Abstract
To better characterize the vaccine potential of Neisseria meningitidis transferrin binding proteins (Tbps), we have overexpressed TbpA and TbpB from Neisseria meningitidis isolate K454 in Escherichia coli. The ability to bind human transferrin was retained by both recombinant proteins, enabling purification by affinity chromotography. The recombinant Tbps were evaluated individually and in combination in a mouse intraperitoneal-infection model to determine their ability to protect against meningococcal infection and to induce cross-reactive and bactericidal antibodies. For the first time, TbpA was found to afford protection against meningococcal challenge when administered as the sole immunogen. In contrast to the protection conferred by TbpB, this protection extended to a serogroup C isolate and strain B16B6, a serogroup B isolate with a lower-molecular-weight TbpB than that from strain K454. However, serum from a TbpB-immunized rabbit was found to be significantly more bactericidal than that from a TbpA-immunized animal. Our evidence demonstrates that TbpA used as a vaccine antigen may provide protection against a wider range of meningococcal strains than does TbpB alone. This protection appears not to be due to complement-mediated lysis and indicates that serum bactericidal activity may not always be the most appropriate predictor of efficacy for protein-based meningococcal vaccines.
Meningococcal disease is a worldwide health problem and is a major cause of meningitis in young children. The causative agent, Neisseria meningitidis, is carried asymptomatically by approximately 10% of the British population, but in rare circumstances it can cause septicemia and/or meningitis. Unless rapidly diagnosed and treated, meningococcal disease can lead to death within a matter of hours (5).
Current meningococcal vaccines are limited in their effectiveness. Polysaccharide vaccines against N. meningitidis serogroups A, C, Y, and W135 offer short-lived protection and are ineffective or less effective in children under the age of 2 years, one of the most susceptible age groups for meningococcal disease. The new serogroup C polysaccharide conjugate vaccines introduced through a United Kingdom-wide vaccination program show great promise (4). In contrast, the serogroup B capsular polysaccharide is poorly immunogenic, due to its similarity to human neural adhesion molecules (33). No vaccine is currently available for this serogroup, which is responsible for over half of all cases of meningococcal disease in Europe and North America. The lack of a serogroup B polysaccharide vaccine has generated much interest in subcapsular antigens. Vaccines consisting of outer membrane vesicles have been assessed in several trials and in general use, with various protection levels being reported (6, 7, 15, 44). However, the protection afforded by these vaccines may be serotype or serosubtype restricted (31). There are a number of candidate protein antigens for inclusion in vaccines against meningococcal disease, including transferrin binding protein B (TbpB) (29), NspA (10), and a variety of candidates arising from the N. meningitidis genome sequence (38).
N. meningitidis transferrin binding proteins (TbpA and TbpB, previously Tbp1 and Tbp2) form a complex responsible for the acquisition of host iron from the human iron transport protein transferrin (hTf) (9, 22). Due to the paucity of free iron available in vivo, this mechanism is critical for the survival and growth of Neisseria in tissues (13). TbpB is a variable, largely extracellular protein anchored to the outer membrane by an N-terminal lipid moiety. TbpA is a more highly conserved, integral membrane protein with homology to TonB-dependent outer membrane porins (22, 25). Functional studies, such as those investigating the ability to discriminate between iron-loaded and iron-depleted transferrin, have implicated TbpB in initial hTf binding (3, 8). Once hTf is bound to TbpB, TbpA interacts with the hTf and energy-dependent iron transport is facilitated (45). Evidence suggests that TbpA forms a 2:1 complex with TbpB and that in such a complex the TbpA dimer may form pores through which iron is transported (8, 9). Their essential function, surface location, and expression in all meningococcal isolates make the Tbps attractive vaccine candidates.
A number of studies have demonstrated that TbpB is a promising vaccine candidate. TbpB is recognized by antibodies in human convalescent-phase sera (16, 21, 24), is protective in a mouse infection model, and elicits a bactericidal antibody response in laboratory animals (29). However, the heterogeneity of TbpB is a potential obstacle to protection against the variety of meningococcal strains in circulation (28, 32, 41, 42). Evidence to date suggests that TbpA would make a poor vaccine antigen, since antibody recognition is strongly conformation dependent and antibodies to the protein are nonbactericidal (14). Protection against experimental meningococcal infection has not been previously demonstrated with TbpA. We have cloned and overexpressed meningococcal tbpA and tbpB genes in Escherichia coli, characterized the recombinant protein, and assessed protection against infection in a murine disease model.
MATERIALS AND METHODS
Cloning and overexpression of tbpA and tbpB.
Genomic DNA from N. meningitidis isolate K454 (B:15:P1.7,16) (30) was prepared using the method of Chen and Kuo (12). Primers for amplifying K454 tbpA were designed after sequencing of the 5′ and 3′ ends. The 5′ primer (TTAGGGAAACCATATGCAACAGCAAC) incorporates an NdeI restriction site at the ATG start codon. The 3′ primer (GACGGATCCGCGTTTGGACGTTTAAAACTTC) includes a BamHI site after the TAA stop codon. PCR products were generated using Hi-Fidelity Taq DNA Polymerase (Boehringer Mannheim) and cloned using the TA Cloning system (Invitrogen). The tbpA NdeI-BamHI and rlpB::tbpB NdeI-EcoRI fragments were subcloned into two of the CAMR pMTL vector series (11) pMTL2000 (in the case of tbpA) or pMTL2010 (for rlpB::tbpB), after sequencing using Big Dye Terminator chemistry (Perkin Elmer Biosystems) and a Prism 377 DNA Sequencer (ABI). The tbp pMTL constructs, which comprise a lac promoter for driving transcription and ampicillin (pMTL2000) or tetracycline (pMTL2010) resistance markers, were used to transform E. coli strain JM109 for expression. As reported previously (26), replacement of the native TbpB leader sequence with that of the E. coli lipoprotein RlpB enhanced the production of mature, lipidated protein. This fusion was constructed in the present study as follows. The rlpB leader sequence was amplified from E. coli strain JM109 using oligonucleotides rlpB 5′ (GGAGGACATATGCGATATCTGGCAAC) and rlpB 3′ (GAAGGATCCGCCTCCGCCCAAACACCCGGCGGTGATTAACAC). The rlpB 5′ oligonucleotide incorporates an NdeI site at the start ATG codon. The rlpB 3′ oligonucleotide incorporates the start of the mature TbpB-encoding sequence and contains silent base changes to introduce a BamHI site. This enables the rlpB sequence to be joined to tbpB where tbpB from strain K454 was amplified using oligonucleotides tbpB 5′ mature (GGAGGCGGATCCTTCGATCTTGATTCTGTCGATACC) and tbpB 3′ (GACGAATTCCGGCAGCCGTGCTTATCGC). The tbpB 5′ mature primer contains a BamHI site. Ligation of the above-described rlpB and tbpB PCR products at their common BamHI sites results in the replacement of the native TbpB leader sequence by that of RlpB. The tbpB 3′ primer contains an EcoRI site for cloning purposes. All rTbpB used in this study was produced using this construct.
