Abstract
Objective
A subanalysis of the multicentre Early Lupus inception cohort was performed to investigate the real-world Glucocorticoids (GCs) Use in newly diagnosed systemic lupus erythematosus (SLE) Patients (GULP).
Methods
Patients starting prednisone (PDN) ≥5 mg/day and concomitant hydroxychloroquine or immunosuppressant within 12 months of SLE classification were enrolled. Core set variables were recorded at baseline and every 6 months, including changes in PDN dose, European Consensus Lupus Activity Measurement (ECLAM) and Systemic Lupus International Collaborating Clinics damage index. Regression models analysed predictors of tapering PDN<5 mg/day at any time and outcomes associated with different patterns of GCs tapering.
Results
The GULP study included 127 patients with SLE; 73 (57.5%) tapered and maintained PDN <5 mg/day, and 17 (13.4%) discontinued PDN within a 2-year follow-up. Renal involvement (HR: 0.41; p=0.009) and lower C3 serum levels (HR: 1.04; p=0.025) predicted a lack of PDN tapering below 5 mg/day. High ECLAM scores were associated with a greater probability of increasing PDN dose (OR: 1.6; p=0.004), independently of daily intake. Disease relapse rate did not statistically differ (p=0.706) between patients tapering PDN <5 mg/day (42/99, 42.4%) and those tapering PDN without dropping below 5 mg/day (13/28, 46.4%). Every month on PDN <5 mg/day associated with lower damage accrual (IRR: 0.96; p=0.007), whereas never tapering PDN <5 mg/day associated with a higher risk of developing GC-related damage (OR 5.9; p=0.014).
Conclusion
Tapering PDN <5 mg/day was achieved and maintained in half of newly diagnosed patients with SLE and may represent a good balance between the need to prevent damage accrual and the risk of disease relapse.
Keywords: systemic lupus erythematosus, glucocorticoids, therapeutics
WHAT IS ALREADY KNOWN ON THIS TOPIC
Glucocorticoids (GCs) are effective in patients with systemic lupus erythematosus (SLE) but should be tapered or discontinued to minimise damaging effects while targeting remission or low disease activity.
Damage develops since the very early stage of SLE, increases steadily over time and is independently associated with the daily and cumulative prednisone (PDN) dose.
Although PDN ≤7.5 mg/day is considered safe in preventing irreversible organ damage, some observations challenge this assumption.
WHAT THIS STUDY ADDS
The Glucocorticoids Use in newly diagnosed SLE Patients (GULP) study provides real-life evidence for successful tapering and maintenance of PDN doses <5 mg/day.
Tapering PDN <5 mg/day was not associated with a higher risk of disease relapse compared with other threshold dose of GCs tapering.
Patients spending much time on PDN <5 mg/day showed lower damage accrual in the short-term.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Tapering PDN dose below 5 mg/day and maintaining it during follow-up is associated with lower damage accrual without substantially increasing the risk of disease relapse compared with patients tapering PDN without dropping below 5 mg/day.
The results of the GULP study support the debate on the need for more stringent maintenance PDN dose thresholds than those commonly applied in SLE.
Future research should define evidence-based approaches to GCs tapering and discontinuation that minimises the risk of disease relapse and damage accrual.
Introduction
Glucocorticoids (GCs) represent a cornerstone in the management of systemic lupus erythematosus (SLE).1 They provide rapid symptom relief and are especially useful in treating moderate to severe manifestations.
Oral GCs should be tapered to maintenance doses targeting remission or low disease activity (LDA)2 and ideally discontinued to minimise the detrimental effects of chronic exposure, particularly the increased risk for irreversible accrual of organ damage.3 GC-related damage develops from the very early stage of the disease, accrues over time and is associated with the daily and cumulative dose of prednisone (PDN).4–6 In addition, organ damage is associated with lower SLE survival,5 and patients with early damage have a fourfold higher mortality rate than those without damage.6 Therefore, to avoid the development of damage and improve short-term and long-term outcomes, the dose reduction or the withdrawal of GCs should be achieved as early as possible in patients with SLE. However, the minimum dose to which GCs can be effectively tapered and whether they can be safely discontinued has not been clearly defined.7
Several attempts to identify the lower acceptable maintenance dose of GC have been performed, but it has remained unclear which daily dose best prevents damage.8 Although an equivalent dose of PDN ≤7.5 mg/day seems to reduce risks, even lower daily doses are associated with significantly increased damage in the long-term.9–12 Observational studies suggest that GC tapering and withdrawal in patients with SLE taking 5 mg/day of PDN is achievable without an increased risk of flare in clinically quiescent disease.13 14 However, a randomized controlled trial (RCT) showed that patients with quiescent SLE who discontinued PDN 5 mg/day experienced significantly more exacerbations than those who maintained it.15 Evidence-based guidelines on schemes, times and modalities of GCs reduction or discontinuation are lacking, and PDN tapering still depends, beyond the clinical features, on the clinician’s experience and the patient’s expectations.16
This study is a subanalysis of the prospectively followed-up Italian Early Lupus inception cohort. The main aim was to report on Glucocorticoid Use in newly diagnosed SLE Patients (GULP) and especially to investigate the feasibility of tapering PDN below 5 mg/day and associated short-term outcomes in the early stage of SLE.
Methods
Study design and participants
Previous research has described the Early Lupus cohort in detail.17–20 A multicentre inception cohort of consecutive patients with SLE enrolled within 12 months since 1997 ACR classification21 was recruited from January 2012 to December 2017. Investigators recorded demographics, symptoms duration and SLE core set variables at the baseline visit and every 6 months. In case of patients attended further visits according to personal clinical needs (eg, extra visits in case of flare), relevant data were recorded at the end of the 6-month observation period. The rheumatologists made the treatment decisions in a real-life setting according to good clinical practice and contemporary recommendations.2 The Independent Ethics Committee AOU of Cagliari approved the study (protocol n. 2012/1312), and all patients provided written informed consent.
