Abstract
Purpose
This study aimed to investigate the incidence rate and risk factors for hepatic encephalopathy (HE) among unresectable hepatocellular carcinoma (uHCC) patients with liver cirrhosis who received sorafenib or lenvatinib treatment.
Patients and Methods
uHCC patients with cirrhosis who received first-line sorafenib or lenvatinib treatment between September 2014 and February 2021 were continually reviewed in our single-center retrospective study. The Hepatic Encephalopathy Scoring Algorithm was used to evaluate the occurrence and grade of HE during treatment, and logistic regression models were used to further explore the risk factors for HE.
Results
A total of 454 eligible patients were enrolled in our study, with 214 and 240 patients in the sorafenib and lenvatinib groups, respectively. At time of data cut-off (2021–12), the incidence of HE in sorafenib group (4.2%, 95% CI:2–7%) was significantly lower than that in lenvatinib group (11.3%,95% CI:7–15%) (p = 0.006), with alcoholic cirrhosis [OR: 5.857 (95% CI: 1.519–22.591)], Child-Pugh >7 [OR: 3.023 (95% CI: 1.135–8.053)], blood ammonia ≥38.65 μmol/L [OR: 4.693 (95% CI: 1.782–12.358)], total bile acid ≥29.5 μmol/L [OR: 11.047 (95% CI: 4.414–27.650)] and duration of treatment ≥5.6 months [OR: 4.350 (95% CI: 1.701–11.126)] to be risk factors for the occurrence of HE during first-line systemic therapy.
Conclusion
In our study, for off-label uHCC patients (Child-Pugh >7) with alcoholic cirrhosis, hyperammonemia, hypercholesterolemia, and estimated longer duration of treatment, the application of lenvatinib has to be cautious, which needs to be confirmed in future clinical trials.
Keywords: hepatic encephalopathy, hepatocellular carcinoma, liver cirrhosis, sorafenib, lenvatinib
Introduction
Hepatocellular carcinoma (HCC) is a malignant tumor with a high mortality rate, especially for unresectable HCC (uHCC).1 Most uHCC patients have a basis of liver cirrhosis. Liver cirrhosis not only promotes the occurrence of liver cancer, but its complications also affect the prognosis of patients with liver cancer.2–4 Hepatic encephalopathy (HE) is a serious complication of portal hypertension secondary to cirrhosis5 that can interfere with antitumor therapy and significantly affect patient prognosis.
Sorafenib and lenvatinib, two traditional tyrosine kinase inhibitors (TKIs) that have shown great anti-tumor activity in uHCC patients, were approved by the US Food and Drug Administration as first-line systemic therapy for uHCC.6 However, safety remains a primary concern. Choice of systemic treatment regimen is still limited for uHCC patients with liver function of Child-Pugh B, for these patients are often outside recommendation of systemic therapies.7 As far, Alessandro Granito and Luigi Bolondi have reviewed safety and efficacy of sorafenib in this group of patients.8 As for lenvatinib, Ogushi et al observed a similar spectrum and similar incidence of adverse events (except for proteinuria) compared with patients with Child-Pugh A.9 But real-world safety data in HBV-dominate patients with Child-Pugh B cirrhosis after lenvatinib treatment was still lacking.
Although previous studies have demonstrated a possible relationship between TKIs and portal hemodynamics in animal models or in explorative small-sample clinical trials,3 whether there was a difference in the incidence of severe complications of portal hypertension (in other words, HE) after systemic therapy with sorafenib or lenvatinib and the risk factors predicting the development of HE during first-line TKIs treatment are unclear.
Patients and Methods
Study Design & Patient Population
Systemic therapy-naïve uHCC patients with confirmed cirrhosis who received first-line sorafenib or lenvatinib treatment were continuously reviewed from the Fifth Medical Centre of the General Hospital of the People’s Liberation Army between September 2014 and February 2021. A standard dose of sorafenib or lenvatinib was recommended to patients. If not tolerable, a tolerable dose was allowed. It was recommended that patients be admitted to the hospital for review every 6 to 8 weeks.
We excluded patients who: [1] suffered from recurrent or persistent HE before treatment; [2] had gastrointestinal bleeding or underwent transjugular intrahepatic portosystemic shunt before treatment; [3] suffered from end-stage HCC (defined as BCLC stage D); and [4] had no effective follow-up.
