Abstract
A man in his 40s with a history of coronary artery disease previously treated with a drug-eluting stent presented for elective craniotomy and resection of an asymptomatic but enlarging meningioma. During his craniotomy, he received desmopressin and tranexamic acid for surgical bleeding. Postoperatively, the patient developed chest pain and was found to have an ST-elevation myocardial infarction (MI). Because of the patient’s recent neurosurgery, standard post-MI care was contraindicated and he was instead managed symptomatically in the intensive care unit. Echocardiogram on postoperative day 1 demonstrated no regional wall motion abnormalities and an ejection fraction of 60%. His presentation was consistent with thrombosis of his diagonal stent. He was transferred out of the intensive care unit on postoperative day 1 and discharged home on postoperative day 3.
Keywords: Drug therapy related to surgery, Adult intensive care, Unwanted effects / adverse reactions, Neurosurgery, Ischaemic heart disease
Background
Craniotomy with mass resection is a common neurosurgical procedure. Complications after craniotomy include bleeding, infection and risk of thromboembolic events including myocardial infarction (MI). MI after craniotomy occurs approximately 0.2% of the time, with a mortality as high as 30%.1
Patients with a history of coronary artery disease (CAD) may be on antiplatelet therapy preoperatively. However, these medications are typically held prior to surgery. Evidence for holding these medications in the perioperative period is conflicting.2–6
Case presentation
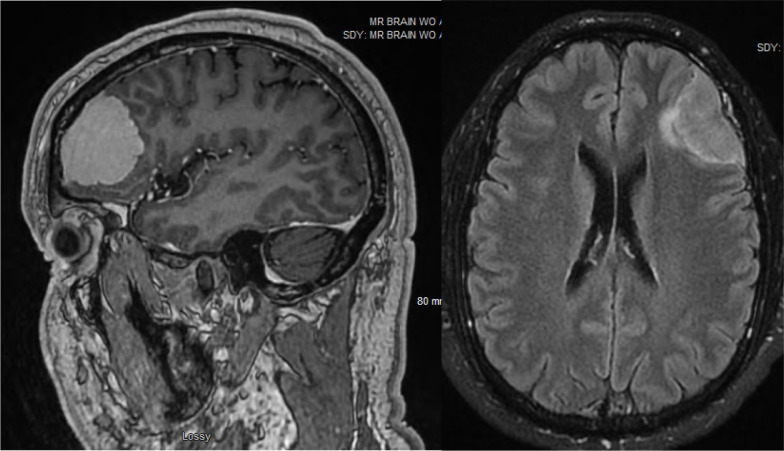
Our patient is a man in his 40s with a history of CAD requiring two drug-eluting stents (DES) to the first diagonal branch of the left anterior descending for 90% stenosis. He presented with an incidentally found brain mass, most consistent with a meningioma. His neurosurgeon recommended surveillance of this lesion with serial axial imaging. Repeat MRI demonstrated that the mass had grown to 2.3 cm × 5.3 cm with new moderate local mass effect on the left frontal and opercular regions (figure 1). Neurosurgery recommended elective craniotomy for surgical resection of his asymptomatic left frontal mass given new signal change adjacent to the tumour on repeat MRI.
Figure 1.
MRI brain demonstrating left frontal extra-axial mass measuring 2.3 cm × 5.3 cm with moderate local mass effect on left frontal and opercular regions.
The patient’s cardiac catheterisation and DES placement were performed 21 months prior and he was no longer requiring dual antiplatelet therapy. His aspirin was held for 10 days before his craniotomy at the direction of his cardiologist. Intraoperatively, he received 0.3 µg/kg of desmopressin (1-deamino-8-D-arginine vasopressin, DDAVP) and 1 g of tranexamic acid (TXA) for treatment of minor surgical bleeding.
