Abstract
Objective:
To identify specific white matter tracts (WMTs) whose disruption is associated with the severity of Neurogenic Lower Urinary Tract Dysfunction (NLUTD) in two independent cohorts of women with MS and NLUTD.
Methods:
Cohort 1 consisted of twenty-eight women with MS and NLUTD. The validation cohort consisted of ten women with MS and NLUTD. Eleven healthy women served as controls. Participants of both MS cohorts had the same inclusion and exclusion criteria. Both MS cohorts and the healthy controls underwent the same clinical assessment and fMRI protocol, except that the validation MS cohort underwent 7-Tesla fMRI scan. Fifteen WMTs (six coursing to relevant brainstem areas) involved in bladder control were a priori regions of interest (ROI). Spearman’s correlation test was performed between each the Fractional Anisotropy (FA) and Mean Diffusivity (MD) of each WMT and the clinical parameters.
Results:
Overall, we found a very high degree of overlap (100% of a priori ROI) in the tracts identified by our correlation analysis as having the greatest contribution to NLUTD symptoms in MS women. The Right Inferior Cerebellar Peduncle, Left Posterior Limb of Internal Capsule, and Left Superior Cerebellar Peduncle displayed significant associations to the greatest number of clinical parameters.
Conclusions:
Our correlation analysis supports the role of specific WMT disruptions in the contribution of symptoms in women with MS and NLUTD, as confirmed in two independent MS cohorts.
Keywords: Multiple Sclerosis, Neurogenic Lower Urinary Tract Dysfunction, Magnetic Resonance Imaging, Diffusion Tensor Imaging
Introduction
Multiple Sclerosis (MS) is an autoimmune inflammatory disease characterized by central nervous system (CNS) damage1. This damage is caused by two main processes, the axonal microstructural injury and demyelination of neuronal axons, and the subsequent inflammation due to this breakdown. Chronic damage prevents neuronal repair, ultimately causing disruption of pre-existing compact white matter pathways and induction of alternative pathways to adapt and compensate to some level for the lost function2. MS has a sporadic pattern of development and progression, resulting in many random lesions involving the CNS which can affect motor, sensory, and even bladder function. The global prevalence of MS is 1 in 10,000, with developed areas of the world such as North America and Europe experiencing higher rates3. Symptoms usually appear between 20 and 40 years of age, and women have a 3 times higher chance of developing MS than men.
One of the common associations of MS is neurogenic lower urinary tract dysfunction (NLUTD)4. In fact, overactive bladder symptoms in MS patients are correlated with longer disease duration and increased physical disability. Furthermore, treatment satisfaction of these symptoms remains low (~33%)5,6. Magnetic Resonance Imaging (MRI) is the most important non-invasive tool for diagnosing, monitoring progression, and assessing treatment in MS. Nevertheless, MRI findings and their association with clinical manifestation remains modest, due to conventional MRI’s lack of specificity for MS lesions, which can be attributed to inaccurate estimates of damage and difficulty defining mechanism of injury and recovery7. Consequently, our understanding of the specific white matter tracts (WMTs) that are involved in higher neural control of the lower urinary tract (bladder, bladder neck and urethral sphincter) function is quite limited. However, with the technological advances in the last 20 years and the development of Diffusion Tensor Imaging (DTI) we can now overcome the shortcomings of standard MRI that often show normal-appearing white matter in MS patients.
The objective of this study is to identify specific white matter tracts whose disruption (as identified on DTI) is associated with the degree of severity of NLUTD in MS (as identified by objective and subjective clinical parameters). Furthermore, we validated our findings in a separate and independent cohort of MS women with NLUTD.
Methods:
Study Design: We conducted post-hoc analysis of two completed pilot trials NCT03033355 and NCT03574610. No power analyses were conducted for these pilot trials, but most fMRI studies evaluating bladder function enroll between 8–12 subjects on average8. Institutional review board approval was obtained.
Study Population:
Cohort 1: Twenty-eight adult women (>18 years of age) with clinically stable MS for ≥3 months before screening and an Expanded Disability Status Score (EDSS) ≤ 6.5 with NLUTD were recruited for the study. Other exclusion criteria included severe debilitating disease, pregnancy or planning to become pregnant, nursing, MRI contraindications, history of augmentation cystoplasty, and presence of other neurological diseases. Patients were screened and recruited by referrals to fellowship-trained neuro-urologists at our tertiary care center and underwent a clinic Urodynamic Study (UDS). Additionally, participants were asked to complete the following questionnaires and assessments: Urogenital Distress Inventory (UDI-6) and Incontinence Impact Questionnaire (IIQ-7), MRI Safety Screening Questionnaire and Hamilton Anxiety Rating Scale, Post-void residual (PVR) volume and urinalysis prior to enrollment.
Cohort 2: Ten women with MS and NLUTD were recruited from our tertiary Neurourology Clinic. The same inclusion and exclusion criteria used in cohort 1 were used for enrollment and all participants underwent the same clinical assessment and fMRI protocol. All neuroimaging data was obtained with a 7-Tesla MRI scanner.
Healthy Controls (HC): Eleven Healthy volunteer women with no lower urinary tract symptoms were recruited for this study. All individuals completed validated LUT questionnaires, clinic urodynamic testing and underwent a similar fMRI scan as the MS women with NLUTD. All control neuroimaging data was obtained with the 3-Tesla MRI scanner.
