Abstract
Sarcopenia, characterized by loss of muscle mass and strength, is common in advanced old age but can be accelerated by chronic disease, malnutrition and physical inactivity. Early initiation of intervention to achieve and maintain a higher peak muscle mass and strength may allow for prevention or delay of sarcopenia and facilitate independent living even in old age. In this context, malnutrition, a significant contributor to sarcopenia, is often overlooked among the Indian population. Maintenance of an optimal energy and protein balance with adequate physical activity level is essential to preserve physical function in the aging population. However, research on the role of micronutrients in muscle maintenance, is still in its infancy. This narrative review, therefore, aims to explore the current status of International and Indian research on the role of nutrition in sarcopenia mitigation and the way forward.
Keywords: Malnutrition, Muscle loss, Chronic disease, Macronutrient, Micronutrient, Nutrient supplementation
1. Introduction
According to the World Health Organization (WHO), worldwide population aging is occurring at an extraordinary rate with a projection of higher growth in the future [1]. Sarcopenia, defined as the progressive skeletal muscle disorder characterized by an accelerated loss of muscle mass and function [2] has traditionally been considered a complication of aging, but in recent times has been shown to be accelerated in the presence of chronic diseases, malnutrition, and physical inactivity [3]. Two main groups working on developing diagnostic criteria for sarcopenia, the European Working Group on Sarcopenia in Older People (EWGSOP) and the Asian Working Group for Sarcopenia (AWGS), have recommended cutoff values for appendicular muscle mass index of 7.0 kg/m2 in men and 5.5 kg/m2 (EWGSOP) and 5.4 kg/m2 (AWGS) in women for diagnosis of sarcopenia among the European and Asian population respectively [4,5]. Sarcopenia is a complex phenomenon resulting from multiple molecular mechanisms that lead to the loss of type II fibers and a decline in muscle regeneration capacity [6]. Presence of sarcopenia with age or disease has been associated with an increased risk of adverse outcomes such as falls, fractures, frailty, hospitalization, and even mortality [2,3]. It is estimated that approximately 5–13% of people in the age range of 60–70 years are sarcopenic [7]. These percentages may increase up to 50% by the time the population reaches 80 years and above [7]. Evidence suggests that muscle mass and strength peak between the age of 20–35 years [8,9] and may be relatively maintained in adulthood. However, initiation of muscle loss may occur as early as 30 years in certain cases at a rate of 3–8% per decade. This can get aggravated to 1-2% per year post the age of 50 years [10,11]. Previous literature has documented a parallel decrease in both muscle mass and strength across the age spectrum from 30 to 93 years [12]. However, recent evidence suggests that muscle strength loss in age may be at a higher rate compared to muscle mass loss. A study on elderly adult Americans demonstrated a 3-fold decrease in strength per year compared to muscle mass when followed up over a 3-year period [13]. Muscle wasting becomes more pronounced in presence of any acute or chronic morbidity and hospitalization which often co-exists in the elderly population, making the elderly more vulnerable to developing sarcopenia. Decreased physical activity among the elderly may act as another contributing factor to skeletal muscle loss [14].
Early initiation of intervention to achieve a higher peak muscle mass and strength may allow for prevention or delay of sarcopenia and facilitate independent living even in old age. Malnutrition, a significant contributor to sarcopenia, is often overlooked among the Indian population in this context. India, along with a large number of low- and middle-income countries, is currently dealing with the dual burden of over and under-nutrition. India has been shown to have the highest share of underweight adults worldwide (19.6% in men and 22.4% in women as per The National Family Health Survey-4 (NFHS-4)) along with a continuing rise in obesity [15]. Moreover, the incidence of malnutrition is common among the elderly population particularly due to anorexia and ensuing decrease in food intake [11,16]. Despite the burden of malnutrition in this country and the possible link of malnutrition with sarcopenia, studies exploring the link between the two have been limited in the Indian population. This narrative review, therefore, aims to explore the current status of International and Indian research on the role of nutrition in sarcopenia mitigation and the way forward.
2. Accelerated sarcopenia in chronic diseases
Sarcopenia may exist independent of aging. Chronic inflammation, advanced organ dysfunction (liver, heart, lung, kidney), endocrine disorder (diabetes mellitus), and malignancy can contribute to the development and acceleration of sarcopenia [[17], [18], [19], [20], [21], [22], [23], [24]]. The EWGSOP has suggested the term “secondary sarcopenia” to encompass etiological factors other than age-related loss such as chronic diseases, physical inactivity, and malnutrition [4]. Sarcopenia in chronic diseases needs to be promptly managed as it directly affects the clinical course of the disease and may result in adverse outcomes.
Sarcopenia is associated with metabolic consequences. Muscle loss contributes to a decrease in metabolically active cells; as a result, there may be a prominent decline in resting energy expenditure (REE) [25]. Additionally, decreased functional capacity attributed to muscle weakness, low muscle store, and easy fatigability may be associated with reduced physical activity [25]. Decline in REE and physical activity may play a key role in the accumulation of visceral fat and ectopic fat contributing to various metabolic complications such as nonalcoholic fatty liver disease (NAFLD), insulin resistance (IR), and increased risk of type 2 diabetes mellitus (T2DM). Recent meta-analyses found that patients with sarcopenia had a 1.3–1.5 fold increased risk of developing NAFLD compared to patients without sarcopenia [26,27]. T2DM has been shown to have a bidirectional relationship with sarcopenia. Loss of lean tissues has been associated with an increased risk of developing T2DM; on the other hand, T2DM itself accelerates the loss of muscle tissues worsening glycemic regulation. The prevalence of sarcopenia among T2DM patients has been observed to be 16.2% compared to only 2.4% in healthy controls [23]. The prevalence of pre-sarcopenia among Asian Indians with diabetes has been observed to be as high as 39.5% [28] while the incidence of sarcopenia has been observed to be 12.5% among males and 5.4% among females [29]. This is a worrisome trend given the immense burden of diabetes in India. Sarcopenia in Chronic Obstructive Pulmonary Disease (COPD) has been associated with increased morbidity, decreased functional capacity and quality of life (QOL), and increased mortality risk, [[30], [31], [32], [33], [34]]. There is evidence of pathophysiological changes in the skeletal muscle associated with COPD such as hypoxemia and shift in muscle fiber from type 1 to type 2 among others. These can accelerate muscle loss and loss of functional capacity [35]. The prevalence of sarcopenia among COPD patients has been reported to range from 4.4 to 27.5% depending on the population studied [36]. Chronic liver disease (CLD) may aggravate muscle loss through specific mechanisms such as endotoxemia, inflammation alongside mitochondrial dysfunction, and denervation that leads to increased risk of decompensation and poor outcomes [37]. Sarcopenia in CLD is usually associated with complications such as malnutrition, inferior QOL, disability, additional metabolic complications, cardiopulmonary deficit, higher health care costs, and increased risk of death [38]. A study on sarcopenia prevalence among Indians with CLD has shown a percentage as high as 61% [39] which is a cause for concern given its implications. Diseases like CLD and chronic kidney disease (CKD) are known to trigger catabolism [40]. An increase in the catabolic state attributed to sarcopenia can wreak havoc on the patient's QOL [41]. Notably, 20-55% of end-stage renal disease patients have been observed to present with sarcopenia. This can remarkably increase the risk of mortality among the patients [42].
Catabolic diseases like cancer cause an increase in the basal metabolic rate which might also increase the energy expenditure in cases. The combination of catabolism with the decrease in food intake common among these patients can put them at risk of developing sarcopenia. The prevalence of sarcopenia among cancer patients has been observed to vary from 16 to 71% depending on the type and stage of cancer [43]. Additionally, the treatment modules usually followed for cancer such as chemotherapy and radiation therapy also contribute to muscle loss to a large extent [43]. Sarcopenia in cancer has been shown to be a predictor of disease progression and survival [43].
Disease-related malnutrition is a cause of concern in such cases as malnutrition itself can aggravate the development of sarcopenia but when combined with the presence of chronic and often catabolic diseases, can significantly affect disease outcome, treatment tolerance, and mortality. Prevalence of malnutrition among chronic disease patients has been observed to be 40% [44]. Therefore, there is a necessity for early detection of malnutrition among patients suffering from any chronic disease to prevent or delay the onset of sarcopenia as well as to improve clinical outcomes.
3. Prevalence of sarcopenia in Asia
Asia has become the region with the fastest growing aging population in the world [45]. South Asian countries including India have observed an exponential growth in the elderly population in the past few decades which is further expected to rise. According to the United Nations World Population Aging, 2019 report, 87 million people in India were aged 65 years and above and the number is expected to reach 128 million by 2030 [46]. With increase in the aging population, the burden of non-communicable diseases such as diabetes and COPD has also increased [47,48]. This can imply that there will be a greater impact of sarcopenia on the Asian population compared to the rest of the world [45]. According to the AWGS 2019 consensus update, the prevalence of sarcopenia was found to range from 5.5 to 25.7% among various Asian populations ≥ 60 years of age [5]. The prevalence of sarcopenia among Indians has been found to be in the range of 5.5–53.95% as evidenced by the available literature, demonstrated in Table 1 [[49], [50], [51], [52], [53], [54]]. Additionally, the elderly and chronic disease patients are more at risk of acute infections as evidenced by the advent of a multi-organ infectious disease like Covid-19. Although the long-term effects of this infection are still unknown, even in the acute phase, patients have been observed to be at risk of losing approximately 5–10% of their body weight, most likely through dehydration and muscle loss [55]. The risk of acute sarcopenia in this situation will be higher among the elderly [55]. Even other common infectious diseases such as tuberculosis are known to cause an exacerbation of sarcopenia [56].
Table 1.