Investigations
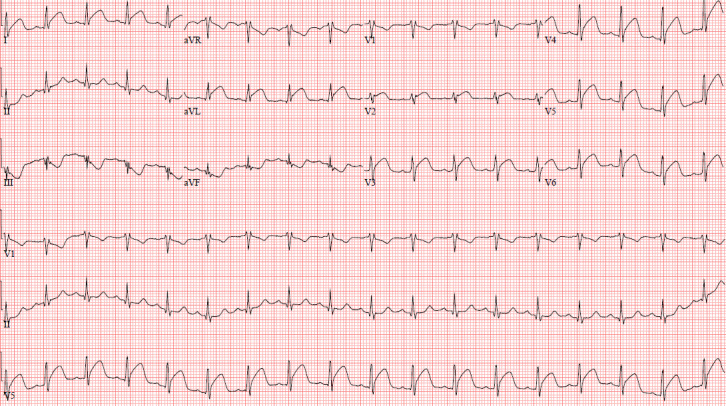
In the postoperative anaesthesia care unit, the patient developed the acute onset of chest pain. Stat ECG and troponin assays were obtained (figure 2). ECG demonstrated ST elevations in leads I, aVL (augmented vector left), V2–V6 and reciprocal ST changes in leads III, aVR (augmented vector right), aVF (augmented vector foot) and V1. His troponin eventually peaked at 10 659 ng/L.
Figure 2.
ECG with ST elevations in leads I, aVL, V2–V6 and reciprocal change in leads III, aVR, aVF and V1.
Treatment
Cardiology was consulted urgently given ST-elevation MI (STEMI). However, due to his recent neurosurgical procedure, systemic anticoagulation or antiplatelet therapy were contraindicated given the risk of intracranial haemorrhage. He was, therefore, deemed not to be a candidate for cardiac catheterisation or stenting. He was instead managed symptomatically with a nitroglycerin infusion, scheduled parenteral metoprolol and as needed morphine. He was admitted to the surgical intensive care unit (SICU) for close haemodynamic and neurological monitoring.
Outcome and follow-up
Repeat echocardiogram on postoperative day 1 demonstrated low-normal right ventricular systolic function, no wall motion abnormalities and an ejection fraction of 60%. His presentation was consistent with in-stent thrombosis of his pre-existing diagonal stent. He was weaned off the nitroglycerin infusion later that day and was safely transferred out of the SICU to the surgical floor. He was instructed to avoid strenuous activity and follow-up in 2 weeks to discuss repeat cardiac catheterisation. He was subsequently discharged home on postoperative day 3.
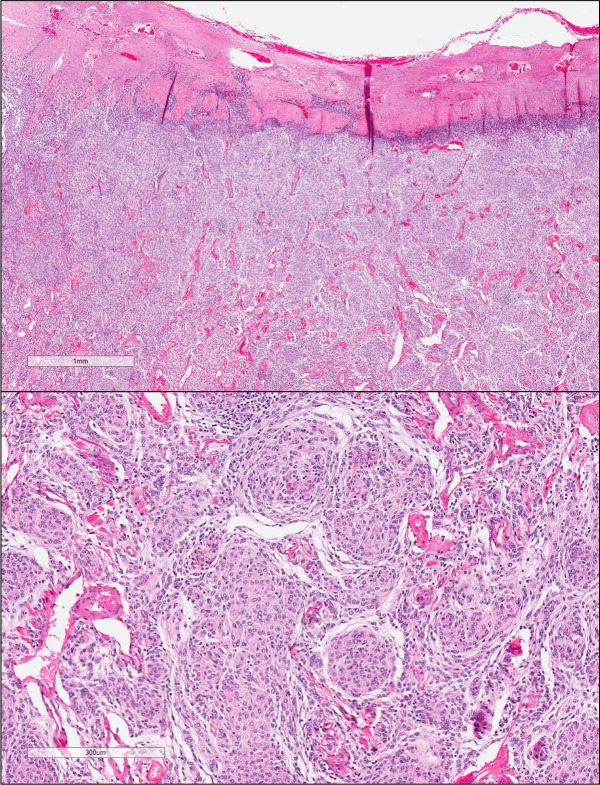
The patient was seen by his cardiologist 1 week after discharge and reported no recurrent chest pain. Repeat head CT imaging 2 weeks after discharge demonstrated improvement in the patient’s postoperative haemorrhage along the surface of the brain, and he was deemed safe to resume his aspirin. He was seen by his neurosurgeon 1 month after discharge and was cleared to advance his activity. Repeat MRI 2 months after discharge demonstrated normal postoperative changes. Histopathology confirmed the resected lesion was a grade 1 meningioma (figure 3). The patient was recently seen for his 3-month follow-up appointment and remains symptom-free from both a cardiac and neurological standpoint.
