Abstract
Background
Tranexamic acid (TXA) is an antifibrinolytic that has shown some promise in improving outcomes in traumatic brain injury (TBI), but only when given early after injury. We examined the association between timing of prehospital TXA administration and outcomes in patients with moderate to severe TBI.
Methods
Patients enrolled in the multi-institutional, double-blind randomized Prehospital TXA for TBI Trial with blunt or penetrating injury and suspected TBI (GCS </=12, SBP >/=90) who received either a 2g TXA bolus or a 1g bolus plus 1g 8h infusion within 2 hours of injury were analyzed. Outcomes were compared between early administration (<45 minutes from injury) and late administration (≥ 45 minutes from injury) using a Chi Square, Fischers Exact Test, t-test, or Mann Whitney U test as indicated. Logistic regression examined time to drug as an independent variable. P < 0.05 was considered significant.
Results
649 Patients met inclusion criteria (354 early and 259 late). 28-day and 6-month mortality, 6-month Glasgow Outcome Scale - Extended (GOSE) and disability rating scale scores were not different between early and late administration. Late administration was associated with higher rates of DVT (0.8 vs 3.4%, p=0.02), cerebral vasospasm (0% vs 2%, p=0.01), as well as prolonged EMS transport and need for a prehospital airway (p<0.01)
Conclusions
In patients with moderate or severe TBI who received TXA within two hours of injury, no mortality benefit was observed in those who received treatment within 45 minutes of injury, although lower rates of select complications were seen. These results support protocols that recommend TXA administration within 45 minutes of injury for patients with suspected TBI.
Level of Evidence
Level 1
Therapeutic
Keywords: Prehospital resuscitation, Tranexamic Acid, Traumatic Brain Injury
Background
Traumatic brain injury (TBI) can have devastating consequences. There have been no new pharmacologic therapies implemented in the past several decades to improve outcomes in patients with head injuries. Tranexamic acid (TXA) is a lysine analogue thought to work by binding to activated plasmin and preventing activation of the fibrinolysis pathway1,2. It was originally discovered in the 1960s3 in a search for a medication to decrease obstetric bleeding. It was approved by the FDA in the 1980s and became a WHO essential medicine in 20094. The WOMEN trial confirmed a mortality benefit in the obstetric population5. In the context of trauma, the CRASH-2 study showed TXA administration within 3 hours of injury improved mortality in patients with traumatic hemorrhage6. The benefit on mortality was greater the earlier TXA was administered. A nested trial within the CRASH-2 study showed a trend towards decreased intracranial hemorrhage (ICH) expansion and mortality in patients with TBI7. Decreased ICH expansion was confirmed by an independent study8. Subsequently the CRASH-3 study showed improvement in head injury related death in patients with mild to moderate TBI, also with greater benefit with earlier administration9. Both the CRASH studies examined estimated time intervals from injury </= 1 hour, >1 hour </= 3 hours and >3 hours with evidence of greater benefit in the earlier time points. However, both of these studies looked at in hospital administration and included many centers without the efficient and advanced prehospital systems found in North America that allow for advanced care to be provided early after injury. Prehospital use of TXA for traumatic hemorrhage has been studied and is generally considered safe and effectively administered2,10–12, data on prehospital TXA for TBI and the optimal timing for administration is limited. The prehospital TXA in TBI trial permitted study of the optimal timing of TXA administration when very early delivery is possible. This study also differs from the CRASH studies because we included a single 2 bolus dose arm and it was this group that showed a survival benefit and 6 month Disability Rating Score benefit. We hypothesized that earlier prehospital administration of TXA would lead to improved outcomes in TBI patients.
Methods
The Prehospital TXA for TBI Trial was a multi-institutional, double-blinded randomized phase II clinical trial performed at 20 centers and 39 emergency medical services agencies in the US and Canada. Data from May 2015 to November 2017 were analyzed. Patients in the prehospital setting who had blunt or penetrating traumatic mechanisms consistent with TBI, GCS =/<12, SBP >/=90 and received TXA (either a 2g bolus or a 1g bolus plus 1g 8h infusion, referred to below as 2g and 1g group respectively) within 2 hours of injury were included in the analysis. The power analysis of the original study was based on the combined dosing groups but a pre-planned secondary analysis also included evaluating the dosing groups separately. The primary paper revealed that the 2-gram dosing group was associated with improved survival and 6 month Disability Rating Scores in patients with intracranial hemorrhage. This follow-up paper is designed the same way as the primary paper justifying evaluating the dosing groups in a combined manner as well as separately. Details of sample size calculation, primary and secondary outcome measure specification and details of data collection have been previously reported13, following CONSORT guidelines. Early administration was defined as less than 45 minutes from the estimated time of injury, and late administration as 45 minutes or greater from time of injury. Forty-five minutes was chosen as the break point because it approximates the median time to delivery of TXA in the study. Time of injury was defined as the time the call was received by EMS dispatch. Outcomes were compared using a Chi Square, Fischers Exact Test, t test, or Mann Whitney U test as indicated. Logistic Binomial and ordinal regression were also performed with time to drug as an independent variable. Holm-Bonferroni multiple comparison correction was utilized due to the large number of variables evaluated. Statistical analysis was performed utilizing SPSS v28 (IBM Corp, Chicago IL). A power analysis was completed for the original study, but was not repeated for this post-hoc sub analysis as post-hoc power analysis is not appropriate in the retrospective analysis of a randomized trial.