Prevalence of sarcopenia among Indians.
| Author, year | Measurement of sarcopenia |
Age Cutoff (in years) | Sample Size (N) | Sarcopenia cut off used | Prevalence, % |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mass | Strength | Gait speed | Others | Total | Men | Women | ||||
| Mohanty et al., 2016 [49] | ≥ 60 y | 678 | SMM below -2SD of the reference young adult population | _ | 15.3% | 20.5% | ||||
| Pal et al., 2020 [50] | DXA | Hand Dynamometer | 4 min walk test | _ | ≥ 20 y | 804 | EWGSOP2, 2018 | 5.5 | 7.3% | 4% |
| Sreepriya P.R. et al., 2020 [51] | Lumbar SMI from CT scan | NA | NA | _ | ≥ 18 y | 152 | 53.95% | NA | NA | |
| Tyrovolas et al., 2015 [52] | Predictive equation proposed by Lee et al. | hand dynamometer | 4 min timed walk | _ | ≥ 65 y | 2441 | Country-specific cut-offs for SMI, < 30 kg for men and < 20 kg for women as weak handgrip and specific cut-offs for slow gait speed | 17.5% | NA | NA |
| Rahman R et al., 2021 [53] | DXA | Hand Dynamometer | 6- meter walk gait speed measured during mid 4 m | _ | ≥ 60 y | 227 | EWGSOP 2010 | 39.2% | 46.8% | 23.3% |
| EWGSOP 2018 | 29.5% | 32.5% | 23.3% | |||||||
| ASWG 2014 and 2019 | 27.8 | 32.5% | 17.8% | |||||||
| Zengin et al., 2021 [54] | DXA | Hand Dynamometer | _ | _ | > 45 y | 1755 | FNIH | _ | 42% | 35% |
| EWGSOP2 | _ | 35% | 20% | |||||||
| AWGS | _ | 31% | 20% | |||||||
Physical activity level, an important contributor to sarcopenia, is generally low in South Asians, including Indians. According to the Health Survey for England, South Asians were observed to be approximately 60% more likely to be physically inactive compared to the white Caucasian population [57]. The overall prevalence of physical inactivity among Indians has been found to be between 18.5% and 88.4% [58]. In general, the Asian population has been shown to have a lower muscle mass, and strength along with a tendency for more body fat compared to the Western population [45] with a comparable BMI, the so-called “thin-fat” phenotype [59]. The low physical activity level observed among Indians might be a contributor to this thin-fat phenotype as well. Increased adiposity may mediate chronic low-grade inflammation and oxidative stress, which by itself can be a contributor to the low muscle mass store and development of sarcopenia [17,60]. This inherent phenotype of low lean mass among the South Asian population, including Indians may be an important determinant of the high prevalence of sarcopenia among this population as they possess a lower baseline level of lean mass, to begin with. This innate low muscle mass of Asian Indians warrants more extensive research.
4. Role of macro and micronutrients in sarcopenia
4.1. Macronutrients: energy
Aging is associated with a myriad of complications that might contribute to decreased food intake and corresponding malnutrition. Prevalence of malnutrition among the elderly in India has been observed to be around 18% and the risk of malnutrition has been observed to be around 48% [61]. Insufficient intake of calories may lead to the catabolism of muscle and fat to meet the need of the body [62]. Therefore, to maintain muscle quality and physical independence, sufficient calorie intake is critical. This poses a challenge among the elderly population on account of anorexia caused due to polypharmacy, hypogeusia, anosmia, dysphagia, chewing difficulties, and delayed gastric emptying [[62], [63], [64]]. Insufficient calorie intake might lead to a negative energy balance which has been shown to contribute to the lowering of muscle protein synthesis (MPS) rates by approximately 20% along with an increase in muscle and fat catabolism [62,65]. Various studies have consistently reported sarcopenia and frailty to be associated with insufficient calorie intake [62,[65], [66], [67]].
Studies have also shown that overfeeding and excess energy intake combined with physical inactivity can lead to excess fat deposition potentially leading to muscle atrophy [68,69]. This muscle atrophy with excess fat deposition has been termed sarcobesity and is often referred to as the double-edged sword [70]. Furthermore, increased lipid accumulation reduces the amino acid incorporation and MPS in skeletal muscle [70,71]. Therefore, balancing the energy intake with the physical activity level to maintain an optimal energy balance is very essential to preserve physical function in the aging population.
4.2. Protein
In concurrence with lower calorie intake, the macronutrient which has most commonly been studied and observed to be lacking in the elderly diet is protein [11], essential for MPS. In general, Indians of different age groups, both in the urban and rural population have shown a considerable risk of low-quality protein intake which varies between 4 and 26% [72] with around 60% of dietary protein being obtained from cereals which are low in digestibility and quality [73].
A study showed that 35% of institutionalized elderly and 10% of community-dwelling elderly had a protein intake that was below the estimated average of 0.7 g/kg body weight/day [62,74]. The average protein consumption among Indians, in general, can be as low as 0.6 g/kg body weight/day [75]. In addition to lower consumption, the requirement for protein among the elderly also seems to be higher than young adults for the same amount of MPS, a phenomenon known as anabolic resistance [76,77]. This might lead to a broadening of the gap between requirement and consumption. This difference may be one of the reasons for the development of sarcopenia among the elderly. Daily protein intake of less than 0.8 g/kg body weight/day has shown difficulty in maintaining muscle mass [78] while Indians have inherently shown to have a low protein intake across all ages. Literature review has shown a protein intake of 1.0–1.2 g/kg body weight/day to be advisable for healthy older adults for optimum maintenance of muscle mass and strength. Even for elderly with chronic diseases the recommended protein intake can go as high as 1.2–1.5 g/kg body weight/day, with an exception of elderly with chronic kidney disease on conservative management [79,80].
Various studies have also suggested that, in addition to preventing sarcopenia, protein plays an important role in sarcopenia management. Studies conducted have shown protein supplementation to be an effective strategy to improve frailty and sarcopenia [81,82]. Administration of whey protein has shown improvements in biomarkers of sarcopenia and frailty through upregulation of mTOR activity that stimulates MPS [83]. These findings are validated in animal and in-vitro models; however, human studies results are still inconclusive [83]. Muscle biopsies have shown promising outcome post administration of 35 g of radio-labeled whey protein where a significant decrease in muscle protein breakdown and increase in MPS was observed 240 minutes post consumption of the whey [83]. Additionally, chronic protein supplementation for 12 weeks has shown an improvement in muscle mass and function independent of the time of administration of the supplement (either pre or post workout) [84]. Among specific amino acids derived from protein, branched-chain amino acids especially leucine and lysine have been linked with a significant increase in MPS by up-regulation of rapamycin complex 1 (mTORC1) activity [85]. A systematic analysis of 16 studies exploring the ‘leucine trigger’ hypothesis re-emphasized the utility of leucine in the regulation of postprandial MPS in older adults, especially when ingested as isolated protein over whole protein food sources [86]. Compared to placebo, 12-week supplementation of leucine enriched protein was shown to significantly improve lean body mass adjusted for weight in Korean healthy adults aged 50 years and above [87]. An improvement in walking time, lean mass, and respiratory muscle function was documented in sarcopenic elderly following 13 weeks of supplementation of leucine compared to placebo [88]. Among post-stroke sarcopenic patients, leucine-rich amino acid supplementation alongside low-intensity resistance exercises for 8 weeks duration demonstrated an improvement in muscle mass, strength, and physical functioning [89]. Healthy Indian adults, following 8-week long high lysine diet demonstrated a significant increase in upper limb muscle strength compared to group following a low lysine diet [90], however effect of the same on the elderly population has not been explored. An additional important component to consider in case of normal protein intake or protein supplementation is the aspect of distribution of the protein throughout the day. Studies have elucidated the requirement of 25–30 g of high-quality protein in each meal, given that this quantity of protein is required for obtaining the threshold dose of 3 g leucine for the optimal stimulation of MPS [91,92]. In general, the protein intake in breakfast and lunch has been observed to be poorer compared to dinner. This is not clear with regard to the Indian population and needs exploration.
However, some contradictory literature is also present with respect to protein. Studies have shown evidence of improvement in muscle mass and strength with protein supplementation in addition to resistance exercise. However, the effect decreases with advancing age [93]. A study by Reidy et al [94] displayed only minimal enhancement in lean body mass and no improvement in muscle function or size with protein supplementation in addition to resistance training compared to only resistance training among healthy young men. Study on elderly community dwellers providing protein supplementation alone illustrated no significant difference in quadriceps size, lean mass, or muscle strength. However, when combined with high load resistance exercise, there was a significant improvement in quadriceps muscle cross-sectional area and muscle strength but not lean mass [95]. The possible reason for a decrease in the effect of protein supplementation on muscle with age may be the presence of anabolic resistance in these individuals. Therefore, going by available literature, more extensive research in large sample sizes is required to elucidate the true effect of protein supplementation on muscle health or sarcopenia and especially given the huge gap of data observed among Indian elderly, especially with the existent low protein intake this becomes even more important in this population.
4.3. Omega 3 fatty acids
A growing body of evidence suggests the potential role of long chain fatty acids particularly, omega 3 (ω3) FA in the regulation of skeletal muscle protein metabolism, maintenance of muscle mass, and function. An early observational study exploring the relationship of diet and grip strength highlighted the potential muscle anabolic action of ω3 FA. A significant dose-dependent positive association was reported between fatty fish consumption and grip strength in this study [96]. Intake of ω3FA has been positively associated with higher appendicular skeletal muscle mass index and lower incidence of sarcopenia [97]. Beneficial role of ω3 FA on muscle is further supported by evidences from interventional studies. A study by Smith et al [98], on healthy elderly adults receiving ω3 FA for 6 months, in a double-blind RCT, reported a significant increment in thigh volume (3.6%), grip strength (2.3 kg), and 1 repetition maximum muscle strength (4.0%) even in absence of an exercise intervention. A randomized control trial (RCT) carried out on older adults has demonstrated an increase in MPS post ω3FA supplementation, possibly reducing anabolic resistance [99]. Although the exact mechanism of action has not been elucidated, the researchers indicated that this increase in MPS may be partially moderated by the activation of the mTOR-p70s6k signaling pathway [99]. Other studies have found a significant lowering of inflammatory markers such as CRP, IL-6, IL-1β, and TNF-α with ω3FA supplementation. This may be relevant as chronic systemic inflammation has been reported as a contributing factor for sarcopenia development [100,101]. The effect of ω3FA on muscle could be in part by modulation of mitochondrial function and partly by moderation of the mTOR pathway [102]. However, the research in this area is still in the initiation phase and no data is available among the Indian population, hence, much more data is necessary to understand the relationship between ω3FA and muscle loss/preservation in different populations.
