Abstract
Objective
Mucormycosis is an opportunistic fungal disease that affects immunocompromised patients. With the advent of SARS-CoV-2, this opportunistic disease has increased.
Methods
A case series of 47 patients with COVID-19 associated mucormycosis have been analyzed. Demographic information, signs, symptoms, laboratory investigations, imaging studies, and their association with ICU admission and 30-day mortality were assessed.
Results
Total number of 47 consecutive rhino-orbital cerebral mucormycosis (ROCM) cases were analyzed. Periorbital swelling was the most common sign among patients. Majority of cases had diabetes. All patients received liposomal Amphotericin B. Debridement was performed for all cases.
Conclusions
SARS-CoV-2 increases the susceptibility to mucormycosis infection in various ways. Uncontrolled level of HbA1c in all patients, even non-diabetic individuals, indicates hyperglycemia over the past three months. Diabetes, orbital exenteration, ptosis, periorbital swelling, DKA, LOC, brain involvement, and mechanical ventilation all correlated with a higher rate of ICU admission and 30-day mortality. In addition, a higher white blood cell count is related to the higher probability of ICU admission. While considering all of the inflammatory laboratory data and HbA1c could help predict 30-day mortality.
Keywords: mucormycosis, COVID-19, rhino-orbito-cerebral Mucormycosis, COVID-19 associated Mucormycosis, SARS-CoV-2
Introduction
Mucormycosis is a fungal infectious disease caused by Mucorales order.1 The most-reported pathogens responsible for mucormycosis are Rhizopus spp, Mucor spp, and Lichthemia spp, respectively.2 This fungal pathogen infects paranasal sinuses, orbit, and even cranial components.1 Mucormycosis usually affects immunodeficient, diabetic, and patients who suffer from malignancies or are receiving immunosuppressive drugs.3
SARS-CoV-2 is a virus that first emerged from Wuhan, China, and then spread throughout the world, causing a pandemic.4 Different studies have confirmed that the incidence of fungal superinfection in COVID-19 patients ranges from 14.8 up to 33% varying based on disease severity.5 In Iran, only 98 cases of mucormycosis were detected in the whole literature until 2015.6 Coronavirus, through various pathways, can predispose the infected patients to mucormycosis infection. SARS-CoV-2 directly causes immune system dysregulation and hyperglycemia. In addition, the use of immune-modulatory drug agents makes patients more susceptible to mucormycosis infection.7-10
Rhino-orbital cerebral mucormycosis (ROCM) is a type of mucormycosis infection in COVID-19 patients. ROCM is a fatal disease with an approximate mortality rate of 40%.11 Mucormycosis infection is manifested by symptoms such as visual deterioration, nasal black discharge or bleeding, facial or periorbital swelling, facial pain, and neurologic symptoms.12-14
There are various modalities to diagnose mucormycosis. The most accurate are histologic specimen and microscopic evaluation.15 In addition, imaging modalities are helpful and less invasive. Magnetic resonance imaging (MRI) with gadolinium contrast is the imaging of choice.16
The management of this disease has 2 components: medical and surgical management. The medical treatment consists of administering Amphotericin B, Psoconazole or Isavuconazole, and glycemic control.2,13,17 Surgical debridement is utilized to control infection spread and manage the disease.18
The aim of this study is to find the relation between symptoms, signs, risk factors, investigations, pathology, and mortality or intensive care unit (ICU) admission of COVID-19 associated mucormycosis (CAM) patients in a single tertiary-center Loghman Hakim Hospital, Tehran, Iran.
Method
Study Design
A case series study was conducted on COVID-19 patients with mucormycosis infection. The inclusion criteria were reverse transcriptase-polymerase chain reaction (RT-PCR)-proven cases of COVID-19 in Loghman Hakim Hospital in Tehran, Iran from 20th of December to 25th of August, plus infection with mucormycosis. The ethics committee of Shahid Beheshti University of Medical Sciences approved this survey. The study protocol was in accordance with the Declaration of Helsinki. All the included patients were informed about the purpose of the study, and written consent was obtained.
Case Definition, Data Collection Laboratory, and Histopathological Surveys
All patients with (RT-PCR) confirmed coronavirus infection, who were diagnosed with histopathological proven sinonasal mucormycosis were included. The interval between 2 infections was not more than 4 weeks. Nasopharyngeal and oropharyngeal swab specimens of patients were evaluated by RT-PCR. Biopsies from paranasal sinuses and nasal mucosa were obtained for histopathological examination.
