Abstract
To investigate the potential immunomodulatory effects of concurrent ascariasis on the cytokine response to a live oral vaccine, we measured cytokine responses to cholera toxin B subunit (CT-B) following vaccination with the live oral cholera vaccine CVD 103-HgR in Ascaris lumbricoides-infected subjects randomized in a double-blind study to receive two doses of either albendazole or placebo prior to vaccination and in a group of healthy U.S. controls. Postvaccination cytokine responses to CT-B were characterized by transient increases in the production of interleukin-2 (IL-2; P = 0.02) and gamma interferon (IFN-γ; P = 0.001) in the three study groups combined; however, postvaccination increases in IFN-γ were significant only in the albendazole-treated A. lumbricoides infection group (P = 0.008). Postvaccination levels of IL-2 were significantly greater in the albendazole-treated group compared with the placebo group (P = 0.03). No changes in levels of Th1 and Th2 cytokines in response to control ascaris antigens were observed over the same period. These findings indicate that vaccination with CVD 103-HgR is associated with a Th1 cytokine response (IL-2 and IFN-γ) to CT-B, that infection with A. lumbricoides diminishes the magnitude of this response, and that albendazole treatment prior to vaccination was able to partially reverse the deficit in IL-2. The potential modulation of the immune response to oral vaccines by geohelminth parasites has important implications for the design of vaccination campaigns in geohelminth-endemic areas.
Geohelminth parasites infect a large proportion of the world's population, particularly children in less socioeconomically privileged regions of the world. The most prevalent intestinal helminth is Ascaris lumbricoides, which is estimated to infect 1.5 billion humans worldwide (3).
Childhood infections with ascariasis are associated with growth stunting (24), deficiencies of macro- and micronutrients (10, 24), and small intestinal mucosal damage and malabsorption (24, 33). The presence of A. lumbricoides parasites in the small bowel may affect also the immune response to oral vaccines and explain the relatively poor immunogenicity of several live oral vaccines in certain populations of Asia, Africa, and Latin America compared with that seen in volunteers from industrialized countries. Such oral vaccines include Sabin oral polio vaccine (12, 25), rotavirus (16, 19), and the oral cholera vaccine CVD 103-HgR (31, 32). Support for a potential effect of concurrent ascariasis on the response to oral vaccines is provided by a recent study that demonstrated enhanced vibriocidal antibody titers to CVD 103-HgR in ascaris-infected children pretreated with albendazole prior to vaccination (7).
A mechanism by which ascariasis might affect the immune response to oral vaccines such as CVD 103-HgR is bystander suppression. Helminth infections are characterized by highly polarized Th2 cytokine responses (e.g., interleukin-4 [IL-4] and IL-5) and have been shown to modulate the immune responses to nonparasite or heterologous antigens by suppressing Th1 cytokine (IL-2 and gamma interferon [IFN-γ]) production and enhancing Th2 cytokine secretion in experimental animal models (1, 14, 26). Evidence for a similar phenomenon occurring in human helminth infections has been more difficult to demonstrate, although helminth-mediated suppression of the IFN-γ response to tetanus toxoid following tetanus vaccination has been shown to occur in infections with Onchocerca volvulus (6) and Schistosoma mansoni (28).
To test the hypothesis that concurrent infections with A. lumbricoides affect the balance of Th1 and Th2 cytokines produced in response to cholera toxin B subunit (CT-B), a secreted antigen of the vaccine CVD 103-HgR, we investigated CT-B-specific cytokine responses following vaccination with CVD 103-HgR in young adults infected with A. lumbricoides, a comparable group of infected controls who had received anthelmintic treatment prior to vaccination, and a group of healthy controls from the United States.
MATERIALS AND METHODS
Subjects and study design.
Healthy adult volunteers were recruited at the Laboratory of Parasitic Diseases, National Institutes of Health. Each volunteer received a dose of 5 × 108 CFU of CVD 103-HgR (Swiss Serum and Vaccine Institute, Berne, Switzerland) administered according to the manufacturer's instructions. Successful vaccination was indicated by consumption of at least 95% of the vaccine suspension, and subjects were instructed not to eat or drink for 90 min before and after vaccination. Study subjects for the A. lumbricoides-infected group were recruited from schools in the district of Pedernales in Manabi Province in Ecuador. The study design chosen was similar to a study described in detail elsewhere in which a younger study group was randomized independently (using separate randomization blocks) to investigate a different research hypothesis (7). The study was performed between October 1997 and April 1998. Briefly, young adults who met all of the following criteria were eligible to enter the study: (i) age, 13 to 17 years; (ii) infection intensity of greater than 1,000 eggs per gram (epg) of A. lumbricoides on both of two consecutive stool samples; (iii) hemoglobin concentration of greater than 10 g/dl; (iv) absence of severe concurrent illness, including clinical evidence of immunodeficiency; and (v) negative urine pregnancy test if female. All individuals with evidence of intestinal helminth infection but who were excluded from the study were offered a single dose of 400 mg of albendazole. Each subject was assessed using standard anthropometric measurements for the presence of malnutrition.
After obtaining informed written consent from a parent or guardian, each subject was randomized, in a double-blind fashion, to receive either 400 mg of albendazole (Zentel; SmithKline Beecham, London, United Kingdom) or identical placebo tablets (SmithKline Beecham) (study day −37). The same dose of albendazole or placebo was repeated after 30 days (study day −7). One week after receiving the second dose of albendazole or placebo (study day 0), all subjects received a single dose of 5 × 108 CFU of CVD 103-HgR (same vaccine lot as for the U.S. control group), which was prepared and administered according to the manufacturer's instructions. All study recruits were offered a single 5 × 109 CFU dose of CVD 103-HgR and 400 mg of albendazole at the end of the study.