5. Micronutrients
The role of macronutrients on the development and treatment of sarcopenia has garnered a lot of attention among the scientific community. However, the role of micronutrients has mostly been overlooked. Furthermore, the studies exploring the role of micronutrients in muscle health are limited and most of the available literature is on animal models and observational human studies. The number of studies exploring the role of nutrients in muscle health among the Indian population is extremely scarce as summarized in Table 2 [90,[103], [104], [105], [106]]. Several micronutrients warrant further investigation in particular in this population as discussed in the next section of this review.
Table 2.
Studies on effect of supplemental nutrition on muscle among the Indian population.
| Author & year | Study design | Participants | Intervention | Outcome |
|---|---|---|---|---|
| Gupta et al., 2010 [103] | A double-blind randomized study | 40 participants (20-40 years) randomized into either supplement arm or placebo arm | Supplementation consisted of cholecalciferol sachet (60000 IU) or placebo (lactose) consumed thrice weekly for 8 weeks followed by once monthly for 4 months along with daily calcium carbonate supplementation | Muscle strength and physical performance |
| Goswami et al., 2012 [104] | A randomized controlled trial with a factorial design | 173 healthy female adults | 4 combinations of intervention were carried out using a combination of cholecalciferol, calcium and placebo: The cholecalciferol dose was set at 60,000 IU/wk for first 8 wk followed by twice a month for 4 months, Calcium carbonate twice daily for 6 months, containing 500 mg elemental calcium and placebo (lactose). | No significant change in muscle strength and physical performance. |
| Wankhede et al., 2015 [105] | A prospective double-blind, randomized, placebo-controlled parallel group trial | Healthy adult males 18-50 years randomized to treatment or placebo | Resistance exercise 3 times per week for 8 weeks with either 300 mg ashwagandha root extract or placebo (300 mg starch). | Significant increase in muscle strength and size in both groups from baseline. Increase significantly higher in treatment group compared to placebo. |
| Saha S et al., 2018 [106] | A randomized controlled trial with a two-by-two factorial design | 228 healthy young males randomized. 180 participants completed the study | 4 intervention groups namely, placebo/placebo, placebo/calcium, cholecalciferol/placebo, and cholecalciferol/calcium. The cholecalciferol dose was set at 60,000 IU/week for 8 weeks followed by fortnightly for 4 months, calcium carbonate consisting of 500 mg elemental calcium, twice daily. | No significant effect observed on muscle strength |
| Unni US et al., 2012 [90] | Randomized controlled trial | 40 young adults (18-35 years) divided equally into undernourished (UN) and well nourished (WN) based on BMI | Participants from each group (UN and WN) were divided into either high lysine or low lysine diet. The high lysine group received 80 mg/kg/day lysine whereas the low lysine group received a range of 25–40 mg/kg/day of lysine. | Significant change observed on upper muscle strength with high lysine diet. Change in muscle strength not accompanied by change in muscle mass or insulin sensitivity |
5.1. Vitamin B complex
Adequate levels of B vitamins is crucial for optimum skeletal muscle function as these play a vital role in the ATP generation process, the essential fuel for muscle contraction. Many of the B vitamins, including vitamins B1, B2, B3, B6, B12, biotin, folic acid, and pantothenic acid catalyzes biochemical reactions in their coenzyme form that are essential for maintaining mitochondrial function. Even in subclinical deficiency of these vitamins, mitochondrial function may be compromised. Mitochondrial dysfunction ie, impaired bioenergetics and turnover may result in increased reactive oxygen species generation and chronic inflammation that affects the skeletal muscle bulk and function [107]. The mitochondrial function related to the level of physical activity, in general, has been observed to decrease with age [108]. In addition to its role in mitochondrial function, vitamin B also plays a role as a neurotrophic agent, in the maintenance of neural integrity and function [109]. Current available literature on vitamin B complex points towards potential beneficial effects on the skeletal muscle. Supplementation of 750 mg/kg B3 has been shown to counteract the muscle fiber shift from oxidative type 1 to glycolytic type 2 fibers and increase the proportion of type 1 fiber in animal models [110,111]. Similarly, 600 mcg of B3-bound chromium supplementation has been shown to preserve muscle mass and induce fat loss in modestly dieting and exercising African-American women [112].
Vitamin B12 deficiency is a common problem among the elderly due to the occurrence of age-related atrophic gastritis changes which can cause malabsorption of vitamin B12 from food [113]. Cross-sectional data have suggested a direct relationship between insufficient vitamin B12, functional decline, and frailty [114]. Homocysteinemia, a consequence of vitamin B12 deficiency has been associated with decreased gait speed and handgrip strength [115,116]. A lower intake of vitamin B12 and a 15% lower serum concentration of the vitamin has been reported in sarcopenic individuals compared to non-sarcopenic ones [117]. However, the suboptimal dietary intake of B12 was not considered clinically significant and vitamin B12 was suggested to be a contributing factor to frailty but not the sole cause [118]. The level of all B vitamins has been shown to severely deplete in the case of protein malnutrition [119], which, as discussed above, is a vital contributor to sarcopenia. Experimental animal studies have demonstrated vitamin B6 supplementation to be able to diminish the negative effects of protein malnutrition by increasing body weight and hemoglobin in the animals [119,120]. A long-term study on the effects of exercise on rats fed protein-deficient diets with or without vitamin B6 or control diets showed a significant increase in body weight even in protein deficient rats in both exercise and non-exercise groups. Vitamin B6 supplementation also exhibited an increase in muscle mass, thus, suggesting a protective effect of vitamin B6 against the negative effects of protein malnutrition [119].
5.2. Vitamin D and calcium
Vitamin D, commonly associated with bone health, has also shown to be significantly associated with muscle strength. A study conducted on post-menarche adolescent girls found a significant positive relationship between serum vitamin D status and muscle velocity, power, force, and jump height [121], thus suggesting a link between vitamin D and muscle contractility. Young women with insufficient vitamin D levels also showed lower muscle attenuation with 24% greater infiltration of fat in muscles independent of body mass, compared to normal level [122], thus reaffirming the link between vitamin D and muscle strength since myosteatosis is a known determinant of muscle strength. Elderly individuals with low serum vitamin D levels have been shown to have a significantly lower handgrip strength compared to vitamin D replete group [123]. Though the underlying mechanism between vitamin D deficiency and sarcopenia is still unclear, expression of Vitamin D receptor (VDR) in skeletal muscle has been postulated to modulate the skeletal muscle mass and function [124]. Reduction in muscle mass, fiber size, grip strength, and increased myostatin expression have been seen in VDR knockout mice compared to wild-type [125]. It is further suggested that vitamin D may have a role in the suppression of atrophy-related genes (FOXO1), up-regulation of mTORC1, and regulation of mitochondrial functions [126]. Among the elderly population, vitamin D insufficiency has been shown to have a direct link to falls and fractures. Although earlier this had been associated with bone health, recent literature reveals an additional role on muscle weakness [127]. A study conducted on elderly women revealed a significant improvement in musculoskeletal function following vitamin D and calcium supplementation for 12 weeks, reiterating the link [127]. Previously Sorensen et al [128] had demonstrated a small but significant increase in type 1 muscle fiber area post vitamin D and calcium supplementation.
A significantly high prevalence of insufficient intake of calcium has also been observed among the sarcopenic population [129,130]. Calpains, the calcium-dependent protease may have a role in the regulation of myogenesis and muscle dysfunction, thus, could explain the link between calcium and sarcopenia [131]. There is evidence of 34% to even 100% vitamin D deficiency among the general Indian population in various age groups [132] and 40.6% prevalence of dietary calcium deficiency during critical growth periods [133]. Therefore, given the inherent low muscle strength observed among Indians as well as the high apparent prevalence of vitamin D and calcium deficiency, this relationship of vitamin D and calcium with muscle needs to be studied extensively in this population. Studies conducted on calcium and vitamin D supplementation among Indians have produced mixed results. While Gupta et al [103] demonstrated an increase in muscle strength and performance post calcium and vitamin D supplementation among young adults in the age range of 20–40 years for 6 months, a similar dosage of supplementation for a similar duration among healthy young females did not elicit any change in the muscle strength or performance [104]. A similar study conducted by Saha et al [106] among healthy young males also elicited no significant change in muscle strength. Thus, the reports from the Indian population is necessary to be critically evaluated to understand the true relationship between the vitamin D status and muscle strength.
5.3. Iron
Deficiency of iron is a common phenomenon occurring in the elderly population, most likely as a consequence of both multi-morbid conditions and poor dietary intake. There has been report of a significantly low dietary intake of iron among sarcopenic elderly compared to non-sarcopenic ones [129]. Dietary iron intake had a significant positive association with appendicular lean mass, muscle strength, and physical performance [129,134,135]. A study found 41% of hospitalized elderly to have iron deficiency [136]. They reported significant improvement in isometric knee extension after iron supplementation (100 mg/day) compared to non-supplementation group [136]. Hence, iron supplementation appears to be a promising intervention to improve skeletal muscle function, especially among elderly suffering from iron insufficiency.
Iron is an important component of mitochondrial enzymes, thus, participates in the regulation of energy metabolism [137]. However, excess of iron has the potential to increase the oxidative stress in the body [138,139] through the production of extremely reactive oxygen species. Animal studies have displayed an elevation and accumulation of iron in skeletal muscle with aging [140]. Study on mice model has demonstrated an elevation of non-heme iron levels with aging [138]. However, administration of an iron chelating agent to the same mice did not show a significant improvement in muscle function [138]. Therefore, it was unclear whether iron accumulation in skeletal muscle has an active role in the impairment of muscle function or it simply overlaps with aging and onset of sarcopenia. Nevertheless, more research is warranted as the current findings are limited and contradictory in nature.
