Abstract
Effective protection against intestinal pathogens requires both mucosal and systemic immune responses. Intranasal administration of antigens induces these responses but generally fails to trigger a strong protective immunity. Mucosal adjuvants can significantly enhance the immunogenicities of intranasally administered antigens. Cholera toxin (CT) and heat-labile enterotoxin (LT) are strong mucosal adjuvants with a variety of antigens. Moreover, the toxicities of CT and LT do not permit their use in humans. Two nontoxic mutant LTs, LTR72 and LTK63, were tested with Toxoplasma gondii SAG1 protein in intranasal vaccination of CBA/J mice. Vaccination with SAG1 plus LTR72 or LTK63 induced strong systemic (immunoglobulin G [IgG]) and mucosal (IgA) humoral responses. Splenocytes and mesenteric lymph node cells from mice immunized with LTR72 plus SAG1, but not those from mice immunized with LTK63 plus SAG1, responded to restimulation with a T. gondii lysate antigen in vitro. Gamma interferon and interleukin 2 (IL-2) production by splenocytes and IL-2 production by mesenteric lymph node cells were observed in vitro after antigen restimulation, underlying a Th1-like response. High-level protection as assessed by the decreased load of cerebral cysts after a challenge with the 76K strain of T. gondii was obtained in the group immunized with LTR72 plus SAG1 and LTK63 plus SAG1. They were as well protected as the mice immunized with the antigen plus native toxins. This is the first report showing protection against a parasite by using combinations of nontoxic mutant LTs and SAG1 antigen. These nontoxic mutant LTs are now attractive candidates for the development of mucosally delivered vaccines.
The intracellular protozoan parasite Toxoplasma gondii infects all mammalian cells and is responsible for toxoplasmosis. Toxoplasmosis is generally harmless in an immunocompetent host, but it can cause severe damage to the fetus during pregnancy and is often lethal for immunodeficient subjects unless they are treated (22). T. gondii infection is also an important problem in animal breeding because it causes the deaths of many fetuses in cattle and sheep, for example (6). The natural site of infection for T. gondii is the mucosal surface of the intestine. Protective immunity obtained after a natural infection with T. gondii points to the importance of developing a vaccine that stimulates mucosal defenses. One major mechanism of protection against toxoplasma is considered to be systemic cell-mediated immunity with gamma interferon (IFN-γ) induction (3, 4, 33, 34, 46). SAG1 is a vaccine candidate of interest for a protective immune response in toxoplasmosis. Partial protective immunity has been produced by immunization with the SAG1 antigen, the major T. gondii surface antigen, which accounts for 3 to 5% of the total parasite protein (5, 13, 14, 35). The ability to increase systemic and mucosal responses against T. gondii may be of great importance for the development of an efficient vaccine. Furthermore, the intranasal route requires less antigen than the oral route because there is much less proteolytic activity in the nasal cavity. This route effectively promotes the production of both systemic and mucosal immune responses to an antigen (49, 61–63). Many if not most antigens trigger only weak or poor mucosal immune responses when given alone. The use of a mucosal adjuvant such as cholera toxin (CT) from Vibrio cholerae or heat-labile enterotoxin (LT) from toxigenic strains of Escherichia coli is necessary to enhance an immune response (14, 20, 21, 29, 39). The amino acid sequences of CT and LT are 80% identical (12). They have two functional domains, the A subunit, with ADP ribosyltransferase activity, and the pentameric B subunit, which is responsible for the toxin binding to the GM1 ganglioside receptor at the cell surface (28). The A subunit is toxic for eucaryotic cells, as it activates the Gs protein that binds to GTP. The Gs protein regulates the intracellular production of cyclic AMP. An increase in cyclic AMP stimulates the secretion of electrolytes and the osmotic movement of water in the gut lumen, which is responsible for profuse watery diarrhea (60).
CT and LT cannot be included in vaccine formulations for use in humans because of their toxicity. Therefore, the construction of nontoxic mutant of E. coli is important for the development of mucosal vaccines. Several mutant LTs have been constructed by site-directed mutagenesis (48). Two of these have been tested in our model of vaccination against T. gondii. The mutant toxin LTR72 (substitution of Arg for Ala at position 72) has reduced enzymatic and toxic activities (25). The other mutant, LTK63 (substitution of Ala for Ser at position 63 of the catalytic site) has neither enzymatic nor toxic activities (15, 25). LTR72 and LTK63 act as appropriate mucosal adjuvants following oral and intranasal immunization with various antigens and trigger local and systemic immune responses (15, 47). Intranasal immunization with influenza virus hemagglutinin in combination with LTR72 induces serum immunoglobulin G (IgG) and mucosal IgA antibodies and neutralization of the virus with the development of a systemic Th1 response (2). These adjuvants have also been used with bacterial antigen and protect against infection with Helicobacter pylori after intragastric vaccination (41), with Streptococcus pneumoniae after nasal vaccination (30), and with Bordetella pertussis as strongly as bacterial antigen with LT (52). These results indicate that ADP ribosyltransferase activity is not necessary for adjuvant activity.
In previous studies, we have shown that intranasal vaccination with SAG1 plus CT protects mice against T. gondii (14). Since CT is too toxic to be used in humans, we have now investigated the capacities of mutant LTR72 and LTK63 to enhance the immunogenicity of intranasally administered SAG1.
MATERIALS AND METHODS
Animals.
Pathogen-free female inbred CBA/J mice were used at 6 to 8 weeks of age (Janvier, Le Genest St. Isle, France).
Parasites.
Tachyzoites of the RH strain of T. gondii were harvested from the peritoneal fluid of Swiss OF1 mice that had been intraperitoneally infected 3 to 4 days earlier. They were used to prepare T. gondii lysate antigen (TLA). Cysts of the 76K strain of T. gondii were obtained from the brains of CBA/J mice infected 1 month previously.
Adjuvants and antigen.
Wild-type CT and LT were purchased from Sigma, and the nontoxic mutant LTs (LTR72 and LTK63) were kindly provided by Chiron (Siena, Italy). They were used as adjuvants in combination with SAG1 protein purified from TLA by immunoaffinity (14). The mutant LTs LTR72 and LTK63 were obtained as previously described (25).
Immunization.
The mucosal immunogenicities and adjuvant activities of mutant toxins were tested by immunizing groups of 10 mice intranasally two times at 28-day intervals with SAG1 (10 μg), LTR72 (1 μg), LTK63 (1 μg), LT (1 μg), or CT (1 μg) alone (defined as control groups) or with the combination SAG1 plus LTR72, SAG1 plus LTK63, SAG1 plus LT, or SAG1 plus CT (10 μg of protein and 1 μg of toxin). Each dose of immunogen was diluted to a final volume of 16 μl in phosphate-buffered saline (10 mM phosphate, 140 mM NaCl [PBS]) and was instilled into the nostrils of anesthetized mice with a micropipettor (8 μl/nostril). The experimental design included a group of untreated mice. The day before and 10 days after the boost, blood was collected by retro-orbital puncture. All samples were kept frozen (−20°C) until assayed for antibody activity.
