Abstract
Primary prevention trials have shifted their focus to the earliest stages of Alzheimer’s disease (AD). Autopsy data indicates that the neuromodulatory subcortical systems’ (NSS) nuclei are specifically vulnerable to initial tau pathology, indicating that these nuclei hold great promise for early detection of AD in the context of the aging brain. The increasing availability of new imaging methods, ultra-high field scanners, new radioligands, and routine deep brain stimulation implants has led to a growing number of NSS neuroimaging studies on aging and neurodegeneration. Here, we review findings of current state-of-the-art imaging studies assessing the structure, function, and molecular changes of these nuclei during aging and AD. Furthermore, we identify the challenges associated with these imaging methods, important pathophysiologic gaps to fill for the AD NSS neuroimaging field, and provide future directions to improve our assessment, understanding, and clinical use of in vivo imaging of the NSS.
Keywords: Neuromodulators, Neuroimaging, Brain aging, Alzheimer’s Disease, (Functional) magnetic resonance imaging, Diffusion-weighted imaging, Positron emission tomography, Electrophysiology
Introduction
Sporadic Alzheimer’s Disease (AD) is the most common cause of dementia, affecting over 5 million people in the United States alone and over 30 million people worldwide, with its prevalence expected to further increase (Alzheimer’s Association, 2022; Haque and Levey, 2019). AD is characterized by two pathologic hallmarks: amyloid-beta (Aβ) and tau proteinopathies. Despite numerous ongoing research efforts, clinical trials targeting AD have had very limited success in delaying clinical progression of the disease (Cao et al., 2018; Jeremic et al., 2021). The majority of these clinical trials have focused on the clearance of Aβ plaques in individuals with cognitive impairment (Cao et al., 2018). However, it has become increasingly clear that the accumulation and aggregation of Aβ and tau start two to three decades prior to the onset of clinical symptoms (Braak and Del Tredici, 2015; Braak et al., 2011b), marking AD’s long preclinical course (Theofilas et al., 2015). The limited clinically relevant advances in therapeutics may reflect that the current window of intervention is suboptimal and consequently, the field’s focus has shifted from symptomatic to asymptomatic individuals. To ensure that interventions in these earlier stages are effective and target individuals who are at risk of AD, there is an urgent need for markers predicting early accumulation of AD pathology as well as markers that track subtle clinical progression.
Structural or functional readouts of the neuromodulatory subcortical systems (NSS) may hold promise as a marker of the earliest stages of the disease process (Grinberg et al., 2011). These NSS correspond to the isodendritic core network and consist of a group of nuclei that synthesize monoamines (such as catecholamines and serotonin) and acetylcholine, and have widespread projections to the cortex. As these phylogenetically conserved subcortical nuclei are vulnerable to the earliest AD-related tau depositions even before tau reaches the transentorhinal cortex, this review is restricted to the isodendritic nuclei and the nucleus of the lateral hypothalamus, which has similar transmission characteristics to the isodendritic nuclei (Avery and Krichmar, 2017; Grinberg et al., 2011; Theofilas et al., 2015). The neurotransmitters provided by these nuclei act as neuromodulators, regulating the activity of neuronal and non-neuronal cells, and are essential for higher-order cognitive functioning, which emphasizes the far-reaching consequences of functional or structural alterations in the NSS (Avery and Krichmar, 2017). So far, several NSS nuclei have been identified to accumulate tau pathology prior to cortical involvement (Rüb et al., 2017; Stratmann et al., 2016). These include the noradrenergic locus coeruleus (LC), the serotonergic dorsal raphe nucleus (DRN), the dopaminergic ventral tegmental area (VTA), the orexinergic lateral hypothalamus (LH), and the cholinergic nucleus basalis of Meynert (NbM) (Fig. 1).
Figure 1. Location of the NSS and their most important projections and interactions.
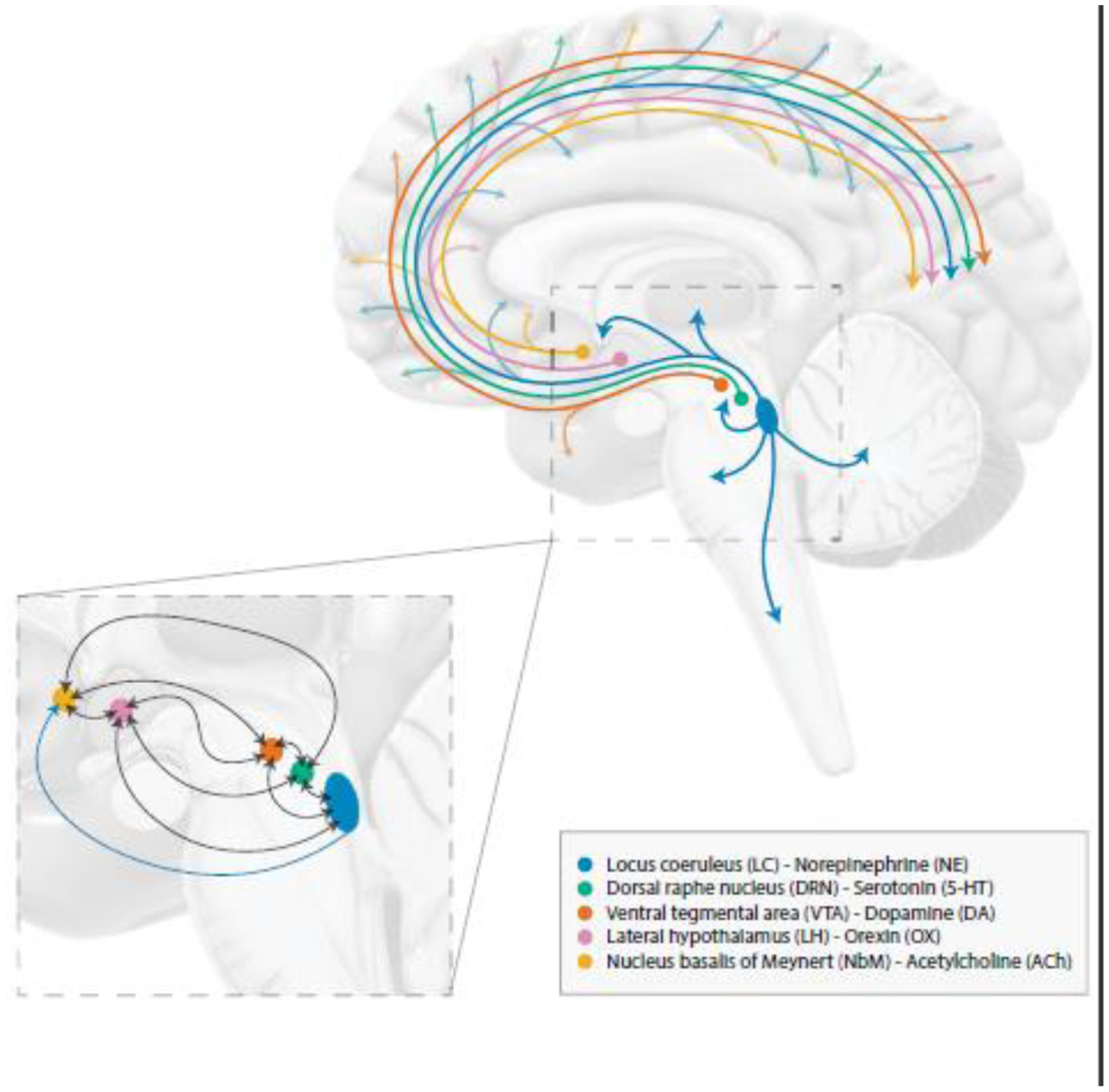
NSS modulate a wide range of cognitive functions and behaviors, as illustrated by their widespread projections to (sub)cortical structures. Blue: the noradrenergic LC, green: the serotonergic DRN, orange: the dopaminergic VTA, pink: the orexinergic LH, yellow: the cholinergic NbM. For simplification purposes, visualization has been limited to main projections and interactions between the NSS. Abbreviations: DRN, dorsal raphe nucleus; LC, locus coeruleus; LH, lateral hypothalamus; NbM, nucleus basalis of Meynert; NSS, neuromodulatory subcortical systems; VTA, ventral tegmental area.
The NSS nuclei are among the very earliest regions accumulating pathologic hyperphosphorylated aggregates of tau or pretangles (Moloney et al., 2021). For instance, the number of LC neurons containing hyperphosphorylated tau increases significantly when moving from Braak stage 0 to I (Ehrenberg et al., 2017) and LC volume decreases by ~8% per Braak stage (Theofilas et al., 2017). Yet no significant LC neuronal loss is observed until Braak stage III-IV, which corresponds to the emergence of clinical symptoms (Theofilas et al., 2017). The volumetric changes prior to Braak stage IV likely reflect reductions in dendritic arborization and synaptic inputs, and retraction of LC innervations to cortical regions, indicating that changes to the functioning and morphology of the LC span the different disease stages of AD. Similar to the LC, the other NSS accumulate hyperphosphorylated tau prior to the transentorhinal cortex (Grinberg et al., 2009; Hampel et al., 2018; Stratmann et al., 2016; Vana et al., 2011), although for the DRN this has been estimated to be at a slightly later time point than the LC (Ehrenberg et al., 2017). These accumulations of pretangle material and the morphological changes in NSS nuclei have been associated with the earliest clinical phenotypes of AD (Ehrenberg et al., 2017; Kelly et al., 2017) and the reported lack of age-correlations during Braak stages 0-I suggest that these changes are likely not merely age-related (Theofilas et al., 2017).
These findings suggest that the NSS undergo significant changes at the very early stages of AD and could serve as indicators of AD-related risk. According to autopsy work, while pathological tau accumulation in the NSS occurs at a very early point in time, amyloid plaques in the brainstem can only be detected at later stages of AD (Braak et al., 2011a; Parvizi et al., 2001). The identification of earlier and less fibrillar forms of amyloid is hampered by the detection limits of our current methods. By identifying the earliest changes in vivo in humans, this could create a promising window of opportunity for early interventions. Recent developments in neuroimaging methods have now opened up exciting possibilities to examine these nuclei in greater detail and from multiple perspectives (Betts et al., 2019b; Billot et al., 2020; Priovoulos et al., 2018; Teipel et al., 2005). However, imaging the NSS has its own challenges and these limitations need to be considered when interpreting the data. In this review, we aim to discuss the current state-of-the-art of findings related to in vivo neuroimaging of the NSS nuclei to improve the understanding of early AD-related changes. Particular emphasis was placed on structural, functional, diffusion-weighted, positron emission tomography (PET), and electrophysiology imaging. We cover recent contributions and associated challenges, and future perspectives as NSS imaging holds great promise to improve the very early detection of AD, advance differential diagnostics, and aid in the identification of individuals suited for prevention trials.
General challenges in imaging the NSS
Neuroimaging is an excellent non-invasive tool to investigate the brain in vivo, facilitating the recording of changes over time in both healthy and disease populations. Advances in neuroimaging have paved the way for research investigating structural, functional, and molecular characteristics of small nuclei. The brainstem nuclei, but also the LH and NbM, are notoriously hard to image due to their small size compared to the spatial resolution of the imaging methods. The small size of these nuclei is particularly challenging in MRI and PET imaging and results in lower spatial resolution of the images, as these methods need larger voxel sizes to obtain a reasonable signal-to-noise ratio (SNR) even at higher MRI (magnetic resonance imaging) field strengths (Fig 2). Moreover, head motion has a profound effect on small structures in the brain, contributing substantially to lower SNR values. Additionally, the proximity of several NSS to non-neuronal tissue can lead to a high variance in the measured signal due to physiological noise, which can be unrelated or confounded with the signal of interest. The physiological noise arises primarily from respiratory and cardiac pulsations in arteries, veins, and cerebrospinal fluid (CSF)- and air-filled cavities, which in turn distort the images. The proximity of the NSS to the CSF in combination with their small size can result in partial-volume effects when voxels that include multiple tissue types are classified incorrectly in the segmentation process. Furthermore, many studies account for multiple comparisons by utilizing cluster-wise corrections, which are geared towards larger structures, over voxel-wise corrections, which are more suited for the small-sized NSS. Consequently, the size and shape of the NSS are not taken into account, and this can introduce type I errors to the data (Sclocco et al., 2018).
Figure 2. Imaging method comparison pertaining to NSS imaging.
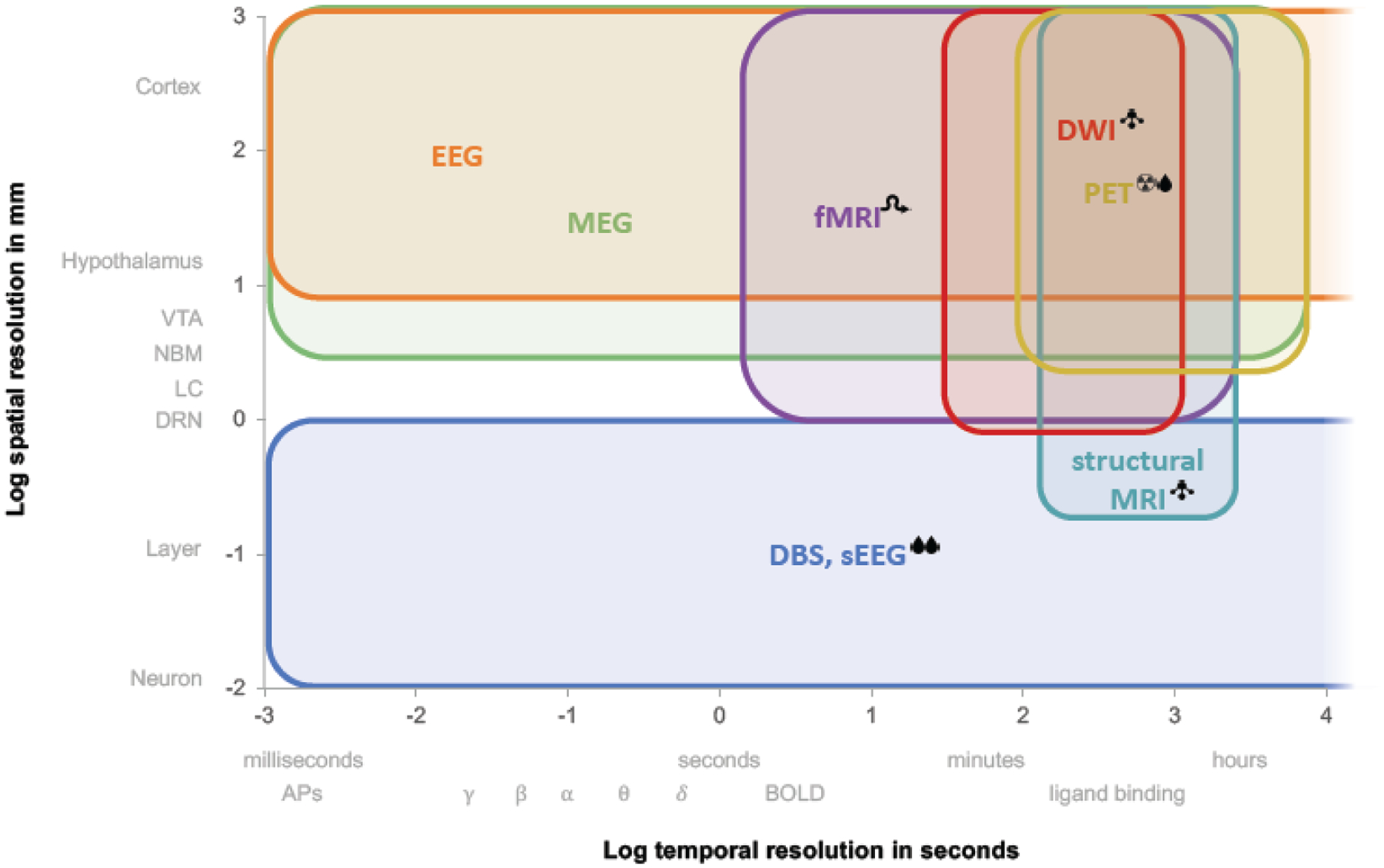
Current neuroimaging tools differ greatly in spatial and temporal resolution, as well as in their invasiveness and the type of measurements taken. Legend:  , invasive;
, invasive;  , radioactive;
, radioactive;  , structural only;
, structural only;  , indirect measure of neuronal activity; δ θ α β γ, EEG bands. Abbreviations: APs, action potentials; BOLD, blood oxygen dependent level; DBS, deep brain stimulation; DWI, diffusion-weighted imaging; EEG, electroencephalogram; fMRI, functional magnetic resonance imaging; MEG, magnetoencephalogram; MRI, magnetic resonance imaging; PET, positron emission tomography; sEEG, stereoencephalography.
, indirect measure of neuronal activity; δ θ α β γ, EEG bands. Abbreviations: APs, action potentials; BOLD, blood oxygen dependent level; DBS, deep brain stimulation; DWI, diffusion-weighted imaging; EEG, electroencephalogram; fMRI, functional magnetic resonance imaging; MEG, magnetoencephalogram; MRI, magnetic resonance imaging; PET, positron emission tomography; sEEG, stereoencephalography.
Individual or group comparisons of the measured signal in these NSS warrant the transformation or warping of the images to a common space, either a population-specific template or a standard space image. Existing standard-space templates do not adjust for individual variability in size or location of the NSS, and minor registration errors can result in significant distortions of the anatomy of these nuclei in subsequent processing steps, introducing partial volume effects and biasing the extracted signal of interest; in particular in aging populations and populations with varying degrees of pathology. As the field of neuroimaging is relatively young, there is an urgent need for further validation of the neuroimaging measures in terms of test-retest reliability, registration, and anatomical accuracy.
Another important practical limitation of in vivo neuroimaging methods is the preclusion of individuals based on surgical interventions or co-morbid illnesses. In MRI, signal distortion can occur due to the forces exerted by the static magnetic field on ferromagnetic materials, and in extreme cases, radio frequency (RF) induced heating and displacement of the implant occur (Espiritu et al., 2021; Jungmann et al., 2017). In PET imaging, interference of certain medical conditions with the uptake of the PET tracer needs to be considered. For instance, in fluordeoxyglucose (FDG)-PET, brain cells can take up high blood sugar levels instead of the injected radioactive glucose (Sprinz et al., 2018). However, diabetes and metal implants (both oral and within the body) are common in the elderly population, and excluding individuals based on age-related comorbidities may result in samples that are less representative of the population.
Imaging the NSS using structural magnetic resonance imaging
A myriad of structural MRI research demonstrated unequivocally that aging and AD are characterized by overlapping as well as distinct patterns of gradual cortical and subcortical gray matter tissue loss. The gray matter changes observed in aging or the early phases of AD most likely reflect a reduction in the dendritic arborization of neurons, while loss of neuronal soma occurs later in the disease (Risacher and Saykin, 2013; Symms et al., 2004). Given that the NSS exhibit a particular vulnerability to tau pathology early in the adult lifespan (Braak and Del Tredici, 2011a, b; Ehrenberg et al., 2017), it remains unknown which and when neurons and neurites in these nuclei are vulnerable or resistant to AD-related pathology. The contrast and spatial resolution of standard T1-weighted imaging are too poor to provide clear boundaries of these nuclei. During the last decade, novel optimized high-resolution MRI sequences and templates were developed that provide a unique opportunity to investigate structural properties of these nuclei.