5.4. Trace minerals
Limited literature is available on the role of trace minerals such as magnesium, selenium, and zinc in the prevention or management of sarcopenia. A study investigating the effect of 300 mg/day magnesium supplementation over a period of 12 weeks on healthy elderly women, showed a significant improvement in the physical performance measures including Short Physical Performance Battery (SPPB), chair stand times, and 4-m walking speeds in the treatment group compared to placebo, however, the effect of the intervention on lower and upper limb muscle function was not significant [141]. Intake of minerals such as magnesium, selenium, and phosphorus among sarcopenic individuals has been observed to be lower than the non-sarcopenic ones despite having a comparable energy consumption in both the groups [117,142]. A prospective cohort study has also found a significant positive association between magnesium intake and appendicular lean mass [135].
Chen et al [143] in their study on elderly adults found that there was a positive relationship between selenium and muscle mass with a lower serum selenium level being independently associated with a higher risk of developing low muscle mass with age. On displaying the muscle strength with the lowest quartiles of plasma selenium, a consistent decrease in muscle strength (hip, knee, and grip strength) has been observed with the decrease in selenium, thus, showing a positive association [144]. Both these studies have demonstrated selenium levels to be an important independent factor affecting muscle mass and strength. Borg et al [142] also showed a lower intake of selenium among sarcopenic subjects when compared to non-sarcopenic. In a study conducted in the UK on the effect of different diet variations on physical performance, a significant effect of low selenium intake on physical performance was identified for women only [145]. Selenium has been shown to be a strong antioxidant in our body; hence, a low intake of selenium may increase susceptibility to oxidative stress, a putative determinant of sarcopenia. Zinc is also a powerful antioxidant having the potential to prevent or delay oxidative damage giving rise to muscle decline with age [134]. Similar to magnesium and selenium, zinc intake has been associated with gait speed and physical performance in older adults [134,146]. In patients with chronic liver disease, zinc has been found to be an independent predictor of sarcopenia. Serum zinc concentration was inversely related to the prevalence of sarcopenia among these patients [147]. Zinc deficiency itself is a promoter of anorexia and taste change [148], a problem widespread among sarcopenic elderly.
Given the positive early evidence towards the possible role of micronutrients in sarcopenia, more randomized controlled trials are required to utilize nutrition in combatting this multi-factorial disorder. In the interim, screening and correction of individuals at risk of sarcopenia for underlying nutritional deficiencies is advisable to allow for the effective utilization of protein or amino acid supplementation. This seems extremely important for the Indian population where deficiency of multiple micronutrients including iron, vitamin B12, and vitamin D is widely prevalent across the age strata [132,149,150]. Even among the elderly Indians, the prevalence of anemia has been found to be about 32% between the ages of 60–65 years; this has been observed to double after 75 years of age [151]. Vitamin B12 deficiency has also been studied among elderly Indians and has been found to be in the range of about 16–62% in different studies [150,152]. Deficiency of micronutrients even among the apparently healthy Indian adult population is evidently high [149]. Evidence evaluating the relationship between micronutrient deficiency and the onset of sarcopenia in Indian population is scarce, however, considering the widespread deficiency of multiple micronutrients; this indeed warrants further exploration.
5.5. Probiotics and prebiotics
In addition to usual nutrients, it may be prudent to explore other nutritional approaches that might help to improve the overall absorption of nutrients such as proteins. Probiotics are one such item that has shown evidence to improve anabolic resistance with age and improve the absorption of proteins from diet or supplements. Murine study with the administration of a probiotic for 4 weeks demonstrated significant improvement in muscle strength and endurance post-supplementation [153]. Even among humans, research on sportspeople has displayed a favorable outcome on skeletal muscle mass among men whereas no significant improvement has been displayed among women post probiotic administration [154]. Administration of prebiotics also has shown to decrease exhaustion and increase hand grip strength among frail elderly individuals [155]. Therefore, probiotics or prebiotics may be considered as an add-on to protein supplementation since there is evidence that the gut microbiota may be a determinant of anabolic resistance. Hence, targeting the gut microbiome through probiotic or prebiotic may help in improving the anabolic resistance and better utilization of the protein supplement. However, such nutritional measures are still in the preliminary stage, and research on the elderly population is very scarce, but given the promising results, may be worthwhile to explore.
6. Conclusions
Throughout the years there has been emerging evidence on the role of nutrition in the prevention and treatment of sarcopenia. However, these have not been done in large enough scales to be incorporated into the dietary guidelines. Even though the role of protein in the management and development of sarcopenia has been given ample attention, the same cannot be said for other vital nutrients, especially micronutrients. There is a strong body of evidence indicating the crucial role of micronutrients such as vitamin B, D, omega 3 fatty acids, magnesium, selenium, and zinc in the pathophysiology and management of sarcopenia majorly coming from either animal studies or observational studies. Therefore, large-scale future randomized control trials are necessary to ascertain these findings and lay the foundation for adopting multi-nutrient supplementation strategies in the management of sarcopenia.
Fig. 1.
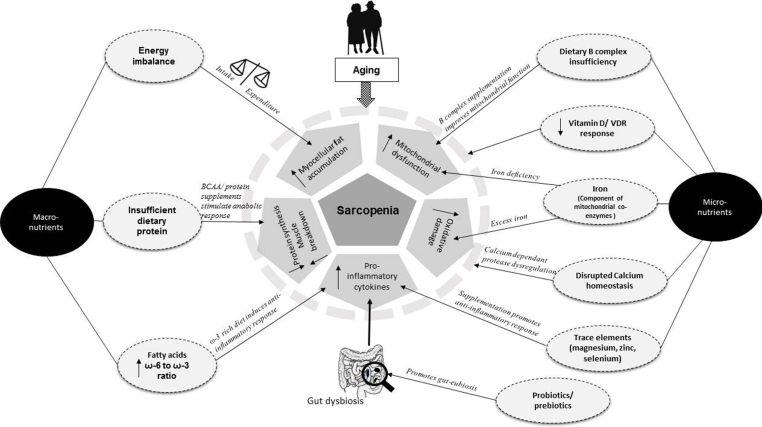
Depicting the potential nutritional factors impacting sarcopenia among Asian Indians Fig. 1 illustrates the contribution of macro- and micro-nutrients in development and management of sarcopenia. Among macronutrients, positive energy balance is a contributor to excess muscle fat deposition. Insufficient dietary protein intake catalyzes muscle protein breakdown and decreases the rate of protein synthesis. Protein and branched-chain amino acids (BCAA) supplementation have shown improvement in skeletal muscle outcomes. Proportion and composition of fatty acids in diet is associated with pro-inflammatory and anti-inflammatory response and is an important consideration in management of muscle dysfunction. Among micronutrients, vitamin B complex, vitamin D, calcium, iron are probably most studied elements. Probiotics/prebiotics improve gut bacterial composition and help lower inflammatory response and may improve digestion and utilization of protein.
CRediT author statement
Shinjini Bhattacharya: Writing – original draft, Writing – review & editing. Rohini Bhadra: Writing – original draft, Writing – review & editing. Annemie M.W.J. Schols: Writing – review & editing, Supervision. Ardy van Helvoort: Writing – review & editing, Supervision. Sucharita Sambashivaiah: Writing – review & editing, Supervision.
Conflicts of interest
The authors declare no competing interests.
Acknowledgments
ORCID Shinjini Bhattacharya: 0000-0001-6796-3213. Rohini Bhadra: 0000-0002-3818-455X. Annemie M.W.J Schols: 0000-0001-9878-0428. Ardy van Helvoort: 0000-0002-3585-0676. Sucharita Sambashivaiah: 0000-0003-2407-383X.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.Ageing and Health [Internet]. World Health Organization. Updated October 4, 2021 [Cited 2022 Feb 24]. Available from https://www.who.int/news-room/fact-sheets/detail/ageing-and-health.