Image-Acquisition:
Neuroimaging data for cohort 1 and HCs was collected using a 3-Tesla MRI scanner, while data for cohort 2 was obtained with a 7-Tesla MRI scanner.
3-Tesla: DTI data were acquired (32 uniformly-distributed diffusion gradient pulse orientations, one b0 image) for each subject using the standard MRI single shot echo planar imaging pulse sequence available on the Philips 3 Tesla Ingenia scanner. (Matrix was 128 × 128, slick thickness 2.5 mm, Field-of-View (FOV) was 224 × 224 cm2, 16 directions, b-value = 800 s/mm2, total scan time of 7 minutes and 22 seconds).
7-Tesla: DTI were acquired (Matrix was 158 × 158, slick thickness 1.4 mm, Field-of-View (FOV) was 220 × 220 cm2, 64 directions, b-value = 1000 s/mm2, total scan time was 12 minutes and 14 seconds) on the 7-Tesla Siemens MAGNETOM Terra MRI scanner with 32 channel single transmit head coil (3T Siemens MAGNETOM Vida MRI scanner was used in two subjects with contraindications for 7T scanner).
DTI:
DTI uses magnetic field gradients and measures water molecule random translation to construct 3D directional architecture of white matter tracts in the CNS. The major values obtained are Mean Diffusivity (MD), which measures the average displacement of water molecules, and Fractional Anisotropy (FA), which quantifies the fraction of water displacement due to the orientation of the fiber1. In general, a low FA value indicates disorganization of the axon fiber, and low MD indicates well-organized and preserved structure. Water movement parallel to the axon is defined as Axial Diffusivity (AD) and water movement perpendicular to the tract is defined as Radial Diffusivity (RD)1.
ICBM DTI-81 Atlas:
DTI maps were constructed and individual patients were aligned onto the ICBM-DTI-81 white matter atlas, which is a reference coordinate system containing the probabilistic locations of 50 white matter tracts obtained from 81 different individuals9.
WMTs Identified in Literature for their Role in Lower Urinary Tract (LUT) function: A literature search was performed with the key words “white matter tracts” AND “bladder” OR “micturition” OR “voiding” and articles that met the following criteria were selected: published in the English language, within the last 15 years, and that had full text available. Expert opinion was then provided by the senior authors on this manuscript regarding the most relevant articles related to the aim/focus of our manuscript. A four-year follow-up study by Kolsasa et al found that in a cohort of 46 MS patients and 10 healthy controls, all DTI markers (FA, MD, AD, RD) were significantly different in the corpus callosum (CC) and internal capsule, with MS patients generally having a higher MD and lower FA than the healthy controls. The strongest differences were seen in the CC, with these changes remaining 1 year later10. The authors suggest that the CC splenium might be particularly sensitive since it has the highest density of thin axons that are susceptible to injury by MS.10 However, the changes observed were not found to predict disability progression over the 4 year follow-up10.
Tadic et al discovered white matter hyper intensities in the anterior thalamic radiation (ATR) are particularly critical in maintaining continence11. Specifically, the ATR was found to account for 55% of the global white matter hyper-intensity burden in older women with urinary incontinence. The role of ATR in maintaining continence is further supported by its’ course through the medial prefrontal cortex, an area cited by previous studies for its role in maintaining continence11. Similarly, the superior longitudinal fasciculus (SLF) was also found to have 8% of the global White Matter Hyperintensity (WMH) burden and connect to regions activated during bladder filling10. Similarly, Tran et al found a lower mean FA and higher mean MD in both the ATR and SLF in MS patients with voiding dysfunction when compared to MS patients without voiding dysfunction12.
Kunchel et al conducted a study in a cohort of 100 individuals at least 75 years of age with varying levels of incontinence13. They concluded that the Anterior Corona Radiata predicted severity and degree of bother, cingulate gyrus predicted incontinence and severity, whereas cingulate (hippocampal) and superior fronto-occipital fasciculs predicted severity13.
Finally, it is currently thought that key brainstem areas such as the periaqueductal gray (PAG) and Pontine Micturition Center (PMC) play a critical role in the higher neural of the bladder cycle14. Therefore, the 6 tracts listed under the “Tracts in the Brainstem” in the ICMB-DTI-81 atlas (to be discussed later) are also WMTs of interest (Table 2)14.
Table 2.
Specific WMTs of Interest Due to their Course through the Brainstem9
| Corticospinal tracts |
| Medial Lemniscus |
| Medial Longitudinal Fasciculus |
| Inferior Cerebellar Peduncle |
| Middle Cerebellar Peduncle |
| Superior Cerebellar Peduncle |
Statistical analysis:
The open-source software Analysis of Functional NeuroImages (AFNI) was utilized to extract FA and MD values. Spearman’s correlation test was performed between each WMT and clinical parameters (both objective and subjective). In the 3-Tesla cohort, only Spearman’s correlation coefficient magnitudes > 0.3 and statistically significant (p < 0.05) were reported. In the 7-Tesla cohort, only Spearman’s correlation coefficient magnitudes > 0.6 and statistically significant (p<0.05) were reported. For direct cohort vs cohort comparison, a Wilcoxon Ranked Sum test was used as normality could not be assumed. P-values < 0.05 were considered statistically significant.