For the GULP study, the inclusion criteria were: (1) enrolment in the Early Lupus cohort; (2) first starting PDN doses ≥5 mg/day at the baseline visit; (3) concomitant treatment with hydroxychloroquine (HCQ) and/or immunosuppressants; and (4) complete data in the 24 months of follow-up. The exclusion criteria were: (1) GCs prescribed for comorbidities other than SLE; (2) GCs initiation before study enrollment without sufficient clinical data.
Patients and clinical assessment
Demographic features and the time elapsed since the onset of the first symptom related to SLE were recorded at baseline. Active clinical manifestations (ie, categorised as present/absent but not differentiated in scoring A, B or C) according to the British Isles Lupus Assessment Group’s (BILAG) index glossary22 and disease activity according to the validated European Consensus Lupus Activity Measurement (ECLAM)23 were collected. According to previous studies, ECLAM >2 was indicative of active disease.24 With the meaning of clinically quiescent disease, remission was defined as ECLAM=0 or no activity in all BILAG domains (ie, scoring D or E).25 The SLICC/ACR Damage Index (SDI) was recorded,26 and damage items were subdivided as GC-related or GC-unrelated as described elsewhere (online supplemental table 1).4 The patient’s quality of life (QoL) was evaluated using a 10 cm global health visual analogue scale (GH-VAS).
rmdopen-2022-002701supp001.pdf (202.1KB, pdf)
Laboratory findings were tested in local laboratories at each centre. Anti-dsDNA, C3 and C4 serum complement fractions, and 24-hour proteinuria were recorded at every visit. C3 and C4 levels were included in the analysis. In addition, hypertension, dyslipidaemia, smoking habits, diabetes and obesity were recorded. Ongoing treatment with GCs, HCQ, conventional immunosuppressants, biologics or a combination of these agents was recorded.
GCs use
The oral mean daily PDN doses taken between visits and the cumulative doses were recorded. The mean daily PDN doses were categorised into high dose (>30 mg), medium dose (>7.5 mg but ≤30 mg) and low dose (≤7.5 mg). For a more sensitive analysis, moderate and low doses were further subcategorised into: (1) ≤30 mg but >15 mg/day, (2) ≤15 mg but >7.5 mg/day, (3) ≤7.5 but ≥5 mg/day, (4) <5 but ≥2.5 mg/day and (5) >2.5 mg/day but >0. Tapering and increasing PDN were defined as changes ≥1 mg/day. Discontinuation of PDN was complete GC withdrawal. The patterns of GCs tapering were defined as follows. Reducing the PDN dose below 5 mg/day and maintaining it below 5 mg/day in the subsequent visits until the end of follow-up was defined as successful GC tapering. Failed tapering was defined as increasing the PDN dose to ≥5 mg/day after tapering below 5 mg/day or retaking GC after discontinuation. Tapering PDN without dropping below 5 mg/day defined the third pattern.
Statistical analysis
Continuous variables are presented as mean (SD) or median and IQR. Absolute and relative frequencies are reported for categorical variables. The rates of remission and PDN dose changes were analysed using the Cochran-Armitage test. Trends for mean PDN daily dose, ECLAM score and the number of BILAG active domains during the study period were analysed in the whole cohort using appropriate analysis of variance models. Multinomial logistic regression models with the physician’s decision to modify GC dose as dependent variables and ECLAM score, ECLAM remission, number of active BILAG domains and BILAG remission as covariates analysed factors influencing the physician’s decision to modify GC dose, using the patient’s identification code variable as a random effect. A Cox regression model was built with time to tapering PDN <5 mg/day and maintaining it during follow-up as the time variable, using Lasso as the variables selection method and adjusting for age and sex. Poisson regression with SDI as the dependent variable and ECLAM, SDI, baseline PDN dose, age and sex as covariates was used to analyse the incidence rate ratio (IRR) of damage measured by the SDI for every month spent taking PDN daily dose <5 mg/day. Logistic regression analysed the OR to develop GC-related and GC-unrelated damage across the different patterns of GCs tapering. Linear regression with GH-VAS as the dependent variable and ECLAM and SDI as covariates, adjusted for age, gender, baseline GH-VAS and PDN daily dose, was used to analyse the effect of PDN daily dose <5 mg/day on QoL measured by GH-VAS.
Kaplan-Meier curves calculated the cumulative probability of successful GC tapering <5 mg/day and GC discontinuation. 95% CIs are reported where relevant, and p values <0.05 were considered to represent statistical significance. All the analyses were done by R software or SPSS. Microsoft Excel 2010 and GraphPad Prism were used to produce the graphs.
Patient and public involvement
The Italian lupus patient association, Gruppo LES Italiano ODV, endorsed and supported the Early Lupus project kick-off and disseminated the research findings through its official magazine and social media.