Results
649 patients met inclusion criteria. 354 received TXA less than 45 minutes from injury and 295 received it between 45 minutes and 2 hours from time of injury. The mean time from injury was 47 minutes for both 1g and 2g groups. There were 309 patients in the 1g group and 340 in the 2g group. For the combined (1g and 2g) groups, discharge disposition, Glasgow Outcome Scale - Extended (GOSE) and disability rating scores were not significantly different between early and later intervention. Seizures were increased in the early administration group which remained significant when head AIS was used as a covariate (4.5 vs 1.7%, p=0.048). Patients who suffered seizures did not have worse outcomes with respect to mortality or long-term neurologic outcomes. Late administration was associated with higher rates of development of DVT (0.8 vs 3.4%, p=0.021), cerebral vasospasm (0% vs 2%, p=0.007), as well as both prolonged EMS transport time and need for a prehospital airway (p<0.001) indicating that need for emergent interventions were often the cause for delay of TXA administration. Ventilator-free days were decreased in the earlier administration group (21+/− 10 vs 20+/−10 p = 0.01). In the 1g group, results were similar but seizures and DVTs, although similar in proportions, failed to achieve significance. 2g groups had similar outcomes to the 1g analysis but maintained significance in the DVT rates (1.1 vs 5.3% p = 0.025) and did not have significantly different Ventilator-free days. Upon multiple comparison correction, only transport time and prehospital airway needs maintained significance.
Discussion
In this study we examined patients with moderate or severe TBI who received TXA within two hours of injury. No mortality or functional neurologic benefit was observed in those who received treatment within 45 minutes compared to those who received treatment between 45 minutes and 2 hours after injury but a significantly lower rate of DVT and cerebral vasospasm were seen.
Previous studies have examined TXA in the context of traumatic injury6,14,15. The largest study, CRASH-2, showed improved mortality with TXA administration in early stages of traumatic hemorrhage6. Subgroup analysis raised interest in potential benefits in TBI patients7, which led to the investigations in CRASH-3 into the effects of TXA in TBI9. This study did not show improvements in outcome with the exception of a significant reduction in head injury related death within 24h of injury in mild to moderate TBI. TXA has also been shown to reduce expansion of ICH in the trauma population8.
Our group previously showed no difference in functional outcomes or mortality with prehospital TXA given within 2 hours after TBI in the combined dosing groups and in all enrolled patients, but did show improved mortality and 6-month Disability Rating Scores in a pre-determined post hoc subgroup analysis of patients with confirmed ICH who received the 2g bolus dose13. The percentage of patients with confirmed ICH in CRASH-3 was likely higher as administration in hospital allowed enrollment after imaging and ICH found before enrollment was one of the inclusion criteria.
With the limitations of CRASH-2 and growing conflicting data on the benefits of TXA in hemorrhage, the answer to when and to whom to give TXA remains unclear. As there is more data on hemorrhagic shock than TBI, principles learned from this group have been applied to TBI particularly in terms of timing of administration. CRASH-2 showed worse outcomes with administration of TXA more than 3 hours after injury6. Mechanistically this makes sense; tissue plasminogen activator (tPA) is activated immediately following tissue injury and reaches peak concentrations about 30 minutes later. Plasminogen and plasmin levels peak at 1 hour post trauma. At 2 hours after injury, plasminogen activator inhibitor-1 (PAI-1) levels increase and peak at approximately 3 hours, causing inhibition of fibrinolysis which may lead to DIC16. As such, TXA administration should theoretically be most beneficial within the first 30–60 minutes up to 2 hours after injury to prevent excessive acceleration of fibrinolysis early and excessive inhibition of fibrinolysis later. This could also affect inflammatory cascades that may affect complications and outcomes throughout the course of recovery14. In TBI compared to hemorrhagic shock, hyperfibrinolysis has been showed to be less pronounced17 and as such the therapeutic window in TBI may be even more narrow than in traumatic hemorrhage.