Results
Cohort 1 versus Healthy Controls
When comparing MS women in cohort 1 as a whole (n=28) to the HC cohort (n=11), the Wilcoxon Ranked Sum test revealed that both the MD and FA of all 50 tracts were significantly different between the two groups (α = 0.05). With regards to FA, the Left Anterior Limb of the Internal Capsule displayed the greatest difference between the two groups, whereas the Right Medial Lemniscus had the greatest difference when measuring MD.
Cohort 1: Association between WMT Disruption and Symptom Severity
Spearman’s correlation analysis revealed that the Left Medial Lemniscus, Middle Cerebellar Peduncle, Left Anterior Limb of the Internal Capsule, and Right Superior Corona Radiata had statistically significant associations to the most number of clinical parameters (Table 5). Figure 2 illustrates scatter plots of two representative tracts. Of note, a significant association with regards to MD did not necessarily co-exist with a significant association with regards to FA in the same tract.
Table 5.
WMTs with Significant Associations to the Greatest Number of Clinical Parameters in Cohort 1.
| WMTs with Correlation to Highest Number of Clinical Variables | |
|---|---|
| Fractional Anisotropy (FA) | |
| White Matter Tract (WMT) | # of Clinical Parameters with Strong Correlation |
| Left Medial Lemniscus | 3 |
| Middle Cerebellar Peduncle | 2 |
| Left Anterior Limb of Internal Capsule | 3 |
| Mean Diffusivity (MD) | |
| Right Superior Corona Radiata | 2 |
Figure 2.
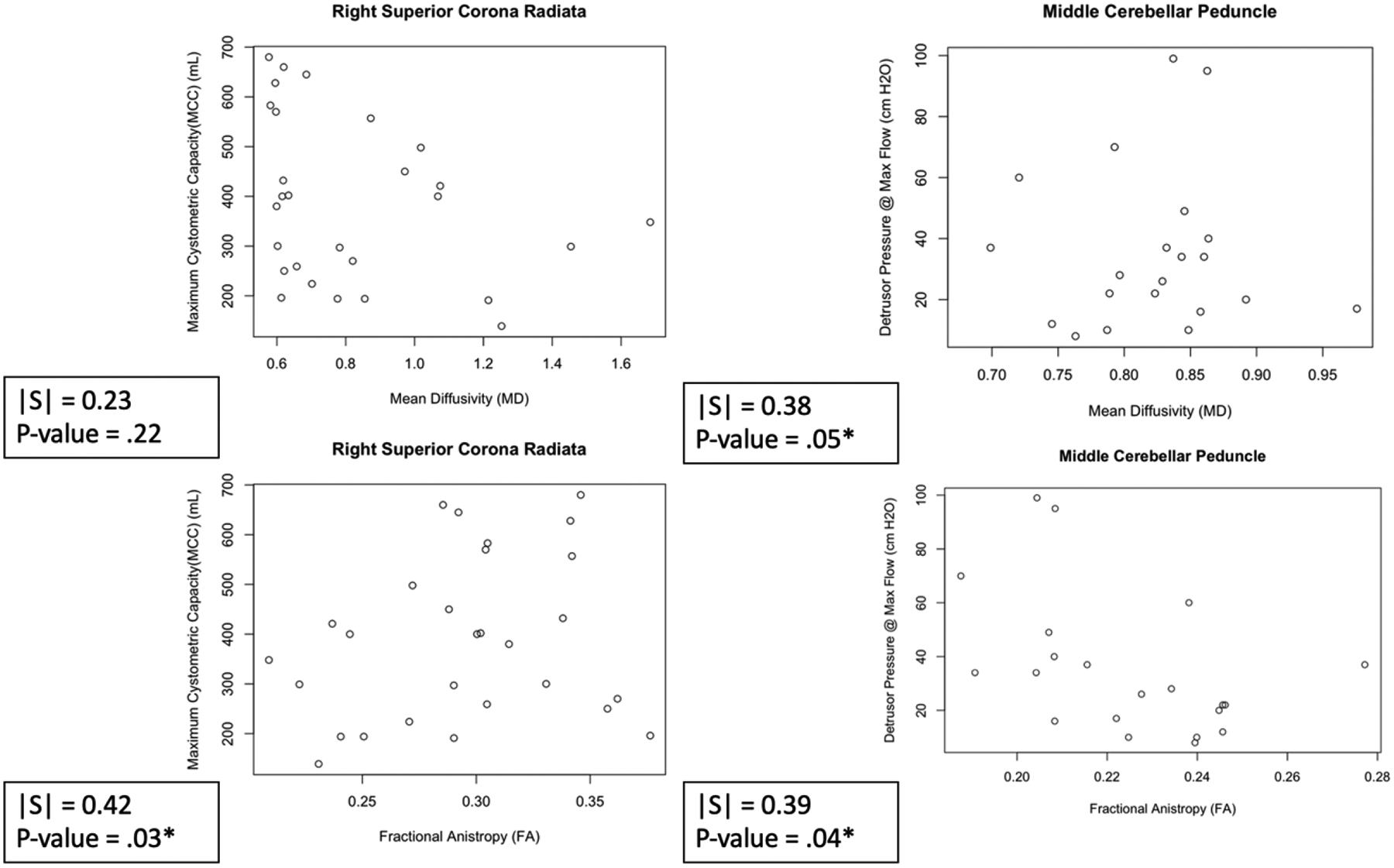
Scatter plots of Representative WMTs and their Relation to Clinical Parameters in Cohort 1. Right Superior Corona Radiata Vs Maximum Cystometric Capacity (Left). Middle Cerebellar Peduncle vs Detrusor Pressure @ Max Flow (Right). On the left, we see the association of white matter integrity and the clinical parameter MCC when using both MD (top) and FA (left). On the right, we see the assocation of white matter integriety and the clinical parameter Detressur Pressure at Max flow when using both MD (top) and FA (left).