Antigen-driven cell-proliferative responses.
Three mice per group were sacrificed at day 42. Spleens, mesenteric lymph nodes, and nasal-associated lymphoid tissue (NALT) were harvested under steril conditions and pressed through a nylon mesh. Single-cell suspensions were obtained by filtration through nylon mesh to remove tissue debris. Hypotonic shock (0.155 M NH4Cl [pH 7.4]) was used to remove splenic erythrocytes. The cells were then suspended in RPMI 1640 medium (GIBCO) supplemented with 5% fetal calf serum, HEPES (25 mM; Sigma), l-glutamine (1 mM; BioWhittaker), sodium pyruvate (1 mM; Sigma), β-mercaptoethanol (50 μM), and penicillin-streptomicin (1 mM; Sigma) and seeded at 5 × 105 cells per well in triplicate in flat-bottomed 96-well microtiter plates (Costar) in 200 μl of culture medium alone or with various concentrations of TLA or 10 μg of concanavalin A per ml as a positive control of proliferation. The plates were incubated in 5% CO2 at 37°C for 4 days, and 1 μCi of [3H]thymidine (NEN, Paris, France) was added for the final 18 h of culture. The cells were harvested on glass fiber filters using an automatic cell harvester (Tomtec; Wallac), and the amounts of [3H]thymidine incorporated into the DNAs of proliferating cells were determined in a liquid scintillation β-counter (Microbeat Trilux: Wallac). Proliferation was expressed as the stimulation index (SI) (counts per minute for unstimulated cells /counts per minute for stimulated cells).
Nasal and lung washes were performed on day 42 by repeated flushing and aspiration of 1 ml of PBS containing 1 mM phenylmethylsulfonyl fluoride (Sigma). Intestinal washes were performed with a syringe by passing 5 ml of PBS–1 mM phenylmethylsulfonyl fluoride through the gut.
Detection of cytokines in cell supernatants.
Cytokines released from spleen and mesenteric lymp node cells stimulated in vitro with 15 μg of TLA per ml were measured in the culture supernatants collected at 48 h for interleukin 2 (IL-2) and IL-4, 72 h for IL-10 and IL-5, and 96 h for IFN-γ detection. Levels of cytokines were determined with a commercial enzyme-linked immunosorbent assay (ELISA) kit (Opteia set: R&D Systems). In brief, 96-well (flat-bottomed) plates (Nunc) were coated with anti-mouse cytokine in PBS overnight at room temperature. Free sites were then blocked with block buffer, and supernatants (1/2 diluted) were added and incubated for 2 h at room temperature. A standard curve was also created with the recombinant cytokine. Cytokine was detected using biotinylated anti-mouse cytokine. Horseradish peroxidase-streptavidin was added and incubated for 20 min. Bound antibodies were visualized with a tetramethylbenzidine substrate (Sigma). The enzymatic reaction was stopped with 2 N sulfuric acid, and absorbances were read at 450 nm (1420 multilabel counter Victor; Wallac).
SAG1-specific antibody responses.
Serum IgG antibody to SAG1 was measured by ELISA. Flat-bottomed 96-well plates (Nunc) were coated with TLA (10 μg/ml) in sodium carbonate buffer overnight. The plates were washed and blocked with PBS–4% bovine serum albumin (BSA). Serial dilutions of serum, in PBS, were added, and the plates were incubated for 1 h at 37°C. The plates were then washed in PBS–0.05% Tween 20 and incubated with alkaline phosphatase-conjugated goat anti-mouse IgG (γ-chain specific) diluted 1/1,000 in PBS–4% BSA for 2 h at 37°C. The plates were washed with 1 mg of p-nitrophenylphosphate per ml in diethanolamine buffer (pH 9.8), and the optical density (OD) of each sample was read at 405 nm (1420 multilabel counter Victor; Wallac). The antigen-specific antibody titer was given as the reciprocal of the highest dilution whose absorbance was 2.5-fold greater than the absorbance of the sera of control mice at the same dilution. Results are expressed as the means of log2 titers ± standard deviations (SD).
The Ig subclasses of the antibodies were determined with the alkaline phosphatase conjugates IgG1, IgG2a, IgG2b, and IgG3 (1:500; Cappel) and developed as described above. Reactions were stopped when the OD at 405 nm for total IgG was 2 U and compared between groups.
IgA from nasal, lung, and intestinal washes were detected by Western blotting. Proteins of TLA were separated on a sodium dodecyl sulfate–12% polyacryamide gel and transferred onto a nitrocellulose membrane. The membrane was blocked by incubation for 1 h at 37°C with TNT (0.1 M Tris, 0.15 M NaCl, and 0.05% Tween 20) and 5% low-fat milk. Nasal washes (dilution 1/2), lung washes (dilution, 1/10), and intestinal washes (dilution, 1/2) were then added, and the mixtures were incubated overnight at 4°C. IgA-bound antibodies were detected using a 1:1,000 dilution of alkaline phosphatase-conjugated goat anti-mouse IgA (Sigma), with washes in TNT after each step. An alkaline phosphatase substrate (Sigma) in 10 mM Tris-HCl–100 mM NaCl–5 mM MgCl2 was added to detect IgG.
Measurement of ASC by ELISPOT assay.
Nasal lymphocytes were obtained by dissecting out the NALT, identified as two small longitudinal strips of tissue (57). Cell suspensions were obtained as described above. Cells were suspended in culture medium containing 1% supernatant from concanavalin A-stimulated rat spleen cells. B cells secreting Ig specific for the SAG1 protein of T. gondii were detected by enzyme-linked immunospot (ELISPOT) assay (11). Briefly, a 96-well plate (Costar) was coated overnight at 4°C with TLA at 10 μg/ml in sodium carbonate buffer. The wells were blocked with PBS–1% BSA at 37°C. Lymphocytes were suspended (5 × 105 to 1.25 × 105 in 100 μl), and the diluted lymphocytes were added to each well, centrifuged, and incubated for 24 h at 37°C in 5% CO2. The plate was washed three times in H2O and three times in PBS–0.05% Tween 20 and incubated overnight at 4°C with alkaline phosphatase-conjugated goat anti-mouse IgG or goat anti-mouse IgA. The plate was washed three times in PBS–0.05% Tween 20, and spots representing antibody-secreting cells (ASC) were developed with 5-bromo-4-chloro-3-indolylphosphate disodium salt (Sigma). The spots were counted with a microscope. Results are expressed as numbers of ASC from 106 cells.
Challenge infection.
Two weeks after the last immunization, mice were infected orally with 70 cysts of the 76K strain. The mice were killed 1 month after the challenge, and their brains were removed. Each brain was homogenized in 5 ml of PBS. The number of cysts per brain was determined microscopically by counting eight samples (10 μl each) of each homogenate and expressed as a mean number ± SD for each group.
