Abstract
Introduction
Photobiomodulation (PBM) represents a potential treatment for non-exudative age-related macular degeneration (AMD). PBM uses wavelengths of light to target components of the mitochondrial respiratory chain to improve cellular bioenergetic outputs. The aim of this study was to further investigate the effects of PBM on clinical, quality of life (QoL) and anatomical outcomes in subjects with intermediate stage non-exudative AMD.
Methods
The multicenter LIGHTSITE II study was a randomized clinical trial evaluating safety and efficacy of PBM in intermediate non-exudative AMD. The LumiThera Valeda® Light Delivery System delivered multiwavelength PBM (590, 660 and 850 nm) or sham treatment 3 × per week over 3–4 weeks (9 treatments per series) with repeated treatments at baseline (BL), 4 and 8 months. Subjects were enrolled with 20/32 to 20/100 best-corrected visual acuity (BCVA) and no central geographic atrophy (GA) within the central fovea (500 μm).
Results
LIGHTSITE II enrolled 44 non-exudative AMD subjects (53 eyes). PBM-treated eyes showed statistically significant improvement in BCVA at 9 months (n = 32 eyes, p = 0.02) with a 4-letter gain in the PBM-treated group versus a 0.5-letter gain in the sham-treated group (ns, p < 0.1) for patients that received all 27 PBM treatments (n = 29 eyes). Approximately 35.3% of PBM-treated eyes showed ≥ 5-letter improvement at 9 months. Macular drusen volume was not increased over time in the PBM-treated group but did show increases in the sham-treated group. While PBM and sham groups both showed GA lesion growth in the trial period, there was 20% less growth in the PBM group over 10 months, suggesting potential disease-modifying effects. No safety concerns or signs of phototoxicity were observed.
Conclusion
These results confirm previous clinical testing of multiwavelength PBM and support treatment with Valeda as a novel therapy with a unique mechanism of action as a potential treatment for non-exudative AMD.
Trial registration
Clinicaltrial.Gov Registration Identifier: NCT03878420.
Keywords: Photobiomodulation, Multiwavelength, Age related macular degeneration, Mitochondria, Ocular disease, Vision
Key Summary Points
| Why carry out this study? |
| Non-exudative age-related macular degeneration (AMD) is a significant retinal disease that impacts on visual outcomes and demonstrates mitochondrial dysfunction as a key pathological underpinning |
| Photobiomodulation (PBM) therapy uses wavelengths of light in the 500–1000 nm spectrum to activate components of the electron transport chain in the mitochondria. Improvements in mitochondrial output and subsequent cellular functioning may improve clinical outcomes in non-exudative AMD |
| The LIGHTSITE II study aimed to determine the impact of multiwavelength PBM (590 nm, 660 nm and 850 nm) on clinical and anatomical outcomes in subjects with non-exudative AMD |
| What was learned from the study? |
| LIGHTSITE II investigated the effects of repeated multiwavelength PBM therapy over the course of 10 months in subjects with intermediate non-exudative AMD on clinical and anatomical outputs. While COVID-19 had a significant interference on study design, improvement in visual outcomes following PBM was observed supporting previous studies |
| Improvements in visual function and potential disease-modifying effects support further research into PBM as a potential treatment for non-exudative AMD. The noninvasive and unique modality of PBM is posed to have high impact in this patient population |
Introduction
Age-related macular degeneration (AMD) is a retinal disease that results in irreversible and severe loss of vision. Disease progression inevitably leads to significant visual dysfunction and serious compromises in quality of life (QoL). AMD is characterized by degeneration in retinal photoreceptors, retinal pigment epithelium (RPE) and Bruch's membrane, with some degree of alterations observed in choroidal capillaries [1]. The non-exudative form of AMD accounts for 85% to 90% of patients and shows disruption in RPE and outer retinal atrophy. Contributing factors to RPE cell degeneration include mitochondrial dysfunction, oxidative stress, inflammation and genetic disposition [2].
There are no approved treatments for non-exudative AMD (with the exception of guidelines on vitamin supplementation); thus, it represents a high-impact patient population with a significant unmet medical need. Patients who progress to the wet form of AMD may be treated with ophthalmic drugs to specifically target vascular endothelial growth factor (VEGF). Photobiomodulation (PBM) involves targeted use of selected wavelengths of visible light to near infrared (NIR) light (500–1000 nm) produced by a laser or a noncoherent light source such as light-emitting diodes (LEDs). The enzyme cytochrome C oxidase (CCO), referred to as complex four in the respiratory electron transport chain (ETC), has been identified as the major photoacceptor underlying the mechanism of action for PBM. A multitude of benefits are observed following treatment with PBM that parallel the molecular underpinnings contributing to non-exudative AMD pathology including inflammatory and mitochondrial distress [3–6]. Mitochondrial activation at CCO elicited by PBM enhances ETC function and promotes adenosine triphosphate (ATP) production, the cell’s major source of energy. PBM can also modulate intracellular signaling molecules, such as reactive-oxygen species (ROS) and nitric oxide (NO) production and, by extrapolation, ROS- and NO-activated signaling pathways involving NfkB activation and cell death that subserve pleotropic signaling cascades that modulate multiple downstream events to effect sustained changes in cell function and viability. In the context of cell death signaling, PBM can prevent cell death following hypoxic, traumatic or toxic insults by activating downstream pathways and upregulating photon-mediated cytoprotective gene products. These include antioxidant enzymes, heat shock proteins and anti-apoptotic/anti-necrotic proteins. The effects of PBM on apoptosis/necrosis further highlight its unique effects on mitochondrial function [7–10]. Previous studies, both preclinical and clinical, show benefit in anatomical and clinical outcome measures following PBM treatment in ocular indications [11–19].
The LumiThera Valeda® Light Delivery System is a medical device that delivers multiwavelength PBM at 590, 660 and 850 nm wavelengths. These wavelengths were selected based on their biological targets which comprehensively act on multiple molecular substrates within the mitochondrial ETC and other biological systems relevant to the indication [3, 20]. The LIGHTSITE I study was the first randomized, controlled clinical trial using Valeda in subjects with non-exudative AMD [21]. Subjects showed improvements in clinical [best-corrected visual acuity (BCVA) and contrast sensitivity (CS)], anatomical [macular drusen volume, drusen thickness and geographic atrophy (GA)] and quality of life (QoL) outcomes. The current LIGHTSITE II study further investigates the safety and efficacy of PBM treatment using Valeda in subjects with intermediate non-exudative AMD in a double-masked, randomized, sham-controlled, parallel group, multi-center prospective design.