Growth of recombinant E. coli strains.
Recombinant E. coli strains (JM109 containing CAMR pMTL vectors with either tbpA or rlpB::tbpB gene inserted) were cultured in 8-liter fermentors. A soytone-based medium containing the appropriate antibiotic (either 1.25 mg of tetracycline per liter or 100 mg of ampicillin per liter) was used. The fermentors were maintained at 37°C with an air flow of 0.5 vessel volume min−1 and a pH of 6.8 to 7.0. The dissolved oxygen tension was maintained at >40% by agitation. The cultures were allowed to grow until an absorbance at 600 nm of approximately 10 was reached, at which point Tbp expression was induced by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to a final concentration of 1.0 mM. The cultures were then allowed to grow for a further 6 to 8 h. Cells were harvested by centrifugation, and the wet weight was determined.
Purification of rTbpA and rTbpB.
Recombinant E. coli cells were resuspended in 100 mM Tris-HCl–0.5 M NaCl buffer (pH 8.0) to 10% (wt/vol) using a glass homogenizer to obtain an even suspension. An equal volume of the same buffer containing 4% (vol/vol) Elugent detergent (Calbiochem) was added to the whole-cell suspension, which was mixed thoroughly. The suspension was incubated with gentle stirring at 4°C for 16 h and then centrifuged at 39,000 × g for 40 min to remove bacterial debris, and the supernatant was retained. Supernatants containing rTbpA or rTbpB were then loaded onto a 10-ml hTf-Sepharose 4B column, as described previously (8), at a flow rate of 1 ml/min. For rTbpB purification, the column was previously saturated with iron by washing with 200 ml of iron saturation buffer (40 mM Tris, 2 mM NaHCO3, 25 mM sodium citrate, 1 mM FeSO4 · 7H2O [pH 7.2]). The affinity columns were then washed with 20 column volumes of wash buffer (100 mM Tris-HCl, 0.5 M NaCl buffer [pH 8.0]) to remove nonspecifically bound material. rTbps were recovered from the column using elution buffer (50 mM glycine, 0.5 M NaCl, 2% [vol/vol] Elugent detergent [pH 2.0]). Fractions containing rTbps were located by monitoring the absorbance at 280 nm. Since Elugent also absorbs at 280 nm, the presence of rTbps was confirmed by hTf-horseradish peroxidase (hTf-HRP) ligand blotting (8) and sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis. Fractions containing rTbps were pooled and applied to a HiPrep desalting column (Sephadex G-25; Amersham Pharmacia) to partially remove glycine and free Elugent. The protein concentration was then determined using a BCA kit (Pierce) with bovine serum albumin as the standard.
SDS-PAGE and Western blotting.
SDS-PAGE was carried out as described previously (8) except that gels were stained with Gelcode Blue (Pierce). For Western blotting, membranes were probed with anti-native Tbp (TbpA or TbpB) serum prepared in mice or with hTf-HRP. Binding of mouse sera was detected by using anti-mouse immunoglobulin G (IgG)-HRP (whole molecule) conjugate and developed using 4-chloro-1-naphthol.
Immunizations.
NIH mice (6 to 8 weeks old) (Harlan) were used in immunogenicity and protection studies. Vaccines were prepared with an equal volume of either Freund's complete adjuvant (first immunization) or Freund's incomplete adjuvant (subsequent immunizations). Each mouse received 0.2 ml, containing 10 μg of protein, by subcutaneous injection. A single New Zealand White rabbit (2 to 3 kg) was also immunised with either 60 μg of TbpA or TbpB in Freund's adjuvant. All animals were immunized on days 1, 21, and 28. On day 35, the mice were infected for protection studies or terminal sera were obtained for enzyme-linked immunosorbent assay (ELISA) and bactericidal antibody assay.
Infection of mice with N. meningitidis.
Mice were infected by intraperitoneal injection of N. meningitidis at several challenge doses (46). Bacteria were grown for 4 h in Mueller-Hinton broth (MHB; Oxoid) made iron-limited by the addition of 5 μg of ethylenediamine dihydroxyphenylacetic acid (EDDHA) per ml, adjusted to the required density with the same medium, and mixed with an equal volume of sterile hTf (40 mg/ml; Sigma) in phosphate-buffered saline. Mice received 0.5 ml of the appropriate challenge dose, and 24 h later a second intraperitoneal injection of 0.2 ml of saline containing hTf (50 mg ml−1) was administered. The health of the mice was monitored for 4 days after infection. All procedures involving animals were conducted according to the requirements of the United Kingdom Home Office Animals (Scientific Procedures) Acts, 1986.
Statistics.
For each protection test as described above, groups of immunized animals were compared with the unimmunized groups for each time point. Contingency tables were analyzed using the chi-squared test after Yates correction for small sample numbers. The P values were categorized as not significant (P > 0.05), significant (P ≤ 0.05), or highly significant (P ≤ 0.01).
ELISA.
ELISA was performed using the standard ELISA method. Briefly, proteins were coated at concentration of 1 μg/ml onto Maxisorb 96-well ELISA plates (Nunc) overnight at 4°C. Mouse antibody binding was detected using a goat anti-mouse IgG whole-molecule–HRP conjugate (Sigma) in combination with TM Blue (Intergen). Whole-bacterial-cell ELISA was carried out as described previously (1), with the following modifications: clinical meningococcal isolates obtained from A. K. Lehmann, University of Bergen (27) were grown in MHB made iron limited by the addition of 5 μg of EDDHA per ml and dried onto Maxisorb ELISA plates (Nunc).
