Abstract
Objective
SLE, primary Sjögren’s syndrome (pSS) and systemic sclerosis (SSc) are heterogeneous autoimmune diseases with a dysregulated type I interferon (IFN) system. The diseases often show overlapping clinical manifestations, which may result in diagnostic challenges. We asked to which extent SSc-associated autoantibodies are present in SLE and pSS, and whether these link to serum IFN-α, clinical phenotypes and sex. Samples with clinical data from patients with SSc and healthy blood donors (HBDs) served as controls. Finally, the diagnostic performance of SSc-associated autoantibodies was evaluated.
Methods
Samples from well-characterised subjects with SLE (n=510), pSS (n=116), SSc (n=57) and HBDs (n=236) were analysed using a commercially available immunoassay (EuroLine Systemic Sclerosis Profile (IgG)). IFN-α was quantified by ELISA. Self-reported data on Raynaud’s phenomenon (RP) were available.
Results
With exceptions for anti-Ro52/SSA and anti-Th/To, SSc-associated autoantibodies were more frequent in SSc than in SLE, pSS and HBDs regardless of sex. IFN-α levels correlated with the number of positive SSc-associated autoantibodies (r=0.29, p<0.0001) and associated with Ro52/SSA positivity (p<0.0001). By using data from SLE, SSc and HBDs, RP was significantly associated with topoisomerase I, centromere protein (CENP)-B, RNA polymerase III 11 kDa, RNA polymerase III 155 kDa and PM-Scl100 whereas Ro52/SSA associated inversely with RP. In SLE, CENP-A was associated with immunological disorder, CENP-B with serositis and Ku with lupus nephritis. By combining analysis of ANA (immunofluorescence) with SSc-associated autoantibodies, the diagnostic sensitivity reached 98% and the specificity 33%.
Conclusions
The 13 specificities included in the EuroLine immunoassay are commonly detected in SSc, but they are also frequent among individuals with other diseases imprinted by type I IFNs. These findings are valuable when interpreting serological data on patients with suspected SSc, especially as patients may present with disease manifestations overlapping different rheumatological diseases. In SLE, we observed associations between manifestations and SSc-associated autoantibodies which have not previously been reported.
Keywords: Sjogren's Syndrome; Scleroderma, Systemic; Lupus Erythematosus, Systemic; Interferon Type I; Autoantibodies
WHAT IS ALREADY KNOWN ON THIS TOPIC
Although SLE, primary Sjögren’s syndrome (pSS) and systemic sclerosis (SSc) constitute different diagnostic entities, organ involvement and disease mechanisms may overlap and result in significant diagnostic challenges.
Autoantibodies are important diagnostic tools in the three diseases.
WHAT THIS STUDY ADDS
Autoantibodies associated with SSc can be found in different diseases imprinted by type I interferons (IFNs).
These autoantibodies show association with separate clinical phenotypes as well as with increased IFN-α.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
SSc-associated antibodies alone are of limited value in the differential diagnosis of patients with new-onset autoimmune disease and must be interpreted together with the clinical picture.
The link between IFN-α and SSc-associated autoantibodies highlights the interplay between type I IFNs and B cells, and motivates further investigation of type I IFN-blocking regimens in SLE, pSS and SSc.
Introduction
SLE, primary Sjögren’s syndrome (pSS) and systemic sclerosis (SSc) are heterogeneous chronic autoimmune conditions, which primarily affect women.1 2 These diseases can present with clinically separate features but the organ involvement and disease mechanisms may overlap and result in significant diagnostic challenges. In addition, secondary Sjögren’s syndrome is estimated to be present in >20% of patients with both established SLE and SSc.3 4
Another common clinical feature of these conditions is the presence of Raynaud’s phenomenon (RP). Yet, presence of RP is not restricted to autoimmune disorders. The idiopathic primary form of RP has a prevalence of approximately 5%–15% in the general population (higher in women than in men), whereof the vast majority have a benign course.5 6 Secondary RP may develop in association with use of various drugs or presence of hypothyroidism, diabetes, haematological abnormalities or autoimmune diseases.7 8 RP is common in rheumatic diseases, including SLE (30%–40%), pSS (15%–25%) and especially in SSc, where at least 95% of patients develop this manifestation. Occasionally, RP may precede the onset of autoimmune diseases and RP is the most common debut symptom of SSc.9 10 Because of this, early testing of antibodies associated with SSc/rheumatic disease is recommended in adult-onset of RP, as well as nailfold capillaroscopy.
Of aetiopathogenetic relevance, activation of the type I interferon (IFN) response constitutes a common denominator of SLE, pSS and SSc.11 Recent data from randomised controlled trials show that blocking the type I IFN receptor by anifrolumab decreases global disease activity in SLE.12 Corresponding studies in pSS are lacking but a phase I open-label study in SSc has been conducted, and recent studies indicate that type I IFN signalling is important also in SSc.13–15 Another similarity between the three diseases is the high prevalence of autoantibodies, for example, antibodies targeting cellular antigens referred to as ANA usually detected by immunofluorescence (IF) microscopy on HEp-2 cells and/or antigen-specific assays.16–18
The EuroLine Systemic Sclerosis Profile is a commercial immunoblot test including a panel of 13 target antigens for SSc-associated antibodies and has been launched to aid the identification of patients with recent-onset SSc and to stratify patients into more homogeneous subsets.19 Some established associations between these autoantibodies with organ involvement and disease progression exist.20 21 These autoantibody specificities include centromere protein (CENP) A and B, topoisomerase I (Scl-70) and RNA polymerase III. Others, like Ku, have been described in the context of idiopathic inflammatory myopathies and recently in SLE, whereas autoantibodies against platelet-derived growth factor receptor (PDGFR) and NOR90 are rare and their clinical significance remains uncertain.22 23 Nowadays, this panel of SSc-associated antibodies is available for use in clinical practice. Still, the panel has not been systemically evaluated using large control groups of resembling conditions.
Herein, we investigated to what extent SSc-associated autoantibodies can be found in patients with SLE and pSS, with and without detectable IFN-α, and to whether these antibodies link to clinical phenotypes, including RP. In addition, we employed samples and concomitant clinical data from patients with confirmed SSc as well as from a large group of healthy blood donors (HBDs). Finally, the diagnostic performance of the SSc-associated autoantibodies in relation to SSc diagnosis in a clinical setting was evaluated.
Patients and methods
Clinical characterisation
SLE: discovery cohort
This cohort consisted of samples obtained from 282 patients (243 women, 39 men), mean age 48.6 years, classified with SLE according to the 1982 American College of Rheumatology (ACR) and/or the 2012 Systemic Lupus International Collaborating Clinics criteria as detailed in table 1.24 25 All subjects were included in the prospective and observational research programme Clinical Lupus Register in North-Eastern Gothia (Swedish acronym ‘KLURING’) at the Rheumatology Unit, Linköping University Hospital.26
Table 1.
Characteristics of the included patients with SLE
| SLE: discovery cohort (n=282) | SLE: replication cohort (n=228) | |
| Female, n (%) | 243 (86.2) | 207 (90.7) |
| Age (years), mean (range) | 48.6 (18–82) | 47.8 (17–81) |
| Caucasian race/ethnicity, n (%) | 260 (92.2) | 217 (95.2) |
| SLEDAI, mean (range) | 3.2 (0–24) | 2.3 (0–24) |
| Classification criteria (1982 ACR definitions) | ||
| Malar rash, n (%) | 114 (40.4) | 130 (57.0) |
| Discoid lupus, n (%) | 42 (14.9) | 74 (32.5) |
| Photosensitivity, n (%) | 144 (51.1) | 136 (59.6) |
| Oral ulcers, n (%) | 34 (12.1) | 73 (32.0) |
| Arthritis, n (%) | 217 (77.0) | 190 (83.3) |
| Serositis, n (%) | 107 (37.9) | 121 (53.1) |
| Renal disorder, n (%) | 81 (28.7) | 91 (39.9) |
| Neurological disorder, n (%) | 16 (5.7) | 17 (7.5) |
| Haematological disorder, n (%) | 173 (61.4) | 154 (67.5) |
| Immunological disorder, n (%) | 147 (52.1) | 172 (75.4) |
| IF-ANA, n (%) | 279 (98.9) | 227 (99.6) |
| Other manifestations | ||
| Raynaud, n (%) | 75 (28.7)* | 75 (44.1)† |
| PAH, n (%) | 4 (1.4) | 9 (3.9) |
*Data available for 261 of 282.