Cohort 2 (Validation of our findings in cohort 1): Association between WMTs and Symptom Severity
Spearman’s correlation analysis revealed that in MS Cohort 2, the Left Posterior Limb of the Internal Capsule, Right Superior Fronto-Occipital Fasciculus, and the Right Anterior Limb of Internal Capsule and Left Tapatum had statistically significant associations in their MD/FA with the greatest number of clinical variables. Figure 3 displays the scatterplots of two representative tracts. Of note, the magnitude of Spearman’s correlation was strong in significant tracts (> 0.7) compared to MS Cohort 1.
Figure 3.
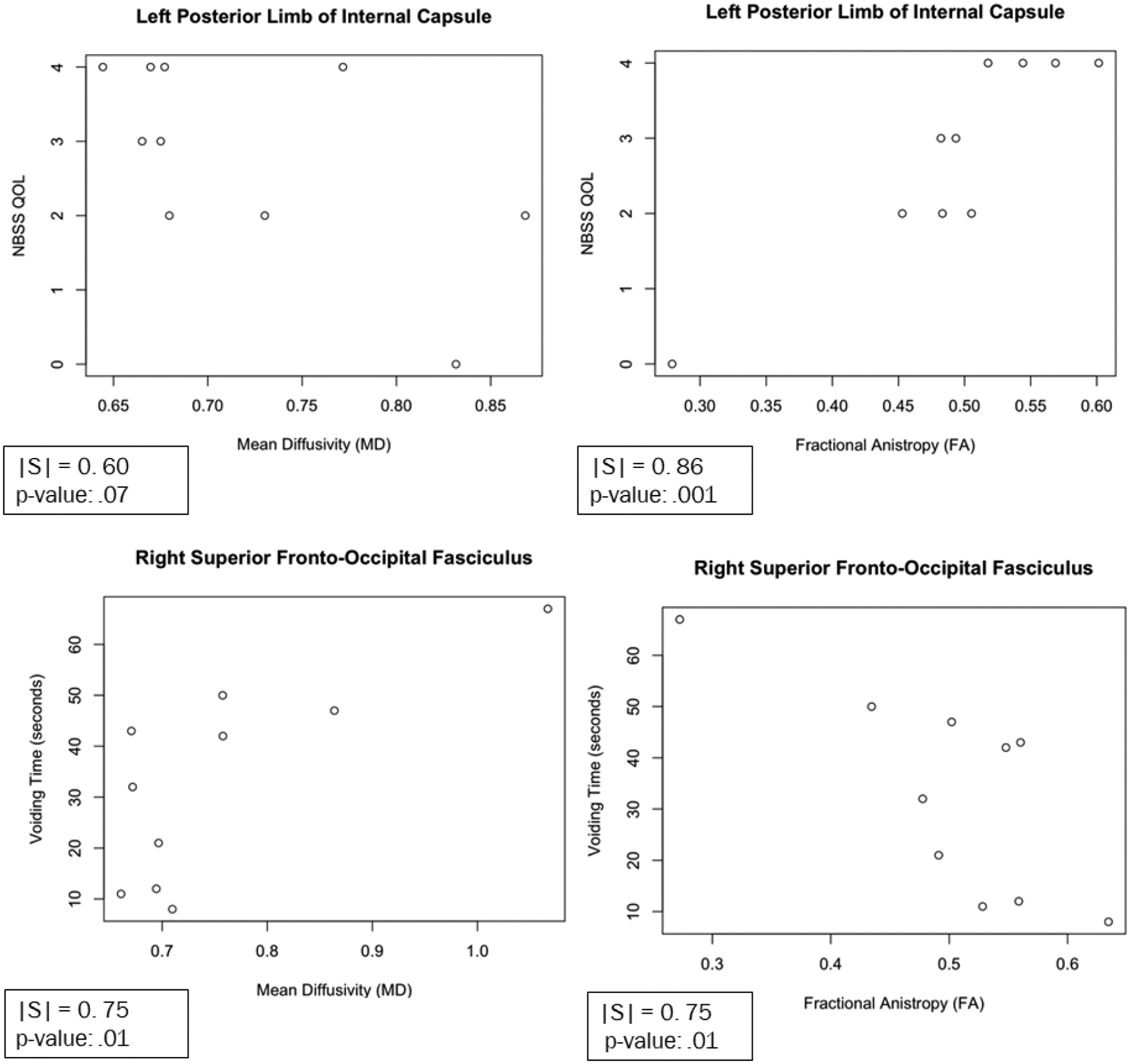
Scatter plots of Representative WMTs and their Relation to Clinical Parameters. Left Posterior Limb of Internal Capsule vs Neurogenic Bladder Symptom Score (NBSS) QOL Score (Top). Right Superior Fronto-Occipital Fasciculus Vs Voiding Time (seconds) (Bottom).
