Abstract
Objective
Pulmonary infarction is a common clinical and radiographic finding in acute pulmonary embolism (PE), yet the clinical relevance and prognostic significance of pulmonary infarction remain unclear. The study aims to investigate the clinical features, radiographic characteristics, impact of reperfusion therapy and outcomes of patients with pulmonary infarction.
Design, setting and participants
A retrospective cohort study of 496 adult patients (≥18 years of age) diagnosed with PE who were evaluated by the PE response team at a tertiary academic referral centre in the USA. We collected baseline characteristics, laboratory, radiographic and outcome data. Statistical analysis was performed by Student’s t-test, Mann-Whitney U test, Fischer’s exact or χ2 test where appropriate. Multivariate logistic regression was used to evaluate potential risk factors for pulmonary infarction.
Results
We identified 143 (29%) cases of pulmonary infarction in 496 patients with PE. Patients with infarction were significantly younger (52±15.9 vs 61±16.6 years, p<0.001) and with fewer comorbidities. Most infarctions occurred in the lower lobes (60%) and involved a single lobe (64%). The presence of right ventricular (RV) strain on CT imaging was significantly more common in patients with infarction (21% vs 14%, p=0.031). There was no significant difference in advanced reperfusion therapy, in-hospital mortality, length of stay and readmissions between groups. In multivariate analysis, age and evidence of RV strain on CT and haemoptysis increased the risk of infarction.
Conclusions
Radiographic evidence of pulmonary infarction was demonstrated in nearly one-third of patients with acute PE. There was no difference in the rate of reperfusion therapies and the presence of infarction did not correlate with poorer outcomes.
Keywords: thromboembolism, respiratory medicine (see thoracic medicine), thoracic medicine
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study represents the largest cohort describing the clinical characteristics and outcomes of patients with pulmonary infarction.
This study highlights the potential role of pulmonary infarction in the risk stratification of acute pulmonary embolism.
Cases of pulmonary infarction were identified based on CT findings and there was no histological correlation to differentiate between true necrosis (infarction) versus alveolar haemorrhage.
Not all patients underwent CT imaging postdischarge and thus we are unable to comment on the precise timing of the resolution of infarction and its long-term significance.
Introduction
Acute pulmonary embolism (PE) is the third-leading cause of cardiovascular death following myocardial infarction and stroke, with approximately 100 000 annual deaths in the USA.1 2 Pulmonary infarction is a common complication of acute PE with a reported radiographic prevalence of up to 36%.3 The dual blood supply of lungs has been thought to be protective against ischaemic insults, with the bronchial circulation and other collateral vessels undergoing hypertrophy or remodelling to maintain blood flow to ischaemic lung tissues through anastomoses at the level of alveoli and respiratory bronchioles.4 5 Nevertheless, obstruction of the pulmonary artery by acute PE can cause infarction. In the past, the presence of infarction has been regarded as a sign of poor outcomes due to its association with compromised cardiac function, which leads to increased pulmonary venous pressure and impairment of forwarding flow through the bronchial circulation.6 7 Contrarily, recent studies have suggested that younger, healthier patients are at the highest risk for infarction, because of their less robust collateral blood supply.8 9 The clinical relevance and prognostic significance of pulmonary infarction remain unclear. The aim of this study is to investigate the clinical features, radiographic characteristics, impact of reperfusion therapy and outcomes of patients with pulmonary infarction.
