Abstract
Heart failure (HF) is one of the leading causes of maternal mortality and morbidity in the United States. Peripartum cardiomyopathy (PPCM) constitutes up to 70% of all HF in pregnancy. Cardiac angiogenic imbalance caused by cleaved 16kDa prolactin has been hypothesized to contribute to the development of PPCM, fueling investigation of prolactin inhibitors for the management of PPCM. We conducted a systematic review and meta-analysis to assess the impact of prolactin inhibition on left ventricular (LV) function and mortality in patients with PPCM. We included English language articles from PubMed and EMBASE published upto March 2022. We pooled the mean difference (MD) for left ventricular ejection fraction (LVEF) at follow-up, odds ratio (OR) for LV recovery and risk ratio (RR) for all-cause mortality using random-effects meta-analysis. Among 548 studies screened, 10 studies (3 randomized control trials (RCTs), 2 retrospective and 5 prospective cohorts) were included in the systematic review. Patients in the Bromocriptine + standard guideline directed medical therapy (GDMT) group had higher LVEF% (pMD 12.56 (95% CI 5.84-19.28, I2=0%) from two cohorts and pMD 14.25 (95% CI 0.61-27.89, I2=88%) from two RCTs) at follow-up compared to standard GDMT alone group. Bromocriptine group also had higher odds of LV recovery (pOR 3.55 (95% CI 1.39-9.1, I2=62)). We did not find any difference in all-cause mortality between the groups. Our analysis demonstrates that the addition of Bromocriptine to standard GDMT was associated with a significant improvement in LVEF% and greater odds of LV recovery, without significant reduction in all-cause mortality.
Introduction
Heart failure (HF) is one of the leading causes of maternal morbidity and mortality in the United States1. Peripartum cardiomyopathy (PPCM) accounts for nearly 70% of all HF in pregnant women.1,2 The European Society of Cardiology defines PPCM as an idiopathic cardiomyopathy that presents as HF with left ventricular (LV) systolic dysfunction in late pregnancy through postpartum period.3 Significant geographical and racial variations have been noted in the incidence of PPCM, with the lowest in Japan (1 in 20,000 live births)4 and the highest in Nigeria (1 in 100 live births).5 The clinical course varies from mild disease with spontaneous recovery to persistent myocardial dysfunction and severe HF, with death occurring in roughly 10% of patients.6,7
While the etiology of PPCM is not entirely understood, multiple hypotheses such as nutritional deficiencies, autoantibodies, genetic mutations, infectious, and vascular processes have been proposed.8,9 Current evidence favors a “double hit” hypothesis involving a vascular insult in addition to genetic predisposition.10,11 The casual role of the cleaved prolactin fragment in the cardiac angiogenic imbalance and development of PPCM has been established in various pre-clinical and clinical models of PPCM.11,12
The current management for PPCM centers around the standard guideline-directed medical therapy (GDMT) for HF with reduced ejection fraction.13 The discovery of prolactin as a potential mediator of PPCM pathophysiology has motivated interest in investigating prolactin inhibition as a potential targeted treatment for PPCM.14 Here, we review the role of prolactin inhibition in the management of PPCM. While a few studies have assessed the efficacy of the prolactin inhibitor, bromocriptine, on the improvement of LV function and mortality, the data are mixed and are limited to small underpowered studies. Moreover, the use of bromocriptine is restricted in the postpartum population due to the increased risk of thromboembolism and disruption of lactation.15,16 In this context, we executed a systematic review and meta-analysis to examine the impact of prolactin inhibitors on LV function and mortality in patients with PPCM.
Pathophysiological Mechanisms
The last decade has witnessed a significant progress in understanding the pathophysiological mechanisms underlying PPCM (Fig 1). Various genetic, environmental, inflammatory, and vascular processes in addition to the physiologic peripartum stress have been postulated in the development of PPCM. Next-generation DNA sequencing of 466 patients with PPCM showed that 10.4% express pathologic truncating mutations in TTN gene encoding titin, a large structural sarcomeric protein that contributes to force generation in the heart.17 The prevalence of these mutations in PPCM are comparable to those of dilated cardiomyopathy (DCM) and roughly 20 times higher than reference population.17,18 Similarly mutations seen in DSP, FLCN, and BAG3 genes further support genetic predisposition in PPCM.17 Autoantibodies to cardiac myocyte-specific proteins such as myosin and Troponin I have also been identified in PPCM, highlighting the involvement of autoimmune and inflammatory processes.19 The peripartum period is characterized by enhanced oxidative stress and anti-angiogenic signals such as PRL, soluble fms-like tyrosine kinase-1 (sFLT1) and angiotensin II.20,21 In a normal pregnancy, these anti-angiogenic signals are countered by pathways such as signal transducer and activator of transcription 3 (STAT3), peroxisome proliferator-activated receptor c coactivator 1α (PGC-1 α) and phosphoinositid-3-kinase (PI3) and protein kinase B (Akt) which aid in up-regulation of anti-oxidants and angiogenesis.22,23 In patients with pre-eclampsia and PPCM, dysregulation of protective STAT3, PGC-1α, and PI3/Akt pathways and abnormal elevations in anti-angiogenic signals (sFLT1, PRL), cause imbalance and contributes to cardiomyopathy.12,14,24
FIG 1. Pathophysiologic mechanisms of peripartum cardiomyopathy.