The cholinergic basal forebrain (or NbM) was one of the first NSS where neuroimaging methods were used to assess its structural properties. A combination of proton density MRI and histological sections of post-mortem brains enabled the localization of the basal forebrain and measurement of its volume (Teipel et al., 2005). By registering the resulting template to study populations, it was demonstrated that basal forebrain volume starts declining from early adulthood on and further aggravates during aging and progression of AD (Grothe et al., 2014). Volume of the basal forebrain was lower in patients with subjective cognitive decline, mild cognitive impairment (MCI), and AD compared to controls (Grothe et al., 2014; Scheef et al., 2019; Teipel et al., 2005; Teipel et al., 2011). Despite substantial multicenter variability, atrophy patterns were similar across studies, showing the most pronounced volume differences between controls and AD patients in the posterior NbM (Kilimann et al., 2014). Individuals progressing from control to amnestic MCI or from MCI to AD exhibited lower basal forebrain volume prior to progression (Brueggen et al., 2015; Kerbler et al., 2015), indicating early involvement of this nucleus in AD’s pathogenesis. In both the ADNI and AIBL cohorts, greater Aβ-PET burden was associated with lower posterior basal forebrain volume in Aβ positive controls and lower anterior basal forebrain volume in those with early mild cognitive impairment, but not in those with late mild cognitive impairment (Grothe et al., 2014; Kerbler et al., 2015). When comparing the basal forebrain to the earliest cortical region accumulating tau pathology, the entorhinal cortex, predictive models revealed that atrophy in the basal forebrain preceded atrophy of the entorhinal cortex in the ADNI cohort, which emphasizes the critical role of subcortical changes in initial disease stages (Fernandez-Cabello et al., 2020; Schmitz et al., 2016). These structural changes in the basal forebrain in prodromal AD have been related to declining cognition, and lower basal forebrain volume has been associated with lower memory performance and attentional control in patients with MCI, and worse spatial pattern separation performance in AD (Parizkova et al., 2020). Reductions in basal forebrain volume in preclinical AD individuals were associated with increased microglial inflammation as measured with sTREM2 and C3 expression, and this effect was more pronounced in individuals carrying the APOE-ε4 allele: the strongest and most common genetic risk factor for AD (Schmitz et al., 2020).
Generally, in vivo structural information on the LC is obtained with the turbo-spin-echo MRI sequence, either without (Keren et al., 2009; Keren et al., 2015) or with additional magnetization transfer (MT) contrast (Jacobs et al., 2021a; Trujillo et al., 2019), gradient-echo sequences with MT (Chen et al., 2014) and T1-weighted Fast Low Angle Shot (FLASH) sequences at 3T (Betts et al., 2017), or the MT-turbo flash sequence at 7T MRI providing near-isotropic resolution, critical for the rod-shaped nature of the LC (Priovoulos et al., 2018). These sequences generate a hyperintense signal in the LC (and the substantia nigra, not discussed in this review). Since neuromelanin cells in the LC sequester metals, including paramagnetic elements such as iron and copper, it was assumed that the hyperintense MRI signal reflects neuromelanin cell density (Keren et al., 2015). However, more recent MRI modeling and animal imaging studies suggest a more complex biological source is underlying this contrast (Trujillo et al., 2017). Components contributing to this hyperintense signal may include lipids (Priovoulos et al., 2020), water (Watanabe et al., 2019) and tau aggregates (Jacobs et al., 2021a), and the relative contribution of these components may vary with age, disease or location (Pamphlett et al., 2020). This variation is reflected in the inverted U-shaped relationship between LC intensity (also referred to as integrity) and age across the entire adult lifespan (Jacobs et al., 2021a; Liu et al., 2019; Shibata et al., 2006). However, several other studies were not able to detect a relationship between age and LC integrity (Betts et al., 2017; Maki-Marttunen and Espeseth, 2021; Ye et al., 2021), which may be due to sample size or selection biases towards very healthy individuals, as a negative age-association in older individuals may be explained by the presence of initial AD pathology (Jacobs et al., 2021a). Initial studies with smaller sample sizes demonstrated that LC integrity is lower in AD patients (Betts et al., 2019a; Hou et al., 2021; Olivieri et al., 2019; Takahashi et al., 2015) and MCI patients (Dordevic et al., 2017) relative to healthy older individuals. In the majority of these studies, AD was based on clinical parameters and not on underlying AD pathologic change biomarkers. Olivieri and colleagues (2019) included Aβ pathology in the diagnostic make-up and found no correlation between LC integrity and Aβ. In contrast, Betts and colleagues (2019) reported an unexpected negative correlation between CSF Aβ and LC integrity and no correlation between CSF ptau and LC integrity, though the sample size was modest (n=44) and consisted of a combination of healthy controls, patients with subjective cognitive impairment, MCI, and dementia.
Recently, in well-characterized and larger cohorts of preclinical autosomal dominant and sporadic AD, lower LC integrity has been associated with initial cortical tau and Aβ pathology, starting several years before the preclinical stages of the disease manifest (Dahl et al., 2021; Jacobs et al., 2021a; Jacobs et al., 2022). Lower LC integrity also correlates with cognitive and behavioral changes. In older individuals, lower LC integrity is associated with poor memory for negative emotional events (Hammerer et al., 2018), retrieval of episodic information (Jacobs et al., 2021a), or attentional shifting, specifically in individuals with low cognitive reserve (Clewett et al., 2016). In particular, the rostral part of the LC is susceptible to age-related learning changes (Dahl et al., 2019b). Furthermore, in sporadic preclinical AD lower LC integrity predicted Aβ-related cognitive decline over a time period of 8 years, and at levels below the established Aβ threshold (Jacobs et al., 2021a); neuropsychiatry symptom severity, particularly impulse dyscontrol (Cassidy et al., 2022); daytime sleep-related dysfunction (Elman et al., 2021a); and a higher degree of tau-related nocturnal awakenings (Van Egroo et al., 2021). In prodromal AD, lower LC integrity, especially in the rostral part, has been associated with worse processing speed and episodic memory (Elman et al., 2021a; Olivieri et al., 2019). LC integrity also correlates with imaging markers obtained with other sequences or modalities. In older individuals, lower rostral LC integrity was associated with lower free water diffusion in the cortex and lower global as well as frontoparietal cortical thickness (Bachman et al., 2021; Elman et al., 2021b).
So far, considerably less structural imaging work has been performed on the VTA, raphe nucleus (RN), or the hypothalamic subunits (Billot et al., 2020). In the ADNI cohort, individuals who increased on the clinical dementia rating scale total score from zero to 0.5 exhibited lower T1-weighted VTA signal at baseline compared to those who remained stable at 0, but this did not correlate with any of the CSF biomarkers (Venneri and De Marco, 2020). The authors did not find group differences for the LC, basal forebrain, or the RN. Other work by the same authors showed a positive relation between VTA volume and both hippocampal volume and memory performance in older individuals (De Marco and Venneri, 2018). These findings suggest that the VTA can be an early marker of incipient memory decline, but more work is needed to determine whether this is specific to AD. In the hypothalamic subunits, lower volume in the anterior hypothalamus in AD patients compared to controls has been reported (Baron et al., 2001), while more recent work using the FreeSurfer parcellation reported large group differences in volume between controls and AD patients in both anterior and posterior subunits (Billot et al., 2020). Recently, a high-resolution hypothalamic atlas has been released, but caution is warranted when deriving conclusions in the context of aging as the template is based on a young population (Neudorfer et al., 2020). The dearth of structural imaging work on the RN, VTA, and hypothalamic subunits underscores the need for dedicated sequences that can identify these small nuclei at the individual level.
Challenges pertaining to structural imaging of the NSS and potential solutions
The majority of the structural imaging work on NSS has focused on the LC, and this has highlighted the need to validate the underlying biological substrates that contribute to the variability in MRI signals when imaging the small nuclei of the NSS. The test-retest reliability of TSE sequences that are used to image the LC were moderate, with Dice coefficients between 0.65 and 0.74 and inter-rater reliability between 0.54 and 0.64 (Tona et al., 2017). Inadequate reproduction of findings can have far-reaching implications that hamper the disentanglement of measurement error from actual meaningful change over time. Additionally, registration and anatomical accuracy of NSS imaging are in similar need of validation. The identification of the LC on MRI images is often based on intensity values extracted from normalized images and this segmentation is performed in native space or after warping the images to a template or a standard space image. These warps can introduce partial volume effects, distort the anatomy, and bias the exact size of the structure. This warrants caution when providing anatomical localization or inferring volumetric measures from MRI scans, particularly since post-mortem data has shown that neuroimaging methods tend to underestimate the length of the LC, as the caudal part is anatomically much more diffuse. Furthermore, templates derived from dedicated LC sequences acquired within a study sample should be distinguished from templates that are based on other imaging domains with insufficient resolution and contrast to provide accurate anatomical information. Creating sample-specific templates derived from dedicated sequences has the advantage that it is less dependent on the operator, and previous work indicated good correlations with manual identification of the LC (Giorgi et al., 2022; Maki-Marttunen and Espeseth, 2021). Another method employed to improve the SNR and consequently the registration is the upsampling of images (Betts et al., 2017; Dahl et al., 2019a), but even the preferred sinc approach can introduce minor blurring and interpolation effects, particularly in small regions as the NSS nuclei. This could result in false positives when aiming to detect associations between voxel intensities and other variables of interest. Therefore, visual inspection of image registration is required to ensure that the warping of the template to each individual image is accurate. The use of existing templates as a tool to identify the LC or other NSS via other imaging modalities not providing sufficient anatomical detail facilitates comparison and standardization across studies. However, caution is warranted because minor registration issues can result in significant changes to these tiny structures, and templates do not account for individual variability in size or location of these regions (Fig. 3). For example, the LC position can differ by at least 2–3mm within the axial plane of the brainstem from one individual to another (Keren et al., 2009). This variability was also reported by a recent study comparing various published templates (Dahl et al., 2021). Even with the ongoing improvements in spatial resolution of neuroimaging methods, validation against post-mortem data, the ultimate ground truth, is needed to confirm the accuracy of localization and volumetric measurements of the LC obtained with MRI.
Figure 3. Multiplanar visualization of the spatial discrepancies among several published atlases of the LC and the VTA.
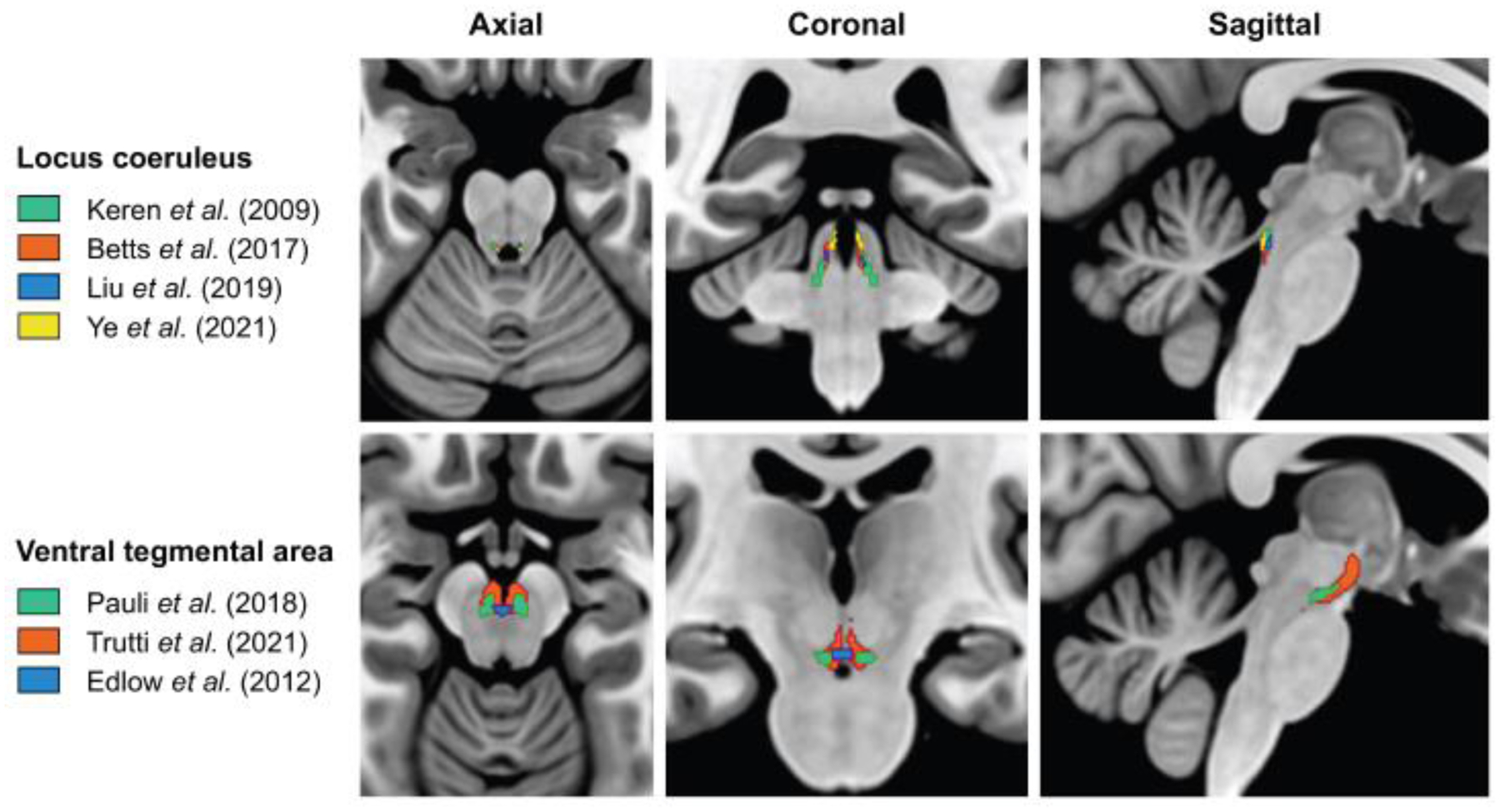
Differences in the data acquisition approach (e.g., in vivo vs. post-mortem, 3T vs. 7T), in the characteristics of the investigated population (e.g., lifespan vs. specific age range; healthy controls vs. patients), and/or in the image processing methods (e.g., registration/normalization pipeline) may lead to significant discrepancies in the location and spatial coverage of a generated atlas for a given NSS. Top: LC atlases retrieved from Keren et al. (2009) (green), Betts et al. (2017) (orange), Liu et al. (2019) (blue), and Ye et al. (2021) (yellow). Bottom: VTA atlases retrieved from Pauli et al. (2018) (green), Trutti et al. (2021) (orange), and Edlow et al. (2012) (blue). All atlases are superimposed on the MNI152 template in MRIcroGL software (https://www.nitrc.org/projects/mricrogl). Abbreviations: LC, locus coeruleus; VTA, ventral tegmental area.
Imaging the NSS using functional magnetic resonance imaging
Blood-oxygenation-level-dependent functional MRI (BOLD-fMRI) is a valuable imaging technique to investigate the function of the NSS in vivo. Recent advances in ultra-high field MRI have facilitated the measurement of functional changes in small structures with considerably higher spatiotemporal resolution than most other neuroimaging techniques (Filippi et al., 2019; Sclocco et al., 2018; Singh et al., 2022), providing unique information relevant to the development of accurate functional biomarkers of AD pathology and cognitive decline (Schumacher et al., 2019; Wang et al., 2021).
The BOLD-fMRI signal depends on the hemodynamic response, making it an indirect measure of neuronal activity (Buxton et al., 2004). In addition to detecting derivatives of neuronal activation, BOLD-fMRI has been extensively used to quantify functional connectivity (FC): the degree of temporal co-variation in the BOLD-fMRI signal between spatially distributed brain regions (Varangis et al., 2021). In the context of AD, BOLD-fMRI has frequently been used to evaluate AD-related disruption in FC between NSS and other brain regions and to investigate the potential of these functional changes to improve early diagnostics of AD.
Resting-state (rs) FC analyses of the basal forebrain in healthy individuals have revealed two distinct anatomical subdivisions with different connectivity profiles: the anterior-medial subdivision, which is characterized by connectivity with the ventromedial prefrontal and retrosplenial/posterior cingulate cortices, and the posterior-lateral subdivision, which is characterized by connectivity with the insula and the dorsal attention network (Fritz et al., 2019). These distinct functional subdivisions of the basal forebrain have been reproduced in clinical populations (Chiesa et al., 2019; Herdick et al., 2020; Li et al., 2017). Even though initial studies reported a decrease in the strength of these basal forebrain FC networks in MCI patients compared to healthy individuals (Li et al., 2017), a more recent investigation including a larger sample spanning all diagnostic groups of the AD spectrum failed to reproduce these results (Herdick et al., 2020). In the latter study, the results obtained with rsFC contrasted with the basal forebrain volume decline and structural connectivity changes across diagnostic groups, and the neuropathologic studies suggesting cholinergic depletion in AD (Bohnen et al., 2018).
RsFC analysis of the LC, in young and healthy individuals, revealed strong FC with the entire neocortex, hippocampus, amygdala, thalamus, pallidum, and many brainstem nuclei (Singh et al., 2022; Zhang et al., 2016). With aging, the LC demonstrated similar connectivity patterns with most of these regions. However, areas such as the lingual and parahippocampal gyri showed diverse connectivity patterns that varied across different age ranges. For example, negative correlations with age were reported for the FC between the LC and the lingual and parahippocampal gyri in a group of young adults between 18 and 49 years of age (Zhang et al., 2016). In contrast, positive correlations were reported for these regions in individuals between 65 and 80 years of age (Jacobs et al., 2015). Curvilinear associations between LC FC and age in a large set of brain regions were reported by a study focusing on the adult lifespan (Jacobs et al., 2018b). The authors speculated that early age-related increases in LC FC might reflect brain maturation, whereas late-life increases might reflect aberrant neuronal firing related to AD pathology.
Whereas FC generally reflects the co-variation of signals in distinct brain areas, whole-brain global connectivity reflects the correlation of time series of gray matter voxels with every other gray matter voxel in the brain. Asymptomatic individuals at risk for familial AD demonstrated lower resting-state whole-brain global connectivity of the LC (Del Cerro et al., 2020b). In line with this finding, lower global task-related modulation of LC FC during the execution of an attention task has been reported in patients with late-life major depressive disorder (Del Cerro et al., 2020a), and in those at increased risk for AD (Byers and Yaffe, 2011). However, these task-related LC FC alterations were not observed in individuals with amnestic MCI, which is reminiscent of the variation in reported results comparing cholinergic basal forebrain FC across diagnostic groups (Herdick et al., 2020). In cognitively healthy older individuals, group baseline fMRI and Aβ-PET imaging with longitudinal cognitive measurements were combined and the results indicated that lower novelty-related LC activity and lower FC between the LC, the amygdala, and the hippocampus are associated with steeper Aβ-related cognitive decline (Prokopiou et al., 2022). These latter findings suggest that before cognitive decline becomes apparent, changes to the LC’s functional properties are associated with markers of AD pathology (Kelberman and Weinshenker, 2022).
RsFC analysis of the VTA in young and healthy individuals revealed significant FC with other brainstem nuclei, including the raphe, laterodorsal tegmental, and periaqueductal gray nuclei (Singh et al., 2022), and with the hippocampus, amygdala, and regions within the prefrontal and cingulate cortices (Kahn and Shohamy, 2013; Murty et al., 2014; Tomasi and Volkow, 2014). From early to middle adulthood, a decrease in connectivity between the VTA and the somatomotor cortex was related to an age-related decline in dopaminergic signaling (Manza et al., 2015). Lower FC of the VTA with the left hippocampus was associated with less hippocampal volume and worse memory performance in a cohort comprised of healthy individuals and AD patients, but this result was not replicated within each diagnostic group, possibly due to lower statistical power (De Marco and Venneri, 2018).
In young and healthy individuals, rsFC analysis of the RN showed significant FC with the substantia nigra, caudate, putamen, and the LC (Singh et al., 2022). In the context of AD, Zhou et al. (2010) reported lower rsFC between the DRN and the default mode network in AD patients. Serotonin projections from the DRN play a critical role in supporting neurogenesis and proper functioning in the hippocampus (Alenina and Klempin, 2015), a key region implicated in learning and memory, suggesting that dysfunction of the serotonin system might be associated with AD-related cognitive and memory decline (Leal and Yassa, 2013). In support of this, lower FC between the CA2, CA3, and subiculum hippocampal subfields has been associated with decreased serotonin transporter density in patients with MCI compared to healthy controls (Barrett et al., 2017).