Methods
Study population
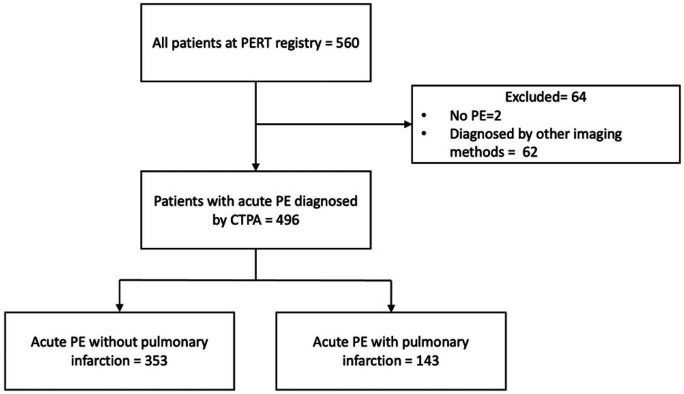
We conducted a single-centre retrospective review of all patients between January 2017 and June 2020 at Temple University Hospital who underwent evaluation by the PE response team (PERT) and included all cases of acute PE diagnosed by CT pulmonary angiography (CTPA). Cases of acute PE diagnosed by other imaging modalities were excluded from the study. Cases of pulmonary infarction were identified by review of the final CT reports by board-certified thoracic radiologists. Figure 1 demonstrates patient selection.
Figure 1.
Patient selection flow diagram. CTPA, CT pulmonary angiography; PERT, pulmonary embolism response team.
Patient and public involvement
Patients or the public were not involved in the design, recruitment, conduct, reporting or dissemination of our research.
Data collection
Clinical data
Patient demographic, clinical features, laboratory data, echocardiographic data, radiographic characteristics and patient outcomes were extracted from electronic medical records. Demographics included age, gender, race and body mass index. Clinical features included symptoms at presentation, comorbid conditions, calculated simplified Pulmonary Embolism Severity Index (sPESI) and PE severity per the European Society of Cardiology guideline at the time of diagnosis (ie, low, intermediate-low, intermediate-high and high risk).10 Laboratory data included B-type natriuretic peptide (BNP; positive if ≥100 pg/mL) and troponin I (positive if ≥0.1 ng/mL). Data regarding treatment modalities, length of stay, in-hospital mortality, readmission within 30 days, new oxygen requirement on discharge, complications including major and minor bleeding using the International Society on Thrombosis and Haemostasis criteria,11 need for transfusion, access site haematoma and pulmonary follow-up were recorded.
Radiographic data
All CTPAs performed at the time of acute PE diagnosis were retrieved. The diagnosis of infarction was based on generally accepted criteria with the presence of a peripheral wedge-shaped consolidation within the region of an obstructed vessel, with or without the presence of the other suggestive findings including: (1) central lucency; (2) vessel signs and (3) air bronchogram.12 In case of discrepancies, a final decision was reached by consensus. A central PE was defined as the presence of thrombus in the main trunk of the pulmonary artery (PA) or the left or the right main PA. A peripheral PE was defined as thrombus in the lobar, segmental or subsegmental PA. Signs of right ventricular (RV) strain were defined as the presence of one, or a combination of the following signs: (1) right-to-left ventricular ratio >0.9; (2) pulmonary artery enlargement; (3) abnormal interventricular septum (flattening of septum or leftward septal bowing) and (4) inferior vena caval contrast reflux. Parameters including PE distribution (central vs distal), infarct location and burden, signs of RV strain and other parenchymal abnormalities were collected. All available follow-up CT chest imaging up to 1-year postdischarge were reviewed by board-certified thoracic radiologists to determine the resolution of infarctions or residual abnormalities on available scans. All echocardiograms were reviewed, and data regarding left ventricular ejection fraction, RV dilation and RV dysfunction were collected.
Statistical analysis
All continuous variables were tested for normality and presented as mean with an SD, or median with an IQR if distribution was skewed. Categorical variables were presented as absolute number (percentage). Comparisons between patients with and without infarction were performed using Student’s t-test or Mann-Whitney U for continuous variables or Fischer’s exact or χ2 test for categorical variables, as appropriate. Univariable and multivariable logistic regression models were used to evaluate risk factors associated with pulmonary infarction. The software SPSS Statistics for Mac, V.26.0 (IBM) was used for statistical analysis. P values of <0.05 (two-sided) were considered statistically significant.
