Abstract
Lipopolysaccharide (LPS) has been known to induce inflammation by interacting with CD14, which serves as a receptor for LPS. Mannose-binding protein (MBP) belongs to the collectin subgroup of the C-type lectin superfamily, along with surfactant proteins SP-A and SP-D. We have recently demonstrated that SP-A modulates LPS-induced cellular responses by interaction with CD14 (H. Sano, H. Sohma, T. Muta, S. Nomura, D. R. Voelker, and Y. Kuroki, J. Immunol. 163:387–395, 2000) and that SP-D also interacts with CD14 (H. Sano, H. Chiba, D. Iwaki, H. Sohma, D. R. Voelker, and Y. Kuroki, J. Biol. Chem. 275:22442–22451, 2000). In this study, we examined whether MBP, a collectin highly homologous to SP-A and SP-D, could bind CD14. Recombinant rat MBP-A bound recombinant human soluble CD14 in a concentration-dependent manner. Its binding was not inhibited in the presence of excess mannose or EDTA. MBP-A bound deglycosylated CD14 treated with N-glycosidase F, neuraminidase, and O-glycosidase, indicating that MBP-A interacts with the peptide portion of CD14. Since LPS was also a ligand for the collectins, we compared the characteristics of binding of MBP-A to LPS with those of binding to CD14. MBP-A bound to lipid A from Salmonella enterica serovar Minnesota and rough LPS (S. enterica serovar Minnesota Re595 and Escherichia coli J5, Rc), but not to smooth LPS (E. coli O26:B6 and O111:B4). Unlike CD14 binding, EDTA and excess mannose attenuated the binding of MBP-A to rough LPS. From these results, we conclude that CD14 is a novel ligand for MBP-A and that MBP-A utilizes a different mechanism for CD14 recognition from that for LPS.
Lipopolysaccharide (LPS) is a major component of the outer membrane of gram-negative bacteria. LPS has been shown to be a potent stimulator of inflammation (34). The cellular responses to LPS are dependent upon membrane CD14, which is phosphatidylinositol anchored to the plasma membrane of myeloid cells (35). A soluble form of CD14 also enhances the responsiveness of the cells to LPS (11, 12). Toll-like receptors have recently been implicated in signaling by LPS and CD14 (14, 24, 36).
Mannose-binding protein (MBP) belongs to the collectin subgroup of the C-type lectin superfamily, along with surfactant proteins SP-A and SP-D and bovine conglutinin (8). Rat MBPs have been isolated from serum and liver, and cDNAs for the proteins have been isolated (10). MBP-A and MBP-C are considered to be the predominant forms of serum MBP and hepatic lectin, respectively. The collectins possess easily discernible characteristic structures consisting of an N-terminal region containing intermolecular disulfide bonding, a collagen-like domain, a neck domain, and a carbohydrate recognition domain (CRD). The collectins prefer binding mannose, glucose, and/or N-acetylglucosamine sugars with high affinity. These proteins are believed to play important roles in the innate immune system that is critical in the first line of host defense. MBP and SP-A, like C1q, interact with C1q receptor (C1qRp) and enhance the phagocytic function of phagocytes (31–33). The collectins also exhibit binding specificities for compound lipids. Human MBP and rat MBP-A bind to glycolipids containing N-acetylglucosamine residues (4, 19). SP-A binds to galactosylceramide, lactosylceramide, and asialo-GM2 (6, 16), and SP-D binds to glucosylceramide (17). SP-A and SP-D specifically interact with phosphatidylcholine and phosphatidylinositol, respectively (15, 22). MBP also interacts with certain phospholipids. Rat MBPs isolated from serum and liver bind to phosphatidylinositol, and liver MBP binds phosphatidylglycerol and phosphatidylserine (18).
We have recently found that SP-A modulates tumor necrosis factor alpha (TNF-α) expression induced by smooth and rough LPS (26). SP-A binds directly to human recombinant CD14, which serves as a receptor for LPS (35). The binding of CD14 to smooth LPS was reduced, but the association of CD14 with rough LPS was augmented in the presence of SP-A. This different effect of SP-A upon distinct serotypes of LPS is likely to occur via the direct interactions of SP-A with CD14. In addition, we have found that SP-D binds CD14 and alters the CD14-LPS interaction (25). The structural and functional homology among the collectins suggests the possibility that MBP binds CD14. In this report, we examined whether MBP bound CD14 by ligand blot analysis and microtiter well binding and investigated the requirement of the oligosaccharide moieties of CD14 for the binding of MBP. Since MBP-A also binds LPS (9, 13), we compared the characteristics of binding of MBP-A to CD14 with those of binding to LPS.