The antibody titer was defined as the reciprocal of the dilution of serum corresponding to the midpoint of the dose-response curve. This was calculated using interpolation software (Genesis; Labsystems) on dose-response curves generated from eight dilutions of each serum (1/100 through seven further threefold dilutions). Interplate variation was corrected for using a pooled serum standard, and sera showing a titer of less than the detection limit were assigned an arbitrary titer of 50 for calculation of geometric mean titers. Each mouse serum was assayed in duplicate. The mean of the duplicate serum titers from each of five different mice was used to generate a geometric mean titer for the group (see Table 2).
TABLE 2.
Meningococcal whole-cell ELISA titers of sera from mice immunized with rTbpA and/or rTbpB
| Meningococcal strain details
|
Whole-cell ELISA titersc in immunization group:
|
|||
|---|---|---|---|---|
| Serotype or group | Isolate sourcea | TbpB typeb | TbpA | TbpB |
| B:15P1.7,16 | 6 | H | 19,676 | 3,050 |
| B:15:P1.7,16 | 8 | H | 20,650 | 3,667 |
| C:15P1.7,16 | 9 | H | 4,987 | 614 |
| C:15P1.7,16 | 12 | H | 9,726 | 1,267 |
| C:15:P1.7,16 | 13 | H | 7,514 | 1,389 |
| C:15:P1.7,16 | 14 | H | 2,301 | 709 |
| B:15:P1.2 | 20 | H | 3,893 | 1,283 |
| B:15:P1.12V | 22 | H | 16,364 | 3,488 |
| B:15:P1.12V | 23 | H | 11,903 | 1,858 |
| C:2a:P1.2 | 29 | H | 7,785 | 3,269 |
| B:NT:P1.12 | 32 | H | 17,385 | 10,226 |
| B:NT:P1.16 | 33 | H | 9,614 | 339 |
| B:4:P1.12 | 37 | H | 18,653 | 7,461 |
| B:19:P1.15 | 39 | H | 18,108 | 1,609 |
| C:2a:P1.2 | 26 | L | 2,539 | 216 |
| C:2a:P1.2 | 27 | L | 12,116 | 1,314 |
| C:2a:P1.2 | 28 | L | 172,779 | 1,130 |
Patient numbers were assigned in reference 27.
The molecular masses of TbpB are grouped as follows: H (high,>80 kDa) or L (low, <70 kDa).
Geometric mean of the reciprocal titer from five separate animals. Where no antibody was detected (e.g., preimmune sera [data not shown]), an arbitrary titer of 50 was assigned. The mean values are 10,408 for the TbpA group and 1,573 for the TbpB group.
Serum bactericidal antibody assays.
Serum bactericidal assays were performed by a standardized method (Centers for Disease Control, Report of the 2nd International Workshop on Meningococcal Immunology and Serology, 1992) utilizing 25% human serum previously screened for lack of antibodies to meningococcal whole cells (by ELISA) as the complement source. Bactericidal titers were expressed as the reciprocal of the final dilution giving ≥50% bactericidal killing at 60 min. N. meningitidis strains used in this assay were grown in MHB with and without the addition of EDDHA.
Nucleotide sequence accession numbers.
The nucleotide sequences of N. meningitidis K454 tbpA and tbpB have been deposited in GenBank under accession numbers AF268474 and AF268475, respectively.
RESULTS
Expression and purification of Tbps.
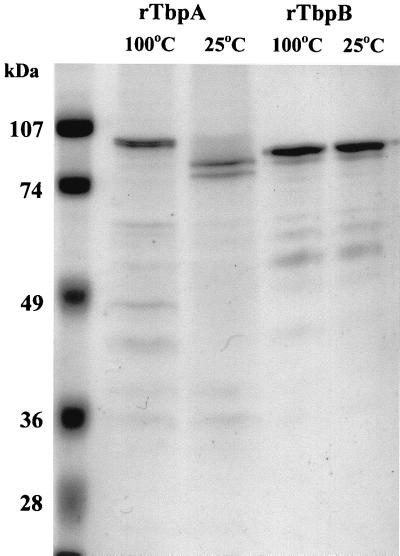
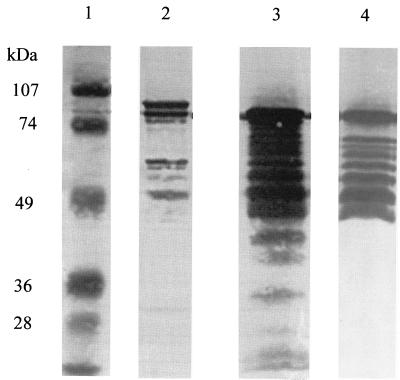
Purified rTbps were assessed for hTf binding by dot blot analysis with hTf-HRP to ensure that eluted protein was active (data not shown). The purity and integrity of Tbps were assessed using SDS-PAGE and Western blotting. The results in Fig. 1 show that the mature forms of both recombinant proteins are of the expected apparent molecular mass (98 kDa for TbpA, 90 kDa for lipidated TbpB). The electrophoretic mobility of rTbpA, unlike that of rTbpB, was affected by boiling in the presence of SDS. A consistent pattern of lower-molecular-mass bands was always observed in preparations of both rTbpA and rTbpB. Western blotting using serum raised against native K454 Tbps isolated from N. meningitidis as described previously (8) (Fig. 2) or hTf-HRP showed that all of these lower-molecular-mass bands were derived from Tbp and that many of the rTbpB products retained transferrin binding activity (note that TbpA does not bind hTf-HRP on Western blots). The observed pattern of banding could not be altered by using an alternative protease-deficient host, by growth at lower temperatures, by including protease inhibitors in the buffers used for purification or size exclusion chromatography (data not shown).
FIG. 1.
SDS-PAGE analysis of purified recombinant TbpA and TbpB. Both proteins were analyzed using denaturing (5 min at 100°C, in loading gel with β-mercaptoethanol) and nondenaturing (no boiling, no β-mercaptoethanol) conditions. The sensitivity of the tertiary structure of TbpA is demonstrated by the change in banding pattern on boiling. The tertiary structure of TbpB withstands this treatment, as evidenced by the similarity of the banding pattern before and after boiling.
FIG. 2.