Results
Patient characteristics
Out of 560 patients in the PERT registry, 62 cases were excluded due to diagnosis by other imaging modalities and 2 cases were excluded after diagnosis of PE was ruled out. Hence, 496 patients were included in the study with a mean age of 58 years and 48% were female. Twenty-nine per cent (143 of 496) of patients had evidence of pulmonary infarction on CTPA. Patients with pulmonary infarction were younger (52±15.9 vs 61±16.6 years, p<0.001) and with a significantly lower prevalence of comorbidities including cardiac disease (30% vs 42%, p=0.014), chronic kidney disease (CKD) (5% vs 42%, p=0.018), diabetes mellitus (17% vs 26%, p=0.032), hypothyroidism (4% vs 9%, p=0.039) and malignancy (16% vs 25%, p=0.032). Although results did not reach statistical significance, chronic obstructive pulmonary disease was less prevalent in the infarction group. Patients with infarction were more likely to present with pleuritic chest pain (32% vs 20%, p=0.004) and haemoptysis (8% vs 3%, p=0.04), while patients without infarction were more likely to present with syncope (6% vs 11%, p=0.05). The baseline characteristics and symptoms of presentation of the study population are reported in table 1.
Table 1.
Baseline characteristics and symptoms of presentation
| All (496) | Without infarction (353) | With infarction (143) | P value | |
| Gender (female) | 238 (48%) | 168 (48%) | 70 (49%) | 0.784 |
| Age, mean (±SD) | 58±16.9 | 61±16.6 | 52±15.9 | <0.001 |
| Race, n (%) | 0.017 | |||
| Black | 249 (50) | 168 (48) | 81 (57) | |
| White | 98 (20) | 81 (23) | 17 (12) | |
| Other | 149 (30) | 104 (30) | 45 (32) | |
| BMI, mean (±SD) | 31.8±9.3 | 31.8±9.5 | 32.0±8.7 | 0.421 |
| Symptoms at presentation, n (%) | ||||
| Dyspnoea | 297 (60) | 207 (59) | 90 (63) | 0.376 |
| Hypoxia | 237 (49) | 173 (50) | 64 (46) | 0.371 |
| Pleuritic chest pain | 114 (23) | 69 (20) | 45 (32) | 0.004 |
| DVT symptoms | 50 (10) | 40 (11) | 10 (7) | 0.146 |
| Syncope | 48 (10) | 40 (11) | 8 (6) | 0.05 |
| Haemoptysis | 21 (4) | 9 (3) | 12 (8) | 0.04 |
| Altered mental status | 38 (8) | 28 (8) | 10 (7) | 0.722 |
| Cardiac arrest | 22 (4) | 15 (4) | 7 (5) | 0.752 |
| Comorbidities, n (%) | ||||
| Cardiac diseases | 191 (39) | 148 (42) | 43 (30) | 0.014 |
| COPD/asthma | 71 (14) | 54 (15) | 17 (12) | 0.326 |
| CKD | 49 (10) | 42 (12) | 7 (5) | 0.018 |
| Diabetes mellitus | 115 (23) | 91 (26) | 24 (17) | 0.032 |
| Hypothyroidism | 36 (7) | 31 (9) | 5 (4) | 0.039 |
| Malignancy | 111 (22) | 88 (25) | 23 (16) | 0.032 |
| Recent surgery | 68 (14) | 52 (15) | 16 (11) | 0.299 |
| Current anticoagulation | 59 (12) | 41 (12) | 18 (13) | 0.762 |
BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DVT, deep vein thrombosis; n, number; SD, standard deviation.
Risk stratification of PE
Troponin elevation (>0.1 ng/mL) was less frequently observed in patients with infarction (39% vs 49%, p=0.031). The presence of RV strain on CT was significantly higher in patients with infarction (58% vs 45%, p=0.009), although no differences in signs of RV dilation or dysfunction on echocardiogram between infarction and non-infarction group were noted. There was no significant difference in sPESI risk group, BNP elevation, PE severity or evidence of RV dysfunction on electrocardiography and echocardiogram between groups. Table 2 describes factors associated with PE severity.
Table 2.