MATERIALS AND METHODS
Expression of recombinant MBP-A.
The cDNA for rat MBP-A was constructed and was inserted into pVL 1392 plasmid vector as described previously (3). Recombinant MBP-A was produced with the baculovirus-insect cell expression system as described by O'Reilly et al. (23). Recombinant baculoviruses were produced by cotransfection of Spodoptera frugiperda (Sf9) cells with linearized Autographa californica virus (Baculogold, Pharmingen) and the pVL 1392 plasmid vector containing MBP-A cDNAs. Plaques containing recombinant baculoviruses were isolated and amplified to 5 × 107 PFU/ml. Recombinant MBP-A protein was expressed in serum-free medium SF900 II (Gibco BRL) by infection of Trichoplusia ni cells with viral stock at a multiplicity of infection of 2. The recombinant MBP-A was purified by an affinity column of mannose-Sepharose 6B from culture medium.
Expression of recombinant CD14 in insect cells and CHO-K1 cells.
The expression and purification of recombinant human CD14 in insect cells and in mammalian cells were described previously (25). Briefly, cDNA for the full-length human CD14 was isolated, and the putative glycosylphosphatidylinositol (GPI)-anchoring site was replaced with a sequence containing a six-histidine tag (CD14H) by a method based on that of Tapping and Tobias (30). The CD14H cDNA was inserted into pVL 1393 vector by using BamHI and NotI sites. CD14H was expressed in the baculovirus-insect cell system as described above for recombinant MBP-A. The recombinant viruses were amplified to 108 PFU/ml and used to infect monolayers of T. ni cells in serum-free insect medium at a multiplicity of infection of 5. The medium was collected after a 3-day incubation and dialyzed against 0.1 M Tris buffer (pH 8.0) containing 0.3 M NaCl. The dialyzed medium was then filtered and applied to a column of nickel-nitrilotriacetic acid beads (Qiagen, Santa Clarita, Calif.). The CD14H protein binding to the beads was finally eluted with 0.1 M Tris buffer (pH 8.0) containing 0.3 M NaCl and 100 mM imidazole. The purified protein was dialyzed against 5 mM Tris buffer (pH 7.4) containing 0.15 M NaCl.
Recombinant CD14H was also expressed in CHO-K1 cells (CHO CD14H) by using the glutamine synthetase amplification system (1). Briefly, the CD14H cDNA was inserted into pEE14 plasmid vector (1) by using the restriction sites of SmaI and EcoRI. The pEE14 vector containing CD14H cDNA was transfected into CHO-K1 cells by using Lipofectamine (Life Technologies, Inc.). Transfected cells were incubated in glutamine-free Glasgow minimum essential medium (GMEM) supplemented with 10% dialyzed fetal bovine serum (complete GMEM) in the presence of 25 μM methionine sulfoximine (Sigma). Colonies were isolated by using cloning cylinders and further incubated with complete GMEM containing higher concentrations of methionine sulfoximine. The stable cell line that secretes CD14H was finally obtained and maintained in the presence of 250 μM methionine sulfoximine. The production of CHO CD14H was carried out with serum-free medium ExCell302 (JRH Biosciences). The medium was collected after a 3-day incubation, and the CHO CD14H protein was finally purified by the nickel-nitrilotriacetic acid column as described above.
Analysis of recombinant MBP-A and CD14H.
Protein concentrations were estimated by the bicinchoninic acid (BCA) assay (Pierce), with bovine serum albumin (BSA) as a standard. Proteins were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (13% polyacrylamide) by the method of Laemmli (20) and stained with Coomassie brilliant blue.
Binding of MBP-A to CD14 used to coat microtiter wells.
The CD14 binding with microtiter wells was performed as described previously (25, 26). Microtiter wells (Immulon 1B; Dynex Laboratories) were coated with CD14H (10 μg/ml, 50 μl/well) produced in insect cells and incubated at 37°C for 5 h with 0 to 10 μg of recombinant MBP-As per ml or BSA in 20 mM Tris buffer (pH 7.4) containing 0.15 M NaCl, 5% (wt/vol) BSA, and 5 mM CaCl2 (buffer A). In some experiments, 0.2 M mannose was included in buffer A, and 1 mM EDTA was used instead of 5 mM CaCl2 in buffer A. The wells were then washed with phosphate-buffered saline (PBS) containing 0.1% (vol/vol) Triton X-100 and 3% (wt/vol) skim milk (buffer B) and incubated at 37°C for 90 min with anti-recombinant MBP-A immunoglobulin G (IgG) (20 μg/ml), followed by incubation with horseradish peroxidase (HRP)-labeled antirabbit IgG (1:1,000). After the wells were washed with PBS containing 0.1% (vol/vol) Triton X-100 (buffer C), the MBP-A binding to solid-phase CD14H was finally detected by the peroxidase reaction with o-phenylenediamine as a substrate.