- 2.Cruz-Jentoft A.J., Sayer A.A. Sarcopenia. Lancet. 2019;393:2636–2646. doi: 10.1016/S0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- 3.Santilli V., Bernetti A., Mangone M., Paoloni M. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11:177–180. [PMC free article] [PubMed] [Google Scholar]
- 4.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L.K., Woo J., Assantachai P., Auyeung T.W., Chou M.Y., Iijima K., et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21:300–307. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Seene T., Kaasik P. Muscle weakness in the elderly: role of sarcopenia, dynapenia, and possibilities for rehabilitation. Eur Rev Aging Phys Act. 2012;9:109–117. [Google Scholar]
- 7.von Haehling S., Morley J.E., Anker S.D. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1:129–133. doi: 10.1007/s13539-010-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batsis J.A., Buscemi S. In: Medical complications of type 2 diabetes. Croniger C., editor. IntechOpen; London: 2011. Sarcopenia, sarcopenic obesity and insulin resistance.https://www.intechopen.com/chapters/19824 Available from. [Google Scholar]
- 9.Metter E.J., Lynch N., Conwit R., Lindle R., Tobin J., Hurley B. Muscle quality and age: cross-sectional and longitudinal comparisons. J Gerontol A Biol Sci Med Sci. 1999;54:B207–B218. doi: 10.1093/gerona/54.5.b207. [DOI] [PubMed] [Google Scholar]
- 10.Volpi E., Nazemi R., Fujita S. Muscle tissue changes with aging. Curr Opin Clin Nutr Metab Care. 2004;7:405–410. doi: 10.1097/01.mco.0000134362.76653.b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sieber C.C. Malnutrition and sarcopenia. Aging Clin Exp Res. 2019;31:793–798. doi: 10.1007/s40520-019-01170-1. [DOI] [PubMed] [Google Scholar]
- 12.Suetta C., Haddock B., Alcazar J., Noerst T., Hansen O.M., Ludvig H., et al. The Copenhagen Sarcopenia Study: lean mass, strength, power, and physical function in a Danish cohort aged 20-93 years. J Cachexia Sarcopenia Muscle. 2019;10:1316–1329. doi: 10.1002/jcsm.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodpaster B.H., Park S.W., Harris T.B., Kritchevsky S.B., Nevitt M., Schwartz A.V., et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 14.Steffl M., Bohannon R.W., Sontakova L., Tufano J.J., Shiells K., Holmerova I. Relationship between sarcopenia and physical activity in older people: a systematic review and meta-analysis. Clin Interv Aging. 2017;12:835–845. doi: 10.2147/CIA.S132940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dutta M., Selvamani Y., Singh P., Prashad L. The double burden of malnutrition among adults in India: evidence from the National Family Health Survey-4 (2015-16) Epidemiol Health. 2019;41 doi: 10.4178/epih.e2019050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans C. Malnutrition in the elderly: a multifactorial failure to thrive. Perm J. 2005;9:38–41. doi: 10.7812/tpp/05-056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng S.J., Yu L.J. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci. 2010 12;11:1509–1526. doi: 10.3390/ijms11041509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Traub J., Bergheim I., Eibisberger M., Stadlbauer V. Sarcopenia and liver cirrhosis-comparison of the European working group on sarcopenia criteria 2010 and 2019. Nutrients. 2020;12:547. doi: 10.3390/nu12020547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasaki K.I., Kakuma T., Sasaki M., Ishizaki Y., Fukami A., Enomoto M., et al. The prevalence of sarcopenia and subtypes in cardiovascular diseases, and a new diagnostic approach. J Cardiol. 2020;76:266–272. doi: 10.1016/j.jjcc.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Jones S.E., Maddocks M., Kon S.S., Canavan J.L., Nolan C.M., Clark A.L., et al. Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax. 2015;70:213–218. doi: 10.1136/thoraxjnl-2014-206440. [DOI] [PubMed] [Google Scholar]
- 21.Limpawattana P., Inthasuwan P., Putraveephong S., Boonsawat W., Theerakulpisut D., Sawanyawisuth K. Sarcopenia in chronic obstructive pulmonary disease: a study of prevalence and associated factors in the Southeast Asian population. Chron Respir Dis. 2018;15:250–257. doi: 10.1177/1479972317743759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu M.D., Zhang H.Z., Zhang Y., Yang S.P., Lin M., Zhang Y.M., et al. Relationship between chronic kidney disease and sarcopenia. Sci Rep. 2021;15 doi: 10.1038/s41598-021-99592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trierweiler H., Kisielewicz G., Jonasson T.H., Petterle R.R., Moreira C.A., Borba V.Z.C. Sarcopenia: a chronic complication of type 2 diabetes mellitus. Diabetol Metab Syndrome. 2018;3:25. doi: 10.1186/s13098-018-0326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pamoukdjian F., Bouillet T., Lévy V., Soussan M., Zelek L., Paillaud E. Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: a systematic review. Clin Nutr. 2018;37:1101–1113. doi: 10.1016/j.clnu.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Greenlund L.J., Nair K.S. Sarcopenia--consequences, mechanisms, and potential therapies. Mech Ageing Dev. 2003;124:287–299. doi: 10.1016/s0047-6374(02)00196-3. [DOI] [PubMed] [Google Scholar]
- 26.Pan X., Han Y., Zou T., Zhu G., Xu K., Zheng J., et al. Sarcopenia contributes to the progression of nonalcoholic fatty liver disease- related fibrosis: a meta-analysis. Dig Dis. 2018;36:427–436. doi: 10.1159/000491015. [DOI] [PubMed] [Google Scholar]
- 27.Wijarnpreecha K., Panjawatanan P., Thongprayoon C., Jaruvongvanich V., Ungprasert P. Sarcopenia and risk of nonalcoholic fatty liver disease: a meta-analysis. Saudi J Gastroenterol. 2018;24:12–17. doi: 10.4103/sjg.SJG_237_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anbalagan V.P., Venkataraman V., Pradeepa R., Deepa M., Anjana R.M., Mohan V. The prevalence of presarcopenia in Asian Indian individuals with and without type 2 diabetes. Diabetes Technol Therapeut. 2013;15:768–775. doi: 10.1089/dia.2013.0068. [DOI] [PubMed] [Google Scholar]
- 29.Palanisami S., Kulkarni V. Association between muscle mass and body mass index in elderly diabetic patients attending tertiary care center in Bangalore, India. Int J Med Stud. 2016;4:96–99. [Google Scholar]
- 30.Schols A.M., Soeters P.B., Dingemans A.M., Mostert R., Frantzen P.J., Wouters E.F. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis. 1993;147:1151–1156. doi: 10.1164/ajrccm/147.5.1151. [DOI] [PubMed] [Google Scholar]
- 31.Vestbo J., Prescott E., Almdal T., Dahl M., Nordestgaard B.G., Andersen T., et al. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med. 2006;173:79–83. doi: 10.1164/rccm.200506-969OC. [DOI] [PubMed] [Google Scholar]
- 32.Lage V.K.S., Silva G.P., Lacerda A.C.R., Paula F.A., Lima L.P., Santos J.N.V., et al. Functional tests associated with sarcopenia in moderate chronic obstructive pulmonary disease. Expet Rev Respir Med. 2021;15:569–576. doi: 10.1080/17476348.2021.1850276. [DOI] [PubMed] [Google Scholar]
- 33.Ansari K., Keaney N., Taylor I., Burns G., Farrow M. Muscle weakness, health status and frequency of exacerbations in chronic obstructive pulmonary disease. Postgrad Med. 2012;88:372–376. doi: 10.1136/postgradmedj-2011-130293. [DOI] [PubMed] [Google Scholar]
- 34.Mador M.J. Muscle mass, not body weight, predicts outcome in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:787–789. doi: 10.1164/rccm.2206003. [DOI] [PubMed] [Google Scholar]
- 35.Bhadra R., Bhattacharya S., D'Souza G.A., Schols A.M.W.J., Sambashivaiah S. Pulmonary rehabilitation in the management of chronic obstructive pulmonary disease among Asian Indians- Current status and moving forward. COPD. 2021;18:476–481. doi: 10.1080/15412555.2021.1962267. [DOI] [PubMed] [Google Scholar]
- 36.van Bakel S.I.J., Gosker H.R., Langen R.C., Schols A.M.W.J. Towards personalized management of sarcopenia in COPD. Int J Chronic Obstr Pulm Dis. 2021;16:25–40. doi: 10.2147/COPD.S280540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barbero-Becerra V.J., López-Méndez I., Romo-Araiza A., Visag-Castillo V., Chávez-Tapia N.C., Uribe M., et al. Sarcopenia in chronic liver diseases: a translational overview. Expet Rev Gastroenterol Hepatol. 2020;14:355–366. doi: 10.1080/17474124.2020.1757427. [DOI] [PubMed] [Google Scholar]
- 38.Hsu C.S., Kao J.H. Sarcopenia and chronic liver diseases. Expet Rev Gastroenterol Hepatol. 2018;12:1229–1244. doi: 10.1080/17474124.2018.1534586. [DOI] [PubMed] [Google Scholar]
- 39.Gajula U. Assessment of sarcopenia in patients with chronic liver disease. Am J Gastroenterol. 2018;113:S537–S538. [Google Scholar]
- 40.Moorthi R.N., Avin K.G. Clinical relevance of sarcopenia in chronic kidney disease. Curr Opin Nephrol Hypertens. 2017;26:219–228. doi: 10.1097/MNH.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noce A., Marrone G., Ottaviani E., Guerriero C., Daniele F.D., Zaitseva A.P., et al. Uremic sarcopenia and its possible nutritional approach. Nutrients. 2021;13:147. doi: 10.3390/nu13010147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilkinson T.J., Miksza J., Yates T., Lightfoot C.J., Baker L.A., Watson E.L., et al. Association of sarcopenia with mortality and end-stage renal disease in those with chronic kidney disease: a UK Biobank study. J Cachexia Sarcopenia Muscle. 2021;12:586–598. doi: 10.1002/jcsm.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chindapasirt J. Sarcopenia in cancer patients. Asian Pac J Cancer Prev APJCP. 2015;16:8075–8077. doi: 10.7314/apjcp.2015.16.18.8075. [DOI] [PubMed] [Google Scholar]
- 44.Cano N., Melchior J.C. Malnutrition in chronic diseases. Rev Prat. 2003;53:268–273. [PubMed] [Google Scholar]
- 45.Wu Y., Hwang A., Liu L., Peng L., Chen L. Sex differences of sarcopenia in Asian populations: the implications in diagnosis and management. J Clin Gerontol Geriatr. 2016;7:37–43. [Google Scholar]
- 46.World Population ageing . 2019. United Nations department of economic and social affairs. [cited 2022 May 02] [Google Scholar]