Stool samples were examined by the modified Kato Katz technique (36) (study days −37, −7, 0, and 14) for assessment of infection with A. lumbricoides and Trichuris trichiura, and stool samples were preserved in 10% Formol and subsequently examined for the presence of hookworm and other intestinal helminth parasites by the formalin-gasoline concentration method (36) (study day −37 only).
Blood sampling.
Because the optimal systemic immune response to orally delivered vaccines is detectable for a brief period following vaccination (13, 20), we performed serial postvaccination measurements of the cellular immune response to CT-B in the group of U.S. healthy controls: 20 ml of blood was drawn into heparinized syringes from all subjects before (three times) and at various time points after vaccination (5, 9, 12, 14, 20, 24, 28, and 32 days). Selection of optimal postvaccination sampling times (study days 14 and 28) for the Ecuador study groups was based on these findings (see Results). In the Ecuador study, 20 ml of blood was drawn into heparinized syringes and processed as described below. Immunologic studies were run in a blinded fashion using coded samples.
CT-B-specific ELISPOT.
Immulon 4 96-well mictotiter plates (Dynex Technologies, Inc., Chantilly, Va.) were coated overnight at 4°C with 10 μg of purified CT-B (Sigma Chemical Corp., St. Louis, Mo.) per ml in carbonate buffer (0.045 M NaHCO3–0.02 M Na2CO3 at pH 9.6). After being washed with phosphate-buffered saline (PBS)-Tween (0.05%), the plates were blocked with 5% bovine serum albumin-PBS-Tween (0.05%) for 2 h at room temperature. Peripheral blood mononuclear cells (PBMC) were added to the plates at a concentration of 2.5 × 106 cells/ml (200 μl/well) in culture medium (RPMI 1640 [BioWhittaker, Walkersville, Md.] supplemented with 10% fetal calf serum [Atlanta Biologicals, Norcross, Ga.], 0.08 mg of gentamicin [Life Technologies/Gibco-BRL, Gaithersburg, Md.] per ml, and 2 mM l-glutamine [Biofluids, Inc., Rockville, Md.]). The plates were incubated at 37°C and 5% CO2 for 6 h. After washing, goat anti-human immunoglobulin G (IgG) Fc (Jackson Immunoreseach, West Grove, Pa.) or goat anti-human IgA (Sigmal Chemical Corp.) was added to the wells. The plates were washed, and BCIP substrate (5-bromo-4-chloro-3-indolylphosphate; Sigma Chemical Corp.)–2.5% agarose (Gibco-BRL) was added. The spots were counted after 24 h using a dissecting microscope. ELISPOT data were expressed as the absolute difference between the number of spots detected in antigen-stimulated cultures and those detected in cultures with medium alone.
PBMC proliferation assays.
PBMC were isolated and cultured as described previously (6). The PBMC were stimulated with recombinant CT-B (kindly provided by Yoshikazu Yuki, JCR Biopharmaceuticals, Inc.) at concentrations of between 1 and 10 μg/ml, a PBS-soluble extract of adult A. lumbricoides worms (8) at 10 μg/ml, and excretory-secretory antigens from Ascaris suum developing from L2 to L3 (L2/L3 antigen) (8) at 1 μg/ml. The recombinant CT-B antigen preparation and ascaris antigens were assayed for the presence of bacterial endotoxin using the Limulus lysate assay (BioWhittaker). Endotoxin levels were undetectable in all samples.
Cytokine ELISA.
Supernatant fluids were harvested from the same cultures used for proliferation assays at 24 h (IL-2 and IL-4) and 5 days (IL-5 and IFN-γ). Cytokine enzyme-linked immunosorbent assay (ELISA) for IFN-γ, IL-2, IL-4, and IL-5 were performed as described previously (22). Cytokine levels were expressed in picograms per milliliter as the absolute difference between protein levels in antigen-stimulated cultures and control medium cultures.
Statistical analysis.
Helminth egg counts were loge transformed before analysis. Associations between categorical variables were analyzed by using Fisher's exact test or the McNemar test (for paired data) (2) where appropriate. Statistical analyses of anthropometric measurements were performed using Epi Info 6 statistical software (Centers for Disease Control and Prevention, Atlanta, Ga.). Assessment of the changes in the levels of cytokines within groups over the observation period was performed by using Friedman's two-way analysis of variance (2). Since significant heterogeneity in the data was observed only at the 14-day observation time, further comparisons of intragroup changes in cytokine levels were performed by comparing the mean of the two prevaccination findings with the findings at 14 days postvaccination using the Wilcoxon signed rank sum test for paired samples. Comparison between study groups was performed by using the Mann-Whitney U test. The protocol was approved by the Institutional Review Boards of the National Institute of Allergy and Infectious Diseases and by the Hospital Vozandes in Ecuador.
RESULTS
Demographic and parasitologic data.
Eight healthy volunteers from the National Institutes of Health were followed up completely (Table 1). None had a recent history of a cholera-like illness or cholera vaccination (e.g., within the past 5 years). In the Ecuador study group, a total of 28 subjects completed follow-up, of whom 13 received placebo and 15 were treated with albendazole: none reported a history of a cholera-like illness or cholera vaccination. The baseline nutritional and parasitologic characteristics of the Ecuador study group are shown in Table 1. Before albendazole or placebo treatment, the infection intensities with A. lumbricoides were greater in the placebo group (median, 7,491 epg; range, 2,166 to 36,814 epg) than in the albendazole group (median, 3,266 epg; range, 1,456 to 9,585 epg) (P = 0.003). There were no other significant differences between the albendazole and placebo groups with respect to age, sex ratios, hematocrit, indices of nutritional status, or the prevalence and intensity of T. trichiura infection. None of the subjects in the infection group had evidence of infection with hookworm or Strongyloides stercoralis by stool examination or had evidence of malnutrition from anthropometric measurements.
