Abstract
The antibody data supporting the use of meningococcal serogroup C conjugate (MCC) vaccines in the United Kingdom were generated by serum bactericidal assay (SBA) using rabbit complement (rSBA). This may give higher titers than those obtained with human complement (hSBA), for which the “gold standard” correlate of protection for meningococcal C disease is a titer of ≥4. Comparison of rSBA and hSBA titers in sera from unvaccinated adults with an rSBA titer of ≥8 showed that for 93% (27 of 29) the titer was ≥4 by hSBA, confirming natural protection. Furthermore, sera from MCC vaccinees showed that an rSBA titer of <8 or ≥128 discriminated susceptibility and protection well (85% with rSBA titers of <8 had hSBA titers of <4, and 99% with rSBA titers of ≥128 had hSBA titers of ≥4). However, discrimination was poor in the rSBA titer range 8 to 64, with only 60% having hSBA titers of ≥4. In such cases we propose that protection can be assumed if there is a fourfold rise in titer between pre- and postvaccination sera or if there is a characteristic booster response to a polysaccharide challenge dose with, if available, evidence of antibody avidity maturation or an hSBA titer of result ≥4. Applying these criteria to toddlers, 10 to 40% of whom had titers in the range 8 to 64 after a single dose of MCC vaccine, showed that 94% had a fourfold rise in titer, including 98% of those in the titer range 8 to 64. In addition, of those with titers of <128 post-MCC vaccination, 90% had titers of ≥128 after a 10-μg polysaccharide booster dose, compared with only 7% of unprimed age-matched toddlers given a full 50-μg dose. Furthermore, the increase in geometric mean avidity index pre- and postbooster was independent of post-primary MCC titer. These results indicated that the majority of toddlers with an rSBA titer between 8 and 64, and some of those with an hSBA result of <4, have mounted a protective immune response with the induction of immunological memory.
Meningococcal serogroup C (MenC) conjugate (MCC) vaccines have been extensively evaluated in phase I and II trials in the United Kingdom (7, 15, 31–34) and elsewhere (2, 10, 11, 23, 24, 36) and have been shown to be highly immunogenic, generating functional antibodies as measured by serum bactericidal assay (SBA). Studies of military recruits during the 1960s had shown that those with naturally acquired SBA titers of ≥4 were protected from MenC disease (20). The United Kingdom Medicines Control Agency therefore took the view that efficacy trials would not be required for MCC vaccines but instead serological correlates utilizing SBA could be relied upon. A similar rationale has also been used to license unconjugated MenC (and MenA) polysaccharide vaccines. Antibody responses to these capsular polysaccharide vaccines have been measured by various serological methods though licensure was gained using data obtained by radioimmunoassay and SBA in the United Kingdom. The original licensure in the United States in the 1970s, however, was based upon classic randomized efficacy trials (4, 16). Serogroup C polysaccharide vaccines have been shown to be efficacious in those over 2 years of age and to reduce carriage of serogroup C meningococci in the short term (4, 12), although they are poorly immunogenic and not protective in those under 2 years of age (35).
The original serological correlate of protection in military recruits was obtained using an SBA in which human sera was the exogenous complement source (hSBA). However, large volumes of suitable human source complement preserved sera are not available or even practical to obtain due to the occurrence of naturally occurring antimeningococcal antibodies induced by oropharyngeal carriage of meningococci or other neisserial species. In the absence of a commercial source of human complement, the standardization of assays between laboratories, each of whom is using their own in-house source, is difficult.
Commercially available heterologous complement has the advantage of being manufactured and supplied to a high standard of consistency and offers the only practical way of achieving standardization of SBA results between laboratories. Therefore, 3- to 4-week-old baby rabbit serum is now recommended as an alternative complement source for the SBA (26, 37).
It is generally accepted, however, that serogroup C meningococci are more susceptible to serogroup C-specific antibodies when using baby rabbit complement as opposed to human complement, resulting in higher SBA titers (21). With the reliance on serological correlates of protection for licensure of MCC vaccines, we have evaluated the use of SBA titers generated with rabbit complement (rSBA) with the aim of defining rSBA responses that predict protection against MenC disease. The objective is to address concerns about specificity without introducing unnecessarily stringent criteria that might obstruct the licensure and use of MCC vaccines elsewhere.
MATERIALS AND METHODS
MCC vaccine.
The MCC vaccines used in this study were the Meningitec vaccine, which contains 10 μg of MenC oligosaccharide coupled to CRM197 mutant diphtheria toxin (Wyeth Lederle Vaccine & Pediatrics [WLVP], Pearl River, N.Y.), the Menjugate vaccine, which contains 10 μg of MenC oligosaccharide linked to CRM197 mutant diphtheria toxin vaccine (Chiron Vaccines, Siena, Italy) and the Neis-vac, a de-O-acetylated MenC polysaccharide (10 μg) coupled to tetanus toxoid (Baxter Hyland Immuno, formerly North American Vaccines Inc. [NAVA], Beltsville, Md.). Booster doses at 6 months following MCC vaccination comprised a 0.1 ml dose of licensed meningococcal AC polysaccharide (MACP) vaccine [Mengivac (A+C); Pasteur Merieux, Lyon, France] containing 10 μg each of MenA and MenC polysaccharides.