- 47.Ramachandran A., Snehalatha C., Shetty A.S., Nanditha A. Trends in prevalence of diabetes in Asian countries. World J Diabetes. 2012;3:110–117. doi: 10.4239/wjd.v3.i6.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhome A.B. COPD in India: iceberg or volcano? J Thorac Dis. 2012;4:298–309. doi: 10.3978/j.issn.2072-1439.2012.03.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohanty L., Sahoo D. Prevalence and risk factors of sarcopenia: a study in a tertiary care centre. Int J Adv Med. 2016;3:364–367. [Google Scholar]
- 50.Pal R., Aggarwal A., Singh T., Sharma S., Khandelwal N., Garg A., et al. Diagnostic cut-offs, prevalence, and biochemical predictors of sarcopenia in healthy Indian adults: the Sarcopenia-Chandigarh Urban Bone Epidemiological Study (Sarco-CUBES) Eur Geriatr Med. 2020;11:725–736. doi: 10.1007/s41999-020-00332-z. [DOI] [PubMed] [Google Scholar]
- 51.Sreepriya P.R., Pillai S.S., Nair A.N.K.K., Rahul A., Pillai S., Nair A.T.S. Prevalence and associated factors of sarcopenia among patients underwent abdominal CT scan in tertiary care hospital of South India. J Frailty Sarcopenia Falls. 2020;5:79–85. doi: 10.22540/JFSF-05-079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tyrovolas S., Koyanagi A., Olaya B., Ayuso-Mateos J.L., Miret M., Chatterji S., et al. Factors associated with skeletal muscle mass, sarcopenia, and sarcopenic obesity in older adults: a multi-continent study. J Cachexia Sarcopenia Muscle. 2016;7:312–321. doi: 10.1002/jcsm.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rahman R., Wilson B.P., Paul T.V., Yadav B., Kango Gopal G., Viggeswarpu S. Prevalence and factors contributing to primary sarcopenia in relatively healthy older Indians attending the outpatient department in a tertiary care hospital: a cross-sectional study. Aging Med. 2021;4:257–265. doi: 10.1002/agm2.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zengin A., Kulkarni B., Khadilkar A.V., Kajale N., Ekbote V., Tandon N., et al. Prevalence of sarcopenia and relationships between muscle and bone in indian men and women. Calcif Tissue Int. 2021;109:423–433. doi: 10.1007/s00223-021-00860-1. [DOI] [PubMed] [Google Scholar]
- 55.Piotrowicz K., Gąsowski J., Michel J.P., Veronese N. Post-COVID-19 acute sarcopenia: physiopathology and management. Aging Clin Exp Res. 2021;33:2887–2898. doi: 10.1007/s40520-021-01942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shin M.K., Choi J.Y., Kim S.Y., Kim E.Y., Lee S.H., Chung K.S., et al. Association of protein consumption and energy intake on sarcopenia in tuberculosis survivors. Ther Adv Chronic Dis. 2021;12 doi: 10.1177/20406223211056712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams E.D., Stamatakis E., Chandola T., Hamer M. Assessment of physical activity levels in South Asians in the UK: findings from the health Survey for England. J Epidemiol Community Health. 2011;65:517–521. doi: 10.1136/jech.2009.102509. [DOI] [PubMed] [Google Scholar]
- 58.Ranasinghe C.D., Ranasinghe P., Jayawardena R., Misra A. Physical activity patterns among South-Asian adults: a systematic review. Int J Behav Nutr Phys Activ. 2013;10:116. doi: 10.1186/1479-5868-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kurpad A.V., Varadharajan K.S., Aeberli I. The thin-fat phenotype and global metabolic disease risk. Curr Opin Clin Nutr Metab Care. 2011;14:542–547. doi: 10.1097/MCO.0b013e32834b6e5e. [DOI] [PubMed] [Google Scholar]
- 60.Pellegrinelli V., Rouault C., Rodriguez-Cuenca S., Albert V., Edom-Vovard F., Vidal-Puig A., et al. Human adipocytes induce inflammation and atrophy in muscle cells during obesity. Diabetes. 2015;64:3121–3134. doi: 10.2337/db14-0796. [DOI] [PubMed] [Google Scholar]
- 61.Kushwaha S., Khanna P., Srivastava R., Jain R., Singh T., Kiran T. Estimates of malnutrition and risk of malnutrition among the elderly (≥ 60 years) in India: a systematic review and meta-analysis. Ageing Res Rev. 2020;63 doi: 10.1016/j.arr.2020.101137. [DOI] [PubMed] [Google Scholar]
- 62.Cruz-Jentoft A.J., Kiesswetter E., Drey M., Sieber C.C. Nutrition, frailty, and sarcopenia. Aging Clin Exp Res. 2017;29:43–48. doi: 10.1007/s40520-016-0709-0. [DOI] [PubMed] [Google Scholar]
- 63.Landi F., Calvani R., Tosato M., Martone A.M., Ortolani E., Savera G., et al. Anorexia of aging: risk factors, consequences, and potential treatments. Nutrients. 2016;8:69. doi: 10.3390/nu8020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laviano A., Gori C., Rianda S. Sarcopenia and nutrition. Adv Food Nutr Res. 2014;71:101–136. doi: 10.1016/B978-0-12-800270-4.00003-1. [DOI] [PubMed] [Google Scholar]
- 65.Bartali B., Frongillo E.A., Bandinelli S., Lauretani F., Semba R.D., Fried L.P., et al. Low nutrient intake is an essential component of frailty in older persons. J Gerontol A Biol Sci Med Sci. 2006;61:589–593. doi: 10.1093/gerona/61.6.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smit E., Winters-Stone K.M., Loprinzi P.D., Tang A.M., Crespo C.J. Lower nutritional status and higher food insufficiency in frail older US adults. Br J Nutr. 2013;110:172–178. doi: 10.1017/S000711451200459X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim J.E., Lee Y.H., Huh J.H., Kang D.R., Rhee Y., Lim S.K. Early-stage chronic kidney disease, insulin resistance, and osteoporosis as risk factors of sarcopenia in aged population: the fourth Korea National Health and Nutrition Examination Survey (KNHANES IV), 2008-2009. Osteoporos Int. 2014;25:2189–2198. doi: 10.1007/s00198-014-2745-y. [DOI] [PubMed] [Google Scholar]
- 68.Hill J.O., Wyatt H.R., Peters J.C. The importance of energy balance. Eur Endocrinol. 2013;9:111–115. doi: 10.17925/EE.2013.09.02.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Biolo G., Agostini F., Simunic B., Sturma M., Torelli L., Preiser J.C., et al. Positive energy balance is associated with accelerated muscle atrophy and increased erythrocyte glutathione turnover during 5 wk of bed rest. Am J Clin Nutr. 2008;88:950–958. doi: 10.1093/ajcn/88.4.950. [DOI] [PubMed] [Google Scholar]
- 70.Parr E.B., Coffey V.G., Hawley J.A. Sarcobesity': a metabolic conundrum. Maturitas. 2013;74:109–113. doi: 10.1016/j.maturitas.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 71.Masgrau A., Mishellany-Dutour A., Murakami H., Beaufrère A.M., Walrand S., Giraudet C., et al. Time-course changes of muscle protein synthesis associated with obesity-induced lipotoxicity. J Physiol. 2012;590:5199–5210. doi: 10.1113/jphysiol.2012.238576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Minocha S., Thomas T., Kurpad A.V. Dietary protein and the health-nutrition-agriculture connection in India. J Nutr. 2017;147:1243–1250. doi: 10.3945/jn.116.243980. [DOI] [PubMed] [Google Scholar]
- 73.Swaminathan S., Vaz M., Kurpad A.V. Protein intakes in India. Br J Nutr. 2012;108:S50–S58. doi: 10.1017/S0007114512002413. [DOI] [PubMed] [Google Scholar]
- 74.Tieland M., Borgonjen-Van den Berg K.J., van Loon L.J., de Groot L.C. Dietary protein intake in community-dwelling, frail, and institutionalized elderly people: scope for improvement. Eur J Nutr. 2012;51:173–179. doi: 10.1007/s00394-011-0203-6. [DOI] [PubMed] [Google Scholar]
- 75.https://www.orfonline.org/expert-speak/indias-protein-deficiency-and-the-need-to-address-the-problem/
- 76.Barclay R.D., Burd N.A., Tyler C., Tillin N.A., Mackenzie R.W. The role of the igf-1 signaling cascade in muscle protein synthesis and anabolic resistance in aging skeletal muscle. Front Nutr. 2019;6:146. doi: 10.3389/fnut.2019.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Casati M., Ferri E., Azzolino D., Cesari M., Arosio B. Gut microbiota and physical frailty through the mediation of sarcopenia. Exp Gerontol. 2019;124 doi: 10.1016/j.exger.2019.110639. [DOI] [PubMed] [Google Scholar]
- 78.Deer R.R., Volpi E. Protein intake and muscle function in older adults. Curr Opin Clin Nutr Metab Care. 2015;18:248–253. doi: 10.1097/MCO.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Deutz N.E., Bauer J.M., Barazzoni R., Biolo G., Boirie Y., Bosy-Westphal A., et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33:929–936. doi: 10.1016/j.clnu.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bauer J., Biolo G., Cederholm T., Cesari M., Cruz-Jentoft A.J., Morley J.E., et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14:542–559. doi: 10.1016/j.jamda.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 81.Beaudart C., Dawson A., Shaw S.C., Harvey N.C., Kanis J.A., Binkley N., et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporos Int. 2017;28:1817–1833. doi: 10.1007/s00198-017-3980-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kang L., Gao Y., Liu X., Liang Y., Chen Y., Liang Y., et al. Effects of whey protein nutritional supplement on muscle function among community-dwelling frail older people: a multicenter study in China. Arch Gerontol Geriatr. 2019;83:7–12. doi: 10.1016/j.archger.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 83.Gilmartin S., O'Brien N., Giblin L. Whey for sarcopenia; can whey peptides, hydrolysates or proteins play a beneficial role? Foods. 2020;9:750. doi: 10.3390/foods9060750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nabuco H.C.G., Tomeleri C.M., Junior P.S., Fernandes R.R., Cavalcante E.F., Antunes M., et al. Effects of whey protein supplementation pre- or post-resistance training on muscle mass, muscular strength, and functional capacity in pre-conditioned older Women: a randomized clinical trial. Nutrients. 2018;10:563. doi: 10.3390/nu10050563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Anthony J.C., Anthony T.G., Kimball S.R., Jefferson L.S. Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. J Nutr. 2001;131:856S–860S. doi: 10.1093/jn/131.3.856S. [DOI] [PubMed] [Google Scholar]