†Data available for 170 of 228.
ACR, American College of Rheumatology; IF-ANA, ANA detected with immunofluorescence microscopy; PAH, pulmonary arterial hypertension; SLEDAI, SLE Disease Activity Index.
SLE: replication cohort
This cohort consisted of samples obtained from 228 patients (207 women, 21 men), mean age 47.8 years, classified with SLE according to the 1982 ACR criteria as demonstrated in table 1.24 The patients attended the Department of Rheumatology in Lund, Skåne University Hospital, during 1982–2022 and were consecutively asked to participate in the cohort.27
For both SLE cohorts, none of the subjects fulfilled classification criteria for SSc. Global disease activity was assessed by the SLE Disease Activity Index (SLEDAI) at the time point of sampling.28
Primary Sjögren’s syndrome
Samples were obtained from 116 patients (111 women, 5 men), mean age 61.6 years, who fulfilled the American-European Consensus Criteria for pSS.29 The subjects with pSS have previously been described in detail and lived in the same geographical area as those with SLE (discovery cohort).17 None of the subjects with pSS fulfilled classification criteria for SSc or SLE. The samples were collected and stored at the Rheumatology Unit, Linköping University Hospital.
Systemic sclerosis
Samples were obtained from 57 patients (43 women, 14 men), mean age 55.2 years, meeting the 2013 European Alliance of Associations for Rheumatology/ACR classification criteria for SSc.30 In total, 19 (33.3%) had diffuse SSc (15 women), while 38 (66.7%) had limited SSc (28 women). All patients had RP and three (5.3%) were diagnosed with pulmonary arterial hypertension (PAH). Subjects with SSc lived in the same region as patients with SLE (replication cohort) and the samples were collected at the Department of Rheumatology in Lund, Skåne University Hospital.
Healthy blood donors
Samples were obtained from 236 HBDs (127 women, 109 men), mean age 43.8 years, who lived in the same region as the patients with SLE (discovery cohort) and pSS. Twenty-five of these sera were selected due to known positive IF-ANA, whereas the remaining 211 were IF-ANA negative. Via questionnaire, 45 (19.1%) reported RP.
In SLE and SSc, data on RP were self-reported and/or observed by a physician, whereas in HBDs, RP was self-reported.
Autoantibody assays
The samples were analysed using the commercially available line immunoblot assay (EuroLine Systemic Sclerosis (Nucleoli) Profile (IgG); Euroimmun, Lübeck, Germany), and performed at the accredited laboratory of Clinical Immunology, Linköping University Hospital. All serum samples were stored at –70°C until the time of testing according to the manufacturer’s instruction. The antibody kit enables simultaneous detection of 13 different antibody specificities (Scl-70, CENP-A, CENP-B, RNA polymerase III 11 kDa, RNA polymerase III 155 kDa, fibrillarin (U3-RNP), NOR90, Th/To, PM-Scl100, PM-Scl75, Ku, PDGFR and Ro52/SSA). The samples were analysed using EUROBlotmaster (Euroimmun, Euroimmun Lübeck, Germany), and after drying, the strips’ signal intensities (SIs) were read by EuroLineScan. The strength of positive reaction was reported in SI units corresponding to ‘weak positive’ (11–25), ‘positive’ (26–50) or ‘strong positive’ (>50). Borderline results (6–10) were classified as ‘negative’.
All positive findings with EuroLine immunoblot assay were evaluated with an immunodot assay (BlueDot Scleroderma12 IgG; D-tek, Mons, Belgium) and a fluorescence enzyme immunoassay (EliA Phadia 250, Thermo-Fisher Scientific, Phadia, Uppsala, Sweden). BlueDot Scleroderma12 assay enables analysis of the following antibody specificities: Scl-70, CENP-A, CENP-B, PM-Scl100, PM-Scl75, Ku, RNA polymerase III (entire complex), U1-RNP, Th/To, fibrillarin, NOR90 and Ro52/SSA. EliA detects the following specificities: Scl-70, CENP-B, RNA polymerase III (entire complex), fibrillarin and PM-Scl100. The analyses were performed according to the manufacturer’s instructions and recommended cut-offs were used, that is, >10 arbitrary units for BlueDot Scleroderma12 and >10 U/mL for EliA. BlueDot Scleroderma12 strips were analysed using Dr DOT software and scanning system provided by D-tek.
ANA
ANA were detected by IF microscopy on HEp-2 cells (IF-ANA), including interpretation of the staining patterns using the International Consensus on ANA Patterns nomenclature, detailed elsewhere.31 32
IFN-α assay
For the IFN-α assay, samples stored at –70°C until analysis were available only from SLE (discovery cohort; n=282), pSS (n=116) and HBDs (n=226). IFN-α was analysed by ELISA according to the manufacturer’s instructions (Human IFN-α (pan-specific) ELISAPRO kit), Mabtech, Nacka Strand, Sweden) and previously detailed.33 34 This ELISA detects subtypes 1/13, 2, 4, 5, 6, 7, 8, 10, 14, 16 and 17 of IFN-α with a standard ranging from 5 to 4000 pg/mL.
Statistics
The data were analysed using SPSS statistics software V.27.0 (IBM) and Prism V.9 (GraphPad Software, La Jolla, USA) for construction of graphs. Concordance was estimated by the sum of double-positive and double-negative samples, divided by the total number of samples, multiplied by 100. The diagnostic performances of the detected autoantibodies for SSc and RP as outcomes were examined with analyses of sensitivity, specificity, accuracy, positive predictive value (PPV) and negative predictive value (NPV), including 95% CIs. Differences between groups were calculated using χ², Mann-Whitney U test or Fisher’s exact test (where appropriate). Correlation analyses between antibody specificities and IFN-α levels were performed by Spearman’s r. P values of ≤0.01 were considered statistically significant.
Patient and public involvement
Patients or the public were not involved in the design, conduct, reporting or dissemination plans of our research.