Study groups.
The study groups were as previously described, comprising infants (34), toddlers (12 to 14 months) (31), preschool children (3 to 4 years) and school leavers (14 to 17 years) (E. Miller, P. Richmond, R. Borrow, E. Kaczmarski, K. Cartwright, R. Morris, and C. Thornton, Abstr. 11th Int. Pathog. Neisseria Conf., p. 57, 1998), 18 to 26 year olds (33), and laboratory staff (R. Borrow, E. Miller, N. Peake, R. Rahim, N. Andrews, M. Acuna, S. Martin, J. Southern, and E. B. Kaczmarski, submitted for publication) who were participants in trials of MCC vaccines in the United Kingdom. The postvaccination serological data in this paper are restricted to recipients of the WLVP MCC vaccine with the exception of the toddlers, for whom data for all three manufacturers' vaccines are included. All study sera were assayed by rSBA as part of the respective trials, with selective sera being reassayed by hSBA.
SBA.
The SBA for infant, toddler, and 18- to 26-year-old cohorts was performed as described previously (2) using broth culture for growth of target strains prior to the SBA assay. The SBA for preschool and school leaver cohorts was performed as described by Maslanka et al. (26), in which agar culture was utilized for target strain growth. The complement source used was either 3- to 4-week-old baby rabbit complement (Pelfreeze Biologicals, Brown Deer, Wis.) or complement-preserved human serum collected from a subject with no intrinsic SBA activity against strain C11. The target strain used throughout was C11 (C:16:P1.7a,1). SBA titers were expressed as the reciprocal of the final serum dilution causing ≥50% killing at 60 min. For computational purposes and the calculation of fourfold rises. SBA titers of <4 were given a value of 2.
Avidity indices.
Serogroup C-specific immunoglobulin G avidity was tested by an elution enzyme-linked immunosorbent assay, using the chaotrophe thiocyanate as described elsewhere (18) and modified for the MenC assay (32).
Statistical methods.
rSBA and hSBA titers determined for the same sera were compared by calculating the proportion with protection by hSBA at different rSBA titers with 95% confidence intervals (95% CIs). Specificity within a population of unvaccinated infants and toddlers was also calculated with a 95% CI. 95% CIs were also calculated for the geometric mean avidity index.
RESULTS
Comparison of rSBA and hSBA titers for the same sera.
Paired SBA titers generated with both rabbit and human complement were generated on a total of 194 post-MCC vaccination serum samples (Table 1). These comprised sera from infants after single or multiple doses of MCC (34) as well as those from toddlers after a single dose (31). An rSBA titer of <8 or ≥128 discriminated well between susceptibility and protection as defined by the “gold standard” hSBA cutoff, since 34 of 40 (85% [95% CI, 70 to 94]) of sera with an rSBA titer of <8 had an hSBA titer of <4 and 123 of 124 (99% [95% CI, 96 to 99.9%]) with an rSBA titer of ≥128 had an hSBA titer of ≥4. However, discrimination was less good in the rSBA titer range 8 to 64, with only 18 of 30 (60% [95% CI, 41 to 77%]) showing a titer of ≥4 by hSBA. Although all three sera with titers of 8 by rSBA had titers of <4 by hSBA, this titer was included in the equivocal region because only three paired results were available. Sensitivity and specificity are not calculated for the post-MCC vaccination sera because they are highly dependent on the proportion of samples that are in the rSBA titer range 8 to 64, which varies depending on the age and dose (Table 2).
TABLE 1.
Comparison of the numbers of sera positive and negative by human complement stratified by rabbit complement titer for subjects who have received MCC vaccination and for unvaccinated adults
| rSBA titer | No. of sera with indicated hSBA titer in:
|
|||
|---|---|---|---|---|
| MCC vaccinees
|
Unvaccinated adultsa
|
|||
| <4 | ≥4 | <4 | ≥4 | |
| <8 | 34 | 6 | ||
| 8 | 3 | 0 | 0 | 2 |
| 16 | 3 | 3 | 0 | 2 |
| 32 | 2 | 5 | 2 | 4 |
| 64 | 4 | 10 | 0 | 5 |
| 128 | 1 | 26 | 0 | 4 |
| ≥256 | 0 | 97 | 0 | 10 |
| Total | 47 | 147 | 2 | 27 |
Note that only those adults with an rSBA titer of ≥8 were tested by the hSBA.