Figure 3.
Low-power and high-power images of mass resection pathology demonstrating meningothelial meningioma grade 1.
Discussion
Although MI after craniotomy is rare, it carries a high mortality as patients are unable to undergo usual post-MI care.1 Patients are not candidates for fibrinolytic therapy and are not able to receive therapeutic anticoagulation given the risk of inducing intracranial haemorrhage. Intracranial haemorrhage is a feared complication of anticoagulation and research regarding safe timing for initiation of such therapy postcraniotomy is lacking. One study in rats suggests that it may be safe to administer therapeutic levels of heparin 10–14 days after craniotomy.7 However, these results have not been validated in human trials. If patients cannot safely receive systemic heparin, then cardiac catheterisation with intervention is not feasible. Furthermore, patients who undergo DES placement require dual antiplatelet therapy for 6–12 months to reduce the risk of in-stent thrombosis.8 Placement of a bare metal stent still requires a minimum of 1 month of treatment with a P2Y12 inhibitor. Drug-coated balloon angioplasty without stent placement also requires a minimum of 1 month of dual antiplatelet therapy.9 Unfortunately, patients with perioperative MI are at high risk for death without usual post-MI care, including fibrinolytic therapy or urgent primary percutaneous coronary intervention. Thus, the in-hospital mortality ranges from 5% to 25% in this patient population.1
Our patient received both TXA and DDAVP intraoperatively for surgical bleeding. TXA is an antifibrinolytic agent used to reduce bleeding in elective surgery and trauma settings. It is a synthetic derivative of arachidonic acid, which blocks the lysine binding sites on plasminogen, therefore, inhibiting fibrinolysis.10 Although there are a number of studies evaluating the safety of TXA, these often exclude patients with a significant cardiac history. In the Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage-2 trial, only young healthy patients were included for administration of TXA. Similarly, in the Aspirin and TXA for Coronary Artery Surgery trial, only 2.5% of the included patients had prior coronary angioplasty or stenting and 1.2%–1.6% had prior cardiac surgery.11 In contrast, some studies in the orthopaedic surgery literature included patients with a significant cardiac history. One large retrospective study of over 26 000 patients with a history of CAD or prior coronary stenting undergoing total joint arthroplasties demonstrated that administration of TXA to minimise blood loss intraoperatively did not increase risk of venous thromboembolism or MI compared with the control group.12
DDAVP is a synthetic analogue of the antidiuretic hormone, vasopressin. It has been shown to increase factor VIII and von Willebrand factor leading to shortened activated partial thromboplastin time and bleeding time.13 Because of its effect on platelet function, DDAVP has utility in reversing the effects of platelet inhibiting medications such as aspirin and clopidogrel.14 It may also have some efficacy in bleeding trauma patients, although the results to date have been controversial.15 One meta-analysis of trauma patients demonstrated reduced blood loss and another demonstrated fewer blood transfusion requirements with the use of DDAVP, although these reductions did not affect overall mortality or clinical outcomes.16 17 However, DDAVP has also been shown to reverse platelet dysfunction in patients with severe traumatic brain injury (TBI).18 As point-of-care testing with thromboelastography (TEG) and rotational thromboelastometry (ROTEM) has become more widespread, these tests are more commonly used in a real time to assist in guiding resuscitation.19 In our practice, DDAVP is typically used when bleeding patients have evidence of platelet dysfunction on these point-of-care assessments.