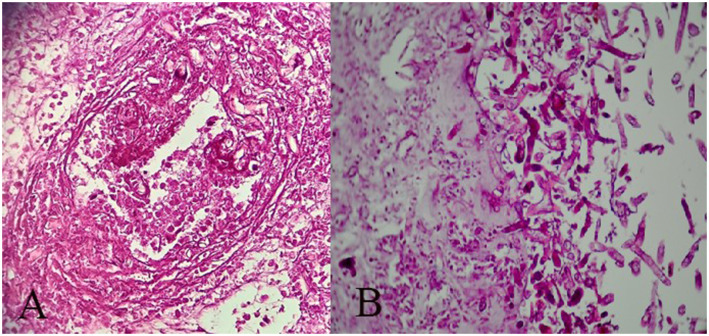
The diagnosis is confirmed by revealing broad non-septate hyphae with rectangle branching and twisting morphology. The fungal hyphae are seen in the tissue with vascular and neural invasion, thrombotic infarction, and variable inflammatory cells in the background (Figure 1).
Figure 1.
Nasal biopsy shows broad non-septate hyphae with rectangle branching and twisting morphology and necrosis (PAS*100).
Demographic, clinical manifestations, and infection sites of each included patient were recorded. Clinical symptoms were divided into 2 groups: primary and secondary symptoms. Secondary manifestations were defined as symptoms that were developed during the disease course. ICU admission, means of oxygen administration, and medical and surgical treatments were all studied. ICU admission was considered as a parameter for disease severity. Moreover, patients were followed up for 30 days to analyze the mortality rate.
Computed tomography (CT) and MRI with gadolinium contrast of orbits, paranasal sinuses, and brain were also performed and the data was analyzed. In addition, pathological and laboratory data including cell blood count (CBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), ferritin, and HbA1c were also analyzed.
The statistical analysis was conducted using SPSS version 22. Chi-square test and binary logistic regression tests were conducted to assess the association of nominal and quantitative variables and ICU admission or 30-day mortality. P-value less than .05 was considered to be statistically significant.
Results
Forty-seven patients were included in the study, 22 (47%) and 25 (53%) were female and male, respectively. This was significantly higher in comparison to the 98 cases reported from 199 to 2015.6 The average age was 57.0 ± 10 (SD) years. The mean interval between early COVID-19 infection and sinonasal mucormycosis was 12.7 ± 4.6 days. Thirty-four (74%) patients suffered from diabetes mellitus as their underlying medical disease. Based on medical documents, the rest 26% did not suffered from diabetes. Interestingly 11 out of 47 patients (23%) had no significant past medical history. Other frequent underlying diseases were hypertension, ischemic heart disease, end-stage renal disease (17%, 4%, and 4%, respectively). Approximately 30% of patients were diagnosed with more than one underlying disease.
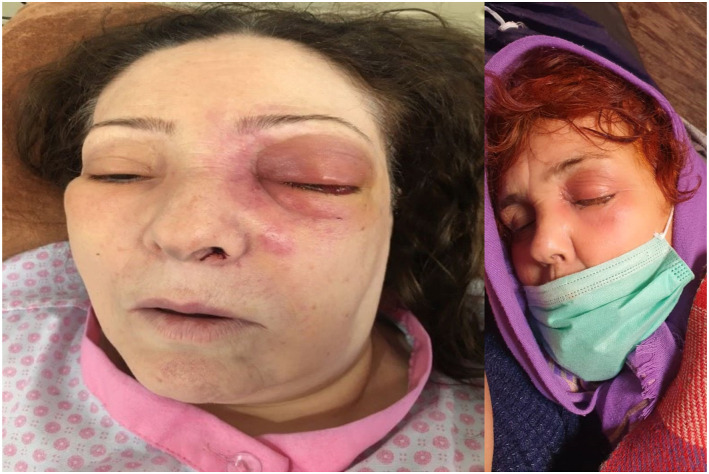
The most frequent symptom noted among cases was headache (29 patients or 62%), 28 patients (59%) had periorbital swelling (Figure 2). Other symptoms frequencies and percentages are reported in Table 1 in detail. Fever was not reported in any of the cases.
Figure 2.
Patients with periorbital edema and proptosis.
Table1.