Results
SSc-associated autoantibodies in SLE, pSS, SSc and HBDs
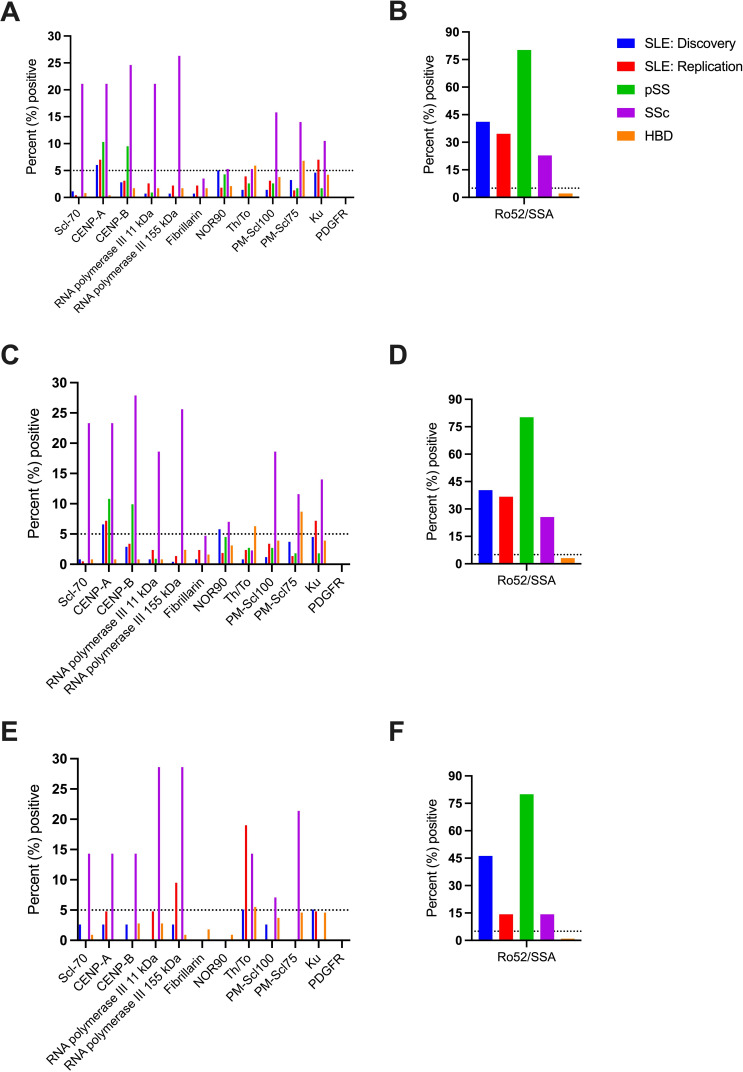
The prevalence of 12 SSc-associated autoantibodies detected by EuroLine is presented in figure 1A. A significantly higher proportion of subjects with SSc than SLE, pSS and HBDs were positive for Scl-70, CENP-A, CENP-B, RNA polymerase III 11 kDa, RNA polymerase III 155 kDa, PM-Scl100 and PM-Scl75 but not for Ku, fibrillarin, NOR90, Th/To or PDGFR. As shown in figure 1B, Ro52/SSA was found in a higher proportion of patients with pSS and SLE than in SSc. Autoantibodies against CENP-A were detected in 6%–10% of patients with SLE (both cohorts) and pSS, CENP-B in approximately 10% of subjects with pSS and Ku in 7% of patients with SLE in the replication cohort. Unexpectedly, high proportions of HBDs (28 of 236) were positive for Th/To and/or PM-Scl75, but in most cases (23 of 28) reactions were ‘weak positive’ (SI 11–25). Autoantibody data were further analysed separately according to sex. As demonstrated, the results essentially remained among both women (figure 1C, D) and men (figure 1E, F).
Figure 1.
Percentage of positive subjects for each autoantibody specificity in SLE, pSS, SSc and HBDs (A, B). Results are divided with regard to sex, for women (C, D) and men (E, F). The dotted line represents 5% positives. A cut-off of ≤5% positives among HBDs is commonly applied for immunoassays. CENP, centromere protein; HBD, healthy blood donor; PDGFR, platelet-derived growth factor receptor; pSS, primary Sjögren’s syndrome; Scl-70, topoisomerase I; SSc, systemic sclerosis.
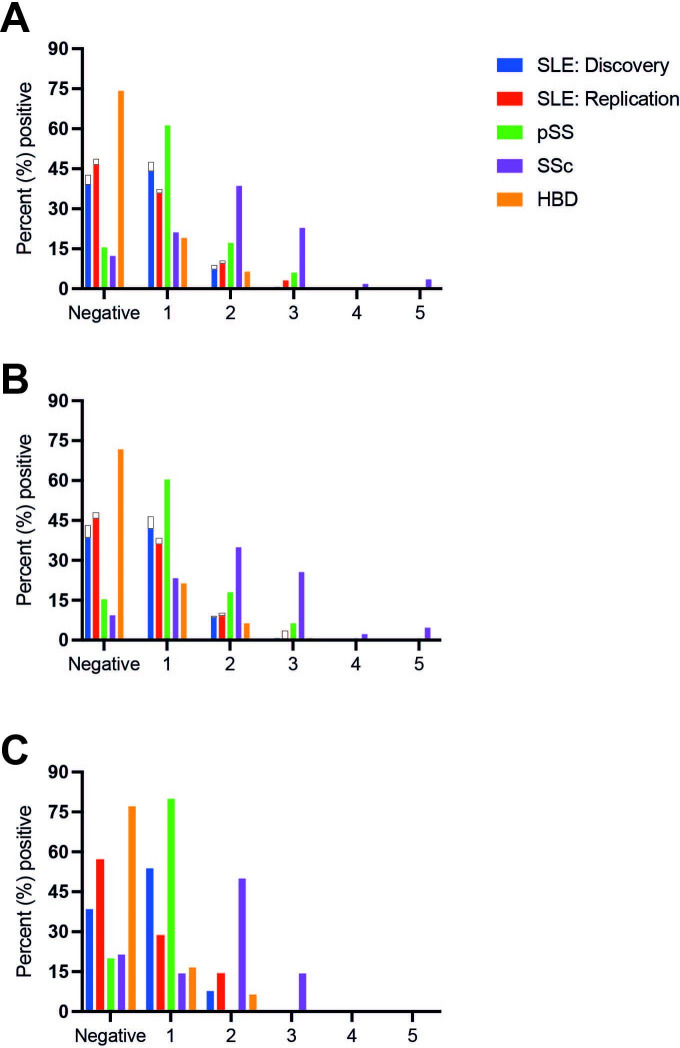
Some individuals were positive for >1 of the antibodies tested. Figure 2A–C illustrates the percentage of individuals positive for multiple autoantibodies, divided according to sex and Caucasian race/ethnicity. Among patients with SSc, 12 (21.1%) were positive for one specificity only, while 22 (38.6%), 13 (22.8%), 1 (1.8%) and 2 (3.5%) were positive for two, three, four and five antibodies, respectively. Seven patients with SSc (12.3%) were negative for all tested SSc-associated antibodies.
Figure 2.
Percentage of individuals (SLE, pSS, SSc and HBDs) positive for multiple autoantibody specificities (A) is shown and divided according to sex (B, C). Non-Caucasian race/ethnicity is illustrated in white (only depicted for SLE). No male patients with SLE had non-Caucasian race/ethnicity (C). HBD, healthy blood donor; pSS, primary Sjögren’s syndrome; SSc, systemic sclerosis.
Among subjects in the SLE discovery cohort, 134 (47.5%) were positive for one specificity only (whereof isolated Ro52/SSA positivity was found in 94 patients), while 25 (8.9%), 2 (0.7%) and 1 (0.4%) were positive for two, three and four antibodies, respectively. Among subjects in the SLE replication cohort, 85 (37.3%) were positive for one specificity only (whereof isolated Ro52/SSA positivity was found in 57 patients), while 24 (10.5%), 7 (3.1%) and 1 (0.4%) were positive for two, three and four antibodies, respectively. The number of positive autoantibodies was not statistically different between Caucasian and non-Caucasian subjects with SLE (p=0.89). Global disease activity, assessed by SLEDAI, was significantly higher in non-Caucasian (p=0.001) than in Caucasian patients. SLEDAI scores showed a borderline significant correlation with the number of positive SSc-associated autoantibodies (r=0.11, p=0.015).
Among individuals with pSS, 71 (61.2%) were positive for one specificity only (whereof isolated Ro52/SSA positivity was found in 68 patients), while 20 (17.2%) and 7 (6.0%) were positive for two and three autoantibodies, respectively. Regarding HBDs, 45 (19.1%) were positive for one specificity and 16 (6.8%) positive for ≥2 SSc-associated autoantibodies. The number of positive autoantibodies was not statistically different between female and male HBDs (p=0.36). Positive nucleolar IF-ANA (AC-8) was detected among four HBDs, of which two also showed an SSc-associated antibody; PM-Scl75 (weak positive) and NOR90 (strong positive), respectively.