Ligand blotting analysis.
Ligand blotting was carried out as described previously (25, 26). Four micrograms of CD14H was electrophoresed and transferred to a polyvinylidene difluoride (PVDF) membrane. The nonspecific binding was blocked with the buffer A containing 1% (wt/vol) polyvinylpyrrolidone (buffer D). The membrane was then incubated at room temperature for 3 h with 2 μg of MBP-A per ml diluted with the buffer D. The membrane was then washed with buffer B and incubated with anti-MBP-A antibody (5 μg/ml) for 1 h, followed by incubation with HRP-labeled antirabbit IgG (1:1,500) for 1 h. After the membrane had been washed with buffer C, the MBP-A binding to the PVDF membrane was visualized by using the ECL enhanced chemiluminescence reagent (Amersham Pharmacia Biotech, Inc.) according to the manufacturer's instructions.
Deglycosylation of CHO CD14H.
Four micrograms of CHO CD14H protein was incubated with 1 U of N-glycosidase F (Boehringer Mannheim), 10 U of neuraminidase (Boehringer Mannheim), and 1 mU of O-glycosidase (Boehringer Mannheim) at 37°C for 90 min in 10 mM Tris buffer (pH 7.4) containing 10 mM EDTA, 2% (vol/vol) β-mercaptoethanol, 0.1% (wt/vol) SDS, and 1% (vol/vol) NP-40.
LPS binding.
The binding of recombinant MBP-As to smooth LPS (Escherichia coli O26:B6, O111:B4 from Sigma), rough LPS [Salmonella enterica serovar Minnesota Re595 and E. coli J5(Rc) from Sigma], and lipid A from S. enterica serovar Minnesota Re595 (List Biologic Laboratories) was performed by the method described previously (25, 26). Briefly, LPS (5 μg/well) or lipid A (5 μg/well) in 20 μl of ethanol was put into the microtiter wells (Immulon 1B; Dynex Laboratories), and the solvent was evaporated in ambient air. After the nonspecific binding was blocked with buffer A, various concentrations of recombinant MBP-As in 50 μl of the buffer A were incubated at 37°C for 5 h. The protein binding in the LPS- and lipid A-coated microtiter wells was detected with anti-rat MBP-A IgG and HRP-labeled antirabbit IgG. The peroxidase reaction was performed by using o-phenylenediamine as described above for CD14 binding.
RESULTS
Recombinant MBP-A and CD14.
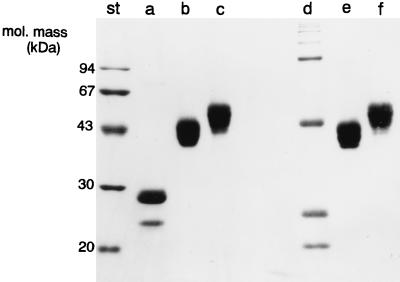
Rat wild-type MBP-A was expressed by using the baculovirus expression system and purified by affinity chromatography on mannose-Sepharose 6B. When analyzed by electrophoresis under reducing conditions, the major form of MBP-A migrated at approximately 30 kDa (Fig. 1), as described previously (3). The proteins formed oligomers under nonreducing conditions. The preservation of lectin activity indicates that the recombinant MBP-As expressed in insect cells exhibit no significant defect in protein folding.
FIG. 1.
Electrophoretic analysis of recombinant MBP-A and CD14. Four micrograms of recombinant MBP-A (lanes a and d), CD14H produced in insect cells (lanes b and e), and CD14H produced in CHO cells (lanes c and f) were subjected to 13% polyacrylamide gel electrophoresis in the presence of SDS under reducing (lanes a to c) and nonreducing (lanes d to f) conditions and visualized by Coomassie brilliant blue staining. St, molecular mass standards.
Recombinant CD14 (CD14H) was also expressed in insect cells and purified by affinity chromatography on nickel-nitrilotriacetic acid beads. Purified CD14H produced in insect cells migrated as bands at approximately 40 to 43 kDa (Fig. 1) as described previously (25), which is consistent with the result reported by Tapping and Tobias (30). The CD14 protein expressed in insect cells reacted with monoclonal anti-human CD14 antibody and also was demonstrated to bind to LPS (30). Human CD14 is N glycosylated (2, 27) and contains O-linked glycosylated oligosaccharides (29). Since the complex oligosaccharide synthesis does not appear to occur in insect cells, although the pentasaccharide core common to N glycosylation is synthesized in insect cells like in mammalian cells (23), we expressed CD14H in CHO cells as well. CD14H produced in CHO cells (CHO CD14) migrated as broad bands of 46 to 56 kDa when analyzed by electrophoresis under denaturing conditions (Fig. 1) (25). The predominant CHO CD14H exhibited molecular masses of 51 to 53 kDa, which is consistent with the results obtained by Stelter et al. (29).