Illustrates the Frequency of Signs and Symptoms in Studied Patients.
| Symptoms and Signs | Number (Percent %) |
|---|---|
| Headache | 29 (46.7) |
| Periorbital swelling | 28 (59.6) |
| Acute vision loss | 20 (42.6) |
| Loss of consciousness | 18 (38.3) |
| Periorbital/facial pain | 17 (36.2) |
| Ptosis | 16 (34) |
| Diabetic ketoacidosis | 15 (31.9) |
| Facial swelling | 14 (29.8) |
| Black crust in nasal cavity | 13 (27.7) |
| Black crust on hard palate | 13 (27.7) |
| Proptosis | 5 (10.5) |
| Cranial nerve palsy | 5 (10.5) |
| Diplopia | 1 (2.1) |
| Orbital swelling | 1 (2.1) |
| Fever | 0 (0) |
| Nasal blockage | 0 (0) |
| Otologic symptoms | 0 (0) |
Diabetic ketoacidosis (DKA) and loss of consciousness (LOC) were developed secondary to sinonasal mucormycosis. Twelve cases (25%) developed both DKA and LOC through the disease course. Isolated LOC and DKA also were reported in 6 and 3 patients, respectively. For all cases, computed tomography was performed. Sinus involvements are illustrated in Table 2.
Table 2.
Indicates the Frequency of Paranasal Sinus Involvement.
| Right frontal sinus involvement | 2 (4.3%) |
| Left frontal sinus involvement | 6 (12.8%) |
| Bilateral frontal sinus involvement | 10 (21.3%) |
| Right ethmoidal sinus involvement | 6 (12.8%) |
| Left ethmoidal sinus involvement | 3 (6.4%) |
| Bilateral ethmoidal sinus involvement | 34 (72.3%) |
| Right sphenoidal sinus involvement | 5 (10.6%) |
| Left sphenoidal sinus involvement | 5 (10.6%) |
| Bilateral sphenoidal sinus involvement | 15 (31.9%) |
| Right maxillary sinus involvement | 9 (19.1%) |
| Left maxillary sinus involvement | 15 (31.9%) |
| Bilateral maxillary sinus involvement | 15 (31.9%) |
| Pansinusitis | 7 (14.9%) |
As illustrated in Table 2, most cases had bilateral ethmoidal involvement. 91% of cases experienced ethmoidal involvement, which was mostly bilateral. In addition, 14.9% of cases had pansinusitis.
Brain complications including infarction and ischemic events (17%), brain involvement (29.8%), and brain abscess (2.1%) were also reported.
Various laboratory investigations were performed for all cases, including cell blood count (CBC) ferritin, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and Hemoglobin A1c (HbA1c).
HbA1c more than 7 was considered high and a sign of uncontrolled diabetes.19 Although nearly 70% of patients had diabetes as their underlying disease, all cases had HbA1c more than 7, indicating that the blood glucose level was high and uncontrolled in all cases through the past three months. The mean HbA1c was 11.24 ± 1.44. All inflammatory markers measured in this survey were high (Table 3).
Table 3.
Illustrates the Laboratory Data of Included Individuals.
| Laboratory Investigation | Minimum-Maximum | Mean ± Standard Deviation |
|---|---|---|
| ESR (mL/hr) | 10-141 | 67.7 ± 30.3 |
| CRP (mg/L) | 1.6-144 | 70.4 ± 41.7 |
| WBC (th/μL) | 4.1-70 | 17.9 ± 12.1 |
| HbA1c (%) | 8.1-14 | 11.24 ± 1.44 |
| Ferritin (μg/L) | 118-1964 | 475.7 ± 261.9 |
All cases were treated with systemic corticosteroids, liposomal Amphotericin B, and antibiotics. The average dexamethasone dose administered during hospitalization was 121.7 ± 79.7 milligrams. Minimum and maximum doses administered were 30.0 and 576.0 milligrams, respectively.
Sinus debridement (endoscopic sinus surgery was used to removed crust and debris) was performed for all patients since none responded solely to antifungal treatment. In addition, orbital exenteration was performed on 14 cases (29.8%).
All patients received oxygen via mask or mechanical ventilation. In addition, 10 patients (21.3%) were mechanically ventilated. Twenty-two (47%) cases were admitted to the ICU.
Chi-square test and binominal logistic regression were conducted to determine if the variables and symptoms listed previously could predict the likelihood of ICU admission as an indicator of disease severity (Table 4).
Table 4.