Signal intensity of EuroLine results in SLE, pSS, SSc and HBDs
SI values for Scl-70, CENP-A, CENP-B, RNA polymerase III 11 and 155 kDa, PM-Scl100 and PM-Scl75 were highest in patients with SSc (online supplemental table 1). For CENP-A and CENP-B, high SI values were also seen in subjects with pSS. Fibrillarin, NOR90 and Th/To overall showed low and similar SI values across the evaluated groups. For Ku, highest SIs were observed among patients with pSS.
lupus-2022-000732supp001.pdf (123.4KB, pdf)
Evaluation of positive EuroLine results with BlueDot and EliA
Positive EuroLine results from subjects with SLE (both cohorts), pSS and HBDs were evaluated with two alternative methods. Samples showing isolated anti-Ro52/SSA positivity were excluded from these analyses. In total, out of 266 positive EuroLine test results, corresponding specificities were confirmed positive in 48 (18%) with BlueDot. EliA was used to evaluate 97 positive EuroLine test results and 18 (19%) of them could be verified as positive. The level of agreement for positive results between EuroLine and the two alternative methods varied between 0% and 57%, depending on the assay and antibody specificity. Overall, the agreement was higher (approximately 75%) at EuroLine SI values >50, while results in the 11–25 interval showed the lowest agreement (2%–3%) (online supplemental table 2).
In addition, subgroups of the SLE discovery cohort (n=153) and the HBD cohort (n=140) were analysed with EliA, and the complete SSc cohort (n=57) was evaluated with both EliA and BlueDot. With EliA, none of the HBD samples tested positive for any specificity; of the SLE samples, only one (0.7%) sample tested positive for fibrillarin, one (0.7%) for Scl-70 and three (2.0%) for CENP-B. Of the 57 patients with SSc, 51 (89.5%) tested positive with BlueDot. The concordance rates between the three immunoassays in the SSc cohort, in total and for each antibody specificity, were convincingly high as shown in online supplemental table 3.
Immunofluorescence-ANA
The percentages of IF-ANA positivity in the groups were as follows: SLE (discovery cohort) 74.5%, pSS 75.0%, SSc 96.5% and HBDs 10.6%. As demonstrated in table 2, homogeneous staining pattern (AC-1) was the most common in SLE followed by homogeneous/speckled (AC-1+AC-4). In pSS, speckled (AC-4) was the most common pattern followed by AC-1.
Table 2.
IF-ANA staining patterns (HEp-2 cells) according to the ICAP nomenclature for the three diseases and as well as for HBDs
| ICAP staining pattern | SLE: discovery cohort (n=282) | pSS (n=116) | SSc (n=57) | HBDs (n=236) |
| AC-0 (negative) | 72 (25.5) | 29 (25.0) | 2 (3.5) | 211 (89.4) |
| AC-1 (homogeneous) | 104 (36.9) | 30 (25.9) | 8 (14.0) | 9 (3.8) |
| AC-3 (centromere) | 2 (0.7) | 1 (0.9) | 12 (21.1) | 0 (0) |
| AC-4 (speckled) | 41 (14.5) | 36 (31.0) | 17 (29.8) | 6 (2.5) |
| AC-6 (multiple nuclear dots) | 2 (0.7) | 0 (0) | 0 (0) | 1 (0.4) |
| AC-8 (nucleolar) | 10 (3.5) | 1 (0.9) | 1 (1.8) | 4 (1.7) |
| AC-1+4 | 39 (13.8) | 18 (15.5) | 7 (12.3) | 5 (2.1) |
| AC-1+4+8 | 2 (0.7) | 0 (0) | 2 (3.5) | 0 (0) |
| AC-1+8 | 9 (3.2) | 1 (0.9) | 0 (0) | 0 (0) |
| AC-4+7 (speckled+few nuclear dots) | 0 (0) | 0 (0) | 1 (1.8) | 0 (0) |
| AC-4+8 | 1 (0.35) | 0 (0) | 7 (12.3) | 0 (0) |
Percentages are given in parentheses.
HBDs, healthy blood donors; ICAP, International Consensus on ANA Patterns; IF-ANA, ANA detected with immunofluorescence microscopy; pSS, primary Sjögren’s syndrome; SSc, systemic sclerosis.
In SSc, speckled (AC-4) was the most common followed by centromere pattern (AC-3). AC-3 was rare among the other diseases than SSc as well as in HBDs. Six of the seven patients with SSc (85.7%), who tested negative for all SSc-associated antibodies by EuroLine, were IF-ANA positive (one individual with AC-1 and five with AC-4).
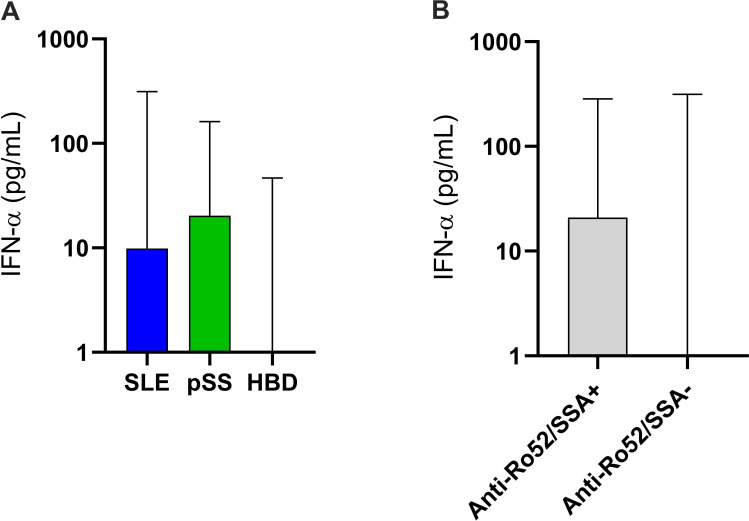
Interferon-α
Detectable levels of IFN-α were found in 178 of 282 patients (63.1%) with SLE (discovery cohort), 77 of 116 (66.4%) pSS and 16 of 226 (7.0%) HBDs (figure 3A). Patients with pSS showed higher levels of IFN-α than those with SLE (p=0.01). IFN-α levels were significantly higher in subjects with ≥1 detected SSc-associated autoantibody (mean 14.1 vs 2.5 pg/mL; p<0.0001) than in negative individuals. In addition, the IFN-α levels correlated with the number of SSc-associated autoantibodies among all (r=0.29, p<0.0001), as well as in separate analyses of SLE (r=0.20, p=0.0006) but unsignificant in HBDs (r=0.16) and pSS (r=0.16). Of all the examined SSc-associated autoantibodies, positive anti-Ro52/SSA showed the strongest association with IFN-α (mean 17.8 vs 3.6 pg/mL; p<0.0001; figure 3B).
Figure 3.
Levels of IFN-α in subjects with SLE, pSS and HBDs (A). The levels were lowest among HBDs, whereas patients with pSS showed significantly higher levels than those with SLE (p=0.01). IFN-α levels were significantly associated with positivity for anti-Ro52/SSA (p<0.0001) (B). HBDs, healthy blood donors; IFN, interferon; pSS, primary Sjögren’s syndrome.