PE severity indices
| All (496) | Without infarction (353) | With infarction (143) | P value | |
| sPESI score | 0.108 | |||
| Low risk (0 points) | 89 (21%) | 57 (19%) | 32 (26%) | |
| High risk (≥1 points) | 327 (79%) | 238 (81%) | 89 (74%) | |
| Elevated BNP | 242 (54%) | 169 (53%) | 73 (55%) | 0.629 |
| Elevated troponin | 228 (46%) | 173 (49%) | 55 (39%) | 0.031 |
| Evidence of RV strain on ECG | 77 (16%) | 48 (14%) | 29 (21%) | 0.137 |
| PE severity | ||||
| Low risk | 132 (27%) | 93 (26%) | 39 (27%) | 0.994 |
| Intermediate low risk | 173 (35%) | 124 (35%) | 49 (34%) | |
| Intermediate high risk | 144 (29%) | 103 (29%) | 41 (29%) | |
| High risk | 47 (10%) | 33 (9%) | 14 (10%) | |
| ECG evidence of RV strain | 77 (16%) | 48 (14%) | 29 (21%) | 0.137 |
| CT evidence of RV strain | 242 (49%) | 159 (45%) | 83 (58%) | 0.009 |
| Echocardiogram | ||||
| RV dilation | 251 (54%) | 173 (52%) | 78 (57%) | 0.302 |
| RV dysfunction | 235 (50%) | 162 (49%) | 73 (54%) | 0.338 |
| LVEF | 38 (8%) | 23 (7%) | 15 (11%) | 0.306 |
| LE DVT | 227 (50%) | 161 (51%) | 66 (50%) | 0.928 |
| UE DVT | 39 (22%) | 27 (22%) | 12 (21%) | 0.916 |
BNP, B type natriuretic peptide; DVT, deep vein thrombosis; ECG, electrocardiography; LE, lower extremity; LVEF, left ventricular ejection fraction; PE, pulmonary embolism; RV, right ventricular; sPESI, simplified Pulmonary Embolism Severity Index; UE, upper extremity.
Radiographic characteristics
There was no difference in PE distribution and clot burden between groups and 50% of patients with infarction had thrombus located at the main PAs including those who had saddle PE. Most infarctions occurred in the lower lobe (60%) and involved a single lobe (64%). Patients with infarction were more likely to have parenchymal abnormalities including consolidation (21% vs 12%, p=0.007), pulmonary oedema (13% vs 7%, p=0.036), pleural effusion (33% vs 24%, p=0.038) and ground glass opacity (20% vs 13%, p=0.042). Table 3 demonstrates the radiographic characteristics of patients with acute PE. Table 4 demonstrates the radiographic characteristics of pulmonary infarction. Fifty-eight (41%) patients had follow-up CT-chest imaging for a variety of reasons performed up to 1-year postdischarge. Resolution of pulmonary infarction was observed in 53% (17 out of 32), 85% (12 out of 14), and 92% (11 out of 12) of patients who had imaging at 3-month, 6-month and 1-year intervals.
Table 3.