Binding of MBP-A to CD14H.
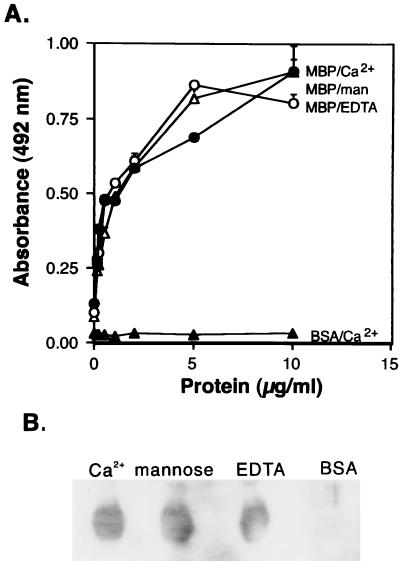
The binding of MBP-A to CD14H produced in insect cells was performed in the presence of 5 mM CaCl2, 0.2 M mannose, or 1 mM EDTA. MBP-A bound in CD14H-coated microtiter wells in a concentration-dependent manner (Fig. 2A). The binding experiments were also carried out in the presence of 5 mM CaCl2 with heat-treated (100°C, 5 min) MBP. The binding of the heat-treated MBP to solid-phase CD14 was reduced to 19% (mean of two experiments) of the binding level of control MBP. Inclusion of excess mannose or EDTA did not inhibit MBP-A binding to the solid-phase CD14H. The effect of excess mannose or EDTA on MBP-A binding to CD14H was also examined by ligand blot analysis (Fig. 2B). The results obtained by ligand blotting were consistent with those obtained by microtiter well binding. Neither excess mannose nor EDTA blocked MBP-A binding to CD14H transferred on the PVDF membranes. Taken together, the results indicate that excess mannose and EDTA did not alter the binding of MBP-A to CD14, suggesting that the lectin activity of MBP-A is not required for the interaction with CD14.
FIG. 2.
Binding of MBP-A to CD14 produced in insect cells. (A) Microtiter well binding. CD14H (10 μg/ml, 50 μl) (●, ▵, ○) produced in insect cells or BSA (▴) was used to coat microtiter wells, which were then incubated with the indicated concentrations of recombinant MBP-A at 37°C overnight in the presence of 5 mM Ca2+ (●). In some experiments, 0.2 M mannose (man) was added to the binding buffer (▵). EDTA (1 mM) was also included instead of Ca2+ (○). The protein binding to CD14H was detected by using polyclonal antibody to MBP-A as described in Materials and Methods. The data shown are means + standard errors of three experiments. (B) Ligand blot analysis. Four micrograms of CD14H produced in insect cells was electrophoresed, and that transferred on the PVDF membranes was incubated with MBP-A (Ca2+) or BSA in the presence of 5 mM Ca2+ at room temperature for 3 h. Mannose (0.2 M) was added to the binding buffer. EDTA (1 mM) was also included instead of 5 mM Ca2+. The MBP-A binding to CD14H was detected with anti-MBP-A IgG as described in Materials and Methods.
Binding of MBP-A to deglycosylated CHO CD14H.
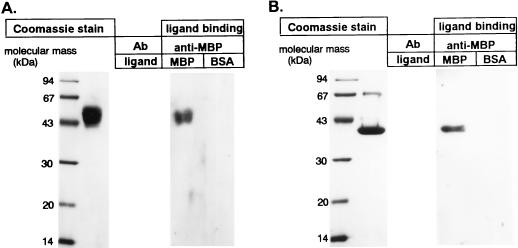
Since the glycosylation pattern of the protein in insect cells is different from that in mammalian cells, we also produced recombinant CD14H in CHO-K1 cells (CHO CD14H) and examined whether the oligosaccharide moieties of CD14 were involved in MBP-A–CD14 interaction. MBP-A clearly bound to CHO CD14H (Fig. 3A). The enzyme treatment of CHO CD14H with N-glycosidase F, neuraminidase, and O-glycosidase resulted in a reduction of molecular mass to 39 kDa (Fig. 3B, Coomassie stain), which corresponds with the result reported by Stelter et al. (29). Ligand blotting analysis revealed that MBP-A bound to deglycosylated CHO CD14H (Fig. 3B, ligand binding). The result clearly demonstrates that MBP-A binds the peptide portion but not the oligosaccharide moieties of CD14.