Our data show that in metropolitan areas with established and effective EMS systems within a window of two hours outcomes, very early administration results in similar outcomes13 and a different complication profile. Both our data on TBI patients and recent studies in hemorrhagic shock10,13,15 seem to indicate that higher bolus doses of TXA with or without maintenance infusion improve outcomes in the subgroup analysis of patients believed to benefit from TXA (TBI patients with confirmed intracranial hemorrhage and patients in severe shock) more than the standard 1g bolus followed by 1g infusion. This standard protocol, used in both CRASH-2 and 3, was initially developed and tested on obstetric and surgical patients where tissue trauma occurs over a prolonged period such as labor or a cardiac surgery. Trauma on the other hand generally has a single tissue insult and subsequent damage is a result of dysregulated coagulation and other physiologic responses as opposed to continued tissue damage. As such, the process on which we believe TXA works can be positively affected for only a few hours after the tissue insult and is potentially harmful after this period of time. In surgeries where injury is prolonged and ongoing an infusion makes theoretical sense while in trauma, we suggest it does not. Additionally, pharmacologic studies have indicated that a 1g bolus is insufficient in many patients to reach therapeutic levels of TXA 1 hour after administration18.
The question of when within the safe window of administration patients obtain the most benefit may differ between etiology. While there is good evidence that earlier prehospital and in hospital administration of TXA within the first 2–3 hours in patients presenting in hemorrhagic shock, our evidence does not support a mortality benefit with very early administration in TBI patients who are not in shock. The balance of risks and benefits is often delicate, and although TXA seems to have a low risk profile6,10,12 the trend to increased seizure rates particularly in higher doses19–21 (likely via GABA inhibition)22 is concerning for our TBI patients who may be more sensitive to this type of neurological effect. For the purpose of this study, a seizure was considered to occur if abnormal movement activity was documented and chemically treated. Seizures were not routinely confirmed and the clinical relevance of having had a seizure in this study is unclear.
Our data show lower rates of DVT and cerebral vasospasm in patients with early TXA administration and these complications have clear negative clinical effects. The absolute rates of these complications were higher in the 2g group which we have previously shown to offer a significant mortality benefit in patients with confirmed ICH. The question that arises is the following: within the 2 hour window where we do not see any difference in outcome benefit, is it better to wait for confirmation of ICH to identify the population which has a proven potential for benefit and accept the higher risk of complications? Alternatively, other studies do show a mortality benefits with very early administration6,15 and the benefit may outweigh the risks of non-fatal complications. Our previous data show that compared to placebo, the 2g dose does not have a significantly different rate of adverse events (conversely, a slight trend toward lower overall non-seizure adverse events)13. As such, with no overall increased risk of clinically significant adverse events, 2g of TXA is safe to administer, and with a lower risk of complication in the first 45 minutes after injury it is safer to administer it earlier. With proven lower risk given early and a proven benefit in general in a subset of patients, we argue that TXA should be given as a 2g bolus within the first 45 minutes from injury. In this study the patients in the >45 minute group were more likely to require advanced airways and have a lower GCS on presentation, indicating that they were sicker and required prioritization of ABCs over the study protocol. The absence of benefit with early administration supports that this prioritization is appropriate. In this population, as we did not see a difference in outcomes overall and the mean GCS of the group (7) put them in the “severe” head injury group based on CRASH-3 criteria which did not show any improved outcomes (reduction in head injury related death was only seen in the mild to moderate group)9, the risk-benefit balance after 45 minutes may favor confirming ICH. Although our study is similar to CRASH-3 in that neurologic outcomes were not different, the analysis in CRASH-3 was limited to very broad timing subgroups (<1h, 1–3h, and >3h) which makes their effect over time analysis difficult to interpret. This is compounded by the facts that how time of injury was determined was not defined and TXA was only administered after evaluation in hospital, making it very unlikely that there were many examples of very early administration in this data set. Additionally, our group showed an overall mortality benefit in patients with confirmed ICH (13), where no mortality benefit was seen in CRASH-3, which may be from dosage differences, much earlier average administration, exclusion of those unlikely to benefit based on CRASH-3 data such as patients with non-reactive pupils, or a combination of these factors. The 2g dosing strategy given very early in the prehospital setting is unique compared to CRASH-3. This strategy oviated the need to give 1g over 8 hours in hospital ensuring a greater likelihood that the patients will receive the full dose of TXA therapy.
