Abstract
Cytokine networking in the lung in response to inhaled Aspergillus fumigatus was assessed using a murine model of primary pulmonary aspergillosis in immunocompetent Crl:CF-1 mice. Inhalation of virulent A. fumigatus (6 × 106 CFU) resulted in the induction of interleukin 18 (IL-18), tumor necrosis factor alpha (TNF-α), IL-12, and gamma interferon (IFN-γ) protein in bronchoalveolar lavage fluid and/or lung tissue. Induction of immunoreactive IL-18 preceded induction of TNF-α protein, which preceded induction of immunoreactive IL-12 and IFN-γ. Real-time reverse transcriptase (RT) PCR analysis of infected lung tissue demonstrated that induction of IL-18 protein also preceded induction of pulmonary TNF-α, IL-12, and IFN-γ mRNAs. Mice were subsequently treated with cytokine-specific neutralizing monoclonal antibodies (MAbs) to the IL-18 receptor (anti-IL-18R MAb), TNF-α (anti-TNF-α MAb), IL-12 (anti-IL-12 MAb), and/or IFN-γ (anti-IFN-γ MAb), and effects on intrapulmonary cytokine activity and growth of A. fumigatus were assessed in infected lung homogenates. Simultaneous neutralization of IL-12 and IL-18 resulted in decreased levels of immunoreactive TNF-α, while neutralization of IL-18, TNF-α, or IL-12 alone or of IL-18 and IL-12 together resulted in decreased levels of immunoreactive IFN-γ. Simultaneous neutralization of IL-12 and IL-18 or neutralization of TNF-α alone or in combination with IL-12, IL-18, or IFN-γ also resulted in a significant increase in A. fumigatus CFU in lung tissue. Taken together, these results demonstrate that endogenous IL-18, IL-12, and TNF-α, through their modulatory effects on both intrapulmonary cytokine activity and growth of A. fumigatus, play key roles in host defense against primary pulmonary aspergillosis.
Aspergillus fumigatus is a saprophytic fungus and a causative agent of invasive pulmonary aspergillosis, a predominant life-threatening opportunistic infection in immunocompromised patients (16, 50, 51). Host defense against primary A. fumigatus pulmonary infection is mediated by phagocytic cells of the innate immune system (11, 15, 25, 38). Alveolar macrophages ingest and kill inhaled Aspergillus conidia, while polymorphonuclear leukocytes destroy Aspergillus hyphae that germinate from conidia that have escaped macrophage killing (25, 45, 49). Polymorphonuclear leukocyte-mediated killing of Aspergillus hyphae is critical for prevention of hyphal invasion and destruction of pulmonary tissue (2, 12–14, 25, 44, 45, 49). The effectiveness of this dual macrophage-neutrophil-mediated host defense against A. fumigatus is evidenced by the paucity of serious Aspergillus infections in immunocompetent persons, despite environmental exposure to the fungus.
Previous studies have demonstrated that T-helper (Th) cytokines contribute to phagocytic cell-mediated host defense against A. fumigatus. Stimulation of cultured phagocytic cells with Th1 cytokines, including gamma interferon (IFN-γ), enhanced fungicidal activity, while stimulation of similarly cultured cells with Th2 cytokines, including interleukin 4 (IL-4) and IL-10, had the opposite effect (15, 37, 41, 42). Similarly, murine resistance to experimentally induced A. fumigatus infections was correlated with induction of cytokines, including tumor necrosis factor alpha (TNF-α), IL-12, and IFN-γ, while susceptibility to infection was associated with the production of IL-4 and/or IL-10 (6–8, 29). Likewise, administration of exogenous cytokines, including TNF-α, IL-12, or IFN-γ, or neutralization of endogenous IL-4 and/or IL-10 enhanced murine resistance to infection, while neutralization of endogenous IFN-γ and/or TNF-α enhanced susceptibility to infection (5, 6, 29).
While these studies suggest a directive role of Th1 cytokines in leukocyte-mediated defense against A. fumigatus infections, the relative contribution of each cytokine to innate immunity in pulmonary aspergillosis has not been thoroughly investigated. Likewise, a recently described cytokine, IL-18 (30–32), has been shown to play a key role in Th1-mediated defense against other pathogens, including Mycobacterium tuberculosis (46) and Cryptococcus neoformans (36), likely due, at least in part, to immunomodulatory effects of IL-18 on other cytokines, including TNF-α and IFN-γ (1, 30, 40, 47, 53). The role of endogenous IL-18 in host resistance to A. fumigatus pulmonary infection has not been investigated.
In the current study, immunocompetent Crl:CF-1 mice were infected with aerosolized, virulent A. fumigatus, and the temporal induction of cytokines, including IL-18, TNF-α, IL-12, and IFN-γ, was determined in bronchoalveolar lavage fluid (BALF) and in lung homogenates by cytokine-specific murine enzyme-linked immunosorbent assays (ELISAs) and by real-time reverse transcriptase (RT) PCR. Subsequently, by using cytokine-specific neutralizing monoclonal antibodies (MAbs), the immunomodulatory role and biological relevance of each cytokine in host resistance to primary A. fumigatus pulmonary infection were assessed.
MATERIALS AND METHODS
Mice.
Male specific-pathogen-free outbred immunocompetent Crl:CF-1 mice (11 to 13 g; Charles River) were used for all experiments. Animals were housed in microisolator cages and were cared for in accordance with standard guidelines.
Inhalation model of murine pulmonary aspergillosis.
Mice were infected with A. fumigatus ATCC 201795 in an inhalation chamber using a 30-s exposure as previously described (26, 34). Briefly, the inhalation chamber consisted of a 1-liter Pyrex flask with eight tubular glass side arms, each at a 30° angle from the horizontal and spaced equidistantly around the flask. Prior to infection, the bottom of the flask was filled with malt agar, seeded with a suspension of A. fumigatus conidia, and incubated at 28°C for 13 days to produce a fungal lawn. Mice were subsequently individually placed in each side arm (eight mice per flask), pushed to the bottom of the arm (so that the nose filled and extended beyond the open end of the tube), and held in place by cotton plugs. The top of the flask was then closed with a rubber stopper, which was transversed by a glass rod, the end of which extended directly above the fungal lawn. To generate an aerosol, turbulence was created over the culture via the forceful expulsion of 60 cm3 of air through the end of the glass rod, using a 60-ml syringe which was connected to the stoppered end of the glass rod by a piece of plastic tubing. This method resulted in the uniform dispersion of conidia and infection of mice throughout the chamber.