TABLE 2.
rSBA titers 4 weeks after vaccination with MCC vaccine (10-μg dose)a
| Vaccine manufacturer and age at first dose | No. of doses | No. tested | GMTb | No. (%) of subjects with indicated postvaccination rSBA titer:
|
||
|---|---|---|---|---|---|---|
| ≤4 | 8–64 | ≥128 | ||||
| Wyeth | ||||||
| 2 mo | 1 | 50 | 33 | 16 (32) | 15 (30) | 19 (38) |
| 2 mo | 2 (1 mo apart) | 56 | 776 | 1 (2) | 3 (5) | 51 (93) |
| 2 mo | 3 (1 mo apart) | 53 | 1,011 | 1 (2) | 1 (2) | 51 (96) |
| 12–14 mo | 1 | 71 | 141 | 6 (8) | 17 (24) | 48 (68) |
| 3–4 yr | 1 | 87 | 908 | 1 (1) | 10 (11) | 76 (88) |
| 14–17 yr | 1 | 81 | 3,890 | 0 (0) | 0 (0) | 81 (100) |
| 18–26 yr | 1 | 86 | 1,425 | 0 (0) | 6 (7) | 80 (93) |
| Chiron | ||||||
| 12–14 mo | 1 | 72 | 123 | 6 (8) | 29 (40) | 37 (52) |
| NAVA | ||||||
| 12–14 mo | 1 | 72 | 564 | 0 (0) | 7 (10) | 65 (90) |
Data from United Kingdom trials.
GMT, geometric mean titer.
In unvaccinated adults with rSBA titers of ≥8 (Table 1), 27 of 29 (93%) had an hSBA titer of ≥4. Of these 27, only 14 (52%) had an rSBA titer of ≥128. This shows that a high proportion of individuals with natural exposure have an rSBA titer in the equivocal range of 8 to 64. It also shows that in a population of unvaccinated adults, an rSBA of 8 to 64 predicts positivity (natural exposure) well with 13 of 15 also positive by hSBA (87% [95% CI, 60 to 98%]).
Age groups in which equivocal rSBA results occur after MCC vaccine.
The rSBA responses in different age groups and with different immunization schedules using the WLVP MCC vaccine are shown in Table 2. Over 90% of infants have rSBA titers of ≥128 after two doses, with a similar proportion being found in 14 to 17 year olds and 18 to 26 year olds after a single dose. However, a substantial proportion of children under 5 years of age have rSBA titers in the equivocal titer range 8 to 64 after a single dose. The proportions of toddlers given a single dose of Chiron or NAVA MCC vaccine who had rSBA titers in the equivocal range were 40 and 10%, respectively (Table 2).
Resolution of the immune status of children with equivocal titers was essential in order to decide whether a one- or two-dose schedule was required for children of 1 to 4 years in the United Kingdom catch-up MCC immunization program.
Specificity of rabbit complement in the SBA in unvaccinated infants and toddlers.
Prevaccination sera from 212 toddlers (31) and 54 infants (34) participating in MCC vaccine trials in the United Kingdom were tested by rSBA. None of these children had a history of prior meningococcal infection and should therefore be susceptible to MenC unless protective SBA antibodies were acquired through carriage or, in infants, through transfer of maternal antibodies.
Of the 212 toddler sera, 200 (94%) had rSBA titers of <4, with the remaining 12 distributed as follows: for six sera, titer = 4; for two sera, titer = 16; for three sera, titer = 32; and for one serum, titer = 512. For the 54 prevaccination infant sera, 46 (85%) had rSBA titers of <4; of the remaining 8 sera, six had titers of 4, one had a titer of 8, and one had a titer of 64. Thus, only one prevaccination toddler serum with a titer of 512 would have been categorized as protected based solely on the rSBA result being ≥128. The hSBA titer with human complement for this serum was 64, confirming protection. The specificity of the rSBA test in a population of unvaccinated uninfected individuals is therefore estimated to be 97% (258 of 265 [95% CI, 95 to 99%]) with a cutoff of ≥8 and 100% (265 of 265 [95% CI, 98.6 to 100%]) with a cutoff of ≥128. This shows that the rSBA does not measure nonspecific functional antibody in nonimmune subjects.
Fourfold rises.
In the United Kingdom toddler study, 1 month after the primary dose of MCC vaccine, 94% of subjects, had a rise of four fold or more in rSBA titer compared with their prevaccination titer (for the Chiron vaccine, 91% [60 of 66]; for the WLVP vaccine, 89% [56 of 63]; for the NAVA vaccine, 100% [71 of 71]). For individuals in whom the post-MCC vaccine rSBA titer was between 8 and 64, 98% (50 of 51) had a fourfold rise in rSBA titer compared with prevaccination levels, regardless of the MCC vaccine administered.