Antibodies versus RP
Data on RP were available for all groups except pSS. None of the autoantibodies associated significantly with RP in SLE or HBDs. None of the HBD subjects reporting RP combined with positive RNA polymerase III 155 kDa and/or PM-Scl100 had been diagnosed with SSc 4 years after sampling. When data from all groups were merged (n=711), antibodies against Scl-70 (p<0.0001), CENP-B (p<0.0001), RNA polymerase III 11 kDa (p<0.001), RNA polymerase III 155 kDa (p<0.0001) and PM-Scl100 (p=0.002) were significantly associated with RP, whereas Ro52/SSA (p=0.004) associated inversely with RP.
Antibodies versus manifestations of SSc
We observed significant associations between pulmonary fibrosis and presence of Scl-70 (EuroLine, p=0.008; EliA, p=0.009), whereas antibodies against RNA polymerase III 11 kDa (EuroLine, p=0.003) and RNA polymerase III 155 kDa (EuroLine, p=0.003) were inversely associated with pulmonary fibrosis. PAH showed a non-significant trend of association with CENP-A (EuroLine, p=0.05). None of the investigated SSc-associated antibodies were associated with arthritis.
The diffuse type of SSc was associated with antibodies against Scl-70 (EuroLine, p=0.001; EliA, p=0.001; BlueDot, p<0.001), RNA polymerase III 11 kDa (EuroLine, p=0.01) and the entire RNA polymerase III complex (BlueDot, p=0.004), whereas the limited type of SSc associated with antibodies against CENP-A (EuroLine, p=0.005; BlueDot, p=0.01) and CENP-B (EuroLine, p=0.002; EliA, p=0.005; BlueDot, p=0.005).
Antibodies versus SLE classification criteria
In SLE, presence of antibodies against CENP-B was significantly associated with serositis in the discovery cohort (p=0.005) and in both cohorts merged (p=0.007) but did not reach statistical significance in the replication cohort. Similarly, anti-Ku associated significantly with lupus nephritis (p=0.007) in the discovery cohort and in both cohorts merged (p=0.01) but not in the replication cohort. Finally, anti-CENP-A was significantly associated with immunological disorder in the discovery cohort (p=0.002) and in both cohorts merged (p=0.001) but was only borderline significant in the replication cohort.
Diagnostic performance of the EuroLine Systemic Sclerosis Profile
The sensitivity, specificity, accuracies, as well as PPV and NPV for SSc diagnosis and presence of RP as outcomes were calculated (table 3). Antibodies included in the 2013 ACR criteria; that is, antibodies targeting Scl-70, RNA polymerase III 11 kDa and RNA polymerase III 155 kDa, achieved best accuracy regarding SSc diagnosis.30 For the entire panel (≥1 positive antibody), the diagnostic sensitivity was estimated to 98% and the diagnostic specificity to 33%.
Table 3.
Diagnostic performance with SSc as outcome based on data from individuals with SLE, pSS, SSc and HBDs
| Sensitivity | Specificity | Accuracy | PPV | NPV | |
| IF-ANA | 0.96 (0.88 to 0.99) | 0.49 (0.45 to 0.53) | 0.66 (0.63 to 0.69) | 0.15 (0.11 to 0.19) | 0.99 (0.98 to 1) |
| Ro52/SSA | 0.23 (0.14 to 0.35) | 0.66 (0.62 to 0.70) | 0.77 (0.74 to 0.79) | 0.06 (0.03 to 0.10) | 0.91 (0.88 to 0.93) |
| Scl-70 | 0.21 (0.12 to 0.33) | 0.99 (0.98 to 1) | 0.96 (0.95 to 0.97) | 0.71 (0.47 to 0.87) | 0.93 (0.91 to 0.95) |
| CENP-A | 0.21 (0.12 to 0.33) | 0.95 (0.93 to 0.97) | 0.94 (0.93 to 0.95) | 0.29 (0.17 to 0.44) | 0.93 (0.91 to 0.95) |
| CENP-B | 0.25 (0.15 to 0.37) | 0.96 (0.95 to 0.98) | 0.95 (0.94 to 0.96) | 0.38 (0.24 to 0.54) | 0.93 (0.91 to 0.95) |
| RNA polymerase III 11 kDa | 0.21 (0.12 to 0.33) | 0.99 (0.98 to 0.99) | 0.96 (0.95 to 0.97) | 0.63 (0.41 to 0.81) | 0.93 (0.91 to 0.95) |
| RNA polymerase III 155 kDa | 0.26 (0.17 to 0.39) | 0.99 (0.98 to 1) | 0.96 (0.95 to 0.97) | 0.71 (0.50 to 0.86) | 0.94 (0.92 to 0.95) |
| Fibrillarin | 0.04 (0.01 to 0.12) | 0.99 (0.98 to 1) | 0.95 (0.94 to 0.96) | 0.25 (0.07 to 0.59) | 0.92 (0.90 to 0.94) |
| NOR90 | 0.05 (0.02 to 0.14) | 0.96 (0.94 to 0.97) | 0.94 (0.93 to 0.95) | 0.11 (0.04 to 0.28) | 0.92 (0.90 to 0.94) |
| Th/To | 0.05 (0.02 to 0.14) | 0.97 (0.95 to 0.98) | 0.94 (0.93 to 0.95) | 0.12 (0.04 to 0.31) | 0.92 (0.90 to 0.94) |
| PM-Scl100 | 0.16 (0.09 to 0.27) | 0.97 (0.96 to 0.98) | 0.95 (0.94 to 0.96) | 0.36 (0.20 to 0.55) | 0.93 (0.91 to 0.95) |
| PM-Scl75 | 0.14 (0.07 to 0.25) | 0.96 (0.94 to 0.97) | 0.94 (0.93 to 0.95) | 0.23 (0.12 to 0.39) | 0.93 (0.90 to 0.94) |
| Ku | 0.11 (0.05 to 0.21) | 0.96 (0.94 to 0.97) | 0.94 (0.93 to 0.95) | 0.19 (0.09 to 0.36) | 0.92 (0.90 to 0.94) |
| PDGFR | 0 (0 to 0.06) | 1 (0.99 to 1) | 0.96 (0.94 to 0.97) | – | 0.92 (0.89 to 0.94) |
| ≥1 antibody specificity | 0.98 (0.91 to 1) | 0.33 (0.29 to 0.36) | 0.49 (0.46 to 0.53) | 0.12 (0.09 to 0.15) | 1 (0.97 to 1) |
Sensitivity, specificity, accuracy, PPV and NPV are detailed including 95% CIs (in parentheses).
CENP, centromere protein; HBDs, healthy blood donors; IF-ANA, ANA detected with immunofluorescence microscopy; NPV, negative predictive value; PDGFR, platelet-derived growth factor receptor; PPV, positive predictive value; pSS, primary Sjögren’s syndrome; Scl-70, topoisomerase I; SSc, systemic sclerosis.
For identifying RP in SLE and HBDs, antibodies against Scl-70, CENP-A, CENP-B, RNA polymerase III 11 kDa, RNA polymerase III 155 kDa, fibrillarin and PM-Scl100 performed best (online supplemental table 4). The entire panel (≥1 positive antibody) showed a sensitivity for RP of 71% and a specificity of 41%.
Discussion
The objective of this cross-sectional study was to investigate the prevalence of SSc-associated antibodies in sera from well-characterised patients with three different type I IFN-dependent diseases and HBDs by using the EuroLine Systemic Sclerosis Profile kit. This immunoassay is commercially available and has been thoroughly evaluated in SSc, but to our knowledge, large groups of disease controls have not been included.19 35–37 Our study propounds caution when using Euroimmun’s immunoassay in the differential diagnosis of patients with recent-onset IFN-mediated rheumatic disease.