Quantification of A. fumigatus in infected lung homogenates.
At specific times postinfection (p.i.), mice were euthanatized and their lungs were excised and homogenized in 10 ml of sterile phosphate-buffered saline. Lung homogenates from individual mice were serially diluted on Sabouraud dextrose agar plates and were incubated for 48 h at 30°C prior to quantification of A. fumigatus CFU. Results are expressed as log10 CFU per lung.
Collection of lung homogenate supernatant and BALF for cytokine analysis.
Lung homogenate supernatant was procured by filtering lung homogenates prepared as described above through a 0.22-μm-pore-size filter (Gelman Sciences, Ann Arbor, Mich.) to remove bacteria. Alternatively, for collection of BALF, mice were humanely euthanatized and their lungs were lavaged with 1.6 ml of phosphate-buffered saline (3). The resultant lavage fluid was subsequently filtered as described above. Filtered lung homogenates and BALF were stored at −80°C until use for cytokine analysis.
Cytokine analysis.
Immunoreactive IL-18, IL-12, TNF-α, and IFN-γ protein levels in BALF and/or lung homogenate supernatants were measured by commercially available cytokine-specific murine ELISA kits (Quantikine mouse-IL-18, mouse IL-12p70, mouse TNF-α, and mouse IFN-γ; R & D, Minneapolis, Minn.) according to the manufacturer's directions.
Quantitation of cytokine transcripts by real-time RT-PCR.
Real-time RT-PCR assays were performed to specifically quantify mouse IL-18, TNF-α, IL-12, and IFN-γ transcripts. Briefly, lungs were excised from A. fumigatus-infected mice at specific times p.i. and were flash frozen in liquid nitrogen. Total RNA was extracted using TriReagent (Molecular Research Center, Cincinnati, Ohio) according to the manufacturer's directions and was stored at −80°C in nuclease-free water containing 0.1 mM EDTA. Isolated RNA (5 μg) was incubated with 10 U of DNase I (Boehringer Mannheim) in the presence of RNasin (Promega) for 30 min at 37°C. The samples were then heat inactivated at 70°C for 10 min, chilled, and reverse transcribed with Superscript II RT (Gibco/BRL) with 1 μg each of random hexamers and oligo(dT)(12–18). PCR primers were obtained as predeveloped assay reagents (TNF-α, IL-12, and IFN-γ; Perkin-Elmer) or were generated with Primer Express version 1.5 software (Perkin-Elmer) and were ordered from Perkin-Elmer. Whenever possible, primer pairs were designed to span intron-exon borders. Samples were then subjected to 40 cycles of amplification at 95° for 15 s followed by 1 min at 60° using an ABI Geneamp 7700 Sequence Detection System (Perkin-Elmer) according to the manufacturer's instructions. PCR amplification of the housekeeping gene ubiquitin was performed for each sample to control for sample loading and to allow normalization between samples according to instructions by the manufacturer (Perkin-Elmer). Water controls were included to ensure specificity. Each data point was examined for integrity by analysis of the amplification plot. The ubiquitin-normalized data were expressed as the fold induction of gene expression in A. fumigatus-infected mice compared to that in uninfected mice.
Interventional studies.
Endogenous intrapulmonary IL-18, TNF-α, IL-12, and/or IFN-γ activities were blocked by pretreatment of the mice with cytokine-specific neutralizing MAbs. Previous studies had demonstrated that responsiveness to IL-18 is conferred by IL-18 binding to its cognate receptor, which consists of the IL-R-related protein 1 chain (IL-1Rrp1, also known as IL-1R5) and the IL-1R accessory protein-like chain (IL-1RAcPL, also known as IL-1R7 [4, 10, 48, 52]). Recent studies have also demonstrated that IL-18-mediated cell activation could be prevented by inhibiting IL-18 ligand receptor interaction, through administration of anti-IL-18 antibody (31) or of MAbs which recognize either the IL-1R5 chain (52) or the IL-1R7 chain (10) of the IL-18 receptor. Therefore, the following cytokine-specific MAbs were used: anti-IL-18 receptor MAb (anti-IL-18R MAb) directed towards the IL-1R7 chain of the IL-18R (10), anti-IL-12 MAb (33), anti-TNF-α MAb (22, 23), and anti-IFN-γ MAb (9). Each neutralizing MAb was administered intraperitoneally at a dose of 1 mg of antibody/mouse, 2 h prior to infection with A. fumigatus. Similarly infected mice that were administered an isotype-matched immunoglobulin G1a (IgG1a) or IgG2a served as controls.
Statistical analysis.
Analysis of variance was used to compare differences between treatment groups. P values of <0.05 were considered significant.
RESULTS
Pathogenesis of pulmonary A. fumigatus infection in immunocompetent Crl:CF-1 mice.
The rate of A. fumigatus clearance from lungs of infected mice was determined by culture of lung homogenates. Results of a representative experiment (five mice per time point) are shown in Fig. 1. All mice survived the infection. Approximately 6.0 × 106 organisms were recovered from the lungs of mice 30 min after infection with A. fumigatus. There was no significant difference in recovery of A. fumigatus over the first 12 h p.i. (hpi) compared to that at 30 min p.i. At 24 to 168 hpi, A. fumigatus was gradually cleared from the lung, with detection of approximately 10 CFU/lung at 7 days p.i. In agreement with previously published studies (7, 14), these results demonstrate that the innate immune response is sufficient to resolve primary A. fumigatus pulmonary infections in immunocompetent mice.
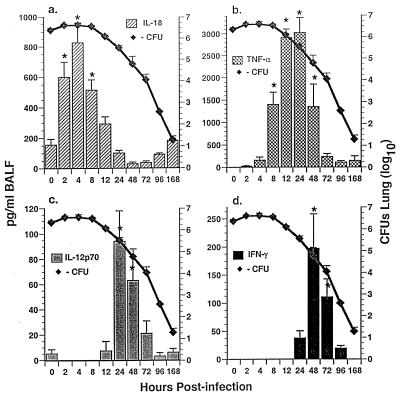
FIG. 1.