Limitations of this study
The timing of administration of TXA by the prehospital providers in this group was rapid with an average of 49 minutes overall (29 minutes in the early group and 69 minutes in the late group). Although this speaks to the efficacy of our prehospital systems, the difference in administration times is not large and as such may have led us to miss a significant difference in outcomes between administration at the extremes of our time points. The time of injury estimated as the time the call was received by EMS dispatch is also subject to error as there can be delays from time of injury to the time EMS services are notified of the injury. Due to the small number of patients in the subgroups dividing by time points in addition to the approximate median did not allow for subgroups large enough for meaningful analysis. Additionally, the nature of prehospital patient management may lead to slight inaccuracies in recording drug administration times which could make a large difference in the relatively small window we examined. In terms of limitations of the analysis, examining these timing groups was done retrospectively in this prospective study. This resulted in a high degree of heterogeneity in timing of treatment. Additionally, similar to other studies6,9,10,14,15 the benefits our group has shown with TXA has been in subgroup analysis. Although examining complications in patients that receive TXA based on protocols with wide inclusion criteria is logical as it includes the entire population at risk for complications, the proportionally low number of patients in larger studies that gain the most benefit from TXA makes it difficult to confidently delineate the magnitude of positive effects of the intervention. Studies such as the MATTERS trial14 that specifically examined patients requiring blood transfusion in the setting of traumatic hemorrhage show much greater effects. As we gain further insight into optimal timing and dosing regimens of TXA in TBI, larger studies are warranted to elucidate more subtle benefits that may be missed in subgroup analysis. In terms of complications, definitions and institutional policies on screening varied between study centers. For example, approximately half of the centers performed routine screening duplex for DVT and the other half performed duplex only on symptomatic patients. This may have led to confounders in the rates of complications.
In the late administration group, it is possible that because the patients were sicker based on lower GCS needing more advanced procedures, the difference in complication rates could be due to the injury rather than the timing of the medication. However, we did not see a difference in head AIS scores or ISS between the early and late administration groups. If a difference in injury severity exists between these groups that is not reflected in these scores, it would not change recommendations because whether the etiology of the increased risk with delayed administration is related to TXA or to the injury, the increased risk of complications may be balanced by improved survival in appropriately selected patients.
