs. Their relationship to psychiatric disorder and intelligence in childhood and adolescence. Arch Gen Psychiatry. 1985;42(4):342–351. doi: 10.1001/archpsyc.1985.01790270028003 [DOI] [PubMed] [Google Scholar]
- 2.Linna SL, Moilanen I, Ebeling H, et al. Psychiatric symptoms in children with intellectual disability. Eur Child Adolesc Psychiatry. 1999;8 Suppl 4:77–82. doi: 10.1007/pl00010704 [DOI] [PubMed] [Google Scholar]
- 3.Burdick KE, Gunawardane N, Woodberry K, Malhotra AK. The role of general intelligence as an intermediate phenotype for neuropsychiatric disorders. Cogn Neuropsychiatry. 2009;14(4–5):299–311. doi: 10.1080/13546800902805347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sánchez de Ribera O, Kavish N, Katz IM, Boutwell BB. Untangling Intelligence, Psychopathy, Antisocial Personality Disorder, and Conduct Problems: A Meta–Analytic Review. Eur J Pers. 2019;33(5):529–564. doi: 10.1002/per.2207 [DOI] [Google Scholar]
- 5.Goodman R The relationship between normal variation in IQ and common childhood psychopathology: a clinical study. Eur Child Adolesc Psychiatry. 1995;4(3):187–196. doi: 10.1007/BF01980457 [DOI] [PubMed] [Google Scholar]
- 6.Batty GD, David Batty G, Mortensen EL, Andersen AMN, Osler M. Childhood intelligence in relation to adult coronary heart disease and stroke risk: evidence from a Danish birth cohort study. Paediatric and Perinatal Epidemiology. 2005;19(6):452–459. doi: 10.1111/j.1365-3016.2005.00671.x [DOI] [PubMed] [Google Scholar]
- 7.Keyes KM, Platt J, Kaufman AS, McLaughlin KA. Association of Fluid Intelligence and Psychiatric Disorders in a Population-Representative Sample of US Adolescents. JAMA Psychiatry. 2017;74(2):179–188. doi: 10.1001/jamapsychiatry.2016.3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emerson E Prevalence of psychiatric disorders in children and adolescents with and without intellectual disability. J Intellect Disabil Res. 2003;47(Pt 1):51–58. doi: 10.1046/j.1365-2788.2003.00464.x [DOI] [PubMed] [Google Scholar]
- 9.Platt JM, Keyes KM, McLaughlin KA, Kaufman AS. Intellectual disability and mental disorders in a US population representative sample of adolescents. Psychol Med. 2019;49(6):952–961. doi: 10.1017/S0033291718001605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Ruiter KP, Dekker MC, Verhulst FC, Koot HM. Developmental course of psychopathology in youths with and without intellectual disabilities. J Child Psychol Psychiatry. 2007;48(5):498–507. doi: 10.1111/j.1469-7610.2006.01712.x [DOI] [PubMed] [Google Scholar]
- 11.Melby L, Indredavik MS, Løhaugen G, Brubakk AM, Skranes J, Vik T. Is there an association between full IQ score and mental health problems in young adults? A study with a convenience sample. BMC Psychol. 2020;8(1):7. doi: 10.1186/s40359-020-0372-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koenen KC, Moffitt TE, Roberts AL, et al. Childhood IQ and adult mental disorders: a test of the cognitive reserve hypothesis. Am J Psychiatry. 2009;166(1):50–57. doi: 10.1176/appi.ajp.2008.08030343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacCabe JH, Lambe MP, Cnattingius S, et al. Excellent school performance at age 16 and risk of adult bipolar disorder: national cohort study. Br J Psychiatry. 2010;196(2):109–115. doi: 10.1192/bjp.bp.108.060368 [DOI] [PubMed] [Google Scholar]
- 14.Gale CR, Batty GD, McIntosh AM, Porteous DJ, Deary IJ, Rasmussen F. Is bipolar disorder more common in highly intelligent people? A cohort study of a million men. Mol Psychiatry. 2013;18(2):190–194. doi: 10.1038/mp.2012.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matson JL, Shoemaker M. Intellectual disability and its relationship to autism spectrum disorders. Res Dev Disabil. 2009;30(6):1107–1114. doi: 10.1016/j.ridd.2009.06.003 [DOI] [PubMed] [Google Scholar]
- 16.Tillmann J, San José Cáceres A, Chatham CH, et al. Investigating the factors underlying adaptive functioning in autism in the EU-AIMS Longitudinal European Autism Project. Autism Res. 2019;12(4):645–657. doi: 10.1002/aur.2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheung CHM, Rijdijk F, McLoughlin G, Faraone SV, Asherson P, Kuntsi J. Childhood predictors of adolescent and young adult outcome in ADHD. J Psychiatr Res. 2015;62:92–100. doi: 10.1016/j.jpsychires.2015.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Debes NMMM, Lange T, Jessen TL, Hjalgrim H, Skov L. Performance on Wechsler intelligence scales in children with Tourette syndrome. Eur J Paediatr Neurol. 2011;15(2):146–154. doi: 10.1016/j.ejpn.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 19.Abramovitch A, Anholt G, Raveh-Gottfried S, Hamo N, Abramowitz JS. Meta-Analysis of Intelligence Quotient (IQ) in Obsessive-Compulsive Disorder. Neuropsychol Rev. 2018;28(1):111–120. doi: 10.1007/s11065-017-9358-0 [DOI] [PubMed] [Google Scholar]