Western blot analysis of recombinant Tbps. Lanes: 1, molecular mass markers (sizes as indicated); 2, rTbpA probed with anti-native meningococcal TbpA antisera; 3, rTbpB probed with anti-native meningococcal TbpB antisera; 4, rTbpB probed with hTf-HRP. Certain epitopes on TbpA must retain their native conformation; hence, TbpA and its degradation products are detected. The abundance of truncated TbpB derivatives is evident. All of these are recognized by antibodies to native TbpB (lane 3), and most are functional, as demonstrated by probing with hTf-HRP (lane 4).
Mouse protection studies.
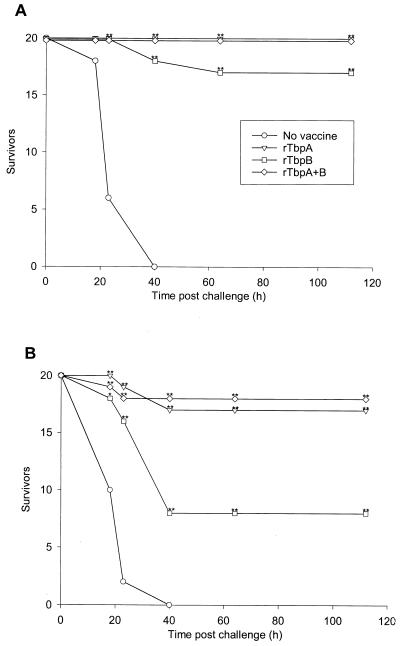
Groups of 20 mice were vaccinated with rTbpA alone, rTbpB alone, or rTbpA plus rTbpB and then challenged with two different doses of the homologous N. meningitidis isolate (strain K454). Analysis of the results in Fig. 3 shows that at both challenge doses, the progression of disease in unimmunized mice was rapid and there were no survivors by day 3 postchallenge. The data also show that 100% protection was afforded by vaccination with rTbpA and rTbpA plus rTbpB at the 2 × 107 CFU challenge dose whereas 85% of the rTbpB-vaccinated group survived. At the 2 × 108 CFU challenge dose, the rTbpA-vaccinated group had an 85% survival rate, the rTbpA-plus-rTbpB-vaccinated group had a 90% survival rate, and the rTbpB-vaccinated group had a 40% survival rate.
FIG. 3.
Immunization of mice with rTbpA, rTbpB, or rTbpA plus rTbpB protects against both low-dose (2 × 107 CFU) (A) and high-dose (2 × 108 CFU) (B) intraperitoneal challenge with N. meningitidis K454. Mice were immunized as follows: no vaccine, rTbpA, rTbpB, or rTbpA plus rTbpB. Significant differences compared with the unimmunized control group are indicated for P ≤ 0.05 (∗) and P ≤ 0.01 (∗∗).
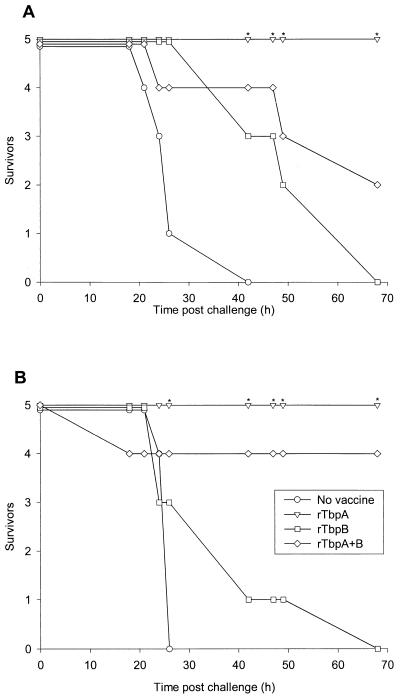
Cross-protection experiments were carried out using smaller groups of mice (five per group). The results (Fig. 4) demonstrated that rTbpA provided 100% protection against challenge with a heterologous serogroup B isolate, B16B6, and serogroup C isolate, L91/543, at a challenge dose of 3 × 106 CFU. In contrast, none of the mice vaccinated with rTbpB alone survived challenge with this dose after 4 days. The survival rate of rTbpA-plus-rTbpB-immunized mice was 40% following challenge with B16B6 and 80% following challenge with the serogroup C isolate. A higher challenge dose (3 × 107 CFU) was also used for each isolate, and no survivors were seen with any vaccine; however, there was a marked delay in the time of death for TbpA- and TbpA-plus-TbpB immunized mice challenged with L91543 (data not shown). The lower levels of protection seen in groups immunized with TbpA plus TbpB compared with TbpA alone is probably because the TbpA-plus-TbpB groups received 5 μg of each antigen (10 μg total) per dose whereas the TbpA-alone vaccine contained 10 μg of TbpA per dose.
FIG. 4.
Immunization of mice with rTbpA, rTbpB, or rTbpA plus rTbpB, survivors following intraperitoneal challenge with N. meningitidis isolates B16B6 4(A) and L91-543 (B). Mice were immunized as follows: no vaccine, rTbpA, rTbpB, or rTbpA plus rTbpB. Significant differences compared with the unimmunized control group are indicated for P ≤ 0.05 (∗).
Serum bactericidal activity.
Assays were carried out using sera from groups of mice which were immunized but not challenged during the protection experiment and from single rabbits immunized with the same antigens. Bactericidal activity could not be detected in mouse sera (Table 1). Rabbit sera from both rTbpA- and rTbpB-vaccinated animals demonstrated bactericidal activity against the homologous iron-starved N. meningitidis strain. Bactericidal titers for antiserum raised against rTbpB were much higher than those for antiserum raised against rTbpA.
TABLE 1.
Bactericidal titers of animal sera against N. meningitidis strain K454
| Serum | Antigen | Iron-limited growth of target bacteria? | Serum bactericidal titera |
|---|---|---|---|
| Mouse | TbpA | Yes | <4 |
| TbpA | No | <4 | |
| TbpB | Yes | <4 | |
| TbpB | No | <4 | |
| TbpA + TbpB | Yes | <4 | |
| TbpA + TbpB | No | <4 | |
| Rabbit | TbpA | Yes | 8, 16 |
| TbpA | No | 4 | |
| TbpB | Yes | 512 | |
| TbpB | No | 4 |
Bactericidal titers are expressed as the reciprocal of the last dilution of sera at which ≥50% of the inoculum is lysed. A pool of sera from five mice or serum from a single rabbit was tested for each antigen. Assays were performed twice. Where the repeat result did not match that of the first, both results are listed.