Data Collection & Outcome
Demographic data, physical examination results, liver function, carcinoma characteristics, treatment history, and serum laboratory examinations were recorded at baseline. Physical and pathological conditions of liver cirrhosis were estimated using the end-stage liver disease (MELD) model.10 Tumor response and clinical decisions were evaluated or made in accordance with the Response Evaluation Criteria in Solid Tumors, version 1.1. The duration of treatment (DOT) was defined as the time from initiation of treatment to the end of treatment or the end of study, whichever came first. Serum total bile acid (TBA) and ammonia levels were repeatedly recorded 4–12 weeks after the initiation of sorafenib or lenvatinib treatment, if available.
Hepatic Encephalopathy Scoring Algorithm (HESA), refined according to West Haven Criteria, has proven to be more sensitive in clinical practice.11,12 In our study, HE diagnosis was dependent on the judgement of neurological or mental symptoms according to the HESA. The consequences of HE, including therapeutic procedures, therapeutic effects, and continuation of sorafenib or lenvatinib treatment, were recorded.
Statistical Analyses
The incidence of sorafenib-related HE has been reported to be nearly 5.2% in HCC patients.13 For lenvatinib, we selected the results of a Japanese cohort study (incidence rate of HE,13%) that most closely matched the characteristics of patients in our center as a reference.14 Therefore, 454 samples provided a power of 84% to detect statistical differences in HE morbidity between the sorafenib and lenvatinib groups.
All continuous variables were expressed as mean with standard deviation and all categorical variables were summarized using numbers and percentages. Student’s T test or Mann–Whitney U-test was used for comparisons of continuous variables, and Pearson’s chi-square test or continuity correction or Fisher’s exact test was used for categorical variables. The Kaplan–Meier method with Log rank testing was used to estimate the DOT between the two cohorts.
For these continuous variables (Child-Pugh, TBA, ammonia, and DOT), receiver operating characteristic (ROC) curves and Youden index were used to determine the optimal cut-off value for predicting HE.15 Risk factors for developing HE were described as odd ratios (OR) with a 95% confidence interval (CI) using a logistic regression model. The variables showing P < 0.1 in univariate logistic regression were incorporated into multivariate logistic regression model using the “Backward: Wald” method. Log-transformation and paired-samples T test were performed for repeated-measured variances.
All statistical tests were two-sided, and statistical significance was set at P < 0.05. All data calculations were performed using SPSS Medical Pack for Windows (version 25.0; SPSS Inc., Chicago, IL, USA). Forest plots and boxplots were constructed using R language version 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria) with the help of ggplot2 and the viridis package to visually display the outcome.16,17
Results
Characteristics and Outcomes
As of February 2021, 214 eligible patients were enrolled in the sorafenib group, while 240 eligible patients were enrolled in the lenvatinib group. No statistical difference was found at baseline (Table 1). At the time of data cut-off (2021–12), the median DOT in the sorafenib and lenvatinib group were 5.3 (95% CI: 4.4–6.2) months and 5.9 (95% CI: 5.0–6.8) months, respectively, without a significant difference (p = 0.26). A total of 194 (90.7%) patients in the sorafenib group and 224 (93.3%) in the lenvatinib group discontinued antitumor treatment. Disease progression was the main reason for the occurrence of adverse events (Table S1). More than half of the patients received regional therapy during the TKIs treatment (Table S1).
Table 1.
Baseline Characteristics of the Study Population
| Variables | Sorafenib Group | Lenvatinib Group | P value |
|---|---|---|---|
| (n = 214) | (n = 240) | ||
| Sex | 0.56 | ||
| Male | 196 (91.6%) | 216 (90.0%) | |
| Female | 18 (8.4%) | 24 (10.0%) | |
| Age (years) | 54 (28–77) | 54 (28–78) | 0.855 |
| Type of Cirrhosis | |||
| HBV-related | 196 (91.6%) | 215 (89.6%) | 0.422 |
| HCV-related | 8 (3.7%) | 17 (7.1%)a | 0.119 |
| Alcoholic | 10 (4.7%) | 12 (5.0%) | 0.871 |
| Maximum size of intrahepatic lesions | 0.757 | ||
| ≤5cm | 64 (29.9%) | 75 (31.3%) | |
| >5cm | 150 (70.1%) | 165 (68.8%) | |
| Macrovascular Invasion | 138 (64.5%) | 149 (62.1%) | 0.596 |
| Extrahepatic Metastasis | 69 (32.2%) | 74 (30.8%) | 0.747 |
| BCLC Stage | 0.728 | ||
| B | 47 (22.0%) | 56 (23.3%) | |
| C | 167 (78.0%) | 184 (76.7%) | |
| AFP level (ng/mL) | 0.768 | ||
| ≥400 | 93 (43.5%) | 101 (42.1%) | |
| <400 | 121 (56.5%) | 139 (57.9%) | |
| Child-Pugh | 0.17 | ||
| A | 152 (71.0%) | 156 (65.0%) | |
| B | 62 (29.0%) | 84 (35.0%) | |
| MELD Grade | 1 | ||
| <10 | 150 (70.1%) | 167 (69.6%) | |
| [10,19] | 64 (29.9%) | 72 (30.0%) | |
| >19 | 0 (0) | 1 (0.4%) | |
| Ammonia (μmol/L) | 39.8 ± 17.2 | 38.0 ± 17.3 | 0.193 |
| Total Bile Acid (μmol/L) | 19.8 ± 28.3 | 17.1 ± 23.5 | 0.126 |
| Previous Regional Therapyb | 199 (93.0%) | 227 (94.6%) | 0.481 |
Notes: aHBV/HCV Coinfection happened among 4 patients. bPrevious regional therapy included surgical resection, ablation, transcatheter arterial chemoembolization, transcatheter arterial embolization and/or hepatic arterial infusion chemotherapy.