Results
Patients characteristics
Overall, 127 consecutive patients with SLE were eligible for the GULP study, and all completed the 24-month follow-up (table 1). Online supplemental figure 1 shows the study flow diagram, and online supplemental table 2 reports attrition analysis with descriptive data for available baseline variables on included versus excluded patients showing no relevant differences between groups. The PDN daily dose prescribed at baseline was low in 40 (31.5%) patients, moderate in 65 (51.2%) patients and high in 22 (17.3%) patients (figure 1A). At the end of follow-up, 106 (83.7%) and 21 (16.3%) patients took low and moderate PDN doses, respectively. The changes in PDN doses are depicted in figure 1B. At baseline, 98 (77.2%) patients received HCQ, 81 (63.8%) received conventional immunosuppressants and 57 (44.9%) received a combination of them. The conventional immunosuppressants prescribed at baseline were mycophenolate (n=32; 25.2%), methotrexate (n=21; 16.5%), azathioprine (n=17; 13.4%), cyclophosphamide (n=11; 8.7%) and calcineurin inhibitors (n=3; 2.4%). In addition, belimumab was prescribed in 5 (3.9%) and rituximab in 4 (3.1%) patients. Medications ongoing after 24 months of follow-up were HCQ in 101 (79.5%) patients, conventional immunosuppressants in 80 (70.9%) patients and biologics in 14 (11.0%) patients (online supplemental table 3).
Table 1.
Baseline characteristics of the 127 patients enrolled in the GULP study cohort
| Value | |
| Age, mean (±SD) | 36.7 (± 13.4) |
| Female | 105 (82.7%) |
| Caucasian | 109 (85.8%) |
| Disease duration (months), median (IQR) | 6.1 (1.3–11.5) |
| Time elapsed since the onset of SLE symptoms (<1 year) | 97 (76.4%) |
| School education <8 years | 5 (3.9%) |
| 8–13 years | 85 (67.0%) |
| >13 years | 37 (29.1%) |
| Clinical phenotypes according to BILAG domains | |
| Constitutional | 58 (45.7%) |
| Mucocutaneous | 67 (53.2%) |
| Neuropsychiatric | 11 (8.7%) |
| Musculoskeletal | 78 (61.4%) |
| Cardiorespiratory | 26 (20.5%) |
| Gastrointestinal | 7 (5.5%) |
| Ophthalmic | 0 (0%) |
| Renal | 46 (36.2%) |
| Haematological | 55 (43.3%) |
| Serologic features | |
| Anti-dsDNA | 101 (80.8%) |
| Anti-Ro/SSA | 41 (35.3%) |
| Anti-La/SSB | 20 (17.4%) |
| Anti-RNP | 30 (26.1%) |
| Anti-Sm | 31 (26.7%) |
| Antiphospholipid antibodies* | 35 (27.6%) |
| C3 complement fraction(mg/dL), median (IQR) | 69 (50–91) |
| C4 complement fraction (mg/dL), median (IQR) | 11 (6–15) |
| ECLAM, median (IQR) | 3 (2–5) |
| SDI, mean (±SD) | 0.2 (±0.6) |
| Comorbidities | |
| Smoking (ever) | 30 (23.6%) |
| Diabetes | 4 (3.1%) |
| Hypertension | 17 (13.4%) |
| Dyslipidaemia | 17 (13.4%) |
| Obesity (BMI >30) | 6 (4.7%) |
| Medications | |
| Hydroxychloroquine† | 98 (77.2%) |
| Conventional immunosuppressants† | 81 (63.8%) |
| Biologics† | 9 (7.0%) |
| Prednisone (prior to enrolment) | 21 (16.5%) |
Data were complete for all participants.
Unless otherwise expressed, numbers are absolute values (percentage).
*Lupus anticoagulant (LA) and/or anticardiolipin antibodies (aCl) IgM/IgG and/or anti-B2-glicoprotein I (aB2GPI) IgM/IgG.
†Treatment prescribed at baseline visit.
BILAG, British Isles Lupus Assessment Group; BMI, body mass index; ECLAM, European Consensus Lupus Activity Measurement; GULP, Glucocorticoids Use in newly diagnosed SLE Patients; SDI, SLICC/ACR Damage Index; SLE, systemic lupus erythematosus.
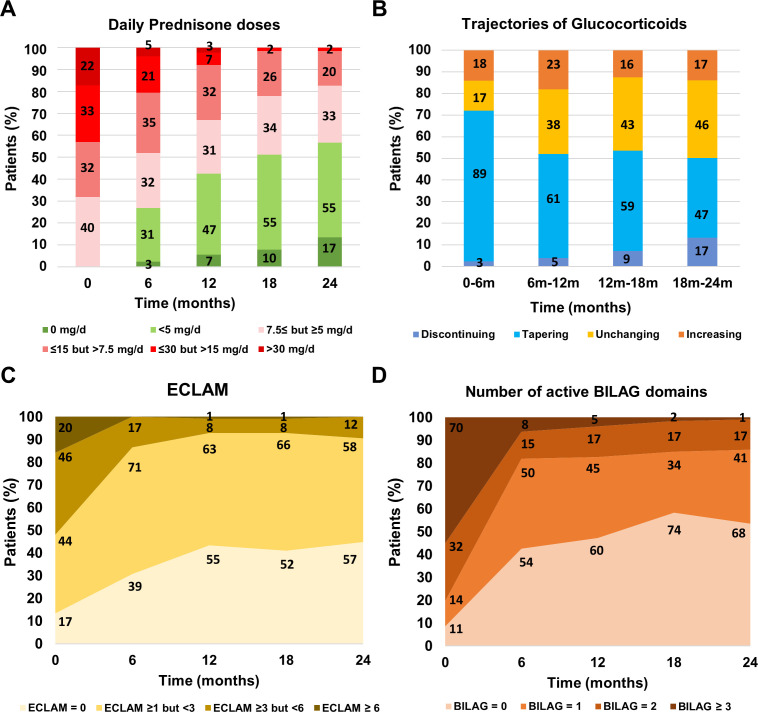
Figure 1.
