Abstract
An immunoglobulin G (IgG)-coated surface, such as that found on helminth parasites, is one of the most effective physiologic stimuli for eosinophil activation. The cysteine proteases secreted by tissue-invasive helminth larvae play an important role in evasion of the immune response by degrading the host immunoglobulins. In this study, we investigated whether cysteine proteases in the excretory-secretory product (ESP) produced by Paragonimus westermani newly excysted metacercariae (PwNEM), which cause pulmonary or extrapulmonary paragonimiasis in human beings, could modify effector functions of human eosinophils stimulated with IgG. We coated 96-well plates with human IgG in the absence or presence of the ESP produced by PwNEM. When eosinophils were incubated in the wells coated with IgG in the presence of the ESP, eosinophil degranulation and superoxide production were significantly reduced compared with results for cells incubated in wells coated with IgG alone. This inhibitory effect of the ESP on IgG-induced superoxide production was dose dependent and was significantly abolished by pretreatment of the ESP with heat. These findings suggest that the cysteine proteases secreted by PwNEM attenuate both activation and degranulation of eosinophils stimulated with IgG. Thus, the cysteine proteases produced by tissue-invasive helminth larvae play crucial roles in evasion of IgG-dependent eosinophil helminthotoxicity and in reduction of eosinophil-associated tissue inflammation during the migratory period.
Eosinophils are known to be important effector cells in the host defense against helminth parasites (15). They can damage or kill helminth worms by antibody-dependent cellular cytotoxicity mechanisms in in vitro cultures (5, 12, 28). Although the exact mechanisms by which eosinophils kill helminth parasites in vivo are not completely understood, degranulation of adhering eosinophils has been suggested to play a major role (13, 25). For example, eosinophil granule proteins, such as major basic protein, eosinophil peroxidase, and eosinophil cationic protein, directly damage a variety of helminth parasites (6, 16, 22, 37). Once the eosinophil has migrated into inflamed tissue in vivo, it becomes activated and releases various mediators, such as reactive oxygen intermediates, lipid mediators, and cytotoxic granular proteins (14). Recently the activators and regulators of eosinophil functions have been demonstrated. Several in vitro studies suggest that immobilized immunoglobulin G (IgG) (21, 27), secretory IgA (1), platelet-activating factor (24), and cytokines, such as interleukin 5 (IL-5), IL-3, granulocyte-macrophage colony-stimulating factor, and RANTES (19), are effective stimuli for activation of human eosinophils. Although the activated eosinophils are clearly involved in the killing of the worms in vitro, it is interesting to note that tissue-dwelling helminth parasites adapted for the human host can reinfect and/or survive for many years even in the activation of the host immune responses. Therefore, tissue-invading helminthic worms may have an immune escape mechanism of down-regulation of eosinophil effector functions, thus enabling the worm to pass through host immune defenses unmolested.
Excretory-secretory products (ESP) produced by tissue-invasive helminth larvae contain a large quantity of proteolytic enzymes, which are essential for worm maturation (35), migration in host tissues (29), and modulation of the immune response (3, 7, 8, 23). In vitro cleavage of IgG by cysteine proteases in the ESP secreted by tissue-invasive helminth larvae has been correlated with immune escape from antibody-dependent cellular toxicity. For example, cysteine proteases produced by Paragonimus westermani newly excysted metacercariae (PwNEM) are capable of degrading host IgG in vitro (8). Cysteine proteases of invasive larvae of other helminths, such as Fasciola hepatica (3) or Spirometra mansoni (23), have also been known to cleave IgG molecules. Moreover, cysteine proteases secreted by F. hepatica in vitro prevent parasite-specific antibody-mediated eosinophil attachment to newly excysted juvenile worms (7). Therefore, these findings led us to speculate that cysteine protease secreted by the tissue-invasive helminth parasites may modify the effector functions of eosinophils in the presence of parasite-specific IgG.
Freshly isolated eosinophils express only FcγRII (18), and eosinophil activation induced by immobilized IgG is mediated through FcγRII (20). IgG bound to Sepharose beads (1) or IgG applied to tissue culture plates (21) triggers degranulation and superoxide production of human eosinophils. In contrast to these responses of eosinophils to solid-phase IgG, little is known regarding the roles of parasite-secreted cysteine proteases that might alter the effector functions of eosinophils stimulated with IgG. The understanding of mechanisms used by cysteine proteases secreted by the PwNEM to moderate IgG-induced effector functions of eosinophils provides a key clue that eosinophils may not serve as strong effector cells in tissue helminth infections. To prove this hypothesis, we investigated whether cysteine proteases released by the PwNEM, which cause pulmonary or extrapulmonary paragonimiasis in human beings, could attenuate degranulation and superoxide production of eosinophils stimulated with IgG.
MATERIALS AND METHODS
Preparation of ESP produced by PwNEM.
