Abstract
Sirtuins play an important role in signalling pathways associated with various metabolic regulations. They possess mono-ADP-ribosyltransferase or deacylase activity like demalonylase, deacetylase, depalmitoylase, demyristoylase and desuccinylase activity. Sirtuins are histone deacetylases which depends upon nicotinamide adenine dinucleotide (NAD) that deacetylate lysine residues. There are a total of seven human sirtuins that have been identified namely, SIRT1, SIRT2, SIRT3, SIRT4, SIRT5, SIRT6 and SIRT7. The subcellular location of mammalian sirtuins, SIRT1, SIRT6, and SIRT7 are in the nucleus; SIRT3, SIRT4, and SIRT5 are in mitochondria, and SIRT2 is in cytoplasm. Structurally sirtuins contains a N-terminal, a C-terminal and a Zn+ binding domain. The sirtuin family has been found to be crucial for maintaining lipid and glucose homeostasis, and also for regulating insulin secretion and sensitivity, DNA repair pathways, neurogenesis, inflammation, and ageing. Based on the literature, sirtuins are overexpressed and play an important role in tumorigenicity in various types of cancer such as non-small cell lung cancer, colorectal cancer, etc. In this review, we have discussed about the different types of human sirtuins along with their structural and functional features. We have also discussed about the various natural and synthetic regulators of sirtuin activities like resveratrol. Our overall study shows that the correct regulation of sirtuins can be a good target for preventing and treating various diseases for improving the human lifespan. To investigate the true therapeutic potential of sirtuin proteins and their efficacy in a variety of pathological diseases, a better knowledge of the link between the structure and function of sirtuin proteins would be necessary.
Keywords: Ageing, Deacetylase, Nicotinamide adenine dinucleotide, Rossmann fold, Sirtuins
Introduction
The human sirtuins are a highly conserved family of NAD+-dependent histone deacetylases, which play a critical role in the regulation of a large number of metabolic pathways involved in stress response and ageing. They are also known as Silent information regulator 2 (Sir2) proteins. They possess mono-ADP-ribosyltransferase or deacylase activity like demalonylase, deacetylase, depalmitoylase, demyristoylase and desuccinylase activity. Sirtuins are nicotinamide adenine dinucleotide (NAD+) dependent proteins that deacetylate lysine residues (Imai et al. 2000). They convert NAD+ (nicotinamide adenine dinucleotide) into NAM (nicotinamide) which acts like an inhibitor of sirtuins. This hydrolysis also releases O-acetyl-ADP-ribose (Grabowska et al. 2017). NAM is a known potent inhibitor of several classes of ribosylase enzymes that require NAD for their activity, as well as sirtuin (SIRT1), class III NAD+-dependent-histone-deacetylase. SIRT1, one of the mammalian sirtuins, catalyzes the deacetylation of acetyl-lysine residues by a mechanism whereby NAD+ is cleaved. The reaction results in the release of NAM, which acts as an end-product noncompetitive inhibitor of SIRT1 by binding to a conserved pocket adjacent to NAD+, thereby blocking NAD+ hydrolysis (Peled et al. 2012). The sirtuin family has been found to be crucial for maintaining lipid and glucose homeostasis, and also for regulating insulin secretion and sensitivity, DNA repair pathways, neurogenesis, inflammation, and ageing. They are also responsible for the regulation of transcription repression, cell cycle regulation and microtubule organization. These proteins are ancient in evolution and have a highly conserved structure throughout wide variety of organisms. Sirtuins are also detected in many gram positive and gram negative bacteria. For the first time sirtuin was recognised in yeast and named as sir2 (Michan and Sinclair 2007; Ivy et al. 1986). Mammals have seven sirtuins, SIRT1 to SIRT7 which are found at different places in the cell. SIRT1, SIRT 6 and SIRT7 are found in the nucleus, SIRT2 is found in the cytoplasm whereas, sirtuins SIRT3, SIRT4 and SIRT5 are localised in the mitochondria.
Sirtuins consists of a highly conserved structure containing a core domain made up of approximately 275 amino acid residues and is flanked by N- and C- terminal regions of different lengths. The catalytic core domain has classical open α/β Rossmann-fold structure which is found in most of NAD+/NADH binding proteins and a small globular domain that has two insertions, one for binding to zinc ion and other is helical module. The enzyme’s active site is located between the two domains in a deep cleft. It binds to both NAD+ and acetyl-lysine substrates. When the substrate binds in the active site, the main chain makes β sheet-like interactions with two flanking strands. The Rossmann fold contains one of those strands. The other is situated between the Rossmann fold and the Zn2+-binding module, in a loop that consists the highly conserved FGExL motif. This formation is called the “β staple” interaction, in which the substrate connects the Rossmann fold and the Zn2+-binding module, positions the acetyl-lysine side chain of the substrate towards the conserved hydrophobic tunnel and consequently changes the enzyme´s conformation from an open to a closed form. This closed form helps in the correct binding of NAD+ into the hydrophobic conserved C pocket that is close to the acyl-lysine-binding tunnel. This binding order is very important as it restricts the binding conformation of NAD+ when the acetyl-lysine-binding tunnel is occupied. As a result it attains the productive conformation in which its adenine ring establishes numerous hydrogen bonds and van der Waals interactions with the Rossmann-fold domain and its nicotinamide ring is placed into the C pocket (Teixeira et al. 2020). The various positions of amino acid residues binding to Zn2+ cofactor and positions of amino acid residues making deacetylase sirtuin-type domain are shown in Table 1. The Root Mean Square Deviation (RMSD) values predicted by structure superpositions of SIRT1, SIRT2, SIRT3, SIRT5, SIRT6 and SIRT7 using mTM-align (https://yanglab.nankai.edu.cn/mTM-align/) also corroborate with the conserved core domain present in the sirtuins (Dong et al. 2018) as shown in Table 2.
Table 1.
Different domain positions of mammalian sirtuins
| Sr no | Name of Protein (UniProt ID) | No. of amino acids | Deacetylase sirtuin-type Domain (Position) | Cofactor binding (Zn+2) Positions |
|---|---|---|---|---|
| 1 |
SIRT1 (Q96EB6) |
747 | 244–498 | 371,374, 395, 398 |
| 2 |
SIRT2 (Q8IXJ6) |
389 | 65–340 | 195, 200, 221, 224 |
| 3 |
SIRT3 (Q9NTG7) |
399 | 126–382 | 256, 259, 280, 283 |
| 4 |
SIRT4 (Q9Y6E7) |
314 | 45–314 | 256, 259, 280, 283 |
| 5 |
SIRT5 (Q9NXA8) |
310 | 41–309 | 166, 169, 207, 212 |
| 6 |
SIRT6 (Q8N6T7) |
355 | 35–274 | 141, 144, 166, 167 |
| 7 |
SIRT7 (Q9NRC8) |
400 | 90–331 | 195, 198, 225, 228 |
Table 2.
RMSD values(Å) predicted by structure superposition of sirtuins using mTM-align
| Sirtuin ⇒ ⇓ |
SIRT 5 (2NYR) | SIRT 3 (3GLS) | SIRT 6 (3K35) | SIRT 2 (3ZGO) | SIRT 1 (4IG9) | SIRT 7 (5IQZ) |
|---|---|---|---|---|---|---|
| SIRT 5 (2NYR) | 0 | 2.672 | 3.464 | 2.589 | 2.657 | 6.129 |
| SIRT 3 (3GLS) | 2.672 | 0 | 3.415 | 2.065 | 1.549 | 4.94 |
| SIRT 6 (3K35) | 3.464 | 3.415 | 0 | 3.319 | 3.224 | 6.939 |
| SIRT 2 (3ZGO) | 2.589 | 2.065 | 3.319 | 0 | 2.352 | 5.664 |
| SIRT 1 (4IG9) | 2.657 | 1.549 | 3.224 | 2.352 | 0 | 6.072 |
| SIRT 7 (5IQZ) | 6.129 | 4.94 | 6.939 | 5.664 | 6.072 | 0 |
Sirtuins are also involved in the deacetylation of histone proteins. Various in vivo experimental data suggests that, activity like deacetylation of histones cause recombination suppression, silencing, and life extension. These studies reveal a molecular basis for NAD-dependent deacetylation of histones in yeast and, possibly, higher eukaryotes that integrates metabolism, genomic silencing, and ageing (Imai et al. 2000).
Classification of sirtuins
There are various types of sirtuins found in different organisms. They are found in prokaryotes, and eukaryotes. Sirtuin was first time detected in the yeast and was named as sir2 (Silent information regulator 2) (Michan and Sinclair 2007; Ivy et al. 1986). The sirtuin family has been classified into five distinct classes (I-IV and U) based on a phylogenetic study of sirtuins from a wide range of prokaryotes and eukaryotes (North and Verdin 2004). SIR-2.1, Drosophila melanogaster Sir2 (D.mel1) orthologues, Sir2 homologue of Sir Two (Hst) 1, and SIRT1 all belong to subclass Ia. Subclass Ib includes Hst2, D.mel2, and various fungi and protozoa sirtuins along with SIRT2 and SIRT3. Both SIRT4 and SIRT5 belong to classes II and III, respectively, and both classes contain sirtuins from bacteria, Archea, nematodes, and protozoans. SIRT6 and SIRT7 belong to Class IV, which can be separated from the first two classes since it only includes sirtuins from eukaryotes. Sir2 homologues from bacteria with undifferentiated motifs constitute class U. The early eukaryotes had all four types of sirtuins, based on this categorization of the sirtuins, and it is thought that sirtuins from classes II, III, and U first appeared in evolution. Later in evolution, certain eukaryotes lost a few classes, which accounts for the diverse distribution of sirtuins in various organisms (Vassilopoulos et al. 2011).
In higher organisms there are seven types of sirtuins which work in similar way as does sir2 in yeast. In bacteria one or two sirtuins are found, that are responsible for regulation of various pathways of metabolism. For the first time bacterial sirtuin was found in Salmonella enterica and it was named as CobB due to its involvement in cobalamin biosynthesis and propionic catabolism (Tsang and Escalante-Semerena 1996). Mammals have seven sirtuins, SIRT1, SIRT2, SIRT3, SIRT4, SIRT5, SIRT6 and SIRT7 found at different places in the cell. SIRT 1, 6 and 7 are found in nucleus, SIRT2 is found in cytoplasm whereas, SIRT3, 4 and 5 are found in mitochondria. Phylogenetic analysis on the basis of amino acid sequence revealed that mammalian sirtuins can be segregated into four classes: SIRT1 to SIRT3 belong to class I, SIRT4 to class II, SIRT5 to III, and SIRT6 and SIRT7 to class IV (Houtkooper et al. 2012) as shown in Table 3.
Table 3.