Methods
Subject Selection
Subjects were eligible for trial enrollment (NCT03878420) if they were at least 50 years of age, had a diagnosis of non-exudative AMD as defined by the presence of drusen and/or geographic atrophy (GA) with BCVA scores as determined by the Early Treatment Diabetic Retinopathy Study (ETDRS) Visual Acuity chart with a letter score between 50 and 80 (Snellen equivalent of 20/25 to 20/100).
Subjects were excluded from enrollment with current or a history of macular neovascularization, presence of center involving GA within the central ETDRS 500 μm diameter, a history of epilepsy, other significant retinal disease or other significant disease. Subjects could use AREDS vitamin supplementation; however, no change in supplements 1 month before the study and during the study trial was allowed. An adapted AREDS classification was used, as each eye was individually assessed for the presence of center involving GA and choroidal neovascularization (CNV). Thus, in the case of center involving GA or CNV in one eye, the fellow eye was not automatically deemed to be AREDS Category 4 but was individually assessed for the presence or absence of drusen, GA and CNV [17]. For example, in a patient with CNV in one eye and one single medium drusen in the other eye, only the eye with CNV was categorized as AREDS 4, while the other eye was categorized as AREDS 2. This allowed for assessment of potential progression in terms of AREDS category independent to each eye during the study.
Study Design
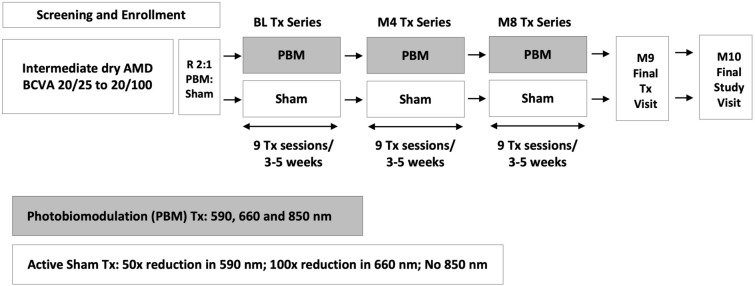
Subjects with non-exudative AMD who met the inclusion criteria, had none of the exclusion criteria and provided their written informed consent were enrolled into the study across seven centers throughout Europe (UK, France, Spain, Germany, Italy). Subjects were treated with the Valeda Light Delivery System (Valeda) and received PBM or sham treatment for a total of nine treatments over a 3- to 5-week period. There was a re-treatment period starting at the 4-month time point to include nine additional treatment visits, repeated at 8 months. Data were collected during 31 visits over the course of the 10-month study (Fig. 1).
Fig. 1.
LIGHTSITE II study design. The study employed a prospective, randomized, double-masked clinical trial design to evaluate the safety and efficacy of PBM in intermediate non-exudative AMD subjects. Data were collected during 31 visits over the course of the 10-month study. AMD age-related macular degeneration, BCVA best-corrected visual acuity, BL baseline, M month, PBM photobiomodulation, Tx treatment
Subjects were assessed for standard visual outcome measurements including ETDRS BCVA, Mars letter CS, Radner reading chart and Visual Function Questionnaire-25 (VFQ-25) test at selected time intervals. Subjects also underwent eye examinations, fundus photographs, Heidelberg optical coherence tomography (OCT) and fundus autofluorescence (FAF) imaging and optional Optos UWF (ultra-widefield) color and UWF autofluorescence imaging of the retina at selected time intervals. All subjects were assessed with 20 × 20-mm high-speed SD-OCT volume scans and FAF imaging using 488-nm wavelength (Spectralis OCT; Heidelberg Engineering, Heidelberg, Germany) as described previously [21]. For the GA area and growth assessment the Heidelberg Engineering Region Finder Analyzer software (Region Finder Software; Heidelberg Engineering, Germany) was used. This software measures the areas of homogeneous FAF hypoautofluorescence in a semiautomated way, which corresponds to the GA lesions [17]. It further allows assessment of the potential growth over time (e.g., Figs. 4, 5). The subRPE macular drusen volume was assessed within the whole 6 × 6-mm ETDRS grid, while the mean central subfield subRPE drusen thickness was analyzed within the central 1-mm ETDRS subfield. The automated and manually corrected alignment of the Bruch’s membrane and the RPE of the inbuilt Heidelberg segmentation software was employed for the assessment of respective parameters. An independent, masked imaging center reviewed and graded all images to determine disease etiology and inclusion/exclusion criterion and to assess the here presented morphological changes.
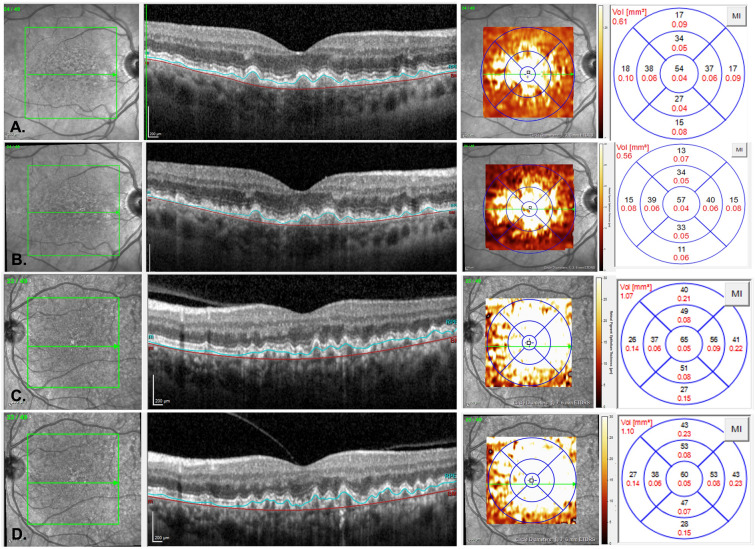
Fig. 4.