- 20.Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379 [DOI] [PubMed] [Google Scholar]
- 21.Caspi A, Moffitt TE. All for One and One for All: Mental Disorders in One Dimension. Am J Psychiatry. 2018;175(9):831–844. doi: 10.1176/appi.ajp.2018.17121383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calkins ME, Merikangas KR, Moore TM, et al. The Philadelphia Neurodevelopmental Cohort: constructing a deep phenotyping collaborative. J Child Psychol Psychiatry. 2015;56(12):1356–1369. doi: 10.1111/jcpp.12416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flouri E, Papachristou E, Midouhas E, Joshi H, Ploubidis GB, Lewis G. Early adolescent outcomes of joint developmental trajectories of problem behavior and IQ in childhood. Eur Child Adolesc Psychiatry. 2018;27(12):1595–1605. doi: 10.1007/s00787-018-1155-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexander LM, Salum GA, Swanson JM, Milham MP. Measuring strengths and weaknesses in dimensional psychiatry. J Child Psychol Psychiatry. 2020;61(1):40–50. doi: 10.1111/jcpp.13104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexander LM, Escalera J, Ai L, et al. An open resource for transdiagnostic research in pediatric mental health and learning disorders. Sci Data. 2017;4:170181. doi: 10.1038/sdata.2017.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boschloo L, van Borkulo CD, Rhemtulla M, Keyes KM, Borsboom D, Schoevers RA. The Network Structure of Symptoms of the Diagnostic and Statistical Manual of Mental Disorders. PLoS One. 2015;10(9):e0137621. doi: 10.1371/journal.pone.0137621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexander-Bloch AF, Raznahan A, Shinohara RT, et al. The architecture of co-morbidity networks of physical and mental health conditions in military veterans. Proc Math Phys Eng Sci. 2020;476(2239):20190790. doi: 10.1098/rspa.2019.0790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fried EI, Cramer AOJ. Moving Forward: Challenges and Directions for Psychopathological Network Theory and Methodology. Perspect Psychol Sci. 2017;12(6):999–1020. doi: 10.1177/1745691617705892 [DOI] [PubMed] [Google Scholar]
- 30.Cherry KE, Penn D, Matson JL, Bamburg JW. Characteristics of schizophrenia among persons with severe or profound mental retardation. Psychiatr Serv. 2000;51(7):922–924. doi: 10.1176/appi.ps.51.7.922 [DOI] [PubMed] [Google Scholar]
- 31.Yoo JH, Valdovinos MG, Schroeder SR. Chapter Two - The Epidemiology of Psychopathology in People with Intellectual Disability: A Forty-Year Review. In: Hodapp RM, ed. International Review of Research in Developmental Disabilities. Vol 42. Academic Press; 2012:31–56. doi: 10.1016/B978-0-12-394284-5.00002-4 [DOI] [Google Scholar]
- 32.Underwood L, McCarthy J, Chaplin E, Bertelli MO. Assessment and diagnosis of psychiatric disorder in adults with autism spectrum disorder. Advances in Mental Health and Intellectual Disabilities. 2015;9(5):222–229. doi: 10.1108/AMHID-05-2015-0025 [DOI] [Google Scholar]
- 33.Eaton C, Tarver J, Shirazi A, et al. A systematic review of the behaviours associated with depression in people with severe-profound intellectual disability. J Intellect Disabil Res. 2021;65(3):211–229. doi: 10.1111/jir.12807 [DOI] [PubMed] [Google Scholar]
- 34.Dirks MA, Weersing VR, Warnick E, et al. Parent and youth report of youth anxiety: evidence for measurement invariance. J Child Psychol Psychiatry. 2014;55(3):284–291. doi: 10.1111/jcpp.12159 [DOI] [PubMed] [Google Scholar]
- 35.Lerch JP, Worsley K, Shaw WP, et al. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage. 2006;31(3):993–1003. doi: 10.1016/j.neuroimage.2006.01.042 [DOI] [PubMed] [Google Scholar]
- 36.Lee NR, Raznahan A, Wallace GL, et al. Anatomical coupling among distributed cortical regions in youth varies as a function of individual differences in vocabulary abilities. Hum Brain Mapp. 2014;35(5):1885–1895. doi: 10.1002/hbm.22299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nadig A, Seidlitz J, McDermott CL, et al. Morphological integration of the human brain across adolescence and adulthood. Proc Natl Acad Sci U S A. 2021;118(14). doi: 10.1073/pnas.2023860118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wechsler Intelligence Scale for children. Accessed March 7, 2022. https://www.pearsonassessments.com/store/usassessments/en/Store/Professional-Assessments/Cognition-%26-Neuro/Gifted-%26-Talented/Wechsler-Intelligence-Scale-for-Children-%7C-Fifth-Edition-/p/100000771.html