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; BCLC: Barcelona Clinic Liver Cancer; AFP: α-fetoprotein; MELD: Model for End-Stage Liver Disease; DOT: duration of TKIs treatment; CI: confidence interval.
In the sorafenib group, nine patients developed HE: three in grade 2, five in grade 3, and one in grade 4. In the lenvatinib group, 27 of 240 patients had HE: one in grade 1, 14 in grade 2, four in grade 3, seven in grade 4, and 1 without grading. The incidence of HE in the sorafenib group (4.2%, 95% CI:2–7%) was significantly lower than that in the lenvatinib group (11.3%, 95% CI:7–15%) (P = 0.006) (Table 2). The principle of dealing with HE in the two cohorts was similar. This mainly included reducing ammonia absorption using lactulose, protein restriction, accelerating ammonia elimination by using l-ornithine–l-aspartate, and altering gut microbiota by rifaximin. Among the nine patients who developed HE in the sorafenib group, eight (3.7%) were cured and the last one (0.5%) died. Five (2.3%) patients stopped sorafenib treatment because of HE. In the lenvatinib group, 20 of the 27 (8.3%) HE patients were cured, and the remaining seven (2.9%) died. Eighteen (7.5%) HE patients discontinued lenvatinib treatment (Table 2).
Table 2.
Occurrence of Hepatic Encephalopathy
| Variables | Sorafenib Group | Lenvatinib Group | P-value |
|---|---|---|---|
| (n = 214, %) | (n = 240, %) | ||
| Cases of HE | 9 (4.2%) | 27 (11.3%) | 0.006 |
| Grade of HE | |||
| 1 | 0 (0%) | 1 (0.4%) | – |
| 2 | 3 (1.4%) | 14 (5.8%) | – |
| 3 | 5 (2.3%) | 4 (1.7%) | – |
| 4 | 1 (0.5%) | 7 (2.9%) | – |
| Unknown | 0 (0%) | 1 (0.4%) | – |
| Therapeutic Effect of HE | |||
| Cured | 8 (3.7%) | 20 (8.3%) | – |
| Void | 1 (0.5%) | 7 (2.9%) | – |
| Discontinuation of First-line Sorafenib or Lenvatinib Treatment | 5 (2.3%) | 18 (7.5%) | – |
Note: –, not applicable.
Abbreviation: HE, hepatic encephalopathy.
Possible Risk Factors for HE
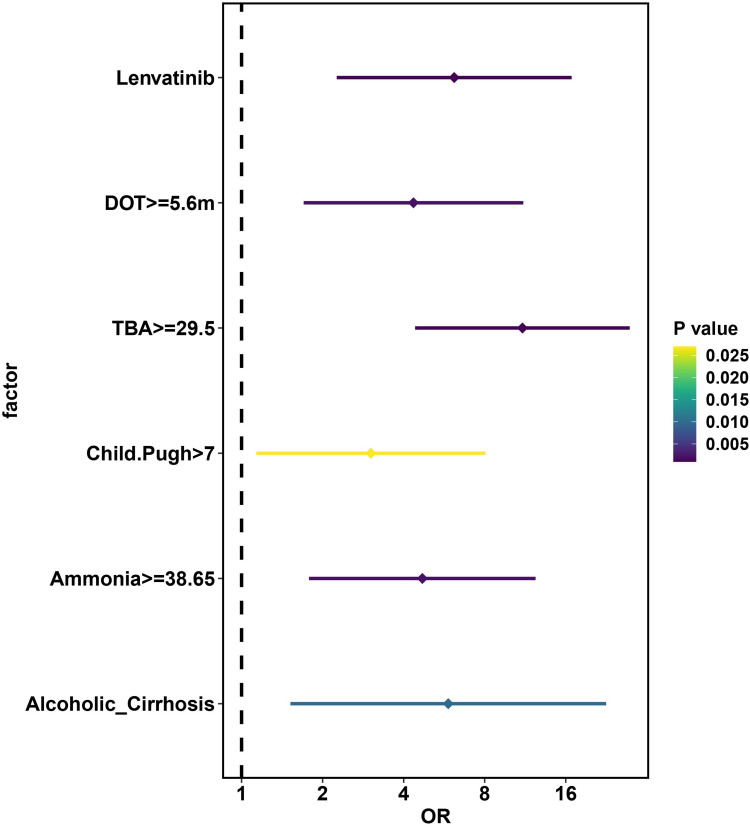
The results of ROC analysis suggested that the optimal cut-off values for ammonia, TBA, Child-Pugh, and DOT were 38.65 μmol/L, 29.5 μmol/L, 7.5 and 5.6 months, respectively, with satisfactory sensitivity and specificity (Table S2 and Figure S1). After multivariate bivariate logistic regression analysis, alcoholic cirrhosis [OR (95% CI): 5.857 (1.519–22.591)] (P = 0.010), Child-Pugh >7 [OR (95% CI): 3.023 (1.135–8.055)] (P = 0.027), blood ammonia ≥38.65 μmol/L [OR (95% CI): 4.693 (1.782–12.358)] (P = 0.002), TBA ≥29.5μmol/L [OR (95% CI): 11.047 (4.414–27.650)] (P < 0.001), LEN treatment [OR (95% CI): 6.162 (2.258–16.818)] (P < 0.001) and DOT ≥5.6 months [OR (95% CI): 4.350 (1.701–11.126)] (P = 0.002) remained significantly correlated with HE development during TKIs treatment (Table 3 and Figure 1).