Radiographic characteristics of patients with acute pulmonary embolism
| All (496) | Without infarction (353) | With infarction (143) | P value | |
| RV strain on CT | 242 (49%) | 159 (45%) | 83 (58%) | 0.009 |
| RV to LV ratio ≥1 | 155 (31%) | 106 (30%) | 49 (34%) | 0.64 |
| Dilated PA | 99 (20%) | 59 (17%) | 40 (28%) | 0.011 |
| Septal position | 0.03 | |||
| Rightward bowing | 131 (27%) | 102 (29%) | 29 (20%) | |
| Flattened | 112 (23%) | 66 (19%) | 46 (32%) | |
| Leftward bowing | 50 (10%) | 32 (9%) | 18 (13%) | |
| PE distribution | 0.052 | |||
| Central PE | 216 (44%) | 144 (41%) | 72 (50%) | |
| Distal PE | 280 (57%) | 209 (59%) | 71 (50%) | |
| Clot burden | 0.2 | |||
| Main, saddle or proximal | 215 (43%) | 143 (41%) | 72 (50%) | |
| Interlobar to lobar | 124 (25%) | 94 (27%) | 30 (21%) | |
| Segmental | 150 (30%) | 110 (31%) | 40 (28%) | |
| Subsegmental | 7 (1%) | 6 (2%) | 1 (1%) | |
| Parenchymal abnormalities | ||||
| Pulmonary oedema | 42 (9%) | 24 (7%) | 18 (13%) | 0.036 |
| Consolidation | 71 (14%) | 41 (12%) | 30 (21%) | 0.007 |
| Emphysema | 65 (13%) | 48 (14%) | 16 (11%) | 0.469 |
| Fibrosis | 42 (9%) | 30 (9%) | 12 (8%) | 0.969 |
| Pleural effusion | 128 (26%) | 84 (24%) | 47 (33%) | 0.038 |
| Atelectasis | 161 (33%) | 113 (32%) | 48 (34%) | 0.738 |
| Ground glass opacity | 72 (15%) | 44 (13%) | 28 (20%) | 0.042 |
CT, computed tomography; LV, left ventricular; PA, pulmonary artery; PA, pulmonary artery; PE, pulmonary embolism; RV, right ventricular.
Table 4.
Radiographic characteristics of pulmonary infarction
| Infarcts, no (%) | |
| Infarct location | |
| Upper lobe | 31 (22) |
| Middle lobe | 8 (6) |
| Lower lobe | 86 (60) |
| Multiple | 18 (12) |
| Infarct burden | |
| Single lobe | 91 (64) |
| Multiple lobes | 52 (36) |
Treatment and outcomes
There was no significant difference in the number of patients receiving advanced reperfusion therapy between groups. Most patients with pulmonary infarction received anticoagulation alone (69%), followed by catheter-directed thrombolysis (12%) and systemic thrombolysis (11%). There were more patients with infarction who received antimicrobial therapy compared with those without infarction (15% vs 5%, p<0.001). Among the 47 patients who had concomitant infarction and pleural effusion, 6 patients (13%) underwent diagnostic and/or therapeutic thoracocentesis and five had an exudative pleural effusion. Patients with infarction were less likely to require oxygen on discharge (11% vs 19%, p=0.031), and those who required oxygen on discharge were more likely to have multiple lobe infarctions than single lobe infarction (19% vs 7%, p=0.032). There was no significant difference between patients with infarction and without infarction regarding length of stay (10.7±14.7 vs 9.5±12.3, p=0.698), in-hospital death (7% vs 8%, p=0.801), disposition (home: 78% vs 75%, p=0.759), bleeding complications (24% vs 12%, p=0.089) and readmission within 30 days (18% vs 16%, p=0.584) (online supplemental table 1).
bmjopen-2022-067579supp001.pdf (67.6KB, pdf)
Patients with infarction who underwent CDT had a longer length of stay (15±24.4 vs 7±8.4, p=0.044) and a higher rate of readmission within 30 days (18% vs 2%, p=0.047), compared with those without infarction. Readmission diagnoses of patients with infarction who underwent CDT were as follows: one patient developed vaginal bleeding from anticoagulant use, one patient developed acute hypoxic respiratory failure due to multifocal pneumonia, and one patient was admitted for chest pain. There was no significant difference in in-hospital mortality (6% vs 6%, p=0.973) and complications including minor and major bleeding (24% vs 21%, p=0.676), access site haematoma (13% vs 2%, p=0.134) and need for transfusion (18% vs 13%, p=0.649) between those with and without infarction (online supplemental table 2).
In univariable regression analyses, we identified several factors independently associated with pulmonary infarction. These included age, history of cardiac diseases, malignancy, hypothyroidism, diabetes mellitus, CKD, elevated troponin, pleuritic chest pain, haemoptysis and RV strain on CT. A multivariable regression analysis was subsequently performed, and four factors remained significant: Both haemoptysis (OR 3.034; 95% CI 1.162 to 7.924) and presence of RV strain on CT (OR 2.142; 95% CI 1.365 to 3.360) significantly increased the risk of infarction, while age (OR 0.973, 95% CI 0.959 to 0.987) and presence of elevated troponin (OR 0.629; 95% CI 0.398 to 0.993) decreased risk (table 5).