ELISA studies.
Initial analysis revealed that murine antisera raised against rTbpA or rTbpB gave high ELISA titers against the homologous protein (3,550 and 8,725, respectively) with no or little cross-reaction (<100 and 215, respectively).
Whole-cell ELISA studies were carried out using a range of patient isolates collected in Norway (obtained from A. K. Lehmann) (27) to assess the cross-reactivity of antisera raised against rTbpA and rTbpB with Tbps expressed by these isolates (Table 2). Titers against isolates of a variety of different serogroups, serotypes, and serosubtypes were consistently higher for antisera raised against rTbpA than for antisera raised against rTbpB. Three isolates expressing low-molecular-mass TbpB were included, and the rTbpB antiserum showed reaction with these cells. Preimmune sera from these mice showed no reaction with the meningococcal isolates (data not shown).
DISCUSSION
Tbps have been proposed as potential vaccine candidates since their discovery (43). However, TbpA alone has shown little promise, with a previous study failing to detect TbpA-induced bactericidal activity (29). Furthermore, antibodies raised against native TbpA or the TbpA plus TbpB complex fail to react with electroblotted TbpA, and purification of active (i.e., hTf binding) protein has been problematic (2, 17, 29). These findings are related to the difficulties in maintaining the tertiary structure of TbpA when isolated from the membrane (2, 36). Only when care is taken to retain this tertiary structure can antibodies be detected against native TbpA (2, 21, 24). These problems have led to vaccine development focusing on TbpB, which, while variable in sequence, has demonstrated good protection in animal studies and induces a strong bactericidal response (14, 29). A phase I clinical trial of rTbpB has recently been carried out and demonstrated that an immune response to rTbpB is elicited after human vaccination (B. Danve, L. Lissolo, F. Guinet, E. Boutry, D. Speck, M. Cadoz, X. Nassif, and M.-J. Quentin-Millet, Abstr. Eleventh Int. Pathog. Neisseria Conf., 1998, p. 53, 1998). However, this response was limited since only 2 of 11 individuals showed a greater-than-fourfold rise in bactericidal antibody titers.
The results presented in this study represent the first demonstration of protection conferred by immunization of animals with rTbpA alone. Separate recombinant expression and purification from E. coli ensured that the observed protection was not due to contaminating N. meningitidis antigens such as lipopolysaccharide or, in the case of TbpA, contaminating TbpB. One of the arguments for including TbpA in a Tbp-based vaccine is the potential for greater cross-protection, which is inferred from the greater degree of TbpA sequence conservation (95 to 100% identity) between the limited number of isolates examined to date (34) and studies on the cross-reaction of TbpA-specific antibody preparations (20, 24). The present study also provides evidence to support this argument, with results demonstrating protection by rTbpA derived from strain K454 against heterologous strain challenge. This protection was greater than that conferred by rTbpB alone against challenge with B16B6 (a low-molecular-mass TbpB variant isolate) and a serogroup C isolate, but heterologous protection was lower than that seen against the homologous strain.
We have evaluated the protective potential of rTbps using a mouse intraperitoneal challenge model (14, 23). It is recognized that this is not an ideal model of meningococcal disease since it does not follow the natural pathogenesis of the disease in humans and an exogenous iron source is required (23). The differences between the immune responses of different animals and between those of animals and humans have been documented (2, 18), and it is clear that results derived from the mouse model cannot necessarily be extrapolated to humans. However, this model of bacteremic disease allows assessment of active immunization and protection and allows a useful comparison of protection provided by different vaccines against different challenge isolates.
Protection against meningococcal disease is usually associated with the presence of bactericidal antibodies against N. meningitidis (19). The serum bactericidal antibody assay has therefore been widely used for assessment of meningococcal vaccines, particularly those based on capsular polysaccharide. Some bactericidal activity was detected using sera from rabbits immunized with rTbpA, but the activity was much lower than that detected for sera from rTbpB-immunized rabbits. In contrast, bactericidal activity was not detected in sera from mice immunized with rTbpA or rTbpB, which does not correlate with the murine protection observed and implies that a mechanism other than complement-mediated bacterial killing may be responsible for rTbpA-induced protection. Other observations have also pointed to the same conclusion: a lack of correlation between IgG levels and bactericidal titre in previous immunogenicity studies (29) implies the possible involvement of other protective mechanisms. The serum bactericidal assay may therefore not be the best correlate for predicting the efficacy of Tbp-based vaccines in humans.
If other mechanisms besides complement-mediated bactericidal activity are responsible for the protection demonstrated in the present study, it is important to assess the contribution of other aspects of the immune system to the defense against meningococcal disease. Human convalescent-phase serum has been tested for its ability to activate opsonophagocytosis by using Tbp-coated beads (27). Tbp-directed opsonic activity was detected and correlated with overall levels of IgG. Further, this activity was found to be independent of the phenotype of the infecting strain and the TbpB species used to coat the beads (low-molecular-mass and high-molecular-mass TbpB). This indicated that the human opsonic antibodies are cross-reactive with TbpB of different molecular mass or that common epitopes are exposed in the TbpA-TbpB complex. It should be noted that laboratory animal studies have shown a lack of cross-reactivity between sera raised against low-molecular-mass TbpBs and higher-molecular-mass species (40). However, other studies with human sera have shown the presence of antibodies that are cross-reactive (2, 21, 24). The data obtained with convalescent-phase sera are difficult to interpret since the cross-reactivity may be a result of infection of the individuals with a range of meningococcal and commensal Neisseria prior to disease. The results presented here indicate that TbpA may be responsible for some of the previously observed cross-reactivity.
The effect of antibody-mediated blockage of iron uptake is also likely to be important in host defense by limiting the growth and dissemination of the bacteria. In vitro studies have demonstrated the ability of Tbp-specific antibodies to interfere with hTf binding and growth (29), and further work has shown the ability of such antibodies to block iron internalization (36). Antibodies directed against TbpA have been shown to inhibit iron uptake by up to 70% (37). Even if these TbpA-specific antibodies were proven to be neither bactericidal nor opsonic, the blockage of iron uptake by these antibodies would presumably be a critical part of the host defense mechanism, limiting the growth and dissemination of infecting bacteria and providing the other arms of the immune system with a better opportunity to clear invading meningococci.