TABLE 1.
Demographic, nutritional, and parasitologic characteristics of the group of healthy U.S. controls and the A. lumbricoides infection group
| Characteristica | Treatment groupb
|
||
|---|---|---|---|
| U.S. controls (n = 8) | Placebo (n = 13) | Albendazole (n = 15) | |
| Mean age in yrs (SD) | 32 (2.3) | 14 (1.4) | 14 (1.5) |
| Sex (no. male [M]/no. female [F]) | 6 M/2 F | 6 M/7 F | 8 M/7 F |
| Hematocrit (%) (SD) | NDc | 40.9 (4.0) | 39.7 (3.4) |
| Ht (cm) (SD) | ND | 149.3 (7.7) | 149.8 (5.9) |
| Wt (kg) (SD) | ND | 44.8 (19.2) | 42.7 (7.7) |
| SFT (mm) (SD) | ND | 12 (5.0) | 13.7 (5.2) |
| MUAC (cm) (SD) | ND | 23.4 (5.3) | 23.0 (2.8) |
| A. lumbricoides | |||
| Prevalence (%) | ND | 100 | 100 |
| Infection intensity (epg) | 7,491 (2,166–36,814)d | 3,266 (1,456–9,585)d | |
| T. trichiura | |||
| Prevalence (%) | ND | 61.5 | 80.0 |
| Infection intensity (epg) | 71 (0–1,775)d | 213 (0–355)d | |
SFT, skin fold thickness; MUAC, mid-upper-arm circumference.
Data are shown only for those subjects who completed follow-up over the observation period.
ND, not done.
Median (range).
Response to anthelmintic treatment.
There were significant decreases (P < 0.0001) in the prevalence of A. lumbricoides infection in the albendazole group (pretreatment level of 100% versus a posttreatment level of 20%) compared with the placebo group (pretreatment level of 100% versus a posttreatment level of 92.3%) following the administration of two doses of albendazole or placebo. The infection intensities with A. lumbricoides did not change significantly in the placebo group (pretreatment median of 7,491 epg versus a posttreatment median of 5,751 epg) but declined significantly in the albendazole-treated group (pretreatment median of 3,266 epg versus a posttreatment median of 0 epg; P = 0.001). The prevalence of T. trichiura infection did not alter significantly in either placebo (pretreatment level of 61.5% versus a posttreatment level of 23%) or albendazole (pretreatment level of 80% versus a posttreatment level of 33%) groups after administration of two doses, but the infection intensity did decline significantly in the albendazole-treated group (pretreatment median of 213 epg versus a posttreatment median of 0 epg; P = 0.004) but not the placebo group (pretreatment median of 71 epg versus a posttreatment median of 142 epg).
Selection of postvaccination sampling times.
Serial blood samples were collected from the group of U.S. healthy controls before and after vaccination, and CT-B-specific PBMC responses were tested for lymphoproliferation, cytokine production (IL-2, IFN-γ, and IL-5), and the frequency of circulating CT-specific IgG and IgA antibody-secreting cells (ASC). The findings are shown in Fig. 1 and Table 2. Maximal lymphoproliferation was biphasic, with peaks at days 14 and 28, although all postvaccination lymphoproliferative responses measured were significantly elevated compared with prevaccination levels (P < 0.05). Trends of increased postvaccination production of both IFN-γ (Fig. 1) and IL-2 (data not shown) were observed, with a peak at 14 days postvaccination. The kinetics of the appearance in the peripheral blood of IgG CT-B-positive ASC are shown in Fig. 1. Peaks in the frequency of both CT-B-specific IgA and IgG ASC occurred between 9 and 14 days postvaccination: IgG ASC frequencies were significantly elevated compared with baseline levels at days 12 (P = 0.04) and 14 (P < 0.04) postvaccination, and IgA ASC frequencies were significantly elevated at day 12 postvaccination (P = 0.04). No CT-B-positive ASC (IgG or IgA) were detected before vaccination or by 24 days postvaccination. To minimize the number of sampling times in the Ecuador study group, it was decided to sample subjects twice prevaccination (before the first dose of anthelmintic treatment) (study day −37) and after receiving two doses of albendazole or placebo and immediately before receiving CVD 103-HgR (study day 0) and at days 14 and 28 postvaccination.
FIG. 1.
Lymphoproliferative, IFN-γ cytokine, and ASC responses to CT-B in eight healthy controls following vaccination with CVD 103-HgR. (A) Percent change of stimulation indices (SI) compared with prevaccination levels. (B) IFN-γ production. (C) CT-B-specific ASC frequencies. Box plots represent median values (central line), interquartile range (box margins), 95% confidence intervals (bars), and outlying values (circles). The observation times at which median values were significantly greater (P < 0.05) than prevaccination levels are indicated (∗). Numbers within or above boxes show the proportions (percentages) of subjects with a detectable response for each group.
TABLE 2.
