Abstract
Fibroblast activation protein (Fap) is a serine protease that degrades denatured type I collagen, α2-antiplasmin and FGF21. Fap is highly expressed in bone marrow stromal cells and functions as an osteogenic suppressor and can be inhibited by the bone growth factor Osteolectin (Oln). Fap is also expressed in synovial fibroblasts and positively correlated with the severity of rheumatoid arthritis (RA). However, whether Fap plays a critical role in osteoarthritis (OA) remains poorly understood. Here, we found that Fap is significantly elevated in osteoarthritic synovium, while the genetic deletion or pharmacological inhibition of Fap significantly ameliorated posttraumatic OA in mice. Mechanistically, we found that Fap degrades denatured type II collagen (Col II) and Mmp13-cleaved native Col II. Intra-articular injection of rFap significantly accelerated Col II degradation and OA progression. In contrast, Oln is expressed in the superficial layer of articular cartilage and is significantly downregulated in OA. Genetic deletion of Oln significantly exacerbated OA progression, which was partially rescued by Fap deletion or inhibition. Intra-articular injection of rOln significantly ameliorated OA progression. Taken together, these findings identify Fap as a critical pathogenic factor in OA that could be targeted by both synthetic and endogenous inhibitors to ameliorate articular cartilage degradation.
Subject terms: Pathogenesis, Bone
Introduction
Osteoarthritis (OA) is one of the most common orthopedic diseases worldwide,1,2 with an approximately 26.6% prevalence rate among people over 45 years old.3 The most prominent pathological changes of OA include articular cartilage degeneration, osteophyte formation, low-grade inflammation and subchondral bone remodeling.4–8 After initial mechanical erosion during aging, articular cartilage and synovium express a panel of proteolytic enzymes and proinflammatory factors that accelerate cartilage matrix degradation and OA progression.9–11 Early-stage OA patients can be treated by microfracture, osteochondral allograft transplantation, or biomaterial implantation,12,13 while late-stage OA patients can only be treated by replacement plastic surgeries.14 Pharmacological treatments such as nonsteroidal anti-inflammatory drugs and intra-articular glucocorticoid injection are recommended by most guidelines to relieve inflammation and pain.15–17 In contrast, the effectiveness of other drugs, such as glucosamine, chondroitin and hyaluronic acid, remains controversial.15–17 Therefore, drugs with higher efficacy for OA treatment are under intensive investigation.18
Aggrecan (Acan) and type II collagen (Col II) are the two major components of the cartilage matrix.19 Whereas Acan degradation is mainly mediated by a disintegrin and metalloprotease with thrombospondin motifs (ADAMTS) family proteases,20 Col II degradation is mainly catalyzed by matrix metalloproteinases (MMPs).21 Collagenases, such as MMP1/8/13, are responsible for unwinding and cleaving the triple-helical Col II fiber into large fragments,22–25 after which gelatinases, such as MMP2/9, further digest them into smaller peptides.26,27 Although MMPs were initially considered potential drug targets for OA treatment, none of the nonselective MMP inhibitors passed clinical trials due to potential side effects, including joint stiffness, inflammation and pain.28,29 In contrast, selective MMP inhibitors demonstrated low solubility and permeability,23 precluding their clinical applications. In addition to ADAMTS and MMPs, serine proteases, such as thrombin, matriptase and HtrA1, were also implicated in OA progression.30–33 However, no serine protease inhibitors have been approved to treat OA.
Fibroblast activation protein (Fap) is a serine protease with both dipeptidyl peptidase and endopeptidase activities.34,35 It is known to degrade denatured type I collagen (Col I), alpha2-antiplasmin and FGF21 in vivo35–39 as well as several other substrates in vitro.40,41 Fap was upregulated in activated fibroblasts and critically involved in tumor microenvironment formation, inflammation and wound healing.42–47 In the musculoskeletal system, Fap is highly expressed in bone marrow stromal cells (BMSCs) and osteoblasts, which function as osteogenic suppressors.48 Genetic deletion or pharmacological inhibition of Fap promotes bone formation and inhibits bone resorption, suggesting that it is a potential anti-osteoporosis drug target.48 Interestingly, Fap was also detected in the synovium of OA patients, although at a lower level compared to rheumatoid arthritis (RA) patients.49,50 Whether Fap plays an important role in regulating OA progression remains poorly understood.
Osteolectin (Oln), also known as Clec11a or Scgf, was initially identified as a growth factor that promotes hematopoietic colony formation in vitro.51,52 Our previous in vivo studies showed that Oln is highly expressed in osteochondral lineage cells and functions as an anabolic factor that promotes bone formation by activating the Wnt pathway.53,54 We also demonstrated that Oln interacts with Fap and inhibits its protease activity,48 suggesting a novel mechanism by which Oln promotes bone formation and osteogenic differentiation.48 In the present study, we found that synovium-derived Fap exacerbates OA progression. Oln forms a protective barrier on the cartilage surface and shows the opposite effects. A mechanistic study revealed that Fap promotes the degradation of Col II in osteoarthritic cartilage, which could be ameliorated by intra-articular injection of a small molecule inhibitor of Fap or recombinant Oln.
Results
Fap is significantly upregulated in OA synovium
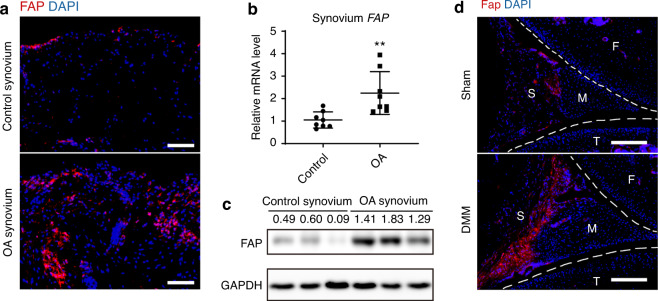
To test whether Fap is involved in OA pathogenesis, we first analyzed its expression in the synovium of patients diagnosed with OA or acute joint injury (control). Immunofluorescence staining showed that human FAP was mainly detected in the OA synovium (Fig. 1a and Supplementary Fig. 1a). Quantitative real-time PCR (qPCR) confirmed that FAP mRNA was significantly upregulated in OA synovium compared to control synovium (Fig. 1b). Western blot analysis showed an even greater increase in FAP protein levels in the OA synovium (Fig. 1c). Consistent with the human data, Fap was only marginally detected in the synovium of sham-operated mice, whereas it was significantly upregulated in OA synovium after surgical destabilization of the medial meniscus (DMM) (Fig. 1d and Supplementary Fig. 1b). To test whether the inflammatory microenvironment induced by OA10 regulates Fap expression, we stimulated primary human synovial fibroblasts with IL-1β ex vivo. We found that FAP mRNA was significantly increased in a time-dependent manner, suggesting that proinflammatory factors promote Fap expression in the synovium (Fig. S1c).
Fig. 1.
Expression analysis of Fap in OA synovium. a Immunostaining of human FAP in the synovium of control and OA patients. DAPI staining indicates the nucleus. Scale bars: 100 μm. b qPCR analysis of human FAP mRNA levels in the synovium of control and OA patients (n = 8 samples per group). c Western blot analysis of human FAP protein levels in the synovium of control and OA patients (n = 3 samples per group). d Immunostaining of mouse Fap in the knee joint of sham and DMM-treated mice. Sham or DMM surgery was performed in 8-week-old FapLacZ/+ mice, which were sacrificed 8 weeks later, and LacZ immunostaining was used to detect Fap expression at the posterior horn of the medial meniscus (F: femur; T: tibia; M: meniscus; S: synovium). DAPI staining indicates the nucleus. Scale bars: 100 μm. The statistical significance was assessed using two-tailed Student’s unpaired t tests. Data are presented as the mean ± SD (**P < 0.01)
Genetic deletion or pharmacological inhibition of Fap ameliorates OA progression
To test whether Fap regulates OA progression, we performed unilateral knee joint DMM surgery in Fap-deficient (Fap KO) and wild-type control mice. Three days after the surgery, we administered weekly intra-articular injections of a Fap-selective small molecule inhibitor (FAPi, 40 μg·kg−1 body weight)55 or vehicle control (PBS) in the DMM-treated knee joints for 8 weeks. Mice were then sacrificed, and knee joint sections of the medial compartment were stained with safranin O and fast green.
Histomorphometry analyses in the contralateral knee joint without DMM surgery showed no significant differences between Fap KO and control mice (Fig. S2a–f), suggesting that Fap is dispensable for knee joint development and maintenance under a steady state. In contrast, the knee joint with DMM surgery showed significant cartilage erosion, subchondral bone thickening and synovitis in control mice (Fig. 2a–f). Notably, these OA symptoms were significantly ameliorated in Fap KO mice (Fig. 2a–f). Intra-articular administration of FAPi in control mice also significantly ameliorated cartilage erosion, subchondral bone thickening and synovitis compared to the vehicle group (Fig. 2a–f). Importantly, FAPi administration showed no therapeutic effects in Fap KO mice, indicating that the inhibitor functioned in a Fap-dependent manner (Fig. 2a–f). Immunofluorescent staining of Col2a1 in the articular cartilage showed similar results (Fig. 2g, h). We also used micro-CT to analyze osteophyte formation and found that the intra-articular administration of FAPi significantly ameliorated osteophyte formation in control but not Fap KO mice (Fig. S3a, b). There was also a trend toward decreased osteophyte formation in Fap KO mice compared to control mice after vehicle treatment (Supplementary Fig. 3a, b).