Although there have been cases of MI after administration of TXA or DDAVP, to our knowledge, there has only been one reported case of in-stent thrombosis after administration of TXA and one after use of DDAVP.20–22 The case of in-stent thrombosis after TXA was a patient undergoing total hip arthroplasty revision with a history of CAD and placement of five DES, the most recent of which were placed 18 months prior to presentation.21 The patient was instructed to stop his clopidogrel 5 days before surgery but to continue his aspirin in the perioperative period. He received a 500 mg bolus of TXA intraoperatively followed by another 500 mg bolus 10 min later for intraoperative bleeding. Three hours after administration, he was noted to have a STEMI requiring cardiac catheterisation and balloon angioplasty. The case of in-stent thrombosis after DDAVP occurred in a patient with persistent subgaleal bleeding from blunt force trauma to the head with a prior history of coronary DES on chronic aspirin and clopidogrel.22 This patient received DDAVP to reverse his antiplatelet agents, but developed severe chest pain 5 hours after administration and was found to have a STEMI requiring thrombectomy and repeat stent placement.
It is prudent to note that most evidence favours a positive side effect profile of DDAVP and TXA when used individually. In contrast to the case reports in the literature, our patient received both TXA and DDAVP empirically for surgical bleeding. Similar to the case described by Kaptein, our patient also developed a STEMI after an elective surgical procedure. However, our patient was only on aspirin for antiplatelet therapy whereas the patient in the case described by Kaptein was taking both aspirin and clopidogrel at the time of total hip arthroplasty.21
Both our patient and the patient in the case by Kaptein were undergoing non-cardiac surgery after recent DES placement. The absolute risk of MI after non-cardiac surgery for patients who have recently undergone DES placement is highest in the first 30 days after stent placement (OR 20.1, 95% CI 8.85 to 45.6).23 This risk falls over time. However, by 12 months after coronary stenting, the risk of postoperative MI is still elevated (OR 2.18, 95% CI 0.89 to 5.14).23 Similar to our patient, the patient in the case described by Kaptein was also beyond 12 months from coronary stenting. Thus, it is less likely that recent stenting contributed to our patient’s postoperative MI, but was more likely secondary to the prothrombotic medications that our patient received intraoperatively.
Preoperative TEG and ROTEM are not commonly recommended for patients undergoing elective craniotomy. There is some literature to support use of ROTEM in TBI patients undergoing craniotomy to guide treatment of coagulopathy and decrease progressive haemorrhagic injury and neurosurgical reintervention.24 In contrast, the cardiothoracic surgery literature strongly advocates for the use of TEG and ROTEM in the perioperative period to decrease use of blood products.25–28 Ghadimi et al propose an algorithm for intraoperative bleeding during noncardiac surgery, with a focus on balanced resuscitation guided by laboratory-based, viscoelastic testing.19
Routine preoperative tests may identify abnormal values which do not necessarily correlate with changes in overall coagulation function. In addition, they lack the ability to identify patients who may be hypercoagulable. Viscoelastic tests provide a holistic view of a patient’s coagulation cascade, reporting clotting time, clot strength, platelet function and fibrinogen levels. By providing a more detailed assessment of the coagulation cascade, viscoelastic tests can predict not only hypocoagulability but can also give insight into patients with hypercoagulability. Therefore, as TEG and ROTEM become more widely available we propose that prothrombotic and antifibrinolytic medications be administered based on the results of these tests performed preoperatively rather than blindly treating perioperative bleeding, especially in patients that are high risk for thombotic events such as patients with a significant cardiac, and in particular those patients with coronary stents.