Production of cytokines IL-2, IL-5, and IFN-γ by PBMC stimulated with recombinant CT-B from the three study groups and all three groups combined
| Group (n) | Mean cytokine production (pg/ml [range])a of:
|
|||||
|---|---|---|---|---|---|---|
| IL-2
|
IFN-γ
|
IL-5
|
||||
| Before | After | Before | After | Before | After | |
| U.S. controls (8) | 9.8 (0–52.7) | 38.0 (0–210.4) | 0 (0–15.3) | 28.3 (0–85.0) | 17.8 (0–50.4) | 0 (0–38.6) |
| A. lumbricoides infected | ||||||
| Placebo (13) | 0 (0–20.9) | 0 (0–252.9) | 0 (0–30.0) | 7.9 (0–298.2) | 0 (0–10.5) | 0 (0–30.3) |
| Albendazole (15) | 0.3 (0–55.8) | 21.8 (0–74.0) | 0 (0–54.0) | 7.7 (0–209.7) | 0 (0–22.7) | 0 (0–25.3) |
| All infected (28) | 0 (0–55.8) | 0 (0–252.9) | 0 (0–54.0) | 7.7 (0–298.2) | 0 (0–22.7) | 0 (0–30.3) |
| Total (all groups) (36) | 0 (0–55.8) | 1.4 (0–252.9) | 0 (0–54.0) | 8.5 (0–298.2) | 0 (0–50.4) | 0 (0–38.6) |
Shown are the median values and ranges for cytokine production levels before vaccination with CVD 103-HgR (before) and at 14 days postvaccination (after). Prevaccination cytokine levels are the median value of the mean of the two prevaccination observation times (before albendazole or placebo treatment and after two doses of albendazole or placebo).
Changes in cytokine secretion by PBMC stimulated with CT-B. (i) Cytokine response to CT-B.
Cytokine production of IL-2, IL-5, and IFN-γ by PBMC stimulated with CT-B are shown in Table 2. IL-4 secretion by PBMC stimulated with CT-B was negligible (data not shown). The CT-B-specific cytokine production was negligible during the prevaccination observation period (Fig. 1 and 2; study days −37 and 0) and became detectable after vaccination at day 14, after which the levels declined. Postvaccination antigen-specific cytokine production was characterized by significant increases in the production of IL-2 (P = 0.04) and IFN-γ (P = 0.005) at day 14. There was no postvaccination IL-5 response in the combined group. In the group of U.S. healthy controls, trends of increased production of IL-2 (P > 0.1) and IFN-γ (P = 0.07) were observed between the prevaccination observation times and day 14 postvaccination (Table 2). In contrast, the levels of IL-5 decreased significantly (P = 0.03) over the same study period.
FIG. 2.
Production of cytokines IL-2, IFN-γ, and IL-5 by PBMC stimulated with CT-B in the Ecuador study groups. Young adults with ascariasis were randomized to receive two sequential doses (days −37 and −7) of either albendazole (400 mg) or placebo, after which all received a single dose of CVD 103-HgR (day 0). PBMC were stimulated with CT-B at day −37 prevaccination, day 0, and days 14 and 28 postvaccination. Shown are box plots of net cytokine production. The observation times at which median values were significantly greater (P < 0.05) than prevaccination levels are shown (+). Times at which intergroup differences were significant (P < 0.05) are shown also (∗). Numbers within or above boxes show the proportions (percentages) of subjects with a detectable response for each group.
(ii) Effect of albendazole treatment of ascariasis on cytokine response to CT-B.
In the Ecuador study group as a whole, significant increases in the production of IFN-γ (P = 0.003) and IL-2 (P = 0.03) were observed after vaccination (day 14), while production of IL-5 did not alter significantly (P > 0.1) (Table 2). The postvaccination increases (day 14) in IL-2 and IFN-γ were significant in the albendazole-treated group (IL-2, P = 0.04; IFN-γ, P = 0.008) but not in the placebo-treated group (Fig. 2 and Table 2). A comparison of day-14 postvaccination cytokine responses between the albendazole- and placebo-treated groups revealed significantly greater levels of IL-2 (P = 0.03) in the former group. The production of IFN-γ was similar between the two groups at the same observation time. Cytokine responses were analyzed also as binary variables (response versus no response) (Fig. 2): among the albendazole-treated group, significant increases in the number of responders at 14 days postvaccination were observed for IL-2 (P < 0.05) and IFN-γ (P < 0.01). No significant changes in rates of response were observed in the placebo group.
Changes in cytokine secretion by PBMC stimulated with ascaris antigens.
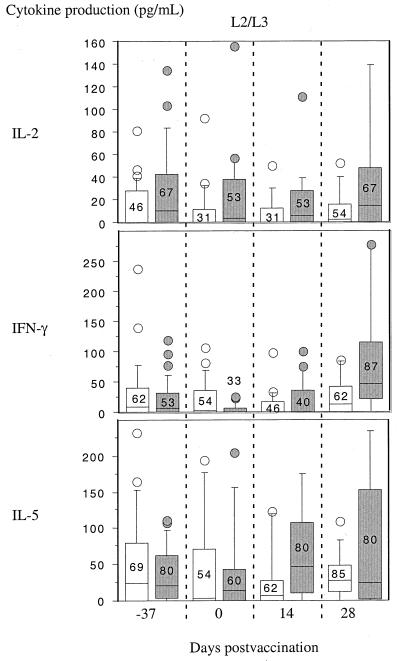
To investigate hypothetical immunopotentiation of postvaccination cellular immunity by albendazole, ascaris antigen-specific cytokine responses were also examined. There were no significant changes in parasite antigen cytokine levels over the four observation times for either adult antigen preparation or L2/L3 larval antigen for IL-2, IL-5, or IFN-γ. The findings for L2/L3 antigen are shown in Fig. 3: no changes were observed before or after the administration of the two doses of albendazole or placebo (study days −37 and 0), 14 days after administration of CVD 103-HgR (day 14), or 28 days postvaccination (day 28).
FIG. 3.
Production of cytokines IL-2, IFN-γ, and IL-5 by PBMC stimulated with L2/L3 ascaris antigen in the Ecuador study groups. Shown are box plots of net cytokine production at the study times described in Fig. 2. There were no significant inter- or intragroup differences over the study period for any of the three cytokines. Numbers within or above boxes show the proportions (percentages) of subjects with a detectable response for each group.