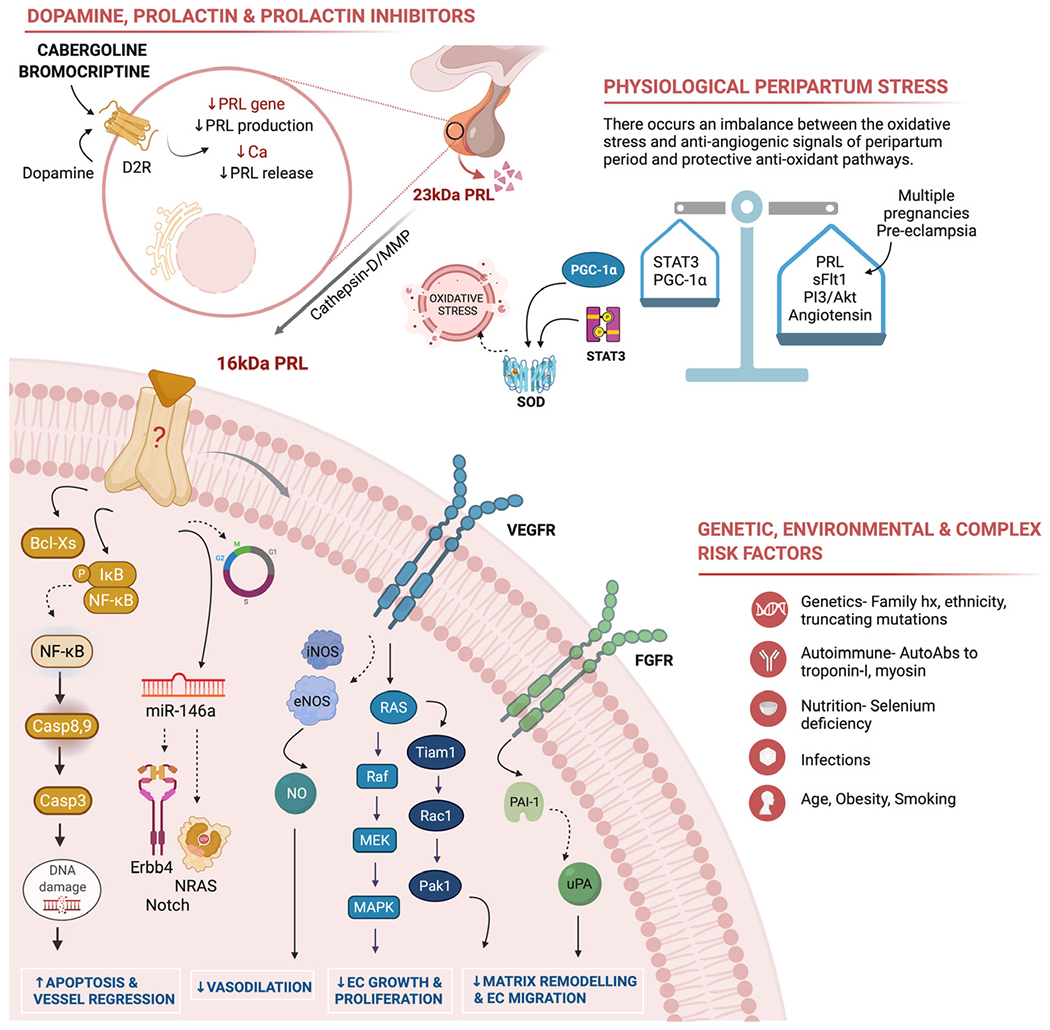
Top left panel – Bromocriptine acts on the G-protein coupled dopamine D2 receptor which in turn suppresses prolactin (PRL) gene expression, inhibits lactotroph proliferation61 and decreases PRL release from secretory granules. Inhibiting PRL release further inhibits 16kDa fragment generation by proteolytic enzymes. Top right panel — Cardiac angiogenic imbalance is caused by the oxidative stress and anti-angiogenic signals associated with the peripartum period [PRL, soluble FLT1 and angiotensin II] and protective antioxidant and angiogenic pathways [signal transducer and activator of transcription 3 (STAT3), peroxisome proliferator-activated receptor c coactivator 1α (PGC-1α) and phosphoinositid-3-kinase (PI3) and protein kinase B (Akt)]. Bottom right panel – Other genetic, inflammatory, autoimmune, infectious, and environmental have been implicated in the development of PPCM. Bottom left circular panel – Demonstrates the anti-angiogenic mechanisms of 16kDa PRL fragment. i) Apoptosis and vessel regression: 16kDa PRL activates nuclear factor kappa B (NFkB) and Bcl-XS which initiates apoptotic pathways causing cell death. Cell cycle arrest is mediated through effect on cyclin D1, B1 and cyclin-dependent kinase inhibitors. Induction of microRNA-146a expression which affects endothelial cell proliferation through downregulation of NRAS (neuroblastoma RAS viral oncogene homolog) and Erb-B2 receptor tyrosine kinase 4 (Erbb4). ii) Inhibition of vasodilation: 16kDa PRL decreases NO (nitric oxide)-mediated vasodilation by blocking both eNOS (endothelial nitric oxide synthase) and iNOS (inducible nitric oxide synthase) expression. iii) Inhibition of endothelial cell (EC) growth and proliferation: 16kDa PRL prevents EC proliferation via inhibition of fibroblast growth factor (FGF)-and vascular endothelial growth factor (VEGF)-mediated activation of the mitogen-activated protein kinases (MAPK) [Ras/Raf/MEK/MAPK signal pathway]. iv) Inhibition of EC migration and vascular remodeling: 16kDa PRL decreases EC migration via downregulation of the Ras-Tiam1-Rac1-Pak1 signaling pathway and plasminogen activator inhibitor-1 (PAI-1)-mediated inhibition of urokinase plasminogen activator (uPA).
One of the key hypothesis includes a cardiac angiogenic imbalance caused by higher oxidative stress and anti-angiogenic signals of the postpartum period in a susceptible host with inadequate protective pro-angiogenic defenses.12 The causal role of 16 kilodalton N-terminal fragment of prolactin hormone (16kDa PRL) in the pathogenesis has been established in various experimental and clinical models.14,25 Through their work on STAT3 knockout mice, Kleiner et al.14 established the role of cathepsin-D in the cleavage of 23kDa PRL to its 16kDa fragment. Unprecedented oxidative stress provoked by many of the above factors triggers this cleavage. The 16kDa PRL is a potent anti-angiogenic and pro-apoptotic peptide initially identified by Ferrarra et al. in bovine brain endothelial cells.26,27 The 16kDa PRL disrupts various steps in angiogenesis including endothelial cell migration, growth and proliferation, cell-cell interactions, vessel remodeling, and maturation.28 Endothelial cell proliferation is affected through inhibition of basal fibroblast growth factor (b-FGF) and vascular endothelial growth factor (VEGF) mediated activation of the mitogen-activated protein kinases (MAPK) [Ras/Raf/MEK/MAPK signal pathway]29. Furthermore, 16kDa PRL induced expression of microRNA-146a has been shown to affect endothelial proliferation and metabolism through down-regulation of NRAS (neuroblastoma RAS viral oncogene homolog) and Erb-B2 receptor tyrosine kinase 4 (Erbb4).30 Lemmens et al. showed that inhibition of cardioprotective ErbB signaling in pregnant mice causes LV dysfunction and premature death.31 In a bovine aortic endothelial cell model, 16kDa PRL caused cell cycle arrest G0 −G1 and the G2 −M phases through its effects on cyclin D1, B1 and cyclin-dependent kinase inhibitors such as p21 and p27.32 Vasoinhibins stimulate plasminogen activator inhibitor-1 (PAI-1), an inhibitor of urokinase plasminogen activator (uPA) thereby affecting endothelial cell migration as well as extracellular matrix remodeling and degradation.33–35 16kDa PRL also inhibits endothelial cell migration via downregulation of the Ras-Tiam1-Rac1-Pak1 signaling pathway.36 The 16kDa prolactin decreases nitric oxide (NO) production by blocking both eNOS and iNOS (endothelial and inducible nitric oxide synthase) expression, thereby inhibiting vasodilation and vascular remodeling.37,38 The vasoinhibins ultimately contribute to anti-angiogenesis through endothelial cell apoptosis and blood vessel regression. The 16kDa prolactin activates nuclear factor kappa B (NFkB) which initiates both intrinsic and extrinsic apoptotic pathways via initiator and effector caspases 8, 9, and 3.39 In addition, anti-apoptotic Bcl-XL is converted to pro-apoptotic Bcl-XS aiding in cell death.40 Hence, therapeutic interventions aimed at blocking the prolactin pathway will prevent 16kDa PRL generation and disease progression in PPCM.
Methods
Search Strategy and Study Selection
We conducted a systematic review and meta-analysis in accordance with the PRISMA reporting guidelines, using the Covidence platform.41 We conducted a comprehensive literature search in electronic databases such as PubMed and Embase from inception to 15th March, 2022 using the search strategy presented in the supplementary document (Supplementary section I). In addition, we manually searched for eligible studies from the references of the included studies and relevant systematic and narrative reviews. Only articles and conference abstracts published in English language were included. We included clinical trials, prospective and retrospective cohorts, and case-control studies. A protocol was submitted to PROSPERO and registered with the number CRD42022316658.