Fig. 2.
Fap promotes OA progression in a mouse DMM model. a–c Safranin O/Fast Green staining in control and Fap KO mice treated with FAPi or vehicle after DMM surgery. Weekly intra-articular administration of FAPi (40 μg/kg body weight) or vehicle (PBS) in 10-week-old mice was started 3 days after the surgery and continued for 8 weeks before paraffin sectioning and safranin O/fast green staining of the knee joints. Representative cartilage erosion (a, top: femur, bottom: tibia), subchondral bone thickening (b) and synovitis (c) images are shown (n = 6–7 mice per genotype in each treatment group). Yellow dotted lines indicate the subchondral bone plate. Arrows indicate the synovium. Scale bars: 100 μm. d–f Quantification of the OARSI score (d), subchondral bone thickness (e) and synovitis score (f). OARSI scores were calculated based on the erosion of the medial tibial plateau cartilage (a, bottom). Subchondral bone thickness was measured as the mean distance of five evenly distributed measuring points between the lower edge of the articular cartilage and the roof of the cancellous bone. Synovitis scores were calculated by summing the enlargement of the synovial lining cell layer, density of the resident cells and inflammatory infiltration. g, h Immunostaining of Col II in the knee joints (g) with quantifications (h) (n = 6 mice per genotype in each treatment group). DAPI staining indicates the nucleus. Scale bars: 100 μm. The statistical significance was assessed using two-way ANOVAs with Sidak’s multiple comparison tests. Data are presented as the mean ± SD (*P < 0.05, **P < 0.01, ***P < 0.001)
To test whether FAPi administration could ameliorate articular symptoms after the onset of OA, we first performed DMM surgery in wild-type mice and waited for 4 weeks. We then administered weekly intra-articular injections of FAPi or vehicle control for another 8 weeks. Compared to sham-operated mice, vehicle-treated mice showed profound cartilage erosion, subchondral bone thickening and synovitis after DMM (Fig. 3a–f), which were significantly ameliorated by FAPi treatment (Fig. 3a–f). Immunofluorescent staining of Col2a1 in the articular cartilage showed similar results (Fig. 3g, h). Taken together, these data showed that the genetic deletion or pharmacological inhibition of Fap significantly ameliorated OA progression.
Fig. 3.
Pharmacological inhibition of Fap ameliorates joint symptoms after the onset of OA. a–c Safranin O/Fast Green staining in wild-type mice treated with FAPi or vehicle after DMM surgery. Weekly intra-articular administration of FAPi (40 μg·kg−1 body weight) or vehicle (PBS) was performed 4 weeks after DMM surgery in 10-week-old mice and continued for 8 weeks before paraffin sectioning and safranin O/fast green staining of the knee joints. Representative cartilage erosion (a, top: femur, bottom: tibia), subchondral bone thickening (b) and synovitis (c) images are shown (n = 6 mice per group). Yellow dotted lines indicate the subchondral bone plate. Arrows indicate the synovium. Scale bars: 100 μm. d–f Quantification of the OARSI score (d), subchondral bone thickness (e) and synovitis score (f). g, h Immunostaining of Col II in the knee joints (g) with quantification (h) (n = 6 mice per group). DAPI staining indicates the nucleus. Scale bars: 100 μm. The statistical significance was assessed using one-way ANOVAs with Tukey’s multiple comparison tests. Data are presented as the mean ± SD (*P < 0.05, **P < 0.01, ***P < 0.001)
Fap does not regulate chondrocyte function in a cell-autonomous manner
Fap is expressed in perichondral mesenchymal cells surrounding the cartilage primordia during mouse embryonic development.56 Consistent with this, Fap was mainly detected in the synovium, but not articular cartilage, of normal and OA knee joints in adult mice (Fig. 1d). To test whether Fap regulates chondrocyte function in a cell-autonomous manner, we cultured primary articular chondrocytes from Fap KO or wild-type newborn mice and stimulated the cells with IL-1β to mimic OA pathogenesis.57–59 Consistent with a previous study,60 qPCR analysis in control chondrocytes showed significantly decreased Acan and Col2a1 mRNA levels and significantly increased Mmp3 mRNA levels after IL-1β stimulation (Fig. S4a–c). Compared to control chondrocytes, Fap KO chondrocytes showed similar Acan, Col2a1 and Mmp3 expression before and after IL-1β stimulation (Fig. S4a–c). Consistent with this, western blot analysis showed significantly decreased Acan and Col2a1 protein levels and significantly increased Mmp3 protein levels after IL-1β stimulation (Fig. S4d, e), with no significant differences between Fap KO and control chondrocytes (Supplementary Fig. 4d, e).
Next, we treated primary wild-type chondrocytes with 10 μg/ml FAPi for different periods ex vivo but still found no significant changes in Acan, Col2a1 or Mmp3 mRNA levels (Supplementary Fig. 5a–c). Different doses of FAPi were also administered with or without IL-1β stimulation during ex vivo culture, which did not significantly affect the expression of Acan, Col2a1 or Mmp3 in primary wild-type chondrocytes (Fig. S5d–f). Taken together, these data suggest that Fap does not autonomously regulate chondrocyte function.
Col II is a novel substrate of Fap both in vitro and in vivo
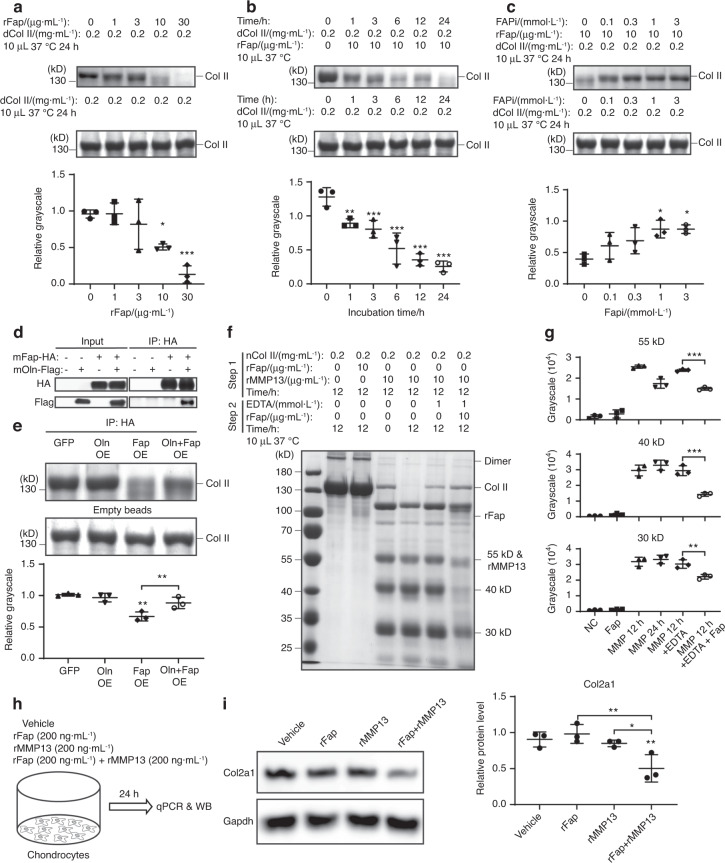
Fap participates in extracellular matrix remodeling in many diseases, such as pancreatic cancer, myocardial infarction, and thin-cap fibroatheroma,61–63 suggesting that it might play similar roles during OA progression. Since Fap is a serine protease that degrades denatured or Mmp1-cleaved Col I,35,63–65 we hypothesized that Fap could also degrade Col II, the key component of the cartilage matrix. Similar to native Col I, native Col II was resistant to digestion by recombinant Fap (rFap) (Supplementary Fig. 6a). Thus, we heat-inactivated Col II at 95 °C for 10 min to depolymerize it into monomers and then incubated it with rFap at 37 °C for 24 h. Remarkably, we observed a dose- and time-dependent degradation of denatured Col II by rFap (Fig. 4a, b). Preincubation with FAPi for 30 min dose-dependently inhibited the degradation of Col II by rFap (Fig. 4c), further validating the efficacy of FAPi. Our recent study demonstrated that Oln functions as an endogenous Fap inhibitor to promote bone formation.48 To test whether Oln inhibits the degradation of denatured Col II by rFap, we overexpressed mouse Fap, alone or together with mouse Oln, in HEK293T cells and immunoprecipitated Fap (Fig. 4d). Consistent with our previous study,48 Oln physically interacts with Fap, which does not affect the amount of Fap that was expressed or precipitated (Fig. 4d). Immunoprecipitated Fap also degraded denatured Col II, which could be partially inhibited by Oln coexpression (Fig. 4e).
Fig. 4.