Immunological memory responses to plain polysaccharide.
In the United Kingdom MCC toddler study (31), the effect of a booster dose with 10 μg of C polysaccharide in toddlers previously immunized with MCC vaccine was investigated. For recipients of the Chiron, WLVP, and NAVA MCC vaccines, 47, 33, and 10%, respectively, had rSBA titers of <128 1 month after the first dose, but only 5, 6, and 3%, respectively, had titers of <128 postbooster (Table 3). For those with a postprimary SBA titer of <128, 90% had a postbooster titer of ≥128. This compares with 1 of 12 (8%) age-matched children (17 to 28 months) vaccinated with 50 μg of MACP vaccine as part of an outbreak control measure (8) (P < 0.0001).
TABLE 3.
Proportion of toddlers with postbooster rSBA titers of ≥128 grouped by postprimary rSBA titer
| Vaccine manufacturer | Proportion of toddlers with the indicated postprimary rSBA titera:
|
||
|---|---|---|---|
| <8 | 8–64 | ≥128 | |
| Chiron | 2/3 | 22/24 | 34/34 |
| WLVP | 6/6 | 11/12 | 41/44 |
| NAVA | 6/7 | 62/63 | |
| Total (%) | 8/9 (89) | 39/43 (91) | 137/141 (97) |
Results are given as number of toddlers with postbooster rSBA titer of ≥128/total number of toddlers.
Avidity indices for assessing immunological memory.
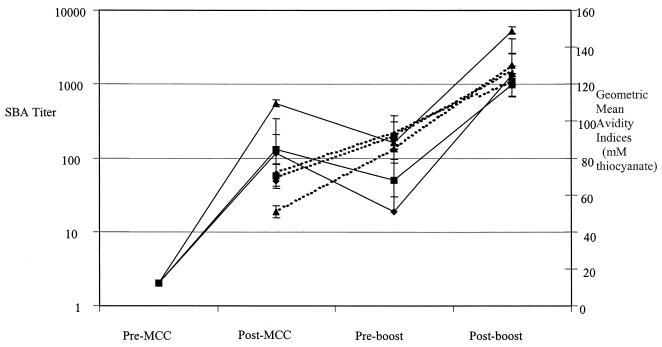
Although the United Kingdom toddler study demonstrated a fall in rSBA titer, the avidity index was shown to increase from postprimary to preboost titers (Fig. 1). There was no significant difference in the avidity index when comparing subjects with postprimary SBA titers of <8, 8 to 64, or ≥128, the geometric mean avidity indices (95% CI) being 119 (90 to 158), 128 (118 to 138), and 128 (123 to 134), respectively, for all three vaccines combined.
FIG. 1.
SBA titers (solid lines) and antibody avidity (dotted lines) in toddlers receiving one dose of MCC followed by a 10-μg dose of MACP 6 months later. The three vaccines used in the study were manufactured by Chiron (diamonds), WLVP (squares), and NAVA (triangles).
Fourfold rises, response to boosting and avidity maturation according to post-MCC rSBA titers.
In Table 4, the United Kingdom MCC toddler study participants were categorized by three different criteria: (i) by the presence of a fourfold rise in SBA titer pre-MCC to 1 month post-MCC, (ii) by the achievement of an rSBA titer of ≥128 after a 10-μg MACP booster, and (iii) by evidence of avidity maturation (i.e., rise in avidity index from 1 month post-MCC to pre-10-μg MACP booster 6 months later). There were no differences in the proportions meeting the above criteria between those with post-primary MCC titers of 8 to 64 compared and those with post-primary MCC titers of ≥128.
TABLE 4.
Categorization of United Kingdom MCC toddler sera post-primary MCC rSBA titers into numbers positive by fourfold rises, response to boosting, and avidity maturation
| Post-MCC rSBA titer | Vaccine | No. with fourfold rise in titer from pre- to post-MCC/ total no. (%) | No. with rSBA titer of ≥128 post-10-μg MACP booster/ total no. (%) | No. with avidity maturationa/ total no. (%) |
|---|---|---|---|---|
| <8 | All | 0/11 (0) | 8/9 (89) | 3/5 (60) |
| 8–64 | All | 48/49 (98) | 39/43 (91) | 25/27 (93) |
| ≥128 | All | 116/117 (99) | 137/141 (97) | 78/86 (91) |
Rise in avidity from post-MCC to pre-MACP booster.