We demonstrate that this immunoassay frequently identifies autoantibodies in patients with SLE and pSS (and also in HBDs), but the majority of samples achieving SI values within the 11–25 interval could not be confirmed with alternative methods. In addition, only very few patients with SLE, and none of the HBD sera, tested positive for any specificity with the EliA assay. Based on these findings, we suggest that Euroimmun’s recommended cut-off should be adjusted, and this is important to consider when using this immunoassay in a clinical setting for patients with suspected new-onset rheumatic disease.
Nevertheless, presence of the SSc antibodies was associated with higher levels of IFN-α, which is in line with a recent observation from the USA and previous data from Southern Sweden in patients with early SSc.38 39 Furthermore, our findings are consistent with the profound effects of type I IFNs on B cells, increased plasma cell differentiation, isotype switch and enhanced autoantibody production.11 Herein, we quantified IFN-α with an ELISA which has previously shown good concordance with type I IFN activity measured in vitro by Wistar Institute Susan Hayflick (WISH) reporter cell assay.34
For the patients with SSc, we observed similar associations between the SSc-associated antibodies and organ manifestations/involvement (eg, PAH, pulmonary fibrosis, diffuse and limited SSc) as have been described previously.20 As demonstrated in online supplemental table 3, the concordance between the assays appeared to be high among subjects with SSc. Our evaluation of diagnostic performance of the EuroLine Systemic Sclerosis Profile kit combined with IF-ANA resulted in an overall (≥1 autoantibody) excellent diagnostic sensitivity, whereas the specificity was considerably lower.
The prevalence of RP in our study is consistent with frequencies in previous reports.5 40 41 We had access to data on RP, which were collected for individuals with SSc, SLE and HBDs. Unfortunately, we had no information on tobacco usage or comorbidities associated with RP, for example, hypothyroidism and diabetes, among the HBDs (individuals with well-controlled hypothyroidism and non-insulin-treated diabetes are accepted as blood donors in Sweden). The evaluation of RP as outcome indicated that antibodies targeting Scl-70, CENP-A, CENP-B, RNA polymerase III 11 kDa, RNA polymerase III 155 kDa, fibrillarin and PM-Scl100 achieved the best accuracy. These findings are line with previous observations by Patterson et al.19 However, we acknowledge that our study was cross-sectional and without systematic follow-up of autoantibody-positive subjects.
SLE is known to present with an array of different autoantibodies, and before onset of disease epitope spreading usually occur and increasing numbers of specificities precede clinical diagnosis.42–44 Also in pSS, data indicate that autoantibodies may appear several years before onset of sicca symptoms.45 Herein, however, apart from autoantibodies against Ro52/SSA, which was significantly more common in pSS and SLE than in SSc, only autoantibodies against CENP-A (SLE both cohorts and pSS), CENP-B (pSS) and Ku (SLE replication cohort) were positive in ≥5% indicating that the cut-offs applied by the manufacturer were mostly acceptable.46 However, surprisingly, many samples from the HBD and SLE discovery cohorts showed positivity for the rare specificities Th/To and NOR90, respectively, and only few of these could be verified with BlueDot and EliA. This does raise the question of not only the cut-off, but also of the source and selection of antigens included in the Euroimmun assay.
Interestingly, similarly to our current findings in SSc, Alkema et al demonstrated high concordance between EuroLine, BlueDot and EliA when a large Dutch SSc cohort (n=347) was analysed.47 Yet, that concordance applied to patients with established SSc while our evaluation is based on a small group of patients with SSc, combined with SLE, pSS and HBD individuals generally showing antibodies with lower SI values. Except for Scl-70 that is affinity purified, the antigens of the panel are produced by recombinant techniques that may generate irrelevant epitopes.48
Autoantibodies targeting Scl-70, CENP-B, RNA polymerase III 11 kDa and RNA polymerase III 155 kDa have previously been shown to be the most common in SSc.19 Also in our hands, these antibody specificities had a significantly higher prevalence in SSc compared with pSS, SLE and HBDs. Other SSc-associated antibodies were less common in SLE and pSS (fibrillarin, NOR90 and Th/To). Consistent with a previous report in SLE, we observed an association between anti-Ku and lupus nephritis, but this association could not be confirmed with EliA.49 Anti-fibrillarin antibodies have previously been linked to non-Caucasian ethnicity and poor survival in SSc.50
A striking finding was that the number of subjects positive for >1 autoantibody specificity (figure 2A) differed between the groups. With Ro52/SSA excluded, patients with SLE, pSS and even HBDs were often positive for one autoantibody while those diagnosed with SSc were frequently positive for two or more autoantibodies. In addition, a clear gender difference was observed with very few or none of the male subjects with SLE and pSS showing positivity for >1 autoantibody (figure 2C). Fifty of 57 patients (87.7%) with SSc had at least one SSc-associated autoantibody and only two (3.5%) were IF-ANA negative. Historically, however, both these patients had tested IF-ANA positive. The frequency of negative IF-ANA in SSc has previously been estimated to 6%–15%.51 52 In SLE and pSS, IF-ANA was positive in approximately 75% of patients. This proportion of IF-ANA positivity may appear low. However, as this study had a cross-sectional design and included patients with different disease duration, it should be emphasised that individuals with SLE may lose ANA over time.32 53 It cannot be excluded that this fact may have had an impact on the diagnostic performance tests for SSc and RP herein.
The EuroLine immunoassay includes 13 specificities ranging from established markers with known high diagnostic values for SSc (ie, Scl-70, CENP-A, CENP-B and RNA polymerase III) to rare specificities like Ku, Th/To, NOR90 and PDGFR for which the clinical value is less well known. This underlines that these specificities should not be included for screening purposes but are of value for subtyping individuals with a confirmed, or strongly suspected, diagnosis within the spectrum of systemic scleroderma disorders. The established clinical importance of autoantibodies against Scl-70 and RNA polymerases relies on decades of experience from less sensitive techniques detecting precipitating antibodies.54 In recent years, most clinical laboratories have shifted to automated and broad testing of autoantibodies with more sensitive methods with the risk for lower diagnostic specificity. Automation and broad testing have advantages, but interpretation of unexpected ‘borderline positive’ findings may be challenging and lead to unnecessary investigations as well as patients’ worries. Antibodies against Ku, Th/To, NOR90 and PDGFR have been described in small proportions of patients with SSc. Anti-NOR90 have mostly been associated with limited scleroderma, is rarely found in other rheumatic diseases and may be associated with malignancy.23 Autoantibodies against Ku are also found in other systemic inflammatory diseases, while the diagnostic specificity of Th/To and PDGFR for SSc is reported to be high.55 56 However, our finding of autoantibodies against Th/To in HBDs questions that view.
Limitations of this study include the limited number of patients with SSc. Also, data on RP were not available for subjects with pSS. Indeed, the female-to-male ratio among HBDs was different than among the other groups, but the number of positive autoantibody findings was similar. The study had a cross-sectional design and no longitudinal analyses were made, which is a possible limitation. However, follow-up data from Patterson et al indicate that the SSc-associated antibodies detected by the EuroLine Systemic Sclerosis Profile kit usually remain stable over time.19 There are several strengths of the study, for example, the well-characterised disease controls and the large group of HBDs with data on RP. Another advantage was that all antibody analyses were performed at the same time by an accredited laboratory.
To conclude, we demonstrate that the 13 autoantibodies included in the EuroLine immunoassay are commonly detected among patients with SSc, but they are also frequent among individuals with other diseases characterised by type I IFNs regardless of sex. Positivity for SSc-associated antibodies—especially anti-Ro52/SSA—was linked to higher levels of IFN-α, and among patients with SLE, we observed associations between clinical manifestations and SSc-associated autoantibodies which have not previously been reported. The Systemic Sclerosis Profile kit showed a decent performance regarding diagnostic accuracy, but the diagnostic specificity was lower. An important observation is that weakly positive antibody results could rarely be confirmed when analysed by two alternative assays (especially in samples originating from subjects without SSc), indicating that some of Euroimmun’s recommended cut-offs are too low and/or that antibodies against irrelevant epitopes are detected.