Fap degrades denatured or MMP13-cleaved Col II. a Fap degrades denatured Col II in a dose-dependent manner. Denatured Col II (boiled at 95 °C for 10 min) was incubated with different amounts of rFap at 37 °C for 24 h. Samples were separated by SDS‒PAGE and quantified by colloidal blue staining (n = 3 independent experiments). b Fap degrades denatured Col II in a time-dependent manner. Denatured Col II was incubated with rFap at 37 °C for 0–24 h (n = 3 independent experiments). c FAPi inhibits Fap-mediated degradation of denatured Col II. Different doses of FAPi were preincubated with rFap for 30 min before denatured Col II was added and further incubated at 37 °C for 24 h (n = 3 independent experiments). d Immunoprecipitation of Fap. HEK293T cells were transfected with GFP control, Oln-Flag, Fap-HA, or Oln-Flag + Fap-HA. Two days after transfection, Fap was immunoprecipitated from total cell lysates with 10 μL anti-HA affinity gel. Five percent total cell lysates were loaded as an input control (n = 3 independent experiments). e Oln inhibits the Fap-mediated degradation of denatured Col II. Col II was coincubated with immunoprecipitated samples (in 10 μL anti-HA affinity gel) at 37 °C for 24 h (n = 3 independent experiments). f Fap degrades MMP13-cleaved Col II. Native Col II was preincubated with rFap or rMMP13 at 37 °C for 12 h. EDTA was then added to the reaction mixture with or without rFap and incubated at 37 °C for another 12 h (n = 3 independent experiments). g Grayscale quantification of the 55, 40 and 30 kDa digestion bands in (f). h Experimental design. Primary chondrocytes were stimulated with vehicle control (PBS), 200 ng·mL−1 rFap, 200 ng·mL−1 rMMP13, or 200 ng·mL−1 rFap plus 200 ng·mL−1 rMMP13 for 24 h. i Western blot analysis of mouse Col2a1 protein levels in primary chondrocytes stimulated as in (h) (n = 3 independent experiments). The statistical significance was assessed using one-way ANOVAs with Tukey’s multiple comparison tests. Data are presented as the mean ± SD (*P < 0.05, **P < 0.01, ***P < 0.001)
Next, we tested whether Fap exhibits gelatinase activity similar to MMP2/9, which further digests MMP13-cleaved Col II into smaller fragments.26,27 Recombinant human MMP13 (rMMP13, 10 μg·mL−1) was able to digest native Col II into 6 major fragments in a time-dependent manner (Fig. 4f). When we terminated the protease activity of MMP13 using EDTA after 12 h of incubation and then added rFap (10 μg·mL−1) for another 12 h, the 55, 40 and 30 kD fragments could be further digested by rFap (Fig. 4f, g). To test whether Fap degrades Col II in a pathological setting, we measured the Fap level in synovial fluids from OA patients by ELISA and found that its average concentration was approximately 150 ng·mL−1 (Fig. S6b). A previous study reported that the concentration of MMP13 in OA synovial fluids is approximately 200 ng·mL−1.66,67 We then cultured primary chondrocytes from newborn mice and administered rFap (200 ng·mL−1), rMMP13 (200 ng·mL−1), or rFap plus rMMP13 for 24 h (Fig. 4h). qPCR analysis showed no significant changes in Col2a1 mRNA levels in any treatment group (Supplementary Fig. 6c). Western blot analysis showed unaltered Col2a1 protein levels in total cell lysates after rFap or rMMP13 treatment (Fig. 4i) but significantly decreased Col2a1 protein levels in the rFap plus rMMP13 group (Fig. 4i). Together, these data indicate that Fap works in concert with MMP13 to degrade Col II both in vitro and ex vivo.
To test whether rFap exacerbates OA progression and Col II degradation in vivo, we administered weekly intra-articular injections of rFap after the onset of OA (4 weeks after DMM surgery) for 8 weeks. Compared to vehicle controls, rFap-treated mice showed significantly increased articular cartilage erosion, subchondral bone thickening and synovitis (Fig. 5a–f). Micro-CT analysis showed increased osteophyte formation after rFap injection (Fig. S7a, b). Immunofluorescent staining of Col2a1 showed a significant decrease in the Col2a1+ area in the articular cartilage of rFap-treated mice (Fig. 5g, h). Together with the fact that the Col II level was significantly elevated in the articular cartilage by genetic or pharmacological inhibition of Fap after DMM (Figs. 2g, h and 3g, h), we concluded that Col II is a novel substrate of Fap in vivo.
Fig. 5.
rFap accelerates joint symptoms after the onset of OA. a–c Safranin O/Fast Green staining in wild-type mice administered rFap or vehicle after DMM surgery. Weekly intra-articular administration of rFap (40 μg/kg body weight) or vehicle (PBS) was performed 4 weeks after DMM surgery in 10-week-old mice and continued for 8 weeks before paraffin sectioning and safranin O/fast green staining of the knee joints. Representative cartilage erosion (a, top: femur, bottom: tibia), subchondral bone thickening (b) and synovitis (c) images are shown (n = 7 mice per group). Yellow dotted lines indicate the subchondral bone plate. Arrows indicate the synovium. Scale bars: 100 μm. d–f Quantification of the OARSI score (d), subchondral bone thickness (e) and synovitis score (f). g, h Immunostaining of Col II in the knee joints (g) with quantification (h) (n = 6 mice per group). DAPI staining indicates the nucleus. Scale bars: 100 μm. The statistical significance was assessed using one-way ANOVAs with Tukey’s multiple comparison tests. Data are presented as the mean ± SD (*P < 0.05, **P < 0.01, ***P < 0.001)
To test whether Fap degrades Acan, another key component of the cartilage matrix, we incubated rFap with recombinant human Acan (G1-IGD-G2 domain) at 37 °C for 24 h. rFap could not digest either native or denatured Acan (Supplementary Fig. 6d, e), indicating that Fap specifically degrades Col II in the cartilage matrix.
Oln is significantly downregulated in OA cartilage
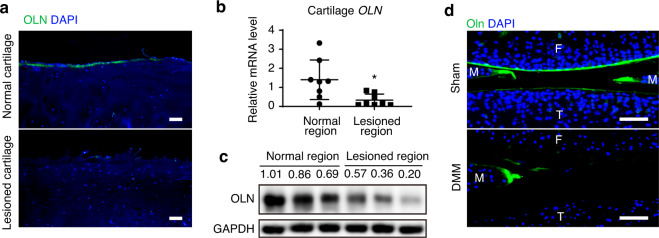
Since Oln functions as an endogenous inhibitor of Fap,48 we tested whether Oln also regulates OA progression. Our previous study showed that Oln is highly expressed in growth plate chondrocytes, osteoblasts and BMSCs.53 Immunofluorescent staining showed that Oln is also expressed in the superficial layer of normal human articular cartilage but absent in the lesioned cartilage from OA patients (Fig. 6a and Supplementary Fig. 8a). qPCR analysis showed that OLN levels were significantly decreased in lesioned OA cartilage compared to adjacent normal cartilage (Fig. 6b), which was confirmed by western blotting (Fig. 6c). In contrast to Fap, Oln was not detected in either normal or OA patient synovium (Supplementary Fig. 8b).
Fig. 6.
Expression analysis of Oln in OA cartilage. a Immunostaining of human OLN in the articular cartilage of control and OA patients. DAPI staining indicates the nucleus. Scale bars: 100 μm. b qPCR analysis of OLN mRNA levels in the articular cartilage of OA patients. Cartilage samples were separated into normal and lesioned regions based on their morphology (n = 8 samples per group). c Western blot analysis of OLN protein levels in the articular cartilage from OA patients (n = 3 samples per group). d Immunostaining of mouse Oln in the knee joints of sham and DMM-treated mice. Sham or DMM surgery was performed in 8-week-old wild-type mice, which were analyzed 8 weeks later (F: Femur; T: Tibia). DAPI staining indicates the nucleus. Scale bars: 100 μm. The statistical significance was assessed using two-tailed Student’s unpaired t tests. Data are presented as the mean ± SD (*P < 0.05)
We also analyzed the localization of Oln in the mouse knee joint and found that it is expressed in the superficial layer of articular cartilage and meniscus (Fig. 6d). Consistent with the human data, mouse Oln was dramatically downregulated after DMM surgery (Fig. 6d). Mouse Oln was also marginally detected in the boundary between the synovium and meniscus, which was not affected by DMM (Supplementary Fig. 8c). No Oln signal could be detected in IgG control or Oln-deficient (Oln KO) mice (Supplementary Fig. 8d). Interestingly, Oln mRNA was significantly decreased in primary chondrocytes after IL-1β stimulation ex vivo (Supplementary Fig. 8e), suggesting that proinflammatory factors inhibit Oln expression during OA progression.