DISCUSSION
We have demonstrated the high specificity of the rSBA in young children who have not been previously vaccinated with MenC polysaccharide or MCC vaccines and could therefore be presumed to be susceptible to MenC infection. Therefore, rSBA titers of <8 predict susceptibility. This conclusion was corroborated in a recent university outbreak of MenC disease in which rSBA titers in students prior to or at the onset of invasive disease were all <4, suggesting that it is the absence of bactericidal activity is that is important irrespective of the complement source used (22). We have also shown that rSBA titers of ≥128 are highly predictive of protection but that many sera with rSBA titers in the range 8 to 64 also come from individuals with protection, particularly for adults with natural exposure. The main issue is therefore the interpretation of rSBA titers in the region of 8 to 64. For groups in whom there is likely to be a large proportion of results in this range, it is important to have other information in order to determine protection. We propose that the other information could be either an hSBA titer (the gold standard), a pre- and postvaccination sample pair that would enable the assessment of fold rises, or a post-polysaccharide booster result that can be compared with the primary response in age-matched controls.
We have shown that evidence of protection, as defined by a fourfold rise in rSBA titer with a characteristic booster response, may be present despite an hSBA titer of <4. We conclude, therefore, that while an hSBA titer of ≥4 indicates protection, an hSBA titer of <4 does not always indicate susceptibility. A similar conclusion was reached by Goldschneider et al. (20), who stated, “The fact that base line sera from cases of meningitis have bactericidal titers of less than 1:4 does not mean that all people with titers below this level are susceptible to infection, although many obviously are.”
Fourfold rises in titer from pre- to postvaccination are widely used as measure of a significant response to vaccination, including the meningococcal polysaccharide vaccine (37). The World Health Organization specified the requirements for the meningococcal polysaccharide vaccine, advocating the use of 3- to 4-week-old baby rabbit serum as an exogenous complement source, and stated that “The antibody titers of the sera from at least 90% of the subjects should show a fourfold or greater rise after immunization.” It is therefore already accepted that fourfold rises in serum bactericidal titers are indicative of the development of a protective response to unconjugated meningococcal vaccines, and extension of this notion to recipients of MCC would seem appropriate. Using this criterion, we showed that 98% of individuals with post-MCC titers of 8 to 64 should be protected against MenC disease.
Another indicator of long-term protection that is proposed is the SBA response to a polysaccharide booster. For toddlers with a postprimary SBA titer of <128, 90% had a postbooster titer of ≥128, compared with only 8% of age-matched naïve controls given a full 50-μg dose of C polysaccharide (8). This difference in response confirms the induction of immunological memory in the MCC vaccines despite postprimary SBA antibody levels in the equivocal range. Response to a plain polysaccharide boost has also been used to demonstrate immunological memory following Haemophilus influenzae type b (Hib) conjugate vaccination, even in the absence of a detectable primary response to the Hib vaccine (14, 19), and it is memory which is thought to contribute to the persistence of protection against Hib in United Kingdom children vaccinated only during infancy (6, 17).
There is now a growing consensus that evidence of successful induction of immunological memory by conjugate vaccines can be obtained by documenting avidity maturation in the months after primary vaccination with a further increase following a booster dose of plain polysaccharide antigen (1, 14, 18). By examining avidity maturation following a 10-μg booster dose of meningococcal polysaccharide, we have demonstrated that vaccinated individuals with SBA titers above and below 128 display similar avidity maturation in their antibody response and by this criterion should therefore have immunological memory established. Data now exist which demonstrate the increase in avidity over time following priming of infants or toddlers with Hib (18, 30), Streptococcus pneumoniae (3), and Neisseria meningitidis serogroup C conjugate vaccines (31). Such data have been generated in the context of boosters of low doses of plain polysaccharide or conjugate vaccine in young infants and are now accepted as indicative that a T-cell-dependent response has been generated.
Concerns have been raised about the relative protection afforded by immunological memory to the different organisms for which conjugate vaccines are available. SBA titers fall dramatically in young children after the primary series, with 25 of 53 (47%) infants with a three-dose schedule (34) and 16 of 65 (25%) toddlers with a one-dose schedule (31) of the WLVP vaccine having SBA titers of <8 6 to 10 months later. This suggests that, as with Hib conjugate vaccines (14), reliance on immunological memory rather than maintenance of SBA titers above a protective threshold will be necessary if MCC vaccines are to protect over the long term. In general, the pathogenesis of the infections caused by N. meningitidis involves invasion following nasopharyngeal colonization (9). Observations during epidemics (13, 20) and in infected laboratory workers (9) indicate that this period is usually short, though a recent case report demonstrated a 7-week interval (28). This suggests that local immunity, induced by carriage, is able to prevent invasion, but in naïve subjects, recent acquisition is associated with invasion because of the delay associated with the mounting of an effective primary response against the relevant strain. The length of this delay is unknown, but is likely to be different in naïve from that in subjects primed by MCC vaccine. For example, with Hib conjugate vaccines, a detectable antibody response post-polysaccharide boosting is present after 4 to 5 days (29), while responses following a primary series take up to 7 days (25). Pneumococcal conjugate vaccines (27), and even MenC polysaccharide vaccines (4, 12), reduce nasopharyngeal carriage of strains contained in the vaccine and therefore clearly provide a degree of mucosal immunity; this is further reinforced by recent pneumococcal conjugate vaccine efficacy data from the United States (5).