DISCUSSION
Geohelminth parasites are highly prevalent in conditions of poor sanitation and hygiene. A. lumbricoides is the most prevalent of the intestinal helminth infections that colonize the small intestine. The presence of parasitic intestinal worms in close association with the intestinal mucosa may affect the immune response to orally delivered antigens, including live oral vaccines, by (i) damaging mucosal integrity, (ii) induction of an inflammatory mucosal immune response that may damage the mucosa itself, and (iii) creating a highly polarized immune environment that may influence the immune response to nonparasite antigens. To investigate the potential modulatory role of ascariasis on the immune response to an orally administered vaccine in this study, we chose as a study site a rural area of Ecuador where A. lumbricoides is highly prevalent and where other small bowel-dwelling geohelminths (hookworm and S. stercoralis) are of low prevalence and used as the model oral vaccine the live attenuated oral cholera vaccine CVD 103-HgR. This vaccine, which induces strong systemic antibody responses in healthy adults (20), has been shown to be poorly immunogenic (at a dose of 5 × 108 CFU) in populations where geohelminths are likely to be highly prevalent (31, 32).
The findings of our study indicate that ascariasis infection in young adults is associated with diminished Th1 response to CT-B following vaccination with CVD 103-HgR. While the postvaccination cytokine responses in the study group as a whole were characterized by the production of IL-2 and IFN-γ, there were trends of impaired secretion of both these Th1 cytokines in the A. lumbricoides infection group as a whole, although none of these differences reached statistical significance. Further, when the A. lumbricoides infection group was analyzed separately, the group that received albendazole prior to vaccination demonstrated significantly enhanced IL-2 production to CT-B after vaccination compared with the placebo group, whereas IFN-γ production was not affected. The reason for this differential effect of albendazole pretreatment on Th1 cytokine secretion to CT-B is not clear. The failure to reach statistical significance in some of the observed effects could be attributed to the relatively small sample sizes of the three study groups, particularly the U.S. control group, and further larger studies are indicated to confirm these observations.
The modulatory role of human helminth infections on the immune response to nonparasite or heterologous antigens is of great potential interest with respect both to the immune responses to vaccines and to the responses to other pathogens. Helminth infections induce potent Th2 cytokine responses that have been shown to influence the responses to nonparasite antigens in both experimental animal models (1, 14, 26) and in humans (6, 28). Typically, such bystander effects in human helminth infections result in impaired secretion of Th1 cytokines (6, 28).
Human infections with A. lumbricoides induce highly polarized Th2 cytokine responses characterized by the relatively greater production of IL-4 and IL-5 compared to IFN-γ in response to adult and larval-stage antigens (8). By inducing a highly polarized Th2 environment in the gastrointestinal (adult parasites and invasive L2/L3-stage parasites) and respiratory mucosa (migratory larval L3/L4-stage parasites), human ascariasis has the potential to modulate the immune responses to antigens presented via the gastrointestinal (e.g., vaccine-derived antigens) or respiratory (e.g., aeroallergens or Mycobacterium tuberculosis) routes. Evidence for an immunomodulatory effect of ascariasis on the immune responses to antigens presented via the mucosal route is suggested by the beneficial effect of anthelmintic treatment on asthma symptoms and requirement for treatment in asthmatic individuals living in a geohelminth-endemic area of Venezuela (21) and by the fact that concurrent A. lumbricoides infections are associated with enhanced and inappropriate production of IL-5 by PBMC stimulated with mycobacterial antigen (PPD) (8). In this study, we provide evidence of immunomodulation by ascariasis of the immune response to an antigenic component of an oral vaccine.
Albendazole is a broad-spectrum drug with efficacy against many helminth and intestinal protozoal infections, including giardiasis. In this study, we have attributed differences in postvaccination cytokine responses between the albendazole and placebo treatment groups to the treatment effects of albendazole against A. lumbricoides. The reasons for this are that (i) the Ecuador subject groups were selected on the basis of significant infection intensities with A. lumbricoides; (ii) T. trichiura, the only other geohelminth parasite in these groups, was of low intensity and resides in the large intestine; and (iii) although we did not examine the stools for the presence of Giardia lamblia (intestinalis) infections, the results of previous surveys in similar rural communities in Ecuador would indicate a prevalence rate of less than 10% (17) and, since the efficacy of single doses of 400 mg of albendazole is low (e.g., 24%) (9), the potential contribution to the postvaccination response of partial treatment is likely to be small.
Benzimidazole drugs such as albendazole have not been associated with immunopotentiating effects following the treatment of human helminth infections, in contrast to anthelmintic drugs such as ivermectin that may induce severe immune-mediated adverse reactions following treatment (5), and are associated with enhanced posttreatment Th1 cytokine responses to parasite antigens (29, 30). The postvaccination increase in the CT-B-specific Th1 cytokine response that we observed is unlikely to be a consequence of albendazole-induced stimulation, because similar responses were observed in both treated and untreated (placebo or U.S. control) individuals, and ascaris-specific cytokine responses were not affected by albendazole treatment (Fig. 3). An increased IL-2 secretion in response to CT-B in the albendazole-treated group compared with the placebo-treated group is unlikely to be an artifact of albendazole treatment for the following reasons: (i) similar but greater responses were observed in the group of U.S. controls who did not receive albendazole and (ii) the first and second doses of albendazole, administered prior to vaccination, had no effect on IL-2 secretion to either CT-B or ascaris antigens.
