Abstract
Background.
Cognitive impairments, which contribute to the profound functional deficits observed in psychotic disorders, have found to be associated with abnormalities in trial-level cognitive control. However, neural tasks operate within the context of sustained cognitive states, which can be assessed with ‘background connectivity’ following the removal of task effects. To date, little is known about the integrity of brain processes supporting the maintenance of a cognitive state in individuals with psychotic disorders. Thus, here we examine background connectivity during executive processing in a cohort of participants with first-episode psychosis (FEP).
Methods.
The following fMRI study examined background connectivity of the dorsolateral prefrontal cortex (DLPFC), during working memory engagement in a group of 43 patients with FEP, relative to 35 healthy controls (HC). Findings were also examined in relation to measures of executive function.
Results.
The FEP group relative to HC showed significantly lower background DLPFC connectivity with bilateral superior parietal lobule (SPL) and left inferior parietal lobule. Background connectivity between DLPFC and SPL was also positively associated with overall cognition across all subjects and in our FEP group. In comparison, resting-state frontoparietal connectivity did not differ between groups and was not significantly associated with overall cognition, suggesting that psychosis-related alterations in executive networks only emerged during states of goal-oriented behavior.
Conclusions.
These results provide novel evidence indicating while frontoparietal connectivity at rest appears intact in psychosis, when engaged during a cognitive state, it is impaired possibly undermining cognitive control capacities in FEP.
Keywords: Cognition, fMRI, functional connectivity, psychosis, schizophrenia, working memory
Introduction
Cognitive deficits are a major contributor to poor psychosocial functioning in individuals with schizophrenia spectrum disorders (Bowie & Harvey, 2006; Green, Kern, Braff, & Mintz, 2000; Shamsi et al., 2011). These deficits impact a variety of cognitive processes, including executive functioning (i.e. attention, working memory (WM) maintenance), higher-order executive function (i.e. manipulation, future planning), processing speed, and episodic memory (Dickinson, Ramsey, & Gold, 2007). Deficits in WM, considered to be central in the pathophysiology of schizophrenia, are observed early in the illness, predating the emergence of psychotic symptoms in individuals with first-episode psychosis (FEP), and are largely persistent throughout the lifespan (Mesholam-Gately, Giuliano, Goff, Faraone, & Seidman, 2009; Rund et al., 2007; Seidman et al., 2016; Szoke et al., 2008).
A robust literature examines functional neuroimaging correlates of impairment in executive functioning, including WM-related processes, in psychotic disorders. Task-based WM deficits have been linked with abnormal neural activation across regions within executive network circuitry, including the dorsolateral prefrontal cortex (DLPFC) and parietal cortex (Minzenberg, Laird, Thelen, Carter, & Glahn, 2009). Studies of intrinsic functional connectivity during resting state have also found impairments in executive networks in schizophrenia (Littow et al., 2015; Manoliu et al., 2014; Woodward, Rogers, & Heckers, 2011). However, findings from these imaging modalities, as well as broader efforts focused on core schizophrenia deficits, have not established clinically useful neural correlates of the cognitive deficits in psychotic disorders (Green, Harris, & Nuechterlein, 2014), suggesting the need for further exploration of novel mechanisms and correlates.
Background connectivity, a neuroimaging measure used to assess connectivity unique to task states, removes transient brain activation in response to individual trial-specific events (i.e. a WM cue) to better characterize state-dependent shifts that extend across the entire task (i.e. an entire run of a WM task) (Al-Aidroos, Said, & Turk-Browne, 2012; Elkhetali et al., 2019; Murty et al., 2018; Norman-Haignere, McCarthy, Chun, & Turk-Browne, 2012). While most analyses of task-based neuroimaging data focus on responses to specific trials on the magnitude of seconds, background connectivity allows for the assessment of executive states unfolding over the course of seconds to minutes. Removing event-evoked activity provides a means to uniquely characterize differences in baseline neural circuitry during a task-related state. In this way, background connectivity provides a qualitatively different characterization of connectivity than can be observed during discrete task epochs. While research has shown the utility of background connectivity as it relates to executive function in healthy populations, relatively little research has utilized this technique to characterize neural deficits related to cognition in psychotic disorders. Beyond impairments in processes supporting the generation of event-specific responses to individual task trials, psychotic disorders may be related to limitations in the ability to maintain sustained control over goal-directed information processing over extended periods of time (Barch & Ceaser, 2012; Braver, Reynolds, & Donaldson, 2003; Cohen, Braver, & Brown, 2002; Reynolds, West, & Braver, 2009). Critically, there may be unexplored links between state-dependent engagement of executive domains and pathological domains of illnesses, such as schizophrenia, possibly in relation to psychopathology or cognition.
In this study, we examined background connectivity of executive networks engaged during a WM task in a cohort of participants presenting with FEP. Background connectivity within executive networks was isolated by regressing task-related events out of fMRI time-courses and characterizing functional connectivity by seeding the DLPFC. To determine if deficits were specific to connectivity during goal-oriented executive function, this signal extracted during a WM task was compared to both task-related activation and intrinsic functional connectivity characterized during resting state. We then examined background connectivity in the context of psychopathology and cognitive performance. Based on the severity of cognitive deficits in schizophrenia and prior work implicating DLPFC dysfunction, as well as a rich literature detailing a role for the DLPFC in maintaining prolonged goal states during executive function (Braver, 2012; Reynolds, O’Reilly, Cohen, & Braver, 2012), we hypothesized that abnormalities exist in the background connectivity of the DLPFC during WM engagement and would be associated with global cognitive impairments.
Methods and materials
Participants and assessments
This study included 43 individuals entering treatment for a first episode of a psychotic disorder, recruited from clinical programs of UPMC Western Psychiatric Hospital. Our FEP group was examined in relation to a group of 35 healthy control (HC) participants matched for age and sex. Clinical details are described in the online Supplementary Methods and Materials.
Comprehensive demographic information was collected for each participant. Ratings of psychopathology were administered via the Brief Psychiatric Rating Scale (BPRS) (Woerner, Mannuzza, & Kane, 1988). Cognition was examined with the Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery (MCCB), which was developed for the cognitive domains impaired in schizophrenia (Nuechterlein et al., 2008). All study procedures were approved by the University of Pittsburgh Institutional Review Board.
WM task and resting state
A description of our task is provided in prior work (Jalbrzikowski et al., 2017; Manivannan et al., 2019). Patients and controls underwent fMRI scanning while performing two runs of a 6 min, event-related, spatial WM task diagrammed in online Supplementary Fig. S1a. Participants were presented with either one or three colored circles on both left and right sides of the screen (two or six total circles). They were instructed to remember the color pattern of the one (low load) or three circles (high load) on the side of the screen indicated by an arrow. Each trial consisted of a cue, 700–1400 ms in length, during which the WM event was presented (encoding phase); a delay period of either 1 or 3 s duration (maintenance phase); and a probe, presented for up to 2 s, while the patient indicated via a button press whether a color change occurred (retrieval phase). Subjects completed 64 full trials within the total 12 min of data acquisition divided into two 6 min runs. The task included ‘catch’ trials with either the cue alone (N = 16) or cue and delay (N = 16) periods, which provided a better estimation of hemodynamic response. The number of correct responses and reaction time of correct responses were used to assess WM performance.
During the resting-state scan, participants were asked to keep their eyes open and look at a fixation cross at the center of the screen for the duration of the scan.
Image acquisition
Imaging data were acquired on a 3.0 Tesla Siemens TIM Trio scanner at the University of Pittsburgh MR research center. Additional details of our image acquisition are described in the online Supplementary Methods and Materials.
Image analysis and preprocessing
Following quality control measures, we underwent a rigorous method for data preprocessing consistent with our prior work (Jalbrzikowski et al., 2017; Manivannan et al., 2019; Murty et al., 2018). Details are described in the online Supplementary Methods and Materials.
Background connectivity
To examine background connectivity of the executive state, independent of task-evoked signals, we implemented background connectivity, an approach that has been adopted by several studies (Al-Aidroos et al., 2012; Murty et al., 2018). We first regressed out task-related components of the time series using a general linear model (GLM). The time series of both WM runs were concatenated for a total of 720 volumes. Task-related events were modeled using 3dDeconvolve in AFNI with a canonical hemodynamic response function. We modeled the following task conditions and events related to all task phases: encoding regressors modeled for the length of the cue presentation (200 ms + variable interval), maintenance regressors modeled for the duration of the delay period, and retrieval regressors modeled from the onset of the target probe to each corresponding reaction time. To further ensure that our background connectivity results did not include residual event-evoked activation, we repeated group comparisons, described below, following high- and lowpass filtering of images at 0.08 and 0.009, respectively.
Connectivity of the DLPFC was then examined on this residual data. We chose the DLPFC given its structural and functional deficits in schizophrenia and associated impairments in executive function (Barch et al., 2001; Karlsgodt et al., 2009; Lewis & Gonzalez-Burgos, 2008; Perlstein, Carter, Noll, & Cohen, 2001; Potkin et al., 2009; Van Snellenberg et al., 2016), as well as its broader role in the maintenance of goal states during executive function (Braver, 2012; Reynolds et al., 2012). These signals are believed to represent state-dependent neural activity that was occurring during the task but not related to responses to specific events. Seed regions of interest capturing the right and left DLPFC regions important for WM were generated based on group-level activation during WM maintenance (online Supplementary Fig. S1b). Peak regions of activation during the WM task across maintenance phases were defined within Brodmann area 9 and spherical seed regions of interest with a 10 mm radius were generated centered around these peaks (online Supplementary Fig. S1c). We confirmed that the location of our DLPFC seeds corresponds with prior work by using Neurosynth (06/20/2018) (Yarkoni, Poldrack, Nichols, Van Essen, & Wager, 2011). The search term ‘working memory’ was entered to generate a reverse inference map representing boundaries of meta-analytic activation of the WM network. We confirmed that our DLPFC seeds were within the meta-analytic WM network map.
For each study participant, the background time series was extracted from each seed (left and right DLPFC) and used to generate whole-brain correlation maps based on a Pearson’s correlation that then underwent a Fisher Z transformation. These maps, representing background connectivity of the DLPFC during WM engagement, were then entered into whole-brain group-level analyses to examine differences between FEP and HC participants.
Significance was defined in our main activation and connectivity analyses by a voxel-wise threshold of p < 0.005, and family-wise error correction at p < 0.05. AFNI’s 3dFWHMx was used to estimate the amount of smoothing present using a spatial auto-correlation function. The resulting values were entered (input parameters: 0.31, 7.92, 16.75) into 3dClustSim to determine, with 10 000 iterations, the number of contiguous voxels needed for cluster correction at p < 0.05. The resulting cluster size was 181 voxels.
Task-based trial-level activation
To ensure that the background state is an independent source of variance form of task-based events, we also compared background connectivity with task activation. Methods are described in our previous work and also in the online Supplementary Methods and Materials (Jalbrzikowski et al., 2017; Manivannan et al., 2019).
Resting-state functional connectivity
To determine if the background connectivity state was specific to task contexts, we compared background DLPFC connectivity with resting-state functional connectivity. Following preprocessing (see online Supplementary Methods and Materials), we generated whole-brain resting-state functional connectivity maps based on a Pearson’s correlation, followed by a Fisher Z transformation, using left and right DLPFC seed ROIs (https://github.com/LabNeuroCogDevel/SzAttWMproc/tree/master/DUP/seed_fc/). Resting-state functional connectivity between DLPFC and each significant cluster that emerged from background connectivity analyses was examined.
Cognitive, behavioral, and post-hoc analyses
Our secondary aim was to examine background connectivity results in relation to cognition. Extracted values for background connectivity and resting-state connectivity were compared with MCCB scores using a GLM approach including with MCCB scores as the dependent variable and connectivity, group (FEP, HC), and their interaction as the independent variables. If an analysis yielded a significant interaction, post-hoc regressions were run separately for each group (FEP, HC). Further, for any clusters that showed significant brain–behavior relationships in either group, we ran a post-hoc test evaluating each of the sub-scores of the MCCB. We included all sub-scores in the same model, in order to account for any covariation across sub-scores measures to account for shared variance across sub-score measures.
In addition, we performed post-hoc analyses with extracted values in relation to psychopathology (total psychopathology, positive symptoms, negative symptoms), medication exposure, age, sex, WM performance measures, and in-scanner movement as measured by frame displacement (FD).
Results
Participant characteristics
We included 43 FEP and 35 HC participants. Demographic and clinical information for all participants is displayed in Table 1. Fourteen patients were naïve to antipsychotic treatment at the time of scanning and the remaining patients had been treated for less than 2 months with antipsychotic drugs. Chlorpromazine equivalents of antipsychotic medication dose at the time of scanning were calculated to account for possible drug effects on imaging data (Andreasen, Pressler, Nopoulos, Miller, & Ho, 2010). Within medicated participants, the mean dose of antipsychotic treatment at the time of scanning in chlorpromazine equivalents was 152.4 ± 101.6 mg.
Table 1.
Baseline demographics and clinical ratings
| FEP (N = 43) | HC (N = 35) | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Mean | s.d. | N | Mean | s.d. | N | T/χ2 | p value |
| Age (years) | 22.37 | 4.66 | 22.22 | 3.52 | −0.156 | 0.8765 | ||
| Sex | 0.026 | 0.8721 | ||||||
| Males | 29 | 23 | ||||||
| Females | 14 | 12 | ||||||
| IQ (WASI) | 105.09 | 13.42 | 108.03 | 8.88 | 1.159 | 0.2503 | ||
| MCCB (total) | 37.59 | 15.75 | 49.57 | 6.33 | 4.374 | 0.00006 | ||
| Percent correct | ||||||||
| Load 1 | 82.12 | 13.93 | 91.61 | 7.34 | −3.856 | 0.00026 | ||
| Load 3 | 67.51 | 14.99 | 76.70 | 10.97 | −3.120 | 0.00256 | ||
| Reaction time (seconds) | ||||||||
| Load 1 | 1053.92 | 158.52 | 973.46 | 191.75 | 1.990 | 0.05077 | ||
| Load 3 | 1119.14 | 191.81 | 1076.67 | 203.60 | 0.940 | 0.3502 | ||
| #Antipsychotic naive | NA | |||||||
| BPRS (total) | 45.29 | 8.87 | NA | |||||
| BPRS (psychotic symptoms) | 8.81 | 2.90 | NA | |||||
| BPRS (negative symptoms) | 6.76 | 2.68 | NA | |||||
| Mean antipsychotic dosage (chlorpromazine equivalent in mg) | 152.4 | 101.6 | NA | |||||
As expected, the FEP group had significantly lower MCCB scores when compared to controls (p = 0.0006), consistent with established findings in schizophrenia (August, Kiwanuka, McMahon, & Gold, 2012; Nuechterlein et al., 2008). Task performance also reflected the core deficits of the disorder, as reported in previous studies. Average WM accuracy during the low and high loads of the task in the FEP group was 82% and 67%, and reaction times were 1054 and 1119 ms, respectively. Accuracy was significantly higher in the HC group 92% (p = 0.003) and 77% (p = 0.003), for low and high loads. Reaction time was significantly lower in the HC group for the low load (973 ms; p = 0.05), but not the high load (1076 ms; p = 0.35).
Background executive connectivity
Our primary aim in this study was to test the hypothesis that background engagement of executive processing contributes to neural deficits in individuals with FEP, independent of intrinsic resting-state connectivity and event-based activation. To address this hypothesis, we examined background connectivity of the DLPFC across the task-regressed visuospatial WM sequence. Whole-brain background connectivity maps were generated for each study participant for left and right DLPFC seed regions of interest based on trial-level task activation peaks. At the group level, whole-brain differences in connectivity were examined between HC and FEP participants.
Patients showed significantly lower background connectivity compared to HCs between the right DLPFC and the right superior parietal lobule (SPL), left SPL, and left inferior parietal lobule (p < 0.05, whole-brain corrected; Table 2; Fig. 1). No significant differences between patients and controls in left DLPFC background connectivity were found. To further confirm that our background connectivity results did not include residual event-evoked signals, we performed group comparisons with bandpass filtered data. Results above were virtually identical to group comparisons with bandpass filtering (online Supplementary Table S1).
Table 2.
Group differences in background connectivity
| Cluster | x | y | Z | T score (max) | K (voxel size) | Brodmann area | Functional connection |
|---|---|---|---|---|---|---|---|
| 1 | 22 | −70 | 47 | −5.47 | 297 | 7 | Right superior parietal lobule |
| 2 | −47 | −51 | 51 | −4.24 | 253 | 7 | Left superior parietal lobule |
| 3 | −24 | −72 | 61 | −4.22 | 198 | 40 | Left inferior parietal lobule |
Fig. 1.
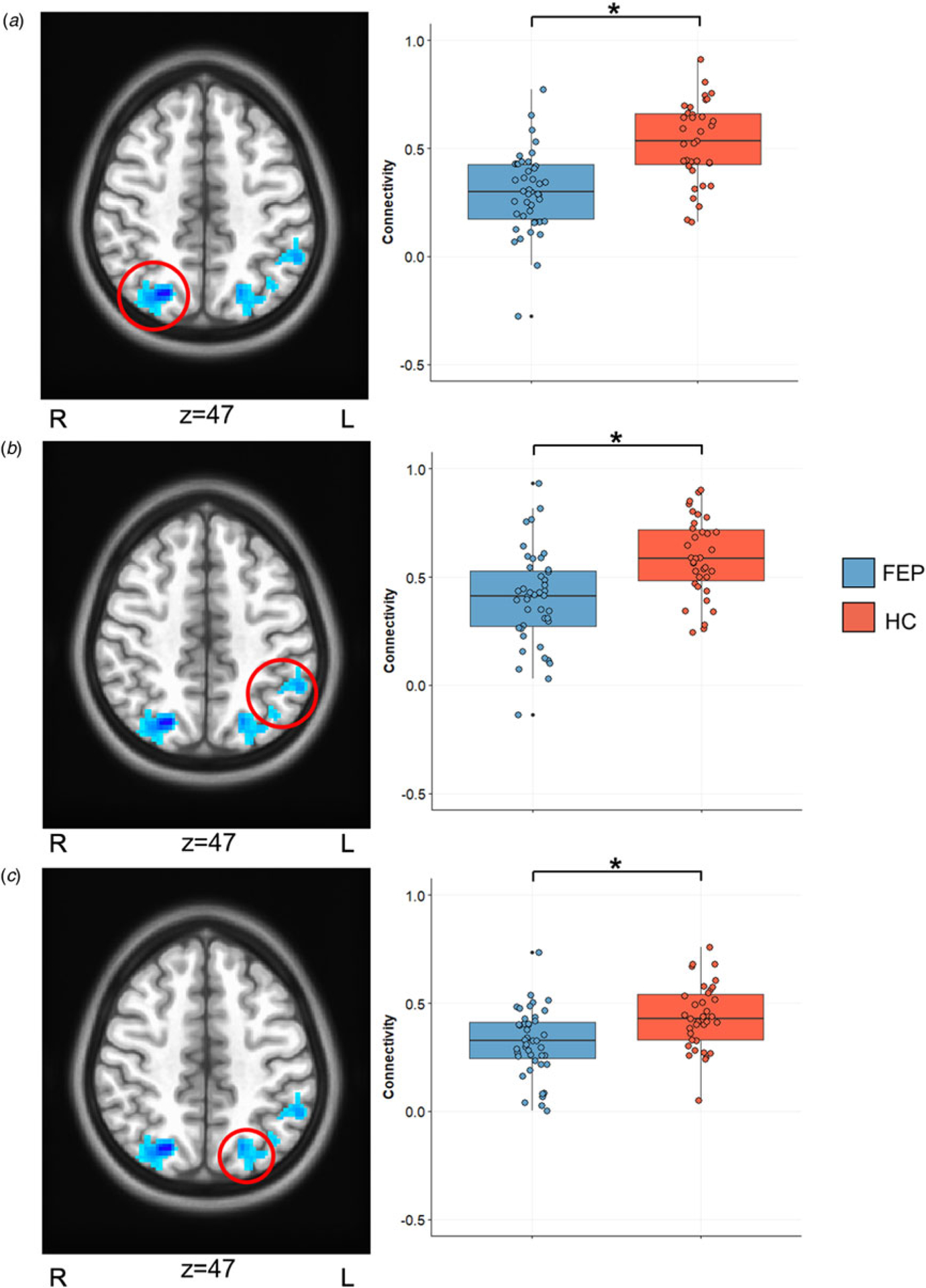
Background connectivity result. Significant whole-brain group differences in background connectivity are displayed in significant clusters listed in Table 2: (a) right superior parietal lobule, (b) left inferior parietal lobule, (c) left superior parietal lobule.
To ensure that our significant background connectivity results were representative of a unique brain state, we examined resting-state connectivity between peak right DLPFC seeds and significant clusters in Table 2. No significant differences between FEP and HC participants were observed in left or right DLPFC resting-state connectivity. Similarly, we examined activation during the WM task’s high load maintenance phase to determine whether activation is related to our background connectivity results. Neither the activation within the clusters was significant in our background connectivity analysis, nor our DLPFC seeds were significantly different between study groups (p > 0.05; online Supplementary Fig. S2). These confirmatory results demonstrate that our background connectivity results represent a unique abnormality in FEP participants, unrelated to intrinsic connectivity or task-based activation.
We performed further analyses to examine significant background DLPFC connectivity findings in relation to resting-state functional connectivity. Resting-state DLPFC connectivity from peaks of our significant clusters (Table 2) was subtracted from background connectivity to examine percent signal change related to task engagement. As displayed in Fig. 2, two out of three of our clusters demonstrate greater increases during task-engagement than rest in HC participants. These results do not survive Bonferroni correction, but are noted to have moderate effect sizes (0.455, 0.545). In further regression analyses, background connectivity in peak regions of our three significant clusters was examined between groups with resting-state connectivity as a covariate. All clusters remained significant (p < 0.001).
Fig. 2.
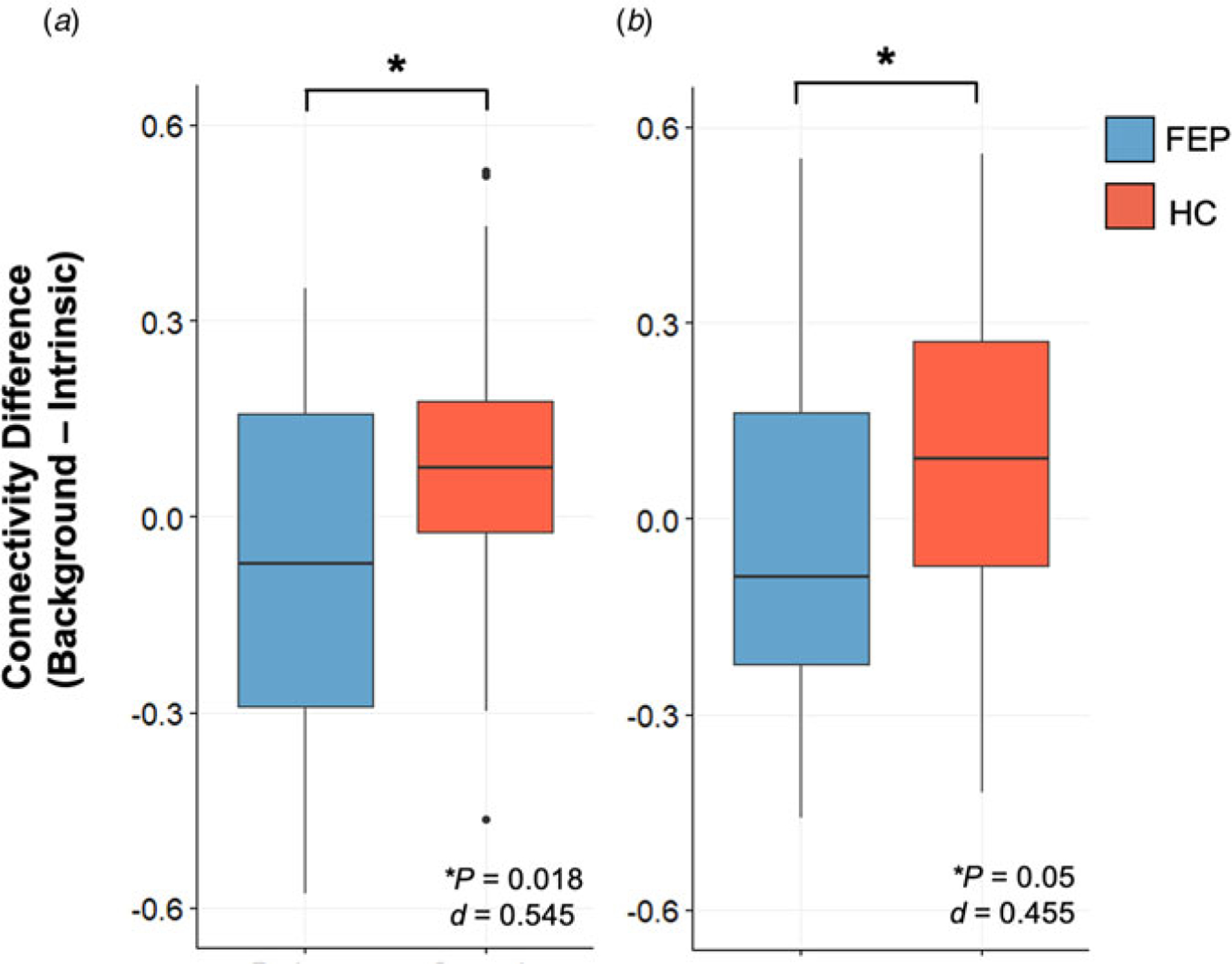
Background connectivity v. intrinsic connectivity. The difference in background and intrinsic connectivity was examined for all three significant clusters. We observed significantly lower differences in FEP than HC participants from peak region within cluster (a) 1 and (b) 3, listed in Table 2. No significant findings were observed in cluster 2.
Finally, we performed an analysis directly comparing differences across task-based background connectivity and resting-state connectivity in the FEP and HC groups. We found a main effect of group across all three clusters (p < 0.001). Further, we found significant group × context interactions in the right SPL [cluster 1, group × context: F(1): 5.94, p = 0.02] and left inferior parietal lobule [cluster 3, group × context: F(1): 4.3, p = 0.03], such that there was greater connectivity in the HC group v. FEP using background connectivity [cluster 1: t(69.5) = 5.3, p < 0.001; cluster 1: t(70.0) = 3.1, p = 0.002], but not resting-state connectivity [cluster 1: t(71.5) = 1.3, p = 0.2; cluster 1: t(70.2) = −0.15, p = 0.88].However, there was no such interaction in the left SPL [cluster 2, F(1): 0.23, p = 0.74], suggesting there were no significant differences in connectivity with this cluster as a function of state.
Background DLPFC connectivity relationship with cognition
In secondary analyses, we examined whether background connectivity during executive processing related to cognition in our study cohort. We examined our background DLPFC connectivity findings (Table 2) with MCCB scores. We observed a significant group-by connectivity-interaction [β (s.e.) = 0.59 (0.23), p < 0.05 Bonferroni-corrected], such that background connectivity between the DLPFC and left SPL (cluster 2) was positively correlated with MCCB scores in FEP [β (s.e.) = 0.37 (0.15), p = 0.02], but not in controls [β (s.e.) = −0.28 (0.16), p = 0.1] (Fig. 3). To further specify the nature of this relationship in FEP, we estimated the relationship of connectivity and MCCB sub-domain scores in the same mode to account for any covariation across sub-scores measures. We found that connectivity positively predicted performance in the visual learning domain [β (s.e.) = 0.79 (0.36), p = 0.04], and showed trend-level effects in the attention [β (s.e.) = 0.93 (0.46), p = 0.05] and WM [β (s.e.) = 0.06 (0.03), p = 0.07] domains, while all other domains were non-significant (p > 0.10). There were no other main effects of background connectivity or group × connectivity interactions for the other significant clusters.
Fig. 3.
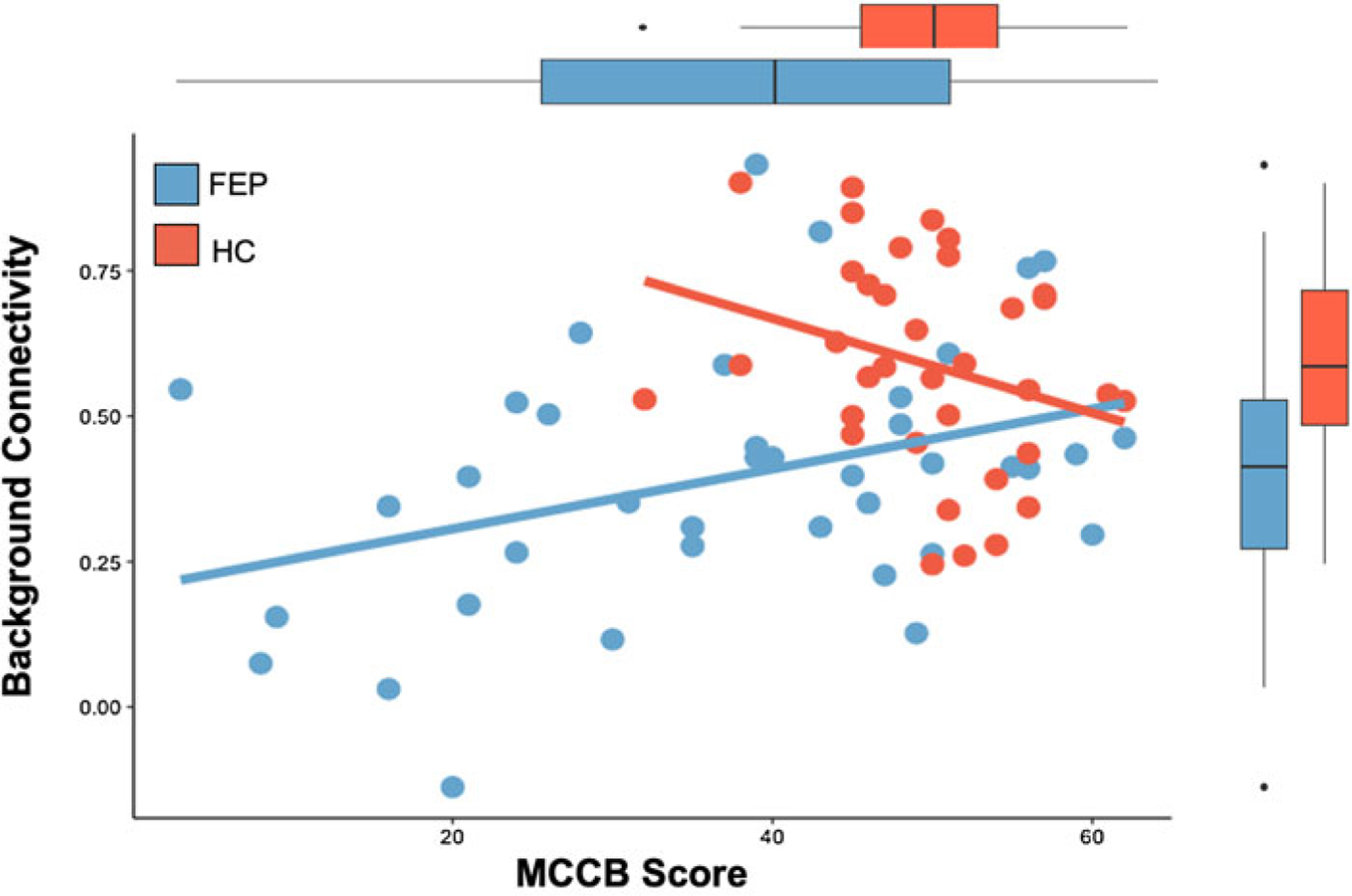
Background DLPFC connectivity results and cognition. Relationship between background connectivity between right DLPFC and left superior parietal lobule (cluster 2 from Table 2) and MCCB composite scores in FEP and HC participants are displayed. The relationship in FEP participants is significant (p = 0.02), but not in the HC group (p = 0.1).
Post-hoc analyses
We also considered the possibility that background connectivity could be related to symptomatology, independent of cognition. Thus, we examined background connectivity findings in relation to psychopathology (overall symptoms, positive symptoms, and negative symptoms): no significant findings were noted (p < 0.05, corrected). In addition, we performed post-hoc analyses to examine whether our background connectivity findings related to task performance were confounded by age, sex, med exposure, or FD. We did not observe significant correlations with our results (Table 2) and any of these variables.
Discussion
Here, we report evidence for aberrant context-specific connectivity of the DLPFC during WM processing in a cohort of participants with FEP and minimal prior treatment. To our knowledge, this is the first study to examine background connectivity of the prefrontal cortex during executive functioning in early psychotic illness. Specifically, we seeded the DLPFC and observed decreased context-specific connectivity during WM processing parietal portions of the central executive network. This finding, which survived whole-brain correction as well as post-hoc analysis utilizing temporal filtering to remove any residual event-evoked responses, was remarkably limited to regions that comprise the widely appreciated central executive network. Differences between resting-state and context-specific connectivity were significantly greater in HCs relative to our FEP group, suggesting that context-specific connectivity represents a unique state separate from intrinsic central executive circuitry. More broadly, we report a relationship between context-specific connectivity of the DLPFC and global cognition, possibly indicating a phenotypic manifestation of this brain state.
Independent of the brain’s intrinsic architecture at rest, or its stimulus-driven functional activation, accumulating evidence suggests that context-specific attentional engagement may represent a distinct brain state. Here, we focused on contextual engagement during WM processing. While prior work has demonstrated that intrinsic networks can be modulated by neural engagement with a cognitive task (Cole, Bassett, Power, Braver, & Petersen, 2014), such task-based modulations in connectivity can be confounded by the activity induced by task-specific stimuli (Arfanakis et al., 2000; Fox & Raichle, 2007). Removal of event-related activity from the BOLD response, via regression, as performed in the present study, results in a ‘background’ state that is context-specific, but free of task-specific activation (Al-Aidroos et al., 2012; Norman-Haignere et al., 2012). Evidence suggests that relevant brain regions important for a specific task enter and maintain a task-specific state to optimize performance (Al-Aidroos et al., 2012; Haynes, Tregellas, & Rees, 2005). Critically, this engagement occurs across the entire task-specific context rather than in response to individual stimuli, and thus reflects the maintenance of goal-states across an entire task period rather than goal states that are evoked in response to individual trials. Prior work shows that the regions engaged are dependent on the nature of the task and the related behavioral state (Chadick & Gazzaley, 2011).
An important piece of our results is that we observe less engagement of this state-dependent network during WM beyond the intrinsic signal, representing an impaired ability to achieve this context-specific brain state in FEP participants. To date, much of the work with ‘background’ states is limited to sensory systems (Al-Aidroos et al., 2012; Elkhetali et al., 2019; Norman-Haignere et al., 2012; Tompary, Al-Aidroos, & Turk-Browne, 2018), and their relationship to higher-order regions (Griffis, Elkhetali, Burge, Chen, & Visscher, 2015). More recent evidence has focused on reward processing by showing that ‘background’ motivational contexts relate to mesolimbic connectivity and task-performance across adolescent development (Murty et al., 2018). We extend this work to executive functioning in the present. Together, these findings suggest that it is critical to assess network connectivity during relevant task states to reveal group differences.
Our finding of reduced context-specific frontoparietal connectivity during WM in participants with FEP contributes to the vast literature on WM deficits in schizophrenia-spectrum disorders (Barch et al., 2001; Jalbrzikowski et al., 2017; Karlsgodt et al., 2009; Minzenberg et al., 2009; Van Snellenberg et al., 2016). While prior reports have focused on active WM engagement in the FEP cohort described here (Jalbrzikowski et al., 2017; Manivannan et al., 2019), the present study illustrates the importance for context-specific brain states during visuospatial WM, and in ability to generate the task states necessary to sub-serve executive function. Overall, WM is a fundamental component of executive functioning (D’Esposito & Postle, 2015), and a complex function, requiring a variety of sub-processes including short-term information storage of visuospatial information, and simultaneous active manipulation of the stored material (Cowan, 2017; Engle, Tuholski, Laughlin, & Conway, 1999). Our finding of decreased context-specific DLPFC connectivity with the parietal lobe reflects limitations in engaging the frontoparietal network, which supports the ability to generate optimal executive responses by linking visuospatial and higher-order processing regions (Marek & Dosenbach, 2018). Within this framework, FEP patients may be able to generate the appropriate sensory representations in the visuospatial cortex but are unable to sustain engagement of goal states throughout the task. This interpretation aligns well with the nature of background connectivity which relates to a prolonged maintenance of goal-states over the magnitude of the entire task, as opposed to individual trials. Alternatively, our finding of ‘background’ connectivity deficits during WM may relate to deficits in storage capacity (Hahn, Robinson, Leonard, Luck, & Gold, 2018). Evidence suggests that posterior parietal cortical engagement during WM may be important for information storage during WM (Hahn et al., 2018; Xu, 2017). The posterior parietal cortex may coordinate stimulus representations or the maintenance of attention of items with features characterized by sensory regions (Hahn et al., 2018; Mitchell & Cusack, 2008; Xu & Chun, 2006). However, this interpretation may be less likely, given that the FEP-related deficits in context-specific connectivity we describe here were not related to WM performance. Although WM performance (accuracy and reaction time) were worsened overall, relative to the HC group, consistent with known cognitive deficits of FEP. Further studies with more robust paradigms that characterize the contents and capacity of WM storage will be important to further breakdown WM performance in relation to task-regressed functional connectivity.
Of note, our background connectivity findings were lateralized to the right DLPFC seed region. This coincides with a previous finding in a sub-cohort of the participants in this study (Jalbrzikowski et al., 2017) that showed an activation decrease during the WM task in the right DLPFC, suggesting lateralized deficits. Previous studies have also found that individuals with schizophrenia activate the right DLPFC less during cognitive control and WM (Perlstein et al., 2001; Ray et al., 2017). Moreover, lateralization across development has been reported (Nagel, Herting, Maxwell, Bruno, & Fair, 2013). Thus, we believe that abnormalities in the engagement of the DLPFC during visuospatial WM may differentially occur on the right side, possibly in relation to abnormal developmental processes.
The differences we report in DLPFC connectivity across groups were not apparent during resting-state fMRI. Prior work has characterized resting-state fMRI within fronto-parietal networks in individuals with psychotic disorders, showing mixed results for a selective DLPFC deficit. Recently, meta-analyses of resting-state fMRI have examined resting-state network connectivity across schizophrenia and normative samples. Across these studies, no consistent differences were observed for DLPFC connectivity with regions within fronto-parietal networks, either in individuals with chronic illness or individuals within early phases of the disorder (Dong, Wang, Chang, Luo, & Yao, 2018; Gong et al., 2020; Li et al., 2019; O’Neill, Mechelli, & Bhattacharyya, 2019). One meta-analytic study did report reliable differences in DLPFC connectivity, but with regions of the thalamus and ventral attention network (Dong et al., 2018). Thus, we suggest that the DLPFC may be a central node contributing to executive function deficits in psychotic disorders, but to accurately assess its connectivity, task states must be considered.
The context-specific abnormalities of the executive network described here represent a unique contribution to overall cognition, which we measured with the MCCB, a targeted assessment of cognitive impairments in schizophrenia and related psychotic disorders. The elucidation of processes that predict the cognitive deficits of the disorder is essential for the development of therapeutic interventions. A few studies report links between MCCB score in patients with chronic schizophrenia and imaging-based markers: an fMRI study at rest demonstrated a negative relationship between hippocampal activity and MCCB score (Tregellas et al., 2014), while a separate magnetic resonance spectroscopy study found related NAA/Cr with composite MCCB score (Jarskog et al., 2013). Meanwhile, a data-driven, multimodal study clustered findings within cortico-striato-thalamic circuits in relation to MCCB scores (Sui et al., 2015). The present study, however, contributes to this literature with evidence linking MCCB deficits and a novel analysis of task-based neural engagement. The findings suggest that an inability to generate task-relevant cognitive states may be core to a broad range of executive function deficits. Given that options for treatment of cognitive impairments in schizophrenia are limited, future studies could focus on interventions that help individuals with psychosis to generate and maintain task-relevant goals. Further, given that our results relate to a broad assessment of cognition, context-specific connectivity could potentially serve to inform interventional studies via neuromodulatory approaches (Dokucu, 2015).
The findings presented here should be considered with several limitations. For one, this study was cross-sectional in nature, and longitudinal changes in context-specific connectivity across treatment for FEP could not be examined. Relatedly, individuals with FEP are typically diagnostically heterogeneous and undifferentiated. It is unclear whether our results represent a shared finding, or if there is variation in context-specific connectivity depending on clinical trajectory and diagnostic specificity. Further studies in various clinical subpopulations, including participants with acute symptomatology and chronic schizophrenia, are necessary. Similarly, given that cognitive deficits predate the onset of psychotic disorders, studies focused on individuals determined to be clinically high risk for the development of psychosis should be evaluated with background connectivity in future work. Finally, replications and extensions of this study will be important for further development of a possible neurocognitive assay for schizophrenia. Subsequent work is necessary to differentiate whether the deficits we characterize are specific to WM states or whether they generalize more broadly to states of cognitive control and/or increased demands on attention. Meanwhile, the underlying neuronal and molecular underpinnings of abnormal context-specific connectivity remain unknown. Multimodal analyses that include EEG and spectroscopic assays may further deconstruct the neurobiology underlying state-dependent connectivity within the executive network.
To conclude, we report the results of an examination of context-specific functional connectivity of the DLPFC during WM engagement in a cohort of participants with FEP, separate from rest or task-based activation. We report novel results that demonstrate network-wide impairments in executive processing. Our findings between frontoparietal regions related globally to cognition. Future work will be important to advance these findings in the context of interventions for neurocognitive deficits observed in psychotic disorders.
Supplementary Material
Acknowledgements.
We thank the faculty and staff of the UPMC WPH Psychosis Recruitment and Assessment Core for their assistance in diagnostic and psychopathological assessments; Dean Salisbury, PhD, Raymond Cho, MD, and Carl Olson, PhD for assistance in task development, and finally, our patients and their families.
Financial support.
The project described was supported by a NARSAD Young Investigator Awards by the Brain & Behavior Research Foundation (Deepak Sarpal, MD, Vishnu Murty PhD); and the National Institutes of Health through grants: K23MH110661 (Deepak Sarpal, MD, PI), K01MH111991 (Vishnu Murty, PhD), P50 MH103204, Conte Center for Translational Mental Health Research (David A. Lewis, MD, Director), and the UL1 TR001857 funded Clinical and Translational Science Institute of the University of Pittsburgh (Steven E. Reis, MD, PI).
Footnotes
Supplementary material. The supplementary material for this article can be found at https://doi.org/10.1017/S0033291720004201.
Conflict of interest. None.
Ethical standards. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All study procedures were approved by the University of Pittsburgh Institutional Review Board.
References
- Al-Aidroos N, Said CP, & Turk-Browne NB (2012). Top-down attention switches coupling between low-level and high-level areas of human visual cortex. Proceedings of the National Academy of Sciences of the USA, 109 (36), 14675–14680. doi: 10.1073/pnas.1202095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, & Ho BC (2010). Antipsychotic dose equivalents and dose-years: A standardized method for comparing exposure to different drugs. Biological Psychiatry, 67(3), 255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfanakis K, Cordes D, Haughton VM, Moritz CH, Quigley MA, & Meyerand ME (2000). Combining independent component analysis and correlation analysis to probe interregional connectivity in fMRI task activation datasets. Magnetic Resonance Imaging, 18(8), 921–930. doi: 10.1016/s0730-725x(00)00190-9. [DOI] [PubMed] [Google Scholar]
- August SM, Kiwanuka JN, McMahon RP, & Gold JM (2012). The MATRICS consensus cognitive battery (MCCB): Clinical and cognitive correlates. Schizophrenia Research, 134(1), 76–82. doi: 10.1016/j.schres.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A 3rd, Noll DC, & Cohen JD (2001). Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Archives of General Psychiatry, 58(3), 280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- Barch DM, & Ceaser A (2012). Cognition in schizophrenia: Core psychological and neural mechanisms. Trends in Cognitive Sciences, 16(1), 27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie CR, & Harvey PD (2006). Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatric Disease and Treatment, 2(4), 531–536. doi: 10.2147/nedt.2006.2.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS (2012). The variable nature of cognitive control: A dual mechanisms framework. Trends in Cognitive Sciences, 16(2), 106–113. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Reynolds JR, & Donaldson DI (2003). Neural mechanisms of transient and sustained cognitive control during task switching. Neuron, 39 (4), 713–726. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- Chadick JZ, & Gazzaley A (2011). Differential coupling of visual cortex with default or frontal-parietal network based on goals. Nature Neuroscience, 14(7), 830–832. doi: 10.1038/nn.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Braver TS, & Brown JW (2002). Computational perspectives on dopamine function in prefrontal cortex. Current Opinions in Neurobiology, 12(2), 223–229. doi: 10.1016/s0959-4388(02)00314-8. [DOI] [PubMed] [Google Scholar]
- Cole MW, Bassett DS, Power JD, Braver TS, & Petersen SE (2014). Intrinsic and task-evoked network architectures of the human brain. Neuron, 83(1), 238–251. doi: 10.1016/j.neuron.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N (2017). The many faces of working memory and short-term storage. Psychonomic Bulletin & Review, 24(4), 1158–1170. doi: 10.3758/s13423-016-1191-6. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, & Postle BR (2015). The cognitive neuroscience of working memory. Annual Review of Psychology, 66, 115–142. doi: 10.1146/annurev-psych-010814-015031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D, Ramsey ME, & Gold JM (2007). Overlooking the obvious: A meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Archives of General Psychiatry, 64(5), 532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- Dokucu ME (2015). Neuromodulation treatments for schizophrenia. Current Treatment Options in Psychiatry, 2(3), 339–348. doi: 10.1007/s40501-015-0055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong D, Wang Y, Chang X, Luo C, & Yao D (2018). Dysfunction of large-scale brain networks in schizophrenia: A meta-analysis of resting-state functional connectivity. Schizophrenia Bulletin, 44(1), 168–181. doi: 10.1093/schbul/sbx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkhetali AS, Fleming LL, Vaden RJ, Nenert R, Mendle JE, & Visscher KM (2019). Background connectivity between frontal and sensory cortex depends on task state, independent of stimulus modality. NeuroImage, 184, 790–800. doi: 10.1016/j.neuroimage.2018.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, & Conway ARA (1999). Working memory, short-term memory, and general fluid intelligence: A latent-variable approach. Journal of Experimental Psychology: General, 128 (3), 309–331. doi: 10.1037/0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Fox MD, & Raichle ME (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience, 8(9), 700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Gong J, Wang J, Luo X, Chen G, Huang H, Huang R, … Wang Y (2020). Abnormalities of intrinsic regional brain activity in first-episode and chronic schizophrenia: A meta-analysis of resting-state functional MRI. Journal of Psychiatry & Neuroscience, 45(1), 55–68. doi: 10.1503/jpn.180245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Harris JG, & Nuechterlein KH (2014). The MATRICS consensus cognitive battery: What we know 6 years later. The American Journal of Psychiatry, 171(11), 1151–1154. doi: 10.1176/appi.ajp.2014.14070936. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, & Mintz J (2000). Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the ‘right stuff’? Schizophrenia Bulletin, 26(1), 119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Griffis JC, Elkhetali AS, Burge WK, Chen RH, & Visscher KM (2015). Retinotopic patterns of background connectivity between V1 and fronto-parietal cortex are modulated by task demands. Frontiers in Human Neuroscience, 9, 338. doi: 10.3389/fnhum.2015.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Robinson BM, Leonard CJ, Luck SJ, & Gold JM (2018). Posterior parietal cortex dysfunction is central to working memory storage and broad cognitive deficits in schizophrenia. The Journal of Neuroscience, 38(39), 8378–8387. doi: 10.1523/JNEUROSCI.0913-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes JD, Tregellas J, & Rees G (2005). Attentional integration between anatomically distinct stimulus representations in early visual cortex. Proceedings of the National Academy of Sciences of the USA, 102(41), 14925–14930. doi: 10.1073/pnas.0501684102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalbrzikowski M, Murty VP, Stan PL, Saifullan J, Simmonds D, Foran W, … Luna B (2017). Differentiating between clinical and behavioral phenotypes in first-episode psychosis during maintenance of visuospatial working memory. Schizophrenia Research, 197, 357–364. doi: 10.1016/j.schres.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarskog LF, Dong Z, Kangarlu A, Colibazzi T, Girgis RR, Kegeles LS, … Lieberman JA (2013). Effects of davunetide on N-acetylaspartate and choline in dorsolateral prefrontal cortex in patients with schizophrenia. Neuropsychopharmacology, 38(7), 1245–1252. doi: 10.1038/npp.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, Sanz J, van Erp TG, Bearden CE, Nuechterlein KH, & Cannon TD (2009). Re-evaluating dorsolateral prefrontal cortex activation during working memory in schizophrenia. Schizophrenia Research, 108(1–3), 143–150. doi: 10.1016/j.schres.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, & Gonzalez-Burgos G (2008). Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology, 33(1), 141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- Li S, Hu N, Zhang W, Tao B, Dai J, Gong Y, … Lui S (2019). Dysconnectivity of multiple brain networks in schizophrenia: A meta-analysis of resting-state functional connectivity. Frontiers in Psychiatry, 10, 482. doi: 10.3389/fpsyt.2019.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littow H, Huossa V, Karjalainen S, Jaaskelainen E, Haapea M, Miettunen J, … Kiviniemi VJ (2015). Aberrant functional connectivity in the default mode and central executive networks in subjects with schizophrenia – a whole-brain resting-state ICA study. Frontiers in Psychiatry, 6, 26. doi: 10.3389/fpsyt.2015.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manivannan A, Foran W, Jalbrzikowski M, Murty VP, Haas GL, Tarcijonas G, … Sarpal DK (2019). Association between duration of untreated psychosis and frontostriatal connectivity during maintenance of visuospatial working memory. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4(5), 454–461. doi: 10.1016/j.bpsc.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoliu A, Riedl V, Zherdin A, Muhlau M, Schwerthoffer D, Scherr M, … Sorg C (2014). Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophrenia Bulletin, 40(2), 428–437. doi: 10.1093/schbul/sbt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S, & Dosenbach NUF (2018). The frontoparietal network: Function, electrophysiology, and importance of individual precision mapping. Dialogues in Clinical Neuroscience, 20(2), 133–140. doi: 10.31887/DCNS.2018.20.2/smarek. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, & Seidman LJ (2009). Neurocognition in first-episode schizophrenia: A meta-analytic review. Neuropsychology, 23(3), 315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, & Glahn DC (2009). Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Archives of General Psychiatry, 66(8), 811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DJ, & Cusack R (2008). Flexible, capacity-limited activity of posterior parietal cortex in perceptual as well as visual short-term memory tasks. Cerebral Cortex, 18(8), 1788–1798. doi: 10.1093/cercor/bhm205. [DOI] [PubMed] [Google Scholar]
- Murty VP, Shah H, Montez D, Foran W, Calabro F, & Luna B (2018). Age-related trajectories of functional coupling between the VTA and nucleus accumbens depend on motivational state. The Journal of Neuroscience, 38(34), 7420–7427. doi: 10.1523/JNEUROSCI.3508-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Herting MM, Maxwell EC, Bruno R, & Fair D (2013). Hemispheric lateralization of verbal and spatial working memory during adolescence. Brain and Cognition, 82(1), 58–68. doi: 10.1016/j.bandc.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman-Haignere SV, McCarthy G, Chun MM, & Turk-Browne NB (2012). Category-selective background connectivity in ventral visual cortex. Cerebral Cortex, 22(2), 391–402. doi: 10.1093/cercor/bhr118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, … Marder SR (2008). The MATRICS consensus cognitive battery, part 1: Test selection, reliability, and validity. The American Journal of Psychiatry, 165(2), 203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- O’Neill A, Mechelli A, & Bhattacharyya S (2019). Dysconnectivity of large-scale functional networks in early psychosis: A meta-analysis. Schizophrenia Bulletin, 45(3), 579–590. doi: 10.1093/schbul/sby094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlstein WM, Carter CS, Noll DC, & Cohen JD (2001). Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. The American Journal of Psychiatry, 158(7), 1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Turner JA, Brown GG, McCarthy G, Greve DN, & Glover GH, … Fbirn. (2009). Working memory and DLPFC inefficiency in schizophrenia: The FBIRN study. Schizophrenia Bulletin, 35(1), 19–31. doi: 10.1093/schbul/sbn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray KL, Lesh TA, Howell AM, Salo TP, Ragland JD, MacDonald AW, … Carter CS (2017). Functional network changes and cognitive control in schizophrenia. NeuroImage: Clinical, 15, 161–170. doi: 10.1016/j.nicl.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JR, O’Reilly RC, Cohen JD, & Braver TS (2012). The function and organization of lateral prefrontal cortex: A test of competing hypotheses. PLoS ONE, 7(2), e30284. doi: 10.1371/journal.pone.0030284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JR, West R, & Braver T (2009). Distinct neural circuits support transient and sustained processes in prospective memory and working memory. Cerebral Cortex, 19(5), 1208–1221. doi: 10.1093/cercor/bhn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rund BR, Melle I, Friis S, Johannessen JO, Larsen TK, Midboe LJ, … McGlashan T (2007). The course of neurocognitive functioning in first-episode psychosis and its relation to premorbid adjustment, duration of untreated psychosis, and relapse. Schizophrenia Research, 91(1–3), 132–140. doi: 10.1016/j.schres.2006.11.030. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Shapiro DI, Stone WS, Woodberry KA, Ronzio A, Cornblatt BA, … Woods SW (2016). Association of neurocognition with transition to psychosis: Baseline functioning in the second phase of the North American prodrome longitudinal study. JAMA Psychiatry, 73 (12), 1239–1248. doi: 10.1001/jamapsychiatry.2016.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsi S, Lau A, Lencz T, Burdick KE, DeRosse P, Brenner R, … Malhotra AK (2011). Cognitive and symptomatic predictors of functional disability in schizophrenia. Schizophrenia Research, 126(1–3), 257–264. doi: 10.1016/j.schres.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J, Pearlson GD, Du Y, Yu Q, Jones TR, Chen J, … Calhoun VD (2015). In search of multimodal neuroimaging biomarkers of cognitive deficits in schizophrenia. Biological Psychiatry, 78(11), 794–804. doi: 10.1016/j.biopsych.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szoke A, Trandafir A, Dupont ME, Meary A, Schurhoff F, & Leboyer M (2008). Longitudinal studies of cognition in schizophrenia: Meta-analysis. The British Journal of Psychiatry, 192(4), 248–257. doi: 10.1192/bjp.bp.106.029009. [DOI] [PubMed] [Google Scholar]
- Tompary A, Al-Aidroos N, & Turk-Browne NB (2018). Attending to what and where: Background connectivity integrates categorical and spatial attention. Journal of Cognitive Neuroscience, 30(9), 1281–1297. doi: 10.1162/jocn_a_01284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Smucny J, Harris JG, Olincy A, Maharajh K, Kronberg E, … Freedman R (2014). Intrinsic hippocampal activity as a biomarker for cognition and symptoms in schizophrenia. The American Journal of Psychiatry, 171(5), 549–556. doi: 10.1176/appi.ajp.2013.13070981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snellenberg JX, Girgis RR, Horga G, van de Giessen E, Slifstein M, Ojeil N, … Abi-Dargham A (2016). Mechanisms of working memory impairment in schizophrenia. Biological Psychiatry, 80(8), 617–626. doi: 10.1016/j.biopsych.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woerner MG, Mannuzza S, & Kane JM (1988). Anchoring the BPRS: An aid to improved reliability. Psychopharmacology Bulletin, 24(1), 112–117. Retrieved from https://pubmed.ncbi.nlm.nih.gov/3387514/. [PubMed] [Google Scholar]
- Woodward ND, Rogers B, & Heckers S (2011). Functional resting-state networks are differentially affected in schizophrenia. Schizophrenia Research, 130(1–3), 86–93. doi: 10.1016/j.schres.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y (2017). Reevaluating the sensory account of visual working memory storage. Trends in Cognitive Sciences, 21(10), 794–815. doi: 10.1016/j.tics.2017.06.013. [DOI] [PubMed] [Google Scholar]
- Xu Y, & Chun MM (2006). Dissociable neural mechanisms supporting visual short-term memory for objects. Nature, 440(7080), 91–95. doi: 10.1038/nature04262. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, & Wager TD (2011). Large-scale automated synthesis of human functional neuroimaging data. Nature Methods, 8(8), 665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