Discussion:
This study assessed the extent of disruption of a number of white matter tracts between two MS cohorts of women with NLUTD and healthy women controls using two different imaging platforms (3-Tesla and 7-Tesla) to identify specific white matter regions contributing to NLUTD in MS women. Our study builds on the findings of Kolasa et al and Tran et al. The overall principle of Kolasa et al’s analysis is similar to ours: we are trying to assess the reliability of utilizing diffusion tensor imaging (DTI) data in assessing the degree of NLUTD. However, our study looked at two separate MS cohorts, on two separate imaging platforms (3T and 7T). Most notably, our analysis focused on specific white matter tracts, rather than bigger regions of the corpus callosum and internal capsule. The 50 WMTs listed in Figure 1, not only look at the CC and IC, but many more regions of the white matter skeleton. Furthermore, Tran et al separated a single MS cohort into voiders and non-voiders based on their need to self-catheterize or have a PVR volume of 40% or more of their MCC. They then evaluated the differences between FA and MD of only 2 WMTs (anterior thalamic radiation and superior longitudinal fasciculus). The ICMB-DTI-81 atlas was not used in Tran et al’s analysis and no correlation analysis was performed. We utilized 2 different MS cohorts, and looked for associations between 50 different WMTs found in the ICMB-DTI-81 atlas and numerous different clinical parameters that can indicate the degree of NLUTD.
Figure 1.
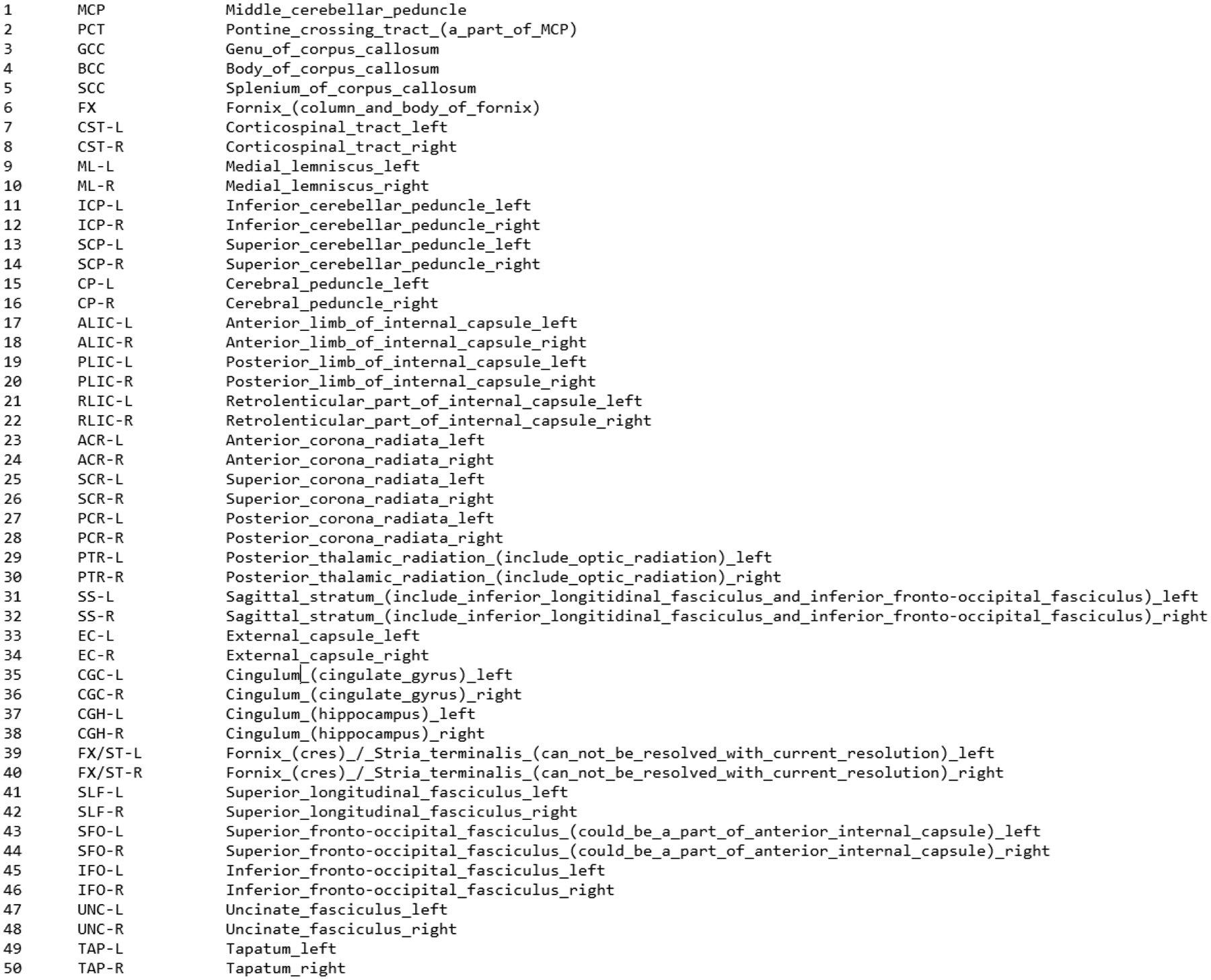
List of the 50 WMTs that are identified as part of the ICMB-DTI-81 Atlas.