Temporal expression of immunoreactive cytokine activity in BALF and growth of A. fumigatus in lungs of immunocompetent Crl:CF-1 mice. Mice were infected with A. fumigatus as described in Materials and Methods. At specific times p.i., mice were euthanatized and growth of A. fumigatus was quantified in the lung homogenates by culture. Alternatively, the lungs were lavaged and immunoreactive cytokine activity was quantified in BALF by cytokine-specific ELISAs for IL-18, TNF-α, IL-12p70, and IFN-γ. Results of a representative experiment (n = 2) are shown and reflect the mean ± standard error of the mean of five animals per time point. Symbol: ∗, significantly greater than in uninfected mice, P < 0.05.
Temporal induction of cytokines in lungs of A. fumigatus-infected mice.
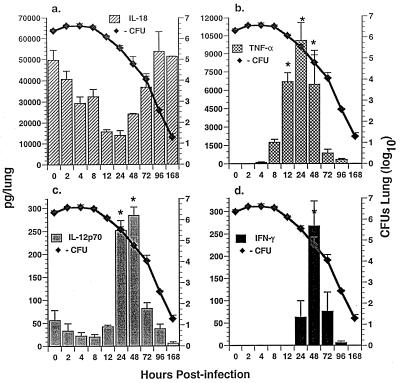
In order to gain insight as to the potential role of IL-18 versus that of IL-12, TNF-α, and IFN-γ in the pathogenesis of pulmonary aspergillus infection, temporal induction of immunoreactive cytokine activity was first assessed in BALF and whole-lung homogenates of A. fumigatus-infected mice by using cytokine-specific ELISAs. Assessment of BALF (Fig. 1) and of lung homogenates (Fig. 2) allowed evaluation of both secreted cytokine activity (i.e., into the intra-alveolar space) and total organ activity, respectively. Results of these studies demonstrated that IL-18 protein was significantly enhanced in BALF within 2 hpi, with maximal induction at 4 hpi (Fig. 1a). In contrast, while IL-18 activity was constitutively expressed in lung homogenates from uninfected mice (T = 0), it was not significantly enhanced in lung homogenates from infected mice at any time p.i. (Fig. 2a). Induction of IL-18 in BALF preceded induction of TNF-α protein in BALF and lung homogenates (Fig. 1b and 2b), which was apparent within 8 to 12 hpi, with maximal induction at 24 hpi. Induction of both IL-18 and TNF-α preceded induction of IL-12p70 and IFN-γ proteins in BALF (Fig. 1c and d) and in lung homogenates (Fig. 2c and d) at 24 to 48 and 48 to 72 hpi, respectively. These studies also demonstrate that induction of IL-18, TNF-α, IL-12, and IFN-γ in BALF and/or in lung homogenates was temporally correlated with clearance of A. fumigatus from the lung.
FIG. 2.
Temporal expression of immunoreactive cytokine activity in lung homogenates and growth of A. fumigatus in lungs of immunocompetent Crl:CF-1 mice. Mice were infected with A. fumigatus. At specific times p.i., mice were euthanatized and intrapulmonary growth of A. fumigatus was determined (as described for Fig. 1). Alternatively, immunoreactive cytokine activity was quantified in lung homogenates by cytokine-specific ELISAs for IL-18, TNF-α, IL-12p70, and IFN-γ. Results of a representative experiment (n = 2) are shown and reflect the mean ± standard error of the mean of five animals per time point. Symbol: ∗, significantly greater than in uninfected mice, P < 0.05.
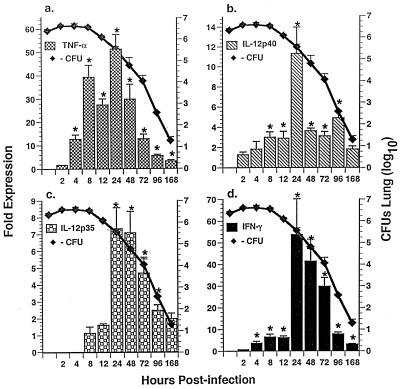
In subsequent studies, temporal expression of IL-18, IL-12, TNF-α, and IFN-γ mRNAs in A. fumigatus-infected lung tissue was evaluated by real-time RT-PCR. This method allows a rapid, accurate, and precise quantitation of gene transcripts (19, 21). As shown in Fig. 3, TNF-α (Fig. 3a), IL-12p40 (Fig. 3b), IL-12p35 (Fig. 3c), and IFN-γ (Fig. 3d) mRNAs were significantly enhanced in the lungs of A. fumigatus-infected mice at ≥4 hpi, with maximal induction at 24 hpi. In contrast, IL-18 mRNA was constitutively expressed and was not significantly induced in the lung in response to A. fumigatus at any time assessed (data not shown). Also, induction of TNF-α, IL-12p40, IL-12p35, and IFN-γ mRNAs was temporally correlated with clearance of A. fumigatus from the lung (Fig. 3). Taken together, these studies demonstrate that in response to inhaled A. fumigatus, IL-18 protein is induced rapidly in BALF, followed by induction of TNF-α, IL-12, and IFN-γ proteins and mRNAs in infected BALF and/or lung tissue.
FIG. 3.
Temporal expression of cytokine mRNA and growth of A. fumigatus in lungs of immunocompetent Crl:CF-1 mice. Mice were infected with A. fumigatus as described in Materials and Methods. At specific times p.i., mice were euthanatized and intrapulmonary growth of A. fumigatus was determined (as described for Fig. 1). Alternatively, the lungs were excised and total RNA was extracted. Transcript levels were quantified by real-time RT-PCR for TNF-α, IL-12p40, IL-12p35, and IFN-γ. Results of a representative experiment (n = 2) are shown and reflect the mean ± standard error of the mean of five animals per time point. Symbol: ∗, significantly greater than uninfected mice, P < 0.05.
Immunomodulatory activity of endogenous IL-18, TNF-α, and IL-12 on cytokine activity in the lungs of A. fumigatus-infected mice.