Illustrates the Evaluation of ICU Admission and Possible Related Comorbidities.
| Nominal Variable*ICU Admission | P-value | Phi |
|---|---|---|
| Orbital exenterationa | .000 | 0.508 |
| Gender | .861 | −00.25 |
| Periorbital/facial pain | .979 | 0.04 |
| Periorbital swellinga | .02 | 0.338 |
| Ptosisa | .03 | 0.316 |
| Acute vision loss | .333 | 0.141 |
| Facial swelling | .355 | 0.135 |
| Headache | .730 | −0.05 |
| Black crust in nasal cavity | .211 | 0.183 |
| Black crust on hard palate | .211 | 0.183 |
| Diabetic ketoacidosisa | .002 | 0.455 |
| Loss of consciousnessa | .000 | 0.664 |
| Brain involvementa | .000 | 0.694 |
| Past medical historya | .022 | 0.556 |
| O2 administrationa | .000 | 0.554 |
| Orbit swelling | .258 | −0.138 |
| Proptosis | .107 | 0.230 |
| Cranial nerve palsy | .532 | 0.001 |
| Diplopia | .214 | 0.157 |
| Ethmoidal sinus involvement | .754 | 0.156 |
| Sphenoidal sinus involvement | .244 | 0.324 |
| Frontal sinus involvement | .196 | 0.289 |
| Maxillary sinus involvement | .377 | 0.255 |
| Pansinusitis | .153 | 0.206 |
| Brain abscess | .214 | 0.157 |
| Brain infarction /ischemia | .560 | −0.084 |
aIndicates statistical significance.
As is illustrated in Table 4, orbital exenteration, ptosis, periorbital swelling, diabetic ketoacidosis, loss of consciousness, brain involvement, past medical history (diabetes), and O2 administration (mechanical ventilation) were all statistically related to ICU admission. Brain involvement had the highest Phi score (0.694), which indicates the highest rate of association with ICU admission.
As shown in Table 5, there was a statistically significant relationship between orbital exenteration, O2 administration, DKA, LOC, past medical history (PMH), and 30-day mortality (P-value less than .05 was considered significant). Among all categorical variables, PMH (diabetes), O2 administration (mechanical ventilation), and DKA had the biggest size effect (0.587, 0.525, and 0.459, respectively).
Table 5.
Shows Evaluation of 30-day Mortality and Possible Related Comorbidities.
| Nominal Variables*30-days Mortality | P-value | Phi |
|---|---|---|
| Orbital exenterationa | .005 | 0.411 |
| Gender | .510 | 0.096 |
| Periorbital/facial pain | .247 | 0.169 |
| Periorbital swellinga | .026 | 0.325 |
| Ptosisa | .027 | 0.323 |
| Acute vision loss | .080 | 0.256 |
| Facial swelling | .384 | 0.127 |
| Headache | .866 | 0.025 |
| Black crust in nasal cavity | .246 | 0.169 |
| Black crust on hard palate | .246 | 0.169 |
| Diabetic ketoacidosisa | .002 | 0.421 |
| Loss of consciousnessa | .004 | 0.411 |
| Brain involvementa | .03 | 0.317 |
| Past medical historya | .009 | 0.587 |
| O2 administrationa | .001 | 0.525 |
| Orbit swelling | .404 | 0.179 |
| Proptosis | .381 | 0.345 |
| Cranial nerve palsy | .984 | −0.003 |
| Diplopia | 1 | −0.121 |
| Ethmoid sinus involvement | .141 | 0.299 |
| Sphenoid sinus involvement | .090 | 0.356 |
| Frontal sinus involvement | .058 | 0.379 |
| Maxillary sinus involvement | .878 | 0.120 |
| Pansinusitis | .417 | 0.329 |
| Brain abscess | 1 | −0.121 |
| Brain infarction /ischemiaa | .047 | 0.29 |
aIndicates statistical significance.
Logistic regression test was conducted to determine if quantitative variables (including white blood cell count, HbA1c, ferritin, ESR, CRP, and the interval between first COVID-19 infection manifestation and mucormycosis) could predict the likelihood of ICU admission and 30-day mortality separately.
Except for white blood cell count (P = .025), none of the quantitative variables had a statistically significant relation with ICU admission.
The result of logistic regression conducted to predict 30-day mortality was statistically significant (P-value = .033, Chi-square (7) = 15.230). Although the combination of all quantitative variables could help predict 30-day mortality, none solely was a significant indicator of 30-day mortality. This model explained 27.7% (Cox and Snell R Square) and 37.4% (NagelkerkeR Square) of variance mortality and correctly classified 68.1% of the cases. Sensitivity and specificity were 57.9 and 75%, respectively.