Classification of mammalian sirtuins
| Sirtuins | Class | Sub-cellular location | Type of activity | Biological role |
|---|---|---|---|---|
| SIRT1 | Class I | Nucleus (euchromatin region of chromosome) | Strong deacetylase activity | Cell survival and lipid metabolism |
| SIRT2 | Class I | Cytoplasm | Both deacetylase and mono-ADP-ribosyl transferase activity | Regulation of cell cycle and cell motility |
| SIRT3 | Class I | Mitochondria | Both deacetylase and mono-ADP-ribosyl transferase activity | Metabolism and thermogenesis |
| SIRT4 | Class II | Mitochondria | Mono-ADP-ribosyl transferase activity | Glucose metabolism and Insulin secretion |
| SIRT5 | Class III | Mitochondria | Weak deacetylase activity | Cellular energy Metabolism |
| SIRT6 | Class IV | Nucleus (heterochromatin region of chromosome) | Mono-ADP-ribosyl transferase activity | DNA repair/ Glucose homeostasis |
| SIRT7 | Class IV | Nucleus (nucleolus) | Deacetylase activity | Metabolism, rDNA transcription |
The subcellular location of mammalian sirtuins varies as well. The nucleus is home to SIRT1, SIRT6, and SIRT7 (whereas SIRT1 also has certain vital cytoplasmic activities). SIRT1 is connected with euchromatin region of a chromosome in the nucleus, whereas the chromosome’s heterochromatin region and nucleolus are the locations for SIRT6 and SIRT7, respectively (Michan and Sinclair 2007). In mitochondria, SIRT3, 4 and 5 are found (Michan and Sinclair 2007). The cytoplasm has the most SIRT2 (North et al. 2003).
SIRT1
Human SIRT1 (UniProt id: Q96EB6) is constituted of 747 amino acid residues. The structure of human SIRT1 shows a catalytic domain in combination with the C-terminal regulatory region (CTR) in an open apo form (Davenport et al. 2014). The catalytic domain ranges from amino acid residues 234 to 510 and the C-terminal regulatory section ranges from amino acid residues 641 to 665. Together they form a heterodimeric complex. The catalytic core of SIRT1 is made up of 277 amino acid residues and has one small and one large sub-domain. The larger domain has a Rossmann fold and it binds to NAD+ whereas the smaller domain has two insertions in the NAD+ binding domain, which is a helical unit made up of amino acid residues spanning from 269 to 324 residues and a Zn2+-binding unit made of amino acid residues spanning from 362 to 419. The Rossmann fold is a three-layered sandwich like structure made up of alternating beta strands and alpha helical segments found in proteins that bind nucleotides. The beta strands are hydrogen bound to one other, forming an extended beta sheet, and the alpha helices surround both faces of the sheet. The NAD+ binding region is made up of a core six-stranded parallel β sheet with strands β1-3 and β7-9, as well as eight helices namely, αA, αB, αG, αH, and αJ-M, that pack against the β sheet core. The helical unit is made up of four α helices namely, αC-F, while the Zn2+-binding unit is made up of three β strands, β4 to β6 and one helix αI. The CTR creates a β hairpin shape that covers a fundamentally unchanging, hydrophobic region, complementing the β sheet of the NAD+ binding domain (Davenport et al. 2014). The smaller SIRT1 sub-domain rotates with respect to the larger NAD+ binding sub-domain in the apo state, resulting in an unique open conformation as shown in Fig. 1.
Fig. 1.
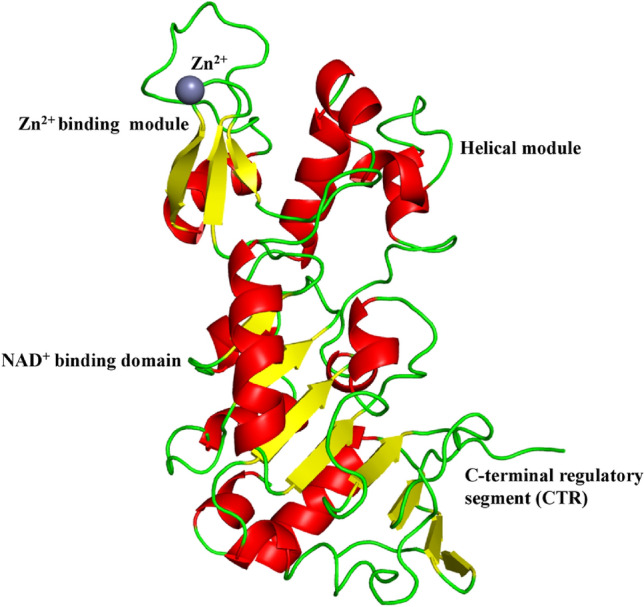
Structure of SIRT1 (PDB ID: 4IG9). The NAD+ binding region is made up of a core six-stranded parallel β sheet with strands β1-3 and β7-9, as well as eight helices namely, αA, αB, αG, αH, and αJ-M, that pack against the β sheet core. The helical module is made up of four α helices namely, αC-F, while the Zn2+binding unit is made up of three β strands, β4 to β6 and one helix αI. The CTR creates a β hairpin shape that covers a fundamentally unchanging, hydrophobic region, complementing the β sheet of the NAD+ binding domain
SIRT1 has strong deacetylase activity (Michan and Sinclair 2007). Modulated Sirt1 along with AMPK has a significant role to play in anti-ageing signalling pathways (Lu et al. 2014a, b; Park et al. 2016). Sirtuin 1 also promotes healthy ageing and protects against cancers linked to the metabolic syndromes (Herranz et al. 2010). SIRT1 activation delays neurodegeneration progression (Gräff et al. 2013). SIRT1 helps to maintain telomeres and promotes homologous recombination (Uhl et al. 2010; Palacios et al. 2010). DNA repair is also regulated by SIRT1 (Fan and Luo 2010). SIRT1 has a significant role in oxidative stress response, increased SIRT1 protein levels counteract the oxidative stress and ageing-related decline in levels of NAD+ (Kilic et al. 2015; Bosch-Presegué et al. 2011). SIRT1 modulates the activity of DNA Methyltransferase 1 by deacetylating it (Peng et al. 2011) and also regulates the p53 activity (Liu et al. 2011). Down regulation of SIRT1 was found to be important in the senescence of different type of endothelial cells (Arunachalam et al. 2014; Vassallo et al. 2014).
SIRT2
Human SIRT2 (UniProt id: Q8IXJ6) is constituted of 389 amino acid residues. SIRT2 comprises of a catalytic core made up of 304 amino acids and a 19 amino acid residue long helical extension at the N-terminal region. The catalytic core of SIRT2 is a prolonged structure, consisting of one larger and one smaller domain. The larger domain is made up of amino acid residues ranging from 53 to 89, 146 to 186, and 241 to 356. This is a type of the Rossmann fold, commonly found in many other NADH/NADPH binding enzymes. The smaller domain is made up of amino acid residues ranging from 90 to 145 and 187 to 240. It also has Zn2+-binding site in it. Four crossovers in the polypeptide chain link the two domains together. A large groove is produced by the four crossovers and three loops of the large domain at the intersection of the two domains (Moniot et al. 2013). The larger domain is comprised of six β-strands with β1–3 and β7–9, which forms a parallel β-sheet and six α-helices namely,α1, α7, α8 and α10–α12, that pack against the β-sheet. The smaller domain is made up of two structural modules that arise from two insertions in the larger domain's Rossmann fold. The primary insertion includes a helical module which is formed by folding four α-helices (α3–α6) together. A short three-stranded antiparallel β-sheet (β4, β5 and β6) is present in the second module, along with an α-helix (α9) and a zinc atom. The zinc-binding region is made up of a three-stranded antiparallel β-sheet and ~ 45° angled α-helix (α9) to the plane of the β-sheet. The small domain primarily binds to one half of the Rossmann fold, forming a large groove perpendicular to the molecule's long axis. The boundaries of the large groove are determined by huge, well-defined loops that are maintained by local secondary structure such as the β-turn in loop L4 and hydrophobic packing (Finnin et al. 2001) as shown in Fig. 2.
Fig. 2.
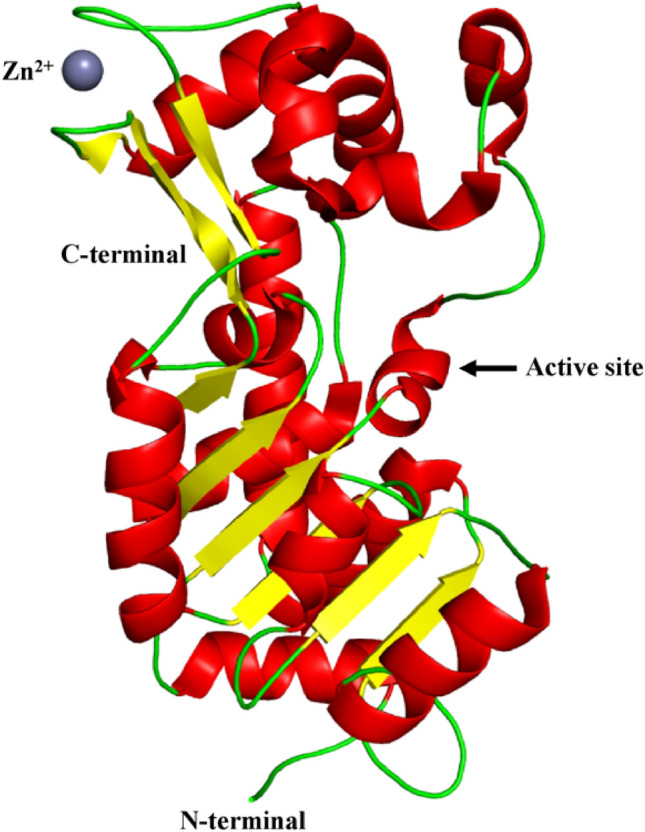
Structure of SIRT2 (PDB ID: 3ZGO). The catalytic core of SIRT2 is a prolonged structure, consisting of one larger and one smaller domain. The larger domain is comprised of six β-strands with β1–3 and β7–9, which forms a parallel β-sheet and six α-helices namely, α1, α7, α8 and α10–α12, that pack against the β-sheet. The smaller domain is made up of two structural modules. The primary insertion includes a helical module which is formed by folding four α-helices (α3–α6) together. A short three-stranded antiparallel β-sheet (β4, β5 and β6) is present in the second module, along with an α-helix (α9) and a zinc atom. The zinc-binding region is made up of a three-stranded antiparallel β-sheet and ~ 45° angled α-helix (α9) to the plane of the β-sheet
SIRT2 have both mono-ADP-ribosyl transferase and deacetylase activity (Michan and Sinclair 2007). By targeting glycolytic enzymes, SIRT2 controls metabolic reprogramming during induced pluripotency (Cha et al. 2017) and it also mediates tubulin acetylation in the presence of NAD+ (Skoge et al. 2014). Enhanced expression of SIRT2 indicate senescence in cells especially in the presence of p53 (Anwar et al. 2016). SIRT2 regulates HIF-1 activity which is important for tumour hypoxia responses. HIF-1 protein stability and transcriptional activity are both negatively influenced by SIRT2 (Seo et al. 2015). The level of SIRT2 is upregulated in non-small cell lung cancers (NSCLC) cell lines (Grbesa et al. 2015), metastatic human melanoma tissues compared to healthy cells and tissues (Wilking-Busch et al. 2017). SIRT2 could be a promising drug target for acute promyelocytic leukaemia therapy (Sunami et al. 2013). SIRT2 also has a role in neurodegeneration and protects neural cells from oxidative damage (Singh et al. 2017).