Metacercariae of P. westermani were collected from naturally infected freshwater crayfish, Cambaroides similis, in an area of endemicity in Korea (32). The soft tissues of crayfish, crushed in a mortar, were emulsified in physiological saline and filtered through a mesh screen, and the sediment was examined under a dissecting microscope. Crude ESP of P. westermani metacercariae was prepared by transferring 5,000 newly excysted metacercariae into 5 ml of physiological saline and incubating at 37°C in a 5% CO2 incubator for 12 h. The incubation medium was dialyzed against distilled water and centrifuged at 1,700 × g for 30 min. The resulting supernatant was lyophilized and diluted with an appropriate medium to the desired concentration immediately before use. The amounts of proteins in the ESP were measured using the bicinchoninic acid protein assay kit (Pierce, Rockford, Ill.). The ESP was analyzed by sodium dodecyl sulfate–7.5 to 15% polyacrylamide gel electrophoresis (SDS–7.5 to 15% PAGE), with a 0.125% (wt/vol) Coomassie blue stain. In some experiments, to examine the relation between the number of PwNEM and protein amounts contained in the ESP secreted by PwNEM, 10, 30, 100, 200, and 300 newly excysted metacercariae were cultured in 48 wells of tissue culture plates for 12 h at 37°C in a 5%-CO2 incubator. After incubation, the culture supernatant was collected for measurement of protein concentrations contained in the ESP. The protein concentrations in the ESP produced by PwNEM were linear between the numbers of 10 and 300 larvae as determined by a standard curve (r2 = 0.937). Two, six, and twenty micrograms of ESP used in this study are equivalent to amounts produced by 9, 36, and 130 excysted metacercariae, respectively.
IgG cleavage assay by the ESP.
Twenty-five microliters of human whole IgG (Sigma, St. Louis, Mo.) diluted at a concentration of 200 μg/ml in phosphate-buffered saline (PBS) were mixed with same volume of the ESP containing 2, 6, and 20 μg of protein or PBS. The mixtures were incubated for 90 min at 37°C, and then the reactants were subjected to SDS–12.5% PAGE. Proteins were electrotransferred to a nitrocellulose membrane. The membrane was then incubated with horseradish peroxidase-conjugated goat anti-human whole IgG (1:1,000 dilution; Sigma) for 3 h at room temperature (RT). The membranes were developed using 4-chloro-1-naphthol (Sigma) solution to visualize degradative products of IgG.
Preparation of IgG-coated wells in the absence or presence of the ESP.
The 96-well tissue culture plates were coated with human IgG, 50 μl, diluted in PBS at three concentrations (10, 30, and 100 μg/ml) in the absence or presence of ESP (2, 6, or 20 μg) for 2 h at 37°C. After 2 h of incubation, the wells were aspirated and washed twice with PBS. In some experiments, the ESP was pretreated with heat for 30 min at 56°C or cysteine protease inhibitor E-64 (Boehringer Mannheim Biochemicals, Mannheim, Germany). In brief, 12.5 μl of ESP (10 μg) diluted in PBS was allowed to react with same volume of E-64 diluted in PBS (final concentration, 5 or 10 μM) for 1 h at RT. The heat- or E-64-treated ESP were mixed with 25 μl of IgG (200 μg/ml) diluted in PBS, 50 μl of the total mixtures was added to wells of 96-well tissue culture plates, and then the plates were incubated for 2 h at 37°C. After incubation, the wells were aspirated and washed twice with 200 μl of PBS before use.
Isolation of peripheral blood eosinophils.
Eosinophils were isolated from the peripheral blood of normal volunteers using a magnetic cell separation system (Miltenyi Biotec, Bergisch Gladbach, Germany), as described previously (17), with minor modifications. In brief, venous blood (30 ml) anticoagulated with 10 U of heparin/ml was diluted with piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) buffer (25 mM PIPES, 50 mM NaCl, 5 mM KCl, 25 mM NaOH, 5.4 mM glucose [pH 7.4]) at a 1:1 ratio. Diluted blood was overlayered on a Histopaque solution (density, 1.083 g/ml) (Sigma) and centrifuged at 100 × g for at 4°C for 30 min. The supernatant and mononuclear cells at the interface were removed carefully. The inside wall of the centrifuge tube was wiped twice with sterile gauze to eliminate mononuclear cells adhering to the walls. Erythrocytes in the sediment were lysed by exposure to two cycles of sterile distilled water. Isolated granulocytes were washed with PIPES buffer containing 1% fetal bovine serum (Gibco, Grand Island, N.Y.), and an approximately equal volume of anti-CD16 antibody (Ab) conjugated with magnetic particles (Miltenyi Biotec) was added to the cell pellet. After 30 min of incubation on ice, cells were loaded onto the separation column positioned in the magnetic cell separation system magnetic field. Cells were eluted with 15 ml of PIPES buffer with 1% fetal bovine serum. The purity of eosinophils counted by Randolph's stain was >95%. The contaminating cells were neutrophils, and no mononuclear cells or basophils were present. Purified eosinophils were used immediately for experiments.