Table 3.
Risk Factors for Hepatic Encephalopathy
| Univariate Logistic Regression Analysis | Multivariate Logistic Regression Analysis | |||
|---|---|---|---|---|
| Variables | OR (95% CI) | P-value | OR (95% CI) | P-value |
| Sex | 0.117 | |||
| Male | 1 (ref) | |||
| Female | 2.122 (0.828–5.437) | |||
| Age (years) | 0.882 | |||
| <60 | 1 (ref) | |||
| ≥60 | 0.944 (0.441–2.017) | |||
| HBV-related Cirrhosis | 0.039 | |||
| NO | 1 (ref) | |||
| YES | 0.390 (0.160–0.954) | |||
| HCV-related Cirrhosis | 0.465 | |||
| NO | 1 (ref) | |||
| YES | 0.469 (0.062–3.571) | |||
| Alcoholic Cirrhosis | 0.002 | 0.01 | ||
| NO | 1 (ref) | 1 (ref) | ||
| YES | 5.025 (1.832–13.782) | 5.857 (1.519–22.591) | ||
| Maximum size of Intrahepatic Lesions > 5cm | 0.457 | |||
| NO | 1 (ref) | |||
| YES | 0.763 (0.375–1.555) | |||
| Macrovascular Invasion | 0.785 | |||
| NO | 1 (ref) | |||
| YES | 0.907 (0.451–1.825) | |||
| Extrahepatic Metastasis | 0.535 | |||
| NO | 1 (ref) | |||
| YES | 1.252 (0.615–2.549) | |||
| BCLC Stage | 0.945 | |||
| B | 1 (ref) | |||
| C | 1.029 (0.454–2.333) | |||
| AFP level (ng/mL) | 0.238 | |||
| <400 | 1 (ref) | |||
| ≥400 | 0.648 (0.316–1.331) | |||
| Child-Pugh | <0.001 | 0.027 | ||
| ≤7 | 1 (ref) | 1 (ref) | ||
| >7 | 7.944 (3.703–17.046) | 3.023 (1.135–8.053) | ||
| MELD grade | 0.003 | |||
| <10 | 1 (ref) | |||
| ≥10 | 2.841 (1.428–5.654) | |||
| Ammonia (μmol/L) | <0.001 | 0.002 | ||
| <38.65 | 1 (ref) | 1 (ref) | ||
| ≥38.65 | 5.644 (2.510–12.688) | 4.693 (1.782–12.358) | ||
| Total bile acid (μmol/L) | <0.001 | <0.001 | ||
| <29.5 | 1 (ref) | 1 (ref) | ||
| ≥29.5 | 11.270 (5.361–23.691) | 11.047 (4.414–27.650) | ||
| Previous regional therapy | 0.208 | |||
| NO | 1 (ref) | |||
| YES | 0.487 (0.159–1.491) | |||
| TKI treatment | 0.008 | <0.001 | ||
| Sorafenib | 1 (ref) | 1 (ref) | ||
| Lenvatinib | 2.887 (1.326–6.288) | 6.162 (2.258–16.818) | ||
| Combination of regional therapy | 0.559 | |||
| NO | 1 (ref) | |||
| YES | 1.246 (0.596–2.603) | |||
| Duration of treatment (months) | <0.001 | 0.002 | ||
| <5.6 | 1 (ref) | 1 (ref) | ||
| ≥5.6 | 4.241 (1.888–9.523) | 4.350 (1.701–11.126) | ||
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; BCLC: Barcelona Clinic Liver Cancer; AFP: α-fetoprotein; MELD: Model for End-Stage Liver Disease; TBA: total bile acid; TKIs: tyrosine kinase inhibitors; DOT: duration of treatment; OR: odds ratio; CI: confidence interval.