Table 5.
Univariate and multivariate analysis of potential risk factors
| Variables | Univariate analysis | Multivariate analysis | ||||||
| Without infarction (353) | With infarction (143) | OR | P value | 95% CI | OR | P value | 95% CI | |
| Age, years | 61±16.6 | 52±15.9 | 0.968 | <0.001 | 0.956 to 0.980 | 0.973 | <0.001 | 0.959 to 0.987 |
| Cardiac diseases | 148 (42%) | 43 (30%) | 0.596 | 0.014 | 0.393 to 0.902 | |||
| CKD | 42 (12%) | 7 (5%) | 0.381 | 0.022 | 0.167 to 0.870 | |||
| DM | 91 (26%) | 24 (17%) | 0.581 | 0.033 | 0.352 to 0.957 | |||
| Malignancy | 88 (25%) | 23 (16%) | 0.577 | 0.034 | 0.348 to 0.958 | |||
| Hypothyroidism | 31 (9%) | 5 (4%) | 0.375 | 0.047 | 0.143 to 0.985 | |||
| Elevated troponin | 173 (49%) | 55 (39%) | 0.647 | 0.032 | 0.435 to 0.962 | 0.629 | 0.047 | 0.398 to 0.993 |
| Pleuritic CP | 69 (20%) | 45 (32%) | 1.890 | 0.005 | 1.217 to 2.935 | |||
| Haemoptysis | 9 (3%) | 12 (8%) | 3.491 | 0.006 | 1.437 to 8.479 | 3.034 | 0.023 | 1.162 to 7.924 |
| RV strain on CT | 159 (45%) | 83 (58%) | 1.688 | 0.009 | 1.140 to 2.500 | 2.142 | <0.001 | 1.365 to 3.360 |
CKD, chronic kidney disease; CP, chest pain; CT, computed tomography; DM, diabetes mellites; RV, right ventricular.
Discussion
In this study, the estimated prevalence of pulmonary infarction was 29%. Patients with infarction were more likely to present with pleuritic chest pain and haemoptysis. Additionally, those with pulmonary infarction were younger and had a lower prevalence of comorbidities. While the presence of RV strain on CT imaging was more common in patients with pulmonary infarction, the rate of reperfusion therapies, complications and outcomes was similar in both groups. The presence of haemoptysis and RV strain on CT significantly increased the risk of infarction, whereas age and elevated troponin decreased the risk. Pulmonary infarction resolved in the majority of patients for whom follow-up imaging was available, which is in concordance with newer studies.8 9 13
The prevalence of pulmonary infarction in our cohort is in keeping with previously reported rates ranging from 9% to 36%.3 13 Regarding clinical presentation, the higher presence of pleuritic chest pain and haemoptysis in patients with infarction likely represents a result of alveolar haemorrhage, leading to pleural inflammation, irritation and necrosis. In addition, the presence of pleural effusion was more prevalent in patients with infarction due to pleural inflammation following infarction, however, most of these effusions were not intervened on and had resolved on follow-up imaging. Although patients with infarction were more likely to present with pleuritic chest pain and haemoptysis, these symptoms were only present in 32% and 8% of patients with infarction, respectively. Thus, the majority did not have these symptoms may not be useful in identifying pulmonary infarction. Interestingly, patients with pulmonary infarction were more likely to be treated with antimicrobial therapy than those without. One possible explanation is that other lung processes, such as pneumonia, pulmonary oedema or atelectasis can produce consolidative changes similar to infarction on CT imaging,14 and as a result, pneumonia cannot be excluded especially when combined with a clinically compatible presentation.