Representative OCT B-scans of macular drusen volume changes in PBM (A,B) and sham (C,D) subjects that completed all study treatments. A PBM subject with overall ETDRS drusen volume of 0.61 mm3 at baseline. B At end of study (month 10), the PBM subject showed a reduction in macular drusen volume to 0.56 mm3. C Sham subject with overall ETDRS drusen volume of 1.07 mm3 at baseline. D At end of study (month 10), the sham subject showed an increase in macular drusen volume to 1.10 mm3. Segmentation of Bruch’s membrane (red line) and the RPE (blue line). ETDRS early treatment diabetic retinopathy study, PBM photobiomodulation
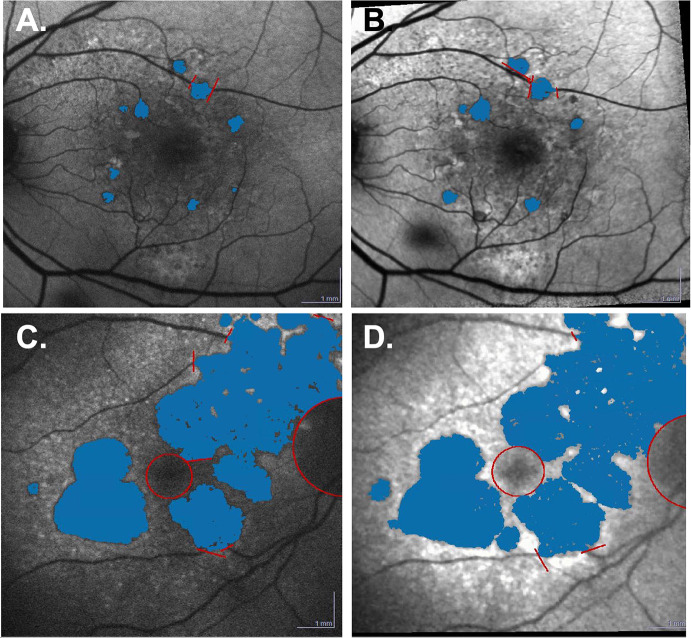
Fig. 5.
Representative FAF scans of geographic lesion growth in PBM and sham subjects that completed all study treatments. The hypoFAF lesions, corresponding to the GA lesions, were semiautomatically assessed using the Heidelberg region finder and marked in blue. A PBM subject showing overall lesions of 0.58 mm2 at baseline. B At end of study (month 10), the lesions had grown to 0.78 mm2. Following PBM treatment, the difference in lesion area was 0.16 mm2. C Sham subject showing overall lesions of 20.53 mm2 at baseline. D At the end of study (month 10), the lesion exceeded the image. The measured area was 25.6 mm2. Following sham treatment, the difference in lesion area was 5.07 mm2. FAF fundus autofluorescence
The primary outcome evaluated PBM effect from baseline to month 9 within PBM-treated subjects. Secondary outcomes included other investigations on patient-reported and imaging outcomes in PBM subjects and between PBM and sham groups at various time points. This study was conducted in compliance with the protocol, Good Clinical Practice guidelines and all other applicable regulatory requirements. This study was performed in adherence to the guidelines of the Declaration of Helsinki.
Photobiomodulation Treatment with Valeda Light Delivery System
Subjects were treated with Valeda, which delivers three distinct wavelengths in the yellow (590 nm; 4 mW/cm2), red (660 nm; 65 mW/cm2) and near infrared (NIR) (850 nm; 0.6 mW/cm2) wavelength range. Treatments last approximately 5 min per eye. Nine treatments were delivered over a 3–4-week period for each treatment series. Subjects underwent 3 treatment series in total at baseline, month 4 and month 8 time points. Valeda delivers PBM and sham treatments, which differ only with respect to light-emitting diode (LED) emissions. The apparent behavior (i.e., the performance/output of all visible and audible indicators including the graphic user interface) of the system is identical for both treatment modalities. Masking of the study was accomplished using the sham treatment, which delivered a lower fluence of selected wavelengths. The sham mode delivered an approximately 50 × and 100 × reduction in treatment fluence compared with the PBM mode of the 590 and 660 nm wavelengths, respectively, and omitted the 850 nm wavelength. Subjects were randomized in a 2:1 fashion (PBM; sham). An independent statistician not associated with the study generated the randomization tables and the codes, which were uploaded in the Valeda by an independent third party. Valeda is CE approved in the EU. Valeda was not FDA-approved in the US at the time of this report.
COVID-19 Interference
The LIGHTSITE III study was underway when the COVID-19 viral outbreak occurred. This global public health emergency significantly impacted the conduct of the clinical trial. Challenges arose due to country-specific quarantines, site closures, travel limitations and other considerations for site personnel or trial subjects becoming infected with COVID-19. These challenges led to difficulties in protocol-specified procedures, including administration of Valeda treatments and adherence to protocol-mandated visits resulting in unavoidable protocol deviations due to COVID-19 illness and/or COVID-19 public health control measures.
In March 2020, all clinical sites were contacted to inform them that LumiThera had decided to suspend new patient enrollment into the LIGHTSITE II study. LumiThera recommended that sites work with enrolled patients to delay the start of the second and third treatment series but encouraged them to remain enrolled, and, when conditions were acceptable, the treatments would resume. Individual guidance was provided to subjects who were in the middle of treatment sessions. The study remained on hold for 11 + weeks. Sites were notified in August 2020 that, after careful consideration, LumiThera decided to terminate new patient enrollment permanently and would convert the study to a feasibility study. The protocol continued to define the earliest time points subjects could be seen for the month 4, 8 and 10 visits, but did not provide a specific window for time points beyond the ideal visit date.
Statistical Analysis
Statistical analyses were performed using Statistical Analysis System (SAS) version 9.4 or R version 3.4.4. All analyses are based on individual eyes rather than individual subjects unless otherwise indicated. All subjects enrolled (n = 44 subjects; n = 53 eyes), modified intent-to-treat (mITT) (n = 42 subjects; n = 51 eyes) and a full series sub-stratification analysis (n = 26 subjects; n = 29 eyes) were conducted across select outcomes and study time points. All subjects randomized to treatment that received all three series of Valeda treatments and attended all 27 study visits per protocol were included in the full series analysis. Analyses of change from baseline following treatment and the treatment effect on the change from baseline used linear mixed effects models that account for correlation between eyes within subject. Covariates were not included in the models. Reported mean values are the least squares means calculated by SAS PROC MIXED. VFQ-25 analysis used a linear regression model.