FIG. 3.
MBP-A binds deglycosylated CHO CD14H. CD14H (4 μg) produced in CHO cells (A) or CHO CD14H treated with N-glycosidase F, neuraminidase, and O-glycosidase (deglycosylated CHO CD14) (B) was electrophoresed and visualized by Coomassie brilliant blue staining. The proteins were also transferred onto PVDF membranes and incubated with MBP-A (2 μg/ml) or BSA at room temperature for 3 h, and those binding to the membranes were detected by anti-MBP-A antibody (Ab) (ligand binding) as described in Materials and Methods.
Binding of MBP-A to LPS.
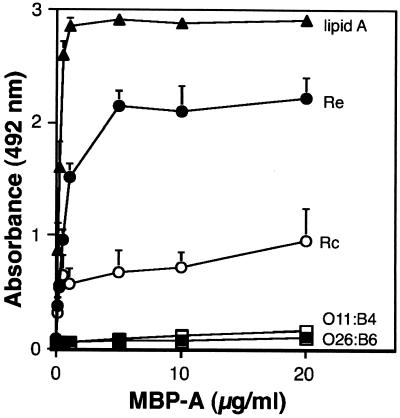
We also examined the binding of recombinant MBP-A to distinct structural types of LPS used to coat microtiter wells (Fig. 4). The wild-type rat MBP-A bound to lipid A and rough strains of LPS in a concentration-dependent manner. The binding of MBP-A to the Rc strain (E. coli J5) was weaker than that to the Re strain (S. enterica serovar Minnesota Re595). In contrast, MBP-A exhibited negligible binding to two strains of smooth LPS (E. coli O26:B6 and O111:B4). In this assay, 5 μg of LPS or lipid A per well was adsorbed onto microtiter wells. When a chromogenic assay of the wells was performed by using the Limulus amebocyte lysate system (ENDOSPECY, Seikagaku Kogyo, Tokyo), 340 ng (mean of two experiments) of O26:B6 LPS, 320 ng of Re LPS, and 290 ng of lipid A were detected in the wells after the washing procedures. The results indicate that similar amounts (by weight) of the solid-phase ligands were present in the microtiter wells.
FIG. 4.
MBP-A binds to lipid A and rough LPS. Five micrograms of E. coli O26:B6 (▪), O111:B4 (□), Re (●), Rc (J5)(○) and lipid A (▴) per well was used to coat microtiter wells, which were then incubated with the indicated concentrations of wild-type MBP-A for 5 h at 37°C. The binding of the protein to LPS and lipid was detected with anti-rat MBP-A IgG and HRP-labeled anti-rabbit IgG as described in Materials and Methods. The data shown are means + standard errors of three experiments.
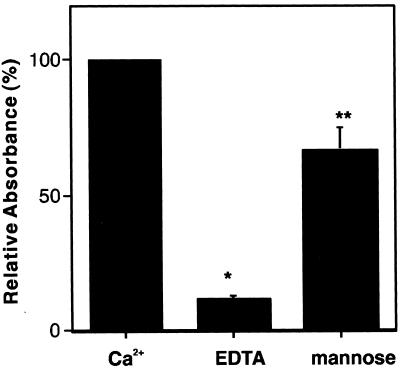
We also examined the effect of EDTA and excess mannose on the binding of MBP-A to rough LPS (Fig. 5). The binding of MBP-A to rough LPS in the presence of 5 mM EDTA was reduced to 11% of the level of binding in the presence of Ca2+. Inclusion of 0.2 M mannose in the binding buffer attenuated the binding of MBP-A to rough LPS to 65% of the level of binding without mannose. These results indicate that the interaction of MBP-A with rough LPS depends on the calcium ion, and the lectin property of MBP-A may be involved in the binding to rough LPS.
FIG. 5.
Effect of EDTA and excess mannose on the binding of MBP-A to Re LPS. Five micrograms of Re LPS was used to coat microtiter wells, which were then incubated with 5 μg of wild-type MBP-A per ml at 37°C for 5 h in 20 mM Tris buffer (pH 7.4) containing 0.15 M NaCl, 5% (wt/vol) BSA, and 5 mM CaCl2 (Ca2+); where indicated, 0.2 M mannose was included. In some experiments, 5 mM EDTA was added instead of CaCl2. The binding of MBP-A to LPS was detected by anti-MBP-A antibody as described in Materials and Methods. The results are expressed as the percentage of relative absorbance compared with that obtained from the binding in the presence of Ca2+ (100%). The data shown are means + standard errors of three experiments. ∗, P < 0.0002; ∗∗, P < 0.05.