Genetic deletion of Oln exacerbates OA progression
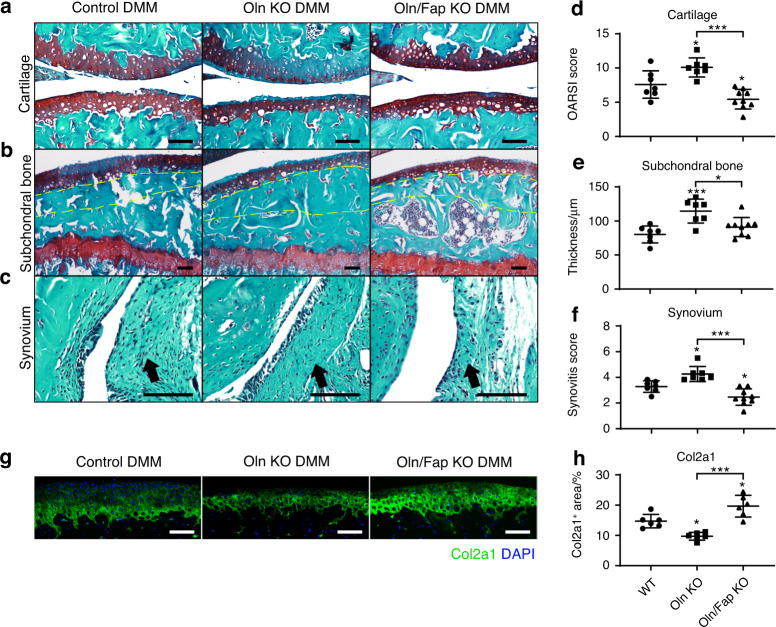
To test whether Oln regulates OA progression, we performed DMM surgery in Oln KO, Oln/Fap KO (double KO) and wild-type control mice. Histomorphometry analysis showed no significant differences among Oln KO, double KO and control mice in the contralateral knee joints without DMM surgery (Fig. S9a–f), suggesting that neither Oln nor Fap are required for articular cartilage development or maintenance under steady state. In the knee joints with DMM surgery, Oln KO mice showed significantly increased cartilage erosion, subchondral bone thickening, and synovitis compared to control mice (Fig. 7a–f). Importantly, these defects were significantly ameliorated in double KO mice (Fig. 7a–f). Col2a1 staining of the articular cartilage showed similar results (Fig. 7g, h).
Fig. 7.
Oln inhibits OA progression in a Fap-dependent manner. a–c Safranin O/Fast Green staining in control, Oln KO and Oln/Fap double KO mouse knee joints 8 weeks after DMM surgery. DMM surgery was performed in 10-week-old mice. Representative cartilage erosion (a, top: femur, bottom: tibia), subchondral bone thickening (b) and synovitis (c) images are shown (n = 7–9 mice per genotype). Yellow dotted lines indicate the subchondral bone plate. Arrows indicate the synovium. Scale bars: 100 μm. d–f Quantification of the OARSI score (d), subchondral bone thickness (e) and synovitis score (f). g, h Immunostaining of Col II in the knee joints (g) with quantification (h) (n = 6 mice per genotype). DAPI staining indicates the nucleus. Scale bars: 100 μm. The statistical significance was assessed using one-way ANOVAs with Tukey’s multiple comparison tests. Data are presented as the mean ± SD (*P < 0.05, **P < 0.01, ***P < 0.001)
Consistent with this finding, weekly intra-articular administration of FAPi also significantly ameliorated OA progression in Oln KO mice (Fig. S10a–h). Oln deficiency did not affect Acan and Col2a1 levels in primary chondrocytes after IL-1β stimulation, while there was a modest but significant increase in Mmp3 levels compared to those in wild-type controls (Fig. S11a–e). Taken together, these findings indicate that the genetic deletion of Oln exacerbates OA progression, which could be partially rescued by Fap deletion or inhibition.
Intra-articular injection of recombinant Oln attenuates OA progression
To test whether recombinant Oln (rOln) showed similar therapeutic effects as those of FAPi, we administered weekly intra-articular injections of recombinant rOln (240 μg·kg−1 body weight) or vehicle control (0.05 mg·mL−1 hyaluronic acid) in wild-type mice 3 days after DMM surgery for a total of 8 weeks. Histomorphometry analysis showed significantly decreased cartilage erosion, subchondral bone thickening, and synovitis compared to vehicle controls (Fig. S12a–f), which was confirmed by Col2a1 staining of articular cartilage (Fig. S12g, h).
To test whether rOln could ameliorate articular symptoms after the onset of OA, we administered weekly intra-articular injections of rOln or vehicle control in wild-type mice 4 weeks after DMM surgery for a total of 8 weeks. The vehicle-treated DMM group showed profound cartilage erosion, subchondral bone thickening and synovitis compared to the sham-operated group, and these effects were significantly ameliorated in the rOln-treated group (Fig. 8a–f). Similar results were obtained by immunostaining of Col2a1 in articular cartilage (Fig. 8g, h). Taken together, these data showed that intra-articular rOln administration significantly attenuated OA progression.
Fig. 8.
rOln ameliorates joint symptoms after the onset of OA. a–c Safranin O/Fast Green staining in wild-type mice treated with rOln or vehicle after DMM surgery. Weekly intra-articular administration of rOln (240 μg·kg−1 body weight) or vehicle (0.05 mg·mL−1 hyaluronic acid) was performed 4 weeks after DMM surgery in 10-week-old mice and continued for 8 weeks before paraffin sectioning and safranin O/fast green staining of the knee joints. Representative cartilage erosion (a, top: femur, bottom: tibia), subchondral bone thickening (b) and synovitis (c) images are shown (n = 6–8 mice per treatment). Yellow dotted lines indicate the subchondral bone plate. Arrows indicate the synovium. Scale bars: 100 μm. d–f Quantification of the OARSI score (d), subchondral bone thickness (e) and synovitis score (f). g, h Immunostaining of Col II in the knee joints (g) with quantification (h) (n = 6 mice per treatment). DAPI staining indicates the nucleus. Scale bars: 100 μm. The statistical significance was assessed using one-way ANOVAs with Tukey’s multiple comparison tests. Data are presented as the mean ± SD (*P < 0.05, **P < 0.01, ***P < 0.001)
Discussion
OA is a degenerative orthopedic disease that severely affects the quality of life of elderly people worldwide. Apart from surgical treatments, very few medicines have been developed to effectively treat OA symptoms by either promoting cartilage regeneration or preventing its degradation.68 In the present study, we found that synovial Fap levels positively correlated with OA progression and that genetic deletion or pharmacological inhibition of Fap significantly ameliorated OA symptoms. Furthermore, we showed that Fap degrades Col II both in vitro and in vivo, and this effect could be inhibited by both FAPi and its endogenous inhibitor Oln. Interestingly, Oln is expressed in the superficial layer of the articular cartilage and meniscus to form a protective barrier, which negatively correlates with OA progression. Genetic deletion of Oln promotes OA progression in a Fap-dependent manner, while intra-articular administration of rOln ameliorates OA. Together, this study identified Fap as a potential drug target to treat OA.
Fap is structurally and functionally similar to Dpp4,69 which is a dipeptidyl peptidase that shares many substrates with Fap.70 DPP4 inhibitors, such as sitagliptin, are orally available drugs that have been approved by the FDA to treat type 2 diabetes.71 Although the FAPi we used in this study is only 9-fold selective over Dpp4,55 it showed no therapeutic effects in Fap KO mice (Fig. 2). This finding suggests that Fap, but not Dpp4, is the downstream target of FAPi. Fap has both membrane-bound and soluble forms.39 Since synoviocytes do not infiltrate the cartilage matrix in OA,9 we reason that the soluble form of Fap secreted from OA synovium contributes to Col II degradation, which was confirmed by our ELISA analysis (Supplementary Fig. 6b). In contrast to OA, RA is characterized by severe inflammation and synoviocyte infiltration.9 Given that Fap+ synovial fibroblasts are known to play key roles in RA72 and that genetic deletion of Fap significantly ameliorates articular cartilage damage in a mouse model of RA,50 it is intriguing to test whether Fap inhibitors could also be used to treat RA in the future.
Previous studies showed that Fap degrades denatured Col I35,63 as well as MMP1-cleaved native Col I.64,65 Here, we provide evidence that Fap also degrades denatured Col II and MMP13-cleaved native Col II in vitro and primary chondrocyte-derived Col II ex vivo, thereby adding a new member to the substrate list of Fap. While MMP13 can cleave native Col II into large fragments, Fap helps to digest them into even smaller peptides (Fig. 4f). It is worth noting that Fap is a prolyl-specific serine protease and that 17% of COL2A1 amino acids are composed of proline. Thus, MMP13 and Fap work together for the efficient degradation of native Col II, the major component of the articular cartilage. Col II is also an in vivo substrate of Fap during OA progression. The Col II level was significantly increased after genetic deletion or inhibition of Fap (Figs. 2h and 3h) and significantly decreased after intra-articular administration of rFap (Fig. 5h). Unfortunately, we were unable to test whether FAPi attenuates OA progression in Col2a1-deficient mice, which died perinatally due to chondrodysplasia.73,74 Therefore, we cannot rule out the possibility that other substrates might also mediate the therapeutic effects of Fap inhibition.
