Abstract
Objectives
This study aims to estimate the association of the often, in daily clinical practice, used biological age-related biomarkers high-sensitivity troponin-T (hs-TnT), C reactive protein (CRP) and haemoglobin (Hb) with all-cause mortality for the purpose of older patient’s risk stratification in the emergency department (ED).
Design
Exploratory, prospective cohort study with a follow-up at 2.5 years after recruitment started. For the predictors, data from the hospital files including the routinely applied biological age-related biomarkers hs-TnT, CRP and Hb were supplemented by a questionnaire.
Setting
A cardiological ED, Chest Pain Unit, University Hospital Heidelberg, Germany.
Participants
N=256 cardiological ED patients with a minimum age of 70 years and the capability to informed consent.
Primary outcome measures
The primary outcome of this study was all-cause mortality which was assessed by requesting registry office information.
Results
Among N=256 patients 63 died over the follow-up period. Positive results in each of the three biomarkers alone as well as the combination were associated with increased all-cause mortality at follow-up. The number of positive age-related biomarkers appeared to be strongly indicative of the risk of mortality, even when controlled for major confounders (age, sex, body mass index, creatinine clearance and comorbidity).
Conclusions
In older ED patients, biomarkers explicitly related to biological ageing processes such as hs-TnT, CRP and Hb were to a certain degree independently of each other as well as combined associated with an increased risk of all-cause mortality. Thus, they may have the potential to be used to supplement the general risk stratification of older patients in the ED. Validation of the results in a large dataset is needed.
Keywords: geriatric medicine, cardiology, risk management, cardiology
Strengths and limitations of this study.
The prospective design and the 2.5 years long follow-up period with few censored observations at early times are strengths of this study.
As information about medical history and diagnoses received from the visit to the emergency department were available, effect estimates could be controlled for major confounders.
Limitations of this study are mainly the exploratory approach, the single-centre design and the relatively small number of events.
Introduction
Older adults are an increasing population and frequent users of the emergency department (ED) setting.1 As a challenging clientele they frequently present with atypical symptoms, polymedication and unclear medical history in the ED2 and physicians then face the challenge of quickly identifying those patients with a high-risk of adverse outcomes. Strategies to combine biomarkers indicative of biological age (see Wagner et al3) may enhance risk stratification of older and geriatric patients in the ED, due to their objectivity and clear clinical implications. From a biomarkers of ageing framework three distinct biomarkers (C reactive protein (CRP), high-sensitive troponin-T (hs-TnT) and haemoglobin (Hb)) are frequently encountered in routine diagnostic procedures.
Established theories of immune-senescence and ‘inflammaging’ describe biological ageing as a chronic, low-grade inflammatory status.4 In accordance with this, markers such as CRP have shown their potential for the prognosis of older people in terms of frailty and mortality5 as well as in terms of events such as myocardial infarction or stroke.6
Similarly, hs-TnT can serve as biomarkers of age. Troponins are regulator proteins of the contractile apparatus and enter the bloodstream in case of cardiomyocyte damage. The hs-TnT showed a notable predictive validity regarding mortality as well as other outcomes such as myocardial infarction or stroke in numerous studies.7–11 It was also found that these effects of elevated troponins are independent of their aetiology.7 They thus mark a general risk factor that must be urgently noticed before any further treatment.
Further, geriatric patients frequently have decreased levels of Hb in the form of anaemia.12 Recently, a study by Han et al found that in apparently healthy older people the occurrence of anaemia is associated with an increased rate of hospitalisation and mortality,13 irrespective of the underlying comorbidities.14
In this study, we therefore aim to estimate the association of the biomarkers CRP, hs-TnT and Hb, with older ED patient’s all-cause mortality. Accordingly, we expect an increased all-cause mortality with elevated cardiac or inflammation markers or low Hb levels, even when age, overall comorbidity and other variables (sex, body mass index (BMI), renal functioning) are controlled for. Given the differential mechanisms in the pathways of these biomarkers, we also hypothesise that the risk of mortality will increase even further when these different markers interact, and a patient therefore shows more than one abnormal result.
Methods
Study design, setting and participants
The study’s design was a single-centre exploratory, prospective cohort study. It was conducted in the Chest Pain Unit (CPU) with 12 beds as part of the cardiological ED of a university hospital (114 beds in the cardiological department overall). In total, 260 cardiological ED patients with a minimum age of 70 years were recruited (participation rate ≈ 76%). Therefore, the study is designed for older CPU patients in order to improve geriatric risk stratification. The first patient was recruited in July 2017 and the last one in May 2018. Patients were recruited only after the first examination by the ED physician. Also, on the basis of this initial examination, patients were excluded in cases of missing informed consent or an expected life expectancy of less than 24 hours. Thus, patients who were in a life-threatening condition and therefore unable to be interviewed or to benefit in any way from a more geriatric risk stratification were excluded. Further, patients who were isolated due to transmission-based precautions had to be excluded from the study. Since all included participants were treated in a CPU, the cohort of this study consists of selected cardiological patients.