In subsequent studies, the relative immunomodulatory effect of IL-18, TNF-α, and IL-12 on pulmonary cytokine activity in A. fumigatus-infected mice was determined. Immunocompetent mice were pretreated with IgG2a (control antibody), anti-IL-18R MAb (10), anti-IL12 MAb (33), or anti-TNF-α MAb (22, 23) alone or in combination 2 h prior to infection with A. fumigatus. The effect of cytokine neutralization on TNF-α activity or on IL-12 and IFN-γ activities too was assessed in lung homogenates at 24 and 48 hpi, respectively (i.e., when these cytokines were maximally induced in lung homogenates of similarly infected immunocompetent mice [Fig. 2]). As shown in Table 1, inhibition of endogenous IL-18 and IL-12 in combination results in significant inhibition (47%) of TNF-α at 24 hpi, when compared to what was found in similarly infected mice that were administered a control MAb. In contrast, inhibition of IL-18 or TNF-α activity did not result in significant inhibition of IL-12 at 48 hpi. However, inhibition of either IL-18, TNF-α, or IL-12 alone or of IL-12 and IL-18 together resulted in significant inhibition (58, 50, 90, or 94%, respectively) of IFN-γ in infected lung tissue at 48 hpi. However, simultaneous inhibition of both IL-18 and IL-12 did not result in significantly greater inhibition of IFN-γ than that caused by inhibition of either cytokine alone. These results demonstrate that endogenous IL-18, IL-12, and TNF-α play key, and in some cases potentially overlapping, roles in modulating cytokine activity in the lung in response to inhaled A. fumigatus.
TABLE 1.
Modulation of intrapulmonary cytokine activity in A. fumigatus-infected mice by endogenous IL-18, TNF-α, and IL-12a
| Cytokine-specific MAb(s) | Cytokine activity (pg/lung) | % Inhibition |
|---|---|---|
| TNF-α | ||
| IgG2a | 6,535 + 525 | |
| Anti-II-18R MAb | 5,122 ± 912 | 22 |
| Anti-IL-12 MAb | 4,832 ± 1,014 | 27 |
| Anti-IL-18R MAb + anti-IL-12 MAb | 3,484 + 475* | 47 |
| IL-12 | ||
| IgG2a | 192 ± 27 | |
| Anti-IL-18R MAb | 122 ± 26 | 37 |
| Anti-TNF-α MAb | 100 + 36 | 48 |
| IFN-γ | ||
| IgG2a | 534 ± 64 | |
| Anti-IL-18R MAb | 229 ± 80* | 58 |
| Anti-TNF-α MAb | 270 ± 101* | 50 |
| Anti-IL-12 MAb | 58 ± 40* | 90 |
| Anti-IL-18R MAb + anti-IL-12 MAb | 37 + 24* | 94 |
Crl:CF-1 mice were administered IgG2a, anti-IL-18R MAb, anti-TNF-α MAb, and/or anti-IL-12 MAb prior to infection with A. fumigatus. At 24 and 48 hrs p.i., mice were euthanatized and their lungs were excised, homogenized, and filtered. Immunoreactive TNF-α was quantified in lung homogenates at 24 hpi, while IL-12 and IFN-γ proteins were quantified at 48 hpi by cytokine-specific ELISAs. Results represent the pooled mean ± standard error of the mean of two separate experiments, 8 to 10 animals per treatment group. Symbol: *, significantly less than in similarly infected mice administered IgG2a, P < 0.05.
Endogenous cytokines and resolution of primary A. fumigatus lung infection.
Because clearance of A. fumigatus from lungs of immunocompetent Crl:CF-1 mice is temporally correlated with local induction of cytokines, including IL-18, TNF-α, IL-12, and IFN-γ (Fig. 1 to 3), the biological relevance of each of these cytokines in the resolution of pulmonary aspergillosis was assessed. Mice were administered cytokine-specific neutralizing MAbs (anti-IL-18R MAb [10], anti-TNF-α MAb [22, 23], anti-IL-12 MAb [33]) or anti-IFN-γ MAb (9) (1 mg/mouse intraperitoneally) alone or in combination 2 h prior to infection with A. fumigatus. At 24, 72, and 144 hpi, the mice were humanely euthanatized and A. fumigatus CFU were quantified in lung homogenates. Preliminary experiments had demonstrated that there was no significant difference between recovery of A. fumigatus from lungs of mice treated with no antibody and recovery from lungs of similarly infected mice administered a control antibody (IgG1a or IgG2a) at all times studied (data not shown). Therefore, intrapulmonary growth of A. fumigatus in mice administered a cytokine-specific neutralizing MAb(s) was compared to that found in similarly infected untreated mice (i.e., control mice).
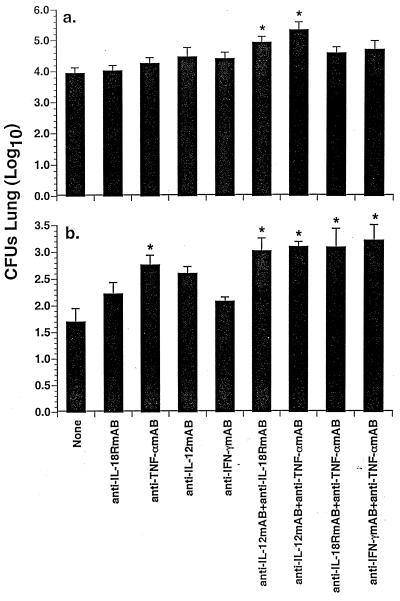
Results of these studies demonstrated that at 24 hpi, there was no significant difference between recovery of A. fumigatus from lungs of mice administered anti-IL-18R MAb, anti-TNF-α MAb, anti-IL-12 MAb, or anti-IFN-γ MAb alone or in combination and recovery from lungs of similarly infected control mice (data not shown). In contrast, at 72 hpi, simultaneous neutralization of endogenous IL-12 activity and either IL-18 or TNF-α activity resulted in a significant increase in A. fumigatus CFU in infected lung homogenates, as compared to similarly infected control mice (Fig. 4a). At 144 hpi, inhibition of endogenous TNF-α alone or in combination with neutralization of other cytokines, including IL-18, IL-12, or IFN-γ, resulted in significantly enhanced recovery of A. fumigatus from the lungs (Fig. 4b). However, there was no significant difference between recovery of A. fumigatus from the lungs of mice treated with an anti-TNF-α MAb alone and recovery from the lungs of mice administered an anti-TNF-α MAb in combination with these other cytokine-specific neutralizing MAbs. Simultaneous neutralization of IL-12 and IL-18 activity also resulted in a significant increase in A. fumigatus CFU in lung homogenates at 144 hpi. In contrast, inhibition of IFN-γ activity did not result in a significant increase in A. fumigatus in lung homogenates at any time p.i. These results demonstrate that intrapulmonary cytokines, including TNF-α, IL-12, and IL-18, likely play critical roles in the clearance of A. fumigatus from lungs of immunocompetent mice, while IFN-γ may not play a major role in host defense against primary pulmonary aspergillosis in this murine model system.