Until more data are available it is difficult to correlate the seriousness or speed of an invasive infection with the relative protection afforded by memory. Furthermore, it is likely that some degree of protection at the early stage of infection is mediated by factors other than antibody (such as complement). In addition, data on the seriousness of invasive infection have been correlated with host factors such as cytokine promoter polymorphisms, which may ultimately be more relevant to the outcome of infection. In the context of our current understanding of memory and protection, the theoretical concerns raised above do not justify unnecessarily stringent criteria for licensing and use of the current MCC vaccines. The United Kingdom postlicensure surveillance program will validate these immunological criteria against measures of clinical effectiveness over the coming years.
ACKNOWLEDGMENTS
We thank Peter Richmond for his enthusiasm and hard work in planning and coordinating many of the MCC studies. We also thank all the staff at the Manchester PHLS Vaccine Evaluation Laboratory, past and present, who have generated SBA data from the United Kingdom MCC studies, namely, Sarah Martin, Francesca Sadler, Jamie Findlow, Marisa Acuna, Emma Longworth, Nick Peake, Rachael O'Connor, Rukhsana Rahim, and Helen Joseph; Maggie Vickers at the Institute of Child Health, London, United Kingdom, for generating avidity index data; and Joanne Southern, Pauline Kaye, and Joan Vurdien at the PHLS Communicable Disease Surveillance Center for assistance with data reports, entry, and analysis. We acknowledge the assistance of Moya Burrage and Carol Thornton (Centre for Applied Microbiology and Research, Salisbury, United Kingdom) in sample processing and assay standardization and Janet Suker and Ian Feavers (National Institute for Biological Standards and Control, Potters Bar, Hertfordshire, United Kingdom) for provision of MenC polysaccharide.
REFERENCES
- 1.Ahman H, Kayhty H, Lehtonen O, Leroy J, Froeschle E, Eskola J. Streptococcus pneumoniae capsular polysaccharide-diphtheria toxoid conjugate vaccine is immunogenic in early infancy and able to induce immunologic memory. Pediatr Infect Dis J. 1998;17:211–216. doi: 10.1097/00006454-199803000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Anderson E L, Bowers T, Mink C M, Kennedy D J, Belshe R B, Harakeh H, Pais L, Holder P, Carlone G M. Safety and immunogenicity of meningococcal A and C polysaccharide conjugate vaccine in adults. Infect Immun. 1994;62:3391–3395. doi: 10.1128/iai.62.8.3391-3395.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anttila M, Eskola J, Ahman H, Kayhty H. Avidity of IgG for Streptococcus pneumoniae type 6B and 23F polysaccharides in infants primed with pneumococcal conjugates and boosted with polysaccharide or conjugate vaccines. J Infect Dis. 1998;177:1614–1621. doi: 10.1086/515298. [DOI] [PubMed] [Google Scholar]
- 4.Artenstein M S, Gold R, Zimmerly J G, Wyle F A, Schneider H, Harkins C. Prevention of meningococcal disease by group C polysaccharide vaccine. N Engl J Med. 1970;282:417–420. doi: 10.1056/NEJM197002192820803. [DOI] [PubMed] [Google Scholar]
- 5.Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen J R, Elvin L, Ensor K M, Hackell J, Siber G, Malinoski F, Madore D, Chang I, Kohb R, Watson W, Austrian R, Edwards K. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr Infect Dis J. 2000;19:187–195. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Booy R, Hodgson S, Carpenter L, Mayon-White R T, Slack M P, Macfar J A, Haworth E A, Kiddle M, Shribman S, Roberts J S, Moxon E R. Efficacy of Haemophilus influenzae type b conjugate vaccine PRP-T. Lancet. 1994;344:362–366. doi: 10.1016/s0140-6736(94)91400-1. [DOI] [PubMed] [Google Scholar]
- 7.Borrow R, Fox A J, Richmond P C, Clark S, Sadler F, Findlow J, Morris R, Begg N T, Cartwright K A V. Induction of immunological memory in UK infants by a meningococcal A/C conjugate vaccine. Epidemiol Infect. 2000;124:427–432. doi: 10.1017/s0950268899003787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borrow R, Richmond P, Kaczmarski E B, Iverson A, Martin S L, Findlow J, Acuna M, Longworth E, O'Connor R, Paul J, Miller E. Meningococcal serogroup C-specific IgG antibody responses and serum bactericidal titers in children following vaccination with a meningococcal A/C polysaccharide vaccine. FEMS Immunol Med Microbiol. 2000;28:79–85. doi: 10.1111/j.1574-695X.2000.tb01460.x. [DOI] [PubMed] [Google Scholar]