We included studies that met all the following criteria: (1) the study included patients with PPCM, defined using the European Society of Cardiology’s definition,3 as the presence of signs and symptoms of HF with LV systolic dysfunction in the late peripartum period without any underlying cause; (2) the study had either of the 2 comparison groups, i) bromocriptine or cabergoline + standard GDMT versus standard GDMT alone ii) short-term versus long-term bromocriptine or cabergoline therapy; (3) the study assessed at least one of the outcomes of interest at follow-up; LVEF%, LV recovery, or all-cause mortality. LVEF% was measured by echocardiography at follow-up and LV recovery was defined as an LVEF>50% at 6 months follow-up.
Literature Screening and Data Extraction
All the studies were imported to the Covidence platform for screening and data extraction. After removing duplicates, 2 independent reviewers screened the title and abstracts, followed by full texts for eligibility. The abstracts and full texts of articles were screened by any 2 of the investigators (AK, RR, RKS, and VC), and conflicts, if any, were resolved by a third investigator (MM). Two reviewers independently extracted data from the studies using a pre-designed data extraction form in the Qualtrics platform,42 and discrepancies were cleared by a third reviewer. Data on study characteristics, sample size, comparison groups, bromocriptine dosing and frequency, and follow-up periods were collected. In addition, baseline patient characteristics, including demographics, medical and obstetric history, and LVEF% were collected. Definitions, time points, effect estimates, and sizes of the outcomes were noted. The Newcastle-Ottawa scale43 and the Cochrane collaboration tool44 for risk of bias assessment were used to assess the quality of non-randomized studies and randomized trials respectively
Statistical Analysis
We used random-effects meta-analysis for our outcomes of interest. We used pooled mean difference (pMD) to assess the difference in LVEF% between the bromocriptine and control groups at the time of follow-up. We examined the association between the LV recovery and bromocriptine use using pooled odds ratio (pOR) with 95% CI. The association between the bromocriptine use and mortality were reported as pooled risk ratio (pRR). Unadjusted effect sizes were calculated from the summary data for the binary exposures. Pre calculated effect sizes were pooled when available. Pooled adjusted effect sizes were unable to be obtained because of the lack of consistency in the reporting of the parameters. Statistical heterogeneity across the studies was assessed by forest plots, I2, and Tau2 statistics. Meta-analyses were stratified based on the study designs. Potential publication bias was assessed using visual inspection of the funnel plot and statistically using Egger’s test when appropriate. All analyses were performed using Stata 16c (StataCorp, version 16).45
Results
Literature Search, Study Characteristics and Quality Assessment
Our search strategy revealed 548 relevant studies from 2 databases (175 from PubMED and 373 from Embase). After excluding 145 duplicates, we performed title and abstract review for 403 articles, of which 382 articles did not meet eligibility criteria and therefore excluded. The study selection algorithm is outlined in Figure 2. Twenty-one studies were selected through the title and abstract review. Among them, three were conference abstracts and did not have full texts. Full texts were assessed for 18 studies, of which a total of 10 studies satisfied the inclusion criteria (three randomized control trials,25,46,47 2 retrospective48,49 and 5 prospective cohorts50–54). A total of 749 patients were studied from the articles included in our systematic review. Among the 10 studies included, 4 studies reported data on LVEF%,25,46,48,52,54 5 studies reported LV recovery,25,50–52,54 and 6 studies assessed mortality risks at follow-up.25,46,48,50,52,53 In addition, 1 study compared 1 week versus 8 weeks bromocriptine therapy47 and 1 study studied cabergoline therapy.49 The characteristics of the included studies are displayed in Table 1. The median age of the patients ranged between 26 and 35 years. The mean baseline LVEF ranged from 25% to 36% in the included studies. Quality assessment for cohort studies using the Newcastle-Ottawa scale (maximum score of 9) revealed that 5/7 studies scored 9, one study scored 6 and the other study score 5. With respect to the RCTs, quality assessment using the Cochrane Risk of bias tool, revealed that one study had low risk of bias while the other 2 studies had intermediate risk of bias (Supplementary Table 1, Supplementary Figure 1). Funnel plots for the individual outcomes were performed to assess publication bias, but Egger’s test was not performed due to the small number (<10) of included studies.
FIG 2.
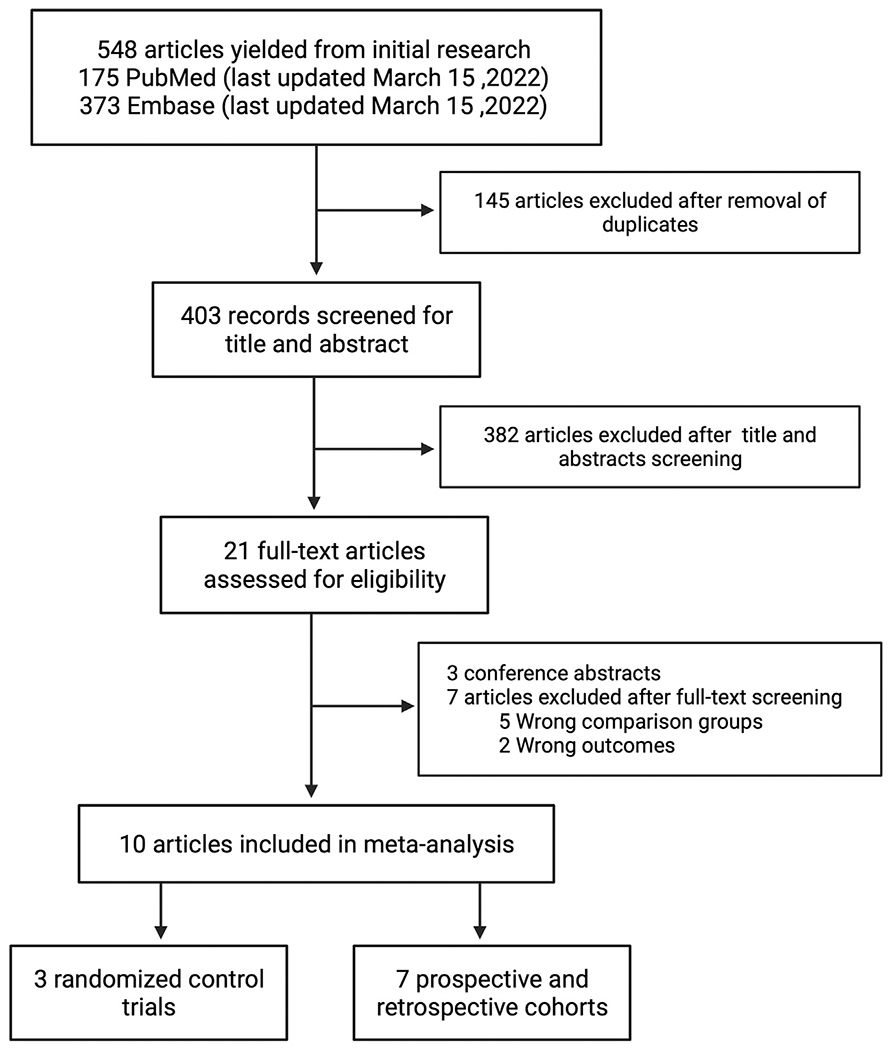
Study selection algorithm.