Assay for eosinophil degranulation.
Freshly purified eosinophils were suspended in Hanks balanced salt solution (pH 7.4; Gibco) supplemented with 10 mM HEPES (Sigma) and 0.03% gelatin (Sigma) at a concentration of 2.5 × 105 cells/ml. Two-hundred-microliter aliquots of cell suspension were added to the wells coated with IgG in the absence or presence of the ESP. For the experiments on eosinophil degranulation, the cells were incubated for 3 h in a humidified incubator at 37°C and 5% CO2. After incubation, supernatants were collected and frozen at −20°C until they were assayed for eosinophil-derived neurotoxin (EDN), as described below.
Assay for EDN.
To quantitate eosinophil degranulation, EDN concentrations in the culture supernatants were measured by radioimmunoassay (RIA), as described previously (1). The RIA is a double Ab competition assay using radioiodinated EDN, rabbit anti-EDN Ab, and burro anti-rabbit IgG. All assays were performed in duplicate.
Superoxide anion generation.
Two hundred microliters of cell suspension (2.5 × 105 cell/ml) in Hanks balanced salt solution with 10 mM HEPES, 0.03% gelatin, and 100 μM cytochrome c (Sigma) were dispensed in the wells coated with IgG in the absence or presence of the ESP. In some experiments, 200 μl of cell suspension was added to wells coated with IgG in the presence of heat- or E-64-treated ESP or PBS-treated ESP. Immediately after the addition of the cells, the absorbance at 550 nm in each well was measured with a microplate autoreader (Thermomax; Molecular Devices, Sunnyvale, Calif.), followed by repeated readings. Between absorbance measurements, the plate was incubated at 37°C. Each reaction was conducted in duplicate, and superoxide anion generation was calculated with an extinction coefficient of 21.1 × 10−3 M/cm for reduced cytochrome c at 550 nm and was expressed as nanomoles of cytochrome c reduction/105 cells.
Statistical analysis.
Statistical significance of difference between the control and treatment groups was assessed with the unpaired Student's t test. Probability values of less than 0.05 or 0.01 were considered significant.
RESULTS
Cleavage effects of ESP produced by PwNEM on human IgG in vitro.
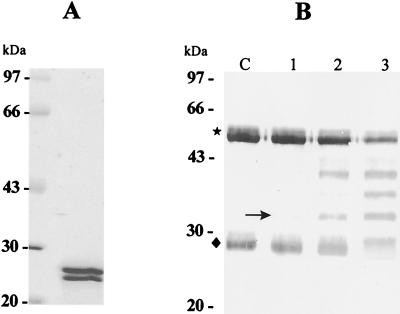
As shown in Fig. 1A, the ESP produced by PwNEM contains two protein bands consisting of two cysteine proteases, 28 and 27 kDa in molecular mass, as previously reported (10). To know what amount of the ESP is required to degrade human IgG, three different doses of the ESP (2, 6, and 20 μg) were mixed with IgG diluted in PBS at a concentration of 100 μg/ml. As shown in Fig. 1B, the ESP cleaved IgG in a dose-dependent manner. Six or twenty micrograms of ESP apparently degraded heavy and light chains of IgG. In contrast, no evident cleavage of IgG by 2 μg of the ESP was shown, although a degradation product of the heavy chain of IgG was faintly observed.
FIG. 1.
Electrophoretic analysis of crude ESP produced by PwNEM and immunoblot analysis for IgG cleavage by the ESP. (A) SDS–7.5 to 15% PAGE of the crude ESP. The protein bands at 28 and 27 kDa in molecular mass are cysteine proteases. (B) Cleavage pattern of human IgG induced by the ESP. Three different amounts (2, 6, and 20 μg at lanes 1, 2, and 3, respectively) of the ESP were incubated with human IgG solution (100 μg/ml) for 90 min at 37°C. After incubation, the reactants were separated by SDS–12.5% PAGE, transferred to a nitrocellulose membrane, and probed with anti-human IgG (whole molecule). A degradation product of heavy chain at 32 kDa (arrow) was first detected. The symbols ★ and ⧫ indicate heavy and light chains of IgG, respectively. Lane C, control IgG.
Effects of cysteine proteases secreted by PwNEM on degranulation of eosinophils stimulated with IgG.