SIRT3
Human SIRT3 (UniProt id: Q9NTG7) is constituted of 399 amino acid residues containing a mitochondrial targeting domain at the N-terminal. Enzymatically, the full-length protein is inactive. Mitochondrial matrix processed peptidase cleaves N-terminal 101 residues, which then activates the enzyme. SIRT3 has a two-domain structure, like other sirtuins, consisting of a large and a smaller domain. Large domain is a type of Rossmann fold for NAD+ binding. The smaller domain consists of a helical bundle and a zinc binding region, which is created by two extending loops from the large domain. A groove formed between zinc binding and helical bundle region takes part in crystal packing. As a result, it might be a location for protein–protein interaction (Jin et al. 2009) as shown in Fig. 3.
Fig. 3.
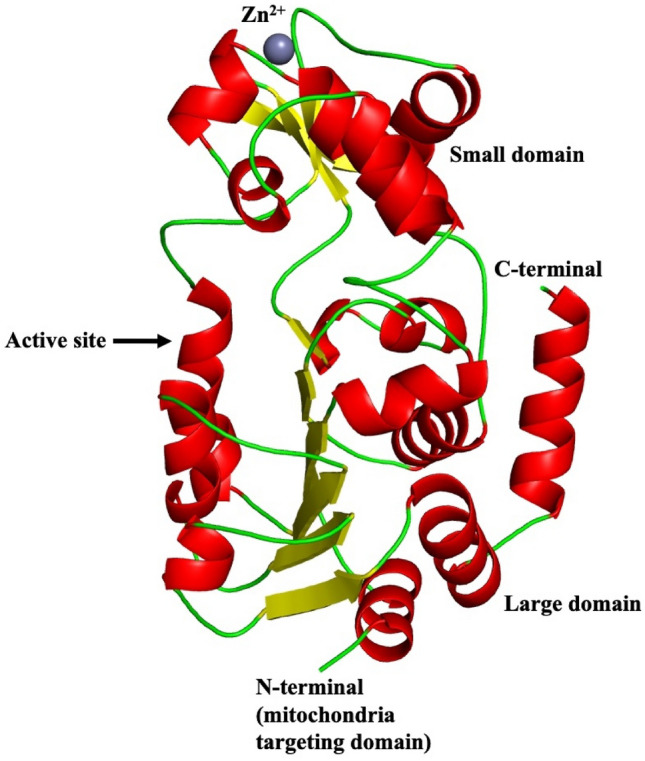
Structure of SIRT3 (PDB ID: 3GLS). SIRT3 has a two-domain structure, like other sirtuins, consisting of a large and a smaller domain. Large domain is a type of Rossmann fold for NAD+ binding. The smaller domain consists of a helical bundle and a zinc binding region, which is created by two extending loops from the large domain
The deacetylase activity of SIRT3 is required for viral suppression, during infection it keeps membrane potential and mitochondrial pH stable (Sheng and Cristea 2021). SIRT3 also has mono-ADP-ribosyl transferase activity (Michan and Sinclair 2007). Through the p53 pathway, SIRT3 prevents dysfunction and senescence caused by high level of glucose in endothelial cells (Chen et al. 2021) by partially abrogating p53 activity (Li et al. 2010). SIRT3 could also be used as a marker for colon cancer (Liu et al. 2014a, b). Sirt3 suppresses tumor growth via regulating hypoxia-inducible factor 1α and suppressing reactive oxygen species (Bell et al. 2011). It also prevents cancer cell metabolic reprogramming by destabilising HIF1α (Finley et al. 2011). SIRT3 activation prevents amyotrophic lateral sclerosis motor neurons from developing metabolic abnormalities (Hor et al. 2021).
SIRT4
Human SIRT4 (UniProt id: Q9Y6E7) is composed of 314 amino acid residues. A domain made up of amino acid residues ranging from 45 to 314, has deacetylase sirtuin-type activity. The crystal structure of SIRT4 from human is not yet reported so far in any of the structural databases.
SIRT4 is a mono-ADP-ribosyl transferase (Michan and Sinclair 2007). By ADP-ribosylation of glutamate dehydrogenase, SIRT4, an enzyme with very low deacetylase activity, could downregulate glutamate metabolism (Mitra and Dey 2020). SIRT4 play crucial role in mitotic cell division regulation (Bergmann et al. 2020), oestrogen receptor (ER) positive breast cancer (Xing et al. 2019), development and metastasis of thyroid cancer cells (Chen et al. 2019) It is also found that SIRT4 is an effective tumour suppressor in non-small cell lung cancers cell (NSCLC) (Fu et al. 2017), human colorectal cancer (Miyo et al. 2015), neuroblastoma (Wang et al. 2018) and gastric cancer (Hu et al. 2019; Sun et al. 2018). According to research, expression of SIRT4 is considerably downregulated in hepatocellular carcinoma tumour tissues (Li et al. 2019) and esophageal squamous cell carcinoma (Nakahara et al. 2016). In humans, SIRT4 inhibits inflammation in endothelial cells of the umbilical vein (Tao et al. 2015) and osteoarthritis (Dai et al. 2020). As an outcome of cellular stresses, SIRT4 controls PTEN stability via the insulin degrading enzyme (IDE) (Liu et al. 2019), it also controls ATP homeostasis and uses AMP-activated protein kinase (AMPK) to mediate retrograde signalling (Ho et al. 2013).
SIRT5
Human SIRT5 (UniProt id: Q9NXA8) is constituted of 310 amino acid residues. The SIRT5 domain structure and fold is similar to those of other sirtuins. SIRT5 is made up of 14 α-helices and 9 β-strands that are grouped in two globular domains: a larger domain and a smaller domain. A large Rossmann fold domain is found in NAD+/NADH binding proteins (Schuetz et al. 2007). It consists of a core β-sheet formed by six parallel β-strands (β1– β3 and β7– β9) and nine α-helices (α1, α2, α6, α7, and α10– α14) that pack against the β-sheet. A smaller zinc-binding domain is made up of five α-helices (α3– α5, α8, and α9) and a three-stranded antiparallel β-sheet (β4, β5, and β6). Various loops bridge the gap between SIRT5's two domains. The smaller zinc binding region shows structural variations between SIRT5 and the crystal structure of other sirtuins. Helix α9 and the 16 amino acid residue loop that precedes the helix form a specific insertion in human SIRT5 (Schuetz et al. 2007) as shown in Fig. 4.
Fig. 4.
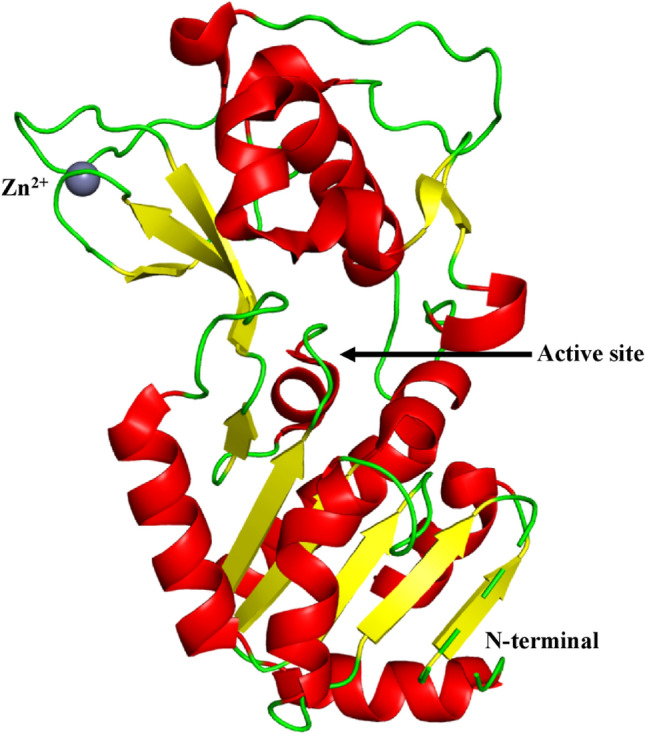
Structure of SIRT5 (PDB ID: 2NYR). SIRT5 is made up of 14 α-helices and 9 β-strands that are grouped in two globular domains: a larger domain and a smaller domain. A large Rossmann fold domain is found in NAD + /NADH binding proteins. It consists of a core β-sheet formed by six parallel β -strands (β1– β3 and β7– β9) and nine α-helices (α1, α2, α6, α7, and α10– α14) that pack against the β-sheet. A smaller zinc-binding domain is made up of five α-helices (α3– α5, α8, and α9) and a three-stranded antiparallel β-sheet (β4, β5, and β6). The smaller zinc binding region shows structural variations between SIRT5 and crystal structure of other sirtuins. Helix α9 and the 16 amino acid residue loop that precedes the helix form a specific insertion in human SIRT5
SIRT5 has weak deacetylase activity (Michan and Sinclair 2007). SIRT5 is important in the growth of breast tumour cells (Greene et al. 2019), hepatocellular carcinoma (Guo et al. 2018; Zhang et al. 2019a, b; Chang et al. 2018) and human non-small cell lung cancer tissues (Lu et al. 2014a, b). SIRT5's deacetylation activity is unusually resistant to nicotinamide inhibition (Fischer et al. 2012) and in lung epithelial cells, Forkhead protein Foxo3A deacetylation inhibits apoptosis induced by cigarette smoke extract (Wang et al. 2015). Desuccinylation of succinate dehydrogenase, serine hydroxy methyltransferase and superoxide dismutase by SIRT5 increases the growth of clear cell renal cell carcinoma tumours (Ma et al. 2019), cancer cell proliferation (Yang et al. 2018), and the elimination of ROS (Lin et al. 2013), respectively. A finding suggests that in cardiomyocytes, SIRT5 is a key moderator in cell death induced by H2O2 (Liu et al. 2013a, b). As a result of the energy stress, SIRT5 modulates cellular energy metabolism and AMP-activated protein kinase activity (Zhang et al. 2019a, b).