Baseline information was assessed by using a questionnaire, in which the Short Portable Mental Status Questionnaire (SPMSQ)15 was integrated, and additionally by extracting relevant data from the hospital files. Follow-up data for this study were collected by requesting registry office information on mortality roughly 2.5 years after recruitment of the first patient.
Based on the cognitive performance on the SPMSQ,15 about 23% of the participants had at least a minor form of cognitive impairment. Participants presented to the cardiological ED with numerous symptoms, including dyspnoea (59%), thorax pain (20%), angina pectoris (18%), vertigo (9%) and palpitations (8%). Frequent primary diagnoses were suspicion/exclusion of myocardial infarction (26%), cardiac decompensation (23%) and heart rhythm disturbances (19%). Further information on primary and secondary diagnoses of the sample are presented in detail in online supplemental tables 1 and 2.
bmjopen-2021-056674supp001.pdf (59KB, pdf)
This research was conducted in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki. The protocol was approved by the ethics committee of the medical faculty at the University of Heidelberg (S-455/2016).
Patient and public involvement
No patient involved.
Study variables
The biomarkers relevant to this study were part of the routinely applied diagnostic procedures in the ED. To classify the lab results as positive or negative, the following cut offs were used.
For CRP a cut-off >5 mg/L was used. This cut-off is an established reference value16 17 and was used for both sexes, as studies showed a similar distribution of this marker among men and women.18
A value of hs-TnT >14 ng/L (ie, exceeding the 99th percentile of a multicentre reference study)19 describes the cut-off for both sexes. Increased levels showed a higher risk for cardiovascular events19 and the prognostic value of including gender difference for hs-TnT was detected only modest in other studies.20
Due to the fact that mild anaemia was defined as a Hb concentration between 10.0 g/dL and 11.9 g/dL in women and between 10.0 g/dL and 12.9 g/dL in men,21 we used a cut-off of <12 g/dL for women and <13 g/dL for men. This identifies anaemia as defined by the WHO.22
To assess the effect of multiple biomarkers of interest being positive in a given patient, a combined predictor variable ranging from 0 to 3 was created, with 0 indicating a positive result in none of the biomarkers, 1 indicating a positive result in either one of them, and so on. This strategy of combining the predictors was chosen as the sample size is relatively small and the pathways of the biomarker effects are assumed to be different. Also, the resulting groups were fairly balanced.
Overall comorbidity and age of the patients as a central control variable was accounted for using the Charlson-Age Comorbidity Index (CACI)23 based on the patient’s medical history which included the diagnoses assigned by the ED physician. These diagnoses, which are important confounding factors, were extracted from the discharge letter after treatment in the CPU, therefore including the diagnosed acute issue for which the patient presented. Higher values of the CACI indicate more severe comorbidity.
In order to more precisely account for patient’s renal functioning as possible confounder, an estimate of the creatinine clearance based on the formula by Cockroft and Gault24 was calculated based on serum creatinine.
For the purpose of sample description, participants’ result (error score) on the cognitive screening SPMSQ is also reported. The theoretical range of the SPMSQ is 0–10 and higher values indicate worse cognitive performance.
The primary outcome examined in this study was the patient’s all-cause mortality (time-to-event, days) after their initial ED visit. This information was collected using registry office information.
Statistical methods
To describe the characteristics of the sample, means, SDs, medians and IQRs were calculated for continuous or discrete variables. Absolute and relative frequencies were calculated for categorical variables.
Kaplan-Meier curves were used to illustrate the associated effect of positive hs-TnT, CRP, anaemia (Hb), as well as the composite predictor of all three on overall survival after the initial ED visit. Cox proportional hazards models were then used to first, get uncontrolled HR estimates for those effects and second, to control those effects for patient’s BMI, age, creatinine clearance, sex and overall comorbidity. Schoenfeld residuals did not show evidence against the proportional hazards assumption.
Missing values were rare (max: 1.5% or 4/260 per variable) and were therefore handled by listwise deletion, which reduced the total sample to N=256 patients.
Uncertainty was quantified using 95% CIs and as this study is of exploratory nature, provided p-values are to be interpreted as descriptive.
All analyses were conducted using R V.3.6.1 (R Core Team, Vienna, Austria).
Results
About 343 patients were eligible, 260 were recruited (participation rate ≈ 76%); 4 patients had missing data for major baseline variables due to them leaving the ED against medical advice.
In total, the final cohort of N=256 participants can be described as a high age but, as measures of dispersion for example for variables such as the number of medications indicate, heterogenous sample (detailed descriptive characteristics in table 1). Bivariate correlations show that there was only a weak association between the results in each of the three biomarkers (hs-TnT with CRP: rφ= 0.30, 95% CI=0.19 to 0.41; hs-TnT with Hb: rφ= 0.35, 95% CI=0.24 to 0.45; and CRP with Hb: rφ =0.24, 95% CI=0.13 to 0.36, respectively).