- 86.Zaromskyte G., Prokopidis K., Ioannidis T., Tipton K.D., Witard O.C. Evaluating the leucine trigger hypothesis to explain the post-prandial regulation of muscle protein synthesis in young and older adults: a systematic review. Front Nutr. 2021;8 doi: 10.3389/fnut.2021.685165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kang Y., Kim N., Choi Y.J., Lee Y., Yun J., Park S.J., et al. Leucine-enriched protein supplementation increases lean body mass in healthy Korean adults aged 50 years and older: a randomized, double-blind, placebo-controlled trial. Nutrients. 2020;12:1816. doi: 10.3390/nu12061816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martínez-Arnau F.M., Fonfría-Vivas R., Buigues C., Castillo Y., Molina P., Hoogland A.J., et al. Effects of leucine administration in sarcopenia: a randomized and placebo-controlled clinical trial. Nutrients. 2020;12:932. doi: 10.3390/nu12040932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yoshimura Y., Bise T., Shimazu S., Tanoue M., Tomioka Y., Araki M., et al. Effects of a leucine-enriched amino acid supplement on muscle mass, muscle strength, and physical function in post-stroke patients with sarcopenia: a randomized controlled trial. Nutrition. 2019;58:1–6. doi: 10.1016/j.nut.2018.05.028. [DOI] [PubMed] [Google Scholar]
- 90.Unni U.S., Raj T., Sambashivaiah S., Kuriyan R., Uthappa S., Vaz M., et al. The effect of a controlled 8-week metabolic ward based lysine supplementation on muscle function, insulin sensitivity and leucine kinetics in young men. Clin Nutr. 2012;31:903–910. doi: 10.1016/j.clnu.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 91.Norton C., Toomey C., McCormack W.G., Francis P., Saunders J., Kerin E., et al. Protein supplementation at breakfast and lunch for 24 weeks beyond habitual intakes increases whole-body lean tissue mass in healthy older adults. J Nutr. 2016;146:65–69. doi: 10.3945/jn.115.219022. [DOI] [PubMed] [Google Scholar]
- 92.Volpi E., Campbell W.W., Dwyer J.T., Johnson M.A., Jensen G.L., Morley J.E., et al. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J Gerontol A Biol Sci Med Sci. 2013;68:677–681. doi: 10.1093/gerona/gls229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morton R.W., Murphy K.T., McKellar S.R., Brad J., Schoenfeld B.J., Henselmans M., et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults [published correction appears in Br J Sports Med. 2020;54:e7] Br J Sports Med. 2018;52:376–384. doi: 10.1136/bjsports-2017-097608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reidy P.T., Borack M.S., Markofski M.M., Dickinson J.M., Deer R.R., Husaini S.H., et al. Protein supplementation has minimal effects on muscle adaptations during resistance exercise training in young men: a double-blind randomized clinical trial. J Nutr. 2016;146:1660–1669. doi: 10.3945/jn.116.231803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mertz K.H., Reitelseder S., Bechshoeft R., Bulow J., Højfeldt G., Jensen M., et al. The effect of daily protein supplementation, with or without resistance training for 1 year, on muscle size, strength, and function in healthy older adults: a randomized controlled trial. Am J Clin Nutr. 2021;113:790–800. doi: 10.1093/ajcn/nqaa372. [DOI] [PubMed] [Google Scholar]
- 96.Robinson S.M., Jameson K.A., Batelaan S.F., Martin H.J., Syddall H.E., Dennison E.M., et al. Diet and its relationship with grip strength in community-dwelling older men and women: the Hertfordshire cohort study. J Am Geriatr Soc. 2008;56:84–90. doi: 10.1111/j.1532-5415.2007.01478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dos Reis A.S., Limirio L.S., Santos H.O., de Oliveira E.P. Intake of polyunsaturated fatty acids and ω-3 are protective factors for sarcopenia in kidney transplant patients. Nutrition. 2021;81 doi: 10.1016/j.nut.2020.110929. [DOI] [PubMed] [Google Scholar]
- 98.Smith G.I., Julliand S., Reeds D.N., Sinacore D.R., Klein S., Mittendorfer B. Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am J Clin Nutr. 2015;102:115–122. doi: 10.3945/ajcn.114.105833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smith G.I., Atherton P., Reeds D.N., Mohammed B.S., Rankin D., Rennie M.J., et al. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr. 2011;93:402–412. doi: 10.3945/ajcn.110.005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dupont J., Dedeyne L., Dalle S., Koppo K., Gielen E. The role of omega-3 in the prevention and treatment of sarcopenia. Aging Clin Exp Res. 2019;31:825–836. doi: 10.1007/s40520-019-01146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tan A., Sullenbarger B., Prakash R., McDaniel J.C. Supplementation with eicosapentaenoic acid and docosahexaenoic acid reduces high levels of circulating proinflammatory cytokines in aging adults: a randomized, controlled study. Prostaglandins Leukot Essent Fatty Acids. 2018;132:23–29. doi: 10.1016/j.plefa.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yoshino J., Smith G.I., Kelly S.C., Julliand S., Reeds D.N., Mittendorfer B. Effect of dietary n-3 PUFA supplementation on the muscle transcriptome in older adults. Phys Rep. 2016;4 doi: 10.14814/phy2.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gupta R., Sharma U., Gupta N., Kalaivani M., Singh U., Guleria R., et al. Effect of cholecalciferol and calcium supplementation on muscle strength and energy metabolism in vitamin D-deficient Asian Indians: a randomized, controlled trial. Clin Endocrinol. 2010;73:445–451. doi: 10.1111/j.1365-2265.2010.03816.x. [DOI] [PubMed] [Google Scholar]
- 104.Goswami R., Vatsa M., Sreenivas V., Singh U., Gupta N., Lakshmy R., et al. Skeletal muscle strength in young Asian Indian females after vitamin D and calcium supplementation: a double-blind randomized controlled clinical trial. J Clin Endocrinol Metab. 2012;97:4709–4716. doi: 10.1210/jc.2012-2340. [DOI] [PubMed] [Google Scholar]
- 105.Wankhede S., Langade D., Joshi K., Sinha S.R., Bhattacharyya S. Examining the effect of Withania somnifera supplementation on muscle strength and recovery: a randomized controlled trial. J Int Soc Sports Nutr. 2015;12:43. doi: 10.1186/s12970-015-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saha S., Goswami R., Ramakrishnan L., Vishnubhatla S., Mahtab S., Kar P., et al. Vitamin D and calcium supplementation, skeletal muscle strength and serum testosterone in young healthy adult males: randomized control trial. Clin Endocrinol. 2018;88:217–226. doi: 10.1111/cen.13507. [DOI] [PubMed] [Google Scholar]
- 107.Coen P.M., Musci R.V., Hinkley J.M., Miller B.F. Mitochondria as a target for mitigating sarcopenia. Front Physiol. 2019;9:1883. doi: 10.3389/fphys.2018.01883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Waters D.L., Brooks W.M., Qualls C.R., Baumgartner R.N. Skeletal muscle mitochondrial function and lean body mass in healthy exercising elderly. Mech Ageing Dev. 2003;124:301–309. doi: 10.1016/s0047-6374(02)00197-5. [DOI] [PubMed] [Google Scholar]
- 109.Aytekin N., Mileva K.N., Cunliffe A.D. Selected B vitamins and their possible link to the aetiology of age-related sarcopenia: relevance of UK dietary recommendations. Nutr Res Rev. 2018;31:204–224. doi: 10.1017/S0954422418000045. [DOI] [PubMed] [Google Scholar]
- 110.Ringseis R., Rosenbaum S., Gessner D.K., Herges L., Kubens J.F., Mooren F.C., et al. Supplementing obese Zucker rats with niacin induces the transition of glycolytic to oxidative skeletal muscle fibers. J Nutr. 2013;143:125–131. doi: 10.3945/jn.112.164038. [DOI] [PubMed] [Google Scholar]
- 111.Khan M., Ringseis R., Mooren F.C., Krüger K., Most E., Eder K. Niacin supplementation increases the number of oxidative type I fibers in skeletal muscle of growing pigs. BMC Vet Res. 2013;9:177. doi: 10.1186/1746-6148-9-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Crawford V., Scheckenbach R., Preuss H.G. Effects of niacin-bound chromium supplementation on body composition in overweight African-American women. Diabetes Obes Metabol. 1999;1:331–337. doi: 10.1046/j.1463-1326.1999.00055.x. [DOI] [PubMed] [Google Scholar]
- 113.Wong C.W. Vitamin B12 deficiency in the elderly: is it worth screening? Hong Kong Med J. 2015;21:155–164. doi: 10.12809/hkmj144383. [DOI] [PubMed] [Google Scholar]
- 114.Pannérec A., Migliavacca E., De Castro A., Michaud J., Karaz S., Goulet L., et al. Vitamin B12 deficiency and impaired expression of amnionless during aging. J Cachexia Sarcopenia Muscle. 2018;9:41–52. doi: 10.1002/jcsm.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vidoni M.L., Pettee Gabriel K., Luo S.T., Simonsick E.M., Day R.S. Vitamin B12 and homocysteine associations with gait speed in older adults: the Baltimore Longitudinal Study of Aging. J Nutr Health Aging. 2017;21:1321–1328. doi: 10.1007/s12603-017-0893-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vidoni M.L., Pettee Gabriel K., Luo S.T., Simonsick E.M., Day R.S. Relationship between homocysteine and muscle strength decline: the baltimore longitudinal study of aging. J Gerontol A Biol Sci Med Sci. 2018;73:546–551. doi: 10.1093/gerona/glx161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Verlaan S., Aspray T.J., Bauer J.M., Cederholm T., Hemsworth J., Hill T.R., et al. Nutritional status, body composition, and quality of life in community-dwelling sarcopenic and non-sarcopenic older adults: a case-control study. Clin Nutr. 2017;36:267–274. doi: 10.1016/j.clnu.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 118.Soh Y., Won C.W. Association between frailty and vitamin B12 in the older Korean population. Medicine (Baltimore) 2020;99 doi: 10.1097/MD.0000000000022327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kalicki B., Lewicka A., Jęderka K., Leśniak M., Marszałkowska-Jakubik J., Lewicki S. Vitamin B6 improves blood parameters in rats fed a protein-deficient diet and subjected to moderate, long-term exercise. Cent Eur J Immunol. 2019;44:23–32. doi: 10.5114/ceji.2019.83266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lewicka A., Lewicki S., Zdanowski R., Rutkowski P., Turkowska M., Kłos A., et al. The effect of vitamin B6 supplementation of protein deficiency diet on hematological parameters in the blood of rats subjected/non subjected to physical exertion – a pilot study. Cent Eur J Immunol. 2012;3:187–192. [Google Scholar]