TABLE 1.
Study characteristics of the included studies
| First author, Year | Country | Study design | Sample size | Inclusion criteria | Intervention group (n) | Control group (n) | Age (ys) (mean ± SD) | Black (%) | Parity(n) | Gest HTN (%) | Baseline LVEF (%) | BR dose | Outcomes and follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biteker53 2018 | Turkey | Prospective cohort | 52 | LVEF< 45 | BR+ Standard treatment (15) | Standard treatment alone (37) | 28±5 | - | 2.6±1 | 15 | 25±6 | 2.5mg bid 2weeks then, 2.5mg po 6weeks | LV recovery (EF>50) at 6 months, All-cause mortality at 39±15 months |
| Haghikia53 2013 | Germany | Prospective cohort | 113 | LVEF< 45 | BR+ Standard treatment (64) | Standard treatment alone (32) | 34±6 | - | 2±7 | 50 | 31±14 | 2.5-5mg po 4weeks | LV recovery (EF>55) at 6±3 months |
| Hilfiker-Kleiner54 2017 | Multi-centric | Prospective cohort | 34 | LVEF< 35 | BR+ Standard treatment (21) | Standard treatment alone (9) | 31±6 | - | 3±4 | 6 | 32±8 | 2.5-5mg po 4weeks | LVEF% and LV recovery (EF>50) at 6 months, All-cause mortality at 6 months |
| Sliwa28 2010 | South Africa | Randomized control trial | 20 | LVEF< 45 | BR+ Standard treatment (10) | Standard treatment alone (10) | 26±8 | - | 1.8±3 | - | 27±8 | 2.5mg bid 2weeks then, 2.5mg po 6weeks | LVEF% and LV recovery (EF>50) at 6 months, All-cause mortality at 6 months |
| Tremblay-Gravel50 2019 | Canada | Retrospective cohort | 76 | LVEF< 45 | BR+ Standard treatment (8) | Standard treatment alone (68) | 31±5 | - | 2±7 | 13 | 29±12 | 2.5mg bid 2weeks then, 2.5mg po 6weeks | LVEF% at 6 months, All-cause mortality at 25±61months |
| Azibani55 2020 | Multi-centric | Prospective cohort | 151 | LVEF< 45 | BR+ Standard treatment (97) | Standard treatment alone (54) | 31±6 | 48 | 2±5 | 26 | 27± 9 | - | All-cause mortality at 6 months |
| Ersboll51 2017 | Denmark | Retrospective cohort | 61 | LVEF< 45 | Cabergoline + Standard treatment (24) | ||||||||
| Standard treatment alone (37) | 32±6 | - | - | 54 | 27±9 | Cabergoline 0.50mg po 2days | LV recovery (EF>55) at 12 months | ||||||
| Kurbanov56 2020 | Uzbekis-tan | Prospective cohort | 43 | LVEF< 45 | BR+ Standard treatment (21) | Standard treatment alone (22) | 30±5 | - | - | 19 | 35±8 | 2.5mg bid 2weeks then, 2.5mg po 2weeks | LVEF% and LV recovery (EF>55) at 12 months |
| Yaméogo47 2017 | Burkina Faso | Randomized control trial | 96 | LVEF< 45 | BR+ Standard treatment (48) | Standard treatment alone (48) | 29±3 | 100 | 3.6±2 | 0 | 36±5 | 2.5mg bid 4weeks | LVEF% at 12 months, All-cause mortality at 12 months |
| Hilfiker-Kleiner49 2017 | Germany | Randomized control trial | 63 | LVEF< 35 | Short-term BR [1week] (32) | Long-term BR [8weeks] (31) | 34±5 | 3 | 1.7±4 | 29 | 28± 9 | 2.5mg po 1week (1W) 5mg po 2weeks then, 2.5mg po 6weeks (8W) | LV recovery (EF>50) at 6 months |
Follow-up LVEF%
A total of 269 patients were included from the 4 studies reporting LVEF% at follow-up. The mean follow-up period during which LVEF% was determined in the study groups ranged from 6 to 12 months. The bromocriptine group had a higher LVEF% when compared to the control group in 2 RCTs (pMD 14.25 (95% CI 0.61-27.89, I2=88.2%)) and in 2 cohort studies (pMD 12.56 (95% CI 5.84-19.28, I2=0%)) (Table 2, Fig 3).
TABLE 2.
Association of bromocriptine use with follow-up LVEF%, LV recovery and all-cause mortality in patients with PPCM using random effects meta-analysis
| Outcome | Number of studies | Effect estimate | Effect size | P-value | I2 statistics |
|---|---|---|---|---|---|
| LVEF% at follow-up (cohorts) | 2 | Mean difference | 12.56 (5.84 to 19.28) | <0.001 | 0% |
| LVEF% at follow-up (RCTs) | 2 | Mean difference | 14.25 (0.61 to 27.89) | 0.04 | 88.2% |
| LVEF recovery (cohorts) | 5 | Odds ratio | 3.55 (1.39 to 9.10) | 0.01 | 62% |
| All-cause mortality (cohorts) | 4 | Relative risk | 0.71 (0.30 to 1.67) | 0.43 | 0% |
| All-cause mortality (RCTs) | 2 | Relative risk | 0.53 (0.26 to 1.07) | 0.08 | 0% |
FIG 3.