Table 1.
Descriptive statistics of the cohort (N=256)
| Variable | N (%) | Mean (SD) | Median (IQR) |
| Age | 79.27 (6.0) | 79.00 (75 to 83) | |
| Sex | |||
| Female | 97 (38%) | ||
| Male | 159 (62%) | ||
| School years | |||
| <9 years | 116 (45%) | ||
| ≥9 years | 140 (55%) | ||
| Falls in the past 12 months | |||
| At least one | 80 (31%) | ||
| None | 176 (69%) | ||
| Smoker | |||
| Current/former | 104 (41%) | ||
| Never | 152 (59%) | ||
| Diabetes | |||
| Yes | 73 (29%) | ||
| No | 183 (71%) | ||
| Malignancy in anamnesis | |||
| Active | 21 (8%) | ||
| Inactive | 19 (7%) | ||
| None | 216 (84%) | ||
| BMI | 26.85 (4.8) | 26.37 (23.74 to 29.76) | |
| SPMSQ | 1.50 (1.6) | 1 (0 to 2) | |
| No of medications | 7.64 (3.8) | 7 (5 to 9.75) | |
| CACI | 5.72 (2.0) | 5 (4 to 7) | |
| Creatinine clearance (mL/min) | 60.42 (24.8) | 60.26 (42.25 to 76.69) | |
| Increased high-sensitivity troponin-T (hs-TnT) | 166 (65%) | ||
| Increased CRP | 115 (45%) | ||
| Decreased haemoglobin | 102 (40%) |
Creatinine clearance as calculated by Cockroft and Gault formula.
BMI, body mass index; CACI, Charlson Age Comorbidity Index; SPMSQ, Short Portable Mental Status Questionnaire (number of errors).
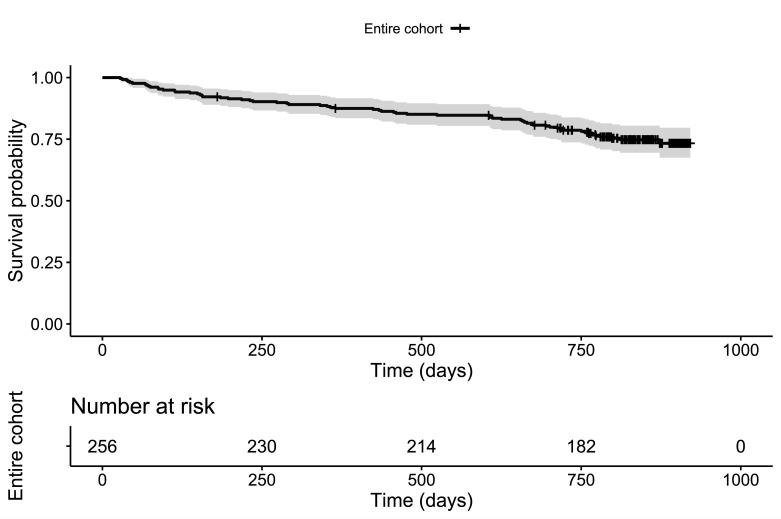
The median follow-up time calculated by reverse Kaplan-Meier method was 835 days. Sixty-three patients (25% of the sample) died during the follow-up period. Separate controlled and uncontrolled estimates of the association between each biomarker and all-cause mortality are presented in table 2, associated Kaplan-Meier curves can be found in online supplemental figure 1. As can be taken from table 2 and online supplemental figure 1, positive results in each of the three biomarkers were associated with increased all-cause mortality at follow-up. Hb showed the weakest association to mortality and did not remain statistically significant when adjusted for confounders.
Table 2.
Uncontrolled and controlled associations (hazard-ratio) of biological ageing-related biomarkers and mortality from separate Cox-regression models (N=256)
| Variable | Crude HR | 95% CI | Adjusted HR | 95% CI |
| Increased high-sensitivity troponin-T (hs-TnT) | 7.28 | (2.92 to 18.16) | 4.41 | (1.71 to 11.43) |
| Increased CRP | 3.67 | (2.12 to 6.34) | 2.97 | (1.69 to 5.23) |
| Decreased haemoglobin | 2.64 | (1.60 to 4.38) | 1.55 | (0.89 to 2.68) |
Adjusted estimates are controlled for sex, body mass index, creatinine clearance, age and comorbidity (Charlson-Age Comorbidity Index Score).
CRP, C reactive protein.