Statistical analyses.
Experimental groups were compared by an analysis of variance test. A P of <0.05 was considered significant.
RESULTS
Proliferative activity after intranasal immunization using LTR72 as the adjuvant.
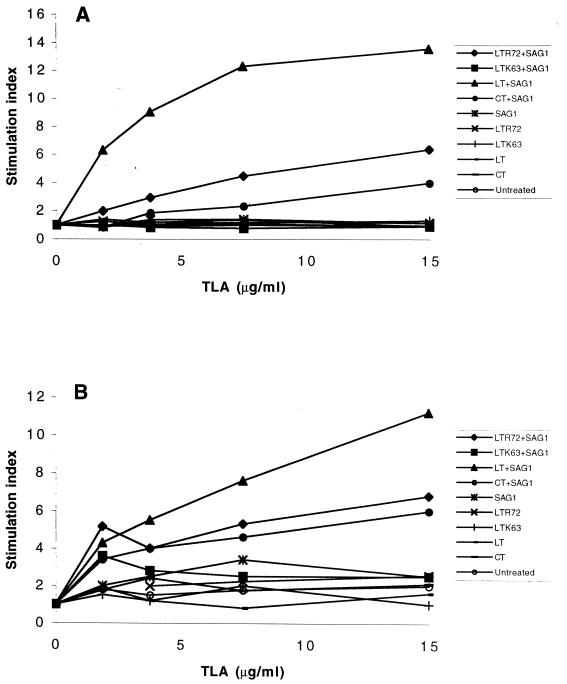
Mice were primed with two intranasal immunizations with SAG1 protein mixed with the LTR72, LTK63, LT, or CT adjuvant. Spleens and mesenteric lymph nodes were removed from three mice of all immunized groups 15 days after the last immunization and prepared as single-cell suspensions. They were then stimulated with various concentrations of TLA in vitro. Cells from untreated mice or mice immunized with SAG1 alone or with toxin alone were also prepared similarly and cultured in parallel. Splenocytes from all groups of mice immunized intranasally with LTR72, LT, or CT combined with SAG1 responded by increased dose-dependent antigen proliferation in vitro. In contrast, cells from mice immunized with LTK63 plus SAG1 and from control mice did not respond to antigen restimulation (Fig. 1A). The SI for LTR72 plus SAG1 (SI = 6.3) was significantly higher than that for CT plus SAG1 (SI = 4) but lower than that for LT plus SAG1 (SI = 13.5) (Fig. 1) (P < 0.001). Splenocytes cultured in the absence of specific antigen showed no enhanced cell proliferation.
FIG. 1.
Splenocyte (A) and mesenteric lymph node cell (B) proliferative responses to T. gondii lysate antigen in vitro. Splenocytes and mesenteric lymph node cells from three mice immunized with LTR72, LTK63, LT, or CT, alone or with SAG1, were isolated and stimulated with different concentrations of T. gondii lysate antigen in triplicate. The optimal concentration was 15 μg/ml. Proliferation were measured by thymidine incorporation and is expressed in counts per minute. The SI is the unstimulated cell counts per minute divided by the stimulated cell counts per minute. Results from one of three similar experiments are shown.
The cellular response in mesenteric lymph nodes was also analyzed. Mesenteric lymph node cells from animals immunized with LTR72 plus SAG1, LT plus SAG1, and CT plus SAG1 (Fig. 1B) were also stimulated dose dependently. The SI for mice immunized with LT plus SAG1 (SI = 11.2) was higher than the SI for mice immunized with LTR72 plus SAG1 (SI = 6.8) or with CT plus SAG1 (SI = 6) (P < 0.01 and P < 0.001, respectively). The mesenteric lymph node cells from mice immunized with LTK63 plus SAG1 did not respond to antigen restimulation. These results demonstrate that intranasal administration of a mixture of SAG1 and LTR72 triggered systemic and mucosal cellular immunity. LTK63, which is devoid of any toxic and enzymatic activity, does not trigger a detectable cellular response. The absence of ADP ribosyltransferase activity might account for its poor stimulation of cell proliferation in our model, and it may be necessary to perform two to three booster immunizations.
Analysis of culture supernatants for cytokines.
The cytokine patterns obtained after cellular stimulation were studied to further explore the immune response. Culture supernatants of splenocytes and mesenteric lymph node cells, unstimulated and stimulated with 15 μg of TLA per ml, were assayed for cytokines. Only groups immunized with SAG1 plus the toxins, except LTK63, produced significant amounts of both IFN-γ and IL-2 compared to levels produced by the control groups (Table 1). Mesenteric lymph node cells from mice immunized with LTR72, LT, or CT with SAG1 (Table 1) produced only IL-2. IL-4, IL-5, and IL-10 were undetectable in any of the supernatants from spleen and mesenteric lymph node lymphocytes stimulated with TLA in vitro (data not shown).
TABLE 1.
Cytokine production by spleen and mesenteric lymph node cells from mice immunized intranasally with SAG1 protein plus toxina
| Group | Amt released (pg/ml)
|
|||
|---|---|---|---|---|
| IFN-γ
|
IL-2
|
|||
| Alone | + TLA | Alone | + TLA | |
| LTR72 + SAG1 | 23 | 1,414 | 20 | 361 |
| LTK63 + SAG1 | 24 | 34 | <15 | <15 |
| LT + SAG1 | 26 | 2,532 | <15 | 163 |
| CT + SAG1 | <8 | 831 | <15 | 141 |
| SAG1 | <8 | <8 | <15 | 16 |
| LTR72 | 31 | 36 | 26 | 24 |
| LTK63 | 29 | 219 | 18 | 30 |
| LT | 138 | 338 | <15 | <15 |
| CT | 119 | 387 | 34 | 35 |
| Untreated | 28 | 37 | 24 | 26 |
| LTR72 + SAG1 | 75 | 198 | <15 | 31 |
| LTK63 + SAG1 | 35 | 209 | <15 | <15 |
| LT + SAG1 | <15 | <15 | <15 | 25 |
| CT + SAG1 | 22 | 344 | <15 | 39 |
| SAG1 | 69 | 116 | <15 | <15 |
| LTR72 | 100 | 235 | 16 | <15 |
| LTK63 | 104 | 235 | <15 | <15 |
| LT | <15 | <15 | <15 | <15 |
| CT | 40 | 180 | <15 | <15 |
| Untreated | 131 | 318 | <15 | <15 |
Mice were primed by two intranasal immunizations and rested for 15 days before being killed. Single-cell suspensions were prepared from spleens and mesenteric lymph nodes and cultured in the presence or absence of T. gondii antigen (15 μg/ml). Amounts released were calculated with recombinant cytokine. Values for IL-2 and IFN-γ were taken from samples at 48 and 96 h, respectively. The results are representative of at least three experiments. Boldface indicates no significant concentration.
Serum antibody reponses.