MMPs play critical roles in cartilage degradation during OA progression,75 while tissue inhibitors of metalloproteinases (TIMPs) protect against OA.76 Whether similar antagonistic mechanisms exist to maintain the homeostasis of the articular cartilage remains poorly understood. Our previous study showed that Oln interacts with Fap and functions as an endogenous inhibitor to promote bone mineralization.48 Here, we found that Oln also localizes to the articular surface, which forms a protective barrier to prevent Fap-dependent degradation of Col II. During OA progression, cartilage erosion first occurs at the articular surface due to mechanical wear,77 which leads to Oln disruption and further deterioration of the cartilage matrix. Notably, Oln KO mice did not exhibit spontaneous OA, possibly because the Fap level is low in the knee joint under steady state (Fig. 1). Unlike FAPi, rOln cannot directly inhibit the serine protease activity of Fap in vitro (data not shown). However, Oln overexpression partially inhibited immunoprecipitated Fap (Fig. 4e), suggesting that they could form an inhibitory complex in vivo together with other protein adaptors. This study falls short of dissecting the detailed molecular mechanism by which Fap is inhibited by Oln, which could be achieved by purification of the inhibitory complex coupled with crystallography/cryo-EM analysis. We also noted that rOln showed limited therapeutic effects in vivo when dissolved in PBS (data not shown). However, HA seemed to be a better vehicle, probably by immobilizing rOln on the articular surface. Future studies are needed to optimize the vehicle and route of administration for FAPi and rOln and to test whether they could ameliorate OA progression in larger animal models and clinical trials.
Materials and methods
Human samples
This study was carried out in compliance with the Helsinki Declaration. Synovium, articular cartilage and synovial fluids were collected from the knee joints of OA patients (Kellgren–Lawrence grade78 3 or 4) during arthroplasty procedures. Cartilage samples were examined under the stereoscope. The smooth regions with no obvious lesions were separated as normal cartilage, while the rough regions were separated as lesioned cartilage. Control synovial tissues were obtained from patients with no radiographic cartilage changes undergoing exploratory arthroscopy. Detailed patient information is summarized in Table S1.
Mice
Fap KO mice (#024288)56 were obtained from the Jackson Laboratory and maintained by crossing with C57BL/6J wild-type mice (#000664). Parts of exons 4 and 5 were replaced by a LacZ-neo cassette in this strain, which led to the genetic ablation of Fap. The generation of Oln KO mice was previously described.53 Wild-type C57BL/6J mice were used for in vivo administration of rOln and FAPi. Male mice at approximately 10 weeks of age were used to perform the DMM surgery.
qPCR
Total RNA was extracted from human samples or mouse primary chondrocytes using TRIzol reagent (Invitrogen) and then reverse transcribed into cDNA using a 5X All-In-One MasterMix kit (ABM). qPCR was performed using iTaq Universal SYBR Green Supermix (Bio-Rad) on a CFX96 real-time system (Bio-Rad). The PCR primers used included hACTB (NM_001101.5): 5’-ATT GGC AAT GAG CGG TTC-3’ and 5’-GGA TGC CAC AGG ACT CCA-3’; hFAP (NM_001291807.3): 5’-TGG CGA TGA ACA ATA TCC TAG A-3’ and 5’-ATC CGA ACA ACG GGA TTC TT-3’; hOLN/CLEC11A (NM_002975.3): 5’-GAG AGG GAG GCC CTG ATG-3’ and 5’-AAC AGT TCC GGC AGG ATT C-3’; mActb (NM_007393.5): 5’-GCT CTT TTC CAG CCT TCC TT-3’ and 5’-CTT CTG CAT CCT GTC AGC AA-3’; mAcan (NM_007424.3): 5’-TGA AGC AGA AGG TCT GGA CA-3’ and 5’-CCA GAA GGA ATC CCA CTA ACA-3’; mCol2a1 (NM_031163.3): 5’-GTC CCC CTG GCC TTA GTG-3’ and 5’-CCA CCA GCC TTC TCG TCA-3’; mMmp3 (NM_010809.2): 5’-TGC AGC TCT ACT TTG TTC TTT GA-3’ and 5’-AGA GAT TTG CGC CAA AAG TG-3’; mOln (NM_009131.3): 5’-AGG TCC TGG GAG GGA GTG-3’ and 5’-GGG CCT CCT GGA GAT TCT T-3’.
Western blot
Protein extracts from human samples or primary chondrocytes were boiled at 95 °C for 10 min in 1× loading buffer and then subjected to SDS‒PAGE. Proteins were transferred onto PVDF blotting membranes, blocked with 5% dry milk in TBST buffer, and probed with anti-FAP (R&D, AF3715), anti-Gapdh (Proteintech, 60004), anti-Acan (Millipore Sigma, AB1031), anti-Col2a1 (Abcam, AB34712), anti-MMP3 (Abcam, AB52915) or anti-OLN/CLEC11A (R&D, BAF1904) antibodies overnight at 4 °C. After three washes in TBST buffer, the membrane was probed with donkey anti-sheep IgG-HRP (R&D, HAF016), goat anti-rabbit IgG-HRP (Absin Bioscience, abs20002) or goat anti-mouse IgG-HRP (Absin Bioscience, abs20001) secondary antibodies for 1 h at room temperature and then washed three times in TBST buffer. Chemiluminescent signals were detected with Pierce ECL Western blotting substrates (Thermo, 32106) or a SuperSignal™ West Femto Substrate Trial Kit (Thermo, 34094).
Cryosection and immunostaining
Human synovium, cartilage or mouse knee joint samples were fixed in 4% paraformaldehyde overnight at 4 °C. Mouse knee joints were decalcified in 10% EDTA (pH 7.4) for 5–7 days. All samples were dehydrated in 30% sucrose for 1 day at room temperature and sectioned at 10 μm using the CryoJane tape-transfer system (Leica). Sections were incubated in blocking buffer (PBS with 5% horse serum) for 1 h at room temperature and then stained with anti-FAP (R&D, AF3715, 1:500), anti-OLN/CLEC11A (R&D, BAF1904, 1:500), anti-Oln/Clec11a (R&D systems, AF3729, 1:500), anti-beta galactosidase (GeneTex, GTX77365, 1:500) or anti-Col2a1 (Boster, BA0533, 1:200) antibodies in blocking buffer overnight at 4 °C. Slides were washed 3 times with PBS and then stained with Alexa Fluor 647 donkey anti-Sheep IgG (H+L) (Thermo Fisher Scientific, A21448, 1:500), Alexa Fluor Plus 555 donkey anti-goat IgG (H+L) (Thermo Fisher Scientific, A32816, 1:500) or Alexa Fluor 488 donkey anti-chicken IgY (IgG) (H+L) (Jackson ImmunoResearch, 703-545-155, 1:500) secondary antibodies in blocking buffer for 1 h at room temperature. Slides were then washed 3 times with PBS, stained with 1 μg/ml DAPI for 1 min and mounted with Anti-fade Prolong Gold (Invitrogen). Images were acquired using an Olympus IX73 microscope.
Genotyping
To genotype Fap KO mice, the following primers were used: 5’-TTT GGG CCA GGG TTT TCC CAG TCA C-3’, 5’-TGG ACA GGG AGG AAG ACA AG-3’ and 5’-GAG GGC AGA GGC TTA GTG TG-3’ (WT: 361 bp; Heterozygous: 230 and 361 bp; KO: 230 bp). To genotype Oln+/+, Oln+/−, and Oln−/− mice, the following primers were used: 5’-TTT GGG TGC TGG GAA GCC C-3’ and 5’-TTG CAC TGA GTC GCG GGT G-3’ (Oln+/+: 910 bp; Oln+/− or Oln−/−: 538 bp). To distinguish between Oln+/− and Oln−/− mice, the following primers were used: 5’-GAG GAA GAG GAA ATC ACC ACA GC-3’ and 5’-TTG CAC TGA GTC GCG GGT G-3’ (Oln+/−: 482 bp; Oln−/−: no amplification product).
DMM model
Mice were anesthetized with 1.5% pentobarbital (80 mg·kg−1 body weight). Ketoprofen (5 mg·kg−1 body weight) was administered subcutaneously for pain relief. Knee joints were shaved and sterilized with Betadine and 70% ethanol, and a 4 mm longitudinal incision was made from the inferior pole of the patellar to the proximal tibial plateau. The joint capsule was opened medial to the patellar tendon with microiris scissors. The fat pad over the intercondylar area was bluntly dissected. For the DMM operation, the medial meniscotibial ligament was incised with microiris scissors, and the mobility of the anterior horn of the medial meniscus was tested. For the sham operation, no other procedure was performed. The joint capsule and the skin were then closed separately with a 7–0 cutting PGA suture line (Jinhuan Medical).
Intra-articular injection
Mice were randomly chosen for each intervention group. Mice were anesthetized with isoflurane, and knee joints were sterilized with 70% ethanol. The needle of the insulin syringe was sagittally inserted into the intercondylar area of the mouse knee, where 10 μl of FAPi (MCE, HY-101801), rFap (R&D Systems, 8647-SE-010) or rOln was injected. For the prevention model, intra-articular injections of FAPi (40 μg·kg−1 body weight, in PBS), rOln (240 μg·kg−1 body weight, in 0.05 mg·mL−1 HA) or the corresponding vehicle were given 3 days after the surgery. For the treatment model, intra-articular injections of FAPi, rFap (40 μg·kg−1 body weight, in PBS), rOln or the corresponding vehicle were given 4 weeks after DMM surgery.