- 121.Ward K.A., Das G., Berry J.L., Roberts S.A., Rawer R., Adams J.E., et al. Vitamin D status and muscle function in post-menarchal adolescent girls. J Clin Endocrinol Metab. 2009;94:559–563. doi: 10.1210/jc.2008-1284. [DOI] [PubMed] [Google Scholar]
- 122.Gilsanz V., Kremer A., Mo A.O., Wren T.A., Kremer R. Vitamin D status and its relation to muscle mass and muscle fat in young women. J Clin Endocrinol Metab. 2010;95:1595–1601. doi: 10.1210/jc.2009-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Visser M., Deeg D.J., Lips P. Longitudinal Aging Study Amsterdam. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metab. 2003;88:5766–5772. doi: 10.1210/jc.2003-030604. [DOI] [PubMed] [Google Scholar]
- 124.Girgis C.M., Mokbel N., Cha K.M., Houweling P.J., Abboud M., Fraser D.R., et al. The vitamin D receptor (VDR) is expressed in skeletal muscle of male mice and modulates 25-hydroxyvitamin D (25OHD) uptake in myofibers. Endocrinology. 2014;155:3227–3237. doi: 10.1210/en.2014-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Girgis C.M., Cha K.M., Houweling P.J., Rao R., Mokbel N., Lin M., et al. Vitamin D receptor ablation and vitamin d deficiency result in reduced grip strength, altered muscle fibers, and increased myostatin in mice. Calcif Tissue Int. 2015;97:602–610. doi: 10.1007/s00223-015-0054-x. [DOI] [PubMed] [Google Scholar]
- 126.Uchitomi R., Oyabu M., Kamei Y. Vitamin D and sarcopenia: potential of vitamin D supplementation in sarcopenia prevention and treatment. Nutrients. 2020;12:3189. doi: 10.3390/nu12103189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bischoff H.A., Stähelin H.B., Dick W., Akos R., Knecht M., Salis C., et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18:343–351. doi: 10.1359/jbmr.2003.18.2.343. [DOI] [PubMed] [Google Scholar]
- 128.Sørensen O.H., Lund B., Saltin B., Lund B., Andersen R.B., Hjorth L., et al. Myopathy in bone loss of ageing: improvement by treatment with 1 alpha-hydroxycholecalciferol and calcium. Clin Sci. 1979;56:157–161. doi: 10.1042/cs0560157. [DOI] [PubMed] [Google Scholar]
- 129.Beaudart C., Locquet M., Touvier M., Reginster J.Y., Bruyère O. Association between dietary nutrient intake and sarcopenia in the SarcoPhAge study. Aging Clin Exp Res. 2019;31:815–824. doi: 10.1007/s40520-019-01186-7. [DOI] [PubMed] [Google Scholar]
- 130.Seo M.H., Kim M.K., Park S.E., Rhee E.J., Park C.Y., Lee W.Y., et al. The association between daily calcium intake and sarcopenia in older, non-obese Korean adults: the fourth Korea National Health and Nutrition Examination Survey (KNHANES IV) 2009. Endocr J. 2013;60:679–686. doi: 10.1507/endocrj.ej12-0395. [DOI] [PubMed] [Google Scholar]
- 131.Dargelos E., Poussard S., Brulé C., Daury L., Cottin P. Calcium-dependent proteolytic system and muscle dysfunctions: a possible role of calpains in sarcopenia. Biochimie. 2008;90:359–368. doi: 10.1016/j.biochi.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 132.G R, Gupta A. Vitamin D deficiency in India: prevalence, causalities and interventions. Nutrients. 2014;6:729–775. doi: 10.3390/nu6020729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Harinarayan C.V., Akhila H. Modern India and the tale of twin nutrient deficiency-calcium and vitamin D-nutrition trend data 50 Years-retrospect, introspect, and prospect. Front Endocrinol. 2019;10:493. doi: 10.3389/fendo.2019.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.van Dronkelaar C., van Velzen A., Abdelrazek M., van der Steen A., Weijs P.J.M., Tieland M. Minerals and Sarcopenia; the role of calcium, iron, magnesium, phosphorus, potassium, selenium, sodium, and zinc on muscle mass, muscle strength, and physical performance in older adults: a systematic review. J Am Med Dir Assoc. 2018 Jan;19:6–11. doi: 10.1016/j.jamda.2017.05.026. e3. [DOI] [PubMed] [Google Scholar]
- 135.Scott D., Blizzard L., Fell J., Giles G., Jones G. Associations between dietary nutrient intake and muscle mass and strength in community-dwelling older adults: the Tasmanian Older Adult Cohort Study. J Am Geriatr Soc. 2010;58:2129–2134. doi: 10.1111/j.1532-5415.2010.03147.x. [DOI] [PubMed] [Google Scholar]
- 136.Neidlein S., Wirth R., Pourhassan M. Iron deficiency, fatigue and muscle strength and function in older hospitalized patients. Eur J Clin Nutr. 2021;75:456–463. doi: 10.1038/s41430-020-00742-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bastian T.W., Rao R., Tran P.V., Georgieff M.K. The Effects of early-life iron deficiency on brain energy metabolism. Neurosci Insights. 2020;15 doi: 10.1177/2633105520935104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.DeRuisseau K.C., Park Y.M., DeRuisseau L.R., Cowley P.M., Fazen C.H., Doyle R.P. Aging-related changes in the iron status of skeletal muscle. Exp Gerontol. 2013;48:1294–1302. doi: 10.1016/j.exger.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Galaris D., Pantopoulos K. Oxidative stress and iron homeostasis: mechanistic and health aspects. Crit Rev Clin Lab Sci. 2008;45:1–23. doi: 10.1080/10408360701713104. [DOI] [PubMed] [Google Scholar]
- 140.Altun M., Edström E., Spooner E., Flores-Moralez A., Bergman E., Tollet-Egnell P., et al. Iron load and redox stress in skeletal muscle of aged rats. Muscle Nerve. 2007;36:223–233. doi: 10.1002/mus.20808. [DOI] [PubMed] [Google Scholar]
- 141.Veronese N., Berton L., Carraro S., Bolzetta F., De Rui M., Perissinotto E., et al. Effect of oral magnesium supplementation on physical performance in healthy elderly women involved in a weekly exercise program: a randomized controlled trial. Am J Clin Nutr. 2014;100:974–981. doi: 10.3945/ajcn.113.080168. [DOI] [PubMed] [Google Scholar]
- 142.Ter Borg S., de Groot L.C., Mijnarends D.M., de Vries J.H.M., Verlaan S., Meijboom S., et al. Differences in nutrient intake and biochemical nutrient status between sarcopenic and nonsarcopenic older adults-results from the Maastricht Sarcopenia Study. J Am Med Dir Assoc. 2016;17:393–401. doi: 10.1016/j.jamda.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 143.Chen Y.L., Yang K.C., Chang H.H., Lee L.T., Lu C.W., Huang K.C. Low serum selenium level is associated with low muscle mass in the community-dwelling elderly. J Am Med Dir Assoc. 2014;15:807–811. doi: 10.1016/j.jamda.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 144.Lauretani F., Semba R.D., Bandinelli S., Ray A.L., Guralnik J.M., Ferrucci L. Association of low plasma selenium concentrations with poor muscle strength in older community-dwelling adults: the InCHIANTI Study. Am J Clin Nutr. 2007;86:347–352. doi: 10.1093/ajcn/86.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Martin H., Aihie Sayer A., Jameson K., Syddall H., Dennison E.M., Cooper C., et al. Does diet influence physical performance in community-dwelling older people? Findings from the Hertfordshire Cohort Study. Age Ageing. 2011;40:181–186. doi: 10.1093/ageing/afq175. [DOI] [PubMed] [Google Scholar]
- 146.Waters D.L., Wayne S.J., Andrieu S., Cesari M., Villareal D.T., Garry P., et al. Sexually dimorphic patterns of nutritional intake and eating behaviors in community-dwelling older adults with normal and slow gait speed. J Nutr Health Aging. 2014;18:228–233. doi: 10.1007/s12603-014-0004-8. [DOI] [PubMed] [Google Scholar]
- 147.Nishikawa H., Enomoto H., Yoh K., Iwata Y., Sakai Y., Kishino K., et al. Serum zinc concentration and sarcopenia: a close linkage in chronic liver diseases. J Clin Med. 2019;8:336. doi: 10.3390/jcm8030336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Suzuki H., Asakawa A., Li J.B., Tsai M., Amitani H., Ohinata K., et al. Zinc as an appetite stimulator - the possible role of zinc in the progression of diseases such as cachexia and sarcopenia. Recent Pat Food, Nutr Agric. 2011;3:226–231. doi: 10.2174/2212798411103030226. [DOI] [PubMed] [Google Scholar]
- 149.Venkatesh U., Sharma A., Ananthan V.A., Subbiah P., Durga R. CSIR Summer Research training team. Micronutrient's deficiency in India: a systematic review and meta-analysis. J Nutr Sci. 2021;10:e110. doi: 10.1017/jns.2021.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sucharita S., Thomas T., Sowmya S., Krishnamachari S., Kurpad A.V., Vaz M. Subclinical vitamin B12 deficiency and heart rate variability across life cycle. Curr Aging Sci. 2016;9:217–223. doi: 10.2174/1874609809666160211125218. [DOI] [PubMed] [Google Scholar]
- 151.Alwar V., Reethi K., Rameshkumar K. Geriatric anemia: an Indian perspective. Indian J Hematol Blood Transfus. 2013;29:126–127. doi: 10.1007/s12288-012-0146-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shobha V., Tarey S.D., Singh R.G., Shetty P., Unni U.S., Srinivasan K., et al. Vitamin B₁₂ deficiency & levels of metabolites in an apparently normal urban south Indian elderly population. Indian J Med Res. 2011;134:432–439. [PMC free article] [PubMed] [Google Scholar]
- 153.Lee M.C., Hsu Y.J., Ho H.H., Hsieh S.H., Kuo Y.W., Sung H.C., et al. Lactobacillus salivarius subspecies salicinius SA-03 is a new probiotic capable of enhancing exercise performance and decreasing fatigue. Microorganisms. 2020;8:545. doi: 10.3390/microorganisms8040545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Smarkusz-Zarzecka J., Ostrowska L., Leszczyńska J., Orywal K., Cwalina U., Pogodziński D. Analysis of the impact of a multi-strain probiotic on body composition and cardiorespiratory fitness in long-distance runners. Nutrients. 2020;12:3758. doi: 10.3390/nu12123758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Buigues C., Fernández-Garrido J., Pruimboom L., Hoogland A.J., Navarro-Martínez R., Martínez-Martínez M., et al. Effect of a prebiotic formulation on frailty syndrome: a randomized, double-blind clinical trial. Int J Mol Sci. 2016;17:932. doi: 10.3390/ijms17060932. [DOI] [PMC free article] [PubMed] [Google Scholar]