Analyses presented used the mITT population, unless otherwise specified. Two subjects (2 eyes) are excluded from the mITT analysis. One subject received a single treatment and discontinued due to personal reasons. The second subject had imaging OCT data indicating significant degenerative photoreceptor pathology at baseline that made the eye ineligible and should have been excluded at enrollment. Efficacy analyses exclude subjects not in the mITT.
Results
Patient Characteristics
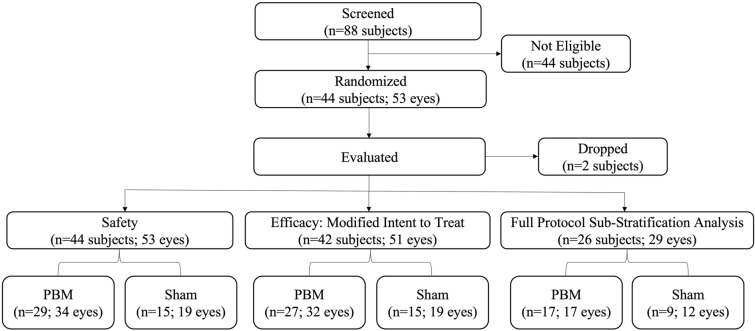
A total of 44 subjects and 53 eyes were enrolled into the study (Fig. 2). Subjects were randomized into two groups such that 15 subjects were given sham treatment and 29 subjects were given PBM treatment to qualifying eyes during each treatment series. The overall mean age at screening was 74.1 (SD 8.0) years with a higher distribution of females (n = 27; 61.4%) than males (n = 17; 38.6%). The median age was 74.5 years. All patients were white (n = 44; 100.0%) with the majority not Hispanic or Latino (n = 34; 77.3%). Most subjects’ eyes had intermediate stages of AMD as classified by a high percentage of AREDS category 3 (n = 35; 66.0%) followed by category 2 (n = 11; 20.8%) and a limited number of category 1 (n = 1; 9.0%) and category 4 (n = 6; 11.3%) at baseline (Table 1).
Fig. 2.
LIGHTSITE II subject disposition. Data from one subject was dropped because of an eligibility violation and one subject discontinued due to personal reasons. The full protocol sub-stratification analysis included all subjects that completed all treatment visits. PBM photobiomodulation
Table 1.
Baseline subject characteristics and clinical outcomes
| Baseline characteristics (all subjects) | PBM | Sham |
|---|---|---|
| No. of subjects: 29 | No. of subjects: 15 | |
| Age at screening visit | ||
| Mean (SD) | 75.6 (7.00) | 71.2 (9.30) |
| Gender | ||
| Male, N (%) | 11 (37.90) | 6 (40.00) |
| Female, N (%) | 18 (62.10) | 9 (60.00) |
| Baseline characteristics (all subjects) | PBM | Sham |
|---|---|---|
| No. of eyes: 34 N (%) |
No. of eyes: 19 N (%) |
|
| AREDS categorya | ||
| Category 1 | 1 (2.90) | 0 (0.00) |
| Category 2 | 10 (29.40) | 1 (5.20) |
| Category 3 | 22 (64.70) | 13 (68.40) |
| Category 4 | 1 (2.90) | 5 (26.30) |
| Baseline characteristics (mITT subgroup) | PBM | Sham |
|---|---|---|
| No. of eyes: 32 Mean (SD) |
No. of eyes: 19 Mean (SD) |
|
| Best-corrected visual acuity | ||
| Baseline | 70.06 (5.76) | 70.53 (5.02) |
| Month 9 | 72.36 (6.81) | 72.57 (4.76) |
| Change from baseline | 2.295 (5.23)* | 2.042 (3.38) |
| Baseline characteristics (full protocol subgroup) | PBM | Sham |
|---|---|---|
| No. of eyes: 17 Mean (SD) |
No. of eyes:12 Mean (SD) |
|
| Best-corrected visual acuity | ||
| Baseline | 70.65 (4.94) | 70.53 (5.02) |
| Month 9 | 74.59 (6.71) | 71.00 (6.70) |
| Change from baseline | 3.94 (7.19) | 0.5 (3.8) |
| Baseline characteristics (full protocol subgroup) | PBM | Sham |
|---|---|---|
| No. of eyes: 17 N (%) |
No. of eyes: 12 N (%) |
|
| Subjects that improved by > 5 letters | ||
| M9 | 6 (35.3) | 2 (16.6) |
| Baseline characteristics (mITT subgroup) | PBM | Sham |
|---|---|---|
| No. of eyes: 32 Mean (SD) |
No. of eyes: 19 Mean (SD) |
|
| Contrast sensitivity (mITT subgroup) | ||
| Baseline | 1.411 (0.18) | 1.13 (0.35) |
| Month 9 | 1.408 (0.19) | 1.24 (0.26) |
| Change from baseline | − 0.003 (0.12) | 0.11 (0.15) |
mITT modified intent-to-treat protocol, PBM photobiomodulation, SD standard deviation
aAREDS category stratified by number of eyes and not subject. Each eye was evaluated independent of the companion eye for AREDS category classification. Full protocol subset: all subjects that completed all treatment visits
*p < 0.05, within PBM-treated group
A total of eight subjects withdrew from the study: four subjects (50.0%) discontinued because of an adverse event (AE) (No AE leading to discontinuation was considered related to the study treatment.) Two subjects (25.0%) discontinued because of concerns related to COVID-19, and two subjects (25.0%) discontinued for personal reasons related to the frequency of protocol specifics. Two subjects were discontinued from the study: one (50.0%) discontinued because of development of wet AMD and one (50.0%) because of development of choroidal neovascular membrane.
COVID-19 Interference
Due to invoked policy and regulations across the EU, subjects were unable to normally participate in the designed study protocol. However, only two subjects withdrew from the study because of COVID-19 concerns. In total, 32.1% of eyes missed data collection at month 4, 17.0% at month 8 and 18.9% at month 10, and 35.9% of subjects did not complete the full treatment protocol. Approximately 9.4% of eyes missed at least one treatment in the first treatment series, 30.2% in the second treatment series and 25.0% in the third treatment series. No eyes missed the entire first series of treatment, 30.2% of eyes missed all treatments in the second treatment series, and 15.1% of eyes missed all treatments in the third treatment series. A total of 151 protocol deviations were reported. COVID-19-related deviations account for 62.9% (n = 95) of all protocol deviations.