Histomorphometry analysis
Dissected mouse knee joints were fixed in 4% paraformaldehyde overnight at 4 °C and decalcified in 10% EDTA for 14 days. Paraffin-embedded knee joints were sectioned at 6-μm thickness and stained with hematoxylin, 0.5% Safranin O solution and 0.2% Fast Green solution. Grading was performed by calculating OARSI scores (medial tibial plateau)79 and synovitis scores (summation of lining layer enlargement, density of resident cells, and inflammatory infiltration).80 Subchondral bone thickness was measured as the mean distance of five evenly distributed measuring points between the lower edge of the articular cartilage and the roof of the cancellous bone.81,82 The average score of 6 knee sections was calculated for statistical analysis.
Micro-CT analysis
Dissected mouse knee joints were fixed in 4% paraformaldehyde overnight at 4 °C and replaced with PBS before micro-CT analysis. Knee joints were scanned at an isotropic voxel size of 7 μm, with a peak tube voltage of 70 kV and current of 0.114 mA (mCT 35; Scanco Medical AG, Bassersdorf, Switzerland). A three-dimensional Gaussian filter (s = 0.8) with a limited, finite filter support of one was used to suppress noise in the images, and a threshold of 220–1 000 was used to segment mineralized bone from the air and soft tissues. The region of interest was selected for calcified osteophyte tissues, and the bone volume was calculated to measure the osteophyte size.
Primary cell culture
Primary chondrocyte culture was performed as previously described.57 Briefly, P5-P7 newborn mice were sacrificed, and cartilage from the knee and shoulder joints was dissected with ophthalmic scissors and micro forceps. The cartilage tissues were rinsed three times with PBS and cut into smaller pieces. Cartilage pieces were incubated in digestion buffer (DMEM, 3 mg/ml collagenase type II, 4 mg·mL−1 Dispase II, 1% penicillin/streptomycin) twice at 37 °C for 45 min each and incubated in diluted digestion buffer (6×) overnight at 37 °C. Dissociated chondrocytes were then filtered, centrifuged, and seeded in 24-well plates at 2 × 105 cells per well. Chondrocytes were cultured with DMEM (Corning, 10-014-CV) plus 10% fetal bovine serum (GIBCO, 10270-106), 2 mmol·L−1 GlutaMAX Supplement (Thermo Fisher Scientific, 35050061) and 1% penicillin/streptomycin. The culture medium was changed after 8–10 h. All experiments were performed within 3 days after seeding. For human synovial fibroblast cultures, synovial tissues (from 1 male and 2 females, average age: 52.33) were rinsed three times with DPBS (1% penicillin/streptomycin) and minced with scissors to smaller pieces. Tissues were then incubated in digestion buffer (DMEM, 1 mg·mL−1 collagenase type I, 1% penicillin/streptomycin) at 37 °C for 45 min and vortexed every 3–5 min. Dissociated synovial fibroblasts were filtered, centrifuged, seeded in 10 cm dishes, and cultured with DMEM (Corning, 10-014-CV) plus 10% fetal bovine serum (GIBCO, 10270-106) and 1% penicillin/streptomycin. The culture medium was changed after 8–10 h. For IL-1β stimulation experiments, synovial fibroblasts were passaged in 48-well plates at 5 × 104 cells per well and stimulated with 10 ng·mL−1 IL-1β for different durations.
Enzymatic digestion
The enzymatic reaction was initiated by adding different doses of rFap (R&D Systems, 8647-SE-010), native or denatured (heated at 95 °C for 10 min) bovine Col II (Chondrex, 20021) into 10 μL PBS buffer and incubated for 1–24 h at 37 °C. For the FAPi inhibition experiment, FAPi and rFap were preincubated for 30 min before denatured Col II was added to the reaction mixture. For the MMP13 predigestion experiment, rMMP13 and native Col II were incubated in assay buffer (50 mmol·L−1 Tris, 1 mmol·L−1 CaCl2, 150 mmol·L−1 NaCl, 0.05% Brij-35) at 37 °C for 12 h, after which EDTA (1 mmol·L−1 final concentration) and rFap were added to the reaction mixture for another 12 h. Different doses of rFap and 10 μg·mL−1 native or denatured (heated at 95 °C for 10 min) recombinant human Aggrecan (G1-IGD-G2 domain, R&D Systems, 1220-PG-025) were incubated in 10 μL PBS buffer at 37 °C for 24 h. Enzymatic reactions were stopped by adding loading buffer (NCM, WB2001) and boiling at 95 °C for 10 min. Then, the samples were subjected to 6% SDS‒PAGE. Gels were stained with a Colloidal Blue Staining Kit (Thermo, LC6025). Grayscales were calculated with ImageJ.
Immunoprecipitation
Fap-HA, Oln-Flag, and control GFP constructs were transfected alone or together into the HEK293T cell line (ATCC) using Lipofectamine 3000 (Thermo Fisher Scientific). Three days after transfection, cells were washed with ice-cold PBS and lysed in 500 μL ice-cold RIPA buffer (100 mmol·L−1 Tris-HCl, 150 mmol·L−1 NaCl, 1% Triton-X-100, 1X protease inhibitor cocktails). After centrifugation at 12 000 r·min−1 for 5 min, the supernatants were incubated with 10 μL anti-HA affinity gel (Sigma) overnight at 4 °C and washed three times with RIPA buffer and three times with PBS. Immunoprecipitated proteins were incubated with denatured Col II at 37 °C for 24 h and then subjected to 6% SDS‒PAGE and colloidal blue staining as described above. Immunoprecipitation efficiency was detected by western blotting with anti-HA (Sigma, H6908) and anti-Flag (Sigma, F1804) antibodies.
ELISA
Secreted FAP levels in human synovial fluids were measured using a Human FAP ELISA Kit (Abcam, ab193701) according to the manufacturer’s instructions. Briefly, human synovial fluids were diluted 1:100 using PBS buffer, and 100 μL of diluted synovial fluid was added to a precoated 96-well ELISA plate at 4 °C for 16 h. The plate was then washed 4 times with 1× wash buffer, and 100 μL of biotinylated human FAP detection antibody (diluted in 1× assay diluent B) was added and incubated at room temperature for 1 h. After washing with 1× wash buffer 4 times, 100 μL of HRP Streptavidin solution (diluted in 1× assay diluent B) was added and incubated at room temperature for 45 min. After washing with 1× wash buffer 4 times, 100 μL of TMB one-step substrate reagent was added to each well and incubated at room temperature in the dark for 30 min. Finally, 50 μL of stop solution was added to each well, and the optical density was measured at 450 nm.
rOln purification
rOln purification has been previously described.53 Briefly, HEK293 cells (ATCC) stably overexpressing mouse Oln-Flag were lysed and immunoprecipitated with anti-Flag M2 affinity gel (Sigma) overnight at 4 °C. The beads were washed three times with RIPA buffer and eluted using 100 μg·mL−1 3× FLAG peptide in elution buffer (50 mmol·L−1 HEPES, 150 mmol·L−1 NaCl and 10% glycerol, pH = 7.5). Eluted protein was concentrated by Amicon Ultra15 Centrifugal Filter Units (Millipore) and quantified by SDS‒PAGE with rOln standards (R&D Systems, 1904-SC-025/CF).
Statistics
The statistical significance of differences between two groups was assessed using two-tailed Student’s t tests. The statistical significance of differences among more than two groups was assessed using one-way ANOVAs with Tukey’s multiple comparison tests or two-way ANOVAs with Sidak’s multiple comparison tests. All data are presented as the mean ± SD. P values less than 0.05 were considered significant (*P < 0.05, **P < 0.01, ***P < 0.001).
Supplementary information
Acknowledgements
This work was supported by grants from the National Key R&D Program of China (2022YFA1103200, 2017YFA0106400, 2021YFA1100900), the Ministry of Science and Technology of China (2020YFC2002804), the National Natural Science Foundation of China (91749124, 81772389, 82070108), the Major Program of Development Fund for Shanghai Zhangjiang National Innovation Demonstration Zone (ZJ2018-ZD-004), the Fundamental Research Funds for the Central Universities (22120190149 and kx0200020173386), and the Peak Disciplines (Type IV) of Institutions of Higher Learning in Shanghai.
Author contributions
A.F. and G.W. performed most of the mouse experiments. A.F. and Jianfang Wang analyzed the human samples. Z. Pang, Z. Pan, Jingyi Wang, Y.W., Laiya Lu, Liangyu Lu and G.F. provided some of the human samples. Z. Pang, Jingyi Wang and Laiya Lu provided some of the reagents. H.W., Y.S., Y.X., C.M., X.Z., M.M., Q.Z., D.C. and J.Q. helped with the experiments and data analysis. F.Y. helped design the experiments and provided human samples. R.Y. designed and interpreted all experiments and wrote the manuscript.
Data availability
All data are available from the corresponding authors upon reasonable request.
Competing interests
R.Y., A.F. and F.Y. have filed a provisional patent on the use of Fap inhibitors as anti-osteoarthritis drugs. The other authors declare no competing interests.
Ethical approval
All human samples were collected in accordance with the ethical guidelines and protocols approved by the Ethics Committee of Shanghai East Hospital. Written informed consent was obtained from all patients before sample collection. All animal procedures were approved by the Tongji University Animal Care and Use Committee.
Footnotes
These authors contributed equally: Aoyuan Fan, Genbin Wu
Contributor Information
Feng Yin, Email: 001yinfeng@sina.com.