Figure 1.
Forest Plot Showing Multivariate Logistic Regression in Exploring Risk Factors of Hepatic Encephalopathy.
Abbreviations: TBA, Total Bile Acid; LEN, lenvatinib; DOT, duration of treatment; OR, odds ratio.
Change of TBA and Ammonia After Treatment
TBA and ammonia were repeatedly measured in 402 patients (88.5%; 191 in the sorafenib arm and 211 in the lenvatinib arm). Paired-samples T test suggested that after 4–12 weeks of treatment, TBA significantly decreased in the sorafenib group (log2(TBA): 3.5 ± 1.6 vs 3.1 ±1.8, p < 0.001), but significantly increased in the lenvatinib group (log2(TBA): 3.3 ± 1.5 vs 3.7 ± 1.7, p < 0.001). While ammonia remained stable in sorafenib group after 4–12 weeks (log2(Ammonia): 5.2 ± 0.6 vs 5.2 ± 0.8, p = 0.358), but significantly increased in lenvatinib group (log2(Ammonia): 5.1 ± 0.7 vs 5.4 ± 0.9, p < 0.001) (Table S3 and Figure S2).
Discussion
HE is a disturbing complication of portal hypertension secondary to liver cirrhosis, which may disturb first-line sorafenib or lenvatinib therapy. Although previous studies have demonstrated a possible relationship between TKIs and portal hemodynamics in animal models or in explorative clinical trials with very limited samples,3 whether there was a difference in the incidence of severe complications of portal hypertension (HE) after first-line systemic therapy with sorafenib or lenvatinib and the risk factors predicting the development of HE during TKIs treatment in the real world remain unclear. In our study, we found that the incidence of HE was significantly higher in the lenvatinib group (11.3%) than that in the sorafenib group (4.2%) in uHCC patients. Moreover, further logistic regression model suggested that alcoholic cirrhosis, Child-Pugh >7, blood ammonia ≥38.65 μmol/L, TBA ≥29.5 μmol/L and DOT ≥5.6 months predicted high risk of HE. The current study may be the first and largest cohort to prove the correlation between TKIs and HE in real-world clinical practice.
The mechanism of ammonia or alcohol in the development of HE or mental disorders has been fully discussed in previous studies.18–20 Baseline liver function characteristics recommended by the FDA for sorafenib are Child-Pugh B7 and Child-Pugh A only for lenvatinib.7 However, considering patients’ intention to treat, the application of TKIs in off-label patients is common in real-world clinical practice. For off-label patients with poor liver function, receiving TKIs often has a poor survival benefit.9,21,22 Ogushi et al found that for patients with Child-Pugh B liver function, the incidence of gastrointestinal or hepatobiliary adverse events, including HE, was higher.9 Our regression model showed that compared with on-label patients, the incidence of HE in off-label patients with Child-Pugh >7 was nearly triple, which supports Ogushi’s result.9 Considering the relatively high risk of HE reported in our study, the direct application of TKIs, especially lenvatinib, to off-label patients must be cautious.
In our study, median DOT in sorafenib group (5.3 months) was higher than that in sorafenib arm of RELFECT clinical trial (3.7 months)23 and Chiu et al’s cohort (range from 2.0 to 2.7 months).13 Although the baseline liver function was more complicated, the median DOT in the lenvatinib group (5.9 months) was similar to that in the lenvatinib arm of the REFLECT (5.7 months).23 A relatively high proportion of locoregional therapies may contribute to prolonged DOT. The efficacy and safety of regional therapies combined with sorafenib or lenvatinib have been reported in previous trials.24–26 However, Scheiner et al observed a significant increase in portal pressure after repeated transcatheter arterial chemoembolization (median: 10 vs 16 mmHg) in 28 HCC patients.27 In our cohort, more than half of uHCC patients (59.3% in the sorafenib group and 70.0% in the lenvatinib group) received locoregional therapies during TKIs, resulting in a similar median DOT between the two groups, but neither univariate nor multivariate logistic regression models showed a direct relationship between locoregional therapies and the development of HE, contrary to the results of Scheine et al.27 This further demonstrates the safety of the combination of locoregional therapies in uHCC patients with cirrhosis who received first-line sorafenib or lenvatinib therapy, which needs to be confirmed in the future.