Efficacy Assessments
Clinical Outcomes
Primary Outcome: Best-Corrected Visual Acuity
The primary efficacy end point was the change from baseline in BCVA among PBM-treated subjects (n = 32 eyes) at month 9. The overall mean BCVA at baseline was 70.06 letters (SD 5.76). A significant 2.30-letter (SD 5.23) improvement from baseline to month 9 in BCVA following PBM treatment was observed (p = 0.02) in PBM-treated subjects (Fig. 2). Secondary analysis of sham-treated (n = 19) vs. PBM-treated (n = 32) eyes on BCVA change from baseline at month 9 showed a letter score change of 2.04 letters (SD 3.34) for sham and 2.3 letters (SD 5.23) for PBM groups. Although the improvement in BCVA was numerically greater in PBM-treated eyes, no significant difference was noted between groups (p = 0.85). Secondary analyses conducted at months 1, 4, 5 and 8 showed no significant difference between sham and PBM groups (p > 0.05) (Fig. 3).
Fig. 3.
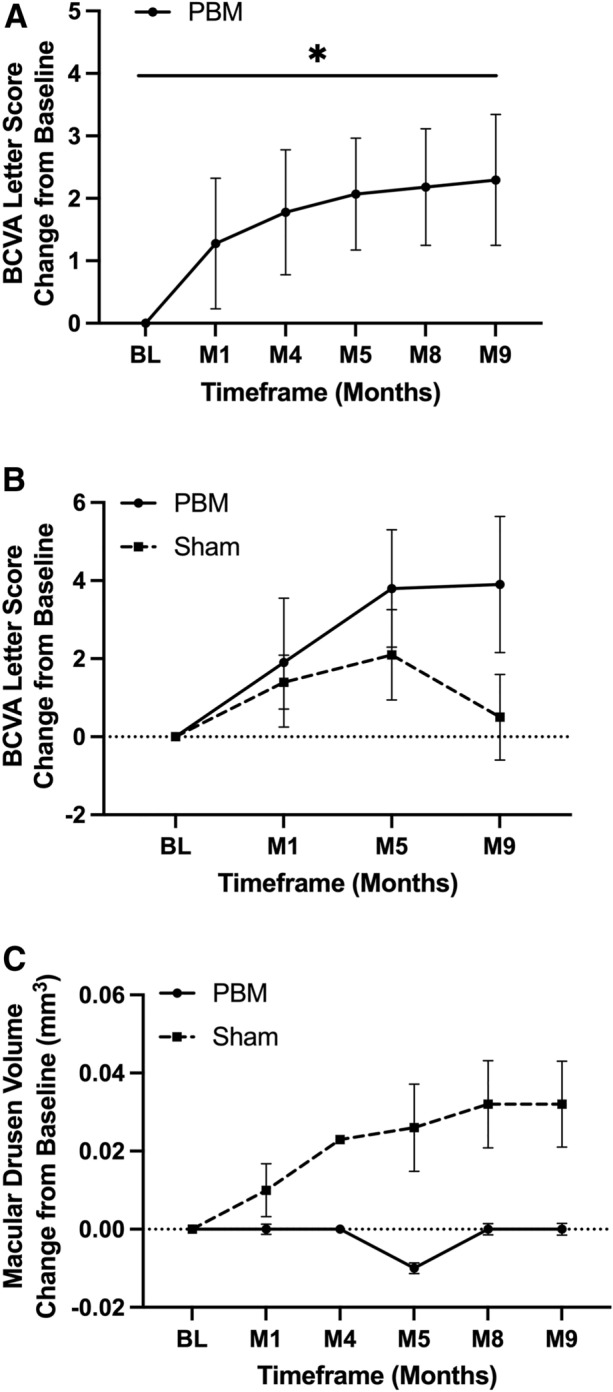
PBM effect on visual and anatomical outcomes. A A statistically significant improvement in BCVA at M9 was seen within PBM-treated subjects (MITT, n = 32 eyes), p = 0.02. B A ~ 4-letter BCVA improvement was seen in PBM-treated subjects vs. 0.5 letters in sham subjects that completed the full protocol (n = 29 eyes). C A numerical increase in macular drusen growth was observed in sham subjects over time. No macular drusen growth was observed in PBM-treated subjects (ns, p > 0.05; n = 36 eyes). Data presented include least squared means plus standard error means. BCVA best-corrected visual acuity, BL baseline, M month, PBM photobiomodulation, Tx treatment
In the full protocol sub-stratification analysis with subjects that received all Valeda treatments (n = 29 eyes: 12 sham; 17 PBM), a significant improvement of 3.94 letters (SD 7.19) in BCVA was observed in the PBM-treated group from baseline (p < 0.01) vs. a 0.5-letter (SD 3.8) gain in the sham-treated group (p = 0.10). Sham vs. PBM group comparison was not statistically significant (Fig. 3). Approximately 35.3% of PBM-treated eyes showed ≥ 5-letter improvement at 9 months compared to 16.6% of sham-treated eyes (Table 1).
Secondary and Exploratory Outcomes
Subjects showed no to mild impairment on secondary and exploratory clinical visual outcomes at baseline. Mars CS analysis was conducted at 40, 80 and 120 cm. The overall baseline and mean log score in the PBM-treated group was numerically higher than in sham groups at baseline, indicating a difference between groups. The impairment in CS was considered mild for this age group. A PBM effect on sham-treated (n = 19) vs. PBM-treated (n = 32) eyes on CS at 40 cm at month 9 showed an average change from baseline of 0.11 (SD 0.15) log units for sham and -0.003 (SD 0.12) log units for PBM groups. No significant effect was noted between baseline and month 9 Mars CS scores at 40 cm between groups (p = 0.10) (Table 1). Assessment at 80 and 120 cm at month 9 and additional analyses for all distances at other time points were non-significant. The overall mean VFQ-25 composite scores in the PBM group were numerically higher than in the sham group at each time point but did not reach statistical significance. No statistically significant differences in change from baseline were observed between groups in VFQ-25-specific category subscales (p > 0.05). No statistically significant effects were observed between PBM and sham groups on logRad score, maximum reading speed or critical print size at month 9 (p > 0.05).