Acknowledgments
Marianne Peterson, Linköping University Hospital, and Birgitta Gullstrand, Lund University, are acknowledged for biobank handling, and Eva Lindbeck at the Clinical Immunology Unit, Linköping University Hospital, and Rui Da Silva Rodrigues at the Clinical Immunology Unit, Karolinska University Hospital, are acknowledged for help with the immunoassays.
Footnotes
RA and AA contributed equally.
Contributors: RA and AA—study design, data collection, statistical analyses, manuscript writing and final approval. PE, LW, CD, RH, AAB, AJ and KA—data collection, manuscript writing and final approval. ÖD—statistical analyses, manuscript writing and final approval. CS—supervision, study design, data collection, manuscript writing and final approval. CS is responsible for the overall content as the guarantor.
Funding: This study was funded by the King Gustaf V and Queen Victoria’s Freemasons Foundation, the King Gustaf V’s 80-year Anniversary Foundation, the Gustafsson Foundation and the Swedish Rheumatism Association, Region Östergötland (ALF grants).
Competing interests: RH is employed by Boehringer Ingelheim. The other authors declare no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Obtained.
Ethics approval
Oral and written informed consent was obtained from all patients and healthy controls. The study was conducted according to the Declaration of Helsinki and the study protocol was approved by the Regional Ethics Boards regarding SLE (Linköping M75-08/2008), SSc (Lund 590/2008) and HBDs (Linköping 2017/474-31), and the Swedish Ethical Review Authority regarding pSS (2020-03287).
References
- 1.Allen ME, Rus V, Szeto GL. Leveraging heterogeneity in systemic lupus erythematosus for new therapies. Trends Mol Med 2021;27:152–71. 10.1016/j.molmed.2020.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Theander E, Jacobsson LTH. Relationship of Sjögren's syndrome to other connective tissue and autoimmune disorders. Rheum Dis Clin North Am 2008;34:viii-ix:935–47. 10.1016/j.rdc.2008.08.009 [DOI] [PubMed] [Google Scholar]
- 3.Avouac J, Sordet C, Depinay C, et al. Systemic sclerosis-associated Sjögren's syndrome and relationship to the limited cutaneous subtype: results of a prospective study of sicca syndrome in 133 consecutive patients. Arthritis Rheum 2006;54:2243–9. 10.1002/art.21922 [DOI] [PubMed] [Google Scholar]
- 4.Ruacho G, Kvarnström M, Zickert A, et al. Sjögren syndrome in systemic lupus erythematosus: a subset characterized by a systemic inflammatory state. J Rheumatol 2020;47:865–75. 10.3899/jrheum.190250 [DOI] [PubMed] [Google Scholar]
- 5.Maricq HR, Carpentier PH, Weinrich MC, et al. Geographic variation in the prevalence of Raynaud's phenomenon: Charleston, SC, USA, vs Tarentaise, Savoie, France. J Rheumatol 1993;20:70–6. [PubMed] [Google Scholar]
- 6.Garner R, Kumari R, Lanyon P, et al. Prevalence, risk factors and associations of primary Raynaud's phenomenon: systematic review and meta-analysis of observational studies. BMJ Open 2015;5:e006389. 10.1136/bmjopen-2014-006389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khouri C, Blaise S, Carpentier P, et al. Drug-induced Raynaud's phenomenon: beyond β-adrenoceptor blockers. Br J Clin Pharmacol 2016;82:6–16. 10.1111/bcp.12912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saraux A, Allain J, Guedes C, et al. Raynaud's phenomenon in rheumatoid arthritis. Br J Rheumatol 1996;35:752–4. 10.1093/rheumatology/35.8.752 [DOI] [PubMed] [Google Scholar]
- 9.Spencer-Green G. Outcomes in primary Raynaud phenomenon: a meta-analysis of the frequency, rates, and predictors of transition to secondary diseases. Arch Intern Med 1998;158:595–600. 10.1001/archinte.158.6.595 [DOI] [PubMed] [Google Scholar]
- 10.Hirschl M, Hirschl K, Lenz M, et al. Transition from primary Raynaud's phenomenon to secondary Raynaud's phenomenon identified by diagnosis of an associated disease: results of ten years of prospective surveillance. Arthritis Rheum 2006;54:1974–81. 10.1002/art.21912 [DOI] [PubMed] [Google Scholar]
- 11.Crow MK, Ronnblom L. Type I interferons in host defence and inflammatory diseases. Lupus Sci Med 2019;6:e000336. 10.1136/lupus-2019-000336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goulden B, Isenberg D. Anti-IFNαR Mabs for the treatment of systemic lupus erythematosus. Expert Opin Biol Ther 2021;21:519–28. 10.1080/14712598.2021.1841164 [DOI] [PubMed] [Google Scholar]
- 13.Goldberg A, Geppert T, Schiopu E, et al. Dose-escalation of human anti-interferon-α receptor monoclonal antibody MEDI-546 in subjects with systemic sclerosis: a phase 1, multicenter, open label study. Arthritis Res Ther 2014;16:R57. 10.1186/ar4492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudnik M, Rolski F, Jordan S, et al. Regulation of monocyte adhesion and type I interferon signaling by CD52 in patients with systemic sclerosis. Arthritis Rheumatol 2021;73:1720–30. 10.1002/art.41737 [DOI] [PubMed] [Google Scholar]
- 15.Assassi S, Li N, Volkmann ER, et al. Predictive significance of serum interferon-inducible protein score for response to treatment in systemic sclerosis-related interstitial lung disease. Arthritis Rheumatol 2021;73:1005–13. 10.1002/art.41627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behmanesh F, Amin R, Khajedaluee M, et al. Autoantibody profile in systemic sclerosis. Acta Med Iran 2010;48:12–20. [PubMed] [Google Scholar]
- 17.Ahmad A, Heijke R, Eriksson P, et al. Autoantibodies associated with primary biliary cholangitis are common among patients with systemic lupus erythematosus even in the absence of elevated liver enzymes. Clin Exp Immunol 2021;203:22–31. 10.1111/cei.13512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamaguchi Y, Takehara K. Anti-nuclear autoantibodies in systemic sclerosis : News and perspectives. J Scleroderma Relat Disord 2018;3:201–13. 10.1177/2397198318783930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patterson KA, Roberts-Thomson PJ, Lester S, et al. Interpretation of an extended autoantibody profile in a well-characterized Australian systemic sclerosis (scleroderma) cohort using principal components analysis. Arthritis Rheumatol 2015;67:3234–44. 10.1002/art.39316 [DOI] [PubMed] [Google Scholar]
- 20.Mahler M, Hudson M, Bentow C, et al. Autoantibodies to stratify systemic sclerosis patients into clinically actionable subsets. Autoimmun Rev 2020;19:102583. 10.1016/j.autrev.2020.102583 [DOI] [PubMed] [Google Scholar]
- 21.Volkmann ER, Andréasson K, Smith V. Systemic sclerosis. Lancet 2022. 10.1016/S0140-6736(22)01692-0. [Epub ahead of print: 25 Nov 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahler M, Swart A, Wu J, et al. Clinical and serological associations of autoantibodies to the Ku70/Ku80 heterodimer determined by a novel chemiluminescent immunoassay. Lupus 2016;25:889–96. 10.1177/0961203316640918 [DOI] [PubMed] [Google Scholar]
- 23.Yamashita Y, Yamano Y, Muro Y, et al. Clinical significance of anti-NOR90 antibodies in systemic sclerosis and idiopathic interstitial pneumonia. Rheumatology 2022;61:1709–16. 10.1093/rheumatology/keab575 [DOI] [PubMed] [Google Scholar]
- 24.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–7. 10.1002/art.1780251101 [DOI] [PubMed] [Google Scholar]