The LH has been reported to have significant connectivity with arousal-related brainstem nuclei, such as the RN and the LC, as well as the striatum, thalamus, orbitofrontal cortex, middle and posterior cingulum, and temporal brain regions (Kullmann et al., 2014; Kullmann and Veit, 2021; Singh et al., 2022). In AD patients with versus without depression, lower rsFC between the LH and the middle temporal and superior temporal gyri has been reported (Liu et al., 2018), indicating that abnormal FC between the hypothalamus and the temporal lobe may reflect the pathophysiology of AD-related depression.
Challenges pertaining to functional imaging of the NSS and potential solutions
So far, FC studies investigating AD-related changes have not resulted in consistent reports on disease stage specific alterations. Nonlinear age effects, as suggested for the LC (Jacobs et al., 2018b), together with varying FC analysis methods and sample sizes are likely contributing to the inconsistency in reported findings. BOLD-fMRI has limited sensitivity compared to structural and diffusion MRI, which partly stems from the proximity of the brainstem to CSF-rich regions as well as the impact of physiological processes (e.g., cardiac pulsation and respiration) on the NSS BOLD-fMRI signal (Brooks et al., 2013). Dissociating the contribution of these non-neuronal contributions to the BOLD-fMRI signal is complicated due to its inherent dependency on blood circulation dynamics and vascular anatomy (Bernier et al., 2018; Bright and Murphy, 2015). Therefore, recording and modeling the contribution of physiology data may improve the sensitivity of the BOLD-fMRI signal in NSS regions (Brooks et al., 2013; Jacobs et al., 2020). Modeling individual- and region-specific hemodynamic response functions showed substantial improvement in signal extraction from the tiny subcortical structures (Lewis et al., 2018; Prokopiou et al., 2022). Furthermore, increases in SNR can be achieved with optimized brainstem-weighted registration techniques (Prokopiou et al., 2022), and using dedicated structural imaging sequences to accurately localize the NSS (Turker et al., 2021). Such dedicated MT-weighted structural sequences are already in use for imaging of the LC (Priovoulos et al., 2018; Trujillo et al., 2019). In general, the sensitivity of the BOLD-fMRI signal to detect pathological changes in the NSS can be significantly improved using multi-echo fMRI, albeit thereby limiting the enhancement of spatial resolution (Markello et al., 2018), and ultra-high-field fMRI (Jacobs et al., 2020; Sclocco et al., 2018; Singh et al., 2022).
Imaging the NSS using diffusion-weighted imaging
Experimental animal and human in vivo studies have provided support for the hypothesis that pathological tau propagates through neuroanatomical connections and spreads via synaptic connectivity (Ahmed et al., 2014; Arnsten et al., 2021; Jacobs et al., 2018a; Khan et al., 2014), resulting in the characteristic topographical pattern of tau progression in AD (Braak et al., 2011b). The trans-entorhinal cortex (TEC) is the first cortical site to harbor hyperphosphorylated tau and neurofibrillary tangles, corresponding to Braak stage I (Braak et al., 2011b; Sanchez et al., 2021). Furthermore, the TEC receives dense projections from several neuromodulatory nuclei, including the LC, DRN, and NbM (Harley et al., 2021). The structural connections between the TEC and these nuclei are therefore of particular interest to investigate the associations between AD pathophysiological progression and the integrity of fiber bundle tracts. In vivo measurement of the axonal fiber bundles that form the structural connections between proximal and distal areas in the human brain is achieved with diffusion-weighted imaging (DWI).
DWI is a magnetic resonance-based technique measuring the restriction of microscopic Brownian motion of water molecules in the brain, based on their displacement within each voxel after applying diffusion gradients. In the axonal fiber bundles, both axonal membranes and myelin sheaths highly restrict the movements of water molecules, causing them to primarily diffuse in parallel with the longitudinal axis of a given fascicle. In turn, the modeling and quantification of such diffusion along axonal bundles yield useful metrics related to axonal density, membrane integrity, and orientation among specific pathways which are often affected by neurodegenerative disorders. Accordingly, one major application of DWI in the context of aging and AD has been the assessment of structural connectivity between brain regions, and most often between cortical areas. In the brainstem, fibers are densely packed and many different pathways run in close proximity, facilitating information transfer between the spinal cord, the cerebellum, and the cortex to support a broad range of physiological and cognitive functions (Zhang et al., 2020). However, this intricate environment poses important challenges to the use of DWI, and studies using DWI to investigate the NSS in the context of aging and AD are relatively scarce.
In a study on individuals across AD stages, the integrity of the LC-TEC structural connections, measured with radial diffusivity (RD), decreased proportionally to disease severity (Sun et al., 2020). RD is a voxel-wise measure of transverse diffusion perpendicular to the principal direction of axonal fiber bundles indicated by the axial diffusivity (AD) in a voxel. The relative length of the principal diffusion direction compared to the transverse diffusion is captured by the fractional anisotropy (FA) metric, which gives a summary indication of the directionality of the diffusion profile within a voxel. In the late mild cognitive impairment (LMCI) group, higher RD values were observed compared to a control group of cognitively unimpaired amyloid-negative participants. In particular, these associations were localized in the laterally bending part of the LC-TEC pathway, past the inferior part of the thalamus. In the AD group, the highest RD values of the LC-TEC pathway were observed, and these changes extended more towards the LC compared to the LMCI group. The authors concluded that the earliest stages of the disease may be related to degradation of the fiber tracts that are in proximity to the TEC, whereas changes closer to the LC could only be detected in more advanced disease stages.
Pathological tau may propagate from the LC to the NbM in the basal forebrain, before reaching the TEC (Braak et al., 2011b; Jethwa et al., 2019; Tiernan et al., 2018). In individuals with early mild cognitive impairment (EMCI) and AD patients, higher free water (FW) diffusivity, reflecting an increase in the volume fraction of extracellular space and thus indicating neurodegenerative processes, was reported among the LC-TEC tract and the NbM compared to cognitively unimpaired participants (Chu et al., 2022; Ofori et al., 2015; Pasternak et al., 2009). After controlling for amyloid status, differences in FW-corrected FA were reported for the NbM and the septum of the forebrain. In the EMCI group, these changes were restricted to the anterior medial portion of the LC-TEC tract, whereas in AD higher FW values were reported throughout the LC-TEC pathway, including the dorsomedial portion of the tract extending from the LC to the basal forebrain. Moreover, higher FW values in the LC-TEC tract and the NbM were associated with lower performance on a series of global cognitive measures (MoCA, MMSE, and LASSI-L). Neurofilament light chain, a cytoplasmic protein that is increased in CSF or blood plasma when neuro-axonal damage or neurodegeneration is present, was positively associated with FW in the LC-TEC tract, the NbM, and the horizontal limb of the diagonal band of the basal forebrain. These results align with the findings reported in the previous study where AD-related alterations to the LC-TEC tract were observed (Chu et al., 2022; Sun et al., 2020).
In another study among 33 cognitively unimpaired older individuals, the LC-TEC pathway was reconstructed with geometric-optics based entropy spectrum pathways, and the proposed metric of equilibrium probability within these pathways showed a negative association with CSF tau (Solders et al., 2021). This indicates that LC-TEC pathway reconstructions with higher probabilities of less anatomical constraints were paired with higher CSF values of p-tau, which was mirrored by the significant negative associations between average LC-TEC FA values and CSF p-tau and total tau, reflecting that elevated tau levels are associated with degradation of the LC-TEC pathway.
Altogether, these DWI studies suggest an association between the disease stage and the degradation of the fiber tracts connecting the LC to the TEC, such that the degradation of the LC-TEC pathway is increasingly more apparent as neuropathological tau accumulates and spreads throughout the brain.
In addition to characterizing the projections originating from the neuromodulatory nuclei, DWI-related contrasts have been used to delineate some of these neuronal populations (Bianciardi et al., 2015; Singh et al., 2021), but also to investigate diffusivity within their boundaries as a proxy measure of their microstructural integrity. Two studies investigating fractional anisotropy (FA) in the LC reported higher FA values in older individuals compared to their younger counterparts (Langley et al., 2020; Porat et al., 2022), whereas lower FA values were observed in the ascending noradrenergic tract of older individuals (Porat et al., 2022). Accordingly, another study showed a decrease in RD and mean diffusivity (MD) values in the LC of older adults, which was further related to memory performance (Langley et al., 2022). These findings may relate to the diffuse projections of the LC, resulting in the fiber bundle tracts that run within the gray matter of the LC to branch out and have highly diffuse orientations. Another caveat is that the majority of these highly branched LC projections are either unmyelinated or thinly myelinated.
Challenges pertaining to diffusion-weighted imaging of the NSS and potential solutions
Similar to brainstem fMRI, technical limitations are inherent to the use of DWI to investigate brainstem neuromodulatory nuclei and their projections in aging and AD. First, an important challenge is that both respiratory and cardiac pulsations can significantly distort brainstem images, such that particular attention should be devoted to the proper preprocessing of DWI images. Second, the inherent poor resolution of diffusion sequences (typically around 2mm3 isotropic) significantly hinders the investigation of DWI metrics within the boundaries of smaller brainstem nuclei, such as the LC. Consequently, these factors have restricted the models that can be used to analyze brainstem diffusion images. Recently, advances in diffusion signal modeling based on biophysical approaches have proposed novel quantitative metrics that aim to significantly refine the DWI-based characterization of tissue microstructure. Among them, the neurite orientation dispersion and density imaging (NODDI) model has attracted particular attention in clinical research (Kamiya et al., 2020; Zhang et al., 2012). By considering the diffusion signal within each voxel and distinguishing between the intra-cellular, extra-cellular, and free water compartments, the NODDI model provides microstructural indices related to the density of neurites and their orientation dispersion in the underlying tissue (Zhang et al., 2012). Combined with the use of dedicated connectome or ≥ 7T MR scanners, the application of these advanced microstructural diffusion models in the context of AD should improve our understanding of the complex and intricate anatomy of fiber bundles in the brainstem area and within the neuromodulatory nuclei of interest, and provide crucial information on how these are affected throughout the disease process (Chu et al., 2022; Lin et al., 2022; Qin et al., 2013).
Imaging the NSS using positron emission tomography
PET imaging has played a major role in advancing our understanding of the trajectory of AD pathology and it has developed into a prominent diagnostic tool. Radiotracers binding to transporters, receptors, and enzymes enable in vivo visualization and quantification of AD pathology and the accompanying characteristic functional metabolic brain changes, such as the accumulation of Aβ plaques, neurofibrillary tau tangles, neuroinflammation, changes in synaptic density and glucose metabolism, as well as neurotransmitter receptor density, synthesis and transport (Bao et al., 2021; Nordberg et al., 2010). In particular, Aβ-PET (Barthel et al., 2011; Curtis et al., 2015; Klunk et al., 2004; Lister-James et al., 2011) and tau-PET (Xia et al., 2013) have revolutionized the understanding of AD pathophysiology. Despite the small anatomical structure of the NSS, their high tracer binding affinity due to high concentrations of molecular targets enables their visualization and quantification using specific PET radioligands and high-resolution PET scanners.
The LC is the earliest site that accumulates detectable phosphorylated tau in AD (Braak et al., 2011b), preceding widespread neurodegeneration. Thus far, PET imaging of pathologic tau in the LC has not been achieved due to constraints related to the high off-target binding of the currently available tau radiotracers to neuromelanin (Aguero et al., 2019; Lee et al., 2018; Lowe et al., 2016; Marquié et al., 2015). Nevertheless, the LC is known to be the primary seat of noradrenergic neurons in the brain, and PET ligands targeting the norepinephrine transporter (NET) have been used to visualize and quantify the LC NET concentration in health and disease (Ding et al., 2006). For example, prior PET studies using [C-11]methylreboxetine (also known as [C-11]MeNer) have demonstrated an upregulation of NET in the LC in cocaine abuse patients (Ding et al., 2010) and a decreased NET concentration in Parkinson’s disease patients with REM sleep behavioral disturbances (Sommerauer et al., 2018). In individuals with AD, a post-mortem autoradiography study demonstrated a decreased NET density in the LC using an F-18 labeled methylreboxetine analogue ([F-18]FMeNER-D2) (Gulyas et al., 2010), but to the best of our knowledge, an in vivo study using a NET PET ligand has not been reported in the AD population.
While the LC is the primary source of noradrenaline release in the brain, it also sends dopaminergic axons to the dorsal hippocampus (Kempadoo et al., 2016). Therefore, one approach to infer the catecholamine synthesis of the LC is the use of [18F]Fluoro-m-tyrosine ([18F]FMT) PET imaging; this radiotracer binds to the enzyme AADC (aromatic amino acid decarboxylase) in monoamine synthesizing pathways. [18F]FMT signal is observed in dopamine synthesizing pathways and in regions synthesizing serotonin and noradrenaline as well (Brown et al., 1999; Ciampa et al., 2022; Kempadoo et al., 2016). Higher LC synthesis of [18F]FMT has been associated with lower rates of pathological tau accumulation in the temporal cortex, measured with [18F]Flortaucipir PET, in individuals with substantial Aβ burden, as quantified with [11C]Pittsburgh compound B PET (Ciampa et al., 2022). The detrimental effect of tau on memory was absent in those with a higher synthesis of [18F]FMT in the LC, indicating a protective effect of NE (Ciampa et al., 2022). In later stages of AD, it has been proposed that neurodegeneration and local hypometabolism are present within cortical and subcortical areas, which can be measured as a reduction in FDG-PET signal (La Joie et al., 2012). Recently, the absence of significant group differences in LC FDG-PET signal were reported in a study comparing individuals with AD and controls (Liu et al., 2021). However, as LC metabolism may not decline in a linear manner over the course of AD, an average FDG-PET value based on individuals in varying stages of the disease may not capture disease-stage specific changes in LC metabolism (Jacobs et al., 2021b; Weinshenker, 2018). Similarly, FDG-PET has been used to investigate glucose synthesis in other NSS. In the VTA, a reduction in FDG-PET signal has been related to apathy, a behavioral symptom often reported in AD (Schroeter et al., 2011). In the NbM, a reduction in FDG-PET signal in the left NbM was reported by two studies (Herholz et al., 2004; Nicolas et al., 2020). At an early stage in the AD disease process, higher uptake values were reported in those with MCI compared to the control group, and this effect was modulated by educational level suggesting that in those with a higher educational level, a compensatory upregulation of cholinergic activity occurs (Nicolas et al., 2020). Overall, these findings suggest that a decline in glucose synthesis is related to later stages of AD, and this association may not be linear in NSS with potential increases in glucose synthesis in the early stages of AD.
In the RN, decreases in transporter and receptor densities in cortical pre-and post-synaptic serotonergic projection areas have been reported in AD patients as assessed with 5-HTT ([11C]DASB; (Ouchi et al., 2009; Smith et al., 2021)), 5-HT1A ([F-18]MPPF; (Kepe et al., 2006; Lanctot et al., 2007; Truchot et al., 2008)) and 5-HT2A ([18F]altanserin; (Blin et al., 1993; Hasselbalch et al., 2008; Marner et al., 2012; Meltzer et al., 1999)) binding tracers (Merlet et al., 2004). In mild AD, neocortical loss of 5-HT2A receptors (Marner et al., 2012) may precede a reduction in 5-HT1A receptors in the RN, as well as decreasing receptor densities in meso- and neocortical postsynaptic serotonergic projection areas, which occur in later disease stages (Kepe et al., 2006). At the early stages of the AD continuum, the reduction in 5-HTT and 5-HT2A but not in 5-HT1A binding suggests a loss of RN projecting serotonergic axons to meso- and neocortical regions while the serotonergic cell bodies in the RN itself are still preserved (Marner et al., 2012).
Cholinergic activity related to the NbM can be measured with PET ligands targeting vesicular acetylcholine transporter (VAChT), acetylcholinesterase enzyme (AChE), α7-nicotinic acetylcholine receptor (α7 nAChR), α4ß2-nicotinic acetylcholine receptor (α4ß2 nAChR), and muscarinic acetylcholine receptor (mAChR) (for a review, see: Tiepolt et al. (2022)). Multiple PET studies showed cholinergic system abnormalities in AD patients in the basal forebrain and cerebral cortex (Tiepolt et al., 2022). Using [F-18]FEOBV-PET, a marker for VAChT, a reduction in VAChT concentration in widespread cortical regions was identified in AD, which correlated with worsening of disease (Aghourian et al., 2017). Further, using (−)-[F-18]Flubatine, a marker for α4ß2 nAChR, AD patients’ executive function and episodic memory were associated with reduced α4ß2 nAChR availability in cortical regions, and in the same study, a decreased basal forebrain α4ß2 nAChR availability correlated with memory scores (Sabri et al., 2018). In addition, reduced α4ß2 nAChR availability in the basal forebrain was associated with lower frontal assessment battery scores in AD patients (Okada et al., 2013). Similarly, an α7 nAChR marker ([C-11]MeQAA) has shown an association between α7 nAChR availability in NbM and cognition deficits in individuals with AD (Nakaizumi et al., 2018). An interesting study combining [C-11]-N-methyl-4-piperidyl acetate (MP4A)-PET (a marker for AChE) with functional MRI demonstrated significantly reduced cortical AChE activity in AD patients as compared to healthy controls, particularly in the lateral temporal lobe (Richter et al., 2018). Further, it was demonstrated that cholinergic integrity in the hippocampus predicted a favorable response to treatment with an AChE inhibitor (rivastigmine), as determined by clinical assessment and fMRI (Richter et al., 2018; Tiepolt et al., 2022). In an older study using [C-11]MP4A, reduced AChE was seen in the amygdala and cerebral cortex but relatively preserved and even increased AChE activity and glucose metabolism were reported for the right NbM in mild to moderate AD (Herholz et al., 2004). This preservation or increase in NbM metabolism as determined with FDG-PET may be accompanied by significant reductions in cortical acetylcholinesterase and may reflect a compensatory phenomenon (Aghourian et al., 2021; Herholz et al., 2004; Richter et al., 2022). These reports underscore the importance of understanding the temporal and mechanistic relationship between the early degeneration of cortical cholinergic axon terminals, which has been hypothesized to reflect cortical Aβ toxicity (Thal et al., 2002), and degeneration of the cholinergic cell bodies in the NbM itself.
Challenges pertaining to PET-based imaging of the NSS and potential solutions
NSS structures are anatomically complex and small in size relative to the spatial resolution of PET, which is limited by physical factors including positron range, annihilation non-collinearity, as well as detector size, inter crystal scatter, and crystal penetration (Brzezinski et al., 2014). To facilitate the accurate detection of tiny brainstem nuclei, the use of high-resolution anatomical imaging of brainstem nuclei using dedicated MR sequences, accurate PET/MRI co-registration algorithms, and point-spread function models are needed (Baete et al., 2004; Jacobs and Cherry, 2001; Mehranian et al., 2017; Song et al., 2020; Song et al., 2019; Sudarshan et al., 2021; Zhu and Zhu, 2019). Furthermore, future studies are required to better understand associations between the capacity of tracer uptake to various monoaminergic pathways (e.g., [18F]FMT), AD-related pathology and clinical symptoms. Additionally, the development of high-field combined MRI/PET systems will help improve our understanding of the NSS. Despite these challenges, the high relative concentration of neurotransmitter receptors and transporters in the NSS leads to a high contrast to noise ratio for various PET radioligands, which facilitates the assessment of molecular changes in NSS in AD.