Percentage of patients on different intervals of daily prednisone dose at each visit (A), the changes in glucocorticoids dose between visits (B), remission rate by ECLAM (C), and number of active BILAG domains (D), in the 127 newly diagnosed patients with systemic lupus erythematosus participating in the Glucocorticoids Use in newly diagnosed SLE Patients study. Data labels (numbers) refer to absolute values. BILAG, British Isles Lupus Assessment Group; ECLAM, European Consensus Lupus Activity Measurement
Changes in PDN dose and disease activity
The median daily dose of PDN at baseline was 12.5 (6.3–25.0) mg/day and significantly decreased to 5.4 (4.3–9.4) mg/day at 12 months, 4.9 (2.9–7.4) mg/day at 18 months and 4.9 (2.5–6.6) mg/day at 24 months (p<0.001), whereas the median cumulative daily dose significantly increased to 4.2 (2.5–6.2) g at 12 months and 5.4 (3.9–8.4) g at 24 months (p<0.001). Disease activity quickly decreased during the first 6 months (p<0.001), resulting in 20 (15.7%) patients with active disease after 6 months and 11 (8.7%) after 24 months, according to ECLAM (figure 1C). The rate of clinically quiescent disease, according to BILAG, was high at 6 months (43.4%, n=55), followed by a linearly increasing rate with marginal fluctuation in the subsequent follow-up period up to 54.3% (n=69) at 24 months (p<0.001). (figure 1D).
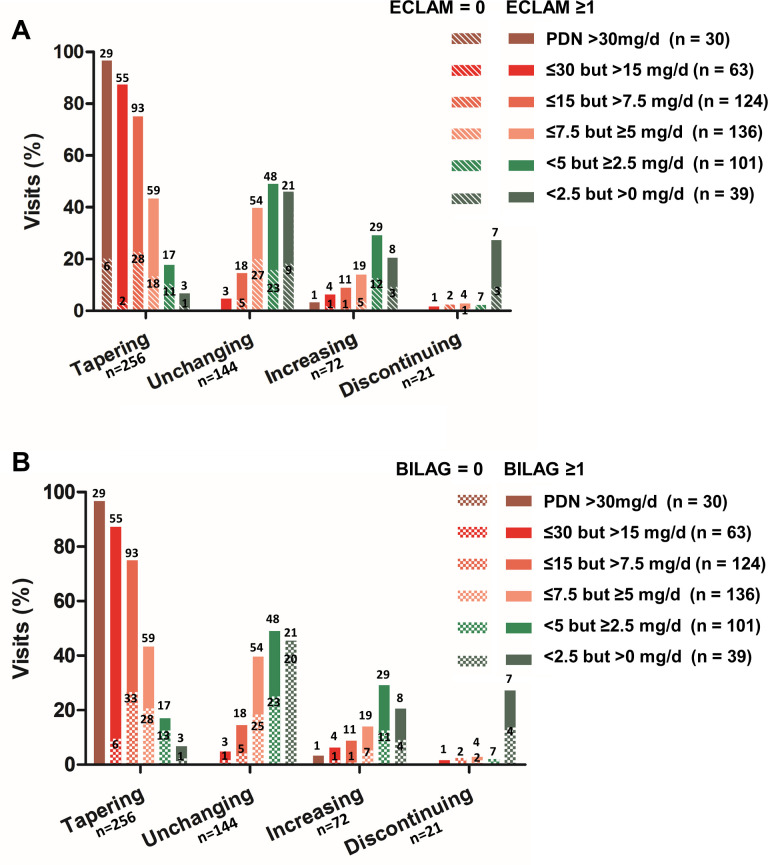
The dose tapering was lesser in patients taking lower PDN daily doses (online supplemental table 4). Regarding the relationship between the changes in PDN doses and the concomitant level of disease activity, a higher ECLAM score was associated with a higher probability (OR: 1.6 per ECLAM point; 95% CI: 1.2 to 2.3; p=0.004) of increasing the PDN dose independently of daily intake (table 2). The percentage of patients requiring increased GCs doses for disease activity relapse did not significantly differ between visits during follow-up (p=0.573) (online supplemental table 5). The rate of disease relapse was higher in patients taking a low dose of PDN compared with those on a moderate or high dose of PDN (p<0.001), but did not statistically differ (p=0.069) between patients taking PDN ≤7.5 but ≥5 mg/day (n=19/136; 14.0%), PDN <5 but ≥2.5 mg/day (n=29/101; 28.7%) and PDN <2.5 mg/day (n=8/39; 20.5%) (online supplemental table 6). The physician decisions regarding GCs changes according to daily PDN dose and remission states are depicted in figure 2, showing that the vast majority of patients taking PDN<5 mg/day received a prescription of unchanging GCs dose despite BILAG remission.
Table 2.