Patient’s perspective
My first memory as I came out of anaesthesia was confusion and chest pain. I’m sure the confusion was normal. I’ve never been fully anaesthetised before. As I became more aware of the world around me, I felt a sharp pain in the centre of my chest, radiating outwards. This was different from my joint pain and occasional sternum aches from osteoarthritis. It was also different from the chest pain that I felt when I first sought treatment for my heart condition. It felt deeper inside of my body and there was nothing that I could do to relieve the pain (stretching, etc.). The medical team must have given me some medication to deal with the pain and I faded back into unconsciousness.
When I awoke in the ICU, I was more comfortable, though my chest still ached. I was able to identify my surroundings but was still very tired. I remember that my head didn’t seem to hurt, but the chest pain would sometimes be a little more intense. I would inform the medical team and occasionally, I was given more pain medication. Generally, I felt relaxed and was not aware of how serious my complications were.
My significant other stayed with me and we talked when my energy allowed. My sibling also visited and stayed much of the evening. I remember having on-and-off conversations with them. After my significant other left to care for our child at home, my chest pain gradually decreased and I requested less medication. The evening after surgery was ‘foggy’, but overall I felt relieved that my meningioma resection was over.
The day following my surgery, I was able to better understand the complications and underwent a number of tests (ECG, bloodwork, etc.) I still felt tired, but given the recent surgery, that seemed normal. I wanted to get out of my bed and move around, but wasn’t quite ready to do anything other than walk to the bathroom by the evening. My activity was limited to visits from the staff, using my laptop and listening to music. I remember eating my meals and visiting with my significant other later that day. Generally, I felt pretty good.
By day three, I was ready to walk around. After breakfast and a check-in from my doctors, I went for a short walk with my significant other and the physical therapy team. Although still tired, we made it around the hallways and back to my room. That walk felt like progress and I started to become eager to go home.
On my final day at the hospital, the results of my tests and imaging looked good. My significant other and I walked around the halls and a stairway before being approved to go home. While I didn’t feel physically weak, I was still tired at times.
After returning home, my significant other (wisely) insisted that I limit movement and get rest. I still spent time on my laptop, watched some television/streaming, and chatted with a few visitors. Over the next few days, I had follow-up imaging, suture removal and post-op appointments with my cardiologist and primary care physician. My cardiologist explained that the medication given to me following my post-op haemorrhaging likely caused the narrowed arteries and stents to contract ‘likely causing a MI’ or ‘reaction equivalent to a MI.’ Discussing the situation with both him and my PCP it was clear that there weren’t many options given the surgical bleeding and my heart condition/medication limitations.
My cardiologist stated that I could gradually resume exercise at a low/moderate level and I began riding a stationary bike and some basic cardio. I was careful to limit the intensity of my exercise and activities until my follow-up appointment with my neurosurgeon where he cleared me to resume activity similar to my pre-surgical routine. I currently exercise 30–60 min, usually 5 days per week. My exercise schedule includes virtual reality boxing (variable intensity with heart rate up to~160–170 bpm), stationary cycling (sustained heart rate between 120–140 bpm) and aerobics (heart rate ranging from 100 to 140 bpm).
Overall, I feel the same or better than before my surgery. My cardiovascular medications have decreased: I no longer take amlodipine, my lisinopril dose is lower (by 50%) and I’ve returned to taking metoprolol succinate 25 mg once per day. My home-measured blood pressure has ranged from 110-130/63-85, but mostly on the lower side of that range. I continue to take my statin in the evening and I’ve maintained a better diet since my initial CAD diagnosis: very rare dairy or eggs (a few times per year) and lean red meat up to once per month (to prevent anaemia).
Learning points
Myocardial infarction (MI) can be a devastating complication following elective craniotomy, as patients are unable to undergo usual post-MI care.
1-deamino-8-D-arginine vasopressin and tranexamic acid for intraoperative bleeding should be used with caution in patients with a history of cardiac stenting given risk for in-stent thrombosis.
Preoperative rotational thromboelastometry or thromboelastography clotting profile and platelet mapping assays may guide judicious use of these agents intraoperatively.