DISCUSSION
MBP-A belongs to the collectin subgroup of the C-type lectin superfamily, along with lung surfactant proteins SP-A and SP-D. This study provides evidence that CD14 is a novel ligand for MBP-A. MBP-A binds the peptide portion of CD14. Since we have recently found that lung collectins bind CD14 (26), the interaction with CD14 may be an important property common to the collectin family.
Since the binding of MBP-A to CD14 is not blocked by excess mannose or EDTA, the lectin activity is not involved in the interaction of MBP-A with CD14. This observation is consistent with the result obtained by ligand blot analysis with deglycosylated CHO CD14. The oligosaccharide moieties of CD14 are not required for the binding of MBP-A to CD14. Although glycosphingolipids and phospholipids are also ligands for MBP, the binding of MBP to these lipids is attenuated by coincubation with EDTA or excess sugars (4, 5, 18). In this study, we showed that the binding of MBP-A to rough LPS is also attenuated in the presence of EDTA or excess mannose, indicating that MBP-A binds rough LPS in a manner similar to that for the recognition of glycosphingolipids and phospholipids. Thus, these studies suggest that MBP-A utilizes a structure for the recognition of CD14 different from that for compound lipids.
Although we have not determined whether the treatment of CD14 with N-glycosidase F, neuraminidase, and O-glycosidase has completely removed the attached carbohydrate, we digested the protein according to the method described by Stelter et al. (29), who could not detect the attached carbohydrate of CD14 by periodate oxidation and incorporation of biotin after treatment with these enzymes. In addition, deglycosylation of CD14 with enzymes requires denaturing detergents, which may destroy MBP binding sites or expose new MBP binding sites. Thus, the limitation of the method we used should be pointed out. However, a recent study (25) from this laboratory has shown that SP-D, but not SP-A, lost the binding activity to CD14 deglycosylated in the same way. Therefore, it is possible to conclude that MBP retains the binding activity to deglycosylated CD14, which SP-D fails to bind to.
Like C1q, MBP and SP-A can enhance mononuclear phagocytic function via the C1q/MBL/SP-A receptor, C1qRp (21, 31–33). SPR210 has also been isolated from U937 cells and has been suggested to be an SP-A receptor for regulation of the functions of alveolar macrophages and alveolar type II cells (7, 31), indicating the existence of multiple receptors for the collectins. This and recent studies from this laboratory have revealed that these three collectins bind CD14 (25, 26). Since CD14 exists as a membrane CD14 that is GPI anchored to the plasma membrane of myeloid cells as well as a soluble form (35), CD14 could be a new receptor for the collectins. Although SP-D utilizes the lectin activity to recognize the oligosaccharide moieties of CD14, SP-A binds the peptide portion of CD14 via its neck domain (25). This study demonstrates that MBP-A binds CD14 in a manner independent of the lectin property. The domain involved in the MBP-A–CD14 interaction remains to be elucidated.
CD14 serves as a receptor for LPS and transmits LPS signals via Toll-like receptors (14, 24, 36). SP-A modulates cellular responses induced by LPS by interaction with CD14 (26). SP-A inhibits CD14 binding to smooth LPS, which is not a ligand for SP-A, and blocks smooth LPS-induced cellular responses. In contrast, SP-A even increases the association of CD14 with rough LPS, which is an SP-A ligand, and failed to inhibit rough LPS-elicited cellular responses. MBP has also been shown to inhibit TNF-α secretion induced by rhamnose glucose polymers (RGPs) of streptococcal cell walls by the direct interaction with RGPs (28). The study also suggests that RGP-MBP complexes are taken up by human monocytes via C1q receptor. SP-A, a structural homologue to MBP, inhibits TNF-α secretion stimulated by smooth LPS in alveolar macrophages (26). The study may suggest one idea: that SP-A functions as an anti-inflammatory molecule against gram-negative bacteria expressing smooth LPS. Since this study indicates that MBP, like SP-A, interacts with CD14 and rough LPS, but not with smooth LPS, it is possible to assume that MBP may possess such an anti-inflammatory function. Whether MBP alters cellular responses elicited by bacterial components via interaction with CD14 is under investigation.
In conclusion, we found that CD14 is a novel ligand for MBP-A and that MBP-A utilizes a different mechanism for CD14 recognition from that used for LPS recognition.
ACKNOWLEDGMENTS
We thank Toyoaki Akino (Sapporo Medical University) for valuable discussions and encouragement.