Multinomial logistic regression models showed the effect of daily PDN dose and disease activity on the physician’s decision to change the PDN dose, taking into account different definitions of active disease measured by ECLAM, the number of active BILAG domains and the lack of ECLAM or BILAG remission
| Unchanging | Tapering | Increasing | Discontinuing | ||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | ||
| Model 1 | Ref. | ||||||
| PDN daily dose* | – | 1.4 (1.3 to 1.5) | <0.001 | 1.0 (0.9 to 1.1) | 0.863 | 1.0 (0.9 to 1.1) | 0.644 |
| ECLAM score† | – | 1.1 (0.9 to 1.3) | 0.299 | 1.6 (1.2 to 2.3) | 0.004 | 1.0 (0.7 to 1.5) | 0.826 |
| Model 2 | Ref. | ||||||
| PDN daily dose* | – | 1.4 (1.3 to 1.5) | <0.001 | 1.0 (0.9 to 1.1) | 0.386 | 1.0 (0.9 to 1.1) | 0.586 |
| ECLAM ≥1 | – | 1.1 (0.7 to 1.9) | 0.607 | 1.8 (0.9 to 4.1) | 0.145 | 0.8 (0.3 to 2.2) | 0.698 |
| Model 3 | Ref. | ||||||
| PDN daily dose* | – | 1.4 (1.3 to 1.5) | <0.001 | 1.0 (0.9 to 1.1) | 0.543 | 1.1 (0.9 to 1.2) | 0.408 |
| Number of active BILAG domains | – | 1.1 (0.8 to 1.3) | 0.687 | 1.9 (1.3 to 3.0) | 0.004 | 0.7 (0.3 to 1.2) | 0.213 |
| Model 4 | Ref. | ||||||
| PDN daily dose* | – | 1.4 (1.3 to 1.5) | <0.001 | 1.0 (0.9 to 1.1) | 0.565 | 1.1 (0.9 to 1.2) | 0.389 |
| Active BILAG domains ≥1 | – | 0.9 (0.5 to 1.4) | 0.599 | 2.5 (1.2 to 6.2) | 0.025 | 0.4 (0.1 to 1.1) | 0.085 |
*Per prednisone mg/day.
†Per ECLAM point.
BILAG, British Isles Lupus Assessment Group; ECLAM, European Consensus Lupus Activity Measurement; PDN, prednisone.
Figure 2.
Bar graphs reporting differences in glucocorticoids changes according to daily PDN dose and (A) the rate of clinically quiescent disease defined by ECLAM=0 (bars with oblique lines), and (B) lack active BILAG domains (chequered bars) in 127 newly diagnosed patients with systemic lupus erythematosus. Data labels (numbers) refer to absolute values. BILAG, British Isles Lupus Assessment Group; ECLAM, European Consensus Lupus Activity Measurement; PDN, prednisone.
Successful tapering or withdrawal of GCs
All patients tapered GCs at least once during follow-up, and 99 (78%) patients tapered the PDN dose below 5 mg/day. However, after tapering, 42 (42.4%) out of 99 patients had to increase the PDN dose ≥5 mg/day because of inadequate disease activity control by the end of the 2-year follow-up, even though 16 of them succeeded in reducing PDN <5 mg/day in a second attempt within the 24 months follow-up. Therefore, 57 (44.9%) patients successfully reduced and maintained PDN doses below 5 mg/day, and the median maintenance dose was 3.0 (2.0–4.0) mg/day. In all, 28 (22.0%) patients tapered PDN but never succeeded in descending below 5 mg/day, their median maintenance dose was 7.0 (5.8–15.0) mg/day and 13 (46.4%) out of 28 had to increase the GCs dose during the observation time because of disease relapse.
After correction for age and gender as confounding factors, regression models showed active renal BILAG domain as independently associated (HR: 0.41; 95% CI: 0.21 to 0.80; p=0.009) with a reduced probability to taper PDN <5 mg/day during the 2-year follow-up. No effect of the baseline PDN dose and ongoing HCQ or immunosuppressant was found. Higher C3 serum levels were weakly associated (HR: 1.04 per 10 mg/dL; 95% CI: 1.01 to 1.08; p=0.025) with an increased probability of tapering PDN doses to<5 mg/day.
During the 2-year follow-up, 21 (16.5%) out of 127 patients discontinued GC, but 4 (19.0%) out of 21 restarted GC in the following months, and only 17 (13.4%) out of 127 finally withdrew GC. The cumulative rate of GC discontinuation was 1.7% (n=2) at 6 months, 3.1% (n=4) at 12 months, 7.1% (n=9) at 18 months and 13.4% (n=17) at 24 months with a significant ascending trend (p<0.001). The mean (±SD) time for GC discontinuation was 18.4 (±6.5) months, and the median dose of PDN before attempting withdrawal was 4.0 mg/day (IQR: 2.0–5.0). No factors predicted successful GCs discontinuation, including HCQ or immunosuppressive use.
Outcomes of the different patterns of GCs tapering
Patients who successfully tapered PDN below 5 mg/day showed a lower risk of GC-related organ damage accrual than patients who tapered PDN without dropping below 5 mg/day (table 3). More interestingly, patients who failed tapering PDN below 5 mg/day had the same risk of either GC-related or unrelated damage as patients with other patterns of PDN tapering. Independently from patterns of GCs tapering, every month spent on PDN <5 mg/day was associated with a lower incidence of damage accrual (IRR: 0.96; 95% CI 0.93 to 0.99; p=0.007) over time.
Table 3.
Risk of GC-related and GC-unrelated organ damage development during 2-year follow-up across different patterns of GC tapering in 127 patients with newly diagnosed SLE starting concomitant hydroxychloroquine or immunosuppressants
|
|
GC-related damage | GC-unrelated damage | ||||||
| Crude OR (95% CI) |
P | Adj OR* (95% CI) |
P | Crude OR (95% CI) | P | Adj OR* (95% CI) |
P | |
| Tapering PDN <5 mg/day | Ref. | Ref. | Ref. | Ref. | ||||
| Fail to taper PDN <5 mg/day | 0.86 (0.21 to 3.22) | 0.824 | 0.98 (0.20 to 4.45) | 0.975 | 0.69 (0.25 to 1.81) | 0.457 | 1.02 (0.32 to 3.24) | 0.967 |
| Never tapered PDN <5 mg/day | 4.54 (1.47 to 15.10) | 0.010 | 5.90 (1.53 to 27.0) | 0.014 | 1.89 (0.71 to 5.03) | 0.197 | 2.28 (0.70 to 7.57) | 0.168 |
*Adjusted for age, gender, ECLAM score, baseline SDI and PDN daily dose.