In our study, patients with infarction were significantly younger and with fewer comorbid conditions, specifically a lower prevalence of cardiac disease and malignancy that have traditionally been regarded as major risk factors for infarction. This discrepancy may be due to a lack of efficient collateral circulation to lung tissues, which presumably develops in the setting of longstanding local tissue hypoxia,8 15 and is unlikely to happen in otherwise healthy, young individuals in the absence of cardiopulmonary diseases.
Our cohort also found that patients with infarction did have higher rates of RV strain, dilated PA and flattened or leftward bowing of the interventricular septum, although signs of RV strain were not reproducible on echocardiography. A potential explanation for this finding is that patients with infarction were younger with less comorbid diseases, thus without pre-existing RV hypertrophy allowing for tolerance of acute RV afterload elevation. This may also explain our finding that troponin elevation was less likely to be observed in patients with infarction. While troponin elevation is indicative of RV strain, RV myocardium might not necessarily be its only source.16 A mismatch between oxygen demand and supply (demand ischaemia) and a decrease in renal clearance could contribute to the higher rates of troponin elevation observed in patients without infarction, as evidenced by their higher prevalence of comorbidities.
Regarding the discrepancy between CT and echocardiographic findings, unlike CTPA which is often the first diagnostic and confirmatory modality for PE, most echocardiography is performed after the initial diagnosis and potentially after reperfusion therapy. In our study, the mean and median time lapse between the availability of CT results to echocardiogram results was 29 hours 40 min and 20 hours 11 min, respectively. Thus, it is possible that the initial signs of RV strain shown on CTPA could have improved or resolved through several potential mechanisms; administration of supplemental oxygen leading to a reversal of hypoxic vasoconstriction, clot burden reduction using reperfusion therapy, or intrinsic thrombolytic activity with anticoagulation support alone.
Importantly, we demonstrated no differences in complication rates and in-hospital mortality in patients who underwent CDT with pulmonary infarction compared with those without, although patients with infarction had a longer length of stay and a higher rate of 30-day readmission for non-PE or CDT-related diagnoses. There remains a theoretical risk of increased bleeding complications in the area of pulmonary infarction during catheter manipulation and local installation of thrombolytics, however, this was not demonstrated in our cohort.
Study limitations
To the best of our knowledge, this study represented the largest cohort of patients with acute PE complicated by pulmonary infarction, however, there are several limitations in our study. Cases of pulmonary infarction were identified based on CT findings and there was no histological correlation to differentiate between true necrosis versus alveolar haemorrhage. Patients with conditions that led to pulmonary hypertension could have pre-existing parenchymal changes that resemble acute RV strain caused by PE. In those scenarios, it would be technically difficult to differentiate whether these CT features are related to pre-existing pulmonary hypertension itself, or the combination. Owing to the retrospective nature of this study, not all patients underwent CT imaging postdischarge, thus we were unable to comment on the precise timing of the resolution of pulmonary infarction. We acknowledge the fact that not all patients in our cohort had echocardiography, in particular, patients with low-risk PE when echocardiography was not deemed necessary to change management or patients with high-risk PE who expired prior to the completion of echocardiography. Other limitations included the retrospective, single-centre nature of the data, which may be prone to selection bias.
In conclusion, pulmonary infarction was demonstrated on CT in nearly one-third of acute PE, and patients with infarction were younger, with fewer comorbidities, and more likely to present with pleuritic chest pain and haemoptysis. Overall, there was no difference in length of stay, in-hospital death, bleeding complication and readmission rate between patients with and without infarction, and patients with infarction were less likely to require oxygen on discharge. The presence of pulmonary infarction did not correlate with poorer outcomes and should not be considered a supporting factor nor a contraindication for advanced reperfusion therapy for PE.
Supplementary Material
Footnotes
Contributors: KUL is the primary author, collected and analysed the data, and is the guarantor of the article, taking responsibility for the integrity of the work as a whole from inception to the published article. OO'C and PR are responsible for the study concept and helped write the manuscript. RB, GC, VL and JP reviewed the imaging studies and treated the patients who underwent catheter-directed thrombolysis. SB and BR-L helped write the manuscript.