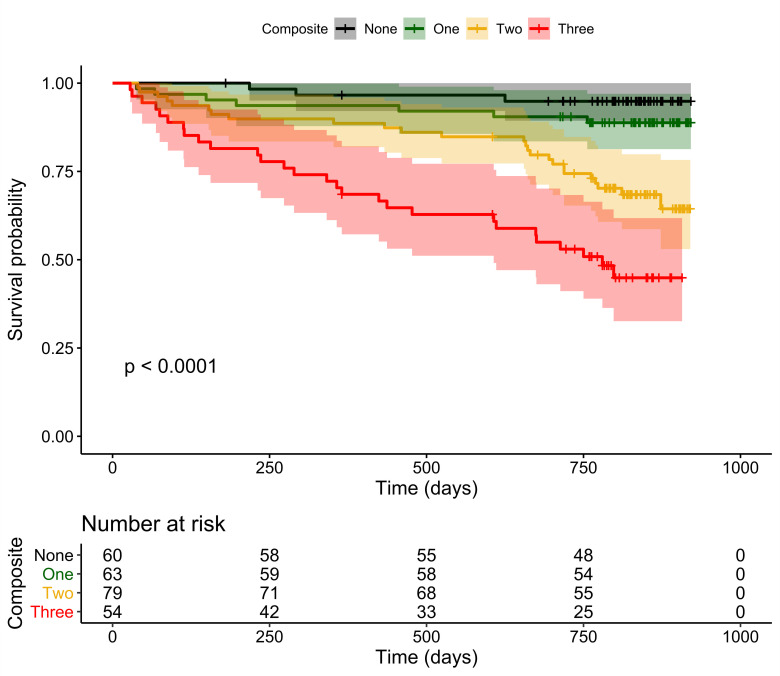
In the main analyses, a summary score was used to predict mortality indicating how many of the three biomarkers showed positive results in a patient (theoretical range: no positive result – three positive results). Kaplan-Meier curves for this grouping are presented in figure 1. Kaplan-Meier curve with 95% CI for the entire cohort is illustrated in figure 2. The number of positive biomarkers appeared to be strongly associated to the patient’s all-cause mortality. Even when controlled for sex, BMI, creatinine clearance, comorbidity and age as scored by the CACI, the summary score of positive biomarkers was still associated with mortality (see table 3). While the point estimates for the effect sizes were notably high, this estimation comes with considerable uncertainty as indicated by the wide 95% CIs (eg, for the patients with three positive biomarkers vs the no positive biomarkers reference HR=7.46 and 95% CI=2.12 to 26.28).
Figure 1.
Kaplan-Meier curves for composite. Kaplan-Meier curves with 95% CIs for patients grouped by the number of positive ageing-related biomarkers (theoretical range: 0–3, N=256).
Figure 2.
Kaplan-Meier curve for the entire cohort. Kaplan-Meier curve with 95% CI for the entire cohort.
Table 3.
Summary of cox-regression model using the composite predictor (number of positive biomarkers, theoretical range 0–3, N=256)
| Variable | Hazard ratio | 95% CI |
| Biomarker composite | ||
| None (n=60) | Ref | Ref |
| One (n=63) | 1.54 | (0.39 to 6.04) |
| Two (n=79) | 4.29 | (1.25 to 14.75) |
| Three (n=54) | 7.46 | (2.12 to 26.28) |
| Sex | ||
| Female | Ref | Ref |
| Male | 1.11 | (0.64 to 1.92) |
| BMI | 0.94 | (0.90 to 1.00) |
| CACI | 1.32 | (1.16 to 1.51) |
| Creatinine clearance | 1.00 | (0.98 to 1.01) |
Note: Creatinine clearance estimation as calculated by Cockroft-Gault formula. Biomarker composite describes the number of biomarkers with a positive result.
BMI, body mass index; CACI, Charlson Age Comorbidity Index; Ref, reference category.
Discussion
This exploratory, prospective cohort study examined the association of the three biomarkers CRP, hs-TnT and Hb with mortality in order to improve older patient’s risk stratification in the ED. The main results of this study can be summarised as follows. First, each marker alone was associated with an increased risk of all-cause mortality during more than 2-year follow-up. Second, these biomarkers were to a certain degree independently associated to patient’s all-cause mortality. Third, the number of positive results on age-related biomarkers was strongly indicative of the risk of patient’s all-cause mortality.
As geriatric patients in the ED represent a complex clientele,2 the quick identification of those with high-risk of adverse outcomes constitutes a considerable challenge. There are few studies which have examined risk stratification of ED patients using a biological ageing markers framework, especially from a dedicated geriatric perspective. As Bahrmann et al25 similar to Wagner et al3 argue on the issue of this approach, combining such biomarkers reflecting different pathophysiological pathways might be better than those which have the same general mechanism.26 Therefore, and based on theoretical considerations such as inflammaging and existent previous research tying CRP, hs-TnT and Hb to biological ageing processes across different pathways, we aimed to provide a joint examination of those biomarkers in terms of their predictive validity regarding the mortality of a heterogeneous sample of older ED patients, including both, frail and well performing older adults.