Finally, this study in itself does not clearly answer questions about how TXA should be used given the small numbers and the nature of sub analysis. We feel that the implications of our results in the context of the larger body of data support our conclusions, but acknowledge that the significant limitations in our study as well as the existing data on TXA continue to impair clarity on the optimal use of TXA. There is much more work to be done.
Conclusion
Initial studies on TXA in trauma found that benefit only occurred if it was administered within 3 hours of trauma but optimal timing within this window has remained unclear. In this analysis of patients with moderate or severe TBI who received TXA within two hours of injury, no mortality or functional neurologic benefit was observed in those who received treatment within 45 minutes compared to those who received treatment between 45 minutes and 2 hours after injury but a significantly lower rate of DVT and cerebral vasospasm were seen. Given the lack of harm and decreased risk, these results support administering TXA within 45 minutes of injury for isolated TBI patients, and consideration for confirming ICH in patients in which administration is delayed beyond 45 but can still be done within 2 hours of injury to optimize the balance of risks and benefits.
Supplementary Material
Figure 1.
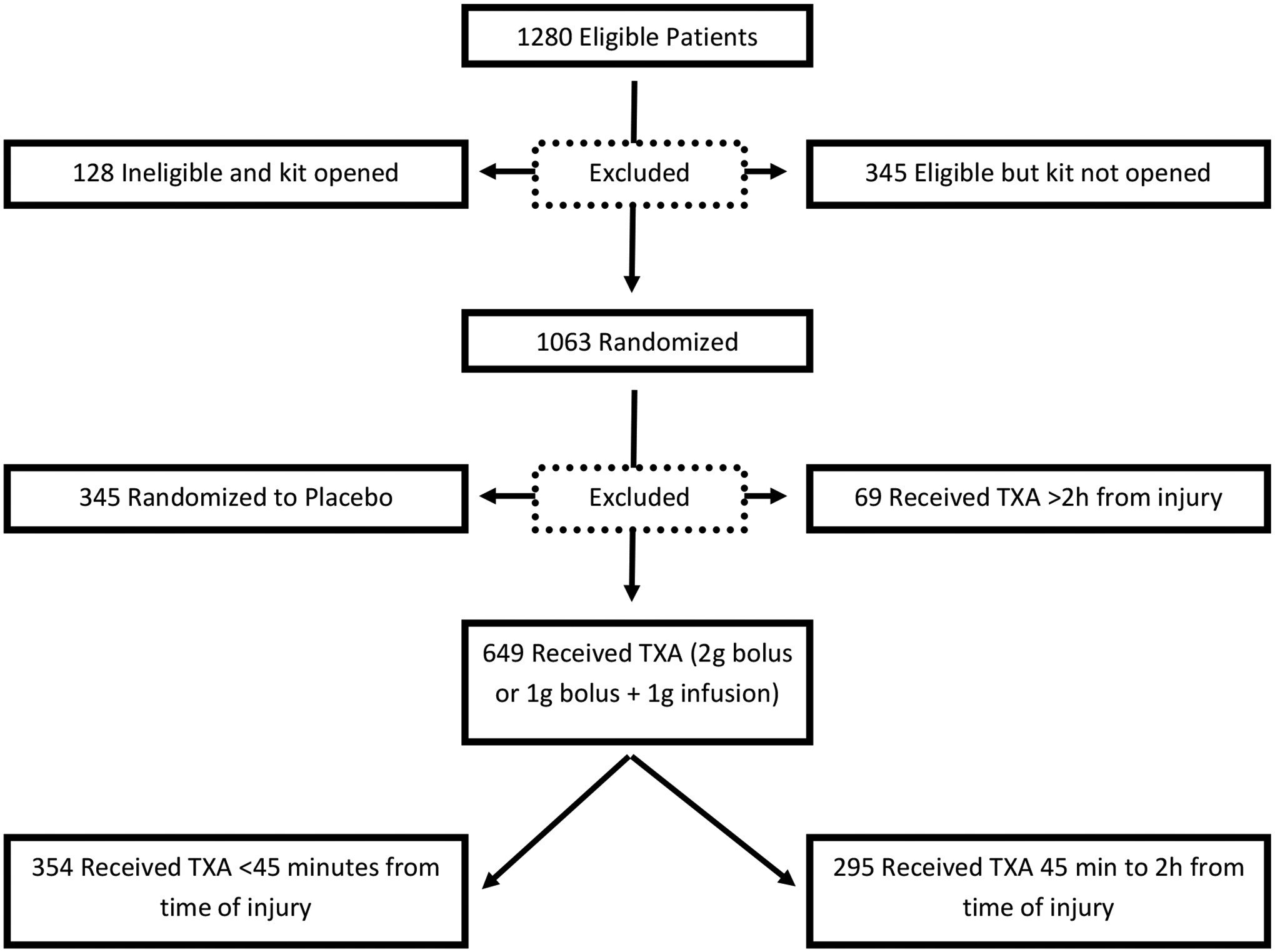
Flow Diagram of Study Participants (this diagram includes the subgroup analysis, for full allocation/randomization/analysis diagram please refer to Rowel et al 2020)
Table 1.
Patient Demographics
| Patient Demographics | Pooled | 2g | 1g | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <45 min (n = 354) | ≥45 min (n = 295) | p | <45 min (n = 190) | ≥45 min (n = 150) | p | <45 min (n = 164) | ≥45 min (n = 145) | p | |||
| Male Gender (Percent) | 72 | 74.6 | 0.467 | 72.6 | 74.7 | 0.711 | 71.3 | 74.5 | 0.536 | ||
| Race (Percent) | <0.001 | <0.001 | 0.001 | ||||||||
| American Indian | 2.2 | 0.4 | 1.8 | 0.8 | 25.7 | 9 | |||||
| Asian | 6.1 | 1.6 | 6.1 | 0 | 6.1 | 3.3 | |||||
| Black/African American | 24.7 | 10 | 23.8 | 10.9 | 25.7 | 9 | |||||
| White | 66.3 | 86.8 | 68.3 | 87.5 | 64.2 | 86.1 | |||||
| Other | 0.6 | 0.4 | 0 | 0 | 1.4 | 0.8 | |||||
| Mean Age | 41+/−18 | 44+/−20 | 0.062 | 41+/−18 | 44+/−19 | 0.086 | 41+/−18 | 43+/−21 | |||
Table 2.
Mechanism of Injury
| Mechanism Data | Pooled | 2g | 1g | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <45 min | ≥45 min | p | <45 min | ≥45 min | p | <45 min | ≥45 min | p | |||
| Blunt Injury Rate (Percent) | 96.3 | 99 | 0.03 | 97.4 | 99.3 | 0.234 | 95.1 | 98.6 | |||
| Mechanism of Injury (Percent) | <0.001 | 0.161 | <0.001 | ||||||||
| MVC | 26.7 | 41.6 | 29.8 | 38.5 | 23.2 | 44.8 | |||||
| Ped vs Auto | 19.6 | 6.5 | 18.6 | 8.8 | 20.7 | 4.1 | |||||
| Bicycle vs Auto | 6.3 | 4.4 | 4.3 | 4.1 | 8.5 | 4.8 | |||||
| MCC or Dirtbike | 9.9 | 14 | 12.2 | 14.2 | 7.3 | 13.8 | |||||
| Suicide Attempt | 2.3 | 1.7 | 1.6 | 1.4 | 3 | 2.1 | |||||
| Assault | 9.7 | 3.8 | 9.6 | 4.7 | 9.8 | 2.8 | |||||
| Ground Level Fall | 13.1 | 13.7 | 12.2 | 14.2 | 14 | 13.1 | |||||
| Fall | 11.6 | 11.9 | 10.6 | 11.5 | 12.8 | 12.4 | |||||
| Other | 0.9 | 2.4 | 1.1 | 2.7 | 0.6 | 2.1 | |||||
Table 3.