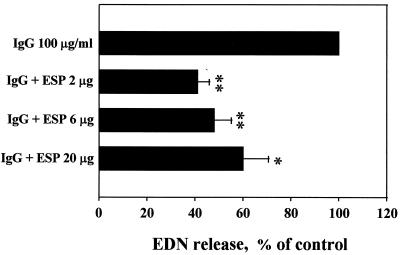
To investigate the effects of cysteine proteases secreted by PwNEM on IgG-induced eosinophil degranulation, eosinophils were incubated in the wells coated with human IgG in the absence or presence of the ESP produced by PwNEM. Table 1 shows degranulation of eosinophils stimulated with immobilized IgG alone. The amount of released EDN was dependent on the concentration of IgG used to coat the wells. When eosinophils were incubated in wells coated with 10, 30, and 100 μg of IgG/ml, EDN release (mean ± the standard error of the mean [SEM]) was 183 ± 86.0, 220 ± 85.2, and 315 ± 106.4 ng/106 cells, respectively. EDN release was about 100 ± 35.6 ng/106 cells when the wells were not coated with IgG. In contrast, when eosinophils were incubated in wells coated with IgG (100 μg/ml) in the presence of the ESP, eosinophil degranulation was reduced about 50% from that of cells incubated in wells coated with IgG alone (Fig. 2). At the same time, we also compared morphologic changes of eosinophils incubated in wells coated with IgG in the absence or presence of the ESP. Most of the eosinophils incubated in the wells coated with IgG alone were fully activated to show an extensive degranulative state, whereas eosinophils cultured in the wells coated with IgG in the presence of the ESP were generally oval and elongated (data not shown). Eosinophils cultured in uncoated plates were spherical and refractile.
TABLE 1.
Degranulation of eosinophils stimulated with various concentrations of human IgGa
| Stimulus (concn) | EDN release (ng/106 cells)
|
|
|---|---|---|
| Mean | Range | |
| None | 100 | 60.0–170.8 |
| IgG (10 μg/ml) | 183 | 86.8–354.4 |
| IgG (30 μg/ml) | 220 | 109.2–386.4 |
| IgG (100 μg/ml) | 315 | 149.2–513.2 |
The wells of tissue culture plates were coated with IgG at the concentrations indicated. After washing, eosinophils were added to the wells and incubated for 3 h at 37°C. Release of EDN into the culture supernatants by degranulation was measured by RIA as described in Materials and Methods. Data were obtained from three independent experiments performed in duplicate.
FIG. 2.
Effects of ESP produced by PwNEM on IgG-induced eosinophil degranulation. Wells were coated with 100 μg of IgG/ml in the absence or presence of the ESP at the doses indicated for 2 h at 37°C. After washing, eosinophils were added to the wells and incubated for 3 h at 37°C. Data are normalized to the mean degranulation of eosinophils incubated in the wells coated with IgG alone, taken as 100% (control values [mean ± SEM], 315 ± 106.4 ng/106 cells). Data are presented as the mean ± SEM from three independent experiments performed in duplicate. Significant differences from the control are as follows: asterisk, P < 0.05; double asterisk, P < 0.01.
Effects of cysteine proteases secreted by PwNEM on superoxide production of eosinophils stimulated with IgG.
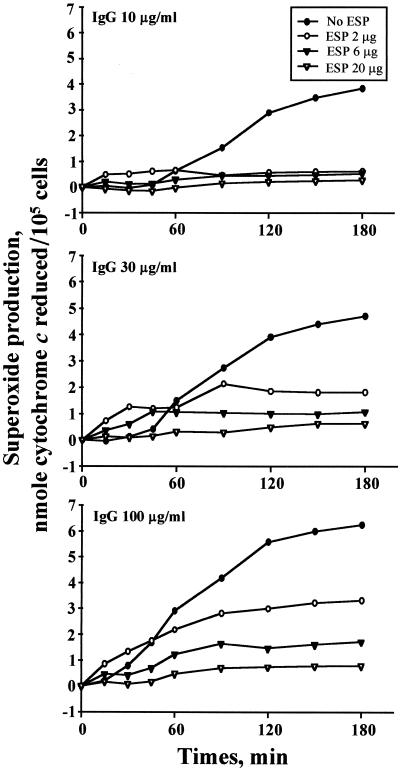
To determine the effects of cysteine proteases secreted by PwNEM on IgG-induced eosinophil superoxide production, eosinophils were incubated in the wells coated with human IgG in the absence or presence of the ESP produced by PwNEM. Figure 3 shows that eosinophils stimulated with 10, 30, and 100 μg of IgG/ml produced superoxide, which is detectable after 90 (1.5 ± 0.18 nmol), 60 (1.5 ± 0.26 nmol), and 45 (1.7 ± 0.36 nmol) min, respectively, and increasingly gradually thereafter. In contrast, superoxide production was strongly decreased when the cells were incubated in the wells coated with IgG in the presence of the ESP (Fig. 3). Consistent with the results of IgG cleavage by the ESP (Fig. 1B), the inhibitory effect of the ESP on IgG-induced eosinophil superoxide production occurred in a dose-dependent fashion.
FIG. 3.
Kinetics of superoxide anion production by eosinophils incubated in the wells coated with IgG in the absence or presence of ESP produced by PwNEM. Wells were coated with IgG (top panel, 10 μg/ml; middle panel, 30 μg/ml; bottom panel, 100 μg/ml) in the absence or presence of the ESP at the doses indicated for 2 h at 37°C. After washing, eosinophils were added to the wells, and superoxide production from eosinophils was measured by superoxide dismutase-inhibitable reduction of cytochrome c, as described in Materials and Methods. Data are shown as nanomoles of cytochrome c reduced/105 cells. The results represent mean values from four independent experiments performed in duplicate.