ECLAM, European Consensus Lupus Activity Measurement; GC, glucocorticoid; PDN, prednisone; SLE, systemic lupus erythematosus.
Patients spending much time on PDN <5 mg/day did not have statistically significant better QoL measured by GH-VAS (beta per month 0.60; 95% CI 0.13 to 1.33; p=0.108).
Discussion
The GULP study provides real-world data on the early GCs tapering and discontinuation in newly diagnosed patients with SLE. It primarily aimed to identify patients who could successfully taper PDN below 5 mg/day in the early disease stage and whether different tapering patterns may influence short-term outcomes. Therefore, it may add new perspectives to the unmet needs of determining what dose of GCs represent thresholds for detrimental effects. Daily doses of PDN ≤7.5 mg/day are recommended as safe in controlling disease activity while preventing the damaging effects of GCs. Some observations challenged this assumption and suggested PDN <5 mg/day as a safer dose.9–12 However, in a multicentre cohort of patients with SLE with no clinical and serological disease activity, time-adjusted GCs dose was independently associated with damage accrual, although the median dose of PDN was only 2.0 mg/day (IQR: 0–5.0).27 In addition, Zen et al reported that patients with long-lasting remission admitting a dose of PDN ≤5 mg/day accrued significantly more organ damage than patients experiencing remission off-GCs.28 These findings point out that even PDN ≤5 mg/day may lead to damage accrual if taken for a prolonged time and support the debate on whether a more stringent PDN dose threshold or drug discontinuation should be recommended in the maintenance treatment of patients with SLE. On the other hand, some authors suggested being more cautious about tapering PDN below 5 mg/day and stopping it from avoiding a disease flare, potentially requiring an increase in PDN dose and raising the risk of damage accrual.29
In the GULP cohort, tapering PDN doses below 5 mg/day and maintaining it over time was associated with a lower incidence of irreversible damage accrual, although feasible in only half of the patients. The probability of tapering PDN doses <5 mg/day was lower in patients with active renal disease and low C3 serum levels. Similarly, two independent inception cohorts reported renal involvement, high anti-dsDNA levels, and low complement fractions among the independent risk factors for failing to achieve Lupus LDA State, with PDN ≤7.5 mg/day as the most frequent unmet criterion in those patients.30 31 Moreover, GCs tapering and withdrawal by themself are not associated with an increased risk of flares, which is influenced by individual features such as active disease and serologic abnormalities.32–34 In the GULP cohort, tapering PDN below 5 mg/day was not associated with a significant risk of later increasing PDN dose due to disease relapse, which rate in these patients was similar (42.4% over 2 years) to that observed in patients tapering PDN without dropping below 5 mg/day (46.4% over 2 years). Noteworthy, the risk of disease relapse was lower in patients taking moderate or high PDN doses than in patients treated with low PDN doses but did not statistically differ in patients taking different PDN dosages lower than 7.5 mg/day. Taken together, the GULP results support that after tapering PDN dosage to 7.5 mg/day, further gradual tapering PDN below 5 mg/day in the early stages of the disease is associated with lower damage accrual without substantially increasing the risk of disease relapse, provided an adequate treatment with HCQ and conventional or biologic immunosuppressants is ensured. Furthermore, we found that the risk of early damage accrual in patients who tapered PDN below 5 mg/day but later required increasing PDN dose did not significantly diverge from patients following different patterns of GCs tapering. Moreover, the risk of developing GC-related damage was higher in those patients who never tapered PDN below 5 mg/day. These findings suggest that tapering PDN below 5 mg/day might be attempted in all patients, being more cautious in patients carrying features associated with a low probability of successful tapering. However, caution in interpreting this result is recommended until further confirmation in more comprehensive follow-up studies.
Finally, we witnessed a lower-than-expected proportion of GCs discontinuation. Surprisingly, remission did not drive the choice of physicians to discontinue GCs. In fact, half of the patients who received a stable low or very low PDN dose were experiencing remission of clinical manifestations. A conceivable explanation for that physician behaviour is the concern of SLE flare primarily related to the short duration of the remission,35 which is supported by the significant increase of GCs discontinuation rate over time. However, in a recent meta-analysis, no significant association was observed between remission duration and the increased risk of flare after GC withdrawal.34 That suggests GCs discontinuation is still strongly driven by the treating physicians' experience in the lack of an evidence-based approach.
Our study has some strengths. First, the GULP inception cohort included patients with SLE starting PDN at the time of enrolment, thus overcoming the confounding effect of GCs exposure during previous years reported in former studies. Second, recording data of interest every 6 months allowed us to adequately model time-varying covariates, including damage accrual, disease activity and daily exposure to PDN between visits. These features contributed to understanding the dynamics of GC use and identifying outcomes for different patterns of GCs tapering. Finally, the present results are generalisable to other cohorts of patients with early SLE, most Caucasian, treated with a high prescription rate of HCQ, conventional immunosuppressants and biologics since the first stage of the disease.
Some limitations of our study need to be acknowledged. First, the observational design of the GULP study may implicate a confounding by indication bias. Indeed, the propensity to prescribe higher GC doses in patients with high disease activity may hamper the chance to discriminate their independent effect on disease outcome, although adjusted regression models were built to minimise this risk. Second, the late effect of low daily PDN dose on damage accrual and the late impact of GCs withdrawal on the risk of disease flare cannot be addressed because of the short follow-up. Third, the sample size might hamper the correct interpretation of some subanalyses when the subgroups shrink too much. That may have determined why factors associated with failing GCs discontinuation were not found. Fourth, we did not apply a specific flare definition to disease relapse but used the increase in PDN dose as a surrogate. Finally, the GULP study did not record pulse therapy with methylprednisolone. Consequently, its known effect on GCs tapering and withdrawal might have been missed.