FIG. 4.
Role of endogenous IL-18, IL-12, TNF-α, and IFN-γ in resolution of primary A. fumigatus lung infection. Crl:CF-1 mice were administered anti-IL-18R MAb, anti-TNF-α MAb, anti-IL-12 MAb, and anti-IFN-γ MAb (1 mg/mouse intraperitoneally) alone or in combination 2 h prior to infection with A. fumigatus. At 24, 72, and 144 hpi, mice were euthanatized and their lungs were excised and homogenized. Intrapulmonary growth of A. fumigatus was quantified by culture of lung homogenates. Results of these studies demonstrated that growth of A. fumigatus in the lung in mice treated with cytokine-specific MAbs at 24 hpi was not significantly different from growth in similarly infected, untreated mice (data not shown). In contrast, significant differences in intrapulmonary growth of the fungus were apparent in mice treated with cytokine-specific MAbs at 72 (a) and 144 (b) hpi, as compared to growth in similarly infected, untreated mice. Results represent the pooled mean ± standard error of the mean of two separate experiments, seven to nine animals per treatment group. Symbol: ∗, significantly greater than similarly infected control mice, P < 0.05.
DISCUSSION
Previous studies have suggested a directive role of Th1 cytokines in leukocyte-mediated defense against primary pulmonary aspergillosis; however, cytokine networking in the lung in response to A. fumigatus has not been thoroughly investigated. In this study, the immunomodulatory role and biological relevance of endogenous cytokines, including IL-18, TNF-α, IL-12, and IFN-γ, in host defense against primary A. fumigatus lung infection were assessed in immunocompetent Crl:CF-1 mice infected with the fungus. Results of our studies demonstrated that IL-18, TNF-α, IL-12, and IFN-γ proteins were induced temporally (in this order) in the lung in response to inhaled A. fumigatus. However, while induction of TNF-α, IL-12, and IFN-γ immunoreactive proteins was associated with induction of the corresponding mRNAs, induction of immunoreactive IL-18 in infected mice occurred in the absence of induction of IL-18 mRNA. These results suggest that regulation of intrapulmonary IL-18 protein expression during A. fumigatus infection occurred, at least in part, by a posttranscriptional mechanism. IL-18 is synthesized as a precursor molecule (pro-IL-18) devoid of a signal sequence and requires the IL-1 β converting enzyme (caspase 1) for cleavage into a mature peptide (18, 20). The potential relationship between intrapulmonary IL-1 β converting enzyme activity and secretion of mature IL-18 during A. fumigatus lung infection remains to be explored.
Temporal analysis of cytokine expression in the lungs of infected mice demonstrated that induction of immunoreactive IL-18 in BALF preceded induction of TNF-α, IL-12, and IFN-γ proteins and corresponding mRNAs. IL-18 has previously been shown to have immunomodulatory effects on other cytokines, including TNF-α and IFN-γ (30, 35). Furthermore, modulation of IFN-γ expression by IL-18 is due in large part to synergistic effects of IL-18 with other cytokines, including IL-12 (1, 28, 30, 40, 53). Consequently, studies using cytokine-specific neutralizing MAbs were conducted to assess immunomodulatory effects of endogenous IL-18, TNF-α, and IL-12 on intrapulmonary cytokine activity in infected mice. Results of these studies (Table 1) demonstrated that IL-18 and IL-12 together regulated intrapulmonary TNF-α activity, while IL-18, TNF-α, and IL-12 regulated IFN-γ activity. These results demonstrate that IL-18, TNF-α, and IL-12 are key modulators of intrapulmonary cytokine activity during A. fumigatus lung infection.
Subsequent studies using cytokine-specific neutralizing antibodies were conducted to identify the potential biological relevance of endogenous IL-18, IL-12, TNF-α, and IFN-γ in host defense against pulmonary aspergillosis. Results of these studies (Fig. 4) demonstrated that IL-12 and IL-18 acted synergistically to facilitate resolution of A. fumigatus pulmonary infection in the immunocompetent host, as simultaneous inhibition of both cytokines but not of either cytokine alone resulted in the enhanced recovery of A. fumigatus from infected lung tissue. The mechanism by which IL-12 and IL-18 mediate the clearance of A. fumigatus from the lungs of immunocompetent Crl:CF-1 mice remains to be explored. Our results suggest that it occurs largely by an IFN-γ-independent mechanism, since inhibition of endogenous IFN-γ alone did not significantly alter clearance of A. fumigatus from the lung. Results of our in vivo studies are in agreement with those of a recent in vitro study which showed that IL-12-induced fungicidal activity of cultured human mononuclear phagocytes against A. fumigatus occurred by means of an IFN-γ-independent mechanism (43). In addition to modulating IFN-γ activity, IL-12 and IL-18 have pleiotropic effects, which include regulation of NK cell cytotoxicity (24, 39). The potential role of cytotoxic NK cells in resistance to primary A. fumigatus lung infection remains to be thoroughly investigated.
Results of our study contrast with those of a previous study, which demonstrated that inhibition of IL-12 alone resulted in enhanced intrapulmonary growth of A. fumigatus in experimentally infected BALB/c mice (8). While the reason for the disparity between results of these two studies is not completely understood, it is likely due, in part, to differences in A. fumigatus strains, inoculum doses, and/or murine strains (i.e., susceptible [BALB/c] versus resistant [Cr:CF1]) used.