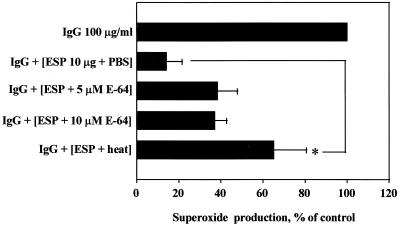
To confirm whether the inhibitory effect of the ESP on superoxide production of eosinophils stimulated with IgG was due to degradation of IgG by cysteine proteases in the ESP, the ESP was pretreated with heat or cysteine protease inhibitor E-64 before reaction with IgG. As shown in Fig. 4, the inhibitory effect of the ESP on IgG-induced superoxide production was partially blocked by pretreatment of the ESP with heat or E-64. However, pretreatment of heat significantly (P < 0.05) prevented the inhibitory effect of the ESP on IgG-induced superoxide production.
FIG. 4.
Effects of heat-treated or cysteine protease inhibitor (E-64)-treated ESP on IgG-induced superoxide production. The ESP (10 μg) was pretreated with heat for 30 min at 56°C or with E-64 at the concentrations indicated for 1 h at RT, and tissue culture plates were coated with IgG (100 μg/ml) in the presence of heat- or E-64-treated ESP for 2 h at 37°C. After aspiration of the solution in the wells, eosinophils were added to the wells and incubated for 180 min at 37°C. Data are normalized to the mean superoxide production (nanomoles of cytochrome c reduced/105 cells) induced by IgG alone, taken as 100% (control; 6.2 ± 0.29 nmol). Data are presented as the mean ± SEM from three independent experiments performed in duplicate. Significant differences from results for PBS-treated ESP are indicated by an asterisk (P < 0.05). The lack of E-64 at the concentrations tested in this experiment had detectable effects on eosinophil superoxide production.
DISCUSSION
This study demonstrates that the cysteine proteases secreted by PwNEM play an important role in attenuation of the effector function of eosinophils stimulated with IgG. The ESP produced by PwNEM, which consists of two cysteine proteases (Fig. 1A), cleaved whole human IgG molecules in a dose-dependent manner in vitro (Fig. 1B). When eosinophils were incubated in the wells coated with IgG in the presence of the ESP, eosinophil degranulation was significantly inhibited compared with results for cells incubated in the wells coated with IgG alone (Fig. 2). This inhibitory effect of the ESP on IgG-induced eosinophil degranulation was well correlated with the morphologic changes of eosinophils incubated in wells coated with IgG in the presence of the ESP (data not shown). In addition, when the cells were incubated in wells coated with IgG in the presence of the ESP, superoxide production of eosinophils was dose-dependently inhibited compared with results for cells cultured in the wells coated with IgG alone (Fig. 3). Furthermore, this inhibitory effect of the ESP on IgG-induced superoxide production was partially abolished by pretreatment of the ESP with heat or E-64 (Fig. 4). However, treatment with heat inhibited the action of the ESP more potently than that with E-64 did. These results suggest that cysteine proteases secreted by PwNEM inhibit activation of eosinophils stimulated with IgG. Taken together, our findings indicate that the cysteine protease secreted by PwNEM moderates the eosinophil's responses to the parasite-specific IgG-coated surface of the larva, resulting in reduced generation of superoxide and diminished degranulation.
In this study, the inhibitory effect of the ESP on eosinophil degranulation was somewhat different from that on superoxide production. For example, the smallest amounts of EDN were released when eosinophils were incubated in the wells coated with IgG (100 μg/ml) in the presence of the lowest dose (2 μg) of the ESP (Fig. 2). This finding is not consistent with the result that the smallest amount of superoxide was produced when cells were cultured in the wells coated with IgG in the presence of the highest dose (20 μg) of the ESP (Fig. 3). The lowest dose of the ESP tested in this study was equivalent to the amounts of proteins produced by nine excysted metacercariae of P. westermani. Therefore, it is suggested that the release of low doses of the ESP secreted by small numbers of PwNEM into host tissues is enough to suppress eosinophil degranulation stimulated with IgG, although the capability of low doses of the ESP for cleaving the IgG is minimal in vitro.