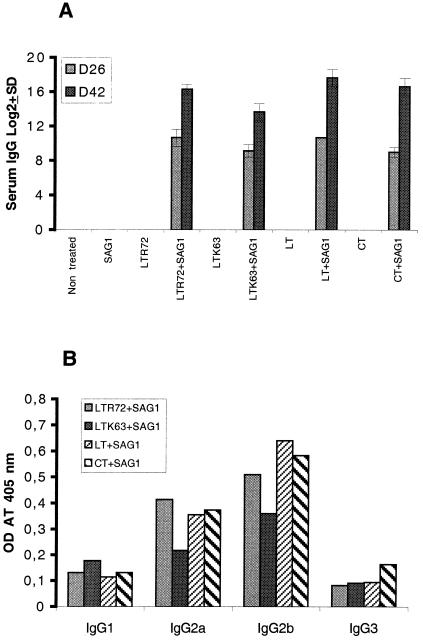
Mice given two 1-μg doses of toxin with 10 μg of SAG1 protein produced a specific IgG antibody response to SAG1 when they were immunized with SAG1 plus LTR72, LTK63, LT, or CT (Fig. 2A) after the first immunization. The second immunization enhanced the antibody response. No antibody was detected in the control group. The titers of anti-SAG1 IgG were high in all groups of mice immunized with one of the toxins plus SAG1. However, the antibody titer in the group given LTK63 plus SAG1 was significantly lower (P < 0.05 compared to the titer with LTR72 plus SAG1 or to that with CT plus SAG1 and p < 0.01 compared to the titer with LT plus SAG1). The IgG isotype pattern of the SAG1-specific antibodies elicited after two immunizations was analyzed. Both IgG2a and IgG2b isotypes were detected in all groups immunized with toxin and SAG1 (Fig. 2B). No IgG1 or IgG3 was detected in significant quantities in any group.
FIG. 2.
(A) Serum IgG titers. Serum was collected 26 days after the first immunization (D26) and 15 days after the last immunization (D42). The antigen-specific antibody titer is given as the reciprocal of the highest dilution whose absorbance was 2.5-fold greater than the absorbance of the sera of control mice at the same dilution. Results are expressed as the means of log2 titers ± SD. (B) A serum IgG subclass profile was performed with serum collected on D42. Results from one of two experiments are shown.
IgA in nasal, lung, and intestinal washes.
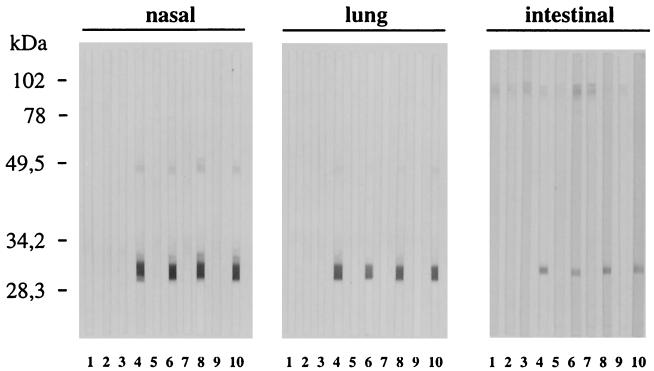
The local humoral IgA response induced by intranasal immunization with the nontoxic mutants LTR72 and LTK63 was analysed using nasal, lung, and intestinal washes taken after the second immunization. IgA responses were visualized by Western blotting on a nitrocellulose membrane of TLA. Bands corresponding to SAG1 protein (30 kDa) were observed in samples from the nasal, lung, and intestinal washes (Fig. 3) of mice immunized with SAG1 plus LTR72 or LTK63. The nasal, lung, and intestinal washes from mice immunized with SAG1 plus wild-type CT and LT showed that they also trigger an IgA response. Control mice and mice immunized with SAG1 alone or toxin alone never showed any nasal, lung, or intestinal antibody responses.
FIG. 3.
Western blot analysis of the local antibody response. Nasal, lung, and intestinal washes were analyzed for IgA specific for SAG1 protein. Washes for two or three mice of each group were done 15 days after the boost. Lanes: 1, untreated; 2, SAG1; 3, LTR72; 4, LTR72 plus SAG1; 5, LTK63; 6, LTK63 plus SAG1; 7, LT; 8, LT plus SAG1; 9, CT; 10, CT plus SAG1. Results from one of two similar experiments are shown.
IgG and IgA ASC in nasal mucosa.
The numbers of IgG and IgA ASC specific for SAG1 were determined in nasal mucosal tissues after the second immunization. Intranasal immunization always induced IgA-specific ASC in all mice immunized with SAG1 plus any of the toxins. The numbers of ASC in the nasal mucosae of mice immunized with LTR72 plus SAG1 and CT plus SAG1 were similar and lower than in mice immunized with LT plus SAG1 (P < 0.01 compared to numbers with LTR72 plus SAG1; P < 0.05 compared to numbers with CT plus SAG1) (Table 2). Mice immunized with LTK63 plus SAG1 had only a few IgA ASC. Mice immunized with SAG1 plus native toxins were the only mice with IgG ASC in the nasal mucosa. There were significantly more ASC in mice immunized with LT plus SAG1 than in mice immunized with CT plus SAG1 (P < 0.01).
TABLE 2.
ASC specific for SAG1 in the nasal mucosaa
| Group | No. of ASC/106 cells ± SD secreting:
|
|
|---|---|---|
| IgA | IgG | |
| Untreated | 0 | 0 |
| SAG1 | 0 | 0 |
| LTR72 | 0 | 0 |
| LTR72 + SAG1 | 142.6 ± 71.6 | 0 |
| LTK63 | 0 | 0 |
| LTK63 + SAG1 | 13 ± 3.6 | 0 |
| LT | 0 | 0 |
| LT + SAG1 | 270 ± 73 | 189 ± 95 |
| CT | 0 | 0 |
| CT + SAG1 | 158 ± 23 | 44 ± 37 |
Fifteen days after the last immunization, the nasal mucosa was remove and ELISPOT analysis was performed for detecting IgA and IgG ASC. Results from one of two similar experiments are shown.
Protection.
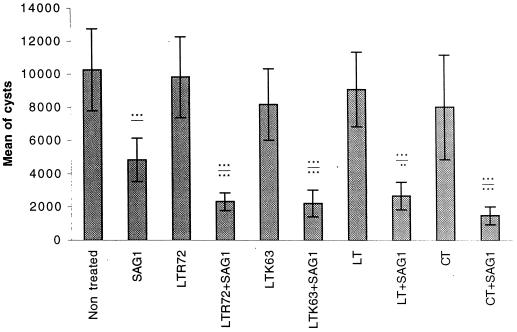
The protection provided by vaccination with these nontoxic mutant toxins as adjuvants was evaluated using T. gondii infection. Mice were challenged orally with 70 cysts of the 76K strain of T. gondii 15 days after the last immunization. CBA/J mice are resistant to the acute phase and sensitive to the chronic phase. Protection was assessed by counting the cysts in the brains of mice 1 month after the challenge. Untreated mice or mice given toxin alone had heavy cyst burdens (between 7,986 ± 3,140 and 10,250 ± 2,475 cysts). Mice immunized with SAG1 alone had slightly fewer cysts (4,833 ± 1,311) than untreated mice (10,250 ± 3,140). A significantly smaller brain cyst load (P < 0.001 versus the cyst load in untreated mice) was obtained in mice immunized with LTR72 plus SAG1 (2,309 ± 530), LTK63 plus SAG1 (2,200 ± 811), LT plus SAG1 (2,667 ± 824), or CT plus SAG1 (1,476 ± 530), which corresponds to 77, 78, 75, or 85% protection, respectively (Fig. 4).