The observation of a lack of effect of curative chemotherapy with albendazole on the cytokine response to ascaris antigens is of interest. As discussed above, the treatment of tissue helminth infections such as filariae is associated with a change in both the magnitude and phenotype of the parasite-specific cytokine response (29, 30). These changes relate to the destruction of parasite larvae, which are the primary targets of the host immune respnse (23). It is probable that larval antigens are the primary target of host immunity in A. lumbricoides infection also, and uninterrupted immune stimulation as a result of continuous exposure to infective eggs in an environment where the organism is endemic may explain the lack of treatment effect on either the magnitude or the phenotype of host immunity despite successful expulsion of adult parasites.
Controversy exists over the nature of the immune response to cholera toxin and its subunit constituents. Research in murine models has consistently shown that oral immunization with CT or CT-B induces a local mucosal and systemic immune response characterized by the predominant production of Th2 cytokines (IL-4 and IL-5) and little or no production of Th1 cytokines (IFN-γ and IL-2) (35, 37, 38), although a few studies have shown either a mixed Th1-Th2 response or strong Th1 (IFN-γ) responses (11, 34). Few human studies have been performed on the cellular response to CT or CT-B following oral immunization (4, 18, 27), and none have examined the human cellular immune response to CVD 103-HgR. The only study to examine cytokine responses following vaccination of healthy volunteers with an oral combined CT-B–whole-cell cholera vaccine was not able to detect IFN-γ in cultures from PBMC but did detect significant IFN-γ levels produced by monocytes that had been isolated from duodenal cellular suspensions (27). The latter study, which did not investigate Th2 cytokine production, examined IFN-γ responses 7 days postvaccination, which may have been too early to detect optimal postvaccination responses in circulating monocytic cells, particularly since T-cell responses (measured as lymphocyte proliferation) tend to peak later (4, 18) (Fig. 1). In this study, in which both Th1 and Th2 cytokine production were investigated, we have been able to demonstrate that the postvaccination response to CT-B in subjects vaccinated with CVD 103-HgR is dominated by the production of the Th1 cytokines IFN-γ and IL-2.
Peripheral blood lymphocytes can be used to measure mucosal responses to oral vaccines because of the phenomenon of lymphocyte trafficking that occurs for a relatively brief period postvaccination and involves an expanded population of antigen-specific lymphocytes leaving mucosal lymph tissues to seed more extensively throughout the mucosa (13, 18). Our observations among the group of healthy controls provide evidence for trafficking of CT-B-specific B and T lymphocytes following vaccination with CVD 103-HgR (Fig. 1) and a sound justification for sampling at day 14 postvaccination to measure CT-B-specific cytokine responses in the Ecuador study group. A bimodal distribution of T-cell reactivity to CT-B was observed in the group of healthy controls and has been reported by others (4), although cytokine (IL-2 and IFN-γ) and IgG ASC responses peaked only at 14 days.
Previous studies have attributed the poor immunogenicity of CVD 103-HgR (at a dose of 5 × 108 CFU) in poor populations in developing countries compared with wealthier populations in the same or industrialized countries to the inhibitory effect of prior or active mucosal immunity on the induction of a measurable systemic response (20, 31, 32) and to small-bowel bacterial overgrowth (15) that may interfere with vaccine replication and/or vaccine attachment to the mucosal surface. In addition, we have demonstrated in a younger study group infected with A. lumbricoides from the same study area that concurrent ascariasis impairs the vibriocidal antibody response to CVD 103-HgR and that pretreatment with albendazole before vaccination results in an improved seroconversion rate (7). The results of the present study provide evidence for a role of ascariasis-associated suppression of Th1 immune responses to CT-B, an important antigenic component of the vaccine. The effect of such an alteration of the cytokine response to CT-B on antitoxic immunity is not clear and requires further investigation.
In conclusion, we have demonstrated that human vaccination with CVD 103-HgR is associated with a postvaccination cytokine response characterized by an elevation of the Th1 cytokines IL-2 and IFN-γ. A. lumbricoides-infected subjects who were pretreated with albendazole prior to vaccination produced a similar Th1-predominated response, while infected subjects who received placebo treatment before vaccination demonstrated a depressed IL-2 response. These results indicate that concurrent ascariasis may modulate the immune response to nonparasite or heterologous antigens by suppression of IL-2 and provide further support for an immunomodulatory role of concurrent helminth infections on immune responses to heterologous antigens. Since geohelminth parasites are ubiquitous in poorer regions of the world, the potential for such infections to alter the immune responses to orally administered vaccines and affect vaccine immunogenicity has important public health implications.
ACKNOWLEDGMENTS
We thank the community representatives and school teachers in the study communities in Canton Pedernales for their cooperation. We thank also the following for their assistance in the completion of this study: Moises Botta, Chief Medical Officer for Canton Pedernales; Marcelo Aguilar, National Director of Health, Ministry of Public Health, Quito, Ecuador; and Ronald Guderian, Hospital Vozandes, Quito, Ecuador. The assistance of Kenneth Farr (U.S. Agency for International Development, Quito, Ecuador) is gratefully acknowledged.
This work was funded in part by a grant from the Wellcome Trust.