To determine the cross-reactivity of mouse sera raised against rTbpA and rTbpB, whole-cell ELISA studies were carried out using a range of meningococcal isolates of different phenotypes. These demonstrated that serum against rTbpA produced higher titers than did serum raised against rTbpB and that the rTbpA serum reacted with all strains tested. This finding is in agreement with previous studies (20, 21, 24, 37), providing further evidence of the potential value of TbpA-induced cross-protection. When interpreting these data, however, it is important to consider that the lower TbpB titers may be simply a result of removal of the more surface-exposed TbpB from the target bacteria by the washing steps of the ELISA. The results of ELISA experiments carried out using recombinant protein as the target antigen eliminate the possibility that the results of the whole-cell ELISA were not simply due to differences in the immune response to the recombinant protein, since titers against the homologous recombinant protein were much higher with antisera raised against rTbpB than with antisera raised against rTbpA.
There are several reasons why we were able to produce rTbpA in a form that was protective. As discussed, retention of tertiary structure is crucial for producing an effective vaccine with this antigen. E. coli was used as a host for expression, since previous studies have shown this to be suitable for surface expression of active TbpA (35), and we extracted active protein from the bacterial surface using a nondenaturing detergent. In addition, the use of affinity purification ensured that only active protein was purified and the elution conditions were such that transferrin binding activity was retained. Additionally, the native conformation was evidently retained when formulated with Freund's adjuvant in this study. Previous studies, including the recent human immunogenicity study of rTbpB, have highlighted the conclusion that the choice of adjuvant and the vehicle used for delivery will be critical for a Tbp-based vaccine (20; Danve et al., Abstr. Eleventh Int. Pathog. Neisseria Conf.).
The argument for inclusion of TbpA in a Tbp-based vaccine is strengthened by the results of this study. Previous results, examining the human immune response to TbpA (24), show that during infection, TbpA is capable of eliciting a strong immune response. The demonstration that TbpA alone can protect against meningococcal infection in the mouse model confirms this finding. Recent studies of Tbps from the bovine pathogen Pasteurella haemolytica provide further evidence of the importance of TbpA (39). It was found that a vaccine containing TbpA and TbpB offered greater protection than did one containing TbpB alone, in spite of an apparently weak immune response to TbpA. This suggests that in addition to being a good vaccine antigen in its own right, TbpA may enhance the efficacy of the TbpB-induced immune response. It might be envisaged that inclusion of TbpA in a TbpB vaccine formulation would help retain the native structure of TbpB and form other discontinuous epitopes which are present in the native transferrin receptor complex but are absent in isolated Tbp molecules. The generation of these new epitopes by the inclusion of TbpA has the potential to enhance the efficacy of a Tbp-based vaccine and may also reduce the requirement for multiple TbpB proteins to generate cross-protection against a wide range of meningococcal strains.
ACKNOWLEDGMENTS
This work was supported by The National Meningitis Trust, The Ralph Sutcliffe Fund for Meningitis Research, and the United Kingdom Department of Health.
REFERENCES
- 1.Abdillahi H, Poolman J T. Typing of group-B Neisseria meningitidis with monoclonal antibodies in the whole-cell ELISA. J Med Microbiol. 1988;26:177–180. [PubMed] [Google Scholar]
- 2.Ala'Aldeen D A A, Stevenson P, Griffiths E, Gorringe A R, Irons L I, Robinson A, Hyde S, Borriello S P. Immune responses in humans and animals to meningococcal transferrin-binding proteins: implications for vaccine design. Infect Immun. 1994;62:2984–2990. doi: 10.1128/iai.62.7.2984-2990.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson J E, Sparling P F, Cornelissen C N. Gonococcal transferrin-binding protein 2 facilitates but is not essential for transferrin utilization. J Bacteriol. 1994;176:3162–3170. doi: 10.1128/jb.176.11.3162-3170.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anonymous. Meningococcal disease falls in vaccine recipients. Commun Dis Rep. 2000;10:133. [PubMed] [Google Scholar]
- 5.Begg N, Cartwright K A V, Cohen J, Kacmarski E B, Innes J A, Leen C L S, Nathwani D, Singer M, Southgate L, Todd W T A, Welsby P D, Wood M J. Consensus statement on diagnosis, investigation, treatment and prevention of acute bacterial meningitis in immunocompetent adults. J Infect. 1999;39:1–15. doi: 10.1016/s0163-4453(99)90095-6. [DOI] [PubMed] [Google Scholar]
- 6.Bjune G, Hoiby E A, Gronnesby J K, Arnesen Ø, Fredriksen J H, Halstensen A, Holten E, Lindbak A-K, Nøkleby H, Rosenqvist E, Solberg L K, Closs O, Eng J, Frøholm L O, Lystad A, Bakketeig L S, Hareide B. Effect of outer membrane vesicle against group B meningococcal disease in Norway. Lancet. 1991;338:1093–1096. doi: 10.1016/0140-6736(91)91961-s. [DOI] [PubMed] [Google Scholar]
- 7.Boslego J, Garcia J, Cruz C, Zollinger W, Brandt B, Ruiz S, Martinez M, Arthur J, Underwood P, Silva W, Moran E, Hankins W, Gilly J, Mays J the Chilean National Committee for Meningococcal Disease. Efficacy, safety and immunogenicity of a meningococcal group B (15:P1.3) outer membrane protein vaccine in Iquique, Chile. Vaccine. 1995;13:821–829. doi: 10.1016/0264-410x(94)00037-n. [DOI] [PubMed] [Google Scholar]