Imaging the NSS using electrophysiology recordings
Unlike other in vivo imaging methods in humans, electrophysiology is a direct measure of neuronal electric activity, and consequently has outstanding temporal sensitivity. A wide variety of invasive and non-invasive electrophysiological recording methods are popular in scientific settings (for reviews, see Frank et al. (2019); He et al. (2011)). In particular, the magnetoencephalogram (MEG) and the electroencephalogram (EEG) have been used extensively to identify general neurophysiological hallmarks of AD (Yener et al., 2022). Unfortunately, little is known about the pathological electrophysiological changes in the human NSS in the context of AD, primarily due to the evident challenge of acquiring electrophysiological data from the human midbrain and brainstem. That is why current research investigating direct recordings of the NSS is limited to deep brain stimulation (DBS) electrodes. However, implantation of DBS electrodes is not without risks, and this procedure is not applied without proven medical effectiveness. Consequently, animal models of AD are often used to directly investigate disease-related alterations in NSS neuronal activity. In humans, studies investigating subcortical DBS in the NbM (Kuhn et al., 2015) and the fornix (Fontaine et al., 2013; Laxton et al., 2010; Leoutsakos et al., 2018) in the context of AD reported no consistent improvements in cognitive scores (Jakobs et al., 2020). Lacking access to direct electrical recordings in the human NSS, often proxy signals like cortical oscillations or event-related potentials (ERPs) that can be recorded with EEG or MEG are investigated through targeted de/activation of specific NSS via tasks, stimulation, or pharmacological intervention (Babiloni et al., 2020; Muthukumaraswamy, 2014; Schumacher et al., 2020). Unfortunately, such studies come with the caveat that these patterns are always subject to other influences as well, e.g., sleep, stress, comorbidities, and medication (Banis et al., 2014; Borghini et al., 2014; Muthukumaraswamy and Liley, 2018; Uhlhaas and Singer, 2010).
To the best of our knowledge, no studies have been published on direct recordings of oscillatory or spiking activity in the human NbM through implanted DBS electrodes in individuals in AD. Patients with MCI displayed a drop in the theta frequency during an oddball task as well as the absence of the beta band component (Lee et al., 2019). In studies using non-invasive electrophysiological methods, the larger group sizes and the inclusion of a healthy control group are mitigating the much lower spatial specificity, and different proxies have been used to establish AD-related changes to the cholinergic system. The EEG-ACh index, derived from pharmacological ACh blocking (Johannsson et al., 2015; Snaedal et al., 2010), shows strong correlations with cortical atrophy across MCI and AD patients (Petrova et al., 2020). Furthermore, NbM volume loss and increased MD along the lateral NbM tract correlated with a slowing of the global alpha peak (Rea et al., 2021; Schumacher et al., 2020). Overall, oddball tasks have revealed disease-specific shifts in low oscillatory EEG components in MCI compared to age-matched healthy individuals (Rosenblum et al., 2022), and oddball-evoked ERP latencies have shown to increase with central cholinergic blocking (Pekkonen et al., 2005) and to decrease under cholinergic therapy (Onofrj et al., 2003; Thomas et al., 2001) and NbM DBS for AD (Dürschmid et al., 2020).
Akin to the NbM, no direct recordings of the VTA have been reported in humans. However, DBS implantation in the neighboring substantia nigra (SN) showed phase-locking of SN firing with prefrontal theta power (Kamiński et al., 2018). Rodent studies have emphasized the functional importance of the VTA/SN theta frequency activity (Shi, 2005; van der Velden et al., 2020), its strong temporal link with prefrontal cortical activity (Mishra et al., 2021), and showed a reduction in theta rhythm firing in dopaminergic VTA neurons in models of familial AD (Vorobyov et al., 2019). As a result, prefrontal theta power appears to be a good candidate for a noninvasive electrophysiological proxy of VTA activity.
Currently, there are no studies reporting on DBS electrodes in the human RN or the LH in the context of AD. Interestingly, even though the dopaminergic system is strongly implicated in attention, oddball ERPs remain largely unaffected by SSRIs, tryptophan depletion or genetic polymorphisms (d’Ardhuy et al., 1999; Enge et al., 2011; Fischer et al., 2015).
LC neurons have two distinct patterns of population firing, phasic and tonic, creating a functional balance across time to allow task engagement and disengagement (Aston-Jones and Cohen, 2005). There are no studies investigating changes in LC firing patterns in humans in vivo (Feinstein et al., 1989). In animals, LC phasic bursts were found connected to slow oscillations in the hippocampus and prefrontal cortex, which in turn modulated the power of cortical theta and gamma rhythms (Durán et al., 2021; Eschenko et al., 2012; Neves et al., 2018; Sara and Hervé-Minvielle, 1995; Totah et al., 2018; Xiang et al., 2022). Injection of preformed synthetic tau fibrils into the mouse LC led to a decrease in alpha peak frequency in the hippocampus and lowered phase-amplitude coupling between hippocampal theta and prefrontal gamma oscillations, indicating that cortical proxies of LC activity can indeed display AD-related changes in the LC (Ahnaou et al., 2019). In humans, norepinephrine release during an attention task (as inferred from pupil size) was associated with cortical alpha and beta band desynchronization events (Dahl et al., 2020). Further, frontal midline beta/theta ratio as well as task-evoked potential changes were recently linked to noradrenergic activity, albeit without the direct measurement of LC activity (Burger et al., 2020; Melnychuk et al., 2021). Specifically, the P3b ERP in oddball tasks has been associated with cognition and showed a decrease in amplitude in MCI and AD (Ashford et al., 2011; Porcaro et al., 2019; Porcaro et al., 2022), but other systems besides the noradrenergic system contribute to the P300 response (Brown et al., 2015). In intervention settings, transcutaneous peripheral nerve stimulation methods, including the vagus nerve and occipital nerve, which are assumed to increase LC activity (Sclocco et al., 2020), resulted in decreased occipital alpha power and increased theta-gamma phase-amplitude coupling as well as increases in the P3b amplitude (Lewine et al., 2019; Sharon et al., 2021; Vanneste et al., 2020) and improved memory function in older individuals (Jacobs et al., 2015).
Challenges pertaining to electrophysiological recordings of the NSS and potential solutions
Technical limitations that apply to electrophysiological recordings to investigate the NSS are primarily related to their size and location. First, the NSS lie far from the surface of the head, hindering the ease with which their electrical activity can be picked up with sensors at or around the head surface. Second, the small size of the NSS translates to their limited contribution to the electrical signals that are captured farther away. These factors limit the utility of non-invasive electrophysiology measures to investigate the NSS directly. Invasive electrophysiology measures evade these challenges, but these come with additional ethical considerations due to the risks attached to brain surgery. Thus, further exploration of the connection between non-invasive electrophysiological signals and the NSS will help our understanding of electrophysiological changes in the human NSS in the context of cognition and AD.
Discussion
AD is characterized by a long and gradually progressive asymptomatic phase characterized by a multitude of silent neuropathologic, morphologic and functional brain changes. As a result, the focus in the AD field has shifted towards earlier intervention with the aim of slowing down and ultimately stopping AD before irreversible damage has occurred (Sperling et al., 2020). Autopsy studies have identified the NSS nuclei as the first regions accumulating tau pathology, highlighting the presence of AD-related pathology well before cortical pathology or clinical symptoms are evident (Braak and Del Tredici, 2011a, b; Braak et al., 2011b). Therefore, reliable in vivo measurements of changes in these systems are critical for the early detection of AD, and in turn, will improve the identification of individuals who are more likely to benefit from prevention trials.
The NSS nuclei are notoriously difficult to image in vivo due to their size, location, and susceptibility to artifacts. However, the development of new methods taking advantage of ultra-high-field imaging to provide better spatial and temporal resolution together with new radioactive isotopes providing insight into molecular and neurotransmitter changes, have revolutionized the in vivo study of the human NSS in the context of neurodegenerative diseases. This progress is evident from the doubling of the number of publications on neuroimaging of the NSS in AD during the last decade. The aim of this review was to discuss the current state of knowledge on neuroimaging these systems in vivo in AD using currently available methods and identify methodological as well as pathophysiological gaps in the literature.
This non-exhaustive review of the literature showed that research investigating the NSS has primarily focused on the LC-norepinephrine and the NbM-cholinergic systems (Table 1). The latter system because of the availability of post-mortem validated templates and the former because of the structural MRI-sequences allowing the identification of the nuclei at the individual level. These studies have consistently shown that morphological changes in these nuclei start early in adulthood and accelerate during aging and AD, consistent with autopsy data. Diffusion data suggest that the extent of white matter degradation of the fiber bundles connecting the LC or NbM with the entorhinal cortex is related to disease progression. Lower structural integrity (volume or intensity) of these nuclei was associated with the earliest accumulations of cortical tau pathology, suggesting that changes in these NSS precede pathology in the cortex. In support of this hypothesis is the observation that lower catecholamine synthesis in the LC, as measured with PET, was associated with greater cortical tau accumulation. Interestingly, both the LC and the NbM nuclei seem to have a specific regional vulnerability to tau pathology. Within the LC, the rostral-dorsal part is more vulnerable to AD-related changes, while the posterior NbM exhibited the earliest volumetric changes, indicating that certain clusters or neuronal ensembles may be more vulnerable to tau. The exact physiologic properties contributing to the vulnerability of these neuronal ensembles remain unclear. Recent animal literature demonstrating that tonic activity is associated with cognitive decline suggest that neurons exhibiting different firing patterns may have a different neurochemical makeup (Noei et al., 2022; Totah et al., 2019). Probing firing patterns with neuroimaging is challenging given its temporal resolution, though recent computational modeling work was able to link phasic LC activity to specific LC-related network dynamics (Munn et al., 2021). Another way to probe phasic activity is through the P300 wave, which is characterized by a lower amplitude in MCI and AD patients compared to clinically normal adults. Cortical EEG measurements such as P300 and P3b combined with saliva alpha-amylase (Ventura-Bort et al., 2018) and data from non-human primate research suggest P3a is more governed by dopamine while P3b seems to be related to the LC norepinephrine system (Polich, 2007), indicating that EEG-measurements can be a promising non-invasive, affordable, and widely available tool to assess the function of these NSS and possibly their interrelationships.
Table 1.
Reported findings of studies included in this review.
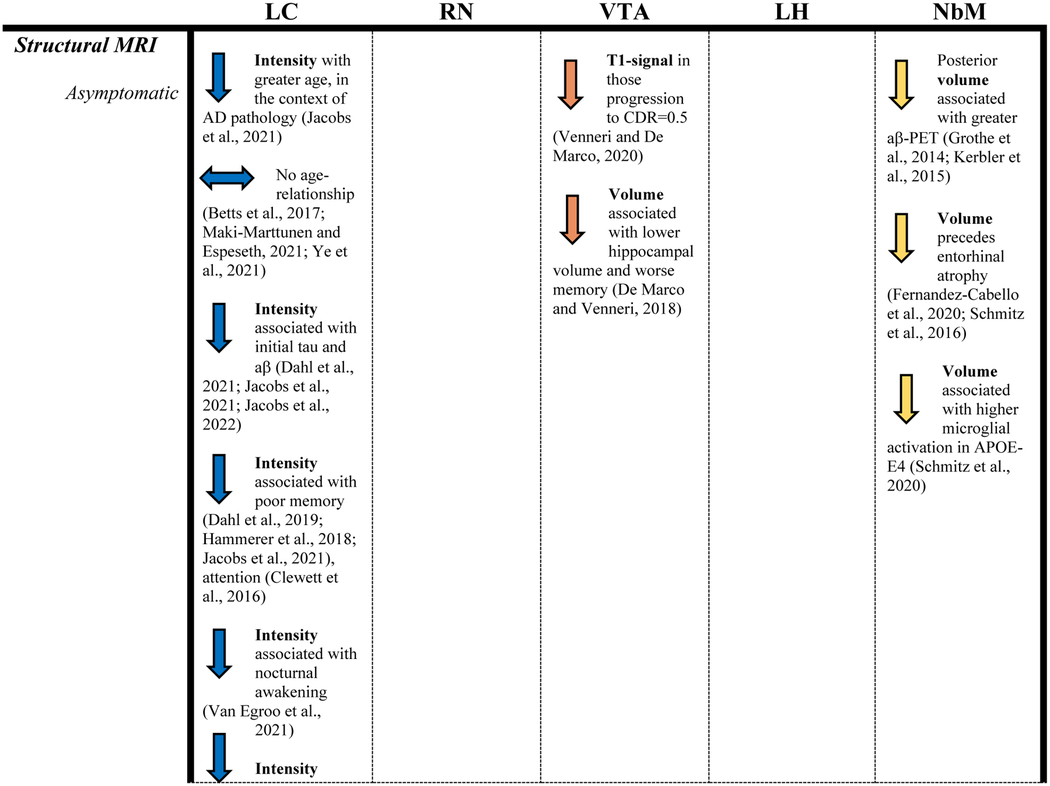
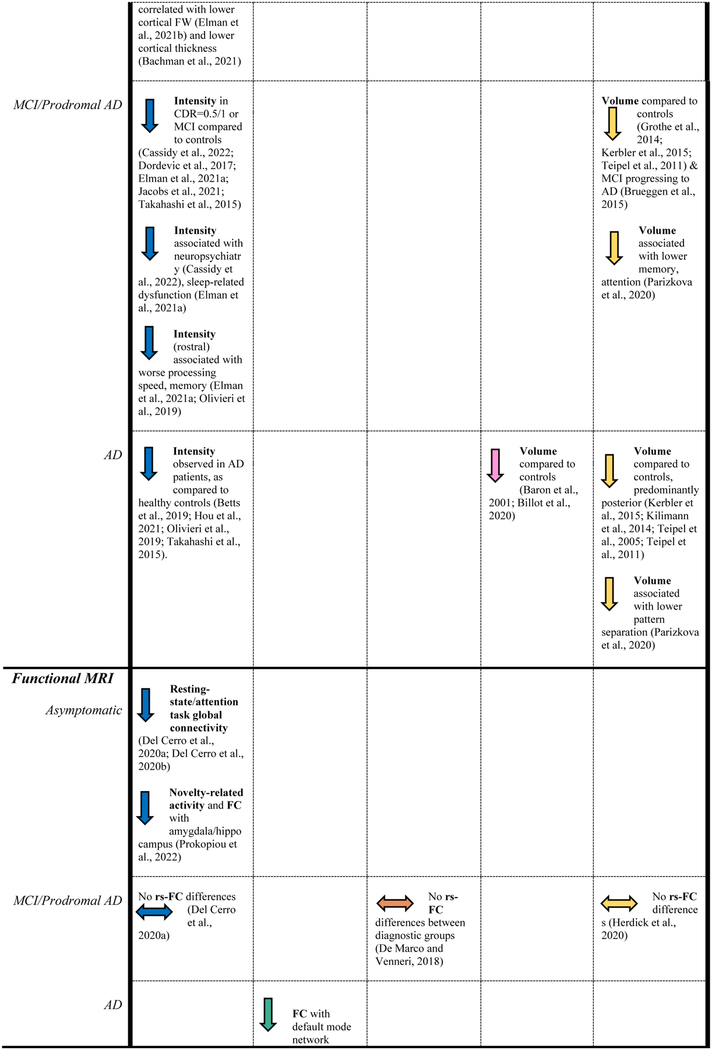
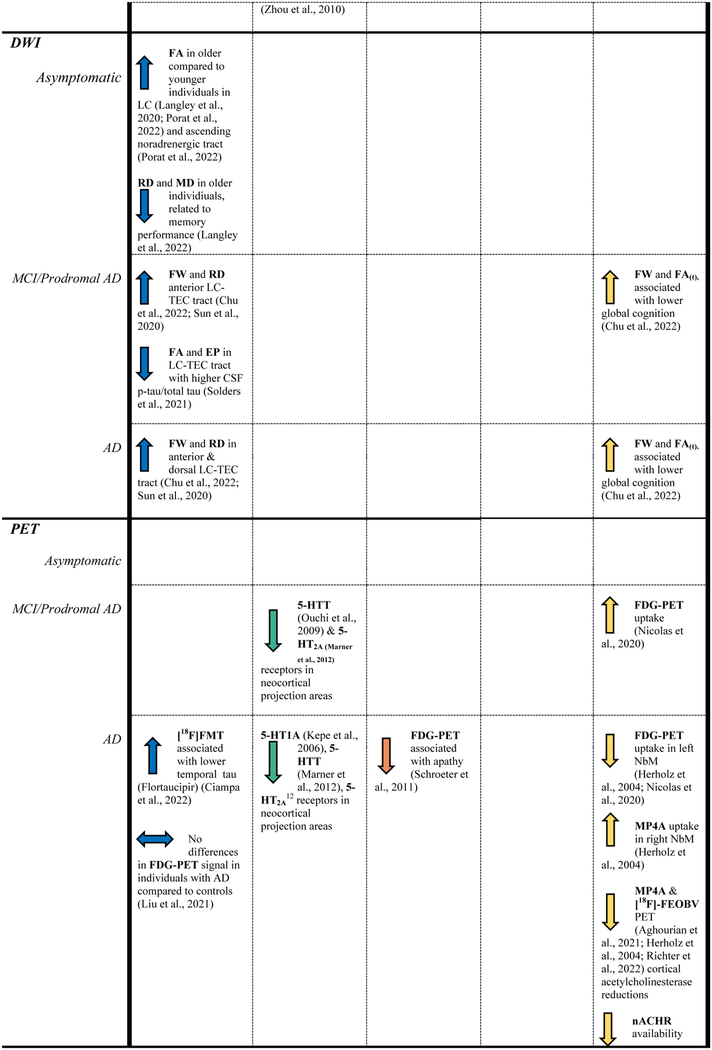
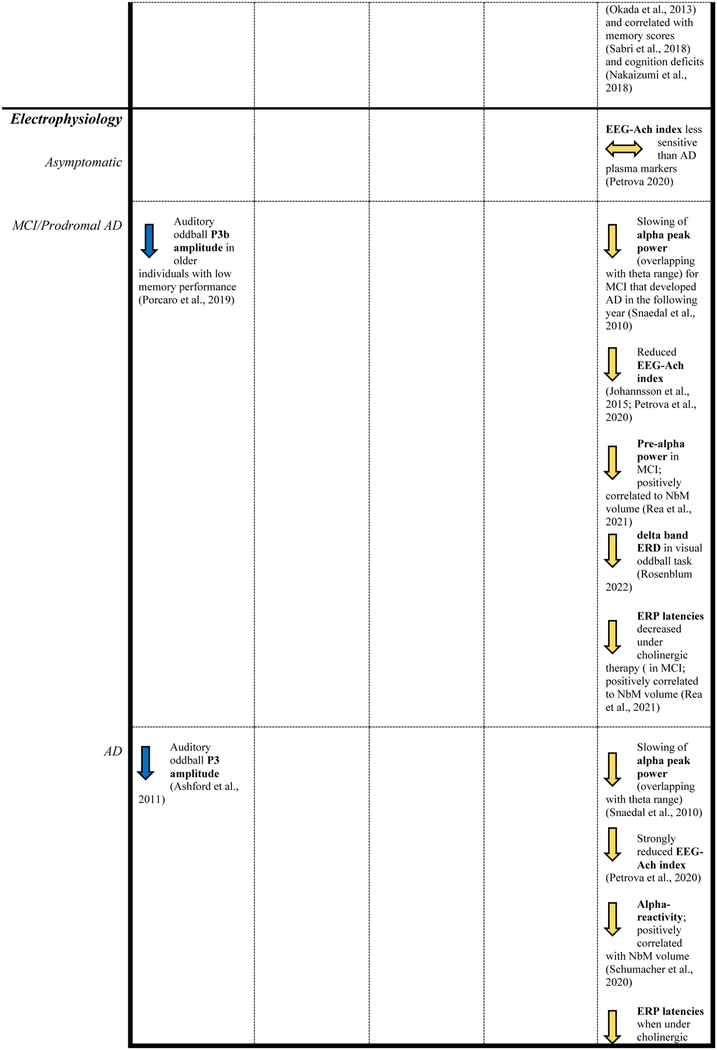

|
So far, the dopaminergic VTA, the orexinergic lateral hypothalamus, and the serotonergic DRN have received much less attention in AD. There is an urgent need for imaging methods that enable the anatomic identification of these nuclei at the individual level to facilitate the study of structural and functional changes in these nuclei. Investigating the NSS nuclei at the individual level is critical for our understanding of interindividual variability and factors that determine progression of versus resilience against AD-related processes. Even though the current spatial resolution of PET is limited given the small size of these nuclei, several tracers allow examination of these neurotransmitter systems in vivo. These tracers revealed that cell bodies in these nuclei remain intact early in the disease course, at which time cortical axonal terminals and projections are degenerating. However, there are currently only a small number of PET imaging studies that report on the NSS, whereas these studies are pivotal to further our understanding of the neurochemical changes within the chronological framework of NSS changes in AD. Similarly, functional MRI studies reported a loss of connectivity between the VTA or DRN and cortical regions in older individuals and patient populations. Whether regions that show strong functional connectivity to the NSS exhibit similar levels of tau pathology accumulation has yet to be investigated. As our field is developing new methods to image different properties of these nuclei, we have the opportunity to answer important pathophysiologic questions to understand why these systems are selectively vulnerable in the early stages of the disease; what the functional consequences are of degeneration of projections; when projections start to degenerate and how they impact NSS neurons, cortical neurons or networks; and finally, whether supporting the NSS nuclei can prevent the retraction of their projections. Such insights can benefit our understanding of AD, improve early detection and even provide novel opportunities for interventions.