Discussion
SARS CoV-2 virus has caused a worldwide disease that, besides diffuse alveolar damage, causes immunological system dysregulation and decreases the CD4 and CD8 T cells, eventually leading to immunosuppression.20 Before the COVID-19, mucor was a rare event.21 Pathogens exist in the environment and commensal on the body surface. By inhalation, the spores enter the nasal cavity and inoculate paranasal sinuses and nasopharynx.1 While Mucorales order does not threaten immunocompetent individuals' health,22 for immunocompromised patients, this infection may lead to pathogen germination in paranasal sinuses, nasopharynx, and eventually spreading to the central nervous system(1). SARS-CoV-2 virus can directly invade pancreatic islet cells or vessels that nourish them, thus causing new-onset diabetes.23,24 Inflammatory reaction also causes insulin resistance which eventually leads to hyperglycemia.8,25 COVID-19 related hyperglycemia, corticosteroid use in COVID-19 management, increase in ferritin level, hypoxia, and COVID-19 induced immune dysregulation predispose patients to mucormycosis co-infection.5,26 The most common form of mucormycosis in patients infected by SARS CoV-2 is ROCM which accounts for 90% of cases.27
Following previous studies, the majority of the cases in our survey were middle-aged with the mean age of 57 ± 10 years. Previous studies have demonstrated that most patients suffering from ROCM after COVID-19 infection were male.28-30 As a result, hypothesizing that estrogen has a protective role. Despite previous studies, no statistically significant difference was observed between the 2 genders in ours. The interval between COVID-19 infection and ROCM ranged from 10 to 14 ± 10 days.29 We have reported a 12.7 ± 4.6 days delay between these 2 infections. Delayed COVID-associated ROCM was also reported, emphasizing the importance of following the mucormycosis susceptible cases for over three-month period.30 Iron overload, malignancies of any type, consuming corticosteroids, organ transplantation, immunosuppressive drugs, immunodeficiencies, and diabetes were all considered classical risk factors of mucormycosis.14,31 Diabetes was reported as the major risk factor in COVID-19 associated ROCM.32-35 Other risk factors included prolonged corticosteroid use, hyperglycemia, hypertension, and chronic renal failure.26,33,36-39 The reason that diabetes makes patients vulnerable to infection could be due to abundant free iron in blood, poor immune response, high level of blood glucose, and acidic PH of the blood.40 In our study, diabetes was an essential risk factor either for ICU admission or 30-day mortality. No relation between the dose of corticosteroid and ICU admission or 30-day mortality was found.
Patel et al. reported that disseminated and ROCM, shorter duration of symptoms, shorter duration of antifungal therapy, using Amphotericin B deoxycholate instead of liposomal Amphotericin B are related to the higher rate of mortality rate in CAM.32 In our study, diabetes, orbital exenteration, ptosis, periorbital swelling, DKA, LOC, brain involvement, and mechanical ventilation were indicators of 30-day mortality and ICU admission. Although 25% of patients had no past medical history, all had HbA1c higher than 7 (with the mean of 11.4 ± 1.44), indicating high blood sugar during the past three months. Naruka et al. performed a single center study in India, including 79 patients with COVID-19 and associated ROCM. In their survey, only 67 patients reported suffering from diabetes, and 12 newly-onset diabetes were recognized.41 This phenomenon could be due to injudicious use of a glucocorticoid, hyper-inflammation status, or COVID-19 new-onset hyperglycemia in patients' bodies.
Although the RECOVERY trial has shown the positive impact of corticosteroids in the management of patients suffering from moderate to severe COVID-19, prolonged and high doses of glucocorticoids predispose patients to mucormycosis infection.42,43 The mean Dexamethasone dose prescribed totally for patients was 121 ± 79 mg. Despite previous studies, there was no meaningful relationship between the dose of dexamethasone and the rate of mortality.
Nasal stuffiness, fuel smell, epistaxis, nasal discharge, nasal mucosal erythema/inflammation/blue or purple discoloration/ eschar or white ulcers, eyelid/periocular/facial edema or discoloration, regional pain, facial pain, worsening headache, sudden loss of vision, facial paresthesia, sudden ptosis, proptosis, ocular motility restriction, diplopia, facial palsy, fever, altered sensorium, focal seizure, ophthalmoplegia, sinuses involvement, cranial nerve palsy, palatine involvements, and even retinal artery occlusion were observed in CAM.2,14,44-48
In an observational study of 2826 patients in India, orbital/facial pain (23%) and periocular/facial edema (33%) were the most frequent primary symptom and sign, respectively.30
In this survey, periorbital edema and headache were most common among affected patients accounting for 59.6% and 46.7% of studied patients, respectively. None of the patients surprisingly had a fever or nasal blockage.