As we found in our study CRP, hs-TnT and Hb were individually all associated with older ED patient’s all-cause mortality, CRP and hs-TnT even when controlled for major confounders such as comorbidity, age, creatinine clearance, sex, and BMI. Our study therefore largely conforms with previous research, which illustrated an association for each marker to mortality.
For instance, Bahrmann et al showed that older patients presenting to the ED with hs-TnT levels of 108 ng/L or higher had a continuous increased risk of cardiovascular death.27 It should be considered that elevated serum troponin concentrations can be caused by any type of heart muscle cell injury, affected by a multitude of factors. Aside from myocardial infarction reasons for an increase can be due to other cardiac as well as non-cardiac diseases, such as for example myocarditis, arrhythmias, sepsis, renal failure, severe acute neurological diseases and critical illness.28 A recent study by Zelis et al also estimated the predictive value of several biomarkers, including hs-TnT, for adverse outcomes in older ED patients. In line with our results, hs-TnT showed one of the strongest associations to mortality.29
CRP as an inflammatory marker was shown to be an independent predictor of overall, cardiovascular and inhospital mortality, as studies by Zimmermann et al5 as well as by Yoshinaga et al have indicated.30 These findings support the theory of inflammaging. The ageing process leads to a paradoxically hyperactivated but at the same time defective immune system. Depending on which of these dominates, inflammatory markers could help to distinguish between healthy ageing and age-associated pathologies or the occurrence of comorbidities.31
Moreover, Han et al recently examined apparently healthy older men and could show the association of decreased levels of Hb with overall and cancer-related mortality.13 Other studies have also linked lowered Hb to mortality.12 32
In our study, we were able to show that positive results on the presumably independent biomarkers CRP, hs-TnT and Hb were indicative of the older ED patient’s risk of all-cause mortality during the follow-up period, even when their effects were controlled for each other. This is in line with the assumption that they exert effects at least in part across their own respective pathways.5 13 33 We could also show that a strong association of the number of positive biomarkers to mortality existed, even when major confounders were controlled for. These results support the idea that risk stratification may especially benefit from the incorporation of a multitude of biological age-related markers that exert their effects over different physiological pathways.26 Our exploratory approach to examine the association of the number of positive results to mortality implies equal weighting of each positive result. However, effect sizes for the separate biomarkers indicate that the relationship might be stronger for selected markers (in our case, hs-TnT). This should be considered in future work, which may build on large multicentre datasets that would resolve the issue of the high uncertainty associated with the estimates and provide the opportunity for construction of clinical risk scores involving these biomarkers.
In this context, it should be considered that this study has an exploratory, hypothesis-generating character. Wagner et al noted there are many theories trying to explain the ageing process but as of yet it is not fully understood,3 though the three biomarkers focused on this study are assumed to be strong candidates for an involvement. However, their elevation or decrease could also be caused by underlying diseases such as of the cardiovascular, oncological or infectious type. As has been noted previously, despite many efforts it remains difficult to identify ‘pure’ biomarkers of ageing due to their overlap with disease markers.34 Hence, it is important to rule out such underlying diseases by using further diagnostic tools. For this purpose, it would be meaningful to expand on the results of this research by examining the biomarkers in a large cohort, where precise health data are available. This health data should be based on a comprehensive health assessment, and include several indicators for latent diseases which may not have yet manifested in a diagnosis.35
Practically one could argue that in case of conspicuous values of the biomarkers an increased risk can be assumed and further diagnostic tools are needed. Therefore, the biomarkers can serve as a good adjunct in risk stratification but they cannot replace further diagnostic measures. As Madhavan et al stated, a biomarker elevation should be considered in clinical context rather than in isolation.36 Still, as numerous studies showed conspicuous values of the used biomarkers reflect an increased risk of adverse outcomes in line with our results,4 13 25 one could argue that regardless of whether the elevations are caused by underlying diseases or by the ageing process, they should be taken into account.
Previous approaches to risk stratification of older patients in the ED frequently employed questionnaire-based methods (eg, Identification of Seniors at Risk37). Although there is quite a body of research on these approaches, they were found to be limited in their predictive validity.38 39 In comparison, the biological age-related biomarkers showed relatively solid associations to the patient’s all-cause mortality in this and numerous other studies.7 13 30 Concerning this matter, the cohort presented in this study was previously examined with regards to the usefulness of a cognitive screening method for the patient’s risk stratification. Even when the timeframe of the current study was restrained to 1 year after initial ED visit, we found the association for biomarkers of ageing to mortality to be considerably stronger than the association of the cognitive measure to mortality.40
Thus, incorporation of biomarkers into the risk stratification of older patients in the ED appears to be a convenient, feasible and low-cost approach, as blood collection is already implemented into the diagnostic routines. In general, the assessment of biomarkers of age can also be considered a much more objective approach than (self-) report-based measures.