FIG. 4.
Protection of mice after challenge with 70 cysts of the 76K strain of T. gondii. Mice were sacrified 1 month after the challenge, and the cysts in each brain were counted. Results are expressed as the mean number of cysts for each group of mice ± SD. Results from one of three similar experiments are shown. Statistics were performed with an analysis of variance test. ∗∗∗, P < 0.001; ∗∗, P < 0.01. P values above the lines indicate statistics from a comparison with nontreated mice. P values below the lines indicate statistics from a comparison with SAG1-treated mice.
DISCUSSION
We have investigated the capacities of two nontoxic mutant LTs, LTR72 and LTK63, to act as mucosal adjuvants. They were mixed with SAG1 protein and used for intranasal immunization of CBA/J mice. They were tested by assessing the protective immune response to a challenge with T. gondii. The protection against T. gondii was measured by the brain cyst load. Mice immunized with LTR72 plus SAG1 or LTK63 plus SAG1 had significantly fewer cysts than controls (Fig. 4). This decrease was as great as with the wild-type toxin and the homolog CT, as previously reported (14). The ADP-ribosylating holotoxins CT and LT are powerful adjuvants. Initially, the A subunit was considered to be crucial for adjuvant activity (40), but the construction of nontoxic mutants devoid of any enzymatic or toxic activity showed that these mutants still had their adjuvant and immunological properties (15, 17, 18, 25). Immunization using these toxins also protected against infections with B. pertussis (52), S. pneumoniae (30), and virus (2).
Nasal delivery of the antigen SAG1 plus LTR72, LT, or CT as the adjuvant produced a cellular response in local (mesenteric lymph node) and systemic (spleen) sites as assessed by antigen restimulation in vitro (Fig. 1). A cellular response has also been obtained by intranasal vaccination with ovalbumin or B. pertussis plus mutant toxins (25, 52). The cellular response was important for protection against infection with T. gondii (31). We have also demonstrated that mesenteric lymph node cells and splenocytes from mice immunized with LTR72 plus SAG1 proliferate strongly in response to TLA, but there was no proliferative response with LTK63 plus SAG1, which is entirely devoid of enzymatic and toxic activity (25). We assume that the quantity of LTK63 (1 μg/mouse) used for immunization in these experiments which was the same as that of LTR72, may not have been sufficient to trigger a detectable in vitro cellular response under our experimental conditions and that three to four immunizations may be required. Giulani et al. noted that a 20× concentration of LTK63 is required to obtain a response similar to that obtained with LT (25). The importance of mesenteric lymph node cell stimulation was recently demonstrated in cell transfer experiments. The adoptive transfer of mesenteric lymph node cells from intranasally immunized mice to naive mice significantly reduced the cyst load (80%) after challenge, compared to that of nontransferred mice (59). Mesenteric lymph nodes drain the gut, the site of natural infection by the parasite. The cells that are important in the gut during infection by T. gondii are intraepithelial lymphocytes, which form the first functional barrier to toxoplasmosis (7–9, 38). Their protective capacity depends on the production of IFN-γ (7). CD4 and CD8 T lymphocytes are also implicated. Experiments with T cells from the spleen (46) and with mice depleted of T cells (23) indicate that CD4 and CD8 T lymphocytes help mediate resistance to T. gondii, probably through the production of IFN-γ.
Several reports indicate that LTR72 elicits a Th2-like response (16, 52). In contrast, the cellular response in our experiments had more of a Th1 pattern in terms of cytokines produced. We detected, by ELISA, IFN-γ and IL-2 in culture supernatants of restimulated cells from mesenteric lymph nodes and splenocytes taken from mice immunized with LTR72 plus SAG1. A response with a Th1 cytokine pattern was also obtained with LT or CT plus SAG1. This observation can be supported by the fact that IFN-γ is an important cytokine in the immune response, conferring resistance to the development of toxoplasmic encephalitis (54–56), and that IFN-γ-mediated cellular immunity is required for the survival of mice in acute and chronic stages of infection with T. gondii (24, 26). Last, T. gondii-specific Th1 cells activate, via IFN-γ production, infected macrophages to kill intracellular parasites (51).
B cells are also crucial in the resistance of the host cell to T. gondii. An experiment with mice lacking B cells showed decreased resistance to infection, indicating that antibody production by B cells prevents the persistent replication of tachyzoites in the brain and lung (32). It has also been reported that antibody can inhibit intracellular proliferation (44) and that antibody-coated tachyzoites are killed by macrophages in vitro (1). In vitro, monoclonal antibodies to SAG1 can inhibit murine enterocyte infection (45). The protection we obtained was correlated with high titers of anti-SAG1 IgG in serum. The specific IgGs produced were IgG2a and IgG2b subclasses (Fig. 2B), as previously observed with CT (14). The IgG2a subclass was specific for a Th1 response. IgG2b production is known to be selectively induced by transforming growth factor β (TGF-β) (53), and this TGF-β can also direct the switching of B cells to the IgA isotype (19).
Intranasal immunization is more effective than intragastric immunization, as it generates an earlier and stronger mucosal immune response (27, 64). Intranasal immunization also delivers the antigen directly to the site of uptake (37). The stimulation of cells from the spleen and mesenteric lymph nodes after nasal immunization points to the existence of a common mucosal immune system. This implies that cells stimulated in the NALT by the antigen presented by antigen-presenting cells can leave this site for mucosal effector sites (36, 42, 50). Intranasal immunization with LTR72 plus SAG1 induced IgA ASC in the nasal mucosa, and significantly fewer were produced by mice immunized with LTK63 plus SAG1. IgA was detected in the nasal, pulmonary, and intestinal washes 15 days after the last immunization with SAG1 plus LTR72 or LTK63 (Fig. 3). Nasal immunization triggers pulmonary immunity. Antigen administered by the nasal route can reach the tracheal area, or dendritic cells loaded with the antigen may have migrated from the NALT to the pulmonary lymph node, where they can initiate an immune response (58). Intranasal immunization triggers both mucosal and systemic T- and B-cell responses (64) and can be used to target pathogens that invade far from the immunization site, such as the gut (10, 14). T. gondii naturally invades the intestine of its host. The intestinal secretory IgA response is most important because IgA antibodies are thought to protect against oral infection with the parasite (43). However, our results are consistent with published reports where the activation of mucosal IgA responses is associated with IFN-γ and IL-2 secretion after vaccination with TLA plus CT (3).