SIRT6
Human SIRT6 (UniProt id: Q8N6T7) is constituted of 355 amino acid residues. It is made up of a potential catalytic sirtuin core and flanking N- and C-terminal extensions. SIRT6 has two globular domains, each with eight α-helices and nine β-strands. A large Rossmann fold domain for NAD+ binding is made up of amino acid residues ranging from 25–128 to 191–266. It is made up of six-stranded (β1, β2, β3, β7, β8, and β9) parallel β-sheet amid between two helices (α6 and α7) on one side and four helices (α1, α4, α5, and α8) on the other side. The smaller domain, which contains a zinc-binding motif is made up of amino acid residues ranging from 129–190. It is formed by two extending loops (linking β3 and α6) from the large domain and includes a three-stranded antiparallel β-sheet (β4, β5, and β6). SIRT6 has the same domain composition as SIRT2, SIRT3, and SIRT5, although there are some variations on the protein's surface. SIRT6 is deprived of the cofactor-binding loop, which has been replaced by a single helix (α3) with numerous NAD+ binding residues. SIRT6's small domain lacks a helix bundle. A short loop replaces the helix bundle, interacting with the loop between α2 and α3, engaging with a small area on the zinc binding unit. One reason for SIRT6's scattered small domain is the absence of a helix bundle to build substantial contacts with the β-sheets in the zinc-binding motif (Pan et al. 2011) as shown in Fig. 5.
Fig. 5.
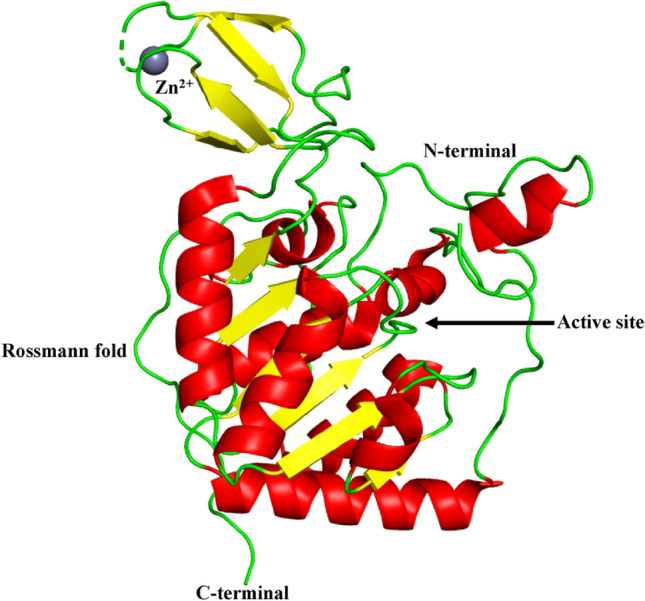
Structure of SIRT6 (PDB ID: 3K35). SIRT6 has two globular domains, each with eight α-helices and nine β-strands. A large Rossmann fold domain for NAD + binding is made up of six-stranded (β1, β2, β3, β7, β8, and β9) parallel β sheet amid between two helices (α6 and α7) on one side and four helices (α1, α4, α5, and α8) on the other side. The smaller domain, which contains a zinc-binding motif is formed by two extending loops (linking β 3 and α6) from the large domain and includes a three-stranded antiparallel β -sheet (β 4, β 5, and β 6). A short loop replaces the helix bundle, interacting with the loop between α2 and α3, engaging with a small area on the zinc binding unit. The dashed line represents the missing residues along the protein backbone
SIRT6 is a mono-ADP-ribosyl transferase (Michan and Sinclair 2007). In humans, SIRT6 is necessary to maintain the telomere position effect (Tennen et al. 2011) and also has a part to play in ageing of human induced pluripotent stem cells as well as their reprogramming (Sharma et al. 2013). Sirtuin 6 inhibits the generation of reactive oxygen species and glycolysis, which decreases the inflammatory response generated by hypoxia in human osteoblasts (Hou et al. 2017). The DNA repair pathway could be activated by human SIRT6's ability to ADP ribosylate PARP1 (Mitra and Dey 2020). The transfer of radiolabel from [32P]NAD, which is also catalysed by other members of the Sir2 family and was previously demonstrated to be mono-ADP-ribosylation, can be catalysed by pure SIRT6. Furthermore, a mono-ADP-ribosylated protein-specific anti-ADP-ribose antibody may identify the enzymatically altered form of human SIRT6 (Liszt et al. 2005). Research has shown senescence of endothelial cell is induced by oxidative stress due to SIRT6 downregulation (Liu et al. 2014a, b). It has also been discovered in humans, that SIRT6 interacts with the nuclear factor erythroid-derived 2-like 2 (NRF2) for the protection of mesenchymal stem cells from oxidative damage (Pan et al. 2016). Compared to healthy cells, SIRT6 overexpression significantly accelerates apoptosis in tumour cells (Van Meter et al. 2011).
SIRT7
SIRT7 (UniProt id: Q9NRC8) is consists of 400 amino acid residues. The DNA activated deacetylase activity of SIRT7 is dependent on both the N- and C-terminal domains. SIRT7-NTD (SIRT7 N-terminal domain) is made up of three helices, α1 to α3, including a short 310 helix connecting α1 and α2. α1 is the longest helix, with 35 amino acid residues ranging from Gly5 to Arg37. The α2 and α3 are short helices, which cover Ala45 to Leu51 and Ser54 to Glu60, respectively, and are almost perpendicular to the α1. According to the findings, SIRT7-NTD has structural similarities to transcription factor regulators and may have a role in DNA binding (Priyanka et al. 2016), as shown in Fig. 6.
Fig. 6.
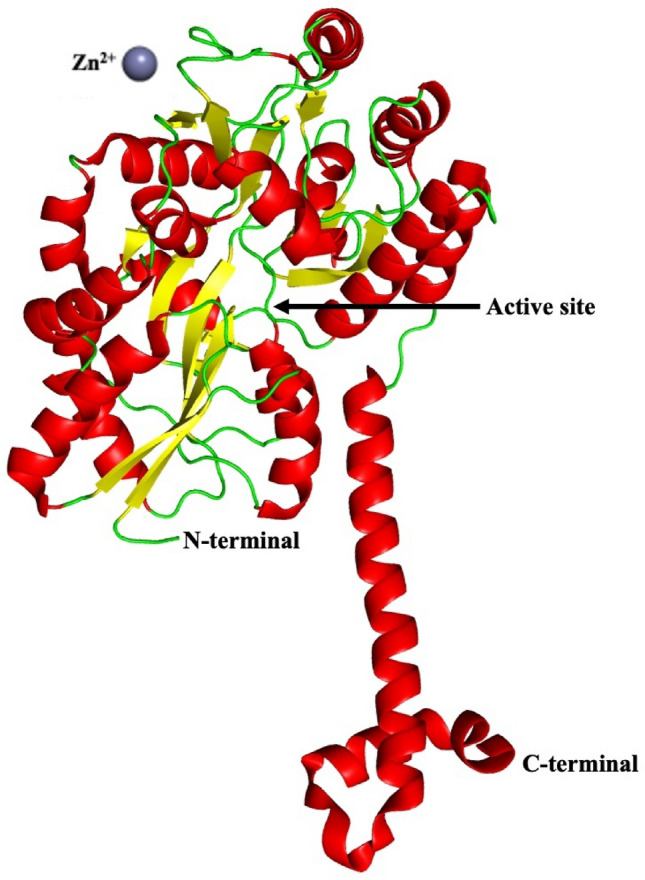
Structure of SIRT7 (PDB ID: 5IQZ). SIRT7-NTD (SIRT7 N-terminal domain) is made up of three helices, α1 to α3, including a short 310 helix connecting α1 & α2. α1 is the longest helix. The α2 & α3 are short helices and are almost perpendicular to the α1
SIRT7 has deacetylase activity, and plays a critical role in ageing and lifespan of hematopoietic cells in humans (Kaiser et al. 2020). SIRT7 arginine methylation maintains energy balance by coordinating glucose availability with mitochondria biogenesis (Yan et al. 2018). Sirtuin 7 is required not only for rDNA transcription but also for particular pre-rRNA cleavage (Chen et al. 2016). SIRT7 depletion decreases DNA repair and compaction of chromatin, rendering cells more susceptible to genotoxic stressors (Li et al. 2016). As a heterochromatin stabiliser, SIRT7 counteracts human stem cell ageing (Bi et al. 2020). SIRT7 inactivation reduces breast cancer metastasis by blocking TGF-β signalling (Tang et al. 2017). In humans, SIRT7 take part in the genesis of colorectal cancer (Yu et al. 2014), gastric cancer (Zhang et al. 2015), and luminal breast cancer (Huo et al. 2020).
Physiological roles of sirtuins
Sirtuins in cancer
Sirtuins are implicated in tumour proliferation and metastasis regulation. SIRT1 and SIRT2 promote cell proliferation and play a pro-tumorigenic role in lung cancer (Grbesa et al. 2015). SIRT5 also enhances lung cancer growth and its overexpression indicates a poor prognosis in human non-small cell lung cancer (Lu et al. 2014a, b). In metastatic human melanoma tissues, SIRT2 levels are increased as compared to tumours of early stage. It highly suggests that there is involvement of SIRT2 in the advancement of metastatic disease (Wilking-Busch et al. 2017). Hypoxia inducible factor-1α (HIF-1α) deacetylation mediated by SIRT2 is necessary for HIF-1α destabilisation and tumour cell hypoxia response (Seo et al. 2015). SIRT3 stops mitochondrial reactive oxygen species generation, which inhibits HIF-1α and tumour growth. When SIRT3 is overexpressed, it resists the growth of established cancer cell lines (Bell et al. 2011). In colon cancer, SIRT3 deficiency reduces cell proliferation, invasion, and migration while increasing apoptosis (Liu et al. 2014a, b). Upregulation of SIRT3 in breast cancer cells suppresses glycolysis and proliferation, offering a metabolic pathway for tumour suppression (Finley et al. 2011). Lower expression of SIRT4 promotes oesophageal squamous cell carcinoma and hepatocellular carcinoma development (Liu et al. 2014a, b). Researchers found that SIRT5 is upregulated during cellular transformation and is involved in cancer and its proliferation by desuccinylating serine hydroxy methyltransferase 2 (Yang et al. 2018). It also advances the growth of hepatocellular carcinoma (Chang et al. 2018; Zhang et al. 2019a, b), clear cell renal cell carcinoma tumorigenesis through desuccinylating SDHA (Ma et al. 2019) and breast cancer progression by maintaining mitochondrial glutaminase (Greene et al. 2019). Lack of SIRT6 decreases the viability of prostate cancer cells while increasing chemotherapeutic sensitivity (Liu et al. 2013a, b). Sirtuins may play pro-tumorigenic roles in some established tumours by aiding in survival under the stressful conditions that characterise the cancer cell state. SIRT7 overexpression has been found in multiple cancer tissues (Barber et al. 2012). Expression of the nuclear sirtuin, SIRT7, promotes the survival of cancer cells and the maintenance of a transformed state. In breast, thyroid and liver cancer, SIRT7 is up-regulated (Huo et al. 2020), also in colorectal cancer (Yu et al. 2014) and gastric cancer (Zhang et al. 2015). Inactivation of SIRT7 significantly reduces cancer cell metastasis in vivo, regardless of changes in original tumour development (Malik et al. 2015) and it is also discovered that SIRT7 deacetylase activity, inhibits metastasis of breast cancer by antagonising transforming growth factor-β signalling (Tang et al. 2017). The importance of SIRT7 in DNA damage repair suggests that this enzyme may have tumour-suppressing abilities. However, SIRT7 is overexpressed in a lot of cancers. SIRT7 may therefore have opposing effects on the emergence and spread of cancer. (Tang et al. 2021).