Limitations
Several limitations of this study must be taken into account. Due to the study being a prospective cohort study with exploratory character, we refrain from causal inferences. While a cohort of older ED patients is difficult to recruit, and the follow-up time in this study was considerably long, this cohort only constitutes a relatively small sample. This rather small sample size, and consequently the low number of overall events, prevented us from examining in more detail the specific interactions of the separate biomarkers. The small number of events also causes the effects found in this study to come with considerable uncertainty, as indicated by the wide CIs. For this reason, weighting approaches required to construct clinical risk scores for application in clinical practice were not feasible. In this context, one could argue that this study has due to the low number of cases more of a pilot character. Also, strong conclusions about whether the values of these biomarkers definitely reflect the biological ageing process or whether the effects are attributable to underlying diseases is not possible, though we made a strong effort to control for possible confounders available in our data. The recruitment of the study sample was also limited to one specific CPU and is therefore as a convenience sample not representative of the more heterogeneous patient populations present in the hospital. The CPU ward relates, among other things, to several specific health problems, associated treatment approaches and reasons of hospital confinement. The preconditioned informed consent procedure also constitutes a selection effect. Finally, while we did not use age-specific cut-offs for hs-TnT as their use is discouraged for convenience in the guideline of the European Society of Cardiology,41 age-related variation of 99th percentile values might provide useful information in risk stratification processes and should be examined in further research.
Conclusions
As the results in this study show, biomarkers with an explicit relation to biological ageing processes such as CRP, hs-TnT and Hb were strongly associated to older ED patient’s all-cause mortality. Therefore, they should be considered as potential candidates to supplement or replace risk stratification approaches based on for example, questionnaires, especially since, unlike questionnaire-related methods, some of these biomarkers are already implemented into the routine diagnostic process within the ED. Given their other properties such as a clear clinical interpretation and objectivity, they could become part of low-cost, feasible risk stratification methods. Future research should address the validation of risk stratification approaches based on biological ageing biomarkers in large, multicentre and across the clinical characteristic diverse samples, which would also allow for a deeper examination of the interactions of the different aging-related biomarkers than it was possible in this small study with pilot character.
Supplementary Material
Footnotes
Contributors: Study concept and design: ALK, AS, H-WW, EG, HAK and AB. Acquisition of data: AS and ALK. Analysis and interpretation of data: AS, ALK, PB and AB. Drafting of the manuscript: ALK, AS, PB, EG, H-WW, HAK, NF and AB. Critical revision of the manuscript for important intellectual content: ALK, AS, PB, EG, H-WW, HAK, NF and AB. Guarantor: AB.
Funding: A.S. is a member of the interdisciplinary Graduate Program “People with Dementia in General Hospitals”, located at the Network Aging Research (NAR), Heidelberg University, Germany, and received a Doctoral Fellowship funded by the Robert Bosch Foundation, Stuttgart, Germany. For the publication fee we acknowledge financial support by Deutsche Forschungsgemeinschaft within the funding programme „Open Access Publikationskosten“ as well as by Heidelberg University.
Competing interests: ALK: no funds, grants or other support to report. AS, MSc: has received research grants from the Robert Bosch Foundation. PB, MD, MHBA: has received no funds, grants or other support for this manuscript. EG, MD: has received research grants and honoraria from Roche Diagnostics, Bayer and Mitsubishi Chemicals. H-WW, PhD: no funds, grants or other support to report. HAK, MD: has received honoraria from AstraZeneca, Daiichi Sankyo, Boehringer Ingelheim, Berlin-Chemie, Bayer Vital and Novo Nordisk. He developed the cTnT assay, but the troponin patent has expired. NF, MD: has received no funds, grants or other support to report. AB, MD: has received research support for her other project the Trade Study (Transport and Delirium in Elderly Study) from the Innovation Committee by the Joint Federal Committee (G-BA), but not for this study. AB has received speaking engagements from Bayer, Pfizer, Lilly, Novartis, Boehringer Ingelheim, Daiichi Sankyo, Novo Nordisk and Sanofi Aventis.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
Ethics approval
This study was approved by Ethics Committee of the medical faculty at the University of Heidelberg reference number of the approval: (S-455/2016). Participants gave informed consent to participate in the study before taking part.