This is the first report showing that mucosal immunization with T. gondii antigen plus mutant LTs protects against a parasite. Our study demonstrates that intranasal immunization with nontoxic mutant toxins as mucosal adjuvants can protect against a challenge with the parasite. The most important finding is a protective immunity obtained without a detectable cellular response by using the association of SAG1 and LTK63. LTR72 in association with SAG1 triggers strong cellular and humoral responses to the protein SAG1. These mutant LTs could thus be attractive mucosal adjuvants to obtain immune responses at systemic and mucosal sites following vaccination against T. gondii. The response had a Th1 cytokine pattern and IgG subclass profile protective against T. gondii infection. Nevertheless, LTK63, which is devoid of toxicity and which produced only a humoral response under our experimental conditions, is as good an adjuvant as LTR72 to promote protection, but more has to be known of the exact immune mechanisms involved in protection. The importance of a humoral or IFN-γ cytokine must be studied in our future work with deficient mice.
ACKNOWLEDGMENTS
We thank Claude Leclerc for helpful discussion. We are indebted to Dany Tabareau for her excellent technical assistance and Remy Magné for assistance in purifying SAG1.
REFERENCES
- 1.Anderson S E, Jr, Bautista S C, Remington J S. Specific antibody-dependent killing of Toxoplasma gondii by normal macrophages. Clin Exp Immunol. 1976;26:375–380. [PMC free article] [PubMed] [Google Scholar]
- 2.Barackman J D, Ott G, O'Hagan D T. Intranasal immunization of mice with influenza vaccine in combination with the adjuvant LT-R72 induces potent mucosal and serum immunity which is stronger than that with traditional intramuscular immunization. Infect Immun. 1999;67:4276–4279. doi: 10.1128/iai.67.8.4276-4279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourguin I, Chardès T, Bout D. Oral immunization with Toxoplasma gondii antigens in association with cholera toxin induces enhanced protective and cell-mediated immunity in C57BL/6 mice. Infect Immun. 1993;61:2082–2088. doi: 10.1128/iai.61.5.2082-2088.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourguin I, Chardès T, Bout D. Peritoneal macrophages from C57BL/6 mice orally immunized with Toxoplasma gondii antigens in association with cholera toxin possess an enhanced ability to inhibit parasite multiplication. FEMS Immunol Med Microbiol. 1995;12:121–126. doi: 10.1111/j.1574-695X.1995.tb00183.x. [DOI] [PubMed] [Google Scholar]
- 5.Bulow R, Boothroyd J C. Protection of mice from fatal Toxoplasma gondii infection by immunization with p30 antigen in liposomes. J Immunol. 1991;147:3496–3500. [PubMed] [Google Scholar]
- 6.Buxton D. Protozoan infections (Toxoplasma gondii, Neospora caninum and Sarcocystis spp.) in sheep and goats: recent advances. Vet Res. 1998;29:289–310. [PubMed] [Google Scholar]
- 7.Buzoni-Gatel D, Debbabi H, Moretto M, Dimier-Poisson I H, Lepage A C, Bout D T, Kasper L H. Intraepithelial lymphocytes traffic to the intestine and enhance resistance to Toxoplasma gondii oral infection. J Immunol. 1999;162:5846–5852. [PubMed] [Google Scholar]
- 8.Buzoni-Gatel D, Lepage A C, Dimier-Poisson I H, Bout D T, Kasper L H. Adoptive transfer of gut intraepithelial lymphocytes protects against murine infection with Toxoplasma gondii. J Immunol. 1997;158:5883–5889. [PubMed] [Google Scholar]
- 9.Chardès T, Buzoni-Gatel D, Lepage A, Bernard F, Bout D. Toxoplasma gondii oral infection induces specific cytotoxic CD8 alpha/beta+ Thy-1+ gut intraepithelial lymphocytes, lytic for parasite-infected enterocytes. J Immunol. 1994;153:4596–4603. [PubMed] [Google Scholar]
- 10.Corthesy-Theulaz I E, Hopkins S, Bachmann D, Saldinger P F, Porta N, Haas R, Zheng-Xin Y, Meyer T, Bouzourene H, Blum A L, Kraehenbuhl J P. Mice are protected from Helicobacter pylori infection by nasal immunization with attenuated Salmonella typhimurium phoPc expressing urease A and B subunits. Infect Immun. 1998;66:581–586. doi: 10.1128/iai.66.2.581-586.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czerkinsky C C, Nilsson L A, Nygren H, Ouchterlony O, Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;65:109–121. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- 12.Dallas W S, Falkow S. Amino acid sequence homology between cholera toxin and Escherichia coli heat-labile toxin. Nature. 1980;288:499–501. doi: 10.1038/288499a0. [DOI] [PubMed] [Google Scholar]
- 13.Darcy F, Maes P, Gras-Masse H, Auriault C, Bossus M, Deslee D, Godard I, Cesbron M F, Tartar A, Capron A. Protection of mice and nude rats against toxoplasmosis by a multiple antigenic peptide construction derived from Toxoplasma gondii P30 antigen. J Immunol. 1992;149:3636–3641. [PubMed] [Google Scholar]
- 14.Debard N, Buzoni-Gatel D, Bout D. Intranasal immunization with SAG1 protein of Toxoplasma gondii in association with cholera toxin dramatically reduces development of cerebral cysts after oral infection. Infect Immun. 1996;64:2158–2166. doi: 10.1128/iai.64.6.2158-2166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Tommaso A, Saletti G, Pizza M, Rappuoli R, Dougan G, Abrignani S, Douce G, De Magistris M T. Induction of antigen-specific antibodies in vaginal secretions by using a nontoxic mutant of heat-labile enterotoxin as a mucosal adjuvant. Infect Immun. 1996;64:974–979. doi: 10.1128/iai.64.3.974-979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Douce G, Giannelli V, Pizza M, Lewis D, Everest P, Rappuoli R, Dougan G. Genetically detoxified mutants of heat-labile toxin from Escherichia coli are able to act as oral adjuvants. Infect Immun. 1999;67:4400–4406. doi: 10.1128/iai.67.9.4400-4406.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douce G, Giuliani M M, Giannelli V, Pizza M G, Rappuoli R, Dougan G. Mucosal immunogenicity of genetically detoxified derivatives of heat labile toxin from Escherichia coli. Vaccine. 1998;16:1065–1073. doi: 10.1016/s0264-410x(98)80100-x. [DOI] [PubMed] [Google Scholar]