In addition, inhibition of TNF-α, either alone or in combination with inhibition of other cytokines, including IL-12, IL-18, and IFN-γ, also resulted in significantly delayed clearance of A. fumigatus from the lung (Fig. 4). These results confirm those of earlier studies (27) and suggest that endogenous TNF-α plays a key role in host resistance to A. fumigatus pulmonary infection. TNF-α likely contributes to host defense against A. fumigatus by means of several mechanisms. Although not directly chemotactic for phagocytic cells, TNF-α induces leukocyte and endothelial cell expression of adhesion molecules, thus influencing phagocytic cell trafficking in the lungs (17, 27). In addition, TNF-α modulates chemokine expression, thereby modulating both the influx and activation of phagocytic cells in the lung (27).
In summary, using a murine model of A. fumigatus pulmonary infection in immunocompetent mice, we have demonstrated that proinflammatory cytokines, including IL-18, TNF-α, and IL-12, play key roles in host defense against pulmonary aspergillosis in the immunocompetent host, due to their effects on both local cytokine activity and clearance of A. fumigatus from the lung. These studies are the first to identify the potential role of IL-18 in innate immunity to pulmonary aspergillosis and to characterize immunomodulatory effects of IL-18 on intrapulmonary cytokine activity during A. fumigatus lung infection. Future studies which elucidate the role of endogenous cytokines in resistance to A. fumigatus lung infection are warranted, as they may provide new strategies for adjunct therapy for prevention and treatment of invasive pulmonary aspergillosis in immunocompromised patients.
ACKNOWLEDGMENTS
We thank Ferous Gheyas and Steven Novick from the Biostatistics Department at Schering Plough Research Institute for their assistance with analysis of the data. We also thank Frank Sabatelli from the Infectious Disease Department at Schering Plough Research Institute for his assistance with graphics.
REFERENCES
- 1.Ahn H J, Maruo S, Tomura M, Mu J, Hamaoka T, Nakanishi K, Clark S, Kurimoto M, Okamura H, Fujiwara H. A mechanism underlying synergy between IL-12 and IFN-gamma-inducing factor in enhanced production of IFN-gamma. J Immunol. 1997;159:2125–2131. [PubMed] [Google Scholar]
- 2.Berenguer J, Allende M C, Lee J W, Garrett K, Lyman C, Ali N M, Bacher J, Pizzo P A, Walsh T J. Pathogenesis of pulmonary aspergillosis. Granulocytopenia versus cyclosporine and methylprednisolone-induced immunosuppression. Am J Respir Crit Care Med. 1995;152:1079–1086. doi: 10.1164/ajrccm.152.3.7663787. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard D K, Djeu J Y, Klein T W, Friedman H, Steward II W E, Stewart E W. Protective effects of tumor necrosis factor in experimental Legionella pneumophila infections of mice via activation of PMN function. J Leukoc Biol. 1988;43:429–435. doi: 10.1002/jlb.43.5.429. [DOI] [PubMed] [Google Scholar]
- 4.Born T L, Thomassen E, Bird T A, Sims J E. Cloning of a novel receptor subunit, AcPL, required for interleukin-18 signaling. J Biol Chem. 1998;273:29445–29450. doi: 10.1074/jbc.273.45.29445. [DOI] [PubMed] [Google Scholar]
- 5.Cenci E, Mencacci A, Fe d'Ostiani C, Montagnoli C, Bacci A, Del Sero G, Perito S, Bistoni F, Romani L. Cytokine and T-helper-dependent immunity in murine aspergillosis. Res Immunol. 1998;149:445–454. doi: 10.1016/s0923-2494(98)80768-2. [DOI] [PubMed] [Google Scholar]
- 6.Cenci E, Mencacci A, Fe d'Ostiani C, Del Sero G, Mosci P, Montagnoli C, Bacci A, Romani L. Cytokine and T helper-dependent lung mucosal immunity in mice with invasive pulmonary aspergillosis. J Infect Dis. 1998;178:1750–1760. doi: 10.1086/314493. [DOI] [PubMed] [Google Scholar]
- 7.Cenci E, Perito S, Enssle K H, Mosci P, Latge J P, Romani L, Bistoni F. Th1 and Th2 cytokines in mice with invasive aspergillosis. Infect Immun. 1997;65:564–570. doi: 10.1128/iai.65.2.564-570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cenci E, Mencacci A, Del Sero G, Bacci A, Montagnoli C, Fe d'Ostiani C, Mosci P, Bachmann M, Bistoni F, Kopf M, Romani L. Interleukin-4 causes susceptibility in invasive pulmonary aspergillosis through suppression of protective type 1 responses. J Infect Dis. 1999;180:1957–1968. doi: 10.1086/315142. [DOI] [PubMed] [Google Scholar]
- 9.Cherwinski H M, Schumacher J H, Brown K D, Mosmann T R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987;166:1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debets R, Timans J C, Churakowa T, Zurawski S, de Waal Malefyt R, Moore K W, Abrams J S, O'Garra A, Bazan J F, Kastelein R A. IL-18 receptors, their role in ligand binding and function: anti-IL-18RAcPL antibody, a potent antagonist of IL-18. J Immunol. 2000;165:4950–4956. doi: 10.4049/jimmunol.165.9.4950. [DOI] [PubMed] [Google Scholar]
- 11.De Repentigny L, Petitbois S, Boushira M, Michaliszyn E, Senechal S, Gendron N, Montplaisir S. Acquired immunity in experimental murine aspergillosis is mediated by macrophages. Infect Immun. 1993;61:3791–3802. doi: 10.1128/iai.61.9.3791-3802.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diamond R D, Krzesicki R, Epstein B, Jao J. Damage to hyphal forms of fungi by human leukocytes in vitro: a possible host defense mechanism in aspergillosis and mucormycosis. Am J Pathol. 1978;91:313–328. [PMC free article] [PubMed] [Google Scholar]