However, prolonged DOT may have led to the occurrence of HE in our study. The effect of sorafenib or lenvatinib on liver function and portal pressure may be an important reason.28–33 In fact, it was observed in previous studies with extremely limited samples that blood ammonia levels decreased after sorafenib administration and increased after lenvatinib administration with a limited time of observation (usually 2 weeks).33–35 Our study extended the observation time window to 4–12 weeks and obtained similar results. TBA, a widely recognized indicator of drug-induced liver damage,36 has no validation data in predicting liver damage and the occurrence of HE for first-line application of TKIs in uHCC patients with cirrhosis. We included this indicator and found that after initiation of TKI therapy of 4–12 weeks, it did not change significantly in the sorafenib group, but significantly increased in the lenvatinib group, which seems to confirm the potential negative impact of lenvatinib on liver function. Moreover, patients with baseline TBA ≥29.5 were more prone to developing HE, suggesting cautious application of TKIs, especially lenvatinib, with the risk factor mentioned above before treatment. Nakano M et al have observed a relatively lower transition rate to secondary treatment and a relatively higher incidence of adverse events in lenvatinib arm compared with patients treated with sorafenib, which further suggested concerns about safety of lenvatinib in clinical practice.37
Our study had several limitations. Our investigation was a retrospective single-center cohort study, and information and admission biases cannot be easily avoided. At the same time, this study only assessed overt HE. Recessive HE needs to be evaluated and recorded in future prospective cohort studies. Finally, the relationship between tumor progression, the relative dose intensity of TKIs or other drugs used in real-world situations, and HE should be investigated in future studies. However, considering its value in real-world clinical practice, especially in the era of sorafenib- or lenvatinib-based combination therapies with multiple drugs or other medical interventions, it provides important guidance for optimizing the safest choice for uHCC patients with cirrhosis.
Conclusion
In conclusion, our study suggests that for off-label uHCC patients (Child-Pugh >7) with alcoholic cirrhosis, hyperammonemia, hypercholesterolemia, and an estimated longer duration of treatment, the application of lenvatinib has to be cautious, which needs to be confirmed in future clinical trials.
Acknowledgments
We acknowledged Editage and Aaron Ge from University of Maryland at College Park, Maryland, USA in language help. Moreover, we thank all patients included in this research and all doctors or nurses who are always willing to help patients. The abstract of this paper was presented at the 2022 The International Liver Congress (ILC) as a poster presentation with interim findings. The poster’s abstract was published in Journal of Hepatology, Volume 77, Supplement 1, 2022, Page S388, ISSN 0168-8278, DOI: 10.1016/S0168-8278(22)01129-1. The poster was available in https://www.postersessiononline.eu/173580348_eu/congresos/ILC2022/aula/-THU_600_ILC2022.pdf.
Funding Statement
Project title: Study on the techniques for comprehensive early diagnosis of liver cancer with clinical application. Program name: Special program of sustainable development. Grant support: Issued by the Science, Technology and Innovation Commission of Shenzhen Municipality. Grant No. KCXFZ202002011006448.
Abbreviations
AFP, Alpha Fetoprotein; AUC, Area Under the ROC Curve; BCLC, Barcelona Clinic Liver Cancer; CI, Confidence Intervals; HE, Hepatic Encephalopathy; HESA, Hepatic Encephalopathy Scoring Algorithm; MELD, Model for End-stage Liver Disease; ROC, Receiver Operating Characteristic curve; TBA, Total Bile Acid; TKIs, Tyrosine Kinase Inhibitors; uHCC, Unresectable Hepatocellular Carcinoma; WHC, West Haven Criteria.
Data Sharing Statement
All the datasets on which the conclusions of this study rely were displayed in the manuscript.
Ethics Approval and Informed Consent
At time before our study, both sorafenib and lenvatinib were standard first-line systemic therapies in unresectable hepatocellular carcinoma approved by Chinese National Medical Products Administration. This study conformed to the Declaration of Helsinki and was approved by the Ethics Committee of the Chinese Clinical Trial Registry (an officially recognized platform) with approval number: ChiECRCT20210406. Informed consent was not obtained from the study participants as this was a retrospective study. The need for informed consent was waived by the Ethics Committee of the Chinese Clinical Trial Registry as specific patient details are not presented here.