MS vs Healthy Control:
In comparing the overall MS Cohort 1 to the Healthy Controls, we found that each of the 50 WMTs were significantly different between the two groups. Such a finding emphasizes that a direct comparison assessing differences in WMT in patients with neurogenic bladder (like MS patients) and healthy controls is not enough, and that correlation studies must be performed in order to truly gain further insight into the specific areas of the white matter skeleton that contribute to NLUTD in patients with neurogenic bladder.
WMT Disruption Association with NLUTD:
MS Cohort 1:
Analysis of WMT disruption in Cohort 1 (Table 5) revealed that the Left Medial Lemniscus, Middle Cerebellar Peduncle, Left Anterior Limb of Internal Capsule, and Right Superior Corona Radiata had associations with at least 2 clinical parameters. It is important to note that 2 out of these 4 WMTs (the left medial lemniscus, and the middle cerebellar peduncle) are part of the “tracts in the brainstem” group of the ICBM-DTI-81 atlas9. This finding supports our current understanding of the key centers of higher neural control of the bladder cycle being housed in the pons.
MS Cohort 2 (validation cohort):
MS Cohort 2 has allowed for a very unique opportunity to validate the findings observed in the larger 3- Tesla MS Cohort 1. Overall, we found a very high degree of overlap (100% of a priori ROIs listed in Table 1) in the tracts identified by our correlation analysis (Tables 5 and 6) as having the greatest contribution to NLUTD in MS women. Here, we again observed that 2 out of 9 tracts (Right Inferior Cerebellar Peduncle and Left Posterior Limb of Internal Capsule) displaying significant associations to the greatest amount of clinical parameters within the FA group, and 1 out of 5 (Left Superior Cerebellar Peduncle) within the MD group were part of the “tracts in the brainstem” group of the ICBM-DTI-81 atlas. In this cohort, significant associations ranged from 0.6–0.9, further reinforcing the applicability and need of the higher resolution and lower signal-to-noise ratio offered by the 7-Tesla platform.
Table 1.
WMTs of Interest as Identified by Previous Studies for their role in the neural control of the bladder cycle.15. Literature search was performed with key words “white matter tracts” AND “bladder” OR “micturition” OR “voiding” and articles were selected for being published in English language, within the last 15 years, and for having full text available. Expert opinion was then provide by the senior authors on this manuscript regarding the most relevant articles related to the aim/focus of our manuscript.
| Region of Interest (ROI) | Known Functions |
|---|---|
| Corpus Callosum | Coordination of sensory, motor, and auditory signaling between hemispheres |
| Internal Capsule | Main tract housing projection fibers that connect cortical areas with the brainstem, cerebellum, and spinal cord |
| Anterior Thalamic Radiation | Relay tract of sensorimotor data to the cortex |
| Superior Longitudinal Fasciculus | Connects frontal lobe to parietal, temporal, and occipital lobe. Thought to coordinate selection of different motor tasks based on appropriate conditions |
| Uncinate Fasciculus | Function unclear; impairment can lead to issues with memory |
| Inferior Fronto-Occipital Fasciculus | Integrates auditory and visual association cortices with prefrontal cortex |
| Inferior Longitudinal Fasciculus | Major player in visual memory |
| Anterior Corona Radiata | Signaling between cerebral cortex and structures within brainstem and spinal cord |
| Cingulum | Visceromotor and skeletomotor control, memory access |
| Superior Fronto-Occipital Fasciculus | Spatial awareness and symmetric processing |
Table 6.
WMTs with Significant Associations to the Greatest Number of Clinical Parameters in Cohort 2.
| Fractional Anisotropy (FA) | |
|---|---|
| White Matter Tract (WMT) | # Of Clinical Parameters with Significant Correlation |
| Right Inferior Cerebellar Peduncle. Right Superior and Right Posterior Corona Radiata, Right Cingulum (Hippocampus), Left Superior Fronto-Occipital Fasciculus | 4 |
| Right Anterior Limb of Internal Capsule | 5 |
| Left Posterior of Limb of Internal Capsule | 9 |
| Right Superior Front-Occipital Fasciculus | 7 |
| Right Tapatum | 5 |
| Mean Diffusivity (MD) | |
| Left Superior Cerebellar Peduncle | 4 |
| Right Posterior Thalamic Radiation | 4 |
| Right Superior Fronto-Occipital Fasciculus | 6 |
| Left Superior Fronto-Occipital Fasciculus | 4 |
| Genu of Corpus Callosum | 4 |
Directionality of Associations:
FA and MD are the two DTI parameters used to assess the level of disruption of the white matter tracts (WMTs) and their association to NLUTD in our patient population. A WMT with a high FA value was considered to have intact transmission of signals along its axons. A tract with a low MD value was considered to have a less dispersed signal transmission. In other words, high FA and low MD values generally are thought to represent lesser disruption of the WMT, and they were used to correlate the white matter disruption of each patient with the severity of NLUTD. However, it is difficult to make generalized statements with regards to positive and negative associations with clinical parameters. FA and MD are the two DTI parameters used to assess the level of disruption of the white matter tracts. For example, with a clinical parameter such as the UDI-6, a higher score is associated with greater severity of symptoms. Therefore, WMTs where MD is positively correlated to the UDI-6 and FA is negatively correlated with UDI-6 is thought to show signs of demyelination and axonal degeneration.