In conclusion, the GULP study describes the GCs tapering and discontinuation in the early stage of SLE. It provides encouraging data that tapering PDN <5 mg/day and maintaining it with concomitant antimalarial or immunosuppressants may represent a good balance between the need to prevent damage accrual and the risk of disease flare. These findings support the debate on the need for more stringent PDN maintenance dose thresholds than commonly applied in SLE and encourage designing effective steroid-sparing strategies. However, further observational studies and RCTs are needed to investigate the long-term prognostic implications of more stringent PDN maintenance dose thresholds and understand the best strategies for GCs tapering and discontinuation.
Footnotes
Twitter: @FRSpin, @RMDclinic
Contributors: Study conception and design: AF, EC, AC and MP. Data collection: AB, FB, EC, LC, RDA, AF, MF, LI, IP, FRS and CT. Analysis and interpretation of results: AC, GC, FC, AD, FF, FI, MG, MM, MP, CAS, GDS and AZ. Draft manuscript preparation: AF and MP. MP has full responsibility for the finished work and/or the conduct of the study, had access to the data, and controlled the decision to publish. All authors reviewed the results and approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request to the corresponding author and the Italian Society of Rheumatology.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involved human participants and was approved by the Independent Ethics Committee AOU of Cagliari under number 2012/1312 and complies with the Declaration of Helsinki. Participants gave informed consent to participate in the study before taking part.
References
- 1.Ruiz-Irastorza G, Ugarte A, Ruiz-Arruza I, et al. Seventy years after Hench's Nobel prize: revisiting the use of glucocorticoids in systemic lupus erythematosus. Lupus 2020;29:1155–67. 10.1177/0961203320930099 [DOI] [PubMed] [Google Scholar]
- 2.van Vollenhoven RF, Mosca M, Bertsias G, et al. Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Ann Rheum Dis 2014;73:958–67. 10.1136/annrheumdis-2013-205139 [DOI] [PubMed] [Google Scholar]
- 3.Fanouriakis A, Kostopoulou M, Alunno A. Update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019;2019:736–45. [DOI] [PubMed] [Google Scholar]
- 4.Piga M, Floris A, Sebastiani GD, et al. Risk factors of damage in early diagnosed systemic lupus erythematosus: results of the Italian multicentre early lupus project inception cohort. Rheumatology 2020;59:2272–81. 10.1093/rheumatology/kez584 [DOI] [PubMed] [Google Scholar]
- 5.Bruce IN, O'Keeffe AG, Farewell V, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the systemic lupus international collaborating clinics (SLICC) inception cohort. Ann Rheum Dis 2015;74:1706–13. 10.1136/annrheumdis-2013-205171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahman P, Gladman DD, Urowitz MB, et al. Early damage as measured by the SLICC/ACR damage index is a predictor of mortality in systemic lupus erythematosus. Lupus 2001;10:93–6. 10.1191/096120301670679959 [DOI] [PubMed] [Google Scholar]
- 7.Felten R, Arnaud L. Is it possible to stop glucocorticoids in systemic lupus? Joint Bone Spine 2020;87:528–30. 10.1016/j.jbspin.2020.03.008 [DOI] [PubMed] [Google Scholar]
- 8.Piga M, Arnaud L. The main challenges in systemic lupus erythematosus: where do we stand? J Clin Med 2021;10:243. 10.3390/jcm10020243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruiz-Arruza I, Ugarte A, Cabezas-Rodriguez I, et al. Glucocorticoids and irreversible damage in patients with systemic lupus erythematosus. Rheumatology 2014;53:1470–6. 10.1093/rheumatology/keu148 [DOI] [PubMed] [Google Scholar]
- 10.Al Sawah S, Zhang X, Zhu B, et al. Effect of corticosteroid use by dose on the risk of developing organ damage over time in systemic lupus erythematosus-the Hopkins lupus cohort. Lupus Sci Med 2015;2:e000066. 10.1136/lupus-2014-000066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thamer M, Hernán MA, Zhang Y, et al. Prednisone, lupus activity, and permanent organ damage. J Rheumatol 2009;36:560–4. 10.3899/jrheum.080828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apostolopoulos D, Kandane-Rathnayake R, Raghunath S, et al. Independent association of glucocorticoids with damage accrual in SLE. Lupus Sci Med 2016;3:e000157. 10.1136/lupus-2016-000157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tani C, Elefante E, Signorini V, et al. Glucocorticoid withdrawal in systemic lupus erythematosus: are remission and low disease activity reliable starting points for stopping treatment? A real-life experience. RMD Open 2019;5:e000916. 10.1136/rmdopen-2019-000916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tselios K, Gladman DD, Su J, et al. Gradual glucocorticosteroid withdrawal is safe in clinically quiescent systemic lupus erythematosus. ACR Open Rheumatol 2021;3:550–7. 10.1002/acr2.11267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathian A, Pha M, Haroche J, et al. Withdrawal of low-dose prednisone in SLE patients with a clinically quiescent disease for more than 1 year: a randomised clinical trial. Ann Rheum Dis 2020;79:339–46. 10.1136/annrheumdis-2019-216303 [DOI] [PubMed] [Google Scholar]