- 13.Diamond R D, Clark R A. Damage to Aspergillus fumigatus and Rhizopus oryzae hyphae by oxidative and nonoxidative microbicidal production of human neutrophils in vitro. Infect Immun. 1982;38:487–495. doi: 10.1128/iai.38.2.487-495.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dixon D M, Polak A, Walsh T J. Fungus dose-dependent primary pulmonary aspergillosis in immunosuppressed mice. Infect Immun. 1989;75:1452–1456. doi: 10.1128/iai.57.5.1452-1456.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Djeu J Y. Modulators of immune responses to fungi. In: Murphy J W, Friedman H, Bendinelli M, editors. Fungal infections and immune responses. New York, N.Y: Plenum Press; 1993. pp. 521–532. [Google Scholar]
- 16.Duong M, Ouellet N, Simard M, Bergeron Y, Olivier M, Bergeron M G. Kinetic study of host defense and inflammatory response to Aspergillus fumigatus in steroid-induced immunosuppressed mice. J Infect Dis. 1998;178:1472–1482. doi: 10.1086/314425. [DOI] [PubMed] [Google Scholar]
- 17.Gamble J R, Harlan J M, Klebanoff S J, Vadas M A. Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proc Natl Acad Sci USA. 1985;82:8667–8671. doi: 10.1073/pnas.82.24.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, Wong W, Kamen R, Tracey D, Allen H. Caspase-1 processes IFN-gamma inducing factor and regulates LPS-induced IFN-γ production. Nature. 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 19.Gibson U E, Heid C A, Williams P M. A novel method for real time quantitative RT-PCR. Genome Res. 1996;6:995–1001. doi: 10.1101/gr.6.10.995. [DOI] [PubMed] [Google Scholar]
- 20.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming M A, Hayashi N, Higashino K, Okamura H, Nakanishi K, Kurimoto M, Tanimoto T, Flavell R A, Sato V, Harding M W, Livingston D J, Su M S. Activation of interferon-γ inducing factor, mediated by interleukin-1B converting enzyme. Science. 1997;275:206–209. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 21.Heid C A, Stevens J, Livak K J, Williams P M. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez-Caselles T, Stutman O. Immune functions of tumor necrosis factor. I. Tumor necrosis factor induces apoptosis of mouse thymocytes and can also stimulate or inhibit IL-6-induced proliferation depending on the concentration of mitogenic costimulation. J Immunol. 1993;151:3999–4012. [PubMed] [Google Scholar]
- 23.Hunter C A, Abrams J S, Beaman M H, Remington J S. Cytokine mRNA in the central nervous system of SCID mice infected with Toxoplasma gondii: importance of T-cell-independent regulation of resistance to T. gondii. Infect Immun. 1993;61:4038–4044. doi: 10.1128/iai.61.10.4038-4044.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyodo Y, Matsui K, Hayashi N, Tsutsui H, Kashiwamura S, Yamauchi H, Hiroishi K, Takeda K, Tagawa Y, Iwakura Y, Kayagaki N, Kuimoto M, Okamura H, Hada T, Yagita H, Akira S, Nakanishi K, Higashino K. IL-18 up-regulates perforin-mediated NK activity without increasing perforin messenger RNA expression by binding to constitutively expressed IL-18 receptor. J Immunol. 1999;162:1662–1668. [PubMed] [Google Scholar]
- 25.Levitz S M, Selsted M E, Ganz T, Lehrer R I, Diamond R D. In vitro killing of spores and hyphae of Aspergillus fumigatus and Rhizopus oryzae by rabbit neutrophil cationic peptides and bronchoalveolar macrophages. J Infect Dis. 1986;154:483–489. doi: 10.1093/infdis/154.3.483. [DOI] [PubMed] [Google Scholar]
- 26.Loebenberg D, Cacciapuoti A, Parmegiani R, Moss E L, Jr, Menzel F, Jr, Antonoacci B, Norris C, Yarosh-Tomaine T, Hare R S, Miller G H. In vitro and in vivo activities of SCH 42427, the active anantiomer of the antifungal agent SCH 39304. Antimicrob Agents Chemother. 1992;36:498–501. doi: 10.1128/aac.36.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehrad B, Strieter R M, Standiford T J. Role of TNF-α in pulmonary host defense in murine invasive aspergillosis. J Immunol. 1999;162:1633–1640. [PubMed] [Google Scholar]
- 28.Micallef M J, Ohtsuki T, Kohno K, Tanabe F, Ushio S, Namba M, Tanimoto T, Torigoe K, Fujii M, Ikeda M, Fukuda S, Kurimoto M. Interferon-γ-inducing factor enhances T helper 1 cytokine production by stimulated human T cells: synergism with interleukin-12 for interferon-γ production. Eur J Immunol. 1996;26:1647–1651. doi: 10.1002/eji.1830260736. [DOI] [PubMed] [Google Scholar]
- 29.Nagai H, Guo J, Choi H, Kurup V. Interferon-γ and tumor necrosis factor-α protect mice from invasive aspergillosis. J Infect Dis. 1995;172:1554–1560. doi: 10.1093/infdis/172.6.1554. [DOI] [PubMed] [Google Scholar]
- 30.Okamura H, Kashiwamura S, Tsutsui H, Yoshimoto T, Nakanishi K. Regulation of interferon-γ production by IL-12 and IL-18. Curr Opin Immunol. 1998;10:259–264. doi: 10.1016/s0952-7915(98)80163-5. [DOI] [PubMed] [Google Scholar]
- 31.Okamura H, Tsutsui H, Komatsu T, Yatsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, Akita K, Namba M, Tanabe F, Knoishi K, Fukuda S, Kurimoto M. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 32.Okamura H, Nagata K, Komatsu T, Tanimoto T, Nukata T, Tanabe Y, Akita K, Torigoe K, Okura T, Fukuda S. A novel costimulatory factor for gamma interferon induction found in the livers of mice causes endotoxic shock. Infect Immun. 1995;63:3966–3972. doi: 10.1128/iai.63.10.3966-3972.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozmen L, Pericin M, Hakimi J, Chizzonite R A, Wysocka M, Trinchieri G, Tagely M, Garotta G. Interleukin 12, interferon gamma, and tumor necrosis factor alpha are the key cytokines of the generalized Shwartzman reaction. J Exp Med. 1994;180:907–915. doi: 10.1084/jem.180.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piggot W R, Emmons C W. Device for inhalation exposure of animals to spores. Proc Soc Exp Biol Med. 1960;103:805–806. doi: 10.3181/00379727-103-25677. [DOI] [PubMed] [Google Scholar]