- 9.Brandtzaeg P. Pathogenesis of meningococcal infections. In: Cartwright K, editor. Meningococcal disease. Chichester, United Kingdom: John Wiley and Sons Ltd.; 1995. pp. 71–114. [Google Scholar]
- 10.Campagne G, Garba A, Fabre P, Schuchat A, Ryall R, Boulanger D, Bybel M, Carlone G, Briantais P, Ivanoff B, Xerri B, Chippaux J-P. Safety and immunogenicity of three doses of a Neisseria meningitidis A + C diphtheria conjugate vaccine in infants from Niger. Pediatr Infect Dis J. 2000;19:144–150. doi: 10.1097/00006454-200002000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Costantino P, Viti S, Podda A, Velmonte M A, Nencioni L, Rappuoli R. Development and phase 1 clinical testing of a conjugate vaccine against meningococci A and C. Vaccine. 1992;10:691–698. doi: 10.1016/0264-410x(92)90091-w. [DOI] [PubMed] [Google Scholar]
- 12.Devine L F, Pierce W E, Floyd T M, Rhode S L, Edwards E A, Siess E E, Peckinpaugh R O. Evaluation of group C meningococcal polysaccharide vaccine in marine recruits, San Diego, California. Am J Epidemiol. 1970;92:25–32. doi: 10.1093/oxfordjournals.aje.a121176. [DOI] [PubMed] [Google Scholar]
- 13.Edwards E A, Devine L F, Sengbusch C H, Ward H W. Immunological investigations of meningococcal disease. III. Brevity of group C acquisition prior to disease occurrence. Scand J Infect Dis. 1977;9:105–110. doi: 10.3109/inf.1977.9.issue-2.09. [DOI] [PubMed] [Google Scholar]
- 14.Eskola J, Ward J, Dagan R, Goldblatt D, Zepp F, Siegriest C A. Combined vaccination of Haemophilus influenzae type b conjugate and diphtheria-tetanus-pertussis containing acellular pertussis. Lancet. 1999;354:2063–2068. doi: 10.1016/S0140-6736(99)04377-9. [DOI] [PubMed] [Google Scholar]
- 15.Fairley C K, Begg N, Borrow R, Fox A J, Jones D M, Cartwright K A V. Reactogenicity and immunogenicity of conjugate meningococcal serogroup A and C vaccine in UK infants. J Infect Dis. 1996;174:1360–1363. doi: 10.1093/infdis/174.6.1360. [DOI] [PubMed] [Google Scholar]
- 16.Gold R, Artenstein M S. Meningococcal infections. 2. Field trial of group C meningococcal polysaccharide vaccine in 1969-70. Bull W H O. 1971;45:279–282. [PMC free article] [PubMed] [Google Scholar]
- 17.Goldblatt D, Miller E, McCloskey N, Cartwright K. Immunological response to conjugate vaccines in infants: follow up study. Br Med J. 1998;316:1570–1571. doi: 10.1136/bmj.316.7144.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldblatt D, Pinto Vas R, Miller E. Antibody avidity as a surrogate marker of successful priming by Haemophilus influenzae type b conjugate vaccine following infant immunization. J Infect Dis. 1998;177:1112–1115. doi: 10.1086/517407. [DOI] [PubMed] [Google Scholar]
- 19.Goldblatt D, Richmond P, Millard E, Thornton C, Miller E. The induction of immunologic memory after vaccination with Haemophilus influenzae type b conjugate and acellular pertussis-containing diphtheria, tetanus, and pertussis vaccine combination. J Infect Dis. 1999;180:538–541. doi: 10.1086/314901. [DOI] [PubMed] [Google Scholar]
- 20.Goldschneider I, Gotschlich E C, Artenstein M S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffiss J M, Goroff D K. IgA blocks IgM and IgG-initiated immune lysis by separate molecular mechanisms by separate molecular mechanisms. J Immunol. 1983;130:2882–2885. [PubMed] [Google Scholar]
- 22.Jones G R, Williams J N, Christodoulides M, Jolley K, Heckels J E. Lack of immunity in university students before an outbreak of serogroup C meningococcal infection. J Infect Dis. 2000;181:1172–1175. doi: 10.1086/315352. [DOI] [PubMed] [Google Scholar]
- 23.Leach A, Twumasi P A, Kumah S, Banya W S, Jaffar S, Forrest B D, Granoff D M, LiButti D E, Carlone G M, Pais L B, Broome C V, Greenwood B M. Induction of immunologic memory in Gambian children by vaccination in infancy with a group A plus group C meningococcal polysaccharide-protein conjugate vaccine. J Infect Dis. 1997;175:200–204. doi: 10.1093/infdis/175.1.200. [DOI] [PubMed] [Google Scholar]