- 8.Boulton I C, Gorringe A R, Allison N, Robinson A, Gorinsky B, Joannou C L, Evans R W. Transferrin-binding protein B isolated from Neisseria meningitidis discriminates between apo and diferric human transferrin. Biochem J. 1998;334:269–273. doi: 10.1042/bj3340269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boulton I C, Gorringe A R, Shergill J K, Joannou C L, Evans R W. A dynamic model of the meningococcal transferrin receptor. J Theor Biol. 1999;198:497–505. doi: 10.1006/jtbi.1999.0928. [DOI] [PubMed] [Google Scholar]
- 10.Cadieux N, Plante M, Rioux C R, Hamel J, Brodeur B R, Martin D. Bactericidal and cross-protective activities of a monoclonal antibody directed against Neisseria meningitidis NspA outer membrane protein. Infect Immun. 1999;67:4955–4959. doi: 10.1128/iai.67.9.4955-4959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers S P, Prior S E, Barstow D A, Minton N P. The pMTL nic− cloning vectors. I. Improved pUC polylinker regions to facilitate the use of sonicated DNA for nucleotide sequencing. Gene. 1988;68:139–149. doi: 10.1016/0378-1119(88)90606-3. [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Kuo T. A simple and rapid method for the preparation of gram-negative bacterial genomic DNA. Nucleic Acids Res. 1993;21:2260. doi: 10.1093/nar/21.9.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornelissen C N, Kelley M, Hobbs M M, Anderson J E, Cannon J G, Cohen M S, Sparling P F. The transferrin receptor expressed by gonococcal FA1090 is required for the experimental infection of human male volunteers. Mol Microbiol. 1998;27:611–616. doi: 10.1046/j.1365-2958.1998.00710.x. [DOI] [PubMed] [Google Scholar]
- 14.Danve B, Lissolo L, Mignon M, Dumas P, Colombani S, Schryvers A B, Quentin Millet M-J. Transferrin-binding proteins isolated from Neisseria meningitidis elicit protective and bactericidal antibodies in laboratory animals. Vaccine. 1993;11:1214–1220. doi: 10.1016/0264-410x(93)90045-y. [DOI] [PubMed] [Google Scholar]
- 15.de Moraes J C, Perkins B A, Camargo M C C, Hidalgo N T R, Barbosa H A, Sacchi C T, Gral I M L, Gattas V L, De H, Vasconcelos G, Plikaytis B D, Wenger J D, Broome C V. Protective efficacy of a serogroup B meningococcal vaccine in Sao Paulo, Brazil. Lancet. 1992;340:1074–1078. doi: 10.1016/0140-6736(92)93086-3. [DOI] [PubMed] [Google Scholar]
- 16.Ferreirós C M, Ferrón L, Craido M T. In vivo human response to transferrin-binding protein 2 and other iron-regulated proteins of Neisseria meningitidis. FEMS Immunol Med Microbiol. 1994;8:63–68. doi: 10.1111/j.1574-695X.1994.tb00426.x. [DOI] [PubMed] [Google Scholar]
- 17.Ferrón L, Ferreirós C M, Craido M T, Andrade M P. Purification of the Neisseria meningitidis transferrin binding protein-2 (TBP2) to homogeneity using column chromotography. FEMS Microbiol Lett. 1993;109:159–166. doi: 10.1111/j.1574-6968.1993.tb06161.x. [DOI] [PubMed] [Google Scholar]
- 18.Ferrón L, Ferreirós C M, Criado M T, Pintor M. Reliability of laboratory models in the analysis of TBP2 and other meningococcal antigens FEMS Immunol. Med Microbiol. 1994;9:299–306. doi: 10.1111/j.1574-695X.1994.tb00365.x. [DOI] [PubMed] [Google Scholar]
- 19.Goldschneider I, Gotschlich E C, Artenstein M S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gómez J A, Hernández E, Criado M T, Ferreirós C M. Effect of adjuvants in the isotypes and bactericidal activity of antibodies against the transferrin-binding proteins of Neisseria meningitidis. Vaccine. 1998;16:1633–1639. doi: 10.1016/s0264-410x(98)00062-0. [DOI] [PubMed] [Google Scholar]
- 21.Gorringe A R, Borrow R, Fox A J, Robinson A. Human antibody response to meningococcal transferrin binding proteins: evidence for vaccine potential. Vaccine. 1995;13:1207–1212. doi: 10.1016/0264-410x(95)00055-6. [DOI] [PubMed] [Google Scholar]
- 22.Gray-Owen S D, Schryvers A B. Bacterial transferrin and lactoferrin receptors. Trends Microbiol. 1996;4:185–191. doi: 10.1016/0966-842x(96)10025-1. [DOI] [PubMed] [Google Scholar]
- 23.Holbein B E. Iron-controlled infection with Neisseria meningitidis in mice. Infect Immun. 1980;29:886–891. doi: 10.1128/iai.29.3.886-891.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson A S, Gorringe A R, Fox A J, Borrow R, Robinson A. Analysis of the human Ig isotype response to individual transferrin binding proteins A and B from Neisseria meningitidis. FEMS Immunol Med Microbiol. 1997;19:159–167. doi: 10.1111/j.1574-695X.1997.tb01085.x. [DOI] [PubMed] [Google Scholar]
- 25.Legrain M, Mazarin V, Irwin S W, Bouchon B, Quentin-Millet M-J, Jacobs E, Schryvers A B. Cloning and characterization of Neisseria meningitidis genes encoding the transferrin-binding proteins Tbp1 and Tbp2. Gene. 1993;130:73–80. doi: 10.1016/0378-1119(93)90348-7. [DOI] [PubMed] [Google Scholar]
- 26.Legrain M, Speck D, Jacobs E. Production of lipidated meningococcal transferrin binding protein 2 in Escherichia coli. Protein Expression Purif. 1995;6:570–578. doi: 10.1006/prep.1995.1075. [DOI] [PubMed] [Google Scholar]
- 27.Lehmann A K, Gorringe A R, Reddin K M, West K, Smith I, Halstensen A. Human opsonins induced during meningococcal disease recognize transferrin binding protein complexes. Infect Immun. 1999;67:6526–6532. doi: 10.1128/iai.67.12.6526-6532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linz B, Schenker M, Zhu P, Achtman M. Frequent interspecific genetic exchange between commensal neisseriae and Neisseria meningitidis. Mol Microbiol. 2000;36:1049–1058. doi: 10.1046/j.1365-2958.2000.01932.x. [DOI] [PubMed] [Google Scholar]