REFERENCES
- 1.Actor J K, Shirai M, Kullberg M C, Buller R M L, Sher A, Berzofsky J A. Helminth infection results in decreased virus-specific CD8+ cytotoxic virus-specific T-cell and Th-1 cytokine responses as well as delayed virus clearance. Proc Natl Acad Sci USA. 1993;90:948–952. doi: 10.1073/pnas.90.3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altman D G. Practical statistics for medical research. London, England: Chapman & Hall; 1991. pp. 334–336. [Google Scholar]
- 3.Bundy D A P. Immunoepidemiology of intestinal helminthic infections. 1. The global burden of intestinal nematode disease. Trans R Soc Trop Med Hyg. 1994;88:259–261. doi: 10.1016/0035-9203(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 4.Castello-Branco L R R, Griffin G E, Poulton T A, Dougan G, Lewis D J M. Characterization of the circulating T-cell response after oral immunization of human volunteers with cholera toxin B-subunit. Vaccine. 1994;12:65–72. doi: 10.1016/0264-410x(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 5.Cooper P J, Awadzi K, Ottesen E A, Remick D, Nutman T B. Eosinophil sequestration and activation are associated with the onset and severity of systemic adverse reactions following the treatment of onchocerciasis with ivermectin. J Infect Dis. 1999;179:738–742. doi: 10.1086/314647. [DOI] [PubMed] [Google Scholar]
- 6.Cooper P J, Espinel I, Paredes W, Guderian R H, Nutman T B. Impaired tetanus-specific cellular and humoral responses following tetanus vaccination in human onchocerciasis: a possible role for interleukin-10. J Infect Dis. 1998;178:1133–1138. doi: 10.1086/515661. [DOI] [PubMed] [Google Scholar]
- 7.Cooper P J, Chico M, Losonsky G, Espinel I, Sandoval C, Aguilar M, Guevara A, Levine M, Griffin G E, Nutman T B. Ascaris lumbricoides infection impairs the immune response to the live oral cholera vaccine CVD 103-HgR. J Infect Dis. 2000;182:1199–1206. doi: 10.1086/315837. [DOI] [PubMed] [Google Scholar]
- 8.Cooper P J, Chico M, Sandoval C, Espinel I, Guevara A, Kennedy M W, Urban J F, Jr, Griffin G E, Nutman T B. Human infection with Ascaris lumbricoides is associated with a polarized Th2 cytokine phenotype. J Infect Dis. 2000;182:1207–1213. doi: 10.1086/315830. [DOI] [PubMed] [Google Scholar]
- 9.Hall A, Anwar K S. Albendazole and infections with Trichuris trichiura and Giardia intestinalis. S E Asian J Trop Med Public Health. 1991;22:84–87. [PubMed] [Google Scholar]
- 10.Hlaing T. Ascariasis and childhood malnutrition. Parasitology. 1993;107:S125–S136. doi: 10.1017/s0031182000075557. [DOI] [PubMed] [Google Scholar]
- 11.Hornquist E, Lycke N. Cholera toxin adjuvant greatly promotes antigen priming of T cells. Eur J Immunol. 1993;23:2136–2143. doi: 10.1002/eji.1830230914. [DOI] [PubMed] [Google Scholar]
- 12.John T J, Jayabal P. Oral polio vaccination of children in the tropics. I. The poor seroconversion rates and absence of viral interference. Am J Epidemiol. 1972;96:263–269. doi: 10.1093/oxfordjournals.aje.a121457. [DOI] [PubMed] [Google Scholar]
- 13.Kantele A, Arvilommi H, Jokinene I. Specific immunoglobulin-secreting human blood cells after peroral vaccination against Salmonella typhi. J Infect Dis. 1986;153:1126–1131. doi: 10.1093/infdis/153.6.1126. [DOI] [PubMed] [Google Scholar]
- 14.Kullberg M C, Pearce E J, Hieny S E, Sher A, Berzofsky J A. Infection with Schistosoma mansoni alters Th1/Th2 cytokine responses to a nonparasite antigen. J Immunol. 1992;148:3264–3270. [PubMed] [Google Scholar]
- 15.Lagos R, Fasano A, Wasserman S S, Prado V, San Martin O, Abrego P, Losonsky G A, Alegria S, Levine M M. Effect of small bowel bacterial overgrowth on the immunogenicity of single-dose live-oral cholera vaccine CVD 103-HgR. J Infect Dis. 1999;180:1709–1712. doi: 10.1086/315051. [DOI] [PubMed] [Google Scholar]
- 16.Lanata C F, Black R E, Burton B, Midthun K, Davidson B. Safety, immunogenicity and efficacy of one or three doses of the rhesus tetravalent rotavirus vaccine in Lima, Peru. Vaccine. 1992;10:273. doi: 10.1093/infdis/174.2.268. [DOI] [PubMed] [Google Scholar]
- 17.Levav M, Mirsky A F, Schantz P M, Castro S, Cruz M E. Parasitic infection in malnourished school children: effects on behaviour and EEG. Parasitology. 1995;110:103–111. doi: 10.1017/s0031182000081105. [DOI] [PubMed] [Google Scholar]
- 18.Lewis D J M, Castello-Branco L R R, Novotny P, Dougan G, Poulton T A, Griffin G E. The early cellular and humoral immune response to primary and booster oral immunization with cholera toxin-B subunit. Vaccine. 1993;11:119–121. doi: 10.1016/0264-410x(93)90005-i. [DOI] [PubMed] [Google Scholar]
- 19.Linhares A C, Gabbay Y B, Mascarenhas J D, de Freitas R B, Oliveira C S, Bellesi N, Monteiro T A, Lins-Lainson Z, Ramos F L, Valente S A. Immunogenicity, safety and efficacy of tetravalent rhesus-human, reassortant rotavirus vaccine in Belem, Brazil. Bull W H O. 1996;74:491–500. [PMC free article] [PubMed] [Google Scholar]