Current disease models and diagnostic criteria position the presence of Aβ deposition as a requirement for AD neuropathologic change (Jack et al., 2018). These models do not yet include early changes in the NSS, reflecting the current debate on whether these early tau depositions in the NSS are age-related or may convey information on AD-risk. Autopsy data and the reviewed studies indicate that the NSS nuclei are affected at an earlier timepoint in the disease process, and the structural and functional changes in the NSS correlate with cognitive decline and disease progression as well as the initial accumulation of AD pathology (Rüb et al., 2000; Rüb et al., 2017; Stratmann et al., 2016). It is important to note that even though by age 50, almost every case at autopsy exhibits tau pathology in the NSS nuclei, not everyone will ultimately develop AD. There is a need to identify factors or properties relating to the NSS that contribute to AD risk. These observations highlight that the AD field needs to move to earlier asymptomatic stages of the disease to gain breakthroughs in the treatment of AD. Correspondingly, these observations emphasize the urgent need to determine the exact sequence of NSS-related changes in the temporal evolution of the pathophysiologic cascade of AD biomarkers, and the value of these NSS changes as biomarkers for early detection and monitoring disease progression or selecting individuals for clinical trials. Multidisciplinary collaboration where neuropathologists, neuroimagers, biofluid researchers, neuropsychologists, and clinicians join forces is necessary to improve the state-of-the-art methods; to understand the evolution of neurobehavioral, cognitive, and pathologic changes by establishing longitudinal cohorts starting from midlife or even earlier on; and to assess new interventions targeting the NSS (Future Directions box).
In concert, related fields can provide insights into critical tools to enlarge the current arsenal available to examine the NSS. For the noradrenergic system, the NET transporter ligand MeNER has been used to give an indication of NET-binding in Parkinson’s Disease (Nahimi et al., 2018; Sommerauer et al., 2018). This tracer would enable the investigation of the cortical correlates of NE-neurochemical changes in AD in vivo, and clarify how these changes relate temporally and spatially to other functional, structural and metabolic changes, as well as to overall amyloid, tau deposition, and neuroinflammation. Already, post-mortem work in AD has demonstrated a correlation between NET transporter density and the cortical Braak stages (Gulyas et al., 2010). Additionally, a recent biomarker-driven, phase II trial of atomoxetine (a NET inhibitor) in individuals with MCI due to AD demonstrated improved metabolic activity in medial temporal lobe circuits, significant reductions in CSF total Tau and pTau181, and improvements in synaptic, metabolic and neuroinflammation markers following treatment with atomoxetine (Levey et al., 2021), revealing that modulating the NSS can be of great therapeutic relevance for AD.
Early pathological changes in subcortical regions have the potential to alter cell firing patterns (Ahnaou et al., 2019), and the ensuing breakdown of physiological networks could be a central driver of AD progression. At present, DBS electrode placement in subcortical regions facilitating in vivo electrophysiology recordings in humans is not practiced for AD. While DBS provides a unique possibility of directly recording field potentials, it is limited by the scarcity of implants that are applied to research and resulting measurements can be confounded by the indication warranting the DBS placement. Nevertheless, implanted electrodes with novel wireless transmitters can record data during daily life, unencumbered by a test setup and situation. Both invasive and non-invasive electrophysiological recordings related to subcortical structures can provide us with specific early functional biomarkers in preclinical AD, prior to our ability to detect structural changes. Overall, longitudinal observations of subcortical structures over the course of AD progression would advance our understanding of pathological changes in NSS activity and this may lead to the development and monitoring of interventions targeting specific physiological functions.
The exploration of neuroscientific and clinical AD-related hypotheses will be facilitated by improving, developing, harmonizing, and integrating multimodal neuroimaging methods with biofluid data, as well as cognitive and neuropsychiatric metrics; but it also necessitates the delineation of the healthy NSS. This work warrants the consideration that AD is often examined in the context of the aging brain, and it can be difficult to separate the effects of aging from those of AD. Interestingly, some of these NSS nuclei are known to be highly resilient against the accumulation of tau. The comprehensive study of the NSS across the lifespan will allow us to assess the temporal order of detrimental or compensatory changes in the NSS compared to modifications that remain within the normative range. Ultimately, this will allow us to map protective as well as risk factors to provide insight into the unanswered question why some individuals progress to AD and other do not (Szot et al., 2006).
Summarizing, recent developments in neuroimaging methods have revived the focus on the NSS in AD as the next frontier for early detection and intervention of AD. This review demonstrated that imaging the NSS in vivo in great detail is feasible and provides important directions to understand the role of the NSS in the earliest pathophysiology and symptomatology of AD. Future research investigating the functional properties of NSS in relation to AD would greatly benefit from large-scale studies, including large clinical cohorts, to overcome statistical power limitations.
Highlights.
Structural and functional alterations of NSS predict AD-related phenotypes.
Multimodal examination of NSS provides complementary information on AD pathogenesis.
Post-mortem validation is crucial for accurate in vivo localization of NSS in humans.
In vivo NSS measurements are essential for early detection and treatment of AD.
Acknowledgments
This work was supported by the National Institutes of Health [R01AG062559, R01AG068062, R21AG074220 (PI Jacobs Heidi, PhD), T32 EB013180 (PI El Fakhri Georges, PhD)]; the Alzheimer’s Association [AARG-22-920434 (PI Jacobs Heidi, PhD)]; and Brightfocus Foundation [A20211016F (PI Van Egroo Maxime, PhD]).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghourian M, Aumont E, Grothe MJ, Soucy JP, Rosa-Neto P, Bedard MA, 2021. FEOBV-PET to quantify cortical cholinergic denervation in AD: Relationship to basal forebrain volumetry. J. Neuroimaging 31, 1077–1081. [DOI] [PubMed] [Google Scholar]
- Aghourian M, Legault-Denis C, Soucy JP, Rosa-Neto P, Gauthier S, Kostikov A, Gravel P, Bedard MA, 2017. Quantification of brain cholinergic denervation in Alzheimer’s disease using PET imaging with [(18)F]-FEOBV. Mol. Psychiatry 22, 1531–1538. [DOI] [PubMed] [Google Scholar]
- Aguero C, Dhaynaut M, Normandin MD, Amaral AC, Guehl NJ, Neelamegam R, Marquie M, Johnson KA, El Fakhri G, Frosch MP, Gomez-Isla T, 2019. Autoradiography validation of novel tau PET tracer [F-18]-MK-6240 on human postmortem brain tissue. Acta Neuropathol Commun 7, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z, Cooper J, Murray TK, Garn K, McNaughton E, Clarke H, Parhizkar S, Ward MA, Cavallini A, Jackson S, Bose S, Clavaguera F, Tolnay M, Lavenir I, Goedert M, Hutton ML, O’Neill MJ, 2014. A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: the pattern of spread is determined by connectivity, not proximity. Acta Neuropathol 127, 667–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahnaou A, Walsh C, Manyakov NV, Youssef SA, Drinkenburg WH, 2019. Early Electrophysiological Disintegration of Hippocampal Neural Networks in a Novel Locus Coeruleus Tau-Seeding Mouse Model of Alzheimer’s Disease. Neural Plast. 2019, e6981268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alenina N, Klempin F, 2015. The role of serotonin in adult hippocampal neurogenesis. Behav. Brain Res 277, 49–57. [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association, 2022. 2022 Alzheimer’s disease facts and figures, Alzheimer’s & Dementia, pp. 700–789. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Datta D, Del Tredici K, Braak H, 2021. Hypothesis: Tau pathology is an initiating factor in sporadic Alzheimer’s disease. Alzheimers Dement 17, 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford JW, Rosen A, Adamson M, Bayley P, Sabri O, Furst A, Black SE, Weiner M, Coburn KL, Rose TL, Bayley PJ, 2011. P300 Energy Loss in Aging and Alzheimer’s Disease. Journal of Alzheimer’s Disease 26, 229–238. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD, 2005. AN INTEGRATIVE THEORY OF LOCUS COERULEUS-NOREPINEPHRINE FUNCTION: Adaptive Gain and Optimal Performance. Annu. Rev. Neurosci 28, 403–450. [DOI] [PubMed] [Google Scholar]
- Avery MC, Krichmar JL, 2017. Neuromodulatory Systems and Their Interactions: A Review of Models, Theories, and Experiments. Front. Neural Circuits 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C, Blinowska K, Bonanni L, Cichocki A, De Haan W, Del Percio C, Dubois B, Escudero J, Fernández A, Frisoni G, Guntekin B, Hajos M, Hampel H, Ifeachor E, Kilborn K, Kumar S, Johnsen K, Johannsson M, Jeong J, LeBeau F, Lizio R, Lopes da Silva F, Maestú F, McGeown WJ, McKeith I, Moretti DV, Nobili F, Olichney J, Onofrj M, Palop JJ, Rowan M, Stocchi F, Struzik ZM, Tanila H, Teipel S, Taylor JP, Weiergräber M, Yener G, Young-Pearse T, Drinkenburg WH, Randall F, 2020. What electrophysiology tells us about Alzheimer’s disease: a window into the synchronization and connectivity of brain neurons. Neurobiol. Aging 85, 58–73. [DOI] [PubMed] [Google Scholar]
- Bachman SL, Dahl MJ, Werkle-Bergner M, Duzel S, Forlim CG, Lindenberger U, Kuhn S, Mather M, 2021. Locus coeruleus MRI contrast is associated with cortical thickness in older adults. Neurobiol. Aging 100, 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baete K, Nuyts J, Van Paesschen W, Suetens P, Dupont P, 2004. Anatomical-based FDG-PET reconstruction for the detection of hypo-metabolic regions in epilepsy. IEEE Trans. Med. Imaging 23, 510–519. [DOI] [PubMed] [Google Scholar]
- Banis S, Geerligs L, Lorist MM, 2014. Acute stress modulates feedback processing in men and women: differential effects on the feedback-related negativity and theta and beta power. PLoS One 9, e95690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao W, Xie F, Zuo C, Guan Y, Huang YH, 2021. PET Neuroimaging of Alzheimer’s Disease: Radiotracers and Their Utility in Clinical Research. Front Aging Neurosci 13, 624330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron JC, Chetelat G, Desgranges B, Perchey G, Landeau B, de la Sayette V, Eustache F, 2001. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer’s disease. NeuroImage 14, 298–309. [DOI] [PubMed] [Google Scholar]
- Barrett FS, Workman CI, Sair HI, Savonenko AV, Kraut MA, Sodums DJ, Joo JJ, Nassery N, Marano CM, Munro CA, Brandt J, Zhou Y, Wong DF, Smith GS, 2017. Association between serotonin denervation and resting-state functional connectivity in mild cognitive impairment. Hum. Brain Mapp 38, 3391–3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel H, Gertz HJ, Dresel S, Peters O, Bartenstein P, Buerger K, Hiemeyer F, Wittemer-Rump SM, Seibyl J, Reininger C, Sabri O, 2011. Cerebral amyloid-β PET with florbetaben (18F) in patients with Alzheimer’s disease and healthy controls: a multicentre phase 2 diagnostic study. Lancet Neurol. 10, 424–435. [DOI] [PubMed] [Google Scholar]
- Bernier M, Cunnane SC, Whittingstall K, 2018. The morphology of the human cerebrovascular system. Hum. Brain Mapp 39, 4962–4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts MJ, Cardenas-Blanco A, Kanowski M, Jessen F, Duzel E, 2017. In vivo MRI assessment of the human locus coeruleus along its rostrocaudal extent in young and older adults. NeuroImage 163, 150–159. [DOI] [PubMed] [Google Scholar]
- Betts MJ, Cardenas-Blanco A, Kanowski M, Spottke A, Teipel SJ, Kilimann I, Jessen F, Düzel E, 2019a. Locus coeruleus MRI contrast is reduced in Alzheimer’s disease dementia and correlates with CSF Aβ levels. Alzheimers Dement (Amst) 11, 281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts MJ, Kirilina E, Otaduy MCG, Ivanov D, Acosta-Cabronero J, Callaghan MF, Lambert C, Cardenas-Blanco A, Pine K, Passamonti L, Loane C, Keuken MC, Trujillo P, Lusebrink F, Mattern H, Liu KY, Priovoulos N, Fliessbach K, Dahl MJ, Maass A, Madelung CF, Meder D, Ehrenberg AJ, Speck O, Weiskopf N, Dolan R, Inglis B, Tosun D, Morawski M, Zucca FA, Siebner HR, Mather M, Uludag K, Heinsen H, Poser BA, Howard R, Zecca L, Rowe JB, Grinberg LT, Jacobs HIL, Duzel E, Hammerer D, 2019b. Locus coeruleus imaging as a biomarker for noradrenergic dysfunction in neurodegenerative diseases. Brain 142, 2558–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianciardi M, Toschi N, Edlow BL, Eichner C, Setsompop K, Polimeni JR, Brown EN, Kinney HC, Rosen BR, Wald LL, 2015. Toward an In Vivo Neuroimaging Template of Human Brainstem Nuclei of the Ascending Arousal, Autonomic, and Motor Systems. Brain Connect 5, 597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billot B, Bocchetta M, Todd E, Dalca AV, Rohrer JD, Iglesias JE, 2020. Automated segmentation of the hypothalamus and associated subunits in brain MRI. NeuroImage 223, 117287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin J, Baron JC, Dubois B, Crouzel C, Fiorelli M, Attar-Lévy D, Pillon B, Fournier D, Vidailhet M, Agid Y, 1993. Loss of brain 5-HT2 receptors in Alzheimer’s disease. In vivo assessment with positron emission tomography and [18F]setoperone. Brain 116 (Pt 3), 497–510. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Grothe MJ, Ray NJ, Müller M, Teipel SJ, 2018. Recent advances in cholinergic imaging and cognitive decline-Revisiting the cholinergic hypothesis of dementia. Curr Geriatr Rep 7, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghini G, Astolfi L, Vecchiato G, Mattia D, Babiloni F, 2014. Measuring neurophysiological signals in aircraft pilots and car drivers for the assessment of mental workload, fatigue and drowsiness. Neurosci. Biobehav. Rev 44, 58–75. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, 2011a. Alzheimer’s pathogenesis: is there neuron-to-neuron propagation? Acta Neuropathol 121, 589–595. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, 2011b. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol 121, 171–181. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, 2015. The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain 138, 2814–2833. [DOI] [PubMed] [Google Scholar]
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K, 2011a. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol 70, 960–969. [DOI] [PubMed] [Google Scholar]
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K, 2011b. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol 70, 960–969. [DOI] [PubMed] [Google Scholar]
- Bright MG, Murphy K, 2015. Is fMRI “noise” really noise? Resting state nuisance regressors remove variance with network structure. NeuroImage 114, 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks JC, Faull OK, Pattinson KT, Jenkinson M, 2013. Physiological noise in brainstem FMRI. Front. Hum. Neurosci 7, 623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SB, van der Wee NJ, van Noorden MS, Giltay EJ, Nieuwenhuis S, 2015. Noradrenergic and cholinergic modulation of late ERP responses to deviant stimuli. Psychophysiology 52, 1620–1631. [DOI] [PubMed] [Google Scholar]
- Brown WD, DeJesus OT, Pyzalski RW, Malischke L, Roberts AD, Shelton SE, Uno H, Houser WD, Nickles RJ, Holden JE, 1999. Localization of trapping of 6-[(18)F]fluoro-L-m-tyrosine, an aromatic L-amino acid decarboxylase tracer for PET. Synapse 34, 111–123. [DOI] [PubMed] [Google Scholar]
- Brueggen K, Dyrba M, Barkhof F, Hausner L, Filippi M, Nestor PJ, Hauenstein K, Kloppel S, Grothe MJ, Kasper E, Teipel SJ, 2015. Basal Forebrain and Hippocampus as Predictors of Conversion to Alzheimer’s Disease in Patients with Mild Cognitive Impairment - A Multicenter DTI and Volumetry Study. J. Alzheimers Dis 48, 197–204. [DOI] [PubMed] [Google Scholar]
- Brzezinski K, Oliver JF, Gillam J, Rafecas M, 2014. Study of a high-resolution PET system using a silicon detector probe. Phys Med Biol 59, 6117–6140. [DOI] [PubMed] [Google Scholar]
- Burger AM, D’Agostini M, Verkuil B, Van Diest I, 2020. Moving beyond belief: A narrative review of potential biomarkers for transcutaneous vagus nerve stimulation. Psychophysiology 57, e13571. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Uludağ K, Dubowitz DJ, Liu TT, 2004. Modeling the hemodynamic response to brain activation. NeuroImage 23 Suppl 1, S220–233. [DOI] [PubMed] [Google Scholar]
- Byers AL, Yaffe K, 2011. Depression and risk of developing dementia. Nat Rev Neurol 7, 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Hou J, Ping J, Cai D, 2018. Advances in developing novel therapeutic strategies for Alzheimer’s disease. Molecular neurodegeneration 13, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy CM, Therriault J, Pascoal TA, Cheung V, Savard M, Tuominen L, Chamoun M, McCall A, Celebi S, Lussier F, Massarweh G, Soucy JP, Weinshenker D, Tardif C, Ismail Z, Gauthier S, Rosa-Neto P, 2022. Association of locus coeruleus integrity with Braak stage and neuropsychiatric symptom severity in Alzheimer’s disease. Neuropsychopharmacology 47, 1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Huddleston DE, Langley J, Ahn S, Barnum CJ, Factor SA, Levey AI, Hu X, 2014. Simultaneous imaging of locus coeruleus and substantia nigra with a quantitative neuromelanin MRI approach. Magn. Reson. Imaging 32, 1301–1306. [DOI] [PubMed] [Google Scholar]
- Chiesa PA, Cavedo E, Grothe MJ, Houot M, Teipel SJ, Potier MC, Habert MO, Lista S, Dubois B, Hampel H, 2019. Relationship between Basal Forebrain Resting-State Functional Connectivity and Brain Amyloid-β Deposition in Cognitively Intact Older Adults with Subjective Memory Complaints. Radiology 290, 167–176. [DOI] [PubMed] [Google Scholar]