Limitations and Conclusions:
Here, we have identified several key WMTs that seem to be directly contributing to NLUTD in MS patients. More notably, there is a very strong overlap between the WMTs identified by our analysis and those identified by previous studies. Our study has multiple strengths: first, an inter-disciplinary team with expertise in MS, neurology, urology, neurosurgery, neuroimaging, and physics have been involved with the hypothesis, protocol design, data analysis, and data interpretation; second, we have been able to find agreement in two well-designed prospective cohorts of MS women with NLUTD on two different imaging platforms (3-T and 7-T); third, it demonstrated the strong associations observed with the 7-Tesla scanner underscore the superior image quality achieved on this imaging platform specifically in MS. Although MS is a dynamic and heterogeneous disorder, here we were able to recruit a more homogenous group of participants with similar gender, age, and MS disability. Additionally, we excluded men from the study to avoid differences between the sexes as a confounding factor such as prostate and bladder outlet pathologies. However, an important limitation exists: We must confirm that the regions of the white matter skeleton that correlate to severity of NLUTD in MS patients are localized to the same areas of the greatest white matter lesion burden (including the size and activity), ensuring that these findings are not in fact due to other confounding factors such as age-related changes, prior trauma, or vascular insults such as stroke. Additionallly, We acknowledge that a cutoff of 0.3 for Spearman’s coefficient is generally considered a weak correlation. However, our rationale for using a low cutoff was that if we can validate that the same tracts found to have a weak association on the 3T cohort, are also the ones to have a strong association on the 7T cohort, then we can feel more strongly that there was in fact a significant association there. Although we acknowledge that this stronger association could be in fact be representative of a smaller sample size, it cannot be excluded that this finding was actually due to the higher resolution of brainstem imaging allowed by the 7T MRI. We also acknowledge that of course the effect size for a Spearman’s test with a smaller sample size will be smaller than one with a larger cohort. Upon literature review of relevant sources, we have found that sample sizes of n=10 can be utilized to perform Spearman’s correlation analysis16. Furthermore, we propose that subsequent studies with a larger cohort on the 7T MRI machine would have to be conducted to confirm our findings. Finally, there is always a level of compelxity associated with the exact interpretation of FA and MD values in DTI analyses. For example, studies have shown that the chronicity of MS has a significant effect on DTI parameters. Early in MS, AD decreases and RD increases due to axonal degeneration and demyelination. However, over time, chronic inflammation, scarring, and gliosis actually lead to increased AD in chronic MS. However, by controlling our cohorts for MS duration and age of participants, we have attempted to minimize the influence of these confounding factors in the interpretation of our MD and FA values. Furthermore, by confirming our findings in two different cohorts, on two different imaging platforms, allows us to further minimize misinterpretation of our findings.
In conclusion, our study has provided strong support for the role of specific WMTs (100% of the WMTs listed in Table 1) in the development and severity of NLUTD in MS women, as well as identified clinical parameters that should be of key interest in assessing the overall and bladder-associated disability of MS. We are one step closer to a stage where we can reliably utilize DTI data as a biomarker for NLUTD diagnosis, progression, and personalization of treatments in MS.
Table 3.
Clinical Parmeters Evaluated in Correlated Analysis
| MS duration (years) | Maximum Cystometric Compliance |
|---|---|
| UDI-6 total score* | % Post-Void Residual/Maximum Cystometric Capacity (PVR/MCC) |
| UDI-6, Q2 (UUI) | Compliance |
| UDI-6, Q5 (voiding) | HAM-Depression* |
| IIQ-7* | AUASS |
| HAM-Anxiety* | AUASS, Q3 (continuous stream)* |
| Detrusor pressure at max flow | AUASS, Q5 (weak stream) |
| Post-Void Residual | AUASS, Q7 (nocturia) |
| AUASS, Q8 (quality of life)* | NBSS Incontinence* |
| Nomogram percentile | NBSS Consequences |
| NBSS Storage & Voiding | NBSS Total |
| NBSS Quality of Life | Uroflow PVR |
| Voided Volume | %PVR/BV |
| Uroflow Bladder Volume | Mean Velocity |
| Maximum Flow Velocity | Flow Time |
| Voiding time | EDSS Score* |
UDI: Urinary Distress Inventory, IIQ-7: Incontinence Impact Questionnaire, HAM: Hamilton Rating Scale, AUASS: AUA Symptom Score, NBSS: Neurogenic Bladder Symptom Score, EDSS: Expanded Disability Status Scale.
Table 4.