It is likely that following tight attachment of eosinophils to IgG-coated worms, the release of EDN may restrict motility of the larvae, thereby preventing the process of shedding of surface antigen and allowing adhering eosinophils to kill the worms by the release of eosinophil-toxic granules (16). On the other hand, actively motile larvae shrink their bodies to decrease their surface area in contact with antibody (28). In addition, the ESP, consisting of two cysteine proteases that are produced by PwNEM, induced a direct time- and concentration-dependent increase in the rate of constitutive apoptosis in mature human eosinophils (31). Annexin-V-positive cells were first apparent 3 h after treatment with the ESP and continued to increase after 6 h of incubation. While only 2.8% of the eosinophils incubated in the medium for 3 h were apoptotic, 7.6, 10.9, and 22.6% of the eosinophils treated with 10, 30, and 100 μg of ESP/ml were apoptotic, respectively. Moreover, treatment of human eosinophils with lower concentrations (0.3 and 1 μg/ml) of the ESP secreted by the PwNEM induced IL-8 production, whereas treatment of the cells with higher concentrations (3 and 10 μg/ml) did not (33). These inhibitory effects of the higher doses of the ESP on IL-8 production were completely abolished by pretreatment of the ESP with heat, and the amount of IL-8 released into culture supernatants was inversely correlated with the rate of eosinophil survival. These findings strongly suggest that the high amount and proteolytic activity of the cysteine proteases secreted by the metacercarial larvae (8, 9) allow the larvae to establish silent migration or residence in their hosts without provoking eosinophil-mediated tissue hypersensitivity reactions during the migratory periods. This suggestion confirms recent findings that eosinophils cannot serve as strong effector cells against tissue helminth parasites in vivo (4, 11, 26, 30).
P. westermani is a lung fluke, which mainly dwells in lung parenchyme in a final host, including humans. Ingested metacercarial larvae excyst in the duodenum by the release of two endogenous cysteine proteases of 27 and 28 kDa in molecular mass (10), and the larva-secreted cysteine proteases pave the way for their safe migration from the intestines to the lungs during the migratory phase. The 27-kDa cysteine protease in the ESP was similar to Fasciola hepatica cathepsin L (7) or sparganum cathepsin S (23) in terms of substrate specificity for Cbz-Phe-Arg-MNA and stability at a neutral pH. On the other hand, the 28-kDa protease shared mammalian cathepsin B-like properties (2) with respect to its molecular mass, selective cleavage of Cbz-Ala-Arg-Arg-MNA, and relatively low activity at pH 7.5. Therefore, the in vivo role of each protease contained in the metacercarial ESP in the behavior of eosinophils may be different. In this regard, it would be interesting to compare the effects of each protease purified from ESP on effector functions of eosinophils.
Until now, at least five species of the cysteine proteases of P. westermani have been identified. For example, 28- and 27-kDa enzymes from the metacercarial larvae (10, 36), 15- and 53-kDa enzymes from the juveniles and adults (9), and 17-kDa cysteine proteases from the adults (34) have been purified. Of the five cysteine proteases, two cysteine proteases from the metacercarial larvae reveal higher levels of proteolytic activity in cleavage of IgG than the others from juveniles and adult worms of P. westermani (8). Furthermore, the amounts of the two cysteine proteases from the metacercariae are dramatically lowered as the worms mature in vivo (9). Thus, different cysteine protease activities in cleaving human IgG during maturation stages of P. westermani may be responsible for the differences in the resident site of the worms during the infection.
In summary, we report that cysteine protease in the ESP produced by newly excysted P. westermani metacercariae plays a crucial role in attenuating effector functions of eosinophils stimulated with IgG. Further studies focused on other biological roles of the cysteine proteases secreted by newly excysted P. westermani metacercariae in behaviors of eosinophils will help us to understand their pathophysiological roles in relation to eosinophil-associated tissue inflammation in helminth parasitic diseases.
ACKNOWLEDGMENTS
We thank Yong-Moo Won, Department of Parasitology, College of Medicine, Ewha Woman's University, for his sincere help in collecting freshwater crayfish and isolating P. westermani metacercariae from the crayfish.
This research was supported by the MOST through the National Research Program (00-N6-01-01-A-05) for Woman's University.