Patient Presenting Characteristics
| Presenting Characteristics | Pooled | 2g | 1g | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <45 min | ≥45 min | p | <45 min | ≥45 min | p | <45 min | ≥45 min | p | |||
| GCS by EMS | 9+/−3 | 7+/−3 | 0.126 | 8+/−3 | 8+/−3 | 0.266 | 9+/−3 | 8+/−3 | 0.32 | ||
| GCS on Presentation | 9+/−3 | 7+/−3 | 0.04 | 8+/−3 | 8+/−3 | 0.156 | 9+/−4 | 8+/−3 | 0.142 | ||
| Max Head Injury (AIS) | 3+/−2 | 3+/−2 | 0.5443 | 3+/−2 | 3+/−2 | 0.98 | 3+/−2 | 3+/−2 | 0.872 | ||
| Injury Severity Score | 17+/−14 | 17+/−13 | 0.47565 | 18+/−14 | 19+/−13 | 0.48 | 17+/−14 | 17.8+/−13 | 0.774 | ||
| Prehospital Advanced Airway | 29.9 | 73.2 | <0.001 | 27.9 | 72.7 | <0.001 | 32.3 | 73.8 | <0.001 | ||
| Air Transport (Percent) | 7.1 | 70.5 | <0.001 | 8.9 | 69.3 | <0.001 | 4.9 | 71.7 | <0.001 | ||
| Advanced Airway Placed (Irrespective of location) | 60.5 | 80.7 | <0.001 | 62.6 | 77.3 | 0.004 | 57.9 | 84.1 | <0.001 | ||
| Time to Drug (minutes) | 29.4+/−8.2 | 69.3+/−19.3 | 29.7+/−7.9 | 71.0+/−20.3 | 29+/−8.5 | 67+/−17.7 | |||||
| Rotterdam Score | 3+/−1 | 3+/−1 | 0.891 | 3+/−1 | 3+/−1 | 0.817 | 3+/−1 | 3+/−1 | 0.617 | ||
Table 4.
Patient Outcomes
| Outcomes | Pooled | 2g | 1g | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <45 min | ≥45 min | p | <45 min | ≥45 min | p | <45 min | ≥45 min | p | |||
| Underwent any Neurosurgical Intervention (Percent) | 20.1 | 21.7 | 0.609 | 21.6 | 21.3 | 1 | 18.3 | 22.1 | 0.477 | ||
| Craniectomy | 4.3 | 4.5 | 0.908 | 5.3 | 3.4 | 0.44 | 3.1 | 5.6 | 0.396 | ||
| Craniotomy | 7.5 | 4.8 | 0.174 | 8 | 5.5 | 0.395 | 6.9 | 4.2 | 0.332 | ||
| ICP Monitor | 14.5 | 18.5 | 0.181 | 15.4 | 17.8 | 0.53 | 13.5 | 19.1 | 0.208 | ||
| Hospital Free Days | 18+/−11 | 16+/−10 | 0.138 | 14+/−10 | 14+/−10 | 0.421 | 18+/−11 | 13+/−10 | 0.217 | ||
| ICU Free Days | 24+/−10 | 23+/−10 | 0.18 | 19+/−10 | 19+/−10 | 0.432 | 24+/−11 | 23+/−10 | 0.279 | ||
| Ventilator Free Days | 26+/−10 | 26+/−10 | 0.011 | 21+/−10 | 21+/−10 | 0.193 | 26+/−11 | 25+/−10 | 0.0215 | ||
| Overall Mortality | 15.7 | 13.7 | 0.49 | 11.1 | 12.3 | 0.732 | 21.1 | 15.1 | 0.224 | ||
| 28-day Mortality | 15.4 | 13.4 | 0.495 | 11.4 | 12.3 | 0.864 | 20 | 14.6 | 0.229 | ||
| 6-month Mortality | 19.9 | 16.7 | 0.337 | 14.8 | 17.1 | 0.628 | 26 | 16.4 | 0.069 | ||
| Gose Upon Discharge | 3+/−2 | 3+/−2 | 0.59 | 4+/−2 | 4+/−2 | 0.876 | 3+/−3 | 3+/−3 | 0.527 | ||
| Gose at 6 Months | 6+/−3 | 6+/−3 | 0.729 | 5+/−2 | 5+/−3 | 0.852 | 6+/−3 | 6+/−3 | 0.722 | ||
| DRS on Discharge | 4+/−11 | 5+/−10 | 0.557 | 8+/−10 | 8+/−10 | 0.795 | 4+/−12 | 6+/−11 | 0.636 | ||
| DRS at 6 Months | 2+/−12 | 2+/−11 | 0.905 | 7+/−11 | 7+/−11 | 0.957 | 2+/−13 | 2+/−11 | 0.814 | ||
| Discharge Status | 0.187 | 0.883 | 0.01 | ||||||||
| Inpatient Center (Rehab, Psych, other) | 21.2 | 24.6 | 23.5 | 27.4 | 18.5 | 21.7 | |||||
| Skilled Nursing Facility | 4.5 | 8.1 | 6.7 | 6.8 | 2 | 9.4 | |||||
| Long-Term Care Facility | 3 | 4.2 | 4.5 | 3.4 | 1.3 | 5.1 | |||||
Table 5.