Footnotes
Twitter: @kwestfall23
Contributors: KMW is the primary author who cared for the patient, contributed to the write up, and edited the manuscript. RNM cared for the patient, contributed to the discussion and edited the manuscript. HLA oversaw the patient’s care and edited the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1. Goel NJ, Mallela AN, Agarwal P, et al. Complications predicting perioperative mortality in patients undergoing elective craniotomy: a population-based study. World Neurosurg 2018;118:e195–205. 10.1016/j.wneu.2018.06.153 [DOI] [PubMed] [Google Scholar]
- 2. Lee AT, Gagnidze A, Pan SR, et al. Preoperative low-dose aspirin exposure and outcomes after emergency neurosurgery for traumatic intracranial hemorrhage in elderly patients. Anesth Analg 2017;125:514–20. 10.1213/ANE.0000000000002053 [DOI] [PubMed] [Google Scholar]
- 3. Li M, Yang S, Liu Q, et al. Evaluating the safety of early surgery for ruptured intracranial aneurysms in patients with long-term aspirin use: a propensity score matching study. Chin Neurosurg J 2020;6:1–6. 10.1186/s41016-020-00216-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hanalioglu S, Sahin B, Sahin OS, et al. Effect of perioperative aspirin use on hemorrhagic complications in elective craniotomy for brain tumors: results of a single-center, retrospective cohort study. J Neurosurg 2019;132:1529–38. 10.3171/2018.12.JNS182483 [DOI] [PubMed] [Google Scholar]
- 5. Rahman M, Donnangelo LL, Neal D, et al. Effects of perioperative acetyl salicylic acid on clinical outcomes in patients undergoing craniotomy for brain tumor. World Neurosurg 2015;84:41–7. 10.1016/j.wneu.2015.02.016 [DOI] [PubMed] [Google Scholar]
- 6. Han H, Koh EJ, Choi H, et al. The effect of preoperative antiplatelet therapy on hemorrhagic complications after decompressive craniectomy in patients with traumatic brain injury. Korean J Neurotrauma 2016;12:61. 10.13004/kjnt.2016.12.2.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schaible KL, Smith LJ, Fessler RG, et al. Evaluation of the risks of anticoagulation therapy following experimental craniotomy in the rat. J Neurosurg 1985;63:959–62. 10.3171/jns.1985.63.6.0959 [DOI] [PubMed] [Google Scholar]
- 8. Helft G. Dual antiplatelet therapy duration after drug-eluting stents: how long? J Thorac Dis 2016;8:E844–6. 10.21037/jtd.2016.07.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Corballis NH, Wickramarachchi U, Vassiliou VS, et al. Duration of dual antiplatelet therapy in elective drug-coated balloon angioplasty. Catheter Cardiovasc Interv 2020;96:1016–20. 10.1002/ccd.28632 [DOI] [PubMed] [Google Scholar]
- 10., Shakur H, Roberts I, et al. , CRASH-2 trial collaborators . Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376:23–32. 10.1016/S0140-6736(10)60835-5 [DOI] [PubMed] [Google Scholar]
- 11. Myles PS, Smith JA, Forbes A, et al. Tranexamic acid in patients undergoing coronary-artery surgery. N Engl J Med 2017;376:136–48. 10.1056/NEJMoa1606424 [DOI] [PubMed] [Google Scholar]
- 12. Zak SG, Tang A, Sharan M, et al. Tranexamic acid is safe in patients with a history of coronary artery disease undergoing total joint arthroplasty. J Bone Joint Surg Am 2021;103:900–4. 10.2106/JBJS.20.01226 [DOI] [PubMed] [Google Scholar]
- 13. Franchini M. The use of desmopressin as a hemostatic agent: a Concise review. Am J Hematol 2007;82:731–5. 10.1002/ajh.20940 [DOI] [PubMed] [Google Scholar]
- 14. Mannucci PM, Desmopressin MPM. Desmopressin (DDAVP) in the treatment of bleeding disorders: the first 20 years. Blood 1997;90:2515–21. 10.1182/blood.V90.7.2515 [DOI] [PubMed] [Google Scholar]