Consent for Publication
All authors gave their consent for publication.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sung H, Ferlay J, Siegel RL., et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5 Suppl 1):S35–S50. doi: 10.1053/j.gastro.2004.09.014 [DOI] [PubMed] [Google Scholar]
- 3.Allaire M, Rudler M, Thabut D. Portal hypertension and hepatocellular carcinoma: des liaisons dangereuses…. Liver Int. 2021;41(8):1734–1743. doi: 10.1111/liv.14977 [DOI] [PubMed] [Google Scholar]
- 4.Meriggi F, Graffeo M. Clinical characterisation and management of the main treatment-induced toxicities in patients with hepatocellular carcinoma and cirrhosis. Cancers. 2021;13(3):584. doi: 10.3390/cancers13030584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Häussinger D, Butz M, Schnitzler A, Görg B. Pathomechanisms in hepatic encephalopathy. Biol Chem. 2021;402(9):1087–1102. doi: 10.1515/hsz-2021-0168 [DOI] [PubMed] [Google Scholar]
- 6.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi: 10.1056/NEJMra1713263 [DOI] [PubMed] [Google Scholar]
- 7.Benson AB, D’Angelica MI, Abbott DE, et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(5):541–565. doi: 10.6004/jnccn.2021.0022 [DOI] [PubMed] [Google Scholar]
- 8.Granito A, Bolondi L. Non-transplant therapies for patients with hepatocellular carcinoma and Child-Pugh-Turcotte class B cirrhosis. Lancet Oncol. 2017;18(2):e101–e12. doi: 10.1016/S1470-2045(16)30569-1 [DOI] [PubMed] [Google Scholar]
- 9.Ogushi K, Chuma M, Uojima H, et al. Safety and efficacy of lenvatinib treatment in child-pugh a and b patients with unresectable hepatocellular carcinoma in clinical practice: a multicenter analysis. Clin Exp Gastroenterol. 2020;13:385–396. doi: 10.2147/CEG.S256691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464–470. doi: 10.1053/jhep.2001.22172 [DOI] [PubMed] [Google Scholar]
- 11.Hassanein TI, Hilsabeck RC, Perry W. Introduction to the Hepatic Encephalopathy Scoring Algorithm (HESA). Dig Dis Sci. 2008;53(2):529–538. doi: 10.1007/s10620-007-9895-0 [DOI] [PubMed] [Google Scholar]
- 12.Hassanein T, Blei AT, Perry W, et al. Performance of the hepatic encephalopathy scoring algorithm in a clinical trial of patients with cirrhosis and severe hepatic encephalopathy. Am J Gastroenterol. 2009;104(6):1392–1400. doi: 10.1038/ajg.2009.160 [DOI] [PubMed] [Google Scholar]
- 13.Chiu J, Tang YF, Yao T-J, et al. The use of single-agent sorafenib in the treatment of advanced hepatocellular carcinoma patients with underlying Child-Pugh B liver cirrhosis: a retrospective analysis of efficacy, safety, and survival benefits. Cancer. 2012;118(21):5293–5301. doi: 10.1002/cncr.27543 [DOI] [PubMed] [Google Scholar]
- 14.Maruta S, Ogasawara S, Ooka Y, et al. Potential of lenvatinib for an expanded indication from the REFLECT trial in patients with advanced hepatocellular carcinoma. Liver Cancer. 2020;9(4):382–396. doi: 10.1159/000507022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47(4):458–472. doi: 10.1002/bimj.200410135 [DOI] [PubMed] [Google Scholar]
- 16.Wilkinson L. ggplot2: elegant graphics for data analysis by WICKHAM, H. Biometrics. 2011;67(2):678–679. doi: 10.1111/j.1541-0420.2011.01616.x [DOI] [Google Scholar]
- 17.Garnier S, Ross N, Rudis R, Camargo AP, Sciaini M, Scherer C. Revision-Colorblind-Friendly Color Maps for R. R Package Version 06. 2021;1:2021. [Google Scholar]
- 18.Weissenborn K. Hepatic encephalopathy: definition, clinical grading and diagnostic principles. Drugs. 2019;79(Suppl1):5–9. doi: 10.1007/s40265-018-1018-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta H, Suk KT, Kim DJ. Gut microbiota at the intersection of alcohol, brain, and the liver. J Clin Med. 2021;10(3):541. doi: 10.3390/jcm10030541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis BC, Bajaj JS. Effects of alcohol on the brain in cirrhosis: beyond hepatic encephalopathy. Alcohol Clin Exp Res. 2018;42(4):660–667. doi: 10.1111/acer.13605 [DOI] [PubMed] [Google Scholar]