- 18.Douce G, Turcotte C, Cropley I, Roberts M, Pizza M, Domenghini M, Rappuoli R, Dougan G. Mutants of Escherichia coli heat-labile toxin lacking ADP-ribosyltransferase activity act as nontoxic, mucosal adjuvants. Proc Natl Acad Sci USA. 1995;92:1644–1648. doi: 10.1073/pnas.92.5.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehrhardt R O, Strober W, Harriman G R. Effect of transforming growth factor (TGF)-beta 1 on IgA isotype expression. TGF-beta 1 induces a small increase in sIgA+ B cells regardless of the method of B cell activation. J Immunol. 1992;148:3830–3836. [PubMed] [Google Scholar]
- 20.Elson C O, Ealding W. Cholera toxin feeding did not induce oral tolerance in mice and abrogated oral tolerance to an unrelated protein antigen. J Immunol. 1984;133:2892–2897. [PubMed] [Google Scholar]
- 21.Elson C O, Ealding W. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J Immunol. 1984;132:2736–2741. [PubMed] [Google Scholar]
- 22.Frenkel J K. Toxoplasmosis. Pediatr Clin North Am. 1985;32:917–932. doi: 10.1016/s0031-3955(16)34862-3. [DOI] [PubMed] [Google Scholar]
- 23.Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol. 1992;149:175–180. [PubMed] [Google Scholar]
- 24.Gazzinelli R T, Hakim F T, Hieny S, Shearer G M, Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991;146:286–292. [PubMed] [Google Scholar]
- 25.Giuliani M M, Del Giudice G, Giannelli V, Dougan G, Douce G, Rappuoli R, Pizza M. Mucosal adjuvanticity and immunogenicity of LTR72, a novel mutant of Escherichia coli heat-labile enterotoxin with partial knockout of ADP-ribosyltransferase activity. J Exp Med. 1998;187:1123–1132. doi: 10.1084/jem.187.7.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hakim F T, Gazzinelli R T, Denkers E, Hieny S, Shearer G M, Sher A. CD8+ T cells from mice vaccinated against Toxoplasma gondii are cytotoxic for parasite-infected or antigen-pulsed host cells. J Immunol. 1991;147:2310–2316. [PubMed] [Google Scholar]
- 27.Hirabayashi Y, Kurata H, Funato H, Nagamine T, Aizawa C, Tamura S, Shimada K, Kurata T. Comparison of intranasal inoculation of influenza HA vaccine combined with cholera toxin B subunit with oral or parenteral vaccination. Vaccine. 1990;8:243–248. doi: 10.1016/0264-410x(90)90053-o. [DOI] [PubMed] [Google Scholar]
- 28.Holmgren J, Lonnroth I, Svennerholm L. Tissue receptor for cholera exotoxin: postulated structure from studies with GM1 ganglioside and related glycolipids. Infect Immun. 1973;8:208–214. doi: 10.1128/iai.8.2.208-214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson R J, Fujihashi K, Xu-Amano J, Kiyono H, Elson C O, McGhee J R. Optimizing oral vaccines: induction of systemic and mucosal B-cell and antibody responses to tetanus toxoid by use of cholera toxin as an adjuvant. Infect Immun. 1993;61:4272–4279. doi: 10.1128/iai.61.10.4272-4279.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jakobsen H, Schulz D, Pizza M, Rappuoli R, Jonsdottir I. Intranasal immunization with pneumococcal polysaccharide conjugate vaccines with nontoxic mutants of Escherichia coli heat-labile enterotoxins as adjuvants protects mice against invasive pneumococcal infections. Infect Immun. 1999;67:5892–5897. doi: 10.1128/iai.67.11.5892-5897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson L L, VanderVegt F P, Havell E A. Gamma interferon-dependent temporary resistance to acute Toxoplasma gondii infection independent of CD4+ or CD8+ lymphocytes. Infect Immun. 1993;61:5174–5180. doi: 10.1128/iai.61.12.5174-5180.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang H, Remington J S, Suzuki Y. Decreased resistance of B cell-deficient mice to infection with Toxoplasma gondii despite unimpaired expression of IFN-gamma, TNF-alpha, and inducible nitric oxide synthase. J Immunol. 2000;164:2629–2634. doi: 10.4049/jimmunol.164.5.2629. [DOI] [PubMed] [Google Scholar]
- 33.Khan I A, Eckel M E, Pfefferkorn E R, Kasper L H. Production of gamma interferon by cultured human lymphocytes stimulated with a purified membrane protein (P30) from Toxoplasma gondii. J Infect Dis. 1988;157:979–984. doi: 10.1093/infdis/157.5.979. [DOI] [PubMed] [Google Scholar]
- 34.Khan I A, Ely K H, Kasper L H. Antigen-specific CD8+ T cell clone protects against acute Toxoplasma gondii infection in mice. J Immunol. 1994;152:1856–1860. [PubMed] [Google Scholar]
- 35.Khan I A, Ely K H, Kasper L H. A purified parasite antigen (p30) mediates CD8+ T cell immunity against fatal Toxoplasma gondii infection in mice. J Immunol. 1991;147:3501–3506. [PubMed] [Google Scholar]
- 36.Kiyono H, Bienenstock J, McGhee J R, Ernst P B. The mucosal immune system: features of inductive and effector sites to consider in mucosal immunization and vaccine development. Reg Immunol. 1992;4:54–62. [PubMed] [Google Scholar]
- 37.Kuper C F, Koornstra P J, Hameleers D M, Biewenga J, Spit B J, Duijvestijn A M, van Breda Vriesman P J, Sminia T. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992;13:219–224. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- 38.Lepage A C, Buzoni-Gatel D, Bout D T, Kasper L H. Gut-derived intraepithelial lymphocytes induce long term immunity against Toxoplasma gondii. J Immunol. 1998;161:4902–4908. [PubMed] [Google Scholar]
- 39.Lycke N, Holmgren J. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology. 1986;59:301–308. [PMC free article] [PubMed] [Google Scholar]
- 40.Lycke N, Tsuji T, Holmgren J. The adjuvant effect of Vibrio cholerae and Escherichia coli heat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur J Immunol. 1992;22:2277–2281. doi: 10.1002/eji.1830220915. [DOI] [PubMed] [Google Scholar]
- 41.Marchetti M, Rossi M, Giannelli V, Giuliani M M, Pizza M, Censini S, Covacci A, Massari P, Pagliaccia C, Manetti R, Telford J L, Douce G, Dougan G, Rappuoli R, Ghiara P. Protection against Helicobacter pylori infection in mice by intragastric vaccination with H. pylori antigens is achieved using a non-toxic mutant of E. coli heat-labile enterotoxin (LT) as adjuvant. Vaccine. 1998;16:33–37. doi: 10.1016/s0264-410x(97)00153-9. [DOI] [PubMed] [Google Scholar]
- 42.McGhee J R, Yamamoto M, Kang D W, Eldridge J H, Mestecky J, Moldoveanu Z, Compans R, Kiyono H, Miller C, Marthas M, et al. Isotype of anti-SIV responses in infected rhesus macaques and in animals immunized by mucosal routes. AIDS Res Hum Retrovir. 1992;8:1389. doi: 10.1089/aid.1992.8.1389. [DOI] [PubMed] [Google Scholar]