REFERENCES
- 1.Abu-Ghazaleh R I, Fujisawa T, Mestecky J, Kyle R A, Gleich G J. IgA-induced eosinophil degranulation. J Immunol. 1989;142:2393–2400. [PubMed] [Google Scholar]
- 2.Barrett A J, Kirschke H. Cathepsin B, cathepsin H, and cathepsin L. Methods Enzymol. 1981;80:535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- 3.Berasain P, Carmona C, Frangione B, Dalton J P, Goni F. Fasciola hepatica: parasite-secreted proteinases degrade all human IgG subclasses: determination of the specific cleavage sites and identification of the immunoglobulin fragments produced. Exp Parasitol. 2000;94:99–110. doi: 10.1006/expr.1999.4479. [DOI] [PubMed] [Google Scholar]
- 4.Brunet L R, Sabin E A, Cheever A W, Kopf M A, Pearce E J. Interleukin 5 (IL-5) is not required for expression of a Th2 response or host resistance mechanisms during murine schistosomiasis mansoni but does play a role in development of IL-4-producing non-T, non-B cells. Infect Immun. 1999;67:3014–3018. doi: 10.1128/iai.67.6.3014-3018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butterworth A E, Sturrock R F, Houba V, Mahmoud A A F, Sher A, Rees P H. Eosinophils as mediators of antibody-dependent damage to schistosomula. Nature. 1975;256:727–729. doi: 10.1038/256727a0. [DOI] [PubMed] [Google Scholar]
- 6.Butterworth A E, Wassom D L, Gleich G J, Loegering D A, David J R. Damage to schistosomula of Schistosoma mansoni induced directly by eosinophil major basic proteins. J Immunol. 1979;122:221–229. [PubMed] [Google Scholar]
- 7.Carmona C, Dowd A J, Smith A M, Dalton J P. Cathepsin L proteinases secreted by Fasciola hepatica in vitro prevent antibody-mediated eosinophil attachment to newly excysted juveniles. Mol Biochem Parasitol. 1993;62:9–17. doi: 10.1016/0166-6851(93)90172-t. [DOI] [PubMed] [Google Scholar]
- 8.Chung Y B, Yang H J, Kang S Y, Kong Y, Cho S Y. Activities of different cysteine proteases of Paragonimus westermani in cleaving human IgG. Korean J Parasitol. 1997;35:139–142. doi: 10.3347/kjp.1997.35.2.139. [DOI] [PubMed] [Google Scholar]
- 9.Chung Y B, Kong Y, Yang H J, Kang S Y, Cho S Y. Cysteine protease activities during maturation stages of Paragonimus westermani. J Parasitol. 1997;83:902–907. [PubMed] [Google Scholar]
- 10.Chung Y B, Kong Y, Joo I J, Cho S Y, Kang S Y. Excystment of Paragonimus westermani metacercariae by endogenous cysteine protease. J Parasitol. 1995;81:137–142. [PubMed] [Google Scholar]
- 11.Dent L A, Daly C M, Mayrhofer G, Zimmerman T, Hallett A, Bignold L P, Creaney J, Parsons J C. Interleukin-5 transgenic mice show enhanced resistance to primary infections with Nippostrongylus brasiliensis but not primary infections with Toxocara canis. Infect Immun. 1999;67:989–993. doi: 10.1128/iai.67.2.989-993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gansmuller A, Anteunis A, Venturiello S M, Bruschi F, Binaghi R A. Antibody-dependent in vitro cytotoxicity of newborn Trichinella spiralis larvae: nature of the cells involved. Parasite Immunol. 1987;9:281–292. doi: 10.1111/j.1365-3024.1987.tb00508.x. [DOI] [PubMed] [Google Scholar]
- 13.Glaert A M, Lammas D A, Duffus W P H. Ultrastructural observations on the interaction between bovine eosinophils and juvenile Fasciola hepatica. Parasitology. 1985;91:459–470. doi: 10.1017/s0031182000062703. [DOI] [PubMed] [Google Scholar]
- 14.Gleich G J. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol. 2000;105:651–663. doi: 10.1067/mai.2000.105712. [DOI] [PubMed] [Google Scholar]
- 15.Gleich G J, Adolphson C R. The eosinophilic leukocytes: structure and functions. Adv Immunol. 1986;39:177–253. doi: 10.1016/s0065-2776(08)60351-x. [DOI] [PubMed] [Google Scholar]
- 16.Hamann K J, Barker R L, Loegering D A, Gleich G J. Comparative toxicity of purified human eosinophil granule proteins for newborn larvae of Trichinella spiralis. J Parasitol. 1987;73:523–529. [PubMed] [Google Scholar]
- 17.Hansel T T, de Vries I J M, Iff T, Rihs S, Wandzilak M, Betz S, Blaser K, Walker C. An improved immunomagnetic procedure for the isolation of highly purified human blood eosinophils. J Immunol. 1991;145:105–110. doi: 10.1016/0022-1759(91)90315-7. [DOI] [PubMed] [Google Scholar]
- 18.Hartnell A, Moqbel R, Walsh G M, Bradley B, Kay A B. Fcγ and CD11/CD18 receptor expression on normal density and low density human eosinophils. Immunology. 1990;69:264–270. [PMC free article] [PubMed] [Google Scholar]
- 19.Horie S, Gleich G J, Kita H. Cytokines directly induce degranulation and superoxide production from human eosinophils. J Allergy Clin Immunol. 1996;98:371–381. doi: 10.1016/s0091-6749(96)70161-6. [DOI] [PubMed] [Google Scholar]