- Chu WT, Wang WE, Zaborszky L, Golde TE, DeKosky S, Duara R, Loewenstein DA, Adjouadi M, Coombes SA, Vaillancourt DE, 2022. Association of Cognitive Impairment With Free Water in the Nucleus Basalis of Meynert and Locus Coeruleus to Transentorhinal Cortex Tract. Neurology 98, e700–e710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciampa CJ, Parent JH, Harrison TM, Fain RM, Betts MJ, Maass A, Winer JR, Baker SL, Janabi M, Furman DJ, D’Esposito M, Jagust WJ, Berry AS, 2022. Associations among locus coeruleus catecholamines, tau pathology, and memory in aging. Neuropsychopharmacology 47, 1106–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewett DV, Lee TH, Greening S, Ponzio A, Margalit E, Mather M, 2016. Neuromelanin marks the spot: identifying a locus coeruleus biomarker of cognitive reserve in healthy aging. Neurobiol. Aging 37, 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis C, Gamez JE, Singh U, Sadowsky CH, Villena T, Sabbagh MN, Beach TG, Duara R, Fleisher AS, Frey KA, Walker Z, Hunjan A, Holmes C, Escovar YM, Vera CX, Agronin ME, Ross J, Bozoki A, Akinola M, Shi J, Vandenberghe R, Ikonomovic MD, Sherwin PF, Grachev ID, Farrar G, Smith AP, Buckley CJ, McLain R, Salloway S, 2015. Phase 3 trial of flutemetamol labeled with radioactive fluorine 18 imaging and neuritic plaque density. JAMA Neurol. 72, 287–294. [DOI] [PubMed] [Google Scholar]
- d’Ardhuy XL, Boeijinga PH, Renault B, Luthringer R, Rinaudo G, Soufflet L, Toussaint M, Macher JP, 1999. Effects of Serotonin-Selective and Classical Antidepressants on the Auditory P300 Cognitive Potential. Neuropsychobiology 40, 207–213. [DOI] [PubMed] [Google Scholar]
- Dahl MJ, Mather M, Duzel S, Bodammer NC, Lindenberger U, Kuhn S, Werkle-Bergner M, 2019a. Rostral locus coeruleus integrity is associated with better memory performance in older adults. Nat. Hum. Behav 3, 1203–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl MJ, Mather M, Duzel S, Bodammer NC, Lindenberger U, Kuhn S, Werkle-Bergner M, 2019b. Rostral locus coeruleus integrity is associated with better memory performance in older adults. Nat. Hum. Behav 3, 1203–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl MJ, Mather M, Sander MC, Werkle-Bergner M, 2020. Noradrenergic Responsiveness Supports Selective Attention across the Adult Lifespan. J. Neurosci 40, 4372–4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl MJ, Mather M, Werkle-Bergner M, Kennedy BL, Guzman S, Hurth K, Miller CA, Qiao Y, Shi Y, Chui HC, Ringman JM, 2021. Locus coeruleus integrity is related to tau burden and memory loss in autosomal-dominant Alzheimer’s disease. Neurobiol. Aging 112, 39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco M, Venneri A, 2018. Volume and Connectivity of the Ventral Tegmental Area are Linked to Neurocognitive Signatures of Alzheimer’s Disease in Humans. Journal of Alzheimer’s disease 63, 167–180. [DOI] [PubMed] [Google Scholar]
- Del Cerro I, Martínez-Zalacaín I, Guinea-Izquierdo A, Gascón-Bayarri J, Viñas-Diez V, Urretavizcaya M, Naval-Baudin P, Aguilera C, Reñé-Ramírez R, Ferrer I, Menchón JM, Soria V, Soriano-Mas C, 2020a. Locus coeruleus connectivity alterations in late-life major depressive disorder during a visual oddball task. Neuroimage Clin. 28, 102482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Cerro I, Villarreal MF, Abulafia C, Duarte-Abritta B, Sanchez SM, Castro MN, Bocaccio H, Ferrer I, Menchon JM, Sevlever G, Nemeroff CB, Soriano-Mas C, Guinjoan SM, 2020b. Disrupted functional connectivity of the locus coeruleus in healthy adults with parental history of Alzheimer’s disease. J Psychiatr Res 123, 81–88. [DOI] [PubMed] [Google Scholar]
- Ding YS, Lin KS, Logan J, 2006. PET imaging of norepinephrine transporters. Curr. Pharm. Des 12, 3831–3845. [DOI] [PubMed] [Google Scholar]
- Ding YS, Singhal T, Planeta-Wilson B, Gallezot JD, Nabulsi N, Labaree D, Ropchan J, Henry S, Williams W, Carson RE, Neumeister A, Malison RT, 2010. PET imaging of the effects of age and cocaine on the norepinephrine transporter in the human brain using (S,S)-[(11)C]O-methylreboxetine and HRRT. Synapse 64, 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dordevic M, Müller-Fotti A, Müller P, Schmicker M, Kaufmann J, Müller NG, 2017. Optimal Cut-Off Value for Locus Coeruleus-to-Pons Intensity Ratio as Clinical Biomarker for Alzheimer’s Disease: A Pilot Study. J Alzheimers Dis Rep 1, 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durán E, Yang M, Neves R, Logothetis NK, Eschenko O, 2021. Modulation of Prefrontal Cortex Slow Oscillations by Phasic Activation of the Locus Coeruleus. Neuroscience 453, 268–279. [DOI] [PubMed] [Google Scholar]
- Dürschmid S, Reichert C, Kuhn J, Freund H-J, Hinrichs H, Heinze H-J, 2020. Deep brain stimulation of the nucleus basalis of Meynert attenuates early EEG components associated with defective sensory gating in patients with Alzheimer disease – a two-case study. Eur. J. Neurosci 51, 1201–1209. [DOI] [PubMed] [Google Scholar]
- Edlow BL, Takahashi E, Wu O, Benner T, Dai G, Bu L, Grant PE, Greer DM, Greenberg SM, Kinney HC, Folkerth RD, 2012. Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J Neuropathol Exp Neurol 71, 531–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenberg AJ, Nguy AK, Theofilas P, Dunlop S, Suemoto CK, Di Lorenzo Alho AT, Leite RP, Diehl Rodriguez R, Mejia MB, Rüb U, Farfel JM, de Lucena Ferretti-Rebustini RE, Nascimento CF, Nitrini R, Pasquallucci CA, Jacob-Filho W, Miller B, Seeley WW, Heinsen H, Grinberg LT, 2017. Quantifying the accretion of hyperphosphorylated tau in the locus coeruleus and dorsal raphe nucleus: the pathological building blocks of early Alzheimer’s disease. Neuropathol. Appl. Neurobiol 43, 393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman JA, Puckett OK, Beck A, Fennema-Notestine C, Cross LK, Dale AM, Eglit GML, Eyler LT, Gillespie NA, Granholm EL, Gustavson DE, Hagler DJ Jr., Hatton SN, Hauger R, Jak AJ, Logue MW, McEvoy LK, McKenzie RE, Neale MC, Panizzon MS, Reynolds CA, Sanderson-Cimino M, Toomey R, Tu XM, Whitsel N, Williams ME, Xian H, Lyons MJ, Franz CE, Kremen WS, 2021a. MRI-assessed locus coeruleus integrity is heritable and associated with multiple cognitive domains, mild cognitive impairment, and daytime dysfunction. Alzheimers Dement 17, 1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman JA, Puckett OK, Hagler DJ, Pearce RC, Fennema-Notestine C, Hatton SN, Lyons MJ, McEvoy LK, Panizzon MS, Reas ET, Dale AM, Franz CE, Kremen WS, 2021b. Associations Between MRI-Assessed Locus Coeruleus Integrity and Cortical Gray Matter Microstructure. Cereb. Cortex [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enge S, Fleischhauer M, Lesch K-P, Strobel A, 2011. On the role of serotonin and effort in voluntary attention: evidence of genetic variation in N1 modulation. Behav. Brain Res 216, 122–128. [DOI] [PubMed] [Google Scholar]
- Eschenko O, Magri C, Panzeri S, Sara SJ, 2012. Noradrenergic Neurons of the Locus Coeruleus Are Phase Locked to Cortical Up-Down States during Sleep. Cereb. Cortex 22, 426–435. [DOI] [PubMed] [Google Scholar]
- Espiritu J, Meier M, Seitz J-M, 2021. The current performance of biodegradable magnesium-based implants in magnetic resonance imaging: A review. Bioactive Materials 6, 4360–4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein B, Gleason CA, Libet B, 1989. Stimulation of Locus Coeruleus in Man. Stereotactic and Functional Neurosurgery 52, 26–41. [DOI] [PubMed] [Google Scholar]
- Fernandez-Cabello S, Kronbichler M, Van Dijk KRA, Goodman JA, Spreng RN, Schmitz TW, Alzheimer’s Disease Neuroimaging I, 2020. Basal forebrain volume reliably predicts the cortical spread of Alzheimer’s degeneration. Brain 143, 993–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M, Spinelli EG, Cividini C, Agosta F, 2019. Resting State Dynamic Functional Connectivity in Neurodegenerative Conditions: A Review of Magnetic Resonance Imaging Findings. Front Neurosci 13, 657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AG, Endrass T, Reuter M, Kubisch C, Ullsperger M, 2015. Serotonin Reuptake Inhibitors and Serotonin Transporter Genotype Modulate Performance Monitoring Functions But Not Their Electrophysiological Correlates. J. Neurosci 35, 8181–8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine D, Deudon A, Lemaire JJ, Razzouk M, Viau P, Darcourt J, Robert P, 2013. Symptomatic treatment of memory decline in Alzheimer’s disease by deep brain stimulation: a feasibility study. J. Alzheimers Dis 34, 315–323. [DOI] [PubMed] [Google Scholar]
- Frank JA, Antonini M-J, Anikeeva P, 2019. Next-generation interfaces for studying neural function. Nat. Biotechnol 37, 1013–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz HJ, Ray N, Dyrba M, Sorg C, Teipel S, Grothe MJ, 2019. The corticotopic organization of the human basal forebrain as revealed by regionally selective functional connectivity profiles. Hum. Brain Mapp 40, 868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi FS, Martini N, Lombardo F, Galgani A, Bastiani L, Della Latta D, Hlavata H, Busceti CL, Biagioni F, Puglisi-Allegra S, Pavese N, Fornai F, 2022. Locus Coeruleus magnetic resonance imaging: a comparison between native-space and template-space approach. J Neural Transm (Vienna) 129, 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinberg LT, Rüb U, Ferretti RE, Nitrini R, Farfel JM, Polichiso L, Gierga K, Jacob-Filho W, Heinsen H, 2009. The dorsal raphe nucleus shows phospho-tau neurofibrillary changes before the transentorhinal region in Alzheimer’s disease. A precocious onset? Neuropathol. Appl. Neurobiol 35, 406–416. [DOI] [PubMed] [Google Scholar]
- Grinberg LT, Rueb U, Heinsen H, 2011. Brainstem: neglected locus in neurodegenerative diseases. Front. Neurol 2, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe MJ, Ewers M, Krause B, Heinsen H, Teipel SJ, Alzheimer’s Disease Neuroimaging I, 2014. Basal forebrain atrophy and cortical amyloid deposition in nondemented elderly subjects. Alzheimers Dement 10, S344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas B, Brockschnieder D, Nag S, Pavlova E, Kasa P, Beliczai Z, Legradi A, Gulya K, Thiele A, Dyrks T, Halldin C, 2010. The norepinephrine transporter (NET) radioligand (S,S)-[18F]FMeNER-D2 shows significant decreases in NET density in the human brain in Alzheimer’s disease: a post-mortem autoradiographic study. Neurochem. Int 56, 789–798. [DOI] [PubMed] [Google Scholar]
- Hammerer D, Callaghan MF, Hopkins A, Kosciessa J, Betts M, Cardenas-Blanco A, Kanowski M, Weiskopf N, Dayan P, Dolan RJ, Duzel E, 2018. Locus coeruleus integrity in old age is selectively related to memories linked with salient negative events. Proc Natl Acad Sci U S A 115, 2228–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H, Mesulam MM, Cuello AC, Farlow MR, Giacobini E, Grossberg GT, Khachaturian AS, Vergallo A, Cavedo E, Snyder PJ, Khachaturian ZS, 2018. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 141, 1917–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque RU, Levey AI, 2019. Alzheimer’s disease: A clinical perspective and future nonhuman primate research opportunities. Proceedings of the National Academy of Sciences of the United States of America 116, 26224–26229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CW, Walling SG, Yuan Q, Martin GM, 2021. The ‘a, b, c’s of pretangle tau and their relation to aging and the risk of Alzheimer’s Disease. Semin. Cell Dev. Biol [DOI] [PubMed] [Google Scholar]
- Hasselbalch SG, Madsen K, Svarer C, Pinborg LH, Holm S, Paulson OB, Waldemar G, Knudsen GM, 2008. Reduced 5-HT2A receptor binding in patients with mild cognitive impairment. Neurobiol. Aging 29, 1830–1838. [DOI] [PubMed] [Google Scholar]
- He B, Yang L, Wilke C, Yuan H, 2011. Electrophysiological Imaging of Brain Activity and Connectivity—Challenges and Opportunities. IEEE Trans. Biomed. Eng 58, 1918–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdick M, Dyrba M, Fritz HJ, Altenstein S, Ballarini T, Brosseron F, Buerger K, Can Cetindag A, Dechent P, Dobisch L, Duezel E, Ertl-Wagner B, Fliessbach K, Dawn Freiesleben S, Frommann I, Glanz W, Dylan Haynes J, Heneka MT, Janowitz D, Kilimann I, Laske C, Metzger CD, Munk MH, Peters O, Priller J, Roy N, Scheffler K, Schneider A, Spottke A, Jakob Spruth E, Tscheuschler M, Vukovich R, Wiltfang J, Jessen F, Teipel S, Grothe MJ, 2020. Multimodal MRI analysis of basal forebrain structure and function across the Alzheimer’s disease spectrum. Neuroimage Clin. 28, 102495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herholz K, Weisenbach S, Zündorf G, Lenz O, Schröder H, Bauer B, Kalbe E, Heiss WD, 2004. In vivo study of acetylcholine esterase in basal forebrain, amygdala, and cortex in mild to moderate Alzheimer disease. NeuroImage 21, 136–143. [DOI] [PubMed] [Google Scholar]
- Hou R, Beardmore R, Holmes C, Osmond C, Darekar A, 2021. A case-control study of the locus coeruleus degeneration in Alzheimer’s disease. Eur Neuropsychopharmacol 43, 153–159. [DOI] [PubMed] [Google Scholar]
- Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, 2018. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14, 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs HI, Priovoulos N, Poser BA, Pagen LH, Ivanov D, Verhey FR, Uludag K, 2020. Dynamic behavior of the locus coeruleus during arousal-related memory processing in a multi-modal 7T fMRI paradigm. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs HIL, Becker JA, Kwong K, Engels-Dominguez E, Prokopiou PC, Papp KV, Properzi M, Hampton OL, D’Oleire Uquillas F, Sanchez JS, Rentz DM, El Fakhri G, Normandin MD, Price JC, Bennett DA, Sperling RA, Johnson KA, 2021a. In vivo and neuropathology data support locus coeruleus integrity as indicator of Alzheimer’s disease pathology and cognitive decline. Science Translational Medicine 13, eabj2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs HIL, Becker JA, Kwong K, Munera D, Ramirez-Gomez L, Engels-Dominguez N, Sanchez JS, Vila-Castelar C, Baena A, Sperling RA, Johnson KA, Lopera F, Quiroz YT, 2022. Waning locus coeruleus integrity precedes cortical tau accrual in preclinical autosomal dominant Alzheimer’s disease. Alzheimers Dement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs HIL, Hedden T, Schultz AP, Sepulcre J, Perea RD, Amariglio RE, Papp KV, Rentz DM, Sperling RA, Johnson KA, 2018a. Structural tract alterations predict downstream tau accumulation in amyloid-positive older individuals. Nat. Neurosci 21, 424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs HIL, Müller-Ehrenberg L, Priovoulos N, Roebroeck A, 2018b. Curvilinear locus coeruleus functional connectivity trajectories over the adult lifespan: a 7T MRI study. Neurobiol. Aging 69, 167–176. [DOI] [PubMed] [Google Scholar]
- Jacobs HIL, Riphagen JM, Ramakers I, Verhey FRJ, 2021b. Alzheimer’s disease pathology: pathways between central norepinephrine activity, memory, and neuropsychiatric symptoms. Mol. Psychiatry 26, 897–906. [DOI] [PubMed] [Google Scholar]
- Jacobs HIL, Wiese S, van de Ven V, Gronenschild EH, Verhey FR, Matthews PM, 2015. Relevance of parahippocampal-locus coeruleus connectivity to memory in early dementia. Neurobiol. Aging 36, 618–626. [DOI] [PubMed] [Google Scholar]
- Jacobs RE, Cherry SR, 2001. Complementary emerging techniques: high-resolution PET and MRI. Curr. Opin. Neurobiol 11, 621–629. [DOI] [PubMed] [Google Scholar]
- Jakobs M, Lee DJ, Lozano AM, 2020. Modifying the progression of Alzheimer’s and Parkinson’s disease with deep brain stimulation. Neuropharmacology 171, 107860. [DOI] [PubMed] [Google Scholar]
- Jeremic D, Jiménez-Díaz L, Navarro-López JD, 2021. Past, present and future of therapeutic strategies against amyloid-β peptides in Alzheimer’s disease: a systematic review. Ageing Research Reviews 72, 101496. [DOI] [PubMed] [Google Scholar]
- Jethwa KD, Dhillon P, Meng D, Auer DP, Alzheimer’s Disease Neuroimaging I, 2019. Are Linear Measurements of the Nucleus Basalis of Meynert Suitable as a Diagnostic Biomarker in Mild Cognitive Impairment and Alzheimer Disease? AJNR Am J Neuroradiol 40, 2039–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannsson M, Snaedal J, Johannesson GH, Gudmundsson TE, Johnsen K, 2015. The Acetylcholine Index: An Electroencephalographic Marker of Cholinergic Activity in the Living Human Brain Applied to Alzheimer’s Disease and Other Dementias. Dementia and Geriatric Cognitive Disorders 39, 132–142. [DOI] [PubMed] [Google Scholar]
- Jungmann PM, Agten CA, Pfirrmann CW, Sutter R, 2017. Advances in MRI around metal. J. Magn. Reson. Imaging 46, 972–991. [DOI] [PubMed] [Google Scholar]
- Kahn I, Shohamy D, 2013. Intrinsic connectivity between the hippocampus, nucleus accumbens, and ventral tegmental area in humans. Hippocampus 23, 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiński J, Mamelak AN, Birch K, Mosher CP, Tagliati M, Rutishauser U, 2018. Novelty-Sensitive Dopaminergic Neurons in the Human Substantia Nigra Predict Success of Declarative Memory Formation. Curr. Biol 28, 1333–1343.e1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya K, Hori M, Aoki S, 2020. NODDI in clinical research. J. Neurosci. Methods 346, 108908. [DOI] [PubMed] [Google Scholar]
- Kelberman MA, Weinshenker D, 2022. A novel link between locus coeruleus activity and amyloid-related cognitive decline. Trends Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SC, He B, Perez SE, Ginsberg SD, Mufson EJ, Counts SE, 2017. Locus coeruleus cellular and molecular pathology during the progression of Alzheimer’s disease. Acta Neuropathologica Communications 5, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER, 2016. Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc Natl Acad Sci U S A 113, 14835–14840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepe V, Barrio JR, Huang SC, Ercoli L, Siddarth P, Shoghi-Jadid K, Cole GM, Satyamurthy N, Cummings JL, Small GW, Phelps ME, 2006. Serotonin 1A receptors in the living brain of Alzheimer’s disease patients. Proc Natl Acad Sci U S A 103, 702–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbler GM, Fripp J, Rowe CC, Villemagne VL, Salvado O, Rose S, Coulson EJ, Alzheimer’s Disease Neuroimaging I, 2015. Basal forebrain atrophy correlates with amyloid beta burden in Alzheimer’s disease. Neuroimage Clin. 7, 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren NI, Lozar CT, Harris KC, Morgan PS, Eckert MA, 2009. In vivo mapping of the human locus coeruleus. NeuroImage 47, 1261–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren NI, Taheri S, Vazey EM, Morgan PS, Granholm AC, Aston-Jones GS, Eckert MA, 2015. Histologic validation of locus coeruleus MRI contrast in post-mortem tissue. NeuroImage 113, 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan UA, Liu L, Provenzano FA, Berman DE, Profaci CP, Sloan R, Mayeux R, Duff KE, Small SA, 2014. Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer’s disease. Nat. Neurosci 17, 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilimann I, Grothe M, Heinsen H, Alho EJ, Grinberg L, Amaro E Jr., Dos Santos GA, da Silva RE, Mitchell AJ, Frisoni GB, Bokde AL, Fellgiebel A, Filippi M, Hampel H, Kloppel S, Teipel SJ, 2014. Subregional basal forebrain atrophy in Alzheimer’s disease: a multicenter study. J. Alzheimers Dis 40, 687–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B, 2004. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann. Neurol 55, 306–319. [DOI] [PubMed] [Google Scholar]