No laboratory examination could solely predict the probability of 30-day mortality in CAM patients, but rather the combination of all mentioned laboratory data to project patients' prognosis.
On the other hand, the only useful laboratory data to predict ICU admission is WBC count.
Ethmoid and maxillary sinuses are the most involved paranasal sinus in CAM.21,33 By previous studies, bilateral ethmoid, maxillary, and sphenoid were mostly affected by mucormycosis among our patients (72.1%, 31.9%, and 31.9%, respectively). Ethmoid sinuses were affected in 91% of cases, making the ethmoid sinuses the most infected among all.
Mucormycosis can spread to the central nervous system causing infarction, brain and cavernous sinus involvement.21 49% of the studied cases suffered from central nervous system involvement.
Early diagnosis and multi-disciplinary management of COVID-19 associated ROCM improve the prognosis. Tight control of predisposing factors especially hyperglycemia, proper antifungal medication (liposomal Amphotericin B), and appropriate debridement are essential in CAM management.30
ROCM is a progressive and opportunistic infection with variable mortality rates from 30 to 90% in cases with brain involvement.30,49 In COVID-associated cases, the overall mortality rate was estimated at 30.1%.26 In this study, 40.4% of cases passed away during 30 days of follow-up.
Limitation
This study was conducted as a single-center survey. A multi-center study with more patients is required to have more precise results. A longer period of follow-up helps to determine the exact rate of mortality and other possible delayed complications.
Conclusion
COVID-19 associated ROCM is a lethal opportunistic infection with a bad prognosis. Therefore, early diagnosing is lifesaving and requires a high level of suspicion. Uncontrolled level of HbA1c in all patients, even non-diabetic individuals, indicates hyperglycemia over the past three months which could be the result of SARS-CoV-2 or injudicious use of steroids. Diabetes, orbital exenteration, ptosis, periorbital swelling, DKA, LOC, brain involvement, and mechanical ventilation all correlated with a higher rate of ICU admission and 30-day mortality. In addition, a higher WBC level is related to the higher probability of ICU admission. While considering all of the inflammatory laboratory data and HbA1c together could help predict 30-day mortality. To contain and control the infection, rapid multi-disciplinary management, including debridement and an adequate amount of proper antifungal medication is required.
Footnotes
Author Contribution: NAD and MK conceptualized the study; NB, MK, and KE acquisition of data and drafting the manuscript; and KE and MF revising for critical intellectual concept and approved the version to be submitted.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Statement: This study was ethically approved by ethical committee of Shahid Beheshti University of Medical Sciences (date of approval: January 21, 2021). Written informed consents were obtained from all included patients.
Data Availability: All analyzed data during this study are included in this article and its supplementary material files. Further enquiries can be directed to the corresponding author.
ORCID iD
Nader Akbari Dilmaghani https://orcid.org/0000-0002-5473-1904
References
- 1.Ravani SA, Agrawal GA, Leuva PA, Modi PH, Amin KD. Rise of the phoenix: Mucormycosis in COVID-19 times. Indian J Ophthalmol. 2021;69(6):1563-1568. doi: 10.4103/ijo.IJO_310_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the mycoses study group education and research consortium. Lancet Infect Dis. 2019;19(12):e405-e421. doi: 10.1016/s1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinbrink JM, Miceli MH. Mucormycosis. Infect Dis Clin North Am. 2021;35(2):435-452. doi: 10.1016/j.idc.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470-473. doi: 10.1016/s0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song G, Liang G, Liu W. Fungal co-infections associated with global COVID-19 pandemic: A clinical and diagnostic perspective from China. Mycopathologia. 2020;185(4):599-606. doi: 10.1007/s11046-020-00462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaezi A, Moazeni M, Rahimi MT, de Hoog S, Badali H. Mucormycosis in Iran: A systematic review. Mycoses. 2016;59(7):402-415. doi: 10.1111/myc.12474. [DOI] [PubMed] [Google Scholar]