- 20.Kaneko M, Swanson M C, Gleich G J, Kita H. Allergen-specific IgG1 and IgG3 through FcγRII induce eosinophil degranulation. J Clin Investig. 1995;95:2813–2821. doi: 10.1172/JCI117986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaneko M, Horie S, Kato M, Gleich G J, Kita H. A crucial role for β2 integrin in the activation of eosinophils stimulated by IgG. J Immunol. 1995;155:2631–2641. [PubMed] [Google Scholar]
- 22.Kephart G G, Gleich G J, Connor D H, Gibson D W, Ackerman S J. Deposition of eosinophil granule major basic protein onto microfilaria of Onchocerca volvulus in the skin of patients treated with diethylcarbamazine. Lab Investig. 1984;50:51–61. [PubMed] [Google Scholar]
- 23.Kong Y, Chung Y B, Cho S Y, Kang S Y. Cleavage of immunoglobulin G by excretory-secretory cathepsin S-like protease of Spirometra mansoni plerocercoid. Parasitology. 1994;109:611–621. doi: 10.1017/s0031182000076496. [DOI] [PubMed] [Google Scholar]
- 24.Kroegel C, Yukawa T, Dent G, Venge P, Chung K F, Barnes P J. Stimulation of degranulation from human eosinophils by platelet activating factor. J Immunol. 1989;142:3518–3526. [PubMed] [Google Scholar]
- 25.McLaren D J, Mackenzie C D, Ramalho-Pinto F J. Ultrastructural observations on the in vitro interaction between rat eosinophils and some parasite helminths (Schistosoma mansoni, Trichinella spiralis, and Nippostrongylus brasiliensis) Clin Exp Immunol. 1977;30:105–118. [PMC free article] [PubMed] [Google Scholar]
- 26.Meeusen E N, Balic A. Do eosinophils have a role in the killing of helminth parasites? Parasitol Today. 2000;16:95–101. doi: 10.1016/s0169-4758(99)01607-5. [DOI] [PubMed] [Google Scholar]
- 27.Moqbel R, MacDonald A J, Cromwell O, Kay A B. Release of leukotriene C4, LTC4, from human eosinophils following adherence to IgE- and IgG-coated schistosomula of Schistosoma mansoni. Immunology. 1990;69:435–442. [PMC free article] [PubMed] [Google Scholar]
- 28.Rainbird M A, Macmillan D, Meeusen E N T. Eosinophil-mediated killing of Haemonchus contortus larvae: effect of eosinophil activation and role of antibody, complement and interleukin-5. Parasite Immunol. 1998;20:93–103. doi: 10.1046/j.1365-3024.1998.00132.x. [DOI] [PubMed] [Google Scholar]
- 29.Rhoads M L, Fetterer R H. Extracellular matrix: a tool for defining the extracorporeal functions of parasite proteases. Parasitol Today. 1997;13:119–122. doi: 10.1016/s0169-4758(96)40011-4. [DOI] [PubMed] [Google Scholar]
- 30.Sher A, Coffman R L, Hiney S, Cheever A W. Ablation of eosinophil and IgE responses with anti-IL-5 or anti-IL-4 antibodies fails to affect immunity against Schistosoma mansoni in the mouse. J Immunol. 1990;145:3911–3916. [PubMed] [Google Scholar]
- 31.Shin M H. Excretory-secretory product of newly excysted metacercariae of Paragonimus westermani directly induces eosinophil apoptosis. Korean J Parasitol. 2000;38:17–23. doi: 10.3347/kjp.2000.38.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin M H, Min D Y. Infection status of Paragonimus westermani metacercariae in crayfish (Cambaroides similis) collected from Bogildo (Islet), Wando-gun, Chollanam-do, Korea. Korean J Parasitol. 1999;37:55–57. doi: 10.3347/kjp.1999.37.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin M H, Lee S Y. Proteolytic activity of cysteine protease in excretory-secretory product of Paragonimus westermani newly excysted metacercariae pivotally regulates IL-8 production of human eosinophils. Parasite Immunol. 2000;22:529–533. doi: 10.1046/j.1365-3024.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 34.Song C Y, Kim T S. Characterization of cysteine proteases from adult worms of Paragonimus westermani. Korean J Parasitol. 1994;32:231–241. doi: 10.3347/kjp.1994.32.4.231. [DOI] [PubMed] [Google Scholar]
- 35.Wasilewski M M, Lim K C, Phillips J, McKerrow J H. Cysteine protease inhibitors block schistosome hemoglobin degradation in vitro and decrease worm burden and egg production in vivo. Mol Biochem Parasitol. 1996;81:179–189. doi: 10.1016/0166-6851(96)02703-x. [DOI] [PubMed] [Google Scholar]
- 36.Yamakami K, Hamajima F. Purification and properties of a neutral thiol protease from larval trematode Paragonimus westermani. Comp Biochem Physiol B. 1987;87:643–648. doi: 10.1016/0305-0491(87)90065-4. [DOI] [PubMed] [Google Scholar]
- 37.Yoshimura K, Uchida K, Sato K, Oya H. Ultrastructural evidence of eosinophil-mediated destruction of Angiostrongylus cantonensis transferred into the pulmonary artery of non-permissive hosts. Parasite Immunol. 1984;6:105–118. doi: 10.1111/j.1365-3024.1984.tb00785.x. [DOI] [PubMed] [Google Scholar]