- Kuhn J, Hardenacke K, Lenartz D, Gruendler T, Ullsperger M, Bartsch C, Mai JK, Zilles K, Bauer A, Matusch A, Schulz RJ, Noreik M, Bührle CP, Maintz D, Woopen C, Häussermann P, Hellmich M, Klosterkötter J, Wiltfang J, Maarouf M, Freund HJ, Sturm V, 2015. Deep brain stimulation of the nucleus basalis of Meynert in Alzheimer’s dementia. Mol. Psychiatry 20, 353–360. [DOI] [PubMed] [Google Scholar]
- Kullmann S, Heni M, Linder K, Zipfel S, Häring HU, Veit R, Fritsche A, Preissl H, 2014. Resting-state functional connectivity of the human hypothalamus. Hum. Brain Mapp 35, 6088–6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann S, Veit R, 2021. Resting-state functional connectivity of the human hypothalamus. Handb Clin Neurol 179, 113–124. [DOI] [PubMed] [Google Scholar]
- La Joie R, Perrotin A, Barre L, Hommet C, Mezenge F, Ibazizene M, Camus V, Abbas A, Landeau B, Guilloteau D, de La Sayette V, Eustache F, Desgranges B, Chetelat G, 2012. Region-specific hierarchy between atrophy, hypometabolism, and beta-amyloid (Abeta) load in Alzheimer’s disease dementia. J. Neurosci 32, 16265–16273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanctot KL, Hussey DF, Herrmann N, Black SE, Rusjan PM, Wilson AA, Houle S, Kozloff N, Verhoeff NP, Kapur S, 2007. A positron emission tomography study of 5-hydroxytryptamine-1A receptors in Alzheimer disease. Am J Geriatr Psychiatry 15, 888–898. [DOI] [PubMed] [Google Scholar]
- Langley J, Hussain S, Flores JJ, Bennett IJ, Hu X, 2020. Characterization of age-related microstructural changes in locus coeruleus and substantia nigra pars compacta. Neurobiol. Aging 87, 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J, Hussain S, Huddleston DE, Bennett IJ, Hu XP, 2022. Impact of Locus Coeruleus and Its Projections on Memory and Aging. Brain Connectivity 12, 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R, Wherrett J, Naglie G, Hamani C, Smith GS, Lozano AM, 2010. A phase I trial of deep brain stimulation of memory circuits in Alzheimer’s disease. Ann. Neurol 68, 521–534. [DOI] [PubMed] [Google Scholar]
- Leal SL, Yassa MA, 2013. Perturbations of neural circuitry in aging, mild cognitive impairment, and Alzheimer’s disease. Ageing Res Rev 12, 823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CM, Jacobs HIL, Marquié M, Becker JA, Andrea NV, Jin DS, Schultz AP, Frosch MP, Gómez-Isla T, Sperling RA, Johnson KA, 2018. 18F-Flortaucipir Binding in Choroid Plexus: Related to Race and Hippocampus Signal. J. Alzheimers Dis 62, 1691–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Milosevic L, Gramer R, Sasikumar S, Al-Ozzi TM, Vloo PD, Dallapiazza RF, Elias GJB, Cohn M, Kalia SK, Hutchison WD, Fasano A, Lozano AM, 2019. Nucleus basalis of Meynert neuronal activity in Parkinson’s disease. J. Neurosurg 132, 574–582. [DOI] [PubMed] [Google Scholar]
- Leoutsakos J-MS, Yan H, Anderson WS, Asaad WF, Baltuch G, Burke A, Chakravarty MM, Drake KE, Foote KD, Fosdick L, Giacobbe P, Mari Z, McAndrews MP, Munro CA, Oh ES, Okun MS, Pendergrass JC, Ponce FA, Rosenberg PB, Sabbagh MN, Salloway S, Tang-Wai DF, Targum SD, Wolk D, Lozano AM, Smith GS, Lyketsos CG, 2018. Deep Brain Stimulation Targeting the Fornix for Mild Alzheimer Dementia (the ADvance Trial): A Two Year Follow-up Including Results of Delayed Activation. Journal of Alzheimer’s Disease 64, 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AI, Qiu D, Zhao L, Hu WT, Duong DM, Higginbotham L, Dammer EB, Seyfried NT, Wingo TS, Hales CM, Gamez Tansey M, Goldstein DS, Abrol A, Calhoun VD, Goldstein FC, Hajjar I, Fagan AM, Galasko D, Edland SD, Hanfelt J, Lah JJ, Weinshenker D, 2021. A phase II study repurposing atomoxetine for neuroprotection in mild cognitive impairment. Brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewine JD, Paulson K, Bangera N, Simon BJ, 2019. Exploration of the Impact of Brief Noninvasive Vagal Nerve Stimulation on EEG and Event-Related Potentials. Neuromodulation: Technology at the Neural Interface 22, 564–572. [DOI] [PubMed] [Google Scholar]
- Lewis LD, Setsompop K, Rosen BR, Polimeni JR, 2018. Stimulus-dependent hemodynamic response timing across the human subcortical-cortical visual pathway identified through high spatiotemporal resolution 7T fMRI. Neuroimage 181, 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Jia X, Qi Z, Fan X, Ma T, Ni H, Li CR, Li K, 2017. Altered Functional Connectivity of the Basal Nucleus of Meynert in Mild Cognitive Impairment: A Resting-State fMRI Study. Front Aging Neurosci 9, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-P, Frigerio I, Boon BDC, Zhou Z, Rozemuller AJM, Bouwman FH, Schoonheim MM, Van De Berg WDJ, Jonkman LE, 2022. Structural (dys)connectivity associates with cholinergic cell density in Alzheimer’s disease. Brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister-James J, Pontecorvo MJ, Clark C, Joshi AD, Mintun MA, Zhang W, Lim N, Zhuang Z, Golding G, Choi SR, Benedum TE, Kennedy P, Hefti F, Carpenter AP, Kung HF, Skovronsky DM, 2011. Florbetapir f-18: a histopathologically validated Beta-amyloid positron emission tomography imaging agent. Semin. Nucl. Med 41, 300–304. [DOI] [PubMed] [Google Scholar]
- Liu KY, Acosta-Cabronero J, Cardenas-Blanco A, Loane C, Berry AJ, Betts MJ, Kievit RA, Henson RN, Duzel E, Cam CAN, Howard R, Hammerer D, 2019. In vivo visualization of age-related differences in the locus coeruleus. Neurobiol. Aging 74, 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KY, Acosta-Cabronero J, Hong YT, Yi YJ, Hämmerer D, Howard R, 2021. FDG-PET assessment of the locus coeruleus in Alzheimer’s disease. Neuroimage Rep 1, 100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Chen W, Tu Y, Hou H, Huang X, Chen X, Guo Z, Bai G, Chen W, 2018. The Abnormal Functional Connectivity between the Hypothalamus and the Temporal Gyrus Underlying Depression in Alzheimer’s Disease Patients. Front Aging Neurosci 10, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe VJ, Curran G, Fang P, Liesinger AM, Josephs KA, Parisi JE, Kantarci K, Boeve BF, Pandey MK, Bruinsma T, Knopman DS, Jones DT, Petrucelli L, Cook CN, Graff-Radford NR, Dickson DW, Petersen RC, Jack CR Jr., Murray ME, 2016. An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol Commun 4, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki-Marttunen V, Espeseth T, 2021. Uncovering the locus coeruleus: Comparison of localization methods for functional analysis. NeuroImage 224, 117409. [DOI] [PubMed] [Google Scholar]
- Manza P, Zhang S, Hu S, Chao HH, Leung HC, Li CR, 2015. The effects of age on resting state functional connectivity of the basal ganglia from young to middle adulthood. NeuroImage 107, 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markello RD, Spreng RN, Luh WM, Anderson AK, De Rosa E, 2018. Segregation of the human basal forebrain using resting state functional MRI. NeuroImage 173, 287–297. [DOI] [PubMed] [Google Scholar]
- Marner L, Frokjaer VG, Kalbitzer J, Lehel S, Madsen K, Baaré WF, Knudsen GM, Hasselbalch SG, 2012. Loss of serotonin 2A receptors exceeds loss of serotonergic projections in early Alzheimer’s disease: a combined [11C]DASB and [18F]altanserin-PET study. Neurobiol. Aging 33, 479–487. [DOI] [PubMed] [Google Scholar]
- Marquié M, Normandin MD, Vanderburg CR, Costantino IM, Bien EA, Rycyna LG, Klunk WE, Mathis CA, Ikonomovic MD, Debnath ML, Vasdev N, Dickerson BC, Gomperts SN, Growdon JH, Johnson KA, Frosch MP, Hyman BT, Gómez-Isla T, 2015. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann. Neurol 78, 787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehranian A, Belzunce MA, Niccolini F, Politis M, Prieto C, Turkheimer F, Hammers A, Reader AJ, 2017. PET image reconstruction using multi-parametric anato-functional priors. Phys Med Biol 62, 5975–6007. [DOI] [PubMed] [Google Scholar]
- Melnychuk MC, Robertson IH, Plini ERG, Dockree PM, 2021. A Bridge between the Breath and the Brain: Synchronization of Respiration, a Pupillometric Marker of the Locus Coeruleus, and an EEG Marker of Attentional Control State. Brain Sci 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer CC, Price JC, Mathis CA, Greer PJ, Cantwell MN, Houck PR, Mulsant BH, Ben-Eliezer D, Lopresti B, DeKosky ST, Reynolds CF 3rd, 1999. PET imaging of serotonin type 2A receptors in late-life neuropsychiatric disorders. Am. J. Psychiatry 156, 1871–1878. [DOI] [PubMed] [Google Scholar]
- Merlet I, Ostrowsky K, Costes N, Ryvlin P, Isnard J, Faillenot I, Lavenne F, Dufournel D, Le Bars D, Mauguiere F, 2004. 5-HT1A receptor binding and intracerebral activity in temporal lobe epilepsy: an [18F]MPPF-PET study. Brain 127, 900–913. [DOI] [PubMed] [Google Scholar]
- Mishra A, Marzban N, Cohen MX, Englitz B, 2021. Dynamics of Neural Microstates in the VTA–Striatal–Prefrontal Loop during Novelty Exploration in the Rat. The Journal of Neuroscience 41, 6864–6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney CM, Lowe VJ, Murray ME, 2021. Visualization of neurofibrillary tangle maturity in Alzheimer’s disease: A clinicopathologic perspective for biomarker research. Alzheimers Dement 17, 1554–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn BR, Müller EJ, Wainstein G, Shine JM, 2021. The ascending arousal system shapes neural dynamics to mediate awareness of cognitive states. Nat Commun 12, 6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Shermohammed M, Smith DV, Carter RM, Huettel SA, Adcock RA, 2014. Resting state networks distinguish human ventral tegmental area from substantia nigra. NeuroImage 100, 580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, 2014. The use of magnetoencephalography in the study of psychopharmacology (pharmaco-MEG). J. Psychopharmacol 28, 815–829. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Liley DT, 2018. 1/f electrophysiological spectra in resting and drug-induced states can be explained by the dynamics of multiple oscillatory relaxation processes. NeuroImage 179, 582–595. [DOI] [PubMed] [Google Scholar]
- Nahimi A, Sommerauer M, Kinnerup MB, Østergaard K, Winterdahl M, Jacobsen J, Schacht A, Johnsen B, Damholdt MF, Borghammer P, Gjedde A, 2018. Noradrenergic Deficits in Parkinson Disease Imaged with (11)C-MeNER. J Nucl Med 59, 659–664. [DOI] [PubMed] [Google Scholar]
- Nakaizumi K, Ouchi Y, Terada T, Yoshikawa E, Kakimoto A, Isobe T, Bunai T, Yokokura M, Suzuki K, Magata Y, 2018. In vivo Depiction of alpha7 Nicotinic Receptor Loss for Cognitive Decline in Alzheimer’s Disease. J. Alzheimers Dis 61, 1355–1365. [DOI] [PubMed] [Google Scholar]
- Neudorfer C, Germann J, Elias GJ, Gramer R, Boutet A, Lozano AM, 2020. A high-resolution in vivo magnetic resonance imaging atlas of the human hypothalamic region. Scientific data 7, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves RM, van Keulen S, Yang M, Logothetis NK, Eschenko O, 2018. Locus coeruleus phasic discharge is essential for stimulus-induced gamma oscillations in the prefrontal cortex. J. Neurophysiol 119, 904–920. [DOI] [PubMed] [Google Scholar]
- Nicolas B, Alessandra D, Daniela P, Osman R, Sara T, Giovanni BF, Valentina G, 2020. Basal forebrain metabolism in Alzheimer’s disease continuum: relationship with education. Neurobiol. Aging 87, 70–77. [DOI] [PubMed] [Google Scholar]
- Noei S, Zouridis IS, Logothetis NK, Panzeri S, Totah NK, 2022. Distinct ensembles in the noradrenergic locus coeruleus are associated with diverse cortical states. Proceedings of the National Academy of Sciences 119, e2116507119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordberg A, Rinne JO, Kadir A, Langstrom B, 2010. The use of PET in Alzheimer disease. Nat Rev Neurol 6, 78–87. [DOI] [PubMed] [Google Scholar]
- Ofori E, Pasternak O, Planetta PJ, Li H, Burciu RG, Snyder AF, Lai S, Okun MS, Vaillancourt DE, 2015. Longitudinal changes in free-water within the substantia nigra of Parkinson’s disease. Brain 138, 2322–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H, Ouchi Y, Ogawa M, Futatsubashi M, Saito Y, Yoshikawa E, Terada T, Oboshi Y, Tsukada H, Ueki T, Watanabe M, Yamashita T, Magata Y, 2013. Alterations in alpha4beta2 nicotinic receptors in cognitive decline in Alzheimer’s aetiopathology. Brain 136, 3004–3017. [DOI] [PubMed] [Google Scholar]
- Olivieri P, Lagarde J, Lehericy S, Valabregue R, Michel A, Mace P, Caille F, Gervais P, Bottlaender M, Sarazin M, 2019. Early alteration of the locus coeruleus in phenotypic variants of Alzheimer’s disease. Ann Clin Transl Neurol 6, 1345–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onofrj M, Thomas A, Iacono D, Luciano AL, Di Iorio A, 2003. The Effects of a Cholinesterase Inhibitor Are Prominent in Patients With Fluctuating Cognition: A Part 3 Study of the Main Mechanism of Cholinesterase Inhibitors in Dementia. Clinical Neuropharmacology 26, 239–251. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Yoshikawa E, Futatsubashi M, Yagi S, Ueki T, Nakamura K, 2009. Altered brain serotonin transporter and associated glucose metabolism in Alzheimer disease. J Nucl Med 50, 1260–1266. [DOI] [PubMed] [Google Scholar]
- Pamphlett R, Mak R, Lee J, Buckland ME, Harding AJ, Jew SK, Paterson DJ, Jones MWM, Lay PA, 2020. Concentrations of toxic metals and essential trace elements vary among individual neurons in the human locus ceruleus. PloS one 15, e0233300–e0233300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parizkova M, Lerch O, Andel R, Kalinova J, Markova H, Vyhnalek M, Hort J, Laczo J, 2020. Spatial Pattern Separation in Early Alzheimer’s Disease. J. Alzheimers Dis 76, 121–138. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Van Hoesen GW, Damasio A, 2001. The selective vulnerability of brainstem nuclei to Alzheimer’s disease. Ann. Neurol 49, 53–66. [DOI] [PubMed] [Google Scholar]
- Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y, 2009. Free water elimination and mapping from diffusion MRI. Magn. Reson. Med 62, 717–730. [DOI] [PubMed] [Google Scholar]
- Pauli WM, Nili AN, Tyszka JM, 2018. A high-resolution probabilistic in vivo atlas of human subcortical brain nuclei. Sci Data 5, 180063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekkonen E, Jääskeläinen IP, Kaakkola S, Ahveninen J, 2005. Cholinergic modulation of preattentive auditory processing in aging. NeuroImage 27, 387–392. [DOI] [PubMed] [Google Scholar]
- Petrova T, Orellana C, Jelic V, Oeksengaard A-R, Snaedal J, Høgh P, Andersen BB, Naik M, Engedal K, Wahlund L-O, Ferreira D, 2020. Cholinergic dysfunction, neurodegeneration, and amyloid-beta pathology in neurodegenerative diseases. Psychiatry Research: Neuroimaging 302, 111099. [DOI] [PubMed] [Google Scholar]
- Polich J, 2007. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol 118, 2128–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porat S, Sibilia F, Yoon J, Shi Y, Dahl MJ, Werkle-Bergner M, Düzel S, Bodammer N, Lindenberger U, Kühn S, Mather M, 2022. Age differences in diffusivity in the locus coeruleus and its ascending noradrenergic tract. NeuroImage 251, 119022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcaro C, Balsters JH, Mantini D, Robertson IH, Wenderoth N, 2019. P3b amplitude as a signature of cognitive decline in the older population: An EEG study enhanced by Functional Source Separation. NeuroImage 184, 535–546. [DOI] [PubMed] [Google Scholar]
- Porcaro C, Vecchio F, Miraglia F, Zito G, Rossini PM, 2022. Dynamics of the “Cognitive” Brain Wave P3b at Rest for Alzheimer Dementia Prediction in Mild Cognitive Impairment. Int. J. Neural Syst 32, 2250022. [DOI] [PubMed] [Google Scholar]
- Priovoulos N, Jacobs HIL, Ivanov D, Uludag K, Verhey FRJ, Poser BA, 2018. High-resolution in vivo imaging of human locus coeruleus by magnetization transfer MRI at 3T and 7T. NeuroImage 168, 427–436. [DOI] [PubMed] [Google Scholar]
- Priovoulos N, van Boxel SCJ, Jacobs HIL, Poser BA, Uludag K, Verhey FRJ, Ivanov D, 2020. Unraveling the contributions to the neuromelanin-MRI contrast. Brain Struct Funct 225, 2757–2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokopiou PC, Engels-Domínguez N, Papp KV, Scott MR, Schultz AP, Schneider C, Farrell ME, Buckley RF, Quiroz YT, El Fakhri G, Rentz DM, Sperling RA, Johnson KA, Jacobs HIL, 2022. Lower novelty-related locus coeruleus function is associated with Aβ-related cognitive decline in clinically healthy individuals. Nat. Commun 13, 1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin YY, Li MW, Zhang S, Zhang Y, Zhao LY, Lei H, Oishi K, Zhu WZ, 2013. In vivo quantitative whole-brain diffusion tensor imaging analysis of APP/PS1 transgenic mice using voxel-based and atlas-based methods. Neuroradiology 55, 1027–1038. [DOI] [PubMed] [Google Scholar]
- Rea RC, Berlot R, Martin SL, Craig CE, Holmes PS, Wright DJ, Bon J, Pirtošek Z, Ray NJ, 2021. Quantitative EEG and cholinergic basal forebrain atrophy in Parkinson’s disease and mild cognitive impairment. Neurobiol. Aging 106, 37–44. [DOI] [PubMed] [Google Scholar]
- Richter N, Beckers N, Onur OA, Dietlein M, Tittgemeyer M, Kracht L, Neumaier B, Fink GR, Kukolja J, 2018. Effect of cholinergic treatment depends on cholinergic integrity in early Alzheimer’s disease. Brain 141, 903–915. [DOI] [PubMed] [Google Scholar]
- Richter N, David LS, Grothe MJ, Teipel S, Dietlein M, Tittgemeyer M, Neumaier B, Fink GR, Onur OA, Kukolja J, 2022. Age and Anterior Basal Forebrain Volume Predict the Cholinergic Deficit in Patients with Mild Cognitive Impairment due to Alzheimer’s Disease. J. Alzheimers Dis 86, 425–440. [DOI] [PubMed] [Google Scholar]