- 24.Macdonald N E, Halperin S A, Law B J, Forrest B, Danzig L E, Granoff D M. Induction of immunologic memory by conjugated vs plain meningococcal C polysaccharide vaccine in toddlers. JAMA. 1998;280:1685–1689. doi: 10.1001/jama.280.19.1685. [DOI] [PubMed] [Google Scholar]
- 25.Madore D V, Johnson-Kraines C L, Rothstein E P, Smith D H. Kinetics of antibody response to Haemophilus influenzae type b vaccines. Pennridge Pediatrics Associates. Curr Med Res Opin. 1999;15:105–112. doi: 10.1185/03007999909113370. [DOI] [PubMed] [Google Scholar]
- 26.Maslanka S E, Gheesling L L, LiButti D E, Donaldson K B J, Harakeh H S, Dykes J K, Arhin F F, Devi S J N, Frasch C E, Huang J C, Kris-Kuzemenska P, Lemmon R D, Lorange M, Peeters C C A M, Quataert S, Tai J Y, Carlone G M. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin Diagn Lab Immunol. 1997;4:156–167. doi: 10.1128/cdli.4.2.156-167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mbelle N, Huebner R E, Wasas A D, Kimura A, Chang I, Klugman K P. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J Infect Dis. 1999;180:1171–1176. doi: 10.1086/315009. [DOI] [PubMed] [Google Scholar]
- 28.Neal K R, Nguyen-van-Tam J S, Slack R C, Kaczmarski E B, White A, Ala'Aldeen D A. Seven-week interval between acquisition of a meningococcus and the onset of invasive disease. A case report. Epidemiol Infect. 1999;123:507–509. doi: 10.1017/s0950268899003192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pichichero M E, Voloshen T, Passador S. Kinetics of booster responses to Haemophilus influenzae type b conjugate after combined diphtheria-tetanus-acellular pertussis Haemophilus influenzae type b vaccination in infants. Pediatr Infect Dis J. 1999;18:1106–1108. doi: 10.1097/00006454-199912000-00019. [DOI] [PubMed] [Google Scholar]
- 30.Pichichero M E, Voloshen T, Zajac D, Passador S. Avidity maturation of antibody to Haemophilus influenzae type b (Hib) after immunization with diphtheria-tetanus-acellular pertussis-hib-hepatatis B combined vaccine in infants. J Infect Dis. 1999;180:1390–1393. doi: 10.1086/314989. [DOI] [PubMed] [Google Scholar]
- 31.Richmond P, Borrow R, Goldblatt D, Findlow J, Martin S, Morris R, Cartwright K, Miller E. Ability of three different meningococcal C conjugate vaccines to induce immunological memory after a single dose in UK toddlers. J Infect Dis. 2001;183:160–163. doi: 10.1086/317646. [DOI] [PubMed] [Google Scholar]
- 32.Richmond P, Goldblatt D, Fusco P C, Fusco J D S, Heron I, Clark S, Borrow R, Michon F. Safety and immunogenicity of a new Neisseria meningitidis serogroup C-tetanus toxoid conjugate vaccine in healthy adults. Vaccine. 1999;18:641–646. doi: 10.1016/s0264-410x(99)00276-5. [DOI] [PubMed] [Google Scholar]
- 33.Richmond P, Kaczmarski E, Borrow R, Findlow J, Clark S, McCann R, Hill J, Barker M, Miller E. Meningococcal C polysaccharide vaccine induces immunologic hyporesponsiveness in adults that is overcome by meningococcal C conjugate vaccine. J Infect Dis. 2000;181:761–764. doi: 10.1086/315284. [DOI] [PubMed] [Google Scholar]
- 34.Richmond P C, Miller E, Borrow R, Clark S, Sadler F, Fox A J, Begg N T, Morris R, Cartwright K A V. Meningococcal serogroup C conjugate vaccine is immunogenic in infancy and primes for memory. J Infect Dis. 1999;179:1569–1572. doi: 10.1086/314753. [DOI] [PubMed] [Google Scholar]
- 35.Taunay A E, Galvao P A, de Morais J S, Gotschlich E C, Feldman R A. Disease prevention by meningococcal serogroup C polysaccharide vaccine in pre-school children: results after eleven months in Sao Paulo, Brazil. Abstr Pediatr Res. 1974;8:429. [Google Scholar]
- 36.Twumasi P A, Kumah S, Leach A, O'Dempsey T J, Ceesay S J, Todd J, Broome C V, Carlone G M, Pais L B, Holder P K, Plikaytis B D, Greenwood B M. A trial of group A and group C meningococcal polysaccharide-protein conjugate vaccine in African infants. J Infect Dis. 1995;171:632–638. doi: 10.1093/infdis/171.3.632. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization. Requirements for meningococcal polysaccharide vaccine. World Health Organization technical report series, no. 594. Geneva, Switzerland: World Health Organization; 1976. [Google Scholar]