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture, Japan.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Amplification using CHO cell expression vectors. New York, N.Y: John Wiley & Sons, Inc.; 1992. [Google Scholar]
- 2.Bazil V, Horejsi V, Baudys M, Kristofova H, Strominger J L, Kostka W, Hilgert I. Biochemical characterization of a soluble form of the 53-kDa monocyte antigen. Eur J Immunol. 1986;16:1583–1589. doi: 10.1002/eji.1830161218. [DOI] [PubMed] [Google Scholar]
- 3.Chiba H, Sano H, Saitoh M, Sohma H, Voelker D R, Akino T, Kuroki Y. Introduction of mannose-binding protein-type phosphatidylinositol recognition into pulmonary surfactant protein A. Biochemistry. 1999;38:7321–7331. doi: 10.1021/bi990353e. [DOI] [PubMed] [Google Scholar]
- 4.Childs R A, Drickamer K, Kawasaki T, Thiel S, Mizuochi T, Feizi T. Neoglycolipids as probes of oligosaccharide recognition by recombinant and natural mannose-binding proteins of the rat and man. Biochem J. 1989;262:131–138. doi: 10.1042/bj2620131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Childs R A, Feizi T, Yuen C-T, Drickamer K, Quesenberry M S. Differential recognition of core and terminal portions of oligosaccharide ligands by carbohydrate-recognition domains of two mannose-binding proteins. J Biol Chem. 1990;265:20770–20777. [PubMed] [Google Scholar]
- 6.Childs R A, Wright J R, Ross G F, Yuen C-T, Lawson A M, Chai W, Drickamer K, Feizi T. Specificity of Lung Surfactant Protein SP-A for both the carbohydrate and the lipid moieties of certain neutral glycolipids. J Biol Chem. 1992;267:9972–9979. [PubMed] [Google Scholar]
- 7.Chroneos Z C, Abdolrasulnia R, Whitsett J A, Rice W R, Shepherd V L. Purification of a cell-surface receptor for surfactant protein A. J Biol Chem. 1996;271:16375–16383. doi: 10.1074/jbc.271.27.16375. [DOI] [PubMed] [Google Scholar]
- 8.Day A J. The C-type carbohydrate recognition domain (CRD) superfamily. Biochem Soc Trans. 1994;22:83–88. doi: 10.1042/bst0220083. [DOI] [PubMed] [Google Scholar]
- 9.Devyatyarova-Johnson M, Rees I H, Robertson B D, Turner M W, Klein N J, Jack D L. The lipopolysaccharide structures of Salmonella enterica serovar Typhimurium and Neisseria gonorrhoeae determine the attachment of human mannose-binding lectin to intact organisms. Infect Immun. 2000;68:3894–3899. doi: 10.1128/iai.68.7.3894-3899.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drickamer K, Dordal M S, Reynolds L. Mannose-binding proteins isolated from rat liver contain carbohydrate-recognition domain linked to collagenous tails. Complete primary structures and homology with pulmonary surfactant apoprotein. J Biol Chem. 1986;261:6878–6887. [PubMed] [Google Scholar]
- 11.Frey E A, Miller D S, Jahr T G, Sundan A, Brazil V, Espevik T, Finlay B B, Wright S D. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176:1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hailman E, Vasselon T, Kelly M, Busse L A, Hu M C, Lichenstein H S, Detmers P A, Wright S D. Stimulation of macrophages and neutrophils by complexes of lipopolysaccharide and soluble CD14. J Immunol. 1996;156:4384–4390. [PubMed] [Google Scholar]
- 13.Jiang G-Z, Sugiyama T, Kato Y, Koide N, Yokochi T. Binding of mannose-binding protein to Klebsiella O3 lipopolysaccharide possessing the mannose homopolysaccharide as the O-specific polysaccharide and its relation to complement activation. Infect Immun. 1995;63:2537–2540. doi: 10.1128/iai.63.7.2537-2540.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirschning C J, Wesche H, Ayres T M, Rothe M. Human Toll-like receptor-2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med. 1998;188:2091–2097. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuroki Y, Akino T. Pulmonary surfactant protein A (SP-A) specifically binds dipalmitoylphosphatidylcholine. J Biol Chem. 1991;266:3068–3073. [PubMed] [Google Scholar]
- 16.Kuroki Y, Gasa S, Ogasawara Y, Makita A, Akino T. Binding of pulmonary surfactant protein A to galactosylceramide and asialo-GM2. Arch Biochem Biophys. 1992;299:261–267. doi: 10.1016/0003-9861(92)90273-y. [DOI] [PubMed] [Google Scholar]