References
- 1.Aminzadeh F DWB. Older adults in the emergency department: a systematic review of patterns of use, adverse outcomes, and effectiveness of interventions; 2002. [Accessed 02 Nov 2019]. [DOI] [PubMed]
- 2.Salvi F, Morichi V, Grilli A, et al. The elderly in the emergency department: a critical review of problems and solutions. Intern Emerg Med 2007;2:292–301. 10.1007/s11739-007-0081-3 [DOI] [PubMed] [Google Scholar]
- 3.Wagner K-H, Cameron-Smith D, Wessner B, et al. Biomarkers of aging: from function to molecular biology. Nutrients 2016;8. 10.3390/nu8060338. [Epub ahead of print: 02 Jun 2016]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Martinis M, Franceschi C, Monti D, et al. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol 2006;80:219–27. 10.1016/j.yexmp.2005.11.004 [DOI] [PubMed] [Google Scholar]
- 5.Zimmermann J, Herrlinger S, Pruy A, et al. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients, 1999. Available: https://www.sciencedirect.com/science/article/pii/S0085253815460090 [Accessed 13 Oct 2019]. [DOI] [PubMed]
- 6.Ridker PM, Cushman M, Stampfer MJ, et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. NEJM 2009. 10.1056/NEJM199704033361401 [DOI] [PubMed] [Google Scholar]
- 7.Meigher S, Thode HC, Peacock WF, et al. Causes of elevated cardiac troponins in the emergency department and their associated mortality. Acad Emerg Med 2016;23:1267–73. 10.1111/acem.13033 [DOI] [PubMed] [Google Scholar]
- 8.Bardaji A, Cediel G, Carrasquer A, et al. Troponin elevation in patients without acute coronary syndrome, 2015. Available: https://www.sciencedirect.com/science/article/pii/S188558571500033X [Accessed 13 Oct 2019]. [DOI] [PubMed]
- 9.de Groot B, Verdoorn RCW, Lameijer J, et al. High-sensitivity cardiac troponin T is an independent predictor of inhospital mortality in emergency department patients with suspected infection: a prospective observational derivation study. Emerg Med J 2014;31:882–8. 10.1136/emermed-2013-202865 [DOI] [PubMed] [Google Scholar]
- 10.deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA 2010;304:2494–502. 10.1001/jama.2010.1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaura A, Panoulas V, Glampson B, et al. Association of troponin level and age with mortality in 250 000 patients: cohort study across five UK acute care centres. BMJ 2019;367:l6055. 10.1136/bmj.l6055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Culleton BF, Manns BJ, Zhang J, et al. Impact of anemia on hospitalization and mortality in older adults. Blood 2006;107:3841–6. 10.1182/blood-2005-10-4308 [DOI] [PubMed] [Google Scholar]
- 13.Han SV, Park M, Kwon Y-M, et al. Mild anemia and risk for all-cause, cardiovascular and cancer deaths in apparently healthy elderly Koreans. Korean J Fam Med 2019;40:151–8. 10.4082/kjfm.17.0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.den Elzen WPJ, Willems JM, Westendorp RGJ, et al. Effect of anemia and comorbidity on functional status and mortality in old age: results from the Leiden 85-plus study. CMAJ 2009;181:151–7. 10.1503/cmaj.090040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc 1975;23:433–41. 10.1111/j.1532-5415.1975.tb00927.x [DOI] [PubMed] [Google Scholar]
- 16.Luppa PB, Steimer W. Referenzwerte der wichtigsten Laborparameter, 2020. Available: https://link.springer.com/chapter/10.1007/978-3-662-54507-2_159#citeas [Accessed 11 Jun 2020].
- 17.Morrow DA, Rifai N, Antman EM, et al. C-reactive protein is a potent predictor of mortality independently of and in combination with troponin T in acute coronary syndromes: a TIMI 11A substudy. thrombolysis in myocardial infarction. J Am Coll Cardiol 1998;31:1460–5. 10.1016/S0735-1097(98)00136-3 [DOI] [PubMed] [Google Scholar]
- 18.Rifai N, Ridker PM. Population distributions of C-reactive protein in apparently healthy men and women in the United States: implication for clinical interpretation. Clin Chem 2003;49:666–9. 10.1373/49.4.666 [DOI] [PubMed] [Google Scholar]
- 19.Giannitsis E, Kurz K, Hallermayer K, et al. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem 2010;56:254–61. 10.1373/clinchem.2009.132654 [DOI] [PubMed] [Google Scholar]
- 20.Mueller-Hennessen M, Lindahl B, Giannitsis E, et al. Diagnostic and prognostic implications using age- and gender-specific cut-offs for high-sensitivity cardiac troponin T - Sub-analysis from the TRAPID-AMI study. Int J Cardiol 2016;209:26–33. 10.1016/j.ijcard.2016.01.213 [DOI] [PubMed] [Google Scholar]
- 21.Riva E, Tettamanti M, Mosconi P, et al. Association of mild anemia with hospitalization and mortality in the elderly: the health and anemia population-based study. Haematologica 2009;94:22–8. 10.3324/haematol.13449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weltgesundheitsorganisation . Research into environmental pollution: report of five WHO scientific groups. Geneva: World Health Organization, 1968. [PubMed] [Google Scholar]