Sirtuins in neurodegeneration
SIRT2 is overexpressed in neurodegenerative disorders and it protects neural cells from oxidative damage caused by ageing and disease (Singh et al. 2017). SIRT2 is found to be overexpressed in Parkinson’s disease. SIRT3 activation and/or NAD+ supplementation can help with amyotrophic lateral sclerosis, which is a neurodegenerative disease caused by systemic NAD+ depletion. The metabolic markers of amyotrophic lateral sclerosis motor neurons are decreased mitochondrial respiration and increased glycolysis. SIRT3 activation has been discovered as a treatment for disease phenotypes in both sporadic and familial amyotrophic lateral sclerosis (Hor et al. 2021).
Sirtuins and epigenetics
In human cells, SIRT1 activation enhances homologous recombination. This function could help to decrease tumours by stabilising chromosomes and/or extending the lifespan by maintaining rDNA arrays (Uhl et al. 2010). A research revealed that SIRT1 controls the activity of DNA methyltransferase 1, a crucial enzyme in DNA methylation. Deacetylation of DNA methyltransferase 1 at certain lysine residues, improves its methyltransferase activity, alters its transcription suppression activity, and impairs its ability to silence tumour suppressor genes (Peng et al. 2011). SIRT1 also controls UV-induced DNA damage repair by deacetylating xeroderma pigmentosum group A, which is required for the nucleotide excision repair pathway to function properly (Fan and Luo 2010). SIRT6 has been discovered as the first enzyme that modifies histones to regulate the telomere position effect in human cells, considering a novel role for SIRT6 in the regulation of age-related genome silencing mechanism and providing a new approach for telomere chromatin silencing (Tennen et al. 2011).
SIRT7 regulates innate immune modulation and ensures geroprotection during stem cell ageing by acting as a heterochromatin stabiliser and safeguarding chromatin structure (Bi et al. 2020). SIRT7 regulates a multitude of essential physiological functions by inducing various genes to improve stress tolerance and cell survival (Chen et al. 2016).
Modulators of sirtuins
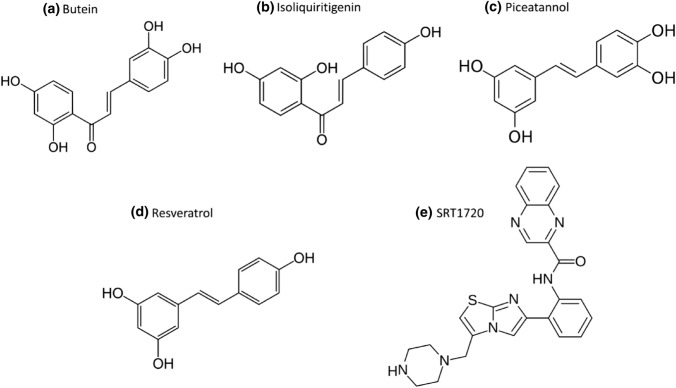
Various physiological functions like energy metabolism, stress response, etc. are regulated by sirtuins in humans. SIRT1-7, the human Sirtuin isoforms, are thought to represent promising therapeutic targets for age-related diseases like type 2 diabetes, inflammatory diseases, and neurodegenerative disorders. Because Sirtuins are appealing therapeutic targets, a lot of work has gone into generating specific sirtuin activators and inhibitors, both as tools for researching sirtuin activity and as prospective therapies for age-related diseases. The mammalian sirtuin family consists of seven proteins, however, not all of them possess deacetylase activity. All sirtuins have a 275-amino-acid catalytic core domain and a stoichiometric need for the cofactor NAD+ to deacetylate substrates including histones and transcriptional regulators. Plant polyphenols notably butein, piceatannol, and isoliquiritigenin (ILQ) have been demonstrated to activate recombinant SIRT1 (Dai et al. 2018), known as sirtuin activating compounds (STACs). One of the most prominent amongst these is resveratrol (3, 5, 4′-trihydroxystilbene) which is found in grapes and red wines. In lower organisms, resveratrol replicates the effects of calorie restriction, and mice fed a high-fat diet have lower insulin resistance. STACs interact directly with SIRT1 and other isoforms, according to substantial mechanistic and structural investigations conducted over the last few years (Shukla and Singh 2011). Many synthetic STACs that are chemically unrelated to resveratrol, such as imidazo[1,2-b]thiazole core, including SRT1720, activate SIRT1 substantially more potently. These activators have been utilised in numerous studies to demonstrate their effect on SIRT1 (Vu et al. 2009). STACs of various chemotypes, such as oxazolo[4,5-b]pyridine, thiazolopyridine, benzimidazole, imidazo[4,5-c]pyridine, and dihydropyridine (DHP) derivatives, have been designed to improve activation potency, physicochemical characteristics, and developability, and they appear to have even larger therapeutic promise (Bemis et al. 2009; Mai et al. 2009; Dai et al. 2018). Some of the activators of sirtuins and their structures are shown in Fig. 7.
Fig. 7.
Activators of sirtuins a butein, b isoliquiritigenin, c piceatannol, d resveratrol and e SRT1720
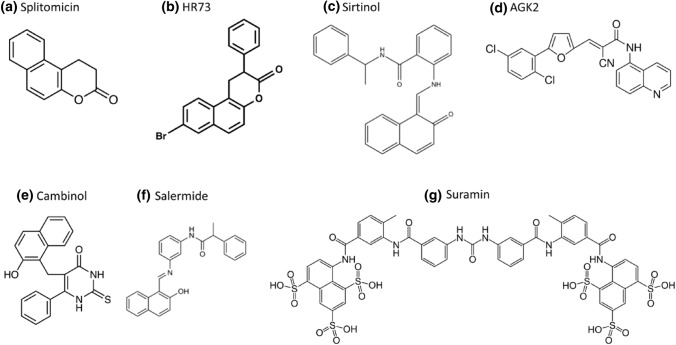
As up-regulated SIRT1 has been found in cancer cell lines, sirtuin inhibiting compounds (STICs) could be effective as therapeutic agents. Sirtuin inhibitors have also been considered for the treatment of Parkinson's disease, in addition to cancer therapy (Abbotto et al. 2022). Unlike the activators, where only SIRT1 activators have been produced, high-throughput and in silico screenings have identified sirtuin inhibitors for SIRT1, SIRT2, SIRT3 and SIRT5. The majority of known sirtuin inhibitors exclusively inhibit SIRT1 and/or SIRT2, however some also inhibit SIRT3 and SIRT5 with reduced affinity. Splitomicin, HR73, Sirtinol, AGK2, Cambinol, Salermide, Tenovin, and Suramin are some of the examples of STICs (Villalba and Alcaín 2012) as shown in Fig. 8. Splitomicin inhibits Sir2 in Saccharomyces cerevisiae, but showed weaker inhibition on human SIRT1. Yeast Sir2 and human SIRT2 are inhibited by sirtinol (Carafa et al. 2012). Suramin is a potent inhibitor of many sirtuins like SIRT1, SIRT2 and SIRT5. Cambinol has the most effective efficacy against Burkitt lymphoma cell lines. Salermide causes apoptosis in cancer cells without relying on p53. Both Cambinol and Salermide have inhibitory effects on SIRT1 and SIRT2 (Bai et al. 2018).
Fig. 8.
Inhibitors of sirtuins a Splitomicin, b HR73, c Sirtinol, d AGK2, e Cambinol, f Salermide and g Suramin
Conclusion
Sirtuins are a family of proteins that are present in all forms of life. Sirtuins have attracted a lot of investigation due to the way they control mammalian physiological processes. This is because they may offer brand-new targets for treating disorders linked to ageing and maybe extending human longevity. Six of the seven mammalian sirtuins have already been linked to biological processes ranging from DNA repair to metabolism, with SIRT1 being the most thoroughly investigated. The human sirtuin family contains seven members (SIRT1-7), which are localised to different cellular compartments and are capable of diverse catalytic activities to modify a great number of proteins, including histone and non-histone proteins. Their biological functions vary from metabolism to cell survival as key regulators and they’re involved in a range of diseases, such as diabetes, neurodegeneration and cancer. Emerging evidence suggests that sirtuins could be placed at the crossroads of stemness, ageing, and cancer.
The catalytic domain of sirtuins is unique to this family, and it requires nicotine adenine dinucleotide (NAD) as a cofactor. Sirtuins are implicated in the pathogenesis of many diseases, including obesity-related metabolic diseases, neurodegenerative diseases, inflammation, cardiovascular diseases, and cancer, amongst others, due to the extensive range of activities in which they participate. Emerging research has recently recognised their role in a wide range of ailments. The modulation of transcriptional repression by sirtuins is mediated by the binding of a complex containing sirtuins and other proteins. The activity of sirtuins can be modulated by various natural as well as synthetic compounds. A natural plant polyphenol resveratrol, can activate SIRT1 and other sirtuins and also enhances their activity. Molecular characterizations of various inhibitors of sirtuins have also been done and is helpful in better understanding of complex role of sirtuins in cellular metabolism. Sirtuin activators have been used in clinical trials to protect against age-related illnesses (Grabowska et al. 2017; Curry et al. 2021). Clinical success of sirtuin-targeting drugs necessitates a comprehensive understanding of the "sirtuin-dependency" of the disease, strong lead compounds that are powerful and selective with perfect drug-like qualities, PK/PD profiling and improvement, as well as advancements in formulation. Growing data suggest that sirtuins are an appealing anti-ageing proteins that improves health by targeting molecules engaged in a variety of biological processes; nonetheless, the significance of sirtuins in lifespan and the longevity effect of calorie restriction remain contentious. To investigate the true therapeutic potential and their efficacy in a variety of pathological diseases, a better knowledge of the link between the structure and function of sirtuin proteins would be necessary.