- 25.Petri M, Orbai A-M, Alarcón GS, et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. 10.1002/art.34473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ighe A, Dahlström Örjan, Skogh T, et al. Application of the 2012 systemic lupus international collaborating clinics classification criteria to patients in a regional Swedish systemic lupus erythematosus register. Arthritis Res Ther 2015;17:3. 10.1186/s13075-015-0521-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nived O, Ingvarsson RF, Jöud A, et al. Disease duration, age at diagnosis and organ damage are important factors for cardiovascular disease in SLE. Lupus Sci Med 2020;7:e000398. 10.1136/lupus-2020-000398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002;29:288–91. [PubMed] [Google Scholar]
- 29.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European consensus group. Ann Rheum Dis 2002;61:554–8. 10.1136/ard.61.6.554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of rheumatology/European League against rheumatism collaborative initiative. Ann Rheum Dis 2013;72:1747–55. 10.1136/annrheumdis-2013-204424 [DOI] [PubMed] [Google Scholar]
- 31.Agmon-Levin N, Damoiseaux J, Kallenberg C, et al. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann Rheum Dis 2014;73:17–23. 10.1136/annrheumdis-2013-203863 [DOI] [PubMed] [Google Scholar]
- 32.Frodlund M, Wetterö J, Dahle C, et al. Longitudinal anti-nuclear antibody (ANA) seroconversion in systemic lupus erythematosus: a prospective study of Swedish cases with recent-onset disease. Clin Exp Immunol 2020;199:245–54. 10.1111/cei.13402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zickert A, Oke V, Parodis I, et al. Interferon (IFN)-λ is a potential mediator in lupus nephritis. Lupus Sci Med 2016;3:e000170. 10.1136/lupus-2016-000170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oke V, Gunnarsson I, Dorschner J, et al. High levels of circulating interferons type I, type II and type III associate with distinct clinical features of active systemic lupus erythematosus. Arthritis Res Ther 2019;21:107. 10.1186/s13075-019-1878-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang WSJ, Schollum J, White DHN, et al. A cross-sectional study of autoantibody profiles in the Waikato systemic sclerosis cohort, New Zealand. Clin Rheumatol 2015;34:1921–7. 10.1007/s10067-015-2981-3 [DOI] [PubMed] [Google Scholar]
- 36.Hamaguchi Y, Kuwana M, Takehara K. Performance evaluation of a line blot assay system for detection of anti-PM-Scl antibody in Japanese patients with systemic sclerosis. Int J Rheum Dis 2019;22:1746–51. 10.1111/1756-185X.13638 [DOI] [PubMed] [Google Scholar]
- 37.Liu C, Hou Y, Yang Y, et al. Evaluation of a commercial immunoassay for autoantibodies in Chinese Han systemic sclerosis population. Clin Chim Acta 2019;491:121–5. 10.1016/j.cca.2019.01.020 [DOI] [PubMed] [Google Scholar]
- 38.Hubbard EL, Pisetsky DS, Lipsky PE. Anti-RNP antibodies are associated with the interferon gene signature but not decreased complement levels in SLE. Ann Rheum Dis 2022;81:632–43. 10.1136/annrheumdis-2021-221662 [DOI] [PubMed] [Google Scholar]
- 39.Wuttge DM, Lood C, Tufvesson E, et al. Increased serum type I interferon activity in early systemic sclerosis patients is associated with antibodies against Sjögren's syndrome antigens and nuclear ribonucleoprotein antigens. Scand J Rheumatol 2013;42:235–40. 10.3109/03009742.2012.736532 [DOI] [PubMed] [Google Scholar]
- 40.Cervera R, Khamashta MA, Font J, et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine 2003;82:299–308. 10.1097/01.md.0000091181.93122.55 [DOI] [PubMed] [Google Scholar]
- 41.Hughes M, Herrick AL. Systemic sclerosis. Br J Hosp Med 2019;80:530–6. 10.12968/hmed.2019.80.9.530 [DOI] [PubMed] [Google Scholar]
- 42.Yaniv G, Twig G, Shor DB-A, et al. A volcanic explosion of autoantibodies in systemic lupus erythematosus: a diversity of 180 different antibodies found in SLE patients. Autoimmun Rev 2015;14:75–9. 10.1016/j.autrev.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 43.Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 2003;349:1526–33. 10.1056/NEJMoa021933 [DOI] [PubMed] [Google Scholar]
- 44.Eriksson C, Kokkonen H, Johansson M, et al. Autoantibodies predate the onset of systemic lupus erythematosus in northern Sweden. Arthritis Res Ther 2011;13:R30. 10.1186/ar3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jonsson R, Theander E, Sjöström B, et al. Autoantibodies present before symptom onset in primary Sjögren syndrome. JAMA 2013;310:1854–5. 10.1001/jama.2013.278448 [DOI] [PubMed] [Google Scholar]
- 46.Damoiseaux J, Potjewijd J, Smeets RL, et al. Autoantibodies in the disease criteria for systemic sclerosis: the need for specification for optimal application. J Transl Autoimmun 2022;5:100141. 10.1016/j.jtauto.2022.100141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alkema W, Koenen H, Kersten BE, et al. Autoantibody profiles in systemic sclerosis; a comparison of diagnostic tests. Autoimmunity 2021;54:148–55. 10.1080/08916934.2021.1907842 [DOI] [PubMed] [Google Scholar]
- 48.Rönnelid J. The choice of laboratory methodology influences autoantibody test results. Front Immunol 2015;6:392. 10.3389/fimmu.2015.00392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sjöwall C, Bentow C, Aure MA, et al. Two-parametric immunological score development for assessing renal involvement and disease activity in systemic lupus erythematosus. J Immunol Res 2018;2018:1–9. 10.1155/2018/1294680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mejia Otero C, Assassi S, Hudson M, et al. Antifibrillarin antibodies are associated with native North American ethnicity and poorer survival in systemic sclerosis. J Rheumatol 2017;44:799–805. 10.3899/jrheum.160574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kavanaugh A, Tomar R, Reveille J, et al. Guidelines for clinical use of the antinuclear antibody test and tests for specific autoantibodies to nuclear antigens. American College of pathologists. Arch Pathol Lab Med 2000;124:71–81. 10.5858/2000-124-0071-GFCUOT [DOI] [PubMed] [Google Scholar]
- 52.Salazar GA, Assassi S, Wigley F, et al. Antinuclear antibody-negative systemic sclerosis. Semin Arthritis Rheum 2015;44:680–6. 10.1016/j.semarthrit.2014.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi MY, Clarke AE, Urowitz M, et al. Longitudinal analysis of ANA in the systemic lupus international collaborating clinics (SLICC) inception cohort. Ann Rheum Dis 2022;81:1143–50. 10.1136/annrheumdis-2022-222168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Basu D, Reveille JD. Anti-scl-70. Autoimmunity 2005;38:65–72. 10.1080/08916930400022947 [DOI] [PubMed] [Google Scholar]
- 55.Satoh M, Ceribelli A, Hasegawa T, et al. Clinical significance of Antinucleolar antibodies: biomarkers for autoimmune diseases, malignancies, and others. Clin Rev Allergy Immunol 2022;63:210–39. 10.1007/s12016-022-08931-3 [DOI] [PubMed] [Google Scholar]
- 56.Baroni SS, Santillo M, Bevilacqua F, et al. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med 2006;354:2667–76. 10.1056/NEJMoa052955 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
lupus-2022-000732supp001.pdf (123.4KB, pdf)
Data Availability Statement
Data are available upon reasonable request.