Anatomical Outcomes
Exploratory analysis evaluated PBM effect in sham (n = 12) and PBM-treated (n = 24) eyes on subRPE macular drusen volume change from baseline. At month 9, the mean change from baseline for sham subjects was 0.032 (SD 0.132) mm3 and 0.0003 (SD 0.035) mm3 for PBM subjects (Table 2). At every time point assessed, no significant growth in macular drusen volume in the PBM subjects was seen but due to the small number of patients, no significant difference was observed between sham and PBM groups (p > 0.05). Although non-significant, over time a numerical increase in macular drusen volume was observed only in sham-treated eyes vs. PBM-treated eyes (Fig. 3). Representative images for changes in macular drusen are presented in Fig. 4.
Table 2.
Anatomical outcomes (mITT subgroup analyses)
| Sham | PBM | |
|---|---|---|
| No. of eyes: 19 Mean (SD) |
No. of eyes: 32 Mean (SD) |
|
| Macular drusen volume (mm3) | ||
| Baseline | 0.59 (0.22) | 0.58 (0.27) |
| Month 9 | 0.62 (0.28) | 0.58 (0.27) |
| Change from baseline | 0.03 (0.14) | 0.003 (0.04) |
| Central subfield drusen thickness (μm) | ||
| Baseline | 42.00 (16.67) | 57.17 (56.05) |
| Month 9 | 46.07 (22.82) | 55.80 (58.13) |
| Change from baseline | 4.07 (14.36) | -0.71 (13.24) |
| Sham | PBM | |
|---|---|---|
| No. of eyes: 8 Mean (SD) |
No. of eyes: 8 Mean (SD) |
|
| Geographic atrophy (mm2) | ||
| Baseline | 6.65 (8.91) | 4.86 (6.32) |
| Month 9 | 7.06(10.0) | 4.601 (6.26) |
| Change from baseline | 1.29 (1.47) | 0.73 (0.56) |
| Geographic atrophy square root analysis (μm) | ||
| Square root | 0.402 | 0.324 |
| Month 9, % difference between sham and PBM | 19.4% | |
mITT modified intent to treat protocol, SD standard deviation
Exploratory analysis evaluated PBM effect in sham (n = 12) and PBM-treated (n = 24) eyes on central subfield subRPE drusen thickness change from baseline. At month 9, the mean change from baseline for sham subjects was 4.07 µm (SD 14.36) and − 0.80 µm (SD 13.24) for PBM subjects (Table 2). Due to the reduced number of subjects, no significant growth in central subfield drusen thickness in the PBM subjects was seen, and no significant difference was observed between sham and PBM groups (p > 0.05). Although non-significant, over time a numerical increase in central subfield drusen thickness was observed in sham-treated eyes vs. PBM-treated eyes.
Exploratory analysis evaluated the effect of PBM in sham- (n = 9) and PBM-treated (n = 10) eyes on GA lesion area changes throughout the study. The mean baseline GA lesion area was 6.6 mm2 (SD 8.9) in the sham group and 4.9 mm2 (SD 6.3) in the PBM group. The change from baseline GA lesion area was numerically smaller at each time point in the PBM treatment group vs. sham treatment group. GA lesion area grew over time in both groups with a slower rate of growth in the PBM-treated group. Although a numerical increase in GA lesion size was observed in sham- vs. PBM-treated eyes, no significant difference was noted between groups at month 9 (p = 0.46). The square root analysis of GA lesion size showed significant growth of GA within each group over time at all time points beginning at the pre-month 4 visit (p < 0.05) excluding month 5 for the sham group (p = 0.175). A numerical difference in GA lesion growth was observed in sham- vs. PBM-treated eyes at month 9 (p = 0.39) but was not significant because of small patient numbers. The percent comparative difference between GA lesion growth of sham-treated vs. PBM-treated eyes was 19.4% at month 9. Representative images for changes in GA are presented in Fig. 5.
Throughout the course of the study, three eyes converted to wet AMD. One eye was in the sham treatment group, one eye was in the PBM treatment group, and one eye was a non-study eye that did not receive either treatment. A total of 16/44 (36.3%) subjects were considered high risk for converting to wet AMD (i.e., the non-study companion eye had a history or current presence of exudative macular neovascularization). Of this high-risk group, 12/16 eyes were in the PBM treatment group and 4/16 eyes were in the sham group. None of the high-risk eyes converted to wet AMD in the PBM-treated group (0.0%) and one eye converted to wet AMD in the sham treatment group (25%).
Safety Assessments
All subjects enrolled were evaluated for the safety analysis. A total of seven serious adverse events (SAEs) were reported during the course of the study. No SAEs were considered related to the Valeda device. A total of 49 AEs were reported in addition to the SAEs. The most common AEs included infections and infestations (n = 12, 24.5%), nervous system disorders (n = 8, 16.3%) and respiratory, thoracic and mediastinal disorders (n = 8, 16.3%). The AEs were consistent with the elderly age of the population of the subjects.
A summary of ocular adverse events by preferred term by eye for treated eyes is presented in Table 3. A total of 19 ocular AEs were reported within normal occurrence in the study population. One subject died prior to study completion. The subject was enrolled into the PBM treatment group and completed all visits except the final exit visit and was thought to be lost to follow-up for several months. It was later determined that the subject passed away from natural causes 8 months after his last PBM treatment. His passing was determined unrelated to the treatment.
Table 3.