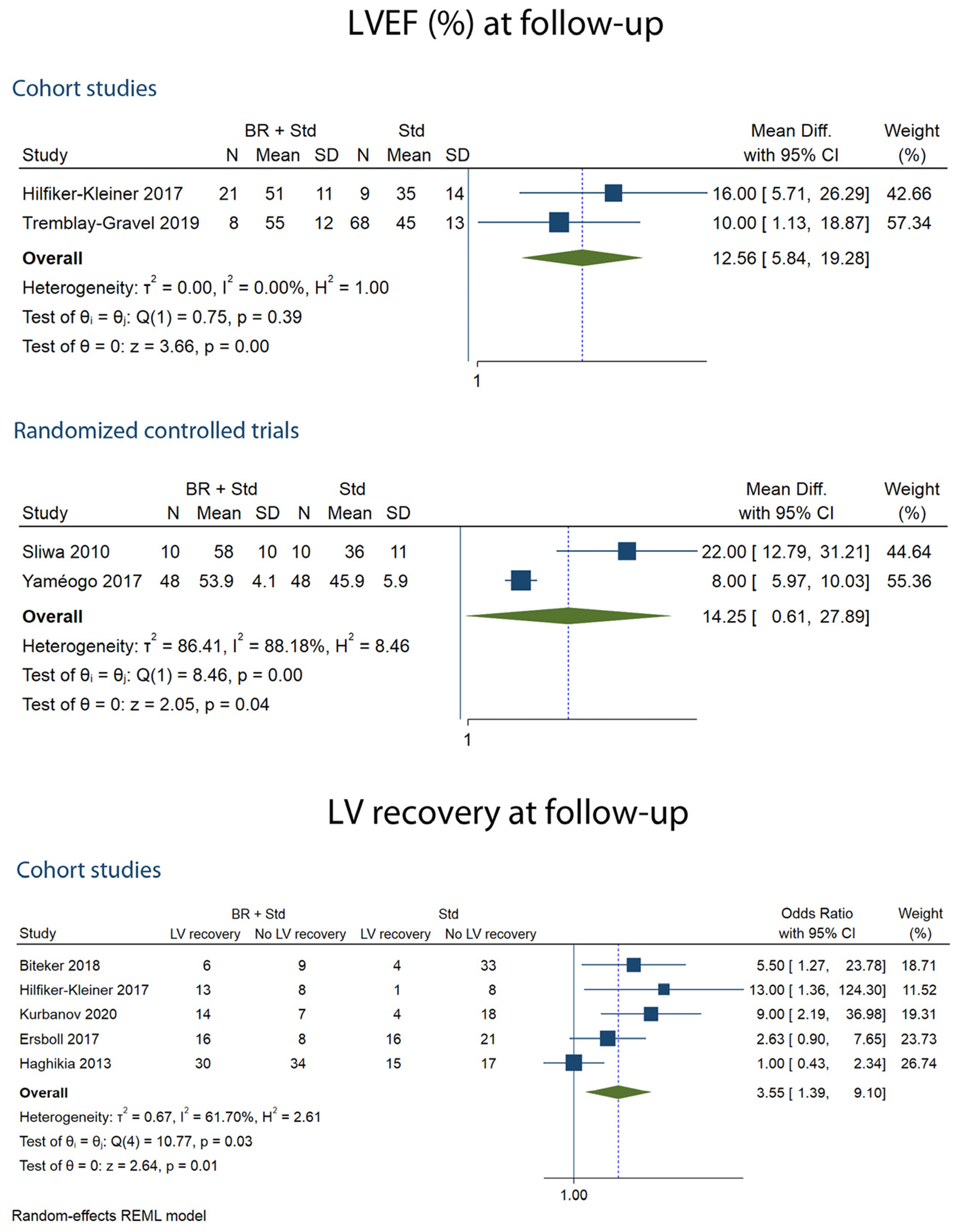
Forest plots showing LVEF% and LV recovery at follow-up.
LV Recovery
Of the 5 studies that determined LV recovery, 386 patients were included. LV recovery was assessed at 6 months follow-up. Compared with control, bromocriptine was associated with greater odds of LV recovery (pOR 3.55 (95% CI, 1.39-9.10, I2=61.7%)) (Table 2, Fig 3). One study of LV recovery reported a higher adjusted HR (aHR 2.26 (95% CI 1.15-5.55)) for the bromocriptine group.50
All-cause Mortality
Of the 6 studies that examined the association between the bromocriptine therapy and risk of mortality, an aggregate of 429 patients were assessed. The mean follow-up duration for the assessment of mortality ranged between 6 months and 5 years. Pooled RR for mortality was obtained from the summary data reported as categorical variables in the studies. Bromocriptine use was not associated with all-cause mortality in the pooled estimates of the two RCTs (pRR 0.53 (95% CI 0.26-1.07, I2=0%)) and the 4 cohorts (pRR 0.71, 95% CI 0.30-1.67, I2=0%)) (Table 2, Fig 4). Of the 8 studies, reporting mortality, none of them reported pre-calculated RR or hazard ratio (HR) for all-cause mortality.
FIG 4.
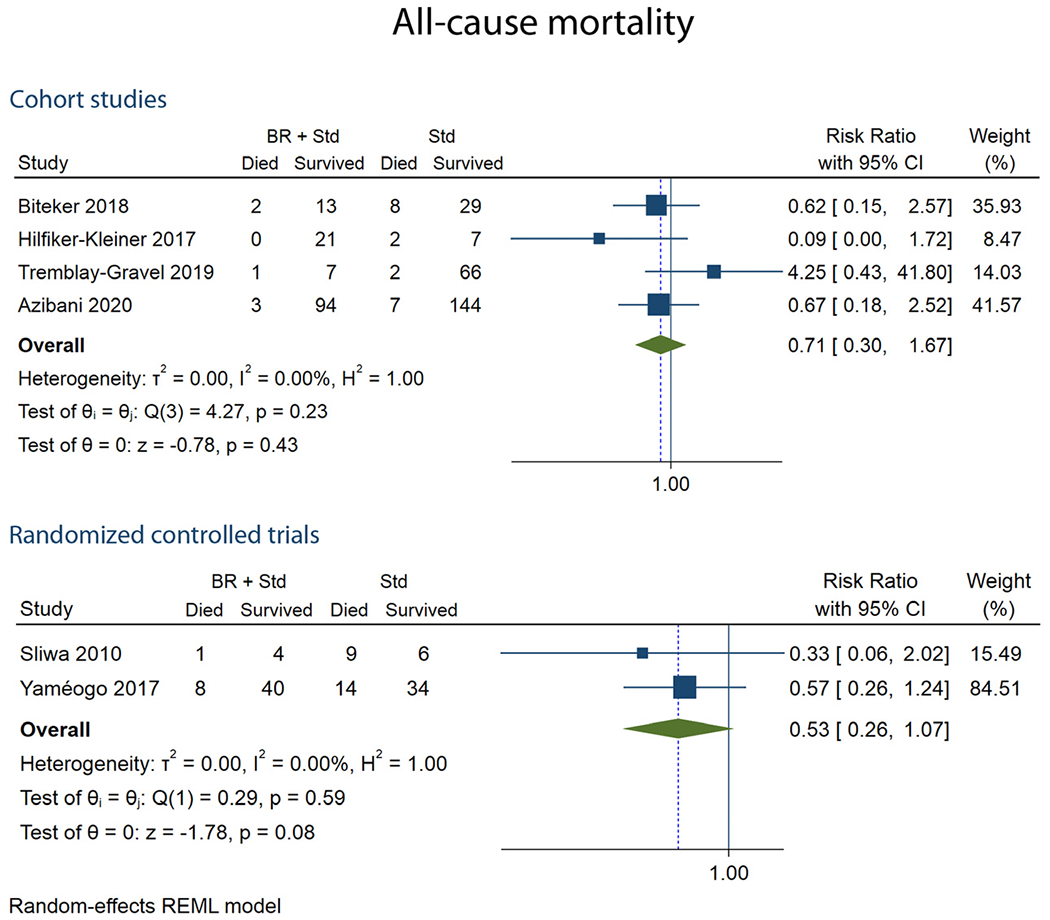
Forest plots showing all-cause mortality at follow-up.
Sensitivity Analysis
For the association of bromocriptine use with the LVEF% at follow-up, sensitivity analysis by including conference abstracts was consistent with the above analysis with a higher pooled mean LVEF% (pMD 8.12 (95% CI 4.14 - 12.10, I2=0%)) in the bromocriptine group. Sensitivity analysis by including conference abstracts did not change the association of bromocriptine use with mortality (pRR 0.74, (95% CI 0.34-1.63, I2=0%)) (Supplementary Table 2, 3 and Supplementary Figure 2).
Cabergoline
In the study by Ersboll et al.49 cabergoline + standard GDMT was compared against standard GDMT, the adjusted OR for recovery for the cabergoline group was 4.64 (95% CI 1.18-18.24).