Rui Yue, Email: ryue@tongji.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41413-022-00243-8.
References
- 1.Safiri S, et al. Global, regional and national burden of osteoarthritis 1990–2017: a systematic analysis of the global burden of disease study 2017. Ann. Rheum. Dis. 2020;79:819–828. doi: 10.1136/annrheumdis-2019-216515. [DOI] [PubMed] [Google Scholar]
- 2.Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet (Lond., Engl.) 2011;377:2115–2126. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 3.Turkiewicz A, et al. Current and future impact of osteoarthritis on health care: a population-based study with projections to year 2032. Osteoarthr. Cartil. 2014;22:1826–1832. doi: 10.1016/j.joca.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Wei Y, Bai L. Recent advances in the understanding of molecular mechanisms of cartilage degeneration, synovitis and subchondral bone changes in osteoarthritis. Connect. Tissue Res. 2016;57:245–261. doi: 10.1080/03008207.2016.1177036. [DOI] [PubMed] [Google Scholar]
- 5.van der Kraan PM, van den Berg WB. Osteophytes: relevance and biology. Osteoarthr. Cartil. 2007;15:237–244. doi: 10.1016/j.joca.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Robinson WH, et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016;12:580–592. doi: 10.1038/nrrheum.2016.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu Y, Chen X, Wang S, Jing Y, Su J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021;9:20. doi: 10.1038/s41413-021-00147-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhen G, Cao X. Targeting TGFbeta signaling in subchondral bone and articular cartilage homeostasis. Trends Pharm. Sci. 2014;35:227–236. doi: 10.1016/j.tips.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pap T, Korb-Pap A. Cartilage damage in osteoarthritis and rheumatoid arthritis-two unequal siblings. Nat. Rev. Rheumatol. 2015;11:606–615. doi: 10.1038/nrrheum.2015.95. [DOI] [PubMed] [Google Scholar]
- 10.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 11.Troeberg L, Nagase H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim. Biophys. Acta. 2012;1824:133–145. doi: 10.1016/j.bbapap.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gracitelli GC, Moraes VY, Franciozi CE, Luzo MV, Belloti JC. Surgical interventions (microfracture, drilling, mosaicplasty, and allograft transplantation) for treating isolated cartilage defects of the knee in adults. Cochrane Database Syst. Rev. 2016;9:Cd010675. doi: 10.1002/14651858.CD010675.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angele P, et al. Chondral and osteochondral operative treatment in early osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2016;24:1743–1752. doi: 10.1007/s00167-016-4047-8. [DOI] [PubMed] [Google Scholar]
- 14.Glyn-Jones S, et al. Osteoarthritis. Lancet (Lond., Engl.) 2015;386:376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 15.Kolasinski SL, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020;72:220–233. doi: 10.1002/art.41142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bannuru RR, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019;27:1578–1589. doi: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Bruyère O, et al. An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) Semin. Arthritis Rheum. 2019;49:337–350. doi: 10.1016/j.semarthrit.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Filardo G, et al. Non-surgical treatments for the management of early osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2016;24:1775–1785. doi: 10.1007/s00167-016-4089-y. [DOI] [PubMed] [Google Scholar]
- 19.Heinegard D, Saxne T. The role of the cartilage matrix in osteoarthritis. Nat. Rev. Rheumatol. 2011;7:50–56. doi: 10.1038/nrrheum.2010.198. [DOI] [PubMed] [Google Scholar]
- 20.Bondeson J, Wainwright S, Hughes C, Caterson B. The regulation of the ADAMTS4 and ADAMTS5 aggrecanases in osteoarthritis: a review. Clin. Exp. Rheumatol. 2008;26:139–145. [PubMed] [Google Scholar]
- 21.Mehana EE, Khafaga AF, El-Blehi SS. The role of matrix metalloproteinases in osteoarthritis pathogenesis: an updated review. Life Sci. 2019;234:116786. doi: 10.1016/j.lfs.2019.116786. [DOI] [PubMed] [Google Scholar]
- 22.Knauper V, Lopez-Otin C, Smith B, Knight G, Murphy G. Biochemical characterization of human collagenase-3. J. Biol. Chem. 1996;271:1544–1550. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- 23.Wan Y, et al. Selective MMP-13 inhibitors: promising agents for the therapy of osteoarthritis. Curr. Med Chem. 2020;27:3753–3769. doi: 10.2174/0929867326666181217153118. [DOI] [PubMed] [Google Scholar]
- 24.Mort JS, Billington CJ. Articular cartilage and changes in arthritis: matrix degradation. Arthritis Res. 2001;3:337–341. doi: 10.1186/ar325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell PG, et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J. Clin. Investig. 1996;97:761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bányai L, Tordai H, Patthy L. The gelatin-binding site of human 72 kDa type IV collagenase (gelatinase A) Biochem. J. 1994;298:403–407. doi: 10.1042/bj2980403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Overall CM, Sodek J. Initial characterization of a neutral metalloproteinase, active on native 3/4-collagen fragments, synthesized by ROS 17/2.8 osteoblastic cells, periodontal fibroblasts, and identified in gingival crevicular fluid. J. Dent. Res. 1987;66:1271–1282. doi: 10.1177/00220345870660071201. [DOI] [PubMed] [Google Scholar]
- 28.Renkiewicz R, et al. Broad-spectrum matrix metalloproteinase inhibitor marimastat-induced musculoskeletal side effects in rats. Arthritis Rheum. 2003;48:1742–1749. doi: 10.1002/art.11030. [DOI] [PubMed] [Google Scholar]
- 29.Krzeski P, et al. Development of musculoskeletal toxicity without clear benefit after administration of PG-116800, a matrix metalloproteinase inhibitor, to patients with knee osteoarthritis: a randomized, 12-month, double-blind, placebo-controlled study. Arthritis Res. Ther. 2007;9:R109. doi: 10.1186/ar2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milner JM, Patel A, Rowan AD. Emerging roles of serine proteinases in tissue turnover in arthritis. Arthritis Rheum. 2008;58:3644–3656. doi: 10.1002/art.24046. [DOI] [PubMed] [Google Scholar]
- 31.Chou PY, Su CM, Huang CY, Tang CH. The characteristics of thrombin in osteoarthritic pathogenesis and treatment. Biomed. Res. Int. 2014;2014:407518. doi: 10.1155/2014/407518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilkinson DJ, et al. Matriptase induction of metalloproteinase-dependent aggrecanolysis in vitro and in vivo: promotion of osteoarthritic cartilage damage by multiple mechanisms. Arthritis Rheumatol. 2017;69:1601–1611. doi: 10.1002/art.40133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akhatib B, et al. Chondroadherin fragmentation mediated by the protease HTRA1 distinguishes human intervertebral disc degeneration from normal aging. J. Biol. Chem. 2013;288:19280–19287. doi: 10.1074/jbc.M112.443010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldstein LA, et al. Molecular cloning of seprase: a serine integral membrane protease from human melanoma. Biochim. Biophys. Acta. 1997;1361:11–19. doi: 10.1016/S0925-4439(97)00032-X. [DOI] [PubMed] [Google Scholar]
- 35.Park JE, et al. Fibroblast activation protein, a dual specificity serine protease expressed in reactive human tumor stromal fibroblasts. J. Biol. Chem. 1999;274:36505–36512. doi: 10.1074/jbc.274.51.36505. [DOI] [PubMed] [Google Scholar]
- 36.Balaziova E, et al. Dipeptidyl peptidase-IV activity and/or structure homologs (DASH): contributing factors in the pathogenesis of rheumatic diseases? Adv. Exp. Med. Biol. 2006;575:169–174. doi: 10.1007/0-387-32824-6_18. [DOI] [PubMed] [Google Scholar]
- 37.Hamson EJ, Keane FM, Tholen S, Schilling O, Gorrell MD. Understanding fibroblast activation protein (FAP): substrates, activities, expression and targeting for cancer therapy. Proteom. Clin. Appl. 2014;8:454–463. doi: 10.1002/prca.201300095. [DOI] [PubMed] [Google Scholar]
- 38.Dunshee DR, et al. Fibroblast activation protein cleaves and inactivates fibroblast growth factor 21. J. Biol. Chem. 2016;291:5986–5996. doi: 10.1074/jbc.M115.710582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee KN, et al. Antiplasmin-cleaving enzyme is a soluble form of fibroblast activation protein. Blood. 2006;107:1397–1404. doi: 10.1182/blood-2005-08-3452. [DOI] [PubMed] [Google Scholar]
- 40.Keane FM, Nadvi NA, Yao TW, Gorrell MD. Neuropeptide Y, B-type natriuretic peptide, substance P and peptide YY are novel substrates of fibroblast activation protein-alpha. FEBS J. 2011;278:1316–1332. doi: 10.1111/j.1742-4658.2011.08051.x. [DOI] [PubMed] [Google Scholar]