LIGHTSITE II ocular adverse events by preferred term by eye (all subjects enrolled)
| Adverse events (by preferred term) | PBM, N (%) No. of eyes: 34 |
Sham, N (%) No. of eyes: 19 |
Non-study, N (%) No. of eyes: 35 |
||
|---|---|---|---|---|---|
| Chalazion | 1 (2.9) | 0 (0.0) | 0 (0.0) | ||
| Macular neovascularization | 0 (0.0) | 1 (5.3) | 0 (0.0) | ||
| Conjunctival edema | 0 (0.0) | 0 (0.0) | 1 (2.9) | ||
| Dry eye | 2 (5.9) | 0 (0.0) | 1 (2.9) | ||
| Excessive eye blinking | 2 (5.9) | 0 (0.0) | 0 (0.0) | ||
| Eye inflammation | 0 (0.0) | 0 (0.0) | 1 (2.9) | ||
| Eye paraesthesia | 0 (0.0) | 2 (10.5) | 0 (0.0) | ||
| Eye pruritus | 0 (0.0) | 1 (5.3) | 0 (0.0) | ||
| Lacrimation increased | 3 (8.8) | 1 (5.3) | 0 (0.0) | ||
| Neovascular age-related macular degeneration | 1 (2.9) | 0 (0.0) | 1 (2.9) | ||
| Swelling of eyelid | 0 (0.0) | 1 (5.3) | 0 (0.0) | ||
| Visual acuity reduced | 1 (2.9) | 1 (5.3) | 2 (5.7) | ||
| Visual impairment | 2 (5.9) | 0 (0.0) | 0 (0.0) | ||
| Total | 12 | 7 | 6 | ||
Total number may reflect adverse events from the same subject
PBM photobiomodulation
Discussion
The LIGHTSITE II study further evaluated the effects of multiwavelength PBM using the Valeda Light Delivery System in subjects with intermediate non-exudative AMD. This randomized, sham-controlled, parallel group study builds upon the previous LIGHTSITE I study, which demonstrated positive PBM benefits on efficacy and safety outcomes in subjects with non-exudative AMD.
Due to the COVID-19 pandemic, the LIGHSITE II study enrollment was truncated and treated as a feasibility study with only 44 subjects (53 eyes) enrolled. The COVID-19 pandemic had a substantial impact on the study design including patient adherence to Valeda PBM and sham treatments and clinical access. In addition, LumiThera paused the study for 11 + weeks during the middle of the study following the initial outbreak in Europe. The majority of subjects missed a number of Valeda treatment visits or were treated outside the initial protocol design windows in a random and non-standardized fashion.
LIGHTSITE II met the primary endpoint of significant BCVA change from baseline within PBM-treated subjects at the end of the three series of Valeda treatments at month 9 with a statistically significant 4-letter increase in BCVA in those that had received all treatments. No statistically significant difference was observed between the PBM and sham treatment groups. Compared with the 4-letter gain in subjects that received all Valeda treatments, the sham-treated group had a mean letter gain of 0.5 letters. Approximately 35.3% of PBM-treated eyes showed ≥ 5-letter improvement at 9 months. Improvements in clinical BCVA outcomes are suggestive of overall improvement in visual function.
Drusen is a risk factor for the development of late complications of AMD including GA, CNV and subsequent central vision loss [22–24]. Previous studies report that the rate of progression to advanced AMD (CNV and GA over 5 years) is 1.3% with many small or few medium drusen, 18% if many medium or any large drusen (= AREDS, category 3) and 43% if unilateral advanced AMD is present. Higher frequency and larger drusen deposits are indicative of disease progression. Currently, there are no approved treatments to treat GA or limit its progression when already present, nor are there treatments that can reverse drusen pathology although investigations have been conducted or are underway [25, 26]. GA and drusen represent appealing targets for treatment. The current study showed no growth in macular drusen volume and central subfield drusen thickness in PBM-treated subjects over the course of the study, whereas an increase was observed in the sham-treated group. A numerical reduction in rate of GA lesion growth over time was also noted in the small number of PBM-treated subjects that presented with GA. Valeda treatment with PBM showed a 19.4% reduction at 9 months when compared to the growth rate in the sham group. While non-significant and in small cohorts of the sample, these anatomical effects provide support for further exploration into the potential disease-modifying effects of PBM on non-exudative AMD pathology.
No safety concerns or signs of phototoxicity were observed during the 10-month study. In addition, patient adherence to the in-clinic treatment visits 3×/week for 3–5 weeks were well attended, when possible, during extreme disruptions due to the COVID-19 pandemic. Repeat in-clinic treatment may prove problematic for patient compliance; however, the high compliance observed in the current study (when not disrupted by COVID-19) mirrors results from the prior LIGHTSITE I study [21]. LIGHTSITE II further highlights the positive safety and tolerability of repeated PBM treatment in the eye.
Limitations exist within the LIGHTSITE II study that need to be considered when interpreting the data. As mentioned, the interference from the COVID-19 pandemic significantly interrupted the study design, adherence to the proposed protocol, data collection and statistical analysis. The study was not sufficiently powered with the recruitment of 44 subjects and was considered a feasibility study with three rounds of treatment. Additionally, the earlier intermediate stage of AMD evaluated showed no to mild disruption in visual dysfunction in several clinical outcomes at baseline that reduced the capacity for treatment effects to be observed.
Conclusions
Overall, the LIGHTSITE II study enabled further exploration into the utility of multiwavelength PBM delivered by Valeda in a randomized, controlled trial with intermediate non-exudative AMD subjects and a time of diagnosis of < 4 years. Consistent improvements in visual function, morphological signs of non-exudative AMD disease activity and maintenance of clinical outcome benefits following repeated PBM treatment with Valeda were observed. These results build on previous clinical testing of multiwavelength PBM and support continued investigation into its potential as a novel treatment for non-exudative AMD. Further studies are necessary to determine an optimized approach for the PBM delivery and treatment intervals specific to ocular indications.
Acknowledgements
Funding
This study, and the journal’s Rapid Service Fee, was supported by LumiThera, Inc.
Medical Writing, Editorial and Other Assistance
LumiThera would like to acknowledge Jing Shi, MD, PhD, MS, for her support in the statistical analysis of the data.
Author Contributions
All authors contributed to the study conception and design, material preparation, data collection and analysis. The first draft of the manuscript was written by Stephanie Tedford and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Ben Burton, Maurizio Battaglia Parodi, Ignasi Jürgens, Xavier Zanlonghi, Dan Hornan, Johann Roider and Katrin Lorenz were PIs in the LIGHTSITE II trial and received support from LumiThera for their efforts. Ben Burton received support from LumiThera for his efforts including honoraria fees, funding/hospitality to support attendance at conferences and support for commercially funded research for LumiThera and uses the LumiThera Valeda in his private practice. Maurizio Battaglia Parodi is a consultant for Novartis. Ignasi Jürgens was a PI in the LT2 trial and received support from LumiThera for his efforts. Dan Hornan is a consultant for Reneo Pharmaceuticals; Eye Department at Peterborough Hospital was lent an Optos imaging device by the Sponsor. Michael Walker is a statistics consultant for LumiThera. Marion R. Munk is a consultant for LumiThera, Ocuterra, RetinAI, Isarna Therapeutics, Bayer, Novartis, Roche, Abbvie, Gensight Therapeutics, Kubota, Helbling and Zeiss. Stephanie Tedford, Cindy L. Croissant, Clark E. Tedford and Rene Ruckert are all employees of LumiThera.