Discussion
Our systematic review and meta-analyses demonstrate that the addition of bromocriptine to the standard GDMT is associated with a significant improvement in LVEF% at follow-up. In addition, we found that the odds of LV recovery (LVEF>50% at follow-up) were higher in the bromocriptine group compared to the non-bromocriptine group, but there was no a significant reduction in all-cause mortality between the 2 groups.
The current recommendations for the management of PPCM mainly focuses on standard GDMT for systolic dysfunction and lack disease-specific therapies.13 Experimental models of PPCM have identified potential disease-specific targets including microRNA-146a, VEGF, TNF-α, relaxin-2, β-adrenergic receptors, and prolactin.12,14,30,55,56 A deeper understanding of PRL in PPCM pathophysiology has motivated interest in prolactin inhibitors for the management of PPCM. Sliwa et al. conducted the first randomized control trial that exploited the prolactin hypothesis and studied bromocriptine addition to standard GDMT.25 Through its prolactin inhibitory effect, bromocriptine, blocks 16kDa fragment generation thereby preventing myocardial damage and subsequent PPCM development.14 Bromocriptine has also been shown to be beneficial in PPCM through its immunomodulatory and cytoprotective effects.57,58 On the other hand, beyond the need for lactation cessation, concerns have been raised with bromocriptine treatment, such as increased risk of thromboembolic events and the potential need for prophylactic anti-coagulation.15 Despite the growing preclinical evidence regarding the critical role of prolactin in the development of PPCM, clinical data on bromocriptine remains scarce and bromocriptine is still considered an experimental therapy.13
We demonstrate a significant improvement in the LVEF at follow-up in the bromocriptine group compared to the non-bromocriptine group in the meta-analysis of estimates from 2 cohort studies. These results were consistent in the meta-analysis from the randomized control trials. In addition, among the studies reporting data on LV recovery, we showed that the bromocriptine group had a significantly higher odds of recovery. In terms of bromocriptine dosing, there were few variations across studies ranging from 2.5 to 5 mg per day for 4 weeks to 2.5 twice daily for 2 weeks, followed by 2.5 mg daily for 6 weeks. Nevertheless, the recent trial comparing 1 week and 8 weeks bromocriptine therapy showed similar LV recovery rates across groups.47 Interestingly, the subgroup analysis performed in the above mentioned trial showed beneficial effects of prolonged treatment in patients with baseline LVEF less than 25%.47 In a prior systematic review, bromocriptine was shown to have survival benefits, but only when RCTs and cohort studies were pooled together in a meta-analysis which is not ideal.59 Our meta-analysis did not show any significant difference in all-cause mortality with the addition of bromocriptine to the standard GDMT. One of the possible reasons is the small sample size and short follow-up periods leading to low event rates in the included studies.
Regarding the efficacy of bromocriptine in prolactin inhibition, 2 studies reported data on serum prolactin levels at follow-up. Kurbanov et al. found a significant reduction in the bromocriptine group compared to the non-bromocriptine group, but Sliwa et al. found no difference between the groups.25,54 The non-availability of lactation data in the control group might confound the above results.25 When considering data on maternal safety, Sliwa et al. did not report any complications with respect to thromboembolic events attributable to bromocriptine in the follow-up period.25 However, Kleiner et al. found 3 possible adverse events related to bromocriptine use, including 2 venous embolisms and a peripheral arterial occlusion.52 Most studies recommended for prophylactic anticoagulation in patients with LVEF less than 25%, while some prophylactically anticoagulated all patients in the bromocriptine group. Only 1 study examined the effect of bromocriptine-mediated lactation cessation on infant growth and mortality and found no differences between the bromocriptine and control lactating group.25
Our systematic review summarizes the current available evidence on the effect of prolactin inhibition in PPCM outcomes and highlights the gaps in clinically relevant data. However, we acknowledge several limitations. Firstly, our meta-analysis is limited by the relatively small number of eligible studies and heterogeneity of study types with only 4 randomized controlled trials. These non-randomized study designs are inherently affected by indication and selection biases. The REBIRTH (Randomized Evaluation of Bromocriptine in Myocardial Recovery Therapy for Peripartum Cardiomyopathy) trail will provide additional information on the use of bromocriptine in this population with an expected completion by the year 2027.60 Secondly, data on key prognostic variables such as ethnicity, time to diagnosis, time to treatment initiation, efficacy, and maternal and fetal safety of bromocriptine were not available in all studies. Many studies solely included women in the postpartum period, skipping a meaningful proportion of PPCM patients in late pregnancy. Thirdly, the duration of follow-up was less than 6 months in most studies, restricting data on long-term prognosis. Finally, our analyses included aggregated study-level data instead of raw patient-level data, constraining our ability to adjust for individual patient characteristics. PPCM being a heterogenous disease, can have significant difference in disease characteristics across studies from difference regions involving various racial and ethnic groups. Hence, clinical data registries with region specific data will aid in better understanding of these differences.
Conclusion
To conclude, we show that the addition of prolactin inhibitors to standard GDMT in patients with PPCM is associated with improvement in LV function. We did not find a significant mortality benefit with prolactin inhibitors, which may be related to the short follow-up and low event rates. Carefully planned multi-center, multinational randomized control trials and data registries with longer follow-up periods will be essential to obtain valid and generalizable data.
Supplementary Material
Funding:
We did not receive any funding for this manuscript
Footnotes
Declaration of Competing Interest: The authors declare no competing interests.
Supplementary materials
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.cpcardiol.2022.101461.