Patient Complications
| Sequelae | Pooled | 2g | 1g | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <45 min | ≥45 min | p | <45 min | ≥45 min | p | <45 min | ≥45 min | p | |||
| Seizures | 4.5 | 1.7 | 0.043 | 6.3 | 2.7 | 0.13 | 2.4 | 0.7 | 0.376 | ||
| Thromboembolic Events | 5.9 | 7.5 | 0.437 | 8.9 | 8.7 | 1 | 2.4 | 6.2 | 0.154 | ||
| Myocardial Infarction | 0.8 | 0.7 | 0.806 | 1.1 | 0 | 0.505 | 0.6 | 1.4 | 0.602 | ||
| Pulmonary Embolism | 1.4 | 1.4 | 0.951 | 2.3 | 1.3 | 0.698 | 0.6 | 1.4 | 0.602 | ||
| Thrombotic Cerebrovascular Accident | 2 | 3.1 | 0.38 | 3.7 | 4 | 1 | 2.4 | 6.2 | 0.154 | ||
| Hemorrhagic Cerebrovascular Accident | 0.8 | 1 | 0.822 | 0.5 | 0.7 | 1 | 1.2 | 1.4 | 1 | ||
| Deep Vein Thrombosis | 0.8 | 3.4 | 0.021 | 1.1 | 5.3 | 0.025 | 0.6 | 1.4 | 0.602 | ||
| Acute Kidney Injury | 4.5 | 1.7 | 0.043 | 3.2 | 6.7 | 0.193 | 7.9 | 2.8 | 0.077 | ||
| Renal Failure | 0.6 | 0.7 | 0.855 | 1.1 | 1.3 | 1 | 0 | 0 | |||
| Acute Respiratory Distress Syndrome | 1.7 | 2 | 0.75 | 2.1 | 0 | 0.133 | 1.2 | 4.1 | 0.153 | ||
| Cerebral Vasospasm | 0 | 2 | 0.007 | 0 | 2.7 | 0.037 | 0 | 1.4 | 0.219 | ||
| Diabetes Insipidus | 0.6 | 0.7 | 0.855 | 1.1 | 1.3 | 1 | 0 | 0 | |||
| Hypernatremia | 2.8 | 3.7 | 0.517 | 3.7 | 4 | 1 | 1.8 | 3.4 | 0.481 | ||
| Pseudomembranous Colitis | 0.6 | 0.3 | 0.673 | 0 | 0 | 0 | 1.2 | 0.7 | 1 | ||
Acknowledgements
The authors would like to express appreciation to all members of the Resuscitation Outcome Consortium who contributed to the Prehospital TXA for TBI Trial
Conflicts of Interest
Dr Rowell has received grants from the US Department of Defense (DoD) and the National Institutes of Health (NIH) during the conduct of the study and personal fees from Portola Pharmaceuticals outside the submitted work. Dr Schreiber reported receiving grants from DoD, HIH, Health Canada, and the American Heart Association and personal fees from Haemonetics during the conduct of the study and personal fees from CSL Behring, Tricol, Velico Medical and Arsenal Medical outside the submitted work.
Funding
The Resuscitation Outcomes Consortium institutions participating in the trial were supported by a series of cooperative agreements from the Natinal heart, Lung and Blood Institute administered by the US Army Medical Reasearch & Material Command (W81XWH-13-2-0090). Including U01HL077863 (University of Washington Data Coordinating Center), U01HL077866 (medical College of Wisconsin), U01HL077871 (University of Pittsburgh), U01HL077873 (Oregon Health and Science University), U01HL07781 (University of Alabama at Birmingham) and U01HL077887 (University of Texas Southwestern Medical Center/Dallas)
Footnotes
Supplemental Digital Content:
CONSORT Checklist.
Presentations
This paper was presented at EAST Annual Scientific Assembly 2022 as a podium presentation
Contributor Information
Dr. Alexandra M.P. Brito, Oregon Health & Science University.
Dr. Martin A. Schreiber, Oregon Health & Science University.
Dr. James El Haddi, Oregon Health & Science University.
Dr. Eric N. Meier, University of Washington.
Dr. Susan E. Rowell, Oregon Health & Science University, University of Chicago School of Medicine.