Acknowledgements
The authors thank Sharda University, Greater Noida, India for support.
Author contributions
MJ conceived the concept of the study. AS, PM and MJ wrote the manuscript. AS, PM and MJ prepared the figures and tables. AS, PM and MJ collected the literature from various resources. MJ, JM and AKS corrected the manuscript. All authors approved the contents of the manuscript.
Funding
Not applicable.
Data availability
The data analyzed during the current study are available from the corresponding author on reasonable request as per the journal guidelines.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Footnotes
Abhishek Sharma and Pragati Mahur authors contributed equally.
References
- Abbotto E, Scarano N, Piacente F, Millo E, Cichero E, Bruzzone S. Virtual screening in the identification of sirtuins' activity modulators. Molecules (Basel, Switzerland) 2022;27(17):5641. doi: 10.3390/molecules27175641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunachalam G, Samuel SM, Marei I, Ding H, Triggle CR. Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through SIRT1. Br J Pharmacol. 2014;171(2):523–535. doi: 10.1111/bph.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X, Yao L, Ma X, Xu X. Small molecules as SIRT modulators. Mini Rev Med Chem. 2018;18(13):1151–1157. doi: 10.2174/1389557516666160620095103. [DOI] [PubMed] [Google Scholar]
- Barber MF, Michishita-Kioi E, Xi Y, Tasselli L, Kioi M, Moqtaderi Z, Tennen RI, Paredes S, Young NL, Chen K, Struhl K, Garcia BA, Gozani O, Li W, Chua KF. SIRT7 links H3K18 deacetylation to maintenance of oncogenic transformation. Nature. 2012;487(7405):114–118. doi: 10.1038/nature11043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell EL, Emerling BM, Ricoult SJ, Guarente L. SirT3 suppresses hypoxia inducible factor 1α and tumor growth by inhibiting mitochondrial ROS production. Oncogene. 2011;30(26):2986–2996. doi: 10.1038/onc.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemis JE, Vu CB, Xie R, Nunes JJ, Ng PY, Disch JS, Milne JC, Carney DP, Lynch AV, Jin L, Smith JJ, Lavu S, Iffland A, Jirousek MR, Perni RB. Discovery of oxazolo[4,5-b]pyridines and related heterocyclic analogs as novel SIRT1 activators. Bioorg Med Chem Lett. 2009;19(8):2350–2353. doi: 10.1016/j.bmcl.2008.11.106. [DOI] [PubMed] [Google Scholar]
- Bergmann L, Lang A, Bross C, Altinoluk-Hambüchen S, Fey I, Overbeck N, Stefanski A, Wiek C, Kefalas A, Verhülsdonk P, Mielke C, Sohn D, Stühler K, Hanenberg H, Jänicke RU, Scheller J, Reichert AS, Ahmadian MR, Piekorz RP. Subcellular localization and mitotic interactome analyses identify SIRT4 as a centrosomally localized and microtubule associated protein. Cells. 2020;9(9):1950. doi: 10.3390/cells9091950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi S, Liu Z, Wu Z, Wang Z, Liu X, Wang S, Ren J, Yao Y, Zhang W, Song M, Liu GH, Qu J. SIRT7 antagonizes human stem cell aging as a heterochromatin stabilizer. Protein Cell. 2020;11(7):483–504. doi: 10.1007/s13238-020-00728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch-Presegué L, Raurell-Vila H, Marazuela-Duque A, Kane-Goldsmith N, Valle A, Oliver J, Serrano L, Vaquero A. Stabilization of Suv39H1 by SirT1 is part of oxidative stress response and ensures genome protection. Mol Cell. 2011;42(2):210–223. doi: 10.1016/j.molcel.2011.02.034. [DOI] [PubMed] [Google Scholar]
- Carafa V, Nebbioso A, Altucci L. Sirtuins and disease: the road ahead. Front Pharmacol. 2012;3:4. doi: 10.3389/fphar.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha Y, Han MJ, Cha HJ, Zoldan J, Burkart A, Jung JH, Jang Y, Kim CH, Jeong HC, Kim BG, Langer R, Kahn CR, Guarente L, Kim KS. Metabolic control of primed human pluripotent stem cell fate and function by the miR-200c-SIRT2 axis. Nat Cell Biol. 2017;19(5):445–456. doi: 10.1038/ncb3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Xi L, Liu Y, Liu R, Wu Z, Jian Z. SIRT5 promotes cell proliferation and invasion in hepatocellular carcinoma by targeting E2F1. Mol Med Rep. 2018;17(1):342–349. doi: 10.3892/mmr.2017.7875. [DOI] [PubMed] [Google Scholar]
- Chen S, Blank MF, Iyer A, Huang B, Wang L, Grummt I, Voit R. SIRT7-dependent deacetylation of the U3–55k protein controls pre-rRNA processing. Nat Commun. 2016;7:10734. doi: 10.1038/ncomms10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Lin J, Feng S, Chen X, Huang H, Wang C, Yu Y, He Y, Han S, Zheng L, Huang G. SIRT4 inhibits the proliferation, migration, and invasion abilities of thyroid cancer cells by inhibiting glutamine metabolism. Onco Targets Ther. 2019;12:2397–2408. doi: 10.2147/OTT.S189536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Ma C, Fan G, Liu H, Lin X, Li J, Li N, Wang S, Zeng M, Zhang Y, Bu P. SIRT3 protects endothelial cells from high glucose-induced senescence and dysfunction via the p53 pathway. Life Sci. 2021;264:118724. doi: 10.1016/j.lfs.2020.118724. [DOI] [PubMed] [Google Scholar]
- Curry AM, White DS, Donu D, Cen Y. Human sirtuin regulators: The "Success" stories. Front Physiol. 2021;12:752117. doi: 10.3389/fphys.2021.752117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H, Sinclair DA, Ellis JL, Steegborn C. Sirtuin activators and inhibitors: Promises, achievements, and challenges. Pharmacol Ther. 2018;188:140–154. doi: 10.1016/j.pharmthera.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Liu S, Li J, Li J, Lan Y, Nie H, Zuo Y. SIRT4 suppresses the inflammatory response and oxidative stress in osteoarthritis. Am J Transl Res. 2020;12(5):1965–1975. [PMC free article] [PubMed] [Google Scholar]
- Davenport AM, Huber FM, Hoelz A. Structural and functional analysis of human SIRT1. J Mol Biol. 2014;426(3):526–541. doi: 10.1016/j.jmb.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong R, Pan S, Peng Z, Zhang Y, Yang J. mTM-align: a server for fast protein structure database search and multiple protein structure alignment. Nucleic Acids Res. 2018;46(W1):W380–W386. doi: 10.1093/nar/gky430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Luo J. SIRT1 regulates UV-induced DNA repair through deacetylating XPA. Mol Cell. 2010;39(2):247–258. doi: 10.1016/j.molcel.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Finley LW, Carracedo A, Lee J, Souza A, Egia A, Zhang J, Teruya-Feldstein J, Moreira PI, Cardoso SM, Clish CB, Pandolfi PP, Haigis MC. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1α destabilization. Cancer Cell. 2011;19(3):416–428. doi: 10.1016/j.ccr.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnin MS, Donigian JR, Pavletich NP. Structure of the histone deacetylase SIRT2. Nat Struct Biol. 2001;8(7):621–625. doi: 10.1038/89668. [DOI] [PubMed] [Google Scholar]
- Fischer F, Gertz M, Suenkel B, Lakshminarasimhan M, Schutkowski M, Steegborn C. Sirt5 deacylation activities show differential sensitivities to nicotinamide inhibition. PloS one. 2012;7(9):e45098. doi: 10.1371/journal.pone.0045098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Dong Q, He J, Wang X, Xing J, Wang E, Qiu X, Li Q. SIRT4 inhibits malignancy progression of NSCLCs, through mitochondrial dynamics mediated by the ERK-Drp1 pathway. Oncogene. 2017;36(19):2724–2736. doi: 10.1038/onc.2016.425. [DOI] [PubMed] [Google Scholar]
- Grabowska W, Sikora E, Bielak-Zmijewska A. Sirtuins, a promising target in slowing down the ageing process. Biogerontology. 2017;18(4):447–476. doi: 10.1007/s10522-017-9685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräff J, Kahn M, Samiei A, Gao J, Ota KT, Rei D, Tsai LH. A dietary regimen of caloric restriction or pharmacological activation of SIRT1 to delay the onset of neurodegeneration. J Neurosci. 2013;33(21):8951–8960. doi: 10.1523/JNEUROSCI.5657-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grbesa I, Pajares MJ, Martínez-Terroba E, Agorreta J, Mikecin AM, Larráyoz M, Idoate MA, Gall-Troselj K, Pio R, Montuenga LM. Expression of sirtuin 1 and 2 is associated with poor prognosis in non-small cell lung cancer patients. PloS one. 2015;10(4):e0124670. doi: 10.1371/journal.pone.0124670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Song X, Guo T, Gu S, Chang X, Su T, Yang X, Liang B, Huang D. Vimentin acetylation is involved in SIRT5-mediated hepatocellular carcinoma migration. Am J Cancer Res. 2018;8(12):2453–2466. [PMC free article] [PubMed] [Google Scholar]
- Herranz D, Muñoz-Martin M, Cañamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, Serrano M. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun. 2010;1:3. doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hor JH, Santosa MM, Lim V, Ho BX, Taylor A, Khong ZJ, Ravits J, Fan Y, Liou YC, Soh BS, Ng SY. ALS motor neurons exhibit hallmark metabolic defects that are rescued by SIRT3 activation. Cell Death Differ. 2021;28(4):1379–1397. doi: 10.1038/s41418-020-00664-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou KL, Lin SK, Chao LH, Hsiang-Hua Lai E, Chang CC, Shun CT, Lu WY, Wang JH, Hsiao M, Hong CY, Kok SH. Sirtuin 6 suppresses hypoxia-induced inflammatory response in human osteoblasts via inhibition of reactive oxygen species production and glycolysis-A therapeutic implication in inflammatory bone resorption. BioFactors (Oxford, England) 2017;43(2):170–180. doi: 10.1002/biof.1320. [DOI] [PubMed] [Google Scholar]
- Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13(4):225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Lin J, Lin Y, Chen X, Zhu G, Huang G. Overexpression of SIRT4 inhibits the proliferation of gastric cancer cells through cell cycle arrest. Oncol Lett. 2019;17(2):2171–2176. doi: 10.3892/ol.2018.9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Q, Li Z, Cheng L, Yang F, Xie N. SIRT7 Is a prognostic biomarker associated with immune infiltration in luminal breast cancer. Front Oncol. 2020;10:621. doi: 10.3389/fonc.2020.00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403(6771):795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Ivy JM, Klar AJ, Hicks JB. Cloning and characterization of four SIR genes of Saccharomyces cerevisiae. Mol Cell Biol. 1986;6(2):688–702. doi: 10.1128/mcb.6.2.688-702.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Wei W, Jiang Y, Peng H, Cai J, Mao C, Dai H, Choy W, Bemis JE, Jirousek MR, Milne JC, Westphal CH, Perni RB. Crystal structures of human SIRT3 displaying substrate-induced conformational changes. J Biol Chem. 2009;284(36):24394–24405. doi: 10.1074/jbc.M109.014928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser A, Schmidt M, Huber O, Frietsch JJ, Scholl S, Heidel FH, Hochhaus A, Müller JP, Ernst T. SIRT7: an influence factor in healthy aging and the development of age-dependent myeloid stem-cell disorders. Leukemia. 2020;34(8):2206–2216. doi: 10.1038/s41375-020-0803-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic U, Gok O, Erenberk U, Dundaroz MR, Torun E, Kucukardali Y, Elibol-Can B, Uysal O, Dundar T. A remarkable age-related increase in SIRT1 protein expression against oxidative stress in elderly: SIRT1 gene variants and longevity in human. PloS one. 2015;10(3):e0117954. doi: 10.1371/journal.pone.0117954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Banck M, Mujtaba S, Zhou MM, Sugrue MM, Walsh MJ. p53-induced growth arrest is regulated by the mitochondrial SirT3 deacetylase. PloS one. 2010;5(5):e10486. doi: 10.1371/journal.pone.0010486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Shi L, Yang S, Yan R, Zhang D, Yang J, He L, Li W, Yi X, Sun L, Liang J, Cheng Z, Shi L, Shang Y, Yu W. SIRT7 is a histone desuccinylase that functionally links to chromatin compaction and genome stability. Nat Commun. 2016;7:12235. doi: 10.1038/ncomms12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Li H, Zhao ZB, Zhu W, Feng PP, Zhu XW, Gong JP. SIRT4 silencing in tumor-associated macrophages promotes HCC development via PPARδ signalling-mediated alternative activation of macrophages. J Experim Clin Cancer Res. 2019;38(1):469. doi: 10.1186/s13046-019-1456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin ZF, Xu HB, Wang JY, Lin Q, Ruan Z, Liu FB, Jin W, Huang HH, Chen X. SIRT5 desuccinylates and activates SOD1 to eliminate ROS. Biochem Biophys Res Commun. 2013;441(1):191–195. doi: 10.1016/j.bbrc.2013.10.033. [DOI] [PubMed] [Google Scholar]
- Liszt G, Ford E, Kurtev M, Guarente L. Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase. J Biol Chem. 2005;280(22):21313–21320. doi: 10.1074/jbc.M413296200. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang D, Zhao Y, Tu B, Zheng Z, Wang L, Wang H, Gu W, Roeder RG, Zhu WG. Methyltransferase Set7/9 regulates p53 activity by interacting with Sirtuin 1 (SIRT1) Proc Natl Acad Sci USA. 2011;108(5):1925–1930. doi: 10.1073/pnas.1019619108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Che W, Zheng C, Liu W, Wen J, Fu H, Tang K, Zhang J, Xu Y. SIRT5: a safeguard against oxidative stress-induced apoptosis in cardiomyocytes. Cellular Physiol Biochem . 2013;32(4):1050–1059. doi: 10.1159/000354505. [DOI] [PubMed] [Google Scholar]
- Liu Y, Xie QR, Wang B, Shao J, Zhang T, Liu T, Huang G, Xia W. Inhibition of SIRT6 in prostate cancer reduces cell viability and increases sensitivity to chemotherapeutics. Protein Cell. 2013;4(9):702–710. doi: 10.1007/s13238-013-3054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Huang Z, Jiang H, Shi F. The sirtuin 3 expression profile is associated with pathological and clinical outcomes in colon cancer patients. BioMed Res Int. 2014 doi: 10.1155/2014a/871263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Liu H, Ha Y, Tilton RG, Zhang W. Oxidative stress induces endothelial cell senescence via downregulation of Sirt6. BioMed Res Int. 2014 doi: 10.1155/2014b/902842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Wang Z, Ren M, Yang X, Liu B, Qi H, Yu M, Song S, Chen S, Liu L, Zhang Y, Zou J, Zhu WG, Yin Y, Luo J. SIRT4 regulates PTEN stability through IDE in response to cellular stresses. FASEB J . 2019;33(4):5535–5547. doi: 10.1096/fj.201801987R. [DOI] [PubMed] [Google Scholar]
- Lu TM, Tsai JY, Chen YC, Huang CY, Hsu HL, Weng CF, Shih CC, Hsu CP. Downregulation of Sirt1 as aging change in advanced heart failure. J Biomed Sci. 2014;21(1):57. doi: 10.1186/1423-0127-21-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Zuo Y, Feng Y, Zhang M. SIRT5 facilitates cancer cell growth and drug resistance in non-small cell lung cancer. Tumour Biol . 2014;35(11):10699–10705. doi: 10.1007/s13277-014-2372-4. [DOI] [PubMed] [Google Scholar]
- Ma Y, Qi Y, Wang L, Zheng Z, Zhang Y, Zheng J. SIRT5-mediated SDHA desuccinylation promotes clear cell renal cell carcinoma tumorigenesis. Free Radical Biol Med. 2019;134:458–467. doi: 10.1016/j.freeradbiomed.2019.01.030. [DOI] [PubMed] [Google Scholar]
- Mai A, Valente S, Meade S, Carafa V, Tardugno M, Nebbioso A, Galmozzi A, Mitro N, De Fabiani E, Altucci L, Kazantsev A. Study of 1,4-dihydropyridine structural scaffold: discovery of novel sirtuin activators and inhibitors. J Med Chem. 2009;52(17):5496–5504. doi: 10.1021/jm9008289. [DOI] [PubMed] [Google Scholar]
- Malik S, Villanova L, Tanaka S, Aonuma M, Roy N, Berber E, Pollack JR, Michishita-Kioi E, Chua KF. SIRT7 inactivation reverses metastatic phenotypes in epithelial and mesenchymal tumors. Sci Rep. 2015;5:9841. doi: 10.1038/srep09841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404(1):1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra N, Dey S. Biochemical characterization of mono ADP ribosyl transferase activity of human sirtuin SIRT7 and its regulation. Arch Biochem Biophys. 2020;680:108226. doi: 10.1016/j.abb.2019.108226. [DOI] [PubMed] [Google Scholar]
- Miyo M, Yamamoto H, Konno M, Colvin H, Nishida N, Koseki J, Kawamoto K, Ogawa H, Hamabe A, Uemura M, Nishimura J, Hata T, Takemasa I, Mizushima T, Doki Y, Mori M, Ishii H. Tumour-suppressive function of SIRT4 in human colorectal cancer. Br J Cancer. 2015;113(3):492–499. doi: 10.1038/bjc.2015.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniot S, Schutkowski M, Steegborn C. Crystal structure analysis of human Sirt2 and its ADP-ribose complex. J Struct Biol. 2013;182(2):136–143. doi: 10.1016/j.jsb.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Nakahara Y, Yamasaki M, Sawada G, Miyazaki Y, Makino T, Takahashi T, Kurokawa Y, Nakajima K, Takiguchi S, Mimori K, Mori M, Doki Y. Downregulation of SIRT4 expression is associated with poor prognosis in oesophageal squamous cell carcinoma. Oncology. 2016;90(6):347–355. doi: 10.1159/000445323. [DOI] [PubMed] [Google Scholar]
- North BJ, Verdin E. Sirtuins: Sir2-related NAD-dependent protein deacetylases. Genome Biol. 2004;5(5):224. doi: 10.1186/gb-2004-5-5-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11(2):437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- Palacios JA, Herranz D, De Bonis ML, Velasco S, Serrano M, Blasco MA. SIRT1 contributes to telomere maintenance and augments global homologous recombination. J Cell Biol. 2010;191(7):1299–1313. doi: 10.1083/jcb.201005160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan PW, Feldman JL, Devries MK, Dong A, Edwards AM, Denu JM. Structure and biochemical functions of SIRT6. J Biol Chem. 2011;286(16):14575–14587. doi: 10.1074/jbc.M111.218990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Guan D, Liu X, Li J, Wang L, Wu J, Zhou J, Zhang W, Ren R, Zhang W, Li Y, Yang J, Hao Y, Yuan T, Yuan G, Wang H, Ju Z, Mao Z, Li J, Qu J, Liu GH. SIRT6 safeguards human mesenchymal stem cells from oxidative stress by coactivating NRF2. Cell Res. 2016;26(2):190–205. doi: 10.1038/cr.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Seong RK, Kim JA, Son SJ, Kim Y, Yokozawa T, Shin OS. Oligonol promotes anti-aging pathways via modulation of SIRT1-AMPK-Autophagy Pathway. Nurs Res Pract. 2016;10(1):3–10. doi: 10.4162/nrp.2016.10.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peled T, Shoham H, Aschengrau D, Yackoubov D, Frei G, Rosenheimer G, Lerrer B, Cohen HY, Nagler A, Fibach E, Peled A. Nicotinamide, a SIRT1 inhibitor, inhibits differentiation and facilitates expansion of hematopoietic progenitor cells with enhanced bone marrow homing and engraftment. Experim Hematol. 2012;40(4):342–55.e1. doi: 10.1016/j.exphem.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Peng L, Yuan Z, Ling H, Fukasawa K, Robertson K, Olashaw N, Koomen J, Chen J, Lane WS, Seto E. SIRT1 deacetylates the DNA methyltransferase 1 (DNMT1) protein and alters its activities. Mol Cell Biol. 2011;31(23):4720–4734. doi: 10.1128/MCB.06147-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyanka A, Solanki V, Parkesh R, Thakur KG. Crystal structure of the N-terminal domain of human SIRT7 reveals a three-helical domain architecture. Proteins. 2016;84(10):1558–1563. doi: 10.1002/prot.25085. [DOI] [PubMed] [Google Scholar]
- Seo KS, Park JH, Heo JY, Jing K, Han J, Min KN, Kim C, Koh GY, Lim K, Kang GY, Uee Lee J, Yim YH, Shong M, Kwak TH, Kweon GR. SIRT2 regulates tumour hypoxia response by promoting HIF-1α hydroxylation. Oncogene. 2015;34(11):1354–1362. doi: 10.1038/onc.2014.76. [DOI] [PubMed] [Google Scholar]