REFERENCES
- 1.Mogos MF, Piano MR, McFarlin BL, Salemi JL, Liese KL, Briller JE. Heart failure in pregnant women: a concern across the pregnancy continuum. Circ Heart Fail 2018;11:e004005. 10.1161/CIRCHEARTFAILURE.117.004005. [DOI] [PubMed] [Google Scholar]
- 2.Ng AT, Duan L, Win T, Spencer HT, Lee MS. Maternal and fetal outcomes in pregnant women with heart failure. Heart 2018:1949–54. 10.1136/heartjnl-2018-313156. Published online. [DOI] [PubMed] [Google Scholar]
- 3.Sliwa K, Hilfiker-Kleiner D, Petrie MC, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail 2010;12:767–78. 10.1093/eurjhf/hfql20. [DOI] [PubMed] [Google Scholar]
- 4.Kamiya CA, Kitakaze M, Ishibashi-Ueda H, et al. Different characteristics of peripartum cardiomyopathy between patients complicated with and without hypertensive disorders: results from the Japanese nationwide survey of peripartum cardiomyopathy. Circ J 2011;75:1975–81. 10.1253/circj.CJ-10-1214. [DOI] [PubMed] [Google Scholar]
- 5.Onyemelukwe GC, Ogunfowokan O, Mbakwem A, et al. Cardiovascular risk factors in adult general out-patient clinics in Nigeria: a country analysis of the Africa and Middle East Cardiovascular Epidemiological (ACE) study. Afr Health Sci 2017;17:1070–81. 10.4314/ahs.vl7i4.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elkayam U, Akhter MW, Singh H, et al. Pregnancy-associated cardiomyopathy: clinical characteristics and a comparison between early and late presentation. Circulation 2005;111:2050–5. 10.1161/01.CIR.0000162478.36652.7E. [DOI] [PubMed] [Google Scholar]
- 7.McNamara DM, Elkayam U, Alharethi R, et al. Clinical outcomes for Peripartum Cardiomyopathy in North America: Results of the IPAC Study (Investigations of Pregnancy-Associated Cardiomyopathy). J Am Coll Cardiol 2015;66:905–14. 10.1016/j.jacc.2015.06.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fett JD, Ansari AA, Sundstrom J, Combs GF. Peripartum cardiomyopathy: a selenium disconnection and an autoimmune connection. Int J Cardiol 2002;86:311–6. 10.1016/S0167-5273(02)00359-5. [DOI] [PubMed] [Google Scholar]
- 9.Bültmann BD, Klingel K, Näbauer M, Wallwiener D, Kandolf R. High prevalence of viral genomes and inflammation in peripartum cardiomyopathy. Am J Obstet Gynecol 2005;193:363–5. 10.1016/j.ajog.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Honigberg MC, Givertz MM. Peripartum cardiomyopathy. BMJ 2019:364. 10.1136/bmj.k5287. [DOI] [PubMed] [Google Scholar]
- 11.Negoro S, Kunisada K, Fujio Y, et al. Activation of signal transducer and activator of transcription 3 protects cardiomyocytes from hypoxia/reoxygenation-induced oxidative stress through the upregulation of manganese superoxide dismutase. Circulation 2001;104:979–81. 10.1161/hc3401.095947. [DOI] [PubMed] [Google Scholar]
- 12.Patten IS, Rana S, Shahul S, et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 2012;485:333–8. 10.1038/naturell040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bozkurt B, Colvin M, Cook J, et al. Current Diagnostic and Treatment Strategies for Specific Dilated Cardiomyopathies: A Scientific Statement from the American Heart Association. 134. 2016. doi: 10.1161/CIR.0000000000000455 [DOI] [PubMed] [Google Scholar]
- 14.Hilfiker-Kleiner D, Kaminski K, Podewski E, et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 2007;128:589–600. 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 15.Dutt S, Wong F, Spurway JH. Fatal myocardial infarction associated with bromocriptine for postpartum lactation suppression. Aust New Zeal J Obstet Gynaecol 1998;38:116–7. 10.1111/j.1479-828X.1998.tb02977.x. [DOI] [PubMed] [Google Scholar]
- 16.Weinstein D, Ben-David M, Polishuk WZ. Serum prolactin and the suppression of lactation. BJOG An Int J Obstet Gynaecol 1976;83:679–82. 10.1111/j.1471-0528.1976.tb00912.x. [DOI] [PubMed] [Google Scholar]
- 17.Goli R, Li J, Brandimarto J, et al. Genetic and phenotypic landscape of peripartum cardiomyopathy. Circulation 2021:1852–62. 10.1161/CIRCULATIONAHA.120.052395. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ware JS, Li J, Mazaika E, et al. Shared genetic predisposition in peripartum and dilated cardiomyopathies. N Engl J Med 2016;374:233–41. 10.1056/nejmoal505517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haghikia A, Kaya Z, Schwab J, et al. Evidence of autoantibodies against cardiac troponin I and sarcomeric myosin in peripartum cardiomyopathy. Basic Res Cardiol 2015;110:60. 10.1007/s00395-015-0517-2. [DOI] [PubMed] [Google Scholar]
- 20.Noori M, Donald AE, Angelakopoulou A, Hingorani AD, Williams DJ. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation 2010;122:478–87. 10.1161/CIRCULATIONAHA.109.895458. [DOI] [PubMed] [Google Scholar]
- 21.Rajakumar A, Cerdeira AS, Rana S, et al. Transcriptionally active syncytial aggregates in the maternal circulation may contribute to circulating soluble fms-like tyrosine kinase 1 in preeclampsia. Hypertension 2012;59:256–64. 10.1161/HYPERTENSIONAHA.111.182170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stapel B, Kohlhaas M, Ricke-Hoch M, et al. Low STAT3 expression sensitizes to toxic effects of β-adrenergic receptor stimulation in peripartum cardiomyopathy. Eur Heart J 2017;38:349–61. 10.1093/eiu-heartj/ehw086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toescu V, Nuttall SL, Martin U, Kendall MJ, Dunne F. Oxidative stress and normal pregnancy. Clin Endocrinol (Oxf) 2002;57:609–13. 10.1046/j.1365-2265.2002.01638.x. [DOI] [PubMed] [Google Scholar]
- 24.Ricke-Hoch M, Bultmann I, Stapel B, et al. Opposing roles of Akt and STAT3 in the protection of the maternal heart from peripartum stress. Cardiovasc Res 2014;101:587–96. 10.1093/cvr/cvu010. [DOI] [PubMed] [Google Scholar]
- 25.Sliwa K, Blauwet L, Tibazarwa K, et al. Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: A proof-of-concept pilot study. Circulation 2010;121:1465–73. 10.1161/CIRCULATIONAHA.109.901496. [DOI] [PubMed] [Google Scholar]
- 26.Ferrara N, Clapp C, Weiner R. The 16K fragment of prolactin specifically inhibits basal or fibroblast growth factor stimulated growth of capillary endothelial cells. Endocrinology 1991;129:896–900. 10.1210/endo-129-2-896. [DOI] [PubMed] [Google Scholar]
- 27.Clapp C, Weiner RI. A specific, high affinity, saturable binding site for the 16-kilodalton fragment of prolactin on capillary endothelial cells. Endocrinology 1992;130:1380–6. 10.1210/endo.130.3.1311239. [DOI] [PubMed] [Google Scholar]
- 28.Corbacho AM, Martínez de la Escalera G, Clapp C. Roles of prolactin and related members of the prolactin/growth hormone/placental lactogen family in angiogenesis. J Endocrinol 2002;173:219–38. 10.1677/joe.0.1730219. [DOI] [PubMed] [Google Scholar]
- 29.D’Angelo G, Martini JF, Iiri T, Fantl WJ, Martial J, Weiner RI. 16K human prolactin inhibits vascular endothelial growth factor-induced activation of Ras in capillary endothelial cells. Mol Endocrinol 1999;13:692–704. 10.1210/mend.13.5.0280. [DOI] [PubMed] [Google Scholar]