- 7.Ceriello A. Hyperglycemia and COVID-19: What was known and what is really new? Diabetes Res Clin Pract. 2020;167:108383. doi: 10.1016/j.diabres.2020.108383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.John TM, Jacob CN, Kontoyiannis DP. When uncontrolled diabetes mellitus and severe COVID-19 converge: The perfect storm for mucormycosis. Journal of fungi (Basel, Switzerland). 2021;7(4). doi: 10.3390/jof7040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: Immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363-374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reiterer M, Rajan M, Gómez-Banoy N, et al. Hyperglycemia in Acute COVID-19 is characterized by adipose tissue dysfunction and insulin resistance. medRxiv: the preprint server for health sciences. 2021. doi: 10.1101/2021.03.21.21254072. [DOI] [Google Scholar]
- 11.Vaughan C, Bartolo A, Vallabh N, Leong SC. A meta-analysis of survival factors in rhino-orbital-cerebral mucormycosis-has anything changed in the past 20 years? Clin Otolaryngol. 2018;43(6):1454-1464. doi: 10.1111/coa.13175. [DOI] [PubMed] [Google Scholar]
- 12.Rupa V, Maheswaran S, Ebenezer J, Mathews SS. Current therapeutic protocols for chronic granulomatous fungal sinusitis. Rhinology. 2015;53(2):181-186. doi: 10.4193/Rhino14.183. [DOI] [PubMed] [Google Scholar]
- 13.Chikley A, Ben-Ami R, Kontoyiannis DP. Mucormycosis of the central nervous system. Journal of fungi (Basel, Switzerland). 2019;5(3). doi: 10.3390/jof5030059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honavar SG. Code mucor: Guidelines for the diagnosis, staging and management of rhino-orbito-cerebral mucormycosis in the setting of COVID-19. Indian J Ophthalmol. 2021;69(6):1361-1365. doi: 10.4103/ijo.IJO_1165_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dilek A, Ozaras R, Ozkaya S, Sunbul M, Sen EI, Leblebicioglu H. COVID-19-associated mucormycosis: Case report and systematic review. Travel Med Infect Dis. 2021;44:102148. doi: 10.1016/j.tmaid.2021.102148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sreshta K, Dave TV, Varma DR, et al. Magnetic resonance imaging in rhino-orbital-cerebral mucormycosis. Indian J Ophthalmol. 2021;69(7):1915-1927. doi: 10.4103/ijo.IJO_1439_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sipsas NV, Gamaletsou MN, Anastasopoulou A, Kontoyiannis DP. Therapy of Mucormycosis. Journal of fungi (Basel, Switzerland). 2018;4(3). July 31 2018. doi: 10.3390/jof4030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malhotra HS, Gupta P, Mehrotra D, et al. COVID-19 associated mucormycosis: Staging and management recommendations (Report of a multi-disciplinary expert committee). Journal of oral biology and craniofacial research. 2021;11(4):569-580. doi: 10.1016/j.jobcr.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dave TV, Gopinathan Nair A, Hegde R, et al. Clinical presentations, management and outcomes of rhino-orbital-cerebral mucormycosis (ROCM) following COVID-19: A multi-centric study. Ophthalmic Plast Reconstr Surg. 2021;37(5):488-495. Sep-Oct 01 2021. doi: 10.1097/iop.0000000000002030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang W, Cao Q, Qin L, et al. Clinical characteristics and imaging manifestations of the 2019 novel Coronavirus disease (COVID-19): A multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80(4):388-393. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhattacharyya A, Sarma P, Sharma DJ, et al. Rhino-orbital-cerebral-mucormycosis in COVID-19: A systematic review. Indian J Pharmacol. 2021;53(4):317-327. doi: 10.4103/ijp.ijp_419_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farmakiotis D, Kontoyiannis DP. Mucormycoses. Infect Dis Clin North Am. 2016;30(1):143-163. doi: 10.1016/j.idc.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS Coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47(3):193-199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Müller JA, Groß R, Conzelmann C, et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nature metabolism. 2021;3(2):149-165. doi: 10.1038/s42255-021-00347-1. [DOI] [PubMed] [Google Scholar]