- 35.Puren A, Fantuzzi G, Gu Y, Su M, Dinarello C. Interleukin-18 (IFN-γ-inducing factor) induces IL-8 and IL-1B via TNF-α production from non-CD14+ human blood mononuclear cells. J Clin Investig. 1998;101:711–721. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qureshi M H, Ahang T, Koguchi Y, Nakashima K, Okamura H, Kurimoto N, Kawakami K. Combined effects of IL-12 and IL-18 on the clinical course and local cytokine production in murine pulmonary infection with Cryptococcus neoformans. Eur J Immunol. 1999;29:643–649. doi: 10.1002/(SICI)1521-4141(199902)29:02<643::AID-IMMU643>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 37.Rex J H, Bennett J E, Galin J I, Malech H L, Decarlo E S, Melnick D A. In vivo interferon-γ augments in vitro ability of chronic granulomatous disease neutrophils to damage Aspergillus hyphae. J Infect Dis. 1991;163:849–852. doi: 10.1093/infdis/163.4.849. [DOI] [PubMed] [Google Scholar]
- 38.Rhodes J C. Aspergillosis. In: Murphy T W, Friedman H, Bendinelli M, editors. Fungal infections and immune responses. New York, N.Y: Plenum Press; 1993. pp. 359–377. [Google Scholar]
- 39.Robertson M J, Soiffer R J, Wolf S F, Manley T J, Donahue C, Young D, Herrmann S H, Ritz J. Response of human natural killer cells to NK cell stimulatory factor (NKSF): cytolytic activity and proliferation of NK cells are differentially regulated by NKSF. J Exp Med. 1992;175:779–788. doi: 10.1084/jem.175.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson D, Shibuya K, Mui A, Zonin F, Murphy E, Sana T, Hartley S B, Menon S, Kastelein R, Bazan F, O'Garra A. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-γ production and activates IRAK and NFκB. Immunity. 1997;7:571–581. doi: 10.1016/s1074-7613(00)80378-7. [DOI] [PubMed] [Google Scholar]
- 41.Roilides E, Dimitriadou A, Kadiltsoglou I, Sein T, Karpouzas J, Pizzo P A, Walsh T J. IL-10 exerts suppressive and enhancing effects on antifungal activity of mononuclear phagocytes against Aspergillus fumigatus. J Immunol. 1997;158:322–329. [PubMed] [Google Scholar]
- 42.Roilides E, Uhlig K, Venzon D, Pizzo P A, Walsh T J. Enhancement of oxidative response and damage caused by human neutrophils to Aspergillus fumigatus hyphae by granulocyte colony-stimulating factor and gamma interferon. Infect Immun. 1993;61:1185–1193. doi: 10.1128/iai.61.4.1185-1193.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roilides E, Tsaparidou S, Kadiltsoglou I, Sein T, Walsh T J. Interleukin-12 enhances antifungal activity of human mononuclear phagocytes against Aspergillus fumigatus: implications for a gamma interferon-independent pathway. Infect Immun. 1999;67:3047–3050. doi: 10.1128/iai.67.6.3047-3050.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roilides E, Katsifa H, Walsh T J. Pulmonary host defenses against Aspergillus fumigatus. Res Immunol. 1998;149:454–465. doi: 10.1016/s0923-2494(98)80769-4. [DOI] [PubMed] [Google Scholar]
- 45.Schaffner A, Douglas H, Braude A. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Investig. 1982;69:617–631. doi: 10.1172/JCI110489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sugawara I, Yamada H, Kaneko H, Mizuno S, Takeda K, Akira S. Role of interleukin-18 (IL-18) in mycobacterial infection in IL-18-gene-disrupted mice. Infect Immun. 1999;67:2585–2589. doi: 10.1128/iai.67.5.2585-2589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomura M, Zhou X Y, Maruo S, Ahn H J, Hamaoka T, Okamura H, Nakanishi K, Tanimoto T, Kurimoto M, Fujiwara H. A critical role for IL-18 in proliferation and activation of NK1.1+ CD3− cells. J Immunol. 1998;160:4730–4746. [PubMed] [Google Scholar]
- 48.Torigoe I, Ushio S, Okura T, Kobayashi S, Taniai M, Kunikata T, Murakami T, Sanou O, Kojima H, Fujii M, Ohta T, Ikeda M, Ikegami H, Kurimoto M. Purification and characterization of the human interleukin-18 receptor. J Biol Chem. 1997;272:25737–25742. doi: 10.1074/jbc.272.41.25737. [DOI] [PubMed] [Google Scholar]
- 49.Waldorf A R, Levitz S M, Diamond R D. In vivo bronchoalveolar macrophage defense against Rhizophus oryzae and Aspergillus fumigatus. J Infect Dis. 1984;150:752–760. doi: 10.1093/infdis/150.5.752. [DOI] [PubMed] [Google Scholar]
- 50.Walsh T. Invasive aspergillosis in patients with neoplastic diseases. Semin Respir Infect. 1990;5:111–122. [PubMed] [Google Scholar]
- 51.Weinberger M, Elattar D, Marshall D, Steinberg S M, Redner R L, Young N S, Pizzo P A. Patterns of infection in patients with aplastic anemia and the emergence of Aspergillus as a major cause of death. Medicine. 1992;71:24–43. doi: 10.1097/00005792-199201000-00003. [DOI] [PubMed] [Google Scholar]
- 52.Xu D, Chan W L, Leung B P, Hunter D, Schulz K, Carter R W, McInnes I B, Robinson J H, Liew F Y. Selective expression and functions of interleukin 18 receptor on T helper (Th) type 1 but not Th2 cells. J Exp Med. 1998;188:1485–1492. doi: 10.1084/jem.188.8.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshimoto T, Takeda K, Tanaka T, Ohkusu K, Kashiwamura S, Okamura H, Akira S, Nakanishi K. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells and B cells: synergism with IL-18 for IFN-γ production. J Immunol. 1998;161:3400–3407. [PubMed] [Google Scholar]