- 29.Lissolo L, Maitre-Wilmotte G, Dumas P, Mignon M, Danve B, Quentin-Millet M-J. Evaluation of transferrin binding protein 2 within the transferrin-binding protein complex as a potential antigen for future meningococcal vaccines. Infect Immun. 1995;63:884–890. doi: 10.1128/iai.63.3.884-890.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mackinnon F G, Borrow R, Gorringe A R, Fox A J, Jones D M, Robinson A. Demonstration of lipooligosaccharide immunotype and capsule as virulence factors for Neisseria meningitidis using an infant mouse intranasal infection model. Microb Pathog. 1993;15:359–366. doi: 10.1006/mpat.1993.1085. [DOI] [PubMed] [Google Scholar]
- 31.Martin S L, Borrow R, van der Ley P, Dawson M, Fox A J, Cartwright K A V. Effect of sequence variation in meningococcal PorA outer membrane protein on the effectiveness of a hexavalent PorA outer membrane vesicle vaccine. Vaccine. 2000;18:2476–2481. doi: 10.1016/s0264-410x(00)00047-5. [DOI] [PubMed] [Google Scholar]
- 32.Mazarin V, Rokbi B, Quentin-Millet M-J. Diversity of the transferrin-binding protein Tbp2 of Neisseria meningitidis. Gene. 1995;158:145–146. doi: 10.1016/0378-1119(95)00151-u. [DOI] [PubMed] [Google Scholar]
- 33.Nedelec J, Boucraut J, Garnier J M, Bernard D, Rougon G. Evidence for autoimmune antibodies directed against embryonic neural adhesion molecules (N-CAM) in patients with group B meningitis. J Neuroimmunol. 1990;29:49–56. doi: 10.1016/0165-5728(90)90146-e. [DOI] [PubMed] [Google Scholar]
- 34.Pajón R, Chinea G, Marrero E, Gonzalez D, Guillén G. Sequence analysis of the structural tbpA gene: protein topology and variable regions within neisserial receptors for transferrin iron acquisition. Microb Pathog. 1997;23:71–84. doi: 10.1006/mpat.1997.0136. [DOI] [PubMed] [Google Scholar]
- 35.Palmer H M, Powell N B L, Ala'Aldeen D A, Wilton J, Borriello S P. Neisseria meningitidis transferrin-binding protein 1 expressed in Escherichia coli is surface exposed and binds human transferrin. FEMS Microbiol Lett. 1993;110:139–146. doi: 10.1111/j.1574-6968.1993.tb06310.x. [DOI] [PubMed] [Google Scholar]
- 36.Pintor M, Ferrón L, Gómez J A, Gorringe A, Criado M T, Ferreirós C M. Blocking of iron uptake by monoclonal antibodies specific for the Neisseria meningitidis transferrin-binding protein 2. J Med Microbiol. 1996;45:252–257. doi: 10.1099/00222615-45-4-252. [DOI] [PubMed] [Google Scholar]
- 37.Pintor M, Gomez J A, Ferron L, Ferreiros C M, Criado M T. Analysis of TbpA and TbpB functionality in defective mutants of Neisseria meningitidis. J Med Microbiol. 1998;47:757–760. doi: 10.1099/00222615-47-9-757. [DOI] [PubMed] [Google Scholar]
- 38.Pizza M, Scarlato V, Masignani V, Giuliani M M, Arico B, Comanducci M, Jennings G T, Baldi L, Bartolini E, Capecchi B, Galeotti C L, Luzzi E, Manetti R, Marchetti E, Mora M, Nuti S, Ratti G, Santini L, Savino S, Scarselli M, Storni E, Zuo P, Broeker M, Hundt E, Knapp B, Blair E, Mason T, Tettelin H, Hood D W, Jeffries A C, Saunders N J, Granoff D M, Venter J C, Moxon E R, Grandi G, Rappuoli R. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science. 2000;287:1816–1820. doi: 10.1126/science.287.5459.1816. [DOI] [PubMed] [Google Scholar]
- 39.Potter A A, Schryvers A B, Ogunnariwo J A, Hutchins W A, Lo R Y C, Watts T. Protective capacity of the Pasturella haemolytica transferrin-binding proteins TbpA and TbpB in cattle. Microb Pathog. 1999;27:197–206. doi: 10.1006/mpat.1999.0297. [DOI] [PubMed] [Google Scholar]
- 40.Rokbi B, Mazarin V, Maitre-Wilmotte G, Quentin-Millet M-J. Identification of two major families of transferrin receptors among Neisseria meningitidis strains based on antigenic and genomic features. FEMS Microbiol Lett. 1993;110:51–58. doi: 10.1111/j.1574-6968.1993.tb06294.x. [DOI] [PubMed] [Google Scholar]
- 41.Rokbi B, Maitre-Wilmotte G, Mazarin V, Fourrichon L, Lissolo L, Quentin-Millet M-J. Variable sequences in a mosaic-like domain of meningococcal tbp2 encode immunoreactive epitopes. FEMS Microbiol Lett. 1995;132:277–283. doi: 10.1016/0378-1097(95)00326-z. [DOI] [PubMed] [Google Scholar]
- 42.Rokbi B, Mignon M, Caugant D A, Quentin-Millet M-J. Heterogeneity of tbpB, the transferrin binding protein B gene, among serogroup B Neisseria meningitidis strains of the ET-5 complex. Clin Diagn Lab Immunol. 1997;4:522–529. doi: 10.1128/cdli.4.5.522-529.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schryvers A B, Morris L J. Identification and characterization of the transferrin receptor from Neisseria meningitidis. Mol Microbiol. 1988;2:281–288. doi: 10.1111/j.1365-2958.1988.tb00029.x. [DOI] [PubMed] [Google Scholar]
- 44.Sierra G V G, Campa H C, Varacel N W, Garcia I L, Izquierdo P L, Sotolongo P F, Casanueva G V, Rico C O, Rodriguez C R, Terry M H. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 1991;14:195–207. [PubMed] [Google Scholar]
- 45.Simonson C, Brener D, DeVoe I W. Expression of a high-affinity mechanism for acquisition of transferrin iron by Neisseria meningitidis. Infect Immun. 1982;36:107–113. doi: 10.1128/iai.36.1.107-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilks K E, Dunn K L R, Farrant J L, Reddin K M, Gorringe A R, Langford P R, Kroll J S. Periplasmic superoxide dismutase in meningococcal pathogenicity. Infect Immun. 1998;66:213–217. doi: 10.1128/iai.66.1.213-217.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]