- 25.Kothandaraman N, Rengaraj A, Xue B, et al. COVID-19 endocrinopathy with hindsight from SARS. Am J Physiol Endocrinol Metab. 2021;320(1):E139-E150. doi: 10.1152/ajpendo.00480.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India. Diabetes Metab Syndr. 2021;15(4):102146. doi: 10.1016/j.dsx.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel A, Agarwal R, Rudramurthy SM, et al. Multicenter epidemiologic study of Coronavirus disease-associated mucormycosis, India. Emerg Infect Dis. 2021;27(9):2349-2359. doi: 10.3201/eid2709.210934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramaswami A, Sahu AK, Kumar A, et al. COVID-19-associated mucormycosis presenting to the emergency Department-an observational study of 70 patients. QJM. 2021;114(7):464-470. doi: 10.1093/qjmed/hcab190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chander J, Kaur M, Singla N, et al. Mucormycosis: Battle with the deadly enemy over a 5-Year period in India. Journal of fungi (Basel, Switzerland). 2018;4(2). doi: 10.3390/jof4020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sen M, Honavar SG, Bansal R, et al. Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India - Collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC), Report 1. Indian J Ophthalmol. 2021;69(7):1670-1692. doi: 10.4103/ijo.IJO_1565_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skiada A, Pavleas I, Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: An update. Journal of fungi (Basel, Switzerland). 2020;6(4). doi: 10.3390/jof6040265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel A, Kaur H, Xess I, et al. A multicentre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin Microbiol Infect. 2020;26(7):944.e9-944.e15. doi: 10.1016/j.cmi.2019.11.021. [DOI] [PubMed] [Google Scholar]
- 33.Sharma S, Grover M, Bhargava S, Samdani S, Kataria T. Post coronavirus disease mucormycosis: A deadly addition to the pandemic spectrum. J Laryngol Otol. 2021;135(5):442-447. doi: 10.1017/s0022215121000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prakash H, Chakrabarti A. Global epidemiology of mucormycosis. Journal of fungi (Basel, Switzerland). 2019;5(1). doi: 10.3390/jof5010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morales-Franco B, Nava-Villalba M, Medina-Guerrero EO, et al. Host-pathogen molecular factors contribute to the pathogenesis of rhizopus spp. in diabetes mellitus. Current tropical medicine reports. 2021;8(1):6-17. doi: 10.1007/s40475-020-00222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13(2):236-301. doi: 10.1128/cmr.13.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torack RM. Fungus infections associated with antibiotic and steroid therapy. Am J Med. 1957;22(6):872-882. doi: 10.1016/0002-9343(57)90023-2. [DOI] [PubMed] [Google Scholar]
- 38.Moorthy A, Gaikwad R, Krishna S, et al. SARS-CoV-2, uncontrolled diabetes and corticosteroids-an unholy trinity in invasive fungal infections of the maxillofacial region? A retrospective, multi-centric analysis. J Maxillofac Oral Surg. 2021;20(3):418-425. doi: 10.1007/s12663-021-01532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarkar S, Gokhale T, Choudhury SS, Deb AK. COVID-19 and orbital mucormycosis. Indian J Ophthalmol. 2021;69(4):1002-1004. doi: 10.4103/ijo.IJO_3763_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khanna M, Challa S, Kabeil AS, et al. Risk of mucormycosis in diabetes mellitus: A systematic review. Cureus. 2021;13(10):e18827. doi: 10.7759/cureus.18827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naruka S, Rana N, Singh N, Kishore A, Nagpal K. COVID-19 associated rhino-orbital-cerebral mucormycosis-an institutional series. Ear Nose Throat J. 2022;18:1455613221077882. doi: 10.1177/01455613221077882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693-704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kontoyiannis DP, Lewis RE. How I treat mucormycosis. Blood. 2011;118(5):1216-1224. doi: 10.1182/blood-2011-03-316430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sen M, Lahane S, Lahane TP, Parekh R, Honavar SG. Mucor in a viral land: A tale of 2 pathogens. Indian J Ophthalmol. 2021;69(2):244-252. doi: 10.4103/ijo.IJO_3774_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehta S, Pandey A. Rhino-orbital mucormycosis associated With COVID-19. Cureus. 2020;12(9):e10726. doi: 10.7759/cureus.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mekonnen ZK, Ashraf DC, Jankowski T, et al. Acute Invasive rhino-orbital mucormycosis in a patient with COVID-19-associated acute respiratory distress syndrome. Ophthalmic Plast Reconstr Surg. 2021;37(2):e40-e80. doi: 10.1097/iop.0000000000001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am J Emerg Med. 2021;42:264.e5-264.e8. doi: 10.1016/j.ajem.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pakdel F, Ahmadikia K, Salehi M, et al. Mucormycosis in patients with COVID-19: A cross-sectional descriptive multicentre study from Iran. Mycoses. 2021;64(10):1238-1252. doi: 10.1111/myc.13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prakash H, Chakrabarti A. Epidemiology of Mucormycosis in India. Microorganisms. 2021;9(3). doi: 10.3390/microorganisms9030523. [DOI] [PMC free article] [PubMed] [Google Scholar]