- 41.Zhang HE, et al. Identification of novel natural substrates of fibroblast activation protein-alpha by differential degradomics and proteomics. Mol. Cell Proteom. 2019;18:65–85. doi: 10.1074/mcp.RA118.001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scanlan MJ, et al. Molecular cloning of fibroblast activation protein alpha, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc. Natl. Acad. Sci. USA. 1994;91:5657–5661. doi: 10.1073/pnas.91.12.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rettig WJ, et al. Cell-surface glycoproteins of human sarcomas: differential expression in normal and malignant tissues and cultured cells. Proc. Natl Acad. Sci. USA. 1988;85:3110–3114. doi: 10.1073/pnas.85.9.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelly T. Fibroblast activation protein-alpha and dipeptidyl peptidase IV (CD26): cell-surface proteases that activate cell signaling and are potential targets for cancer therapy. Drug Resist. Updat. 2005;8:51–58. doi: 10.1016/j.drup.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 45.Garin-Chesa P, Old LJ, Rettig WJ. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc. Natl. Acad. Sci. USA. 1990;87:7235–7239. doi: 10.1073/pnas.87.18.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cortez E, Roswall P, Pietras K. Functional subsets of mesenchymal cell types in the tumor microenvironment. Semin. Cancer Biol. 2014;25:3–9. doi: 10.1016/j.semcancer.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 47.Kou X, et al. The Fas/Fap-1/Cav-1 complex regulates IL-1RA secretion in mesenchymal stem cells to accelerate wound healing. Sci. Transl. Med. 2018;10:eaai8524. doi: 10.1126/scitranslmed.aai8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei H, et al. Identification of fibroblast activation protein as an osteogenic suppressor and anti-osteoporosis drug target. Cell Rep. 2020;33:108252. doi: 10.1016/j.celrep.2020.108252. [DOI] [PubMed] [Google Scholar]
- 49.Bauer S, et al. Fibroblast activation protein is expressed by rheumatoid myofibroblast-like synoviocytes. Arthritis Res. Ther. 2006;8:R171. doi: 10.1186/ar2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waldele S, et al. Deficiency of fibroblast activation protein alpha ameliorates cartilage destruction in inflammatory destructive arthritis. Arthritis Res. Ther. 2015;17:12. doi: 10.1186/s13075-015-0524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hiraoka A, et al. Cloning, expression, and characterization of a cDNA encoding a novel human growth factor for primitive hematopoietic progenitor cells. Proc. Natl. Acad. Sci. USA. 1997;94:7577–7582. doi: 10.1073/pnas.94.14.7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hiraoka A, et al. Stem cell growth factor: in situ hybridization analysis on the gene expression, molecular characterization and in vitro proliferative activity of a recombinant preparation on primitive hematopoietic progenitor cells. Hematol. J. 2001;2:307–315. doi: 10.1038/sj.thj.6200118. [DOI] [PubMed] [Google Scholar]
- 53.Yue R, Shen B, Morrison SJ. Clec11a/osteolectin is an osteogenic growth factor that promotes the maintenance of the adult skeleton. eLife. 2016;5:e18782. doi: 10.7554/eLife.18782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen B, et al. Integrin alpha11 is an Osteolectin receptor and is required for the maintenance of adult skeletal bone mass. eLife. 2019;8:e42274. doi: 10.7554/eLife.42274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edosada CY, et al. Selective inhibition of fibroblast activation protein protease based on dipeptide substrate specificity. J. Biol. Chem. 2006;281:7437–7444. doi: 10.1074/jbc.M511112200. [DOI] [PubMed] [Google Scholar]
- 56.Niedermeyer J, et al. Expression of the fibroblast activation protein during mouse embryo development. Int. J. Dev. Biol. 2001;45:445–447. [PubMed] [Google Scholar]
- 57.Gosset M, Berenbaum F, Thirion S, Jacques C. Primary culture and phenotyping of murine chondrocytes. Nat. Protoc. 2008;3:1253–1260. doi: 10.1038/nprot.2008.95. [DOI] [PubMed] [Google Scholar]
- 58.Goldring MB, Berenbaum F. Human chondrocyte culture models for studying cyclooxygenase expression and prostaglandin regulation of collagen gene expression. Osteoarthr. Cartil. 1999;7:386–388. doi: 10.1053/joca.1998.0219. [DOI] [PubMed] [Google Scholar]
- 59.Liacini A, Sylvester J, Li WQ, Zafarullah M. Inhibition of interleukin-1-stimulated MAP kinases, activating protein-1 (AP-1) and nuclear factor kappa B (NF-kappa B) transcription factors down-regulates matrix metalloproteinase gene expression in articular chondrocytes. Matrix Biol. 2002;21:251–262. doi: 10.1016/S0945-053X(02)00007-0. [DOI] [PubMed] [Google Scholar]
- 60.Robbins JR, et al. Immortalized human adult articular chondrocytes maintain cartilage-specific phenotype and responses to interleukin-1beta. Arthritis Rheum. 2000;43:2189–2201. doi: 10.1002/1529-0131(200010)43:10<2189::AID-ANR6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 61.Lee HO, et al. FAP-overexpressing fibroblasts produce an extracellular matrix that enhances invasive velocity and directionality of pancreatic cancer cells. BMC Cancer. 2011;11:245. doi: 10.1186/1471-2407-11-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nagaraju CK, et al. Global fibroblast activation throughout the left ventricle but localized fibrosis after myocardial infarction. Sci. Rep. 2017;7:10801. doi: 10.1038/s41598-017-09790-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brokopp CE, et al. Fibroblast activation protein is induced by inflammation and degrades type I collagen in thin-cap fibroatheromata. Eur. Heart J. 2011;32:2713–2722. doi: 10.1093/eurheartj/ehq519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Christiansen VJ, Jackson KW, Lee KN, McKee PA. Effect of fibroblast activation protein and alpha2-antiplasmin cleaving enzyme on collagen types I, III, and IV. Arch. Biochem. Biophys. 2007;457:177–186. doi: 10.1016/j.abb.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fan MH, et al. Fibroblast activation protein (FAP) accelerates collagen degradation and clearance from lungs in mice. J. Biol. Chem. 2016;291:8070–8089. doi: 10.1074/jbc.M115.701433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumagai K, et al. Changes of synovial fluid biomarker levels after opening wedge high tibial osteotomy in patients with knee osteoarthritis. Osteoarthr. Cartil. 2021;29:1020–1028. doi: 10.1016/j.joca.2021.03.013. [DOI] [PubMed] [Google Scholar]
- 67.Li L, et al. Profiling of inflammatory mediators in the synovial fluid related to pain in knee osteoarthritis. BMC Musculoskelet. Disord. 2020;21:99. doi: 10.1186/s12891-020-3120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Spil WE, Kubassova O, Boesen M, Bay-Jensen AC, Mobasheri A. Osteoarthritis phenotypes and novel therapeutic targets. Biochem. Pharm. 2019;165:41–48. doi: 10.1016/j.bcp.2019.02.037. [DOI] [PubMed] [Google Scholar]
- 69.Pineiro-Sanchez ML, et al. Identification of the 170-kDa melanoma membrane-bound gelatinase (seprase) as a serine integral membrane protease. J. Biol. Chem. 1997;272:7595–7601. doi: 10.1074/jbc.272.12.7595. [DOI] [PubMed] [Google Scholar]
- 70.Aertgeerts K, et al. Structural and kinetic analysis of the substrate specificity of human fibroblast activation protein alpha. J. Biol. Chem. 2005;280:19441–19444. doi: 10.1074/jbc.C500092200. [DOI] [PubMed] [Google Scholar]
- 71.Deacon CF. Dipeptidyl peptidase 4 inhibitors in the treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020;16:642–653. doi: 10.1038/s41574-020-0399-8. [DOI] [PubMed] [Google Scholar]
- 72.Croft AP, et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature. 2019;570:246–251. doi: 10.1038/s41586-019-1263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Metsäranta M, et al. Chondrodysplasia in transgenic mice harboring a 15-amino acid deletion in the triple helical domain of pro alpha 1(II) collagen chain. J. Cell Biol. 1992;118:203–212. doi: 10.1083/jcb.118.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rintala M, Metsäranta M, Säämänen AM, Vuorio E, Rönning O. Abnormal craniofacial growth and early mandibular osteoarthritis in mice harbouring a mutant type II collagen transgene. J. Anat. 1997;190:201–208. doi: 10.1046/j.1469-7580.1997.19020201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front. Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 76.Liu J, Khalil RA. Matrix metalloproteinase inhibitors as investigational and therapeutic tools in unrestrained tissue remodeling and pathological disorders. Prog. Mol. Biol. Transl. Sci. 2017;148:355–420. doi: 10.1016/bs.pmbts.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hollander AP, et al. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J. Clin. Investig. 1995;96:2859–2869. doi: 10.1172/JCI118357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pritzker KP, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthr. Cartil. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 80.Krenn V, et al. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology. 2006;49:358–364. doi: 10.1111/j.1365-2559.2006.02508.x. [DOI] [PubMed] [Google Scholar]
- 81.Lorenz J, Grässel S. Experimental osteoarthritis models in mice. Methods Mol. Biol. 2014;1194:401–419. doi: 10.1007/978-1-4939-1215-5_23. [DOI] [PubMed] [Google Scholar]
- 82.Yao Z, et al. Reduced PDGF-AA in subchondral bone leads to articular cartilage degeneration after strenuous running. J. Cell. Physiol. 2019;234:17946–17958. doi: 10.1002/jcp.28427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available from the corresponding authors upon reasonable request.