- 16.Little J, Parker B, Lunt M, et al. Glucocorticoid use and factors associated with variability in this use in the systemic lupus international collaborating clinics inception cohort. Rheumatology 2018;57:677–87. 10.1093/rheumatology/kex444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sebastiani GD, Prevete I, Piga M, et al. Early Lupus Project - A multicentre Italian study on systemic lupus erythematosus of recent onset. Lupus 2015;24:1276–82. 10.1177/0961203315585817 [DOI] [PubMed] [Google Scholar]
- 18.Sebastiani GD, Prevete I, Iuliano A, et al. Early Lupus Project: one-year follow-up of an Italian cohort of patients with systemic lupus erythematosus of recent onset. Lupus 2018;27:1479–88. 10.1177/0961203318777112 [DOI] [PubMed] [Google Scholar]
- 19.Prevete I, Espinosa G, Bellisai F, et al. Comparative study between two European inception cohorts of patients with early systemic lupus erythematosus. Clin Exp Rheumatol 2020;38:925–32. [PubMed] [Google Scholar]
- 20.Prevete I, Iuliano A, Cauli A, et al. Similarities and differences between younger and older disease onset patients with newly diagnosed systemic lupus erythematosus. Clin Exp Rheumatol 2022. doi: 10.55563/clinexprheumatol/oo5ymg. [Epub ahead of print: 26 07 2022]. [DOI] [PubMed] [Google Scholar]
- 21.Hochberg MC. Updating the American College of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:40. 10.1002/art.1780400928 [DOI] [PubMed] [Google Scholar]
- 22.Isenberg DA, Rahman A, Allen E, et al. BILAG 2004. development and initial validation of an updated version of the British Isles lupus assessment group's disease activity index for patients with systemic lupus erythematosus. Rheumatology 2005;44:902–6. 10.1093/rheumatology/keh624 [DOI] [PubMed] [Google Scholar]
- 23.Vitali C, Bencivelli W, Isenberg DA, et al. Disease activity in systemic lupus erythematosus: report of the consensus Study group of the European workshop for rheumatology research. II. Identification of the variables indicative of disease activity and their use in the development of an activity score. The European consensus Study Group for disease activity in SLE. Clin Exp Rheumatol 1992;10:541–7. [PubMed] [Google Scholar]
- 24.Doria A, Rinaldi S, Ermani M, et al. Health-related quality of life in Italian patients with systemic lupus erythematosus. II. Role of clinical, immunological and psychological determinants. Rheumatology 2004;43:1580–6. 10.1093/rheumatology/keh392 [DOI] [PubMed] [Google Scholar]
- 25.van Vollenhoven R, Voskuyl A, Bertsias G, et al. A framework for remission in SLE: consensus findings from a large international task force on definitions of remission in SLE (DORIS). Ann Rheum Dis 2017;76:554–61. 10.1136/annrheumdis-2016-209519 [DOI] [PubMed] [Google Scholar]
- 26.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the systemic lupus international collaborating Clinics/American College of rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996;39:363–9. 10.1002/art.1780390303 [DOI] [PubMed] [Google Scholar]
- 27.Apostolopoulos D, Kandane-Rathnayake R, Louthrenoo W, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus with no clinical or serological disease activity: a multicentre cohort study. Lancet Rheumatol 2020;2:e24–30. 10.1016/S2665-9913(19)30105-5 [DOI] [PubMed] [Google Scholar]
- 28.Zen M, Iaccarino L, Gatto M, et al. The effect of different durations of remission on damage accrual: results from a prospective monocentric cohort of Caucasian patients. Ann Rheum Dis 2017;76:562–5. 10.1136/annrheumdis-2016-210154 [DOI] [PubMed] [Google Scholar]
- 29.Mathian A, Pha M, Yssel H, et al. Reducing lupus flares: should we be more careful about stopping glucocorticoids? Expert Rev Clin Immunol 2020;16:539–42. 10.1080/1744666X.2020.1778466 [DOI] [PubMed] [Google Scholar]
- 30.Golder V, Kandane-Rathnayake R, Hoi AY-B, et al. Frequency and predictors of the lupus low disease activity state in a multi-national and multi-ethnic cohort. Arthritis Res Ther 2016;18:260. 10.1186/s13075-016-1163-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piga M, Floris A, Cappellazzo G, et al. Failure to achieve lupus low disease activity state (LLDAS) six months after diagnosis is associated with early damage accrual in Caucasian patients with systemic lupus erythematosus. Arthritis Res Ther 2017;19:247. 10.1186/s13075-017-1451-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zahr ZA, Fang H, Magder LS, et al. Predictors of corticosteroid tapering in SLE patients: the Hopkins lupus cohort. Lupus 2013;22:697–701. 10.1177/0961203313490434 [DOI] [PubMed] [Google Scholar]
- 33.Fasano S, Coscia MA, Pierro L, et al. Which patients with systemic lupus erythematosus in remission can withdraw low dose steroids? results from a single inception cohort study. Lupus 2021;30:991–7. 10.1177/09612033211002269 [DOI] [PubMed] [Google Scholar]
- 34.Ji L, Xie W, Fasano S, et al. Risk factors of flare in patients with systemic lupus erythematosus after glucocorticoids withdrawal. A systematic review and meta-analysis. Lupus Sci Med 2022;9:e000603. 10.1136/lupus-2021-000603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ngamjanyaporn P, McCarthy EM, Sergeant JC, et al. Clinicians approaches to management of background treatment in patients with SLE in clinical remission: results of an international observational survey. Lupus Sci Med 2017;4:e000173. 10.1136/lupus-2016-000173 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2022-002701supp001.pdf (202.1KB, pdf)
Data Availability Statement
Data are available upon reasonable request to the corresponding author and the Italian Society of Rheumatology.