- 20.Losonsky G A, Tacket C O, Wasserman S S, Kaper J B, Levine M M. Secondary Vibrio cholerae-specific cellular antibody responses following wild-type homologous challenge in people vaccinated with CVD 103-HgR live oral cholera vaccine: changes with time and lack of correlation with protection. Infect Immun. 1993;61:729–733. doi: 10.1128/iai.61.2.729-733.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lynch N R, Palenque M, Hagel I, DiPrisco M C. Clinical improvement of asthma after anthelminthic treatment in a tropical situation. Am J Respir Crit Care Med. 1997;156:50–54. doi: 10.1164/ajrccm.156.1.9606081. [DOI] [PubMed] [Google Scholar]
- 22.Mahanty S, Abrams J S, King C L, Limaye A P, Nutman T B. Parallel regulation of IL-4 and IL-5 in human helminth infections. J Immunol. 1992;148:3567–3571. [PubMed] [Google Scholar]
- 23.Ottesen E A. Immune responsiveness and the pathogenesis of human onchocerciasis. J Infect Dis. 1995;171:659–671. doi: 10.1093/infdis/171.3.659. [DOI] [PubMed] [Google Scholar]
- 24.Nesheim M C. Ascariasis and human nutrition. In: Crompton D W T, Nesheim M C, Pawlowski Z S, editors. Ascariasis and its prevention and control. London, England: Taylor & Francis; 1989. pp. 87–100. [Google Scholar]
- 25.Patriarca P A, Wright P F, Jacob J T. Factors affecting immunogenicity of oral poliovirus vaccine in developing countries. Rev Infect Dis. 1991;13:926–939. doi: 10.1093/clinids/13.5.926. [DOI] [PubMed] [Google Scholar]
- 26.Pearlman E, Kazura J W, Hazlett F E, Jr, Boom W H. Modulation of murine cytokine responses to mycobacterial antigens by helminth-induced T helper 2 cell responses. J Immunol. 1993;151:4857–4864. [PubMed] [Google Scholar]
- 27.Quiding M, Nordstrom I, Kilander A, Andersson G, Hanson L A, Holmgren J, Czerkinsky C. Intestinal immune responses in humans: oral vaccination induces strong intestinal antibody responses and interferon-γ production and evokes local immunological memory. J Clin Investig. 1991;88:143–148. doi: 10.1172/JCI115270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabin E A, Araujo M I, Carvalho E M, Pearce E J. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J Infect Dis. 1996;173:269–272. doi: 10.1093/infdis/173.1.269. [DOI] [PubMed] [Google Scholar]
- 29.Soboslay P T, Luder C G K, Hoffman W H, Michaelis I, Helling G, Heuschkel C, Dreweck C M, Blanke C H, Pritze S, Banla M, Schulz-Key H. Ivermectin-facilitated immunity in onchocerciasis: activation of parasite-specific Th1-type responses with subclinical Onchocerca volvulus infection. Clin Exp Immunol. 1994;96:238–244. doi: 10.1111/j.1365-2249.1994.tb06548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steel C S, Lujan-Trangay A, Gonzalez-Peralta C, Zea-Flores G, Nutman T B. Transient changes in cytokine profiles following ivermectin treatment of onchocerciasis. J Infect Dis. 1994;170:962–970. doi: 10.1093/infdis/170.4.962. [DOI] [PubMed] [Google Scholar]
- 31.Su-Arehawatana P, Singharaj P, Taylor D, Hoge C, Trofa A, Kruvanont K, Migasena S, Pitisuttitham P, Leung Lim Y, Losonsky G, Kaper J B, Wasserman S S, Cryz S, Echeverria P, Levine M M. Safety and immunogenicity of different immunization regimes of CVD 103-HgR live oral cholera vaccine in soldiers and civilians in Thailand. J Infect Dis. 1992;165:1042–1048. doi: 10.1093/infdis/165.6.1042. [DOI] [PubMed] [Google Scholar]
- 32.Suharyono, Simanjuntak C, Witham N, Punjabi N, Heppner D G, Losonsky G, Totosudirjo H, Rifai A R, Clemens J, Yeung Lim Y, Burr D, Wasserman S S, Kaper J, Sorenson K, Cryz S, Levine M M. Safety and immunogenicity of single-dose live oral cholera vaccine CVD-103-HgR in 59-year-old Indonesian children. Lancet. 1992;340:689–694. doi: 10.1016/0140-6736(92)92231-4. [DOI] [PubMed] [Google Scholar]
- 33.Tripathy K, Duque E, Bolanos O, Lotero H, Mayoral L G. Malabsorption syndrome in ascariasis. Am J Clin Nutr. 1972;25:1276–1281. doi: 10.1093/ajcn/25.11.1276. [DOI] [PubMed] [Google Scholar]
- 34.Wilson A D, Bailey M, Williams N A, Stokes C R. The in vitro production of cytokines by mucosal lymphocytes immunized by oral administration of keyhole limpet hemocyanin using cholera toxin as an adjuvant. Eur J Immunol. 1991;21:2333–2339. doi: 10.1002/eji.1830211007. [DOI] [PubMed] [Google Scholar]
- 35.Williams N A, Hirst T R, Nashar T O. Immune modulation by the cholera-like enterotoxins: from adjuvant to therapeutic. Immunol Today. 1999;20:95–101. doi: 10.1016/s0167-5699(98)01397-8. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. Diagnostic techniques for intestinal parasitic infections (IPI) applicable to primary health care (PHC) services. WHO/PDP/85.2, 1211. Geneva, Switzerland: WHO; 1985. [Google Scholar]
- 37.Xu-Amano J, Jackson R J, Fujihashi K, Kiyono H, Staats H F, McGhee J R. Helper Th1 and Th2 cell responses following mucosal or systemic immunization with cholera toxin. Vaccine. 1994;12:903–911. doi: 10.1016/0264-410x(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 38.Xu-Amano J, Kiyono H, Jackson R J, Staats H F, Fujihashi K, Burrows P D, Elson C O, Pillai S, McGhee J R. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J Exp Med. 1993;178:1309–1320. doi: 10.1084/jem.178.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]