- 23.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245–51. 10.1016/0895-4356(94)90129-5 [DOI] [PubMed] [Google Scholar]
- 24.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31–41. 10.1159/000180580 [DOI] [PubMed] [Google Scholar]
- 25.Bahrmann P, Bahrmann A, Hofner B, et al. Multiple biomarker strategy for improved diagnosis of acute heart failure in older patients presenting to the emergency department. Eur Heart J Acute Cardiovasc Care 2015;4:137–47. 10.1177/2048872614541904 [DOI] [PubMed] [Google Scholar]
- 26.Manzano L, Escobar C, Cleland JGF, et al. Diagnosis of elderly patients with heart failure. Eur J Heart Fail 2012;14:1097–103. 10.1093/eurjhf/hfs109 [DOI] [PubMed] [Google Scholar]
- 27.Bahrmann P, Christ M, Bahrmann A, et al. A 3-hour diagnostic algorithm for non-ST-elevation myocardial infarction using high-sensitivity cardiac troponin T in unselected older patients presenting to the emergency department. J Am Med Dir Assoc 2013;14:409–16. 10.1016/j.jamda.2012.12.005 [DOI] [PubMed] [Google Scholar]
- 28.Suzuki K, Komukai K, Nakata K, et al. The usefulness and limitations of point-of-care cardiac troponin measurement in the emergency department. Intern Med 2018;57:1673–80. 10.2169/internalmedicine.0098-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zelis N, Hundscheid R, Buijs J, et al. Value of biomarkers in predicting mortality in older medical emergency department patients: a Dutch prospective study. BMJ Open 2021;11:e042989. 10.1136/bmjopen-2020-042989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshinaga R, Doi Y, Ayukawa K, et al. High-sensitivity C reactive protein as a predictor of inhospital mortality in patients with cardiovascular disease at an emergency department: a retrospective cohort study. BMJ Open 2017;7:e015112. 10.1136/bmjopen-2016-015112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larbi A, Franceschi C, Mazzatti D, et al. Aging of the immune system as a prognostic factor for human longevity. Physiology 2008;23:64–74. 10.1152/physiol.00040.2007 [DOI] [PubMed] [Google Scholar]
- 32.Zakai NA, Katz R, Hirsch C, et al. A prospective study of anemia status, hemoglobin concentration, and mortality in an elderly cohort: the cardiovascular health study. Arch Intern Med 2005;165:2214–20. 10.1001/archinte.165.19.2214 [DOI] [PubMed] [Google Scholar]
- 33.Bahrmann P, Bahrmann A, Breithardt O-A, et al. Additional diagnostic and prognostic value of copeptin ultra-sensitive for diagnosis of non-ST-elevation myocardial infarction in older patients presenting to the emergency department. Clin Chem Lab Med 2013;51:1307–19. 10.1515/cclm-2012-0401 [DOI] [PubMed] [Google Scholar]
- 34.American Federation for Aging Research . Biomarkers of aging, 2016. Available: https://www.afar.org/imported/AFAR_BIOMARKERS_OF_AGING_2016.pdf
- 35.Häseler-Ouart K, Arefian H, Hartmann M, et al. Geriatric assessment for older adults admitted to the emergency department: a systematic review and meta-analysis. Exp Gerontol 2021;144:111184. 10.1016/j.exger.2020.111184 [DOI] [PubMed] [Google Scholar]
- 36.Madhavan MV, Gersh BJ, Alexander KP, et al. Coronary artery disease in patients ≥80 Years of Age. J Am Coll Cardiol 2018;71:2015–40. 10.1016/j.jacc.2017.12.068 [DOI] [PubMed] [Google Scholar]
- 37.McCusker J, Bellavance F, Cardin S, et al. Detection of older people at increased risk of adverse health outcomes after an emergency visit: the ISAR screening tool. J Am Geriatr Soc 1999;47:1229–37. 10.1111/j.1532-5415.1999.tb05204.x [DOI] [PubMed] [Google Scholar]
- 38.Galvin R, Gilleit Y, Wallace E, et al. Adverse outcomes in older adults attending emergency departments: a systematic review and meta-analysis of the identification of seniors at risk (ISAR) screening tool. Age Ageing 2017;46:179–86. 10.1093/ageing/afw233 [DOI] [PubMed] [Google Scholar]
- 39.Carpenter CR, Shelton E, Fowler S, et al. Risk factors and screening instruments to predict adverse outcomes for undifferentiated older emergency department patients: a systematic review and meta-analysis. Acad Emerg Med 2015;22:1–21. 10.1111/acem.12569 [DOI] [PubMed] [Google Scholar]
- 40.Schönstein A, Wahl H-W, Katus HA, et al. SPMSQ zur Risikostratifizierung älterer Patienten in der Notaufnahme Eine explorative prospektive Kohortenstudie. Z Gerontol Geriatr 2019;52:222–8. 10.1007/s00391-019-01626-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collet J-P, Thiele H, Barbato E. The 'Ten Commandments' for the 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2020;41:3495–7. 10.1093/eurheartj/ehaa624 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-056674supp001.pdf (59KB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.