References
- 1.Kashuk JL, Moore EE, Sawyer M, Wohlauer M, Pezold M, Barnett C, et al. Primary fibrinolysis is integral in the pathogenesis of the acute coagulopathy of trauma. Ann Surg. 2010;252(3):434–442; discussion 443–434. [DOI] [PubMed] [Google Scholar]
- 2.Stansfield R, Morris D, Jesulola E. The Use of Tranexamic Acid (TXA) for the Management of Hemorrhage in Trauma Patients in the Prehospital Environment: Literature Review and Descriptive Analysis of Principal Themes. Shock. 2020;53(3):277–283. [DOI] [PubMed] [Google Scholar]
- 3.The L Bringing women to the forefront of science and medicine. Lancet. 2012;379(9819):867. [DOI] [PubMed] [Google Scholar]
- 4.Gill R, Ganatra B, Althabe F. WHO essential medicines for reproductive health. BMJ Glob Health. 2019;4(6):e002150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collaborators WT. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389(10084):2105–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts I, Shakur H, Coats T, Hunt B, Balogun E, Barnetson L, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess. 2013;17(10):1–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perel P, Al-Shahi Salman R, Kawahara T, Morris Z, Prieto-Merino D, Roberts I, Sandercock P, et al. CRASH-2 (Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage) intracranial bleeding study: the effect of tranexamic acid in traumatic brain injury--a nested randomised, placebo-controlled trial. Health Technol Assess. 2012;16(13):iii–xii, 1–54. [DOI] [PubMed] [Google Scholar]
- 8.Jokar A, Ahmadi K, Salehi T, Sharif-Alhoseini M, Rahimi-Movaghar V. The effect of tranexamic acid in traumatic brain injury: A randomized controlled trial. Chin J Traumatol. 2017;20(1):49–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collaborators C-t. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet. 2019;394(10210):1713–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guyette FX, Brown JB, Zenati MS, Early-Young BJ, Adams PW, Eastridge BJ, et al. Tranexamic Acid During Prehospital Transport in Patients at Risk for Hemorrhage After Injury: A Double-blind, Placebo-Controlled, Randomized Clinical Trial. JAMA Surg. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huebner BR, Dorlac WC, Cribari C. Tranexamic Acid Use in Prehospital Uncontrolled Hemorrhage. Wilderness Environ Med. 2017;28(2S):S50–S60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawati KA, Sharif S, Maqbali SA, Rimawi HA, Petrosoniak A, Belley-Cote EP, et al. Efficacy and safety of tranexamic acid in acute traumatic brain injury: a systematic review and meta-analysis of randomized-controlled trials. Intensive Care Med. 2021;47(1):14–27. [DOI] [PubMed] [Google Scholar]
- 13.Rowell SE, Meier EN, McKnight B, Kannas D, May S, Sheehan K, et al. Effect of Out-of-Hospital Tranexamic Acid vs Placebo on 6-Month Functional Neurologic Outcomes in Patients With Moderate or Severe Traumatic Brain Injury. JAMA. 2020;324(10):961–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrison JJ, Dubose JJ, Rasmussen TE, Midwinter MJ. Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) Study. Arch Surg. 2012;147(2):113–119. [DOI] [PubMed] [Google Scholar]
- 15.Li SR, Guyette F, Brown J, Zenati M, Reitz KM, Eastridge B, et al. Early Prehospital Tranexamic Acid Following Injury Is Associated With a 30-day Survival Benefit: A Secondary Analysis of a Randomized Clinical Trial. Ann Surg. 2021;274(3):419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts I, Edwards P, Prieto D, Joshi M, Mahmood A, Ker K, et al. Tranexamic acid in bleeding trauma patients: an exploration of benefits and harms. Trials. 2017;18(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meizoso JP, Moore HB, Moore EE, Gilna GP, Ghasabyan A, Chandler J, et al. Traumatic Brain Injury Provokes Low Fibrinolytic Activity in Severely Injured Patients. J Trauma Acute Care Surg. 2022. [DOI] [PubMed] [Google Scholar]
- 18.Grassin-Delyle S, Theusinger OM, Albrecht R, Mueller S, Spahn DR, Urien S, et al. Optimisation of the dosage of tranexamic acid in trauma patients with population pharmacokinetic analysis. Anaesthesia. 2018;73(6):719–729. [DOI] [PubMed] [Google Scholar]
- 19.Lin Z, Xiaoyi Z. Tranexamic acid-associated seizures: A meta-analysis. Seizure. 2016;36:70–73. [DOI] [PubMed] [Google Scholar]
- 20.Kalavrouziotis D, Voisine P, Mohammadi S, Dionne S, Dagenais F. High-dose tranexamic acid is an independent predictor of early seizure after cardiopulmonary bypass. Ann Thorac Surg. 2012;93(1):148–154. [DOI] [PubMed] [Google Scholar]
- 21.Murkin JM, Falter F, Granton J, Young B, Burt C, Chu M. High-dose tranexamic Acid is associated with nonischemic clinical seizures in cardiac surgical patients. Anesth Analg. 2010;110(2):350–353. [DOI] [PubMed] [Google Scholar]
- 22.Kratzer S, Irl H, Mattusch C, Bürge M, Kurz J, Kochs E, et al. Tranexamic acid impairs γ-aminobutyric acid receptor type A-mediated synaptic transmission in the murine amygdala: a potential mechanism for drug-induced seizures? Anesthesiology. 2014;120(3):639–649. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