Participants’ Demographics
| Cohort 1 (n=28) | Cohort 2 (n=10) | Healthy Control (n=11) | |
|---|---|---|---|
| Gender | Female | Female | Female |
| Mean Age | 50.63 | 53.4 | 33.5 |
| Mean IIQ-7 score | 8.57 | 10.4 | 0 |
| Mean UDI-6 Score | 11.25 | 11.6 | 0.1 |
| Mean MS duration (years) | 15.82 | 16.3 | N/A |
Acknowledgments
The datasets generated during and/or analyzed during the current study are available from corresponding author on reasonable request. We conducted post-hoc analysis of two completed pilot trials NCT03033355 and NCT03574610. Institutional Review Board of Houston Methodist Hospital approved both studies. All participants were consented with the understanding that participation was voluntary and they were able to withdraw at any time. Funding provided by K23DK118209, National Institute of Health, NIDDK (RK). Houston Methodist Clinician Scientist Award (RK).
Footnotes
Conflicts of Interest: The authors report no conflict of interest.
References:
- 1.Aung WY, Mar S, Benzinger TL. Diffusion tensor MRI as a biomarker in axonal and myelin damage. Imaging Med. Oct 01 2013;5(5):427–440. doi: 10.2217/iim.13.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chard DT, Alahmadi AAS, Audoin B, et al. Mind the gap: from neurons to networks to outcomes in multiple sclerosis. Nat Rev Neurol. March 2021;17(3):173–184. doi: 10.1038/s41582-020-00439-8 [DOI] [PubMed] [Google Scholar]
- 3.Compston A, Coles A. Multiple sclerosis. Lancet. Apr 06 2002;359(9313):1221–31. doi: 10.1016/S0140-6736(02)08220-X [DOI] [PubMed] [Google Scholar]
- 4.Tornic J, Panicker JN. The Management of Lower Urinary Tract Dysfunction in Multiple Sclerosis. Curr Neurol Neurosci Rep. June 28 2018;18(8):54. doi: 10.1007/s11910-018-0857-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang G, Marrie RA, Fox RJ, et al. Treatment satisfaction and bothersome bladder, bowel, sexual symptoms in multiple sclerosis. Mult Scler Relat Disord. Feb 2018;20:16–21. doi: 10.1016/j.msard.2017.12.006 [DOI] [PubMed] [Google Scholar]
- 6.Mahajan ST, Patel PB, Marrie RA. Under treatment of overactive bladder symptoms in patients with multiple sclerosis: an ancillary analysis of the NARCOMS Patient Registry. J Urol. Apr 2010;183(4):1432–7. doi: 10.1016/j.juro.2009.12.029 [DOI] [PubMed] [Google Scholar]
- 7.Filippi M, Absinta M, Rocca MA. Future MRI tools in multiple sclerosis. J Neurol Sci. Aug 15 2013;331(1–2):14–8. doi: 10.1016/j.jns.2013.04.025 [DOI] [PubMed] [Google Scholar]
- 8.Desmond JE, Glover GH. Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. J Neurosci Methods. Aug 30 2002;118(2):115–28. doi: 10.1016/s0165-0270(02)00121-8 [DOI] [PubMed] [Google Scholar]
- 9.Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. Apr 01 2008;40(2):570–582. doi: 10.1016/j.neuroimage.2007.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolasa M, Hakulinen U, Brander A, et al. Diffusion tensor imaging and disability progression in multiple sclerosis: A 4-year follow-up study. Brain Behav. January 2019;9(1):e01194. doi: 10.1002/brb3.1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tadic SD, Griffiths D, Murrin A, Schaefer W, Aizenstein HJ, Resnick NM. Brain activity during bladder filling is related to white matter structural changes in older women with urinary incontinence. Neuroimage. Jul 15 2010;51(4):1294–302. doi: 10.1016/j.neuroimage.2010.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran K, Karmonik C, Boone TB, Khavari R. Are White Matter Tract Integrities Different in Multiple Sclerosis Women With Voiding Dysfunction? Female Pelvic Med Reconstr Surg. 01 January 2021;27(1):e101–e105. doi: 10.1097/SPV.0000000000000830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuchel GA, Moscufo N, Guttmann CR, et al. Localization of brain white matter hyperintensities and urinary incontinence in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. Aug 2009;64(8):902–9. doi: 10.1093/gerona/glp037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy HA, Griffiths DJ, Aziz TZ, Green AL, Menke RAL. Investigation of urinary storage symptoms in Parkinson’s disease utilizing structural MRI techniques. Neurourol Urodyn. April 2019;38(4):1168–1175. doi: 10.1002/nau.23976 [DOI] [PubMed] [Google Scholar]
- 15.Wycoco V, Shroff M, Sudhakar S, Lee W. White matter anatomy: what the radiologist needs to know. Neuroimaging Clin N Am. May 2013;23(2):197–216. doi: 10.1016/j.nic.2012.12.002 [DOI] [PubMed] [Google Scholar]
- 16.May Justin and Looney Stephen W.. Sample Size Charts for Spearman and Kendall Coefficients. Journal of biometrics & biostatistics 11 (2020): 1–7. doi: 10.37421/jbmbs.2020.11.440 [DOI] [Google Scholar]