Funding: Publication of this article was funded in part by the Temple University Libraries Open Access Publishing Fund.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Our study met approval by the Institutional Review Board (Protocol #26021) at Lewis Katz School of Medicine at Temple University and informed consent was waived due to the retrospective nature of the study. Informed consent was waived due to the retrospective nature of the study.
References
- 1.Barco S, Valerio L, Ageno W, et al. Age-sex specific pulmonary embolism-related mortality in the USA and Canada, 2000-18: an analysis of the who mortality database and of the CDC multiple cause of death database. Lancet Respir Med 2021;9:33–42. 10.1016/S2213-2600(20)30417-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raskob GE, Angchaisuksiri P, Blanco AN, et al. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol 2014;34:2363–71. 10.1161/ATVBAHA.114.304488 [DOI] [PubMed] [Google Scholar]
- 3.Heyer CM, Lemburg SP, Knoop H, et al. Multidetector-CT angiography in pulmonary embolism-can image parameters predict clinical outcome? Eur Radiol 2011;21:1928–37. 10.1007/s00330-011-2125-3 [DOI] [PubMed] [Google Scholar]
- 4.Osiro S, Wear C, Hudson R, et al. A Friend to the airways: a review of the emerging clinical importance of the bronchial arterial circulation. Surg Radiol Anat 2012;34:791–8. 10.1007/s00276-012-0974-3 [DOI] [PubMed] [Google Scholar]
- 5.Pump KK. The bronchial arteries and their anastomoses in the human lung. Dis Chest 1963;43:245–55. 10.1378/chest.43.3.245 [DOI] [PubMed] [Google Scholar]
- 6.Hampton AO, Castleman BL. Correlation of postmortem chest teleroentgenograms with autopsy findings with special reference to pulmonary embolism and infarction, 1940. [Google Scholar]
- 7.Tsao MS, Schraufnagel D, Wang NS. Pathogenesis of pulmonary infarction. Am J Med 1982;72:599–606. 10.1016/0002-9343(82)90458-2 [DOI] [PubMed] [Google Scholar]
- 8.Islam M, Filopei J, Frank M, et al. Pulmonary infarction secondary to pulmonary embolism: an evolving paradigm. Respirology 2018:866–72. 10.1111/resp.13299 [DOI] [PubMed] [Google Scholar]
- 9.Miniati M, Bottai M, Ciccotosto C, et al. Predictors of pulmonary infarction. Medicine 2015;94:e1488. 10.1097/MD.0000000000001488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European respiratory Society (ERS). Eur Heart J 2020;41:543–603. 10.1093/eurheartj/ehz405 [DOI] [PubMed] [Google Scholar]
- 11.Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692–4. 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 12.Revel M-P, Triki R, Chatellier G, et al. Is it possible to recognize pulmonary infarction on multisection CT images? Radiology 2007;244:875–82. 10.1148/radiol.2443060846 [DOI] [PubMed] [Google Scholar]
- 13.Cha S-I, Shin K-M, Lee J, et al. Clinical relevance of pulmonary infarction in patients with pulmonary embolism. Thromb Res 2012;130:e1–5. 10.1016/j.thromres.2012.03.012 [DOI] [PubMed] [Google Scholar]
- 14.Ren H, Kuhlman JE, Hruban RH, et al. CT of inflation-fixed lungs: wedge-shaped density and vascular sign in the diagnosis of infarction. J Comput Assist Tomogr 1990;14:82–6. [PubMed] [Google Scholar]
- 15.Malik AB, Tracy SE. Bronchovascular adjustments after pulmonary embolism. J Appl Physiol Respir Environ Exerc Physiol 1980;49:476–81. 10.1152/jappl.1980.49.3.476 [DOI] [PubMed] [Google Scholar]
- 16.Becattini C, Vedovati MC, Agnelli G. Prognostic value of troponins in acute pulmonary embolism: a meta-analysis. Circulation 2007;116:427–33. 10.1161/CIRCULATIONAHA.106.680421 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-067579supp001.pdf (67.6KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.