- 21.McNamara MG, Slagter AE, Nuttall C, et al. Sorafenib as first-line therapy in patients with advanced Child-Pugh B hepatocellular carcinoma-a meta-analysis. Eur J Cancer. 2018;105:1–9. doi: 10.1016/j.ejca.2018.09.031 [DOI] [PubMed] [Google Scholar]
- 22.Cheon J, Chon HJ, Bang Y, et al. Real-world efficacy and safety of lenvatinib in Korean patients with advanced hepatocellular carcinoma: a multicenter retrospective analysis. Liver Cancer. 2020;9(5):613–624. doi: 10.1159/000508901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1 [DOI] [PubMed] [Google Scholar]
- 24.Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69(8):1492–1501. doi: 10.1136/gutjnl-2019-318934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding X, Sun W, Li W; Ding X, Sun W, Li W, Shen Y, Guo X, Teng Y, et al. Transarterial chemoembolization plus lenvatinib versus transarterial chemoembolization plus sorafenib as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: a prospective randomized study. Cancer. 2021;127(20):3782–3793. doi: 10.1002/cncr.33677 [DOI] [PubMed] [Google Scholar]
- 26.Yang B, Jie L, Yang T, et al. TACE plus lenvatinib versus TACE plus sorafenib for unresectable hepatocellular carcinoma with portal vein tumor thrombus: a prospective cohort study. Front Oncol. 2021;11:821599. doi: 10.3389/fonc.2021.821599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheiner B, Ulbrich G, Mandorfer M, et al. Short- and long-term effects of transarterial chemoembolization on portal hypertension in patients with hepatocellular carcinoma. United Eur Gastroenterol J. 2019;7(6):850–858. doi: 10.1177/2050640619840199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiberger T, Angermayr B, Schwabl P, et al. Sorafenib attenuates the portal hypertensive syndrome in partial portal vein ligated rats. J Hepatol. 2009;51(5):865–873. doi: 10.1016/j.jhep.2009.06.024 [DOI] [PubMed] [Google Scholar]
- 29.Mejias M, Garcia-Pras E, Tiani C, Miquel R, Bosch J, Fernandez M. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology. 2009;49(4):1245–1256. doi: 10.1002/hep.22758 [DOI] [PubMed] [Google Scholar]
- 30.Kong B, Sun R, Huang M, et al. Fibroblast growth factor 15-dependent and bile acid-independent promotion of liver regeneration in mice. Hepatology. 2018;68(5):1961–1976. doi: 10.1002/hep.30041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Q, Zhao Q, Zhang C, et al. The ileal FGF15/19 to hepatic FGFR4 axis regulates liver regeneration after partial hepatectomy in mice. J Physiol Biochem. 2018;74(2):247–260. doi: 10.1007/s13105-018-0610-8 [DOI] [PubMed] [Google Scholar]
- 32.Kudo M. Lenvatinib may drastically change the treatment landscape of hepatocellular carcinoma. Liver Cancer. 2018;7(1):191. doi: 10.1159/000487148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hidaka H, Uojima H, Nakazawa T, et al. Portal hemodynamic effects of lenvatinib in patients with advanced hepatocellular carcinoma: a prospective cohort study. Hepatol Res. 2020;50(9):1083–1090. doi: 10.1111/hepr.13531 [DOI] [PubMed] [Google Scholar]
- 34.Hidaka H, Nakazawa T, Kaneko T, et al. Portal hemodynamic effects of sorafenib in patients with advanced hepatocellular carcinoma: a prospective cohort study. J Gastroenterol. 2012;47(9):1030–1035. doi: 10.1007/s00535-012-0563-6 [DOI] [PubMed] [Google Scholar]
- 35.Ohya K, Kawaoka T, Namba M, et al. Early changes in ammonia levels and liver function in patients with advanced hepatocellular carcinoma treated by lenvatinib therapy. Sci Rep. 2019;9(1):12101. doi: 10.1038/s41598-019-48045-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saran C, Sundqvist L, Ho H, Niskanen J, Honkakoski P, Brouwer KLR. Novel bile acid-dependent mechanisms of hepatotoxicity associated with tyrosine kinase inhibitors. J Pharmacol Exp Ther. 2022;380(2):114–125. doi: 10.1124/jpet.121.000828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakano M, Kuromatsu R, Niizeki T, et al. Primary treatment with molecular-targeted agents for hepatocellular carcinoma: a propensity score-matching analysis. Hepatol Commun. 2020;4(8):1218–1228. doi: 10.1002/hep4.1535 [DOI] [PMC free article] [PubMed] [Google Scholar]