- Risacher SL, Saykin AJ, 2013. Neuroimaging Biomarkers of Neurodegenerative Diseases and Dementia. Seminars in neurology 33, 386–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum Y, Shiner T, Bregman N, Fahoum F, Giladi N, Maidan I, Mirelman A, 2022. Event-related oscillations differentiate between cognitive, motor and visual impairments. Journal of Neurology. [DOI] [PubMed] [Google Scholar]
- Rüb U, Del Tredici K, Schultz C, Thal DR, Braak E, Braak H, 2000. The evolution of Alzheimer’s disease-related cytoskeletal pathology in the human raphe nuclei. Neuropathol. Appl. Neurobiol 26, 553–567. [DOI] [PubMed] [Google Scholar]
- Rüb U, Stratmann K, Heinsen H, Seidel K, Bouzrou M, Korf H-W, 2017. Alzheimer’s Disease: Characterization of the Brain Sites of the Initial Tau Cytoskeletal Pathology Will Improve the Success of Novel Immunological Anti-Tau Treatment Approaches. Journal of Alzheimer’s Disease 57, 683–696. [DOI] [PubMed] [Google Scholar]
- Sabri O, Meyer PM, Graf S, Hesse S, Wilke S, Becker GA, Rullmann M, Patt M, Luthardt J, Wagenknecht G, Hoepping A, Smits R, Franke A, Sattler B, Tiepolt S, Fischer S, Deuther-Conrad W, Hegerl U, Barthel H, Schonknecht P, Brust P, 2018. Cognitive correlates of alpha4beta2 nicotinic acetylcholine receptors in mild Alzheimer’s dementia. Brain 141, 1840–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez JS, Becker JA, Jacobs HIL, Hanseeuw BJ, Jiang S, Schultz AP, Properzi MJ, Katz SR, Beiser A, Satizabal CL, O’Donnell A, DeCarli C, Killiany R, El Fakhri G, Normandin MD, Gomez-Isla T, Quiroz YT, Rentz DM, Sperling RA, Seshadri S, Augustinack J, Price JC, Johnson KA, 2021. The cortical origin and initial spread of medial temporal tauopathy in Alzheimer’s disease assessed with positron emission tomography. Sci Transl Med 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ, Hervé-Minvielle A, 1995. Inhibitory influence of frontal cortex on locus coeruleus neurons. Proceedings of the National Academy of Sciences 92, 6032–6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheef L, Grothe MJ, Koppara A, Daamen M, Boecker H, Biersack H, Schild HH, Wagner M, Teipel S, Jessen F, 2019. Subregional volume reduction of the cholinergic forebrain in subjective cognitive decline (SCD). Neuroimage Clin. 21, 101612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Nathan Spreng R, Alzheimer’s Disease Neuroimaging I, 2016. Basal forebrain degeneration precedes and predicts the cortical spread of Alzheimer’s pathology. Nat. Commun 7, 13249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Soreq H, Poirier J, Spreng RN, 2020. Longitudinal Basal Forebrain Degeneration Interacts with TREM2/C3 Biomarkers of Inflammation in Presymptomatic Alzheimer’s Disease. J. Neurosci 40, 1931–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter ML, Vogt B, Frisch S, Becker G, Seese A, Barthel H, Mueller K, Villringer A, Sabri O, 2011. Dissociating behavioral disorders in early dementia-An FDG-PET study. Psychiatry Res 194, 235–244. [DOI] [PubMed] [Google Scholar]
- Schumacher J, Peraza LR, Firbank M, Thomas AJ, Kaiser M, Gallagher P, O’Brien JT, Blamire AM, Taylor JP, 2019. Dynamic functional connectivity changes in dementia with Lewy bodies and Alzheimer’s disease. Neuroimage Clin. 22, 101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J, Thomas AJ, Peraza LR, Firbank M, Cromarty R, Hamilton CA, Donaghy PC, O’Brien JT, Taylor J-P, 2020. EEG alpha reactivity and cholinergic system integrity in Lewy body dementia and Alzheimer’s disease. Alzheimer’s Research & Therapy 12, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclocco R, Beissner F, Bianciardi M, Polimeni JR, Napadow V, 2018. Challenges and opportunities for brainstem neuroimaging with ultrahigh field MRI. NeuroImage 168, 412–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclocco R, Garcia RG, Kettner NW, Fisher HP, Isenburg K, Makarovsky M, Stowell JA, Goldstein J, Barbieri R, Napadow V, 2020. Stimulus frequency modulates brainstem response to respiratory-gated transcutaneous auricular vagus nerve stimulation. Brain Stimulation 13, 970–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon O, Fahoum F, Nir Y, 2021. Transcutaneous Vagus Nerve Stimulation in Humans Induces Pupil Dilation and Attenuates Alpha Oscillations. J. Neurosci 41, 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W-X, 2005. Slow Oscillatory Firing: A Major Firing Pattern of Dopamine Neurons in the Ventral Tegmental Area. J. Neurophysiol 94, 3516–3522. [DOI] [PubMed] [Google Scholar]
- Shibata E, Sasaki M, Tohyama K, Kanbara Y, Otsuka K, Ehara S, Sakai A, 2006. Age-related changes in locus ceruleus on neuromelanin magnetic resonance imaging at 3 Tesla. Magnetic resonance in medical sciences: MRMS: an official journal of Japan Society of Magnetic Resonance in Medicine 5, 197–200. [DOI] [PubMed] [Google Scholar]
- Singh K, Cauzzo S, García-Gomar MG, Stauder M, Vanello N, Passino C, Bianciardi M, 2022. Functional connectome of arousal and motor brainstem nuclei in living humans by 7 Tesla resting-state fMRI. NeuroImage 249, 118865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Garcia-Gomar MG, Bianciardi M, 2021. Probabilistic Atlas of the Mesencephalic Reticular Formation, Isthmic Reticular Formation, Microcellular Tegmental Nucleus, Ventral Tegmental Area Nucleus Complex, and Caudal-Rostral Linear Raphe Nucleus Complex in Living Humans from 7 Tesla Magnetic Resonance Imaging. Brain Connect 11, 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GS, Workman CI, Protas H, Su Y, Savonenko A, Kuwabara H, Gould NF, Kraut M, Joo JH, Nandi A, Avramopoulos D, Reiman EM, Chen K, 2021. Positron emission tomography imaging of serotonin degeneration and beta-amyloid deposition in late-life depression evaluated with multi-modal partial least squares. Transl Psychiatry 11, 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaedal J, Johannesson GH, Gudmundsson TE, Gudmundsson S, Pajdak TH, Johnsen K, 2010. The use of EEG in Alzheimer’s disease, with and without scopolamine – A pilot study. Clinical Neurophysiology 121, 836–841. [DOI] [PubMed] [Google Scholar]
- Solders SK, Galinsky VL, Clark AL, Sorg SF, Weigand AJ, Bondi MW, Frank LR, 2021. Diffusion MRI tractography of the locus coeruleus-transentorhinal cortex connections using GO-ESP. Magn. Reson. Med, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerauer M, Fedorova TD, Hansen AK, Knudsen K, Otto M, Jeppesen J, Frederiksen Y, Blicher JU, Geday J, Nahimi A, Damholdt MF, Brooks DJ, Borghammer P, 2018. Evaluation of the noradrenergic system in Parkinson’s disease: an 11C-MeNER PET and neuromelanin MRI study. Brain 141, 496–504. [DOI] [PubMed] [Google Scholar]
- Song TA, Chowdhury SR, Yang F, Dutta J, 2020. Super-Resolution PET Imaging Using Convolutional Neural Networks. IEEE Trans Comput Imaging 6, 518–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song TA, Yang F, Chowdhury SR, Kim K, Johnson KA, El Fakhri G, Li Q, Dutta J, 2019. PET Image Deblurring and Super-Resolution with an MR-Based Joint Entropy Prior. IEEE Trans Comput Imaging 5, 530–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Donohue MC, Raman R, Sun C-K, Yaari R, Holdridge K, Siemers E, Johnson KA, Aisen PS, Team, f.t.A.S., 2020. Association of Factors With Elevated Amyloid Burden in Clinically Normal Older Individuals. JAMA Neurol. 77, 735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratmann K, Heinsen H, Korf HW, Del Turco D, Ghebremedhin E, Seidel K, Bouzrou M, Grinberg LT, Bohl J, Wharton SB, den Dunnen W, Rüb U, 2016. Precortical Phase of Alzheimer’s Disease (AD)-Related Tau Cytoskeletal Pathology. Brain Pathol 26, 371–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudarshan VP, Li S, Jamadar SD, Egan GF, Awate SP, Chen Z, 2021. Incorporation of anatomical MRI knowledge for enhanced mapping of brain metabolism using functional PET. NeuroImage 233, 117928. [DOI] [PubMed] [Google Scholar]
- Sun W, Tang Y, Qiao Y, Ge X, Mather M, Ringman JM, Shi Y, for Alzheimer’s Disease Neuroimaging, I., 2020. A probabilistic atlas of locus coeruleus pathways to transentorhinal cortex for connectome imaging in Alzheimer’s disease. NeuroImage 223, 117301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symms M, Jäger HR, Schmierer K, Yousry TA, 2004. A review of structural magnetic resonance neuroimaging. J. Neurol. Neurosurg. Psychiatry 75, 1235–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, Raskind MA, 2006. Compensatory changes in the noradrenergic nervous system in the locus ceruleus and hippocampus of postmortem subjects with Alzheimer’s disease and dementia with Lewy bodies. J. Neurosci 26, 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi J, Shibata T, Sasaki M, Kudo M, Yanezawa H, Obara S, Kudo K, Ito K, Yamashita F, Terayama Y, 2015. Detection of changes in the locus coeruleus in patients with mild cognitive impairment and Alzheimer’s disease: high-resolution fast spin-echo T1-weighted imaging. Geriatr Gerontol Int 15, 334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teipel SJ, Flatz WH, Heinsen H, Bokde AL, Schoenberg SO, Stockel S, Dietrich O, Reiser MF, Moller HJ, Hampel H, 2005. Measurement of basal forebrain atrophy in Alzheimer’s disease using MRI. Brain 128, 2626–2644. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Meindl T, Grinberg L, Grothe M, Cantero JL, Reiser MF, Moller HJ, Heinsen H, Hampel H, 2011. The cholinergic system in mild cognitive impairment and Alzheimer’s disease: an in vivo MRI and DTI study. Hum. Brain Mapp 32, 1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DR, Rub U, Orantes M, Braak H, 2002. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 58, 1791–1800. [DOI] [PubMed] [Google Scholar]
- Theofilas P, Dunlop S, Heinsen H, Grinberg LT, 2015. Turning on the Light Within: Subcortical Nuclei of the Isodentritic Core and their Role in Alzheimer’s Disease Pathogenesis. Journal of Alzheimer’s Disease 46, 17–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilas P, Ehrenberg AJ, Dunlop S, Di Lorenzo Alho AT, Nguy A, Leite REP, Rodriguez RD, Mejia MB, Suemoto CK, Ferretti-Rebustini REL, Polichiso L, Nascimento CF, Seeley WW, Nitrini R, Pasqualucci CA, Jacob Filho W, Rueb U, Neuhaus J, Heinsen H, Grinberg LT, 2017. Locus coeruleus volume and cell population changes during Alzheimer’s disease progression: A stereological study in human postmortem brains with potential implication for early-stage biomarker discovery. Alzheimers Dement 13, 236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Iacono D, Bonanni L, D’Andreamatteo G, Onofrj M, 2001. Donepezil, rivastigmine, and vitamin E in Alzheimer disease: a combined P300 event-related potentials/neuropsychologic evaluation over 6 months. Clinical Neuropharmacology 24, 31–42. [DOI] [PubMed] [Google Scholar]
- Tiepolt S, Meyer PM, Patt M, Deuther-Conrad W, Hesse S, Barthel H, Sabri O, 2022. PET Imaging of Cholinergic Neurotransmission in Neurodegenerative Disorders. J Nucl Med 63, 33S–44S. [DOI] [PubMed] [Google Scholar]
- Tiernan CT, Mufson EJ, Kanaan NM, Counts SE, 2018. Tau Oligomer Pathology in Nucleus Basalis Neurons During the Progression of Alzheimer Disease. J. Neuropathol. Exp. Neurol 77, 246–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, 2014. Functional connectivity of substantia nigra and ventral tegmental area: maturation during adolescence and effects of ADHD. Cereb. Cortex 24, 935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tona KD, Keuken MC, de Rover M, Lakke E, Forstmann BU, Nieuwenhuis S, van Osch MJP, 2017. In vivo visualization of the locus coeruleus in humans: quantifying the test-retest reliability. Brain Struct Funct 222, 4203–4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totah NK, Neves RM, Panzeri S, Logothetis NK, Eschenko O, 2018. The Locus Coeruleus Is a Complex and Differentiated Neuromodulatory System. Neuron 99, 1055–1068.e1056. [DOI] [PubMed] [Google Scholar]
- Totah NKB, Logothetis NK, Eschenko O, 2019. Noradrenergic ensemble-based modulation of cognition over multiple timescales. Brain Res 1709, 50–66. [DOI] [PubMed] [Google Scholar]
- Truchot L, Costes N, Zimmer L, Laurent B, Le Bars D, Thomas-Anterion C, Mercier B, Hermier M, Vighetto A, Krolak-Salmon P, 2008. A distinct [18F]MPPF PET profile in amnestic mild cognitive impairment compared to mild Alzheimer’s disease. NeuroImage 40, 1251–1256. [DOI] [PubMed] [Google Scholar]
- Trujillo P, Petersen KJ, Cronin MJ, Lin YC, Kang H, Donahue MJ, Smith SA, Claassen DO, 2019. Quantitative magnetization transfer imaging of the human locus coeruleus. NeuroImage 200, 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo P, Summers PE, Ferrari E, Zucca FA, Sturini M, Mainardi LT, Cerutti S, Smith AK, Smith SA, Zecca L, Costa A, 2017. Contrast mechanisms associated with neuromelanin-MRI. Magn. Reson. Med 78, 1790–1800. [DOI] [PubMed] [Google Scholar]
- Trutti AC, Fontanesi L, Mulder MJ, Bazin PL, Hommel B, Forstmann BU, 2021. A probabilistic atlas of the human ventral tegmental area (VTA) based on 7 Tesla MRI data. Brain Struct Funct 226, 1155–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turker HB, Riley E, Luh WM, Colcombe SJ, Swallow KM, 2021. Estimates of locus coeruleus function with functional magnetic resonance imaging are influenced by localization approaches and the use of multi-echo data. NeuroImage 236, 118047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W, 2010. Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci 11, 100–113. [DOI] [PubMed] [Google Scholar]
- van der Velden L, Vinck MA, Wadman WJ, 2020. Resonance in the Mouse Ventral Tegmental Area Dopaminergic Network Induced by Regular and Poisson Distributed Optogenetic Stimulation in-vitro. Frontiers in Computational Neuroscience 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Egroo M, van Hooren RWE, Jacobs HIL, 2021. Associations between locus coeruleus integrity and nocturnal awakenings in the context of Alzheimer’s disease plasma biomarkers: a 7T MRI study. Alzheimers Res Ther 13, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vana L, Kanaan NM, Ugwu IC, Wuu J, Mufson EJ, Binder LI, 2011. Progression of Tau Pathology in Cholinergic Basal Forebrain Neurons in Mild Cognitive Impairment and Alzheimer’s Disease. The American Journal of Pathology 179, 2533–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, Mohan A, Yoo HB, Huang Y, Luckey AM, McLeod SL, Tabet MN, Souza RR, McIntyre CK, Chapman S, Robertson IH, To WT, 2020. The peripheral effect of direct current stimulation on brain circuits involving memory. Sci Adv 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varangis E, Habeck CG, Stern Y, 2021. Task-based functional connectivity in aging: How task and connectivity methodology affect discovery of age effects. Brain Behav 11, e01954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneri A, De Marco M, 2020. Reduced monoaminergic nuclei MRI signal detectable in pre-symptomatic older adults with future memory decline. Sci Rep 10, 18707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura-Bort C, Wirkner J, Genheimer H, Wendt J, Hamm AO, Weymar M, 2018. Effects of Transcutaneous Vagus Nerve Stimulation (tVNS) on the P300 and Alpha-Amylase Level: A Pilot Study. Front. Hum. Neurosci 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobyov V, Bakharev B, Medvinskaya N, Nesterova I, Samokhin A, Deev A, Tatarnikova O, Ustyugov AA, Sengpiel F, Bobkova N, 2019. Loss of Midbrain Dopamine Neurons and Altered Apomorphine EEG Effects in the 5xFAD Mouse Model of Alzheimer’s Disease. Journal of Alzheimer’s Disease 70, 241–256. [DOI] [PubMed] [Google Scholar]
- Wang Q, He C, Wang Z, Zhang Z, Xie C, 2021. Dynamic Connectivity Alteration Facilitates Cognitive Decline in Alzheimer’s Disease Spectrum. Brain Connect 11, 213–224. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Tan Z, Wang X, Martinez-Hernandez A, Frahm J, 2019. Magnetic resonance imaging of noradrenergic neurons. Brain Struct Funct 224, 1609–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D, 2018. Long Road to Ruin: Noradrenergic Dysfunction in Neurodegenerative Disease. Trends Neurosci. 41, 211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia CF, Arteaga J, Chen G, Gangadharmath U, Gomez LF, Kasi D, Lam C, Liang Q, Liu C, Mocharla VP, Mu F, Sinha A, Su H, Szardenings AK, Walsh JC, Wang E, Yu C, Zhang W, Zhao T, Kolb HC, 2013. [(18)F]T807, a novel tau positron emission tomography imaging agent for Alzheimer’s disease. Alzheimers Dement 9, 666–676. [DOI] [PubMed] [Google Scholar]
- Xiang L, Harel A, Todorova R, Gao H, Sara SJ, Wiener SI, 2022. Locus cœruleus noradrenergic neurons phase-lock to prefrontal cortical and hippocampal infra-slow rhythms which synchronize with behavioral events. bioRxiv, p. 2022.2005.2012.491630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R, Rua C, O”Callaghan C, Jones PS, Hezemans FH, Kaalund SS, Tsvetanov KA, Rodgers CT, Williams G, Passamonti L, Rowe JB, 2021. An in vivo probabilistic atlas of the human locus coeruleus at ultra-high field. NeuroImage 225, 117487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yener G, Hünerli-Gündüz D, Yıldırım E, Aktürk T, Başar-Eroğlu C, Bonanni L, Del Percio C, Farina F, Ferri R, Güntekin B, Hajós M, Ibáñez A, Jiang Y, Lizio R, Lopez S, Noce G, Parra MA, Randall F, Stocchi F, Babiloni C, 2022. Treatment effects on event-related EEG potentials and oscillations in Alzheimer’s disease. Int. J. Psychophysiol 177, 179–201. [DOI] [PubMed] [Google Scholar]
- Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC, 2012. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage 61, 1000–1016. [DOI] [PubMed] [Google Scholar]
- Zhang S, Hu S, Chao HH, Li CS, 2016. Resting-State Functional Connectivity of the Locus Coeruleus in Humans: In Comparison with the Ventral Tegmental Area/Substantia Nigra Pars Compacta and the Effects of Age. Cereb. Cortex 26, 3413–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Vakhtin AA, Jennings JS, Massaband P, Wintermark M, Craig PL, Ashford JW, Clark JD, Furst AJ, 2020. Diffusion tensor tractography of brainstem fibers and its application in pain. PLOS ONE 15, e0213952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, Kramer JH, Weiner M, Miller BL, Seeley WW, 2010. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain 133, 1352–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Zhu X, 2019. MRI-Driven PET Image Optimization for Neurological Applications. Front Neurosci 13, 782. [DOI] [PMC free article] [PubMed] [Google Scholar]