Compliance with Ethics Guidelines
This study was approved by all institutional ethics committees. This study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments. All subjects provided informed consent to participate in the study. Clinicaltrial.Gov Registration Identifier: NCT03878420.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res. 2009;28(1):1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joachim N, et al. The incidence and progression of age-related macular degeneration over 15 years: The blue mountains eye study. Ophthalmology. 2015;122(12):2482–2489. doi: 10.1016/j.ophtha.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Wong-Riley MT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280(6):4761–4771. doi: 10.1074/jbc.M409650200. [DOI] [PubMed] [Google Scholar]
- 4.Kauppinen A, et al. Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci. 2016;73(9):1765–1786. doi: 10.1007/s00018-016-2147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Passarella S, Karu T. Absorption of monochromatic and narrow band radiation in the visible and near IR by both mitochondrial and non-mitochondrial photoacceptors results in photobiomodulation. J Photochem Photobiol B. 2014;140:344–358. doi: 10.1016/j.jphotobiol.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 6.Karu T. Mitochondrial mechanisms of photobiomodulation in context of new data about multiple roles of ATP. Photomed Laser Surg. 2010;28(2):159–160. doi: 10.1089/pho.2010.2789. [DOI] [PubMed] [Google Scholar]
- 7.Gouveia VA, et al. Photobiomodulation reduces cell death and cytokine production in C2C12 cells exposed to Bothrops venoms. Lasers Med Sci. 2020;35(5):1047–1054. doi: 10.1007/s10103-019-02884-4. [DOI] [PubMed] [Google Scholar]
- 8.da Silva PAV, et al. Photobiomodulation can alter mRNA levels cell death-related. Lasers Med Sci. 2019;34(7):1373–1380. doi: 10.1007/s10103-019-02732-5. [DOI] [PubMed] [Google Scholar]
- 9.Hamblin MR. Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochem Photobiol. 2018;94(2):199–212. doi: 10.1111/php.12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferraresi C, et al. Light-emitting diode therapy in exercise-trained mice increases muscle performance, cytochrome c oxidase activity ATP and cell proliferation. J Biophoton. 2015;8(9):740–754. doi: 10.1002/jbio.201400087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gopalakrishnan S, et al. Photobiomodulation preserves mitochondrial redox state and is retinoprotective in a rodent model of retinitis pigmentosa. Sci Rep. 2020;10(1):20382. doi: 10.1038/s41598-020-77290-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eells JT, Gopalakrishnan S, Valter K. Near-infrared photobiomodulation in retinal injury and disease. Adv Exp Med Biol. 2016;854:437–441. doi: 10.1007/978-3-319-17121-0_58. [DOI] [PubMed] [Google Scholar]
- 13.Albarracin R, Eells J, Valter K. Photobiomodulation protects the retina from light-induced photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2011;52(6):3582–3592. doi: 10.1167/iovs.10-6664. [DOI] [PubMed] [Google Scholar]
- 14.Hall A, Liedhegner E. 670nm photobiomodulation modulates bioenergetics and oxidative stress in an in vitro model of diabetic retinopathy, in Euretina. 2019: Paris, France.
- 15.Ivandic BT, Ivandic T. Low-level laser therapy improves vision in patients with age-related macular degeneration. Photomed Laser Surg. 2008;26(3):241–245. doi: 10.1089/pho.2007.2132. [DOI] [PubMed] [Google Scholar]
- 16.Ivandic BT, Ivandic T. Low-level laser therapy improves vision in a patient with retinitis pigmentosa. Photomed Laser Surg. 2014;32(3):181–184. doi: 10.1089/pho.2013.3535. [DOI] [PubMed] [Google Scholar]
- 17.Ivandic BT, Ivandic T. Low-level laser therapy improves visual acuity in adolescent and adult patients with amblyopia. Photomed Laser Surg. 2012;30(3):167–171. doi: 10.1089/pho.2011.3089. [DOI] [PubMed] [Google Scholar]
- 18.Tang J, Herda AA, Kern TS. Photobiomodulation in the treatment of patients with non-center-involving diabetic macular oedema. Br J Ophthalmol. 2014;98(8):1013–1015. doi: 10.1136/bjophthalmol-2013-304477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng Y, et al. Photobiomodulation inhibits long-term structural and functional lesions of diabetic retinopathy. Diabetes. 2018;67(2):291–298. doi: 10.2337/db17-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ball KA, Castello PR, Poyton RO. Low intensity light stimulates nitrite-dependent nitric oxide synthesis but not oxygen consumption by cytochrome c oxidase: implications for phototherapy. J Photochem Photobiol B. 2011;102(3):182–191. doi: 10.1016/j.jphotobiol.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Markowitz SN, et al. A double-masked, randomized, sham-controlled, single-center study with photobiomodulation for the treatment of dry age-related macular degeneration. Retina. 2020;40(8):1471–1482. doi: 10.1097/IAE.0000000000002632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yehoshua Z, et al. Spectral domain optical coherence tomography imaging of dry age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2010;41(Suppl):S6–s14. doi: 10.3928/15428877-20101031-19. [DOI] [PubMed] [Google Scholar]
- 23.Folgar FA, et al. Drusen volume and retinal pigment epithelium abnormal thinning volume predict 2-year progression of age-related macular degeneration. Ophthalmology. 2016;123(1):39–50.e1. doi: 10.1016/j.ophtha.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Abdelfattah NS, et al. Drusen volume as a predictor of disease progression in patients with late age-related macular degeneration in the fellow eye. Invest Ophthalmol Vis Sci. 2016;57(4):1839–1846. doi: 10.1167/iovs.15-18572. [DOI] [PubMed] [Google Scholar]
- 25.Apellis announces top-line results from phase 3 DERBY and OAKS studies in geographic atrophy (GA) and plans to submit NDA to FDA in the first half of 2022.
- 26.Holz FG, et al. Efficacy and safety of lampalizumab for geographic atrophy due to age-related macular degeneration: chroma and spectri phase 3 randomized clinical trials. JAMA Ophthalmol. 2018;136(6):666–677. doi: 10.1001/jamaophthalmol.2018.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.