- 43.McLeod R, Frenkel J K, Estes R G, Mack D G, Eisenhauer P B, Gibori G. Subcutaneous and intestinal vaccination with tachyzoites of Toxoplasma gondii and acquisition of immunity to peroral and congenital toxoplasma challenge. J Immunol. 1988;140:1632–1637. [PubMed] [Google Scholar]
- 44.Mineo J R, Khan I A, Kasper L H. Toxoplasma gondii: a monoclonal antibody that inhibits intracellular replication. Exp Parasitol. 1994;79:351–361. doi: 10.1006/expr.1994.1097. [DOI] [PubMed] [Google Scholar]
- 45.Mineo J R, McLeod R, Mack D, Smith J, Khan I A, Ely K H, Kasper L H. Antibodies to Toxoplasma gondii major surface protein (SAG-1, P30) inhibit infection of host cells and are produced in murine intestine after peroral infection. J Immunol. 1993;150:3951–3964. [PubMed] [Google Scholar]
- 46.Parker S J, Roberts C W, Alexander J. CD8+ T cells are the major lymphocyte subpopulation involved in the protective immune response to Toxoplasma gondii in mice. Clin Exp Immunol. 1991;84:207–212. doi: 10.1111/j.1365-2249.1991.tb08150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Partidos C D, Salani B F, Pizza M, Rappuoli R. Heat-labile enterotoxin of Escherichia coli and its site-directed mutant LTK63 enhance the proliferative and cytotoxic T-cell responses to intranasally co-immunized synthetic peptides. Immunol Lett. 1999;67:209–216. doi: 10.1016/s0165-2478(99)00013-9. [DOI] [PubMed] [Google Scholar]
- 48.Pizza M, Domenighini M, Hol W, Giannelli V, Fontana M R, Giuliani M M, Magagnoli C, Peppoloni S, Manetti R, Rappuoli R. Probing the structure-activity relationship of Escherichia coli LT-A by site-directed mutagenesis. Mol Microbiol. 1994;14:51–60. doi: 10.1111/j.1365-2958.1994.tb01266.x. [DOI] [PubMed] [Google Scholar]
- 49.Porgador A, Staats H F, Faiola B, Gilboa E, Palker T J. Intranasal immunization with CTL epitope peptides from HIV-1 or ovalbumin and the mucosal adjuvant cholera toxin induces peptide-specific CTLs and protection against tumor development in vivo. J Immunol. 1997;158:834–841. [PubMed] [Google Scholar]
- 50.Porgador A, Staats H F, Itoh Y, Kelsall B L. Intranasal immunization with cytotoxic T-lymphocyte epitope peptide and mucosal adjuvant cholera toxin: selective augmentation of peptide-presenting dendritic cells in nasal mucosa-associated lymphoid tissue. Infect Immun. 1998;66:5876–5881. doi: 10.1128/iai.66.12.5876-5881.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reichmann G, Stachelhaus S, Meisel R, Mevelec M N, Dubremetz J F, Dlugonska H, Fischer H G. Detection of a novel 40,000 MW excretory Toxoplasma gondii antigen by murine Th1 clone which induces toxoplasmacidal activity when exposed to infected macrophages. Immunology. 1997;92:284–289. doi: 10.1046/j.1365-2567.1997.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ryan E J, McNeela E, Murphy G A, Stewart H, O'Hagan D, Pizza M, Rappuoli R, Mills K H. Mutants of Escherichia coli heat-labile toxin act as effective mucosal adjuvants for nasal delivery of an acellular pertussis vaccine: differential effects of the nontoxic AB complex and enzyme activity on Th1 and Th2 cells. Infect Immun. 1999;67:6270–6280. doi: 10.1128/iai.67.12.6270-6280.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Snapper C M, Mond J J. Towards a comprehensive view of immunoglobulin class switching. Immunol Today. 1993;14:15–17. doi: 10.1016/0167-5699(93)90318-F. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki Y, Conley F K, Remington J S. Treatment of toxoplasmic encephalitis in mice with recombinant gamma interferon. Infect Immun. 1990;58:3050–3055. doi: 10.1128/iai.58.9.3050-3055.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suzuki Y, Joh K. Effect of the strain of Toxoplasma gondii on the development of toxoplasmic encephalitis in mice treated with antibody to interferon-gamma. Parasitol Res. 1994;80:125–130. doi: 10.1007/BF00933779. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki Y, Orellana M A, Schreiber R D, Remington J S. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 57.Tamura S, Iwasaki T, Thompson A H, Asanuma H, Chen Z, Suzuki Y, Aizawa C, Kurata T. Antibody-forming cells in the nasal-associated lymphoid tissue during primary influenza virus infection. J Gen Virol. 1998;79:291–299. doi: 10.1099/0022-1317-79-2-291. [DOI] [PubMed] [Google Scholar]
- 58.Trolle S, Caudron E, Leo E, Couvreur P, Andremont A, Fattal E. In vivo fate and immune pulmonary response after nasal administration of microspheres loaded with phosphorylcholine-thyroglobulin. Int J Pharm. 1999;183:73–79. doi: 10.1016/s0378-5173(99)00047-2. [DOI] [PubMed] [Google Scholar]
- 59.Velge-Roussel F, Marcelo P, Lepage A C, Buzoni-Gatel D, Bout D T. Intranasal immunization with Toxoplasma gondii SAG1 induces protective cells into both NALT and GALT compartments. Infect Immun. 2000;68:969–972. doi: 10.1128/iai.68.2.969-972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams N A, Hirst T R, Nashar T O. Immune modulation by the cholera-like enterotoxins: from adjuvant to therapeutic. Immunol Today. 1999;20:95–101. doi: 10.1016/s0167-5699(98)01397-8. [DOI] [PubMed] [Google Scholar]
- 61.Wu H Y, Nikolova E B, Beagley K W, Eldridge J H, Russell M W. Development of antibody-secreting cells and antigen-specific T cells in cervical lymph nodes after intranasal immunization. Infect Immun. 1997;65:227–235. doi: 10.1128/iai.65.1.227-235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu H Y, Russell M W. Induction of mucosal and systemic immune responses by intranasal immunization using recombinant cholera toxin B subunit as an adjuvant. Vaccine. 1998;16:286–292. doi: 10.1016/s0264-410x(97)00168-0. [DOI] [PubMed] [Google Scholar]
- 63.Wu H Y, Russell M W. Induction of mucosal immunity by intranasal application of a streptococcal surface protein antigen with the cholera toxin B subunit. Infect Immun. 1993;61:314–322. doi: 10.1128/iai.61.1.314-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu H Y, Russell M W. Nasal lymphoid tissue, intranasal immunization, and compartmentalization of the common mucosal immune system. Immunol Res. 1997;16:187–201. doi: 10.1007/BF02786362. [DOI] [PubMed] [Google Scholar]