- 15. Rossaint R, Bouillon B, Cerny V, et al. Management of bleeding following major trauma: an updated European guideline. Crit Care 2010;14:R52–29. 10.1186/cc8943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Özal E, Kuralay E, Bingöl H, et al. Does tranexamic acid reduce desmopressin-induced hyperfibrinolysis? J Thorac Cardiovasc Surg 2002;123:539–43. 10.1067/mtc.2002.117281 [DOI] [PubMed] [Google Scholar]
- 17. Crescenzi G, Landoni G, Biondi-Zoccai G, et al. Desmopressin reduces transfusion needs after surgery: a meta-analysis of randomized clinical trials. Anesthesiology 2008;109:1063–76. 10.1097/ALN.0b013e31818db18b [DOI] [PubMed] [Google Scholar]
- 18. Furay EJ, Daley MJ, Satarasinghe P, et al. Desmopressin is a transfusion sparing option to reverse platelet dysfunction in patients with severe traumatic brain injury. J Trauma Acute Care Surg 2020;88:80–6. 10.1097/TA.0000000000002521 [DOI] [PubMed] [Google Scholar]
- 19. Ghadimi K, Levy JH, Welsby IJ. Perioperative management of the bleeding patient. vol. 117, British Journal of anaesthesia. Br J Anaesth 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garg J, Pinnamaneni S, Aronow WS, et al. St elevation myocardial infarction after tranexamic acid: first reported case in the United States. Am J Ther 2014;21:e221–4. 10.1097/MJT.0b013e31828fdb06 [DOI] [PubMed] [Google Scholar]
- 21. Kaptein YE. Acute ST-elevation myocardial infarction due to in-stent thrombosis after administering tranexamic acid in a high cardiac risk patient. BMJ Case Rep 2019;12. 10.1136/bcr-2018-227957. [Epub ahead of print: 08 Apr 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shah SN, Tran HA, Assal A, et al. In-Stent thrombosis following DDAVP administration: case report and review of the literature. Blood Coagul Fibrinolysis 2014;25:81–3. 10.1097/MBC.0b013e328364c232 [DOI] [PubMed] [Google Scholar]
- 23. Thim T, Egholm G, Kristensen SD, et al. Risk of myocardial infarction and death after noncardiac surgery performed within the first year after coronary drug-eluting stent implantation for acute coronary syndrome or stable angina pectoris. Am J Cardiol 2021;160:14–20. 10.1016/j.amjcard.2021.08.040 [DOI] [PubMed] [Google Scholar]
- 24. Rimaitis M, Bilskienė D, Tamošuitis T, et al. Implementation of Thromboelastometry for coagulation management in isolated traumatic brain injury patients undergoing craniotomy. Med Sci Monit 2020;26:e922879–1. 10.12659/MSM.922879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fleming K, Redfern RE, March RL, et al. TEG-Directed transfusion in complex cardiac surgery: impact on blood product usage. J Extra Corpor Technol 2017;49:283-290. [PMC free article] [PubMed] [Google Scholar]
- 26. Westbrook AJ, Olsen J, Bailey M, et al. Protocol based on thromboelastograph (TEG) out-performs physician preference using laboratory coagulation tests to guide blood replacement during and after cardiac surgery: a pilot study. Heart Lung Circ 2009;18:277–88. 10.1016/j.hlc.2008.08.016 [DOI] [PubMed] [Google Scholar]
- 27. Ak K, Isbir CS, Tetik S, et al. Thromboelastography-based transfusion algorithm reduces blood product use after elective CABG: a prospective randomized study. J Card Surg 2009;24:404–10. 10.1111/j.1540-8191.2009.00840.x [DOI] [PubMed] [Google Scholar]
- 28. Weber CF, Görlinger K, Meininger D, et al. Point-Of-Care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology 2012;117:531–47. 10.1097/ALN.0b013e318264c644 [DOI] [PubMed] [Google Scholar]