- 30.Halkein J, Tabruyn SP, Ricke-Hoch M, et al. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J Clin Invest 2013;123:2143–54. 10.1172/JCI64365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lemmens K, Doggen K, de Keulenaer GW. Activation of the neuregulin/ErbB system during physiological ventricular remodeling in pregnancy. Am J Physiol - Hear Circ Physiol 2011;300:931–42. 10.1152/ajpheart.00385.2010. [DOI] [PubMed] [Google Scholar]
- 32.Tabruyn SP, Nguyen NQN, Cornet AM, Martial JA, Struman I. The antiangiogenic factor, 16-kDa human prolactin, induces endothelial cell cycle arrest by acting at both the G0-G1 and the G 2-M phases. Mol Endocrinol 2005;19:1932–42. 10.1210/me.2004-0515. [DOI] [PubMed] [Google Scholar]
- 33.Bacharach E, Itin A, Keshet E. In vivo patterns of expression of urokinase and its inhibitor PAI-1 suggest a concerted role in regulating physiological angiogenesis. Proc Natl Acad Sci U S A 1992;89:10686–90. 10.1073/pnas.89.22.10686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menashi S, Lu H, Soria C, Legrand Y. 2 Endothelial cell proteases: physiological role and regulation. Baillieres Clin Haematol 1993;6:559–76. 10.1016/S0950-3536(05)80188-X. [DOI] [PubMed] [Google Scholar]
- 35.Lee H, Struman I, Clapp C, Martial J, Weiner RI. Inhibition of urokinase activity by the antiangiogenic factor 16K prolactin: activation of plasminogen activator inhibitor 1 expression. Endocrinology 1998;139:3696–703. 10.1210/endo.139.9.6194. [DOI] [PubMed] [Google Scholar]
- 36.Lee SH, Kunz J, Lin SH, Yu-Lee LY. 16-kDa prolactin inhibits endothelial cell migration by down-regulating the Ras-Tiam1-Rac1-Pak1 signaling pathway. Cancer-Res 2007;67:11045–53. 10.1158/0008-5472.CAN-07-0986. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez C, Corbacho AM, Eiserich JP, et al. 16K-prolactin inhibits activation of endothelial nitric oxide synthase, intracellular calcium mobilization, and endothelium-dependent vasorelaxation. Endocrinology 2004;145:5714–22. 10.1210/en.2004-0647. [DOI] [PubMed] [Google Scholar]
- 38.Lee SH, Nishino M, Mazumdar T, et al. 16-kDa prolactin down-regulates inducible nitric oxide synthase expression through inhibition of the signal transducer and activator of transcription 1/IFN regulatory factor-1 pathway. Cancer Res 2005;65:7984–92. 10.1158/0008-5472.CAN-05-0631. [DOI] [PubMed] [Google Scholar]
- 39.Tabruyn SP, Sorlet CM, Rentier-Delrue L, et al. The antiangiogenic factor 16K human prolactin induces caspase-dependent apoptosis by a mechanism that requires activation of nuclear factor-κB. Mol Endocrinol 2003;17:1815–23. 10.1210/me.2003-0132. [DOI] [PubMed] [Google Scholar]
- 40.Martini JP, Piot C, Humeau LM, Struman I, Martial JA, Weiner RI. The antiangiogenic factor 16K PRL induces programmed cell death in endothelial cells by caspase activation. Mol Endocrinol 2000;14:1536–49. 10.1210/mend.14.10.0543. [DOI] [PubMed] [Google Scholar]
- 41.Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation. 2022. [Google Scholar]
- 42.Qualtrics XM //The Leading Experience Management Software, https://www.qualtrics.com/au/?rid=ip&prevsite=en&newsite=au&geo=IN&geomatch=au. Accessed November 17, 2021.
- 43.Wells G, Shea B, O’Connell D, et al. Newcastle-Ottawa scale 1993;113:198. Available at http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 44.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.StataCorp. Stata Statistical Software: Release 16. College Station TSL: Stata | StataCorp LLC; 2019. [Google Scholar]
- 46.Yaméogo NV, Kagambèga LJ, Seghda A, Owona A, Kaboré O. Bromocriptine in management of peripartum cardiomyopathy: a randomized study on 96 women in Burkina Faso. J Cardiol Clin Res 2017;5:1098. [Google Scholar]
- 47.Hilfiker-Kleiner D, Haghikia A, Berliner D, et al. Bromocriptine for the treatment of peripartum cardiomyopathy: a multicentre randomized study. Eur Heart J 2017;38:2671–9. 10.1093/eurheartj/ehx355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tremblay-Gravel M, Marquis-Gravel G, Avram R, et al. The effect of bromocriptine on left ventricular functional recovery in peripartum cardiomyopathy: insights from the BRO-HF retrospective cohort study. ESC Hear Fail 2019;6:27–36. 10.1002/ehf2.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ersbøll AS, Johansen M, Damm P, Rasmussen S, Vejlstrup NG, Gustafsson F. Peripartum cardiomyopathy in Denmark: a retrospective, population-based study of incidence, management and outcome. Eur J Heart Fail 2017;19:1712–20. 10.1002/ejhf.882. [DOI] [PubMed] [Google Scholar]
- 50.Biteker M, Özlek B, Özlek E, et al. Predictors of early and delayed recovery in peripartum cardiomyopathy: a prospective study of 52 Patients. J Matern Neonatal Med 2020;33:390–7. 10.1080/14767058.2018.1494146. [DOI] [PubMed] [Google Scholar]
- 51.Haghikia A, Podewski E, Libhaber E, et al. Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol 2013;108. 10.1007/s00395-013-0366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hilfiker-Kleiner D, Haghikia A, Masuko D, et al. Outcome of subsequent pregnancies in patients with a history of peripartum cardiomyopathy. Eur J Heart Fail 2017;19:1723–8. 10.1002/ejhf.808. [DOI] [PubMed] [Google Scholar]
- 53.Azibani F, Pfeffer TJ, Ricke-Hoch M, et al. Outcome in German and South African peripartum cardiomyopathy cohorts associates with medical therapy and fibrosis markers. ESC Hear Fail 2020;7:512–22. 10.1002/ehf2.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kurbanov RD, Mirzarakhimova ST, Abdullaev TA, Tsoi IA. The effect of bromocriptine on clinical and laboratory parameters in patients with peripartum cardiomyopathy. Kardiologiya 2020;60:58–62. 10.18087/cardio.2020.6.n984. [DOI] [PubMed] [Google Scholar]
- 55.Sliwa K, Skudicky D, Candy G, Bergemann A, Hopley M, Sareli P. The addition of pentoxifylline to conventional therapy improves outcome in patients with peripartum cardiomyopathy. Eur J Heart Fail 2002;4:305–9. 10.1016/S1388-9842(02)00008-9. [DOI] [PubMed] [Google Scholar]
- 56.Nonhoff J, Ricke-Hoch M, Mueller M, et al. Serelaxin treatment promotes adaptive hypertrophy but does not prevent heart failure in experimental peripartum cardiomyopathy. Cardiovasc Res 2017;113:598–608. 10.1093/cvr/cvw245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Borba VV, Zandman-Goddard G, Shoenfeld Y. Prolactin and autoimmunity. Front Immunol 2018;9. Available at 10.3389/hmmu.2018.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McKeon KP, Jiang SH. Treatment of systemic lupus erythematosus. Aust Preset 2020;43(3):85–90. 10.18773/austprescr.2020.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trongtorsak A, Kittipibul V, Mahabir S, et al. Effects of bromocriptine in peripartum cardiomyopathy: a systematic review and meta-analysis. Heart Fail Rev 2022;27:533–43. 10.1007/sl0741-021-10185-8. [DOI] [PubMed] [Google Scholar]
- 60.McNamara DM. Impact of Bromocriptine on Clinical Outcomes for Peripartum Cardiomyopathy. Available at: https://clinicaltrials.gov/ct2/show/study/NCT05180773. Accessed February 20, 2022.
- 61.Ben-Jonathan N, Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocr Rev 2001;22:724–63. 10.1210/edrv.22.6.0451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