- Sharma A, Diecke S, Zhang WY, Lan F, He C, Mordwinkin NM, Chua KF, Wu JC. The regulation of age related genome silencing protein in aging and reprogramming of human induced pluripotent stem cells. J Biol Chem. 2013;288(25):18439–18447. doi: 10.1074/jbc.M112.405928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng X, Cristea IM. The antiviral sirtuin 3 bridges protein acetylation to mitochondrial integrity and metabolism during human cytomegalovirus infection. PLoS Pathogens. 2021;17(4):e1009506. doi: 10.1371/journal.ppat.1009506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla Y, Singh R. Resveratrol and cellular mechanisms of cancer prevention. Ann N Y Acad Sci. 2011;1215:1–8. doi: 10.1111/j.1749-6632.2010.05870.x. [DOI] [PubMed] [Google Scholar]
- Singh P, Hanson PS, Morris CM. Sirtuin-2 protects neural cells from oxidative stress and is elevated in neurodegeneration. Parkinson's Disease. 2017;2017:2643587. doi: 10.1155/2017/2643587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoge RH, Dölle C, Ziegler M. Regulation of SIRT2-dependent α-tubulin deacetylation by cellular NAD levels. DNA Repair. 2014;23:33–38. doi: 10.1016/j.dnarep.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Sun H, Huang D, Liu G, Jian F, Zhu J, Zhang L. SIRT4 acts as a tumor suppressor in gastric cancer by inhibiting cell proliferation, migration, and invasion. Onco Targets Ther. 2018;11:3959–3968. doi: 10.2147/OTT.S156143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunami Y, Araki M, Hironaka Y, Morishita S, Kobayashi M, Liew EL, Edahiro Y, Tsutsui M, Ohsaka A, Komatsu N. Inhibition of the NAD-dependent protein deacetylase SIRT2 induces granulocytic differentiation in human leukemia cells. PloS one. 2013;8(2):e57633. doi: 10.1371/journal.pone.0057633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Shi L, Xie N, Liu Z, Qian M, Meng F, Xu Q, Zhou M, Cao X, Zhu WG, Liu B. SIRT7 antagonizes TGF-β signaling and inhibits breast cancer metastasis. Nat Commun. 2017;8(1):318. doi: 10.1038/s41467-017-00396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Tang H, Tu B, Zhu WG. SIRT7: a sentinel of genome stability. Open Biol. 2021;11(6):210047. doi: 10.1098/rsob.210047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Huang C, Huang Y, Hong L, Wang H, Zhou Z, Qiu Y. SIRT4 suppresses inflammatory responses in human umbilical vein endothelial cells. Cardiovasc Toxicol. 2015;15(3):217–223. doi: 10.1007/s12012-014-9287-6. [DOI] [PubMed] [Google Scholar]
- Teixeira C, Cerqueira N, Gomes P, Sousa SF. A molecular perspective on sirtuin activity. Int J Mol Sci. 2020;21(22):8609. doi: 10.3390/ijms21228609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennen RI, Bua DJ, Wright WE, Chua KF. SIRT6 is required for maintenance of telomere position effect in human cells. Nat Commun. 2011;2:433. doi: 10.1038/ncomms1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theos AC, Tenza D, Martina JA, Hurbain I, Peden AA, Sviderskaya EV, Stewart A, Robinson MS, Bennett DC, Cutler DF, Bonifacino JS, Marks MS, Raposo G. Functions of adaptor protein (AP)-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol Biol Cell. 2005;16(11):5356–5372. doi: 10.1091/mbc.e05-07-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang AW, Escalante-Semerena JC. cobB function is required for catabolism of propionate in Salmonella typhimurium LT2: evidence for existence of a substitute function for CobB within the 1,2-propanediol utilization (pdu) operon. J Bacteriol. 1996;178(23):7016–7019. doi: 10.1128/jb.178.23.7016-7019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl M, Csernok A, Aydin S, Kreienberg R, Wiesmüller L, Gatz SA. Role of SIRT1 in homologous recombination. DNA Repair. 2010;9(4):383–393. doi: 10.1016/j.dnarep.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Vassallo PF, Simoncini S, Ligi I, Chateau AL, Bachelier R, Robert S, Morere J, Fernandez S, Guillet B, Marcelli M, Tellier E, Pascal A, Simeoni U, Anfosso F, Magdinier F, Dignat-George F, Sabatier F. Accelerated senescence of cord blood endothelial progenitor cells in premature neonates is driven by SIRT1 decreased expression. Blood. 2014;123(13):2116–2126. doi: 10.1182/blood-2013-02-484956. [DOI] [PubMed] [Google Scholar]
- Vassilopoulos A, Fritz KS, Petersen DR, Gius D. The human sirtuin family: evolutionary divergences and functions. Hum Genomics. 2011;5(5):485–496. doi: 10.1186/1479-7364-5-5-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba JM, Alcaín FJ. Sirtuin activators and inhibitors. BioFactors (Oxford, England) 2012;38(5):349–359. doi: 10.1002/biof.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu CB, Bemis JE, Disch JS, Ng PY, Nunes JJ, Milne JC, Carney DP, Lynch AV, Smith JJ, Lavu S, Lambert PD, Gagne DJ, Jirousek MR, Schenk S, Olefsky JM, Perni RB. Discovery of imidazo[1,2-b]thiazole derivatives as novel SIRT1 activators. J Med Chem. 2009;52(5):1275–1283. doi: 10.1021/jm8012954. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhu Y, Xing S, Ma P, Lin D. SIRT5 prevents cigarette smoke extract-induced apoptosis in lung epithelial cells via deacetylation of FOXO3. Cell Stress Chaperones. 2015;20(5):805–810. doi: 10.1007/s12192-015-0599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Guo Y, Gao J, Yuan X. Tumor-suppressive function of SIRT4 in neuroblastoma through mitochondrial damage. Cancer Manag Res. 2018;10:5591–5603. doi: 10.2147/CMAR.S172509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Li J, Fu L, Gai J, Guan J, Li Q. SIRT4 enhances the sensitivity of ER-positive breast cancer to tamoxifen by inhibiting the IL-6/STAT3 signal pathway. Cancer Med. 2019;8(16):7086–7097. doi: 10.1002/cam4.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Wang Z, Li X, Liu B, Liu M, Liu L, Chen S, Ren M, Wang Y, Yu M, Wang B, Zou J, Zhu WG, Yin Y, Gu W, Luo J. SHMT2 Desuccinylation by SIRT5 Drives Cancer Cell Proliferation. Can Res. 2018;78(2):372–386. doi: 10.1158/0008-5472.CAN-17-1912. [DOI] [PubMed] [Google Scholar]
- Yu H, Ye W, Wu J, Meng X, Liu RY, Ying X, Zhou Y, Wang H, Pan C, Huang W. Overexpression of sirt7 exhibits oncogenic property and serves as a prognostic factor in colorectal cancer. Clin Cancer Res . 2014;20(13):3434–3445. doi: 10.1158/1078-0432.CCR-13-2952. [DOI] [PubMed] [Google Scholar]
- Zhang S, Chen P, Huang Z, Hu X, Chen M, Hu S, Hu Y, Cai T. Sirt7 promotes gastric cancer growth and inhibits apoptosis by epigenetically inhibiting miR-34a. Sci Rep. 2015;5:9787. doi: 10.1038/srep09787. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhang M, Wu J, Sun R, Tao X, Wang X, Kang Q, Wang H, Zhang L, Liu P, Zhang J, Xia Y, Zhao Y, Yang Y, Xiong Y, Guan KL, Zou Y, Ye D. SIRT5 deficiency suppresses mitochondrial ATP production and promotes AMPK activation in response to energy stress. PloS one. 2019;14(2):e0211796. doi: 10.1371/journal.pone.0211796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Wang C, Tian Y, Yao Y, Mao J, Wang H, Li Z, Xu Y, Ye M, Wang L. SIRT5 promotes hepatocellular carcinoma progression by regulating mitochondrial apoptosis. J Cancer. 2019;10(16):3871–3882. doi: 10.7150/jca.31266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar T, Khosla S, Ramakrishna G (2016) Increased expression of SIRT2 is a novel marker of cellular senescence and is dependent on wild type p53 status. Cell cycle (Georgetown,Tex.), 15(14): 1883–1897. 10.1080/15384101.2016.1189041 [DOI] [PMC free article] [PubMed]
- Greene KS, Lukey MJ, Wang X, Blank B, Druso JE, Lin MJ, Stalnecker CA, Zhang C, Negrón Abril Y, Erickson JW, Wilson KF, Lin H, Weiss RS, Cerione RA (2019) SIRT5 stabilizes mitochondrial glutaminase and supports breast cancer tumorigenesis. Proc Natl Acad Sci USA 116(52), 26625–26632. Advance online publication. 10.1073/pnas.1911954116 [DOI] [PMC free article] [PubMed]
- Ho L, Titus AS, Banerjee KK, George S, Lin W, Deota S, Saha AK, Nakamura K, Gut P, Verdin E, Kolthur-Seetharam U (2013) SIRT4 regulates ATP homeostasis and mediates a retrograde signaling via AMPK. Aging 5(11), 835–849. 10.18632/aging.100616 [DOI] [PMC free article] [PubMed]
- Schuetz A, Min J, Antoshenko T, Wang CL, Allali-Hassani A, Dong A, Loppnau P, Vedadi M, Bochkarev A, Sternglanz R, Plotnikov AN (2007) Structural basis of inhibition of the human NAD+-dependent deacetylase SIRT5 by suramin. Structure (London, England: 1993), 15(3): 377–389. 10.1016/j.str.2007.02.002 [DOI] [PubMed]
- Van Meter M, Mao Z, Gorbunova V, Seluanov A (2011) SIRT6 overexpression induces massive apoptosis in cancer cells but not in normal cells. Cell cycle (Georgetown, Tex.), 10(18): 3153–3158. 10.4161/cc.10.18.17435 [DOI] [PMC free article] [PubMed]
- Wilking-Busch MJ, Ndiaye MA, Huang W, Ahmad N (2017) Expression profile of SIRT2 in human melanoma and implications for sirtuin-based chemotherapy. Cell Cycle (Georgetown, Tex.), 16(6): 574–577. 10.1080/15384101.2017.1288323 [DOI] [PMC free article] [PubMed]
- Yan WW, Liang YL, Zhang QX, Wang D, Lei MZ, Qu J, He XH, Lei QY, Wang YP (2018) Arginine methylation of SIRT7 couples glucose sensing with mitochondria biogenesis. EMBO Rep 19(12): e46377. 10.15252/embr.201846377 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analyzed during the current study are available from the corresponding author on reasonable request as per the journal guidelines.