- 17.Kuroki Y, Gasa S, Ogasawara Y, Shiratori M, Makita A, Akino T. Binding specificity of lung surfactant protein SP-D for glucosylceramide. Biochem Biophys Res Commun. 1992;187:963–969. doi: 10.1016/0006-291x(92)91291-w. [DOI] [PubMed] [Google Scholar]
- 18.Kuroki Y, Honma T, Chiba H, Sano H, Saitoh M, Ogasawara Y, Sohma H, Akino T. A novel type of binding specificity to phospholipids for rat mannose-binding proteins isolated from serum and liver. FEBS Lett. 1997;414:387–392. doi: 10.1016/s0014-5793(97)01022-3. [DOI] [PubMed] [Google Scholar]
- 19.Kyogashima M, Krivan H C, Schweinle J E, Ginsburg V, Holt G D. Glycosphingolipid-binding specificity of the mannose-binding protein from human sera. Arch Biochem Biophys. 1990;283:217–222. doi: 10.1016/0003-9861(90)90634-b. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli U K. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Nepomuceno R R, Henschen-Edman A H, Burgess W H, Tenner A J. cDNA cloning and primary structure analysis of C1qRp, the human C1q/MBL/SP-A receptor that mediates enhanced phagocytosis in vitro. Immunity. 1997;6:119–129. doi: 10.1016/s1074-7613(00)80419-7. [DOI] [PubMed] [Google Scholar]
- 22.Ogasawara Y, Kuroki Y, Akino T. Pulmonary surfactant protein D specifically binds to phosphatidylinositol. J Biol Chem. 1992;267:21244–21249. [PubMed] [Google Scholar]
- 23.O'Reilly D R, Miller L K, Luckow V A. Baculovirus expression vector. A laboratory manual. W. H. New York, N.Y: Freeman and Company; 1992. [Google Scholar]
- 24.Poltorak A, Xiaolong H, Smirnova I, Liu M-Y, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HEJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 25.Sano H, Chiba H, Iwaki D, Sohma H, Voelker D R, Kuroki Y. Surfactant proteins A and D bind CD14 by different mechanisms. J Biol Chem. 2000;275:22442–22451. doi: 10.1074/jbc.M001107200. [DOI] [PubMed] [Google Scholar]
- 26.Sano H, Sohma H, Muta T, Nomura S, Voelker D R, Kuroki Y. Pulmonary surfactant protein A modulates the cellular responses to smooth and rough lipopolysaccharides by interactions with CD14. J Immunol. 1999;163:387–395. [PubMed] [Google Scholar]
- 27.Simmons D L, Tan S, Tenen D G, Nicholson-Weller A, Seed B. Monocytic antigen CD14 is a phospholipid anchored membrane protein. Blood. 1989;73:284–289. [PubMed] [Google Scholar]
- 28.Soell M, Lett E, Holveck F, Scholler M, Wachsmann D, Klein J-P. Activation of human monocytes by streptococcal rhamnose glucose polymers is mediated by CD14 antigen, and mannan binding protein inhibits TNF-α release. J Immunol. 1995;154:851–860. [PubMed] [Google Scholar]
- 29.Stelter F, Pfister M, Bernheiden M, Jack R S, Bufler P, Engelmann H, Schütt C. The myeloid differentiation antigen CD14 is N- and O-glycosylated. Eur J Biochem. 1996;236:457–464. doi: 10.1111/j.1432-1033.1996.00457.x. [DOI] [PubMed] [Google Scholar]
- 30.Tapping R I, Tobias P S. Cellular binding of soluble CD14 requires lipopolysaccharide (LPS) and LPS-binding protein. J Biol Chem. 1997;272:23157–23164. doi: 10.1074/jbc.272.37.23157. [DOI] [PubMed] [Google Scholar]
- 31.Tenner A J. Membrane receptors for soluble defense collagens. Curr Opin Immunol. 1999;11:34–41. doi: 10.1016/s0952-7915(99)80007-7. [DOI] [PubMed] [Google Scholar]
- 32.Tenner A J, Robinson S L, Borchelt J, Wright J R. Human pulmonary surfactant protein (SP-A), a protein structurally homologous to C1q, can enhance FcR- and CR1-mediated phagocytosis. J Biol Chem. 1989;264:13923–13928. [PubMed] [Google Scholar]
- 33.Tenner A J, Robinson S L, Ezekowitz R A B. Mannose binding protein (MBP) enhances mononuclear phagocyte function via a receptor that contains the 126,000 Mr component of the C1q receptor. Immunity. 1995;3:485–493. doi: 10.1016/1074-7613(95)90177-9. [DOI] [PubMed] [Google Scholar]
- 34.Ulevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 35.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;21:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 36.Yang R B, Mark M R, Gray A, Huang A, Xie M H, Zhang M, Goddard A, Wood W I, Gurney A L, Godowski P J. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signaling. Nature. 1998;395:284. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]