- 39.Achenbach TM, Rescorla L. Manual for the ASEBA School-Age Forms & Profiles: An Integrated System of Multi-Informant Assessment. Aseba; Burlington, VT:; 2001. [Google Scholar]
- 40.Connors CK. Conners 3rd Edition: Manual. Toronto, Ontario, Canada: Multi-Health Systems. Published online 2008. [Google Scholar]
- 41.Kimonis ER, Frick PJ, Skeem JL, et al. Assessing callous–unemotional traits in adolescent offenders: Validation of the Inventory of Callous–Unemotional Traits. International Journal of Law and Psychiatry. 2008;31(3):241–252. doi: 10.1016/j.ijlp.2008.04.002 [DOI] [PubMed] [Google Scholar]
- 42.Lam KSL, Aman MG. The Repetitive Behavior Scale-Revised: independent validation in individuals with autism spectrum disorders. J Autism Dev Disord. 2007;37(5):855–866. doi: 10.1007/s10803-006-0213-z [DOI] [PubMed] [Google Scholar]
- 43.Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, Baugher M. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. J Am Acad Child Adolesc Psychiatry. 1999;38(10):1230–1236. doi: 10.1097/00004583-199910000-00011 [DOI] [PubMed] [Google Scholar]
- 44.Goodman R Psychometric properties of the strengths and difficulties questionnaire. J Am Acad Child Adolesc Psychiatry. 2001;40(11):1337–1345. doi: 10.1097/00004583-200111000-00015 [DOI] [PubMed] [Google Scholar]
- 45.Constantino JN, Davis SA, Todd RD, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003;33(4):427–433. doi: 10.1023/a:1025014929212 [DOI] [PubMed] [Google Scholar]
- 46.Draper NR, Smith H. Fitting a straight line by least squares. Applied Regression Analysis. Published online April 9, 1998:15–46. doi: 10.1002/9781118625590.ch1 [DOI] [Google Scholar]
- 47.Betzel RF, Medaglia JD, Bassett DS. Diversity of meso-scale architecture in human and non-human connectomes. Nat Commun. 2018;9(1):346. doi: 10.1038/s41467-017-02681-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leger JB. Blockmodels: A R-package for estimating in Latent Block Model and Stochastic Block Model, with various probability functions, with or without covariates. arXiv [statCO]. Published online February 24, 2016. http://arxiv.org/abs/1602.07587 [Google Scholar]
- 49.Biernacki C, Celeux G, Govaert G. Assessing a mixture model for clustering with the integrated completed likelihood. IEEE Trans Pattern Anal Mach Intell. 2000;22(7):719–725. doi: 10.1109/34.865189 [DOI] [Google Scholar]
- 50.Barratt W The Barratt Simplified Measure of Social Status (BSMSS) measuring SES. 2006. Unpubl Pap Indiana State Univ Terre Ht Indiana Available at http://wbarrattindstateedu/socialclass/BarrattSimplifedMeasureofSocialStatuspdf. [Google Scholar]
- 51.Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc Series B Stat Methodol. 2011;73(1):3–36. doi: 10.1111/j.1467-9868.2010.00749.x [DOI] [Google Scholar]
- 52.Putnick DL, Bornstein MH. Measurement Invariance Conventions and Reporting: The State of the Art and Future Directions for Psychological Research. Dev Rev. 2016;41:71–90. doi: 10.1016/j.dr.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borsboom D, Deserno MK, Rhemtulla M, et al. Network analysis of multivariate data in psychological science. Nature Reviews Methods Primers. 2021;1(1):1–18. doi: 10.1038/s43586-021-00055-w [DOI] [Google Scholar]
- 54.Mewton L, Lees B, Squeglia LM, et al. The relationship between brain structure and general psychopathology in preadolescents. J Child Psychol Psychiatry. Published online September 1, 2021. doi: 10.1111/jcpp.13513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marek S, Tervo-Clemmens B, Calabro FJ, et al. Towards Reproducible Brain-Wide Association Studies. Cold Spring Harbor Laboratory. Published online August 22, 2020:2020.08.21.257758. doi: 10.1101/2020.08.21.257758 [DOI] [Google Scholar]
- 56.Shanmugan S, Wolf DH, Calkins ME, et al. Common and Dissociable Mechanisms of Executive System Dysfunction Across Psychiatric Disorders in Youth. Am J Psychiatry. 2016;173(5):517–526. doi: 10.1176/appi.ajp.2015.15060725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nisbett RE, Aronson J, Blair C, et al. Group differences in IQ are best understood as environmental in origin. Am Psychol. 2012;67(6):503–504. doi: 10.1037/a0029772 [DOI] [PubMed] [Google Scholar]
- 58.Data * Science + R. Matching clustering solutions using the “Hungarian method.” R-bloggers. Published November 19, 2012. Accessed February 14, 2022. https://www.r-bloggers.com/2012/11/matching-clustering-solutions-using-the-hungarian-method/
- 59.Kuhn HW. The Hungarian method for the assignment problem. Nav Res Logist Q. 1955;2(1–2):83–97. doi: 10.1002/nav.3800020109 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.