Abstract
Staphylococcus aureus and Salmonella enterica serovar Dublin invade osteoblasts and are causative agents of human bone disease. In the present study, we examined the ability of S. aureus and Salmonella serovar Dublin to induce the production of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) by normal osteoblasts. Normal mouse and human osteoblasts were cocultured with S. aureus or Salmonella serovar Dublin at different multiplicities of infection. Following initial incubation and examination of TRAIL expression, extracellular bacteria were killed by the addition of media containing the antibiotic gentamicin. Lysates and conditioned media from osteoblast cultures were then collected at various times following invasion and analyzed. The results demonstrated that S. aureus and Salmonella serovar Dublin are potent inducers of TRAIL expression by osteoblasts. Mouse and human TRAIL mRNA expression was induced by bacterial infection and demonstrated a dose-dependent response. Analysis of kinetics suggested that TRAIL mRNA was induced within 30 min after exposure to bacteria and that its level of expression remained relatively constant over the time period examined. mRNA molecules encoding TRAIL receptors were constitutively expressed by osteoblasts. Furthermore, TRAIL protein was detected as early as 45 min and up to 24 h following infection. The quantity of TRAIL protein produced also increased in a dose-dependent manner. Collectively, these findings suggest a mechanism whereby bacterial pathogens mediate bone destruction via osteoblast apoptosis.
Staphylococcus aureus is a common cause of bone and joint infections in humans. Infections can be a complication of septicemia or can follow local trauma to the implicated tissue. The pathogenesis of staphylococcal bone and joint infection is currently poorly understood but is likely to be multifactorial. Bacterial virulence determinants are important in infection, as are host factors, such as immune status and the presence of underlying disease.
S. aureus is a capable bone pathogen in part because it possesses several cell surface adhesion molecules that facilitate its binding to the bone matrix. Binding involves a family of adhesins that interact with extracellular matrix components, and these adhesins have been termed microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) (26). Specific MSCRAMMs are responsible for the localization of S. aureus to bone tissue (14). Once the bacteria adhere to and colonize the bone matrix, they elaborate several virulence factors, such as proteases, which can break down matrix components. The resultant bone destruction facilitates bacterial invasiveness.
S. aureus is generally not considered a significant intracellular pathogen compared to species of genera such as Listeria and Shigella; however, there is growing evidence that S. aureus has the ability to invade epithelial and endothelial cells (1, 2, 3, 22, 34). Regarding infection of bone, S. aureus has the ability to invade mouse and human osteoblast cell lines (7, 16). Experiments with these cell lines indicated that actin microfilaments, microtubules, and receptor-mediated endocytosis are used in the internalization of S. aureus into osteoblasts; however, microfilaments seem to play the most significant role in the invasion process. S. aureus also has the ability to invade normal human osteoblasts. Human osteoblasts infected with S. aureus express high levels of interleukin 6 (IL-6) and IL-12 p75, as indicated by complementary investigations demonstrating S. aureus-induced up-regulation of IL-6, IL-12 p40 mRNA expression, and IL-6 and IL-12 p75 secretion by these cells (6). In addition, a quantitative bioassay demonstrated that IL-12 p75 secreted following infection was biologically active (6). These studies were the first to demonstrate induced IL-12 p75 expression by osteoblasts and suggest a previously unrecognized role for osteoblasts in initiating immune responses following S. aureus infection.
Finally, S. aureus has been shown to invade normal chicken osteoblasts both in vitro and in vivo (15, 29). S. aureus cells were found in approximately 14% of calvarial osteoblasts after subcutaneous injection of chicken embryos and in 11% of calvarial and tibial osteoblasts after intra-allantoic injection. As in in vitro studies, most intracellular bacteria are eventually free in the osteoblast cytoplasm in vivo. S. aureus cells in calvariae and tibiae were also observed in the cytoplasm of approximately 4% of the osteocytes in the mineralized bone matrix. Therefore, osteoblasts containing internalized S. aureus cells continue differentiating into osteocytes.
Observations that S. aureus invades osteoblasts, persists intracellularly, and induces proinflammatory cytokine secretion indicate that the intracellular survival of the bacterium may be involved in bone infection. Evidence that invasion occurs in vivo further justifies this presumption. S. aureus sequestered from the host immune system in the osteoblast intracellular environment may provide a reservoir of bacteria for recurring osteomyelitis and may be more relevant to chronic disease than bacteria associated with the bone matrix.
It has been reported that S. aureus surface-associated proteins are potent stimulators of bone resorption (24) and that stimulation of osteoclast formation by the proteins plays a role in bone destruction (21). Induction of the apoptotic pathway in osteoblasts following internalization (31) likely exacerbates the bone destruction characteristic of infection. In the present study, we examined one potential mechanism of apoptosis induction in normal mouse and human osteoblasts. We report that the bone pathogens S. aureus and Salmonella enterica serovar Dublin are surprisingly potent inducers of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). These results suggest a pathway for bacterium-induced osteoblast apoptosis which has not previously been considered.
MATERIALS AND METHODS
Normal mouse osteoblast cell culture
Normal osteoblast cell cultures were prepared from mouse neonates according to a method previously described for chicken embryos (28). Bone-forming cells were isolated from mouse neonate calvariae by sequential collagenase and protease digestions. The periostea were removed, the frontal bones were harvested free of the suture regions, and the bones were incubated for 10 min at 37°C in 10 ml of digestion medium, containing collagenase (375 U/ml, type VII; Sigma Chemical Company, St. Louis, Mo.) and protease (7.5 U/ml; Sigma). The digestion medium and released cells were removed and discarded. Ten milliliters of fresh digestion medium was added, and incubation was continued for 20 min. Cells were harvested by centrifugation and rinsed three times in 25 mM HEPES-buffered Hanks' balanced salt solution (pH 7.4; HBSS). The digestion step was repeated twice, and the three cell isolates were pooled in mouse osteoblast growth medium (OBGM), consisting of Dulbecco's modified Eagle's medium containing 25 mM HEPES, 10% fetal bovine serum (Sigma), 2 g of sodium bicarbonate per liter, 75 μg of glycine/ml, 100 μg of ascorbic acid/ml, 40 ng of vitamin B12/ml, 2 μg of p-aminobenzoic acid/ml, 200 ng of biotin/ml, and penicillin (100 U/ml)-streptomycin (100 μg/ml)-amphotericin B (Fungizone; 0.25 μg) (pH 7.4) (27). Cells were seeded in six-well cluster plates and incubated at 37°C in a 5% CO2 atmosphere until they reached confluence (6 to 7 days).
Characterization of normal mouse osteoblasts.
Mouse osteoblasts were grown on glass coverslips in 24-well plates until they were confluent; they were then fixed and permeabilized using CytoFix/CytoPerm according to the methods recommended by the manufacturer (PharMingen, San Diego, Calif.). Rabbit antibodies specific for osteocalcin (1:100 dilution; Peninsula Laboratories, Belmont, Calif.), type I collagen (1:40 dilution; Chemicon, Temecula, Calif.), alkaline phosphatase (1:40 dilution; Sigma), or keyhole limpet hemocyanin (1:40 dilution) were incubated on cell preparations for 45 min at 4°C. After unbound antibody was washed off, a phycoerythrin-conjugated goat anti-rabbit immunoglobulin G antibody (1:50 dilution; Sigma) was added for 45 min at 4°C. After the samples were washed, at least 500 cells were scored for positive fluorescence using an Olympus BX60 fluorescence microscope. Osteocalcin, type I collagen, and alkaline phosphatase were selected for analysis, since the expression of these proteins has been used to define osteoblasts as such (9, 20, 30).
Normal human osteoblast cultures.
Normal human osteoblasts (Clonetics, San Diego, Calif.) were propagated according to the guidelines provided by the vendor. Cells were seeded in 25-cm2 flasks and incubated at 37°C in a 5% CO2 atmosphere in medium supplied by the manufacturer; this medium contains 10% fetal bovine serum, ascorbic acid, and gentamicin. After the cells reached approximately 80% confluence (5 to 9 days), they were removed from flasks with 0.025% trypsin–0.01% EDTA, washed in medium, and seeded into six-well plates. Cells were used as described below once they reached approximately 80% confluence (6 to 7 days). These commercially available cells have been extensively characterized as osteoblasts (9, 13).
Bacterial strains and growth conditions
S. aureus strain UAMS-1 (ATCC 49230) (osteomyelitis clinical isolate) and Salmonella serovar Dublin strain 1363 were grown separately overnight in 5 ml of tryptic soy broth at 37°C with aeration. Bacteria were harvested by centrifugation for 10 min at 4,300 × g and 4°C and washed in 5 ml of HBSS. Bacteria were then resuspended in either mouse or human OBGM.
Infection and invasion assay
Following resuspension of bacteria in OBGM, bacterial cell density was determined via spectrophotometric analysis. Cells were then diluted in OBGM to obtain the desired multiplicity of infection (MOI). Osteoblasts were infected with S. aureus at an MOI of 25:1, 75:1, or 250:1 or with Salmonella serovar Dublin at an MOI of 1:1, 3:1, or 10:1. The highest MOIs used for each organism resulted in approximately one internalized bacterium per osteoblast in cultures (data not shown). Following either 30 or 45 min of infection, osteoblasts were either lysed by the addition of 0.1% Triton X-100 with incubation for 5 min at 37°C or washed three times with HBSS and incubated in medium containing 25 μg of gentamicin per ml to kill extracellular bacteria. At various times following bacterial invasion, osteoblasts were lysed, and osteoblast RNA or protein was isolated for further analysis.
RNA isolation, RT, and semiquantitative PCR.
At 30 min as well as 6 h following bacterial infection, RNA was extracted from either normal mouse or normal human osteoblasts, reverse transcribed, and subjected to semiquantitative reverse transcription (RT)-PCR as previously described (4, 5). Briefly, total RNA was isolated using TRIZOL reagent (Gibco-BRL, Gaithersburg, Md.), and 2 μg of total RNA was reverse transcribed in the presence of random hexamers using 200 U of RNase H-negative Moloney murine leukemia virus reverse transcriptase (Superscript II; Gibco-BRL) in the buffer supplied by the manufacturer. PCR was performed on 5% of the total cDNA to quantify the expression of the mRNAs encoding TRAIL, TRAIL receptors, and glyceraldehyde-3-phosphate dehydrogenase (G3PDH). Cycles consisted of 95°C denaturation, 60°C annealing, and 72°C extension (Robocycler 40; Stratagene, La Jolla, Calif.), with the first 3 of 27 total cycles having extended denaturation and annealing times.
PCR primers were derived from published sequences and were designed for optimal amplifications using Oligo 4.0 primer analysis software (National Biosciences, Inc., Plymouth, Minn.). Primers were also selected based on their utility for the amplification of a minimum of 250 bp from the cDNA, their identity with different exons of the genomic sequence for each gene, and the lack of significant homology with other sequences. Positive- and negative-strand primers were as follows: G3PDH, GGAGCCAAACGGGTCATCATCTC and ATGCCTGCTTCACCACCTTCTTG; mouse TRAIL, CAAAGACGGATGAGGATTTCTGGGACT and TGAATGCCCTTTCCGAGAGGACTCC; mouse TRAIL receptor, GGTTCCAGTAGTGCTGCTGATTGGA and CGACCATTCGGATTTGATTGTCTG; human TRAIL, CCAATGACGAAGAGAGTATGAACAGCC and GTTGCTCAGGAATGAATGCCCACTC; human TRAIL receptor 1 (R1), GGGATGGTCAAGGTCAAGGATTGTAC and CTGCTCAGAGACGAAAGTGGACAGC; and human TRAIL receptor 2 (R2), TATAGCACTCACTGGAATGACCTCCTTT and ACCACCACCTGAGCAGATGCCTTTC.
Following PCR, 15% of each amplified sample was electrophoresed in ethidium bromide-stained agarose gels and visualized by UV illumination. PCR amplification of the housekeeping gene, the G3PDH gene, was performed on cDNA from each sample to ensure equal input of RNA and similar efficiencies of RT.
Protein isolation, SDS-PAGE, and Western immunoblot analysis.
At 45 min as well as 6, 12, and 24 h following infection, normal human osteoblasts were lysed using 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) denaturing buffer; proteins were separated via electrophoresis as described previously (18). Supernatants were also collected from some infected osteoblast cultures and concentrated using Centricon YM-30 centrifugal filter devices (Millipore Corporation, Bedford, Mass.). The concentrated supernatant samples were mixed with 2× SDS-PAGE denaturing buffer; proteins were separated via electrophoresis. S. aureus suspensions devoid of osteoblasts and purified TRAIL protein (amino-terminal histidine-tagged recombinant human TRAIL [21 kDa]; R & D Systems, Inc., Minneapolis, Minn.) were also treated as described above, followed by electrophoresis. Proteins were then electrotransferred to polyvinylidene difluoride membranes and incubated with either anti-TRAIL polyclonal antibodies (StressGen, Victoria, British Columbia, Canada) or anti-phosphorylated MKK3 or MKK6 polyclonal antibodies (New England Biolabs, Inc., Beverly, Mass.). A secondary, anti-rabbit horseradish peroxidase-conjugated antibody (New England Biolabs) was used to visualize reactive proteins. All antibodies were diluted prior to use according to company recommendations.
RESULTS
TRAIL and TRAIL receptor mRNA expression.
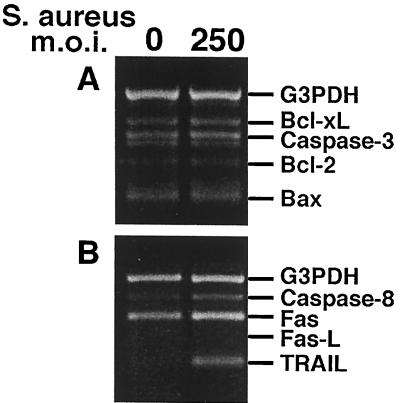
The mechanisms which function to induce apoptosis of osteoblasts during bone infections have not been defined. For this reason, we began an investigation to identify apoptosis-related genes expressed during infection of osteoblasts with bacterial pathogens. Using RT-PCR detection kits (CytoXpress multiplex human apoptosis sets 2 and 3; BioSource International, Camarillo, Calif.), we first screened cultured human osteoblasts for the expression of a variety of apoptosis-related genes which were candidates for induction following infection. Results from these studies (Fig. 1) suggested that TRAIL and perhaps caspase 8 were induced following S. aureus infection of osteoblasts. A detailed analysis of TRAIL and TRAIL receptor expression by infected osteoblasts was then undertaken.
FIG. 1.
RT-PCR analysis of apoptosis-related genes in normal human osteoblasts infected with S. aureus at an MOI of 250:1 (S. aureus organisms to osteoblasts). PCR amplification was performed as recommended by BioSource International. Gene expression was monitored at 6 h following the addition of viable bacteria. Bcl-xL, B-cell lymphoma gene × Long; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated × protein; Fas, CD95; Fas-L, Fas ligand.
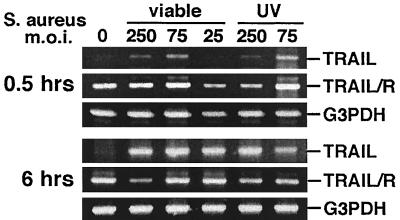
Mouse osteoblasts were exposed to S. aureus at an MOI of 25:1, 75:1, or 250:1. Following a 30-min infection period, osteoblasts were either lysed or incubated in medium containing gentamicin to kill extracellular bacteria prior to osteoblast lysis. At 30 min and at 6 h following bacterial infection, osteoblast RNA was isolated and semiquantitative RT-PCR analysis was performed to detect the expression of TRAIL, TRAIL receptor, or G3PDH mRNA. Surprisingly, the mRNA encoding TRAIL was rapidly and dramatically up-regulated following exposure of mouse osteoblasts to S. aureus (Fig. 2). This increase in TRAIL mRNA expression occurred whether viable or UV-killed bacteria were used (Fig. 2), suggesting that active S. aureus gene expression was not required for induction. TRAIL mRNA expression was absent in uninfected cultures and showed a dose-dependent response to S. aureus at 30 min following infection. In contrast to the inducible nature of TRAIL mRNA, the message encoding the TRAIL receptor was constitutively expressed (Fig. 2). Differences noted in TRAIL mRNA expression could not be attributed to significant differences in input RNA or efficiencies of RT between samples, as indicated by amplification of the G3PDH housekeeping gene from the same cDNA samples.
FIG. 2.
RT-RCR analysis of TRAIL and TRAIL receptor (TRAIL/R) expression in normal mouse osteoblasts infected with S. aureus or UV-killed S. aureus at different MOIs (25:1, 75:1, or 250:1 [S. aureus organisms to osteoblasts]). PCR amplification of G3PDH was performed to ensure equal input of RNA and similar efficiencies of RT. Gene expression was monitored at 0.5 and 6 h following the addition of viable or UV-killed bacteria.
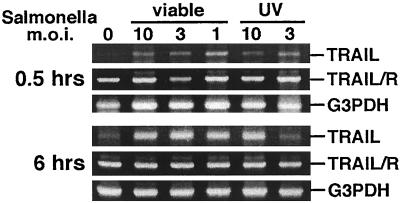
While S. aureus is the primary causative agent of bacterially induced bone and joint diseases, Salmonella species can also be responsible for such infections. We questioned whether these two very different bacterial pathogens could induce similar osteoblast responses. As shown in Fig. 3, the gram-negative bacterium Salmonella serovar Dublin was also able to rapidly up-regulate TRAIL mRNA expression in mouse osteoblasts. As with S. aureus, UV-killed bacteria were capable inducers of TRAIL mRNA expression. Taken together, the results presented in Fig. 2 and 3 demonstrate that both S. aureus and Salmonella serovar Dublin can induce the rapid up-regulation of TRAIL mRNA expression and that mouse osteoblasts constitutively express mRNA encoding the single known TRAIL receptor.
FIG. 3.
RT-RCR analysis of TRAIL and TRAIL receptor (TRAIL/R) expression in normal mouse osteoblasts infected with Salmonella serovar Dublin or UV-killed Salmonella serovar Dublin at different MOIs (10:1, 3:1, or 1:1 [Salmonella serovar Dublin organisms to osteoblasts]). PCR amplification of G3PDH was performed to ensure equal input of RNA and similar efficiencies of RT. Gene expression was monitored at 0.5 and 6 h following the addition of viable or UV-killed bacteria.
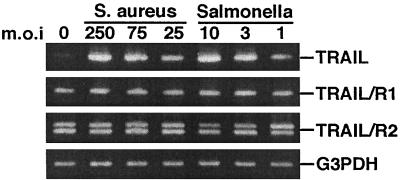
To address whether the TRAIL induction response to infectious agents was conserved in humans, RT-PCR analysis was also performed with normal human osteoblast cultures following exposure to S. aureus or Salmonella serovar Dublin. The results presented in Fig. 4 are qualitatively similar to those obtained using cultured mouse osteoblasts. Specifically, TRAIL mRNA expression was up-regulated in cultured human osteoblasts exposed to viable S. aureus or Salmonella serovar Dublin, whereas the mRNAs encoding TRAIL R1 and R2 were constitutively expressed. Thus, the results from mRNA analyses were clear regarding S. aureus- or Salmonella serovar Dublin-induced TRAIL expression by cultured mouse or human osteoblasts.
FIG. 4.
RT-RCR analysis of TRAIL, TRAIL R1, and TRAIL R2 expression in normal human osteoblasts infected with S. aureus or Salmonella serovar Dublin at different MOIs (25:1, 75:1, or 250:1 [S. aureus organisms to osteoblasts]; 1:1, 3:1, or 10:1 [Salmonella serovar Dublin organisms to osteoblasts]). PCR amplification of G3PDH was performed to ensure equal input of RNA and similar efficiencies of RT. Gene expression was monitored at 6 h following the addition of viable bacteria.
TRAIL protein synthesis and secretion.
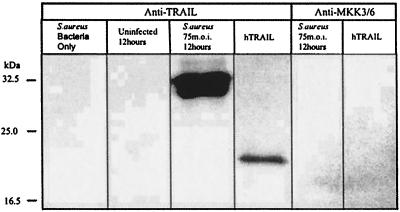
To complement the mRNA analyses, Western blot analyses were performed to examine the up-regulation of TRAIL following infection. These analyses were limited to human osteoblasts, since the antibodies which are commercially available recognize the human protein. An osteoblast protein reacted with polyclonal anti-TRAIL antibody in S. aureus-infected cultures but was absent in uninfected osteoblasts and in preparations from S. aureus cultures devoid of osteoblasts (Fig. 5). The reactive protein present in infected cultures has a mass of approximately 33 kDa, which is the predicted size of at least one isoform of the human TRAIL protein (19). A rabbit polyclonal antibody preparation specific for human phosphorylated MKK3 or MKK6 did not react with proteins from infected osteoblast cultures. This antibody was used as a control, since we have recently demonstrated that osteoblast MKK3 and MKK6 are not phosphorylated in response to S. aureus infection (J. K. Ellington, A. Elhofy, and M. C. Hudson, submitted for publication). Figure 5 demonstrates that the polyclonal anti-TRAIL antibody reacts with recombinant human TRAIL and therefore suggests that the reactive protein present in infected osteoblast cultures is indeed TRAIL.
FIG. 5.
Western immunoblot analysis of cell-associated TRAIL expression by normal human osteoblasts infected with S. aureus. Lanes contained S. aureus devoid of osteoblasts; uninfected osteoblasts 12 h after mock infection; osteoblasts infected with S. aureus at an MOI of 75:1, 12 h after the addition of bacteria; and recombinant human TRAIL (hTRAIL). The first four lanes were probed with polyclonal anti-TRAIL antibody, and the fifth and sixth lanes were probed with polyclonal anti-phosphorylated MKK3 or MKK6 antibody, prior to reaction with the secondary antibody. Two micrograms of total protein was loaded for the first three lanes and the fifth lane; 1 μg of recombinant hTRAIL was loaded for the fourth and sixth lanes.
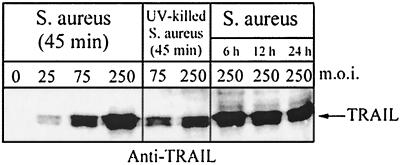
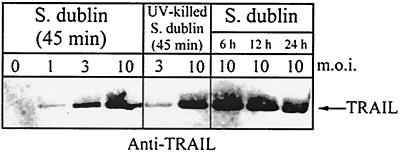
Cell-associated TRAIL protein was detected as early as 45 min and up to 24 h following infection of normal human osteoblasts with S. aureus (Fig. 6). In addition, S. aureus-induced TRAIL expression increased in a dose-dependent manner. Cell-associated TRAIL protein was also detected in normal human osteoblasts within 45 min following exposure to Salmonella serovar Dublin (Fig. 7). As with S. aureus infection, the production of TRAIL by Salmonella serovar Dublin-infected normal human osteoblasts also increased in a dose-dependent manner and was sustained for at least 24 h. Consistent with the results of the RT-PCR analyses, uninfected human osteoblasts did not express significant amounts of TRAIL protein. Finally, UV-killed bacteria induced the expression of TRAIL protein at levels similar to those induced by live bacteria (Fig. 6 and 7).
FIG. 6.
Western immunoblot analysis of cell-associated TRAIL expression by normal human osteoblasts infected with S. aureus or UV-killed S. aureus at different MOIs (25:1, 75:1, or 250:1 [S. aureus organisms to osteoblasts]). TRAIL expression was monitored at 45 min following the addition of viable or UV-killed bacteria and at 6, 12, and 24 h following infection with viable S. aureus cells. Two micrograms of total protein was loaded for each lane.
FIG. 7.
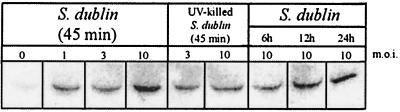
Western immunoblot analysis of cell-associated TRAIL expression by normal human osteoblasts infected with Salmonella serovar Dublin or UV-killed Salmonella serovar Dublin at different MOIs (1:1, 3:1, or 10:1 [Salmonella serovar Dublin organisms to osteoblasts]). TRAIL expression was monitored at 45 min following the addition of viable or UV-killed bacteria and at 6, 12, and 24 h following infection with viable Salmonella serovar Dublin cells. Two micrograms of total protein was loaded for each lane.
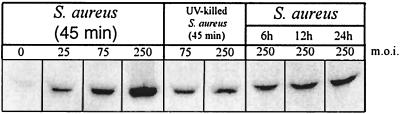
Infected osteoblast cultures are also capable of secreting significant quantities of TRAIL protein. Secreted TRAIL protein was detected as early as 45 min and up to 24 h following infection of normal human osteoblasts with S. aureus (Fig. 8). As with cell-associated TRAIL, S. aureus-induced secretion of TRAIL increased in a dose-dependent manner. Secreted TRAIL protein was also detected within 45 min following exposure to Salmonella serovar Dublin (Fig. 9). Secretion of TRAIL by Salmonella serovar Dublin-infected normal human osteoblasts also increased in a dose-dependent manner and was sustained for at least 24 h. Uninfected human osteoblasts did not secrete significant amounts of TRAIL protein. Finally, UV-killed bacteria induced the secretion of TRAIL protein at levels similar to those induced by live bacteria (Fig. 8 and 9).
FIG. 8.
Western immunoblot analysis of TRAIL secretion by normal human osteoblasts infected with S. aureus or UV-killed S. aureus at different MOIs. Lanes, from left to right, contained protein reactive with anti-TRAIL antibody in culture supernatants 45 min following infection with S. aureus at an MOI of 0, 25:1, 75:1, or 250:1; protein reactive with anti-TRAIL antibody in culture supernatants 45 min following the addition of UV-killed S. aureus at an MOI of 75:1 or 250:1; and protein reactive with anti-TRAIL antibody in culture supernatants at 6, 12, and 24 h following infection with S. aureus at an MOI of 250:1. Ten micrograms of total protein from serum-containing medium was loaded for each lane.
FIG. 9.
Western immunoblot analysis of TRAIL secretion by normal human osteoblasts infected with Salmonella serovar Dublin or UV-killed Salmonella serovar Dublin at different MOIs. Lanes, from left to right, contained protein reactive with anti-TRAIL antibody in culture supernatants 45 min following infection with Salmonella serovar Dublin at an MOI of 0, 1:1, 3:1, or 10:1; protein reactive with anti-TRAIL antibody in culture supernatants 45 min following the addition of UV-killed Salmonella serovar Dublin at an MOI of 3:1 or 10:1; and protein reactive with anti-TRAIL antibody in culture supernatants at 6, 12, and 24 h following infection with Salmonella serovar Dublin at an MOI of 10:1. Ten micrograms of total protein from serum-containing medium was loaded for each lane.
DISCUSSION
TRAIL is believed to induce apoptosis in tumorigenic or transformed cells but not in normal cells (35). mRNA encoding TRAIL has been detected in many tissues, including spleen, prostate, ovary, and colon, and in peripheral blood lymphocytes. The expression of TRAIL in many tissues therefore has suggested that the regulation of TRAIL-induced apoptosis is mediated through the regulation of TRAIL receptor expression. A recent report was the first to demonstrate the induction of TRAIL by an infectious agent (32). Measles virus was shown to induce TRAIL production by human dendritic cells, although the expression of TRAIL receptors was not examined.
The current study demonstrates that bacterial bone pathogens strongly induce TRAIL expression by both normal mouse and normal human osteoblasts, while there is constitutive expression of TRAIL receptors in the same cells. Surprisingly, qualitatively similar results were obtained following interactions of gram-positive and gram-negative bone pathogens with normal osteoblasts from two different mammalian species. Viable and UV-killed S. aureus and Salmonella serovar Dublin caused a rapid and sustained up-regulation of TRAIL mRNA and TRAIL protein expression. This up-regulation of TRAIL mRNA by normal mouse osteoblasts in response to bacterial infection is coupled with the constitutive expression of the single known mouse TRAIL receptor (Fig. 2 and 3). Several human TRAIL receptors are known, including R1, R2, R3, R4, and osteoprotegerin (OPG) (8, 12); however, R1 and R2 are the only TRAIL receptors known to contain complete death domains. Studies with human osteoblasts indicated that the up-regulation of TRAIL mRNA expression is coupled with the constitutive expression of R1 and R2 mRNAs (Fig. 4). The rapid and dose-dependent nature of TRAIL induction in infected normal mouse and human osteoblasts suggests that TRAIL may be a significant factor in osteoblast apoptosis. The observation that UV-killed bacteria induced TRAIL clearly indicates that active bacterial gene expression is not required to induce the osteoblast response.
The present study, the first to demonstrate that bacterial pathogens induce TRAIL expression in any normal cell, suggests that intracellular bacteria mediate the response and that TRAIL likely initiates the apoptosis observed following bacterial infection of osteoblasts (31). TRAIL clearly mediates cell death in other tissues through the activation of specific caspases (11). Caspase 8 activation is observed within minutes of TRAIL addition to human melanoma cells, suggesting that caspase 8 is one of the proximal components of the TRAIL-induced cell death pathway (10). Other reports indicate that caspase 10 is the initial mediator in TRAIL-induced apoptosis (25). Caspase 3 is a substrate for both caspase 8 and caspase 10. The results presented in Fig. 1 suggest that S. aureus infection of osteoblasts might actually result in the up-regulation of caspase 8 expression, while caspase 3 expression remains unchanged. Wesson et al. have recently demonstrated that S. aureus induces apoptosis in epithelial cells via a mechanism involving caspase 8 and caspase 3 (33), although the inducer of caspase activation was not reported. It is currently unclear if TRAIL is the mediator of S. aureus-induced caspase activation in epithelial cells or if TRAIL induces caspase 8 or caspase 10 activation in infected osteoblasts.
Collectively, the findings reported here suggest a mechanism whereby bone pathogens mediate bone destruction via the induction of osteoblast apoptosis. Extracts from Actinobacillus actinomycetemcomitans have recently been demonstrated to induce osteoblast apoptosis (23, 36), but intracellular bacteria also appear to be capable of such destruction (31). The presence of intracellular bacterial cells in osteoblast cultures is most likely required for apoptosis induction by S. aureus and Salmonella serovar Dublin. Normal osteoblasts are evident in infected cultures even at 24 h after the addition of gentamicin (data not shown), so induction of apoptosis by extracellular bacterial products is unlikely. In addition, the presence of gentamicin in the culture medium after 45 min of infection makes it unlikely that any bacteria potentially released from osteoblasts after that time would be responsible for inducing apoptosis. It is unclear if all intracellularly infected osteoblasts express TRAIL or whether the induction of expression is dependent on the numbers of bacteria present inside individual cells.
In addition to potentially inducing osteoblast apoptosis, TRAIL expression likely plays another role in bone pathology. TRAIL binding to the soluble OPG receptor can derepress the OPG inhibition of osteoclastogenesis, thereby increasing bone destruction (8). The induction of TRAIL in osteoblasts following bacterial infection could therefore have a positive osteoclastogenic impact on the bone microenvironment. Further, the demonstration that TRAIL is secreted by infected osteoblasts (Fig. 8 and 9) suggests that TRAIL could influence cells outside the environment of a localized bacterial infection.
In summary, the data indicate that S. aureus and Salmonella serovar Dublin induce TRAIL expression in infected normal osteoblasts. This result indicates that TRAIL-induced cell death may exacerbate bone loss already attributed to the osteoclast-mediated bone resorption characteristic of osteomyelitis. Osteoblasts have been demonstrated to undergo apoptosis in vitro and in vivo, and it has been suggested that the process can be modulated by growth factors and cytokines produced in the bone microenvironment (17). The mechanism of S. aureus and Salmonella serovar Dublin-induced TRAIL expression is currently unclear; however, the S. aureus agr locus influences apoptosis induction in bovine mammary epithelial cells (34). We are currently investigating whether such global regulatory loci control the initiation of osteoblast apoptosis following invasion of osteoblasts by S. aureus and Salmonella species.
ACKNOWLEDGMENTS
This work was supported by grant AI32976 from the National Institutes of Health (to K.L.B.), by the Foundation for the Carolinas (support given to M.C.H.), and by the UNC Charlotte Foundation (support given to M.C.H. and F.M.H.).
REFERENCES
- 1.Almeida R A, Matthews K R, Cifrian E, Guidry A J, Oliver S P. Staphylococcus aureus invasion of bovine mammary epithelial cells. J Dairy Sci. 1996;79:1021–1026. doi: 10.3168/jds.S0022-0302(96)76454-8. [DOI] [PubMed] [Google Scholar]
- 2.Bayles K W, Wesson C A, Liou L E, Fox L K, Bohach G A, Trumble W R. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infect Immun. 1998;66:336–342. doi: 10.1128/iai.66.1.336-342.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beekhuizen H, Van De Gevel J S, Olsson B, Van Benten I J, Van Furth R. Infection of human vascular endothelial cells with Staphylococcus aureus induces hyperadhesiveness for human monocytes and granulocytes. J Immunol. 1997;158:774–782. [PubMed] [Google Scholar]
- 4.Bost K L, Clements J D. In vivo induction of interleukin-12 mRNA expression after oral immunization with Salmonella dublin or the B subunit of Escherichia coli heat-labile enterotoxin. Infect Immun. 1995;63:1076–1083. doi: 10.1128/iai.63.3.1076-1083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bost K L, Mason M J. Thapsigargin and cyclopiazonic acid initiate rapid and dramatic increases in IL-6 mRNA expression and IL-6 secretion in murine peritoneal macrophages. J Immunol. 1995;155:285–296. [PubMed] [Google Scholar]
- 6.Bost K L, Ramp W K, Nicholson N C, Bento J L, Marriott I, Hudson M C. Staphylococcus aureus infection of mouse or human osteoblasts induces high levels of IL-6 and IL-12 production. J Infect Dis. 1999;180:1912–1920. doi: 10.1086/315138. [DOI] [PubMed] [Google Scholar]
- 7.Ellington J K, Reilly S S, Ramp W K, Smeltzer M S, Kellam J F, Hudson M C. Mechanisms of Staphylococcus aureus invasion of cultured osteoblasts. Microb Pathog. 1999;26:317–323. doi: 10.1006/mpat.1999.0272. [DOI] [PubMed] [Google Scholar]
- 8.Emery J G, McDonnell P, Burke M B, Deen K C, Lyn S, Silverman C, Dul E, Appelbaum E R, Eichman C, DiPrinzio R, Dodds R A, James I E, Rosenberg M, Lee J C, Young P R. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998;273:14363–14367. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher J A, Gundle R, Beresford J N. Isolation and culture of bone forming cells (osteoblasts) from human bone. In: Jones G E, editor. Methods in molecular medicine: human cell culture protocols. Totowa, N.J: Humana Press; 1996. pp. 233–241. [DOI] [PubMed] [Google Scholar]
- 10.Griffith T S, Chin W A, Jackson G C, Lynch D H, Kubin M Z. Intracellular regulation of TRAIL-induced apoptosis in human melanoma cells. J Immunol. 1998;161:2833–2840. [PubMed] [Google Scholar]
- 11.Griffith T S, Lynch D H. TRAIL: a molecule with multiple receptors and control mechanisms. Curr Opin Immunol. 1998;10:559–563. doi: 10.1016/s0952-7915(98)80224-0. [DOI] [PubMed] [Google Scholar]
- 12.Griffith T S, Rauch C T, Smolak P J, Waugh J Y, Boiani N, Lynch D H, Smith C A, Goodwin R G, Kubin M Z. Functional analysis of TRAIL receptors using monoclonal antibodies. J Immunol. 1999;162:2597–2605. [PubMed] [Google Scholar]
- 13.Gundle R, Beresford J N. The isolation and culture of cells from explants of human trabecular bone. Calcif Tissue Int. 1995;56(Suppl. 1):S8–S10. [Google Scholar]
- 14.Hudson C M, Ramp W K, Frankenburg K P. Staphylococcus aureus adhesion to bone matrix and bone-associated biomaterials. FEMS Microbiol Lett. 1999;173:279–284. doi: 10.1111/j.1574-6968.1999.tb13514.x. [DOI] [PubMed] [Google Scholar]
- 15.Hudson C M, Ramp W K, Nicholson N C, Williams A S, Nousiainen M T. Internalization of Staphylococcus aureus by cultured osteoblasts. Microb Pathog. 1995;19:409–419. doi: 10.1006/mpat.1995.0075. [DOI] [PubMed] [Google Scholar]
- 16.Jevon M, Guo C, Ma B, Mordan N, Nair S P, Harris M, Henderson B, Bentley G, Meghji S. Mechanisms of internalization of Staphylococcus aureus by cultured human osteoblasts. Infect Immun. 1999;67:2677–2681. doi: 10.1128/iai.67.5.2677-2681.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jilka R L, Weinstein R S, Bellido T, Parfitt A M, Manolagas S C. Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res. 1998;13:793–802. doi: 10.1359/jbmr.1998.13.5.793. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Mariani S M, Krammer P H. Differential regulation of TRAIL and CD95 ligand in transformed cells of the T and B lymphocyte lineage. Eur J Immunol. 1998;28:973–982. doi: 10.1002/(SICI)1521-4141(199803)28:03<973::AID-IMMU973>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 20.Marie P J, Lomri A, Sabbagh A, Basle M. Culture and behavior of osteoblastic cells isolated from normal trabecular bone surfaces. In Vitro Cell Dev Biol. 1989;25:373–380. doi: 10.1007/BF02624601. [DOI] [PubMed] [Google Scholar]
- 21.Meghji S, Crean S J, Hill P A, Sheikh M, Nair S P, Heron K, Henderson B, Mawer E B, Harris M. Surface-associated protein from Staphylococcus aureus stimulates osteoclastogenesis: possible role in S. aureus-induced bone pathology. Br J Rheumatol. 1998;37:1095–1101. doi: 10.1093/rheumatology/37.10.1095. [DOI] [PubMed] [Google Scholar]
- 22.Menzies B E, Kourteva I. Internalization of Staphylococcus aureus by endothelial cells induces apoptosis. Infect Immun. 1998;66:5994–5998. doi: 10.1128/iai.66.12.5994-5998.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morimoto Y, Morimoto H, Murata T, Kobayashi S, Ohba T, Haneji T. Extracts of Actinobacillus actinomycetemcomitans induce apoptotic cell death in human osteoblastic MG63 cells. J Dent Res. 1999;78:735–742. doi: 10.1177/00220345990780030501. [DOI] [PubMed] [Google Scholar]
- 24.Nair S P, Song Y, Meghji S, Reddi K, Harris M, Ross A, Poole S, Wilson M, Henderson B. Surface-associated proteins from Staphylococcus aureus demonstrate potent bone resorbing activity. J Bone Miner Res. 1995;10:726–734. doi: 10.1002/jbmr.5650100509. [DOI] [PubMed] [Google Scholar]
- 25.Pan G, Ni J, Wei Y-F, Yu G I, Gentz R, Dixit V M. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- 26.Patti J M, Allen B L, McGavin M J, Hook M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 27.Ramp W K, Dillaman R M, Lenz L G, Gay D M, Roer R D, Ballard T A. A serum substitute promotes osteoblast-like phenotypic expression in cultured cells from chick calvariae. Bone Miner. 1991;15:1–17. doi: 10.1016/0169-6009(91)90107-b. [DOI] [PubMed] [Google Scholar]
- 28.Ramp W K, Lenz L G, Kaysinger K K. Medium pH modulates matrix, mineral, and energy metabolism in cultured chick bones and osteoblast-like cells. Bone Miner. 1994;24:59–73. doi: 10.1016/s0169-6009(08)80131-6. [DOI] [PubMed] [Google Scholar]
- 29.Reilly S S, Hudson M C, Kellam J F, Ramp W K. In vivo internalization of Staphylococcus aureus by embryonic chick osteoblasts. Bone. 2000;26:63–70. doi: 10.1016/s8756-3282(99)00239-2. [DOI] [PubMed] [Google Scholar]
- 30.Rodan G A, Heath J K, Yoon K, Noda M, Rodan S B. Diversity of the osteoblastic phenotype. Ciba Found Symp. 1988;136:78–91. doi: 10.1002/9780470513637.ch6. [DOI] [PubMed] [Google Scholar]
- 31.Tucker K A, Reilly S S, Leslie C S, Hudson M C. Intracellular Staphylococcus aureus induces apoptosis in mouse osteoblasts. FEMS Microbiol Lett. 2000;186:151–156. doi: 10.1111/j.1574-6968.2000.tb09096.x. [DOI] [PubMed] [Google Scholar]
- 32.Vidalain P-O, Azocar O, Lamouille B, Astier A, Rabourdin-Combe C, Servet-Delprat C. Measles virus induces functional TRAIL production by human dendritic cells. J Virol. 2000;74:556–559. doi: 10.1128/jvi.74.1.556-559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wesson C A, Deringer J, Liou L E, Bayles K W, Bohach G A, Trumble W R. Apoptosis induced by Staphylococcus aureus in epithelial cells utilizes a mechanism involving caspases 8 and 3. Infect Immun. 2000;68:2998–3001. doi: 10.1128/iai.68.5.2998-3001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wesson C A, Liou L E, Todd K M, Bohach G A, Trumble W R, Bayles K W. Staphylococcus aureus Agr and Sar global regulators influence internalization and induction of apoptosis. Infect Immun. 1998;66:5238–5243. doi: 10.1128/iai.66.11.5238-5243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiley S R, Schooley K, Smolak P J, Din W S, Huang C P, Nicholl J K, Sutherland G R, Smith T D, Rauch C, Smith C A, Goodwin R G. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto S, Mogi M, Kinpara K, Ishihara Y, Ueda N, Amano K, Nishihara T, Noguchi T, Togari A. Anti-proliferative capsular-like polysaccharide antigen from Actinobacillus actinomycetemcomitans induces apoptotic cell death in mouse osteoblastic MC3T3-E1 cells. J Dent Res. 1999;78:1230–1237. doi: 10.1177/00220345990780060601. [